-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Dengue Virus Infection of Requires a Putative Cysteine Rich Venom Protein
Dengue virus (DENV) is responsible for serious human disease worldwide and the World Health Organization estimates that over 2 billion people are at risk for disease. There are no vaccines or specific antiviral medications currently available for DENV infection. DENV is transmitted to humans by infected mosquitoes during feeding and probing. By examining the effects of virus infection on gene expression, and interactions between virus and vector, we may be able to find new targets for prevention and treatment. Here we look at a mosquito protein, CRVP379, whose gene expression was highly increased during DENV infection in mosquitoes. We show a requirement for CRVP379 during DENV infection in the mosquito and a correlation between the levels of CRVP379 and levels of infection. Our results indicate that the protein may be acting with a putative DENV receptor in the mosquito, prohibitin protein. These data also suggest that blocking CRVP379 function may be used to block DENV infection in the mosquito.
Published in the journal: . PLoS Pathog 11(10): e32767. doi:10.1371/journal.ppat.1005202
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005202Summary
Dengue virus (DENV) is responsible for serious human disease worldwide and the World Health Organization estimates that over 2 billion people are at risk for disease. There are no vaccines or specific antiviral medications currently available for DENV infection. DENV is transmitted to humans by infected mosquitoes during feeding and probing. By examining the effects of virus infection on gene expression, and interactions between virus and vector, we may be able to find new targets for prevention and treatment. Here we look at a mosquito protein, CRVP379, whose gene expression was highly increased during DENV infection in mosquitoes. We show a requirement for CRVP379 during DENV infection in the mosquito and a correlation between the levels of CRVP379 and levels of infection. Our results indicate that the protein may be acting with a putative DENV receptor in the mosquito, prohibitin protein. These data also suggest that blocking CRVP379 function may be used to block DENV infection in the mosquito.
Introduction
Dengue virus (DENV) is the most important arbovirus in tropical areas leading to substantial pediatric morbidity and mortality worldwide [1–6]. DENV is transmitted to humans via the bite of an infected mosquito of the Aedes spp. Infection with DENV in humans can result in dengue fever (DF), dengue shock symptom (DSS) and dengue hemorrhagic fever (DHF), the latter two that can lead to severe disease and death. There are no specific antivirals or approved vaccines for use in DENV treatment or prevention [4,7–9]. Current dengue control methods rely mostly on activities to reduce vector population [10,11]. The increase in number of cases despite vector control indicates that these strategies are not as effective as expected, and that new tools need be developed to alleviate disease burden in endemic areas [12–14].
During the last five decades, much effort has been invested in the development of vaccines against DENV [15–23]. One of the obstacles in dengue vaccine development is the potential risk of severe disease mediated by the presence of sub-neutralizing antibodies against virus particles. These antibodies can predispose an individual to severe disease through a phenomenon called antibody-dependent enhancement (ADE), where the virus can infect cells via FcR in mononuclear cells [8,9,24–28]. Traditional vaccine approaches have included live attenuated viruses, recombinant subunits, virus-like particles and plasmid or viral vectors. There are live attenuated and chimeric DENV vaccines that have gone into clinical trials but none have proven to provide complete and lasting protection against all four DENV serotypes [21,29].
An attractive complement to traditional vaccines is to induce an immune response in the vertebrate host (infected or non-infected) that will block virus infection of the mosquito vectors. This would successfully interrupt transmission by inducing antibody responses against non-viral antigens [30]. These type of vaccines are called transmission-blocking vaccines (TBV), since they aim to interfere with pathogen development within the vector, thereby blocking transmission to human hosts [31]. The majority of TBVs designed to inhibit malaria infection are based on the mammalian immune response to pathogen proteins [31]. Another category of TBVs in development are based on arthropod molecules able to reduce pathogen infection in vector tissues [32]. For arboviruses, vector molecules able to interact directly with the pathogen (i.e. ligands/receptors) are highly suitable candidates for blocking transmission [33,34].
The main global transmission vector for DENV is Ae. aegypti. Extensive research has shown that DENV infection of Ae. aegypti induces many varied changes in gene expression [35–44]. Our hypothesis is that genes upregulated during DENV infection are required for virus survival or are related with defense against infection [37]. Consequently, a better understanding of the role of mosquito proteins regulated by DENV infection will reveal important insights into DENV biology and transmission as well as be helpful to the design of an effective TBV against DENV. For example, antibodies directed against mosquito molecules involved in steps of the pathogen life cycle are promising candidates for TBV. In addition, a recent study demonstrated that antibodies against a mosquito C-type lectin, mosGCTL1, effectively interrupts the infection of Ae. aegypti mosquitoes with DENV [34]. Other proteins which genes are unregulated upon infection also show promising capacity of interrupting infection since they are considered important for the microorganism survival. One of these proteins is the tick histamine release factor (tHRF) from Ixodes scapularis upregulated during Borrelia burgdorferi infection. Previous work showed that expression of tHRF is associated with the tick blood feeding and that the silencing its gene by RNA interference or antibodies not only effectively impairs tick feeding but subsecuently decreases B. burgdorferi burden [45].
Using comprehensive microarray analysis to identify key alterations in the Ae. aegypti transcriptome during flavivirus infection, we previously identified 203 mosquito genes that were up - and 202 genes that were down-regulated during infection [35]. Comparative analysis revealed that at least 15 of these genes had differential expression during infection with DENV, Yellow fever (YFV) and West Nile virus (WNV) [35]. One of these conserved, up-regulated genes was a putative cysteine-rich venom protein (AAEL000379), which we named CRVP379. Cysteine-rich venom proteins (CRVPs) are expressed in multiple mosquito tissues including the salivary glands [37,46,47]. Examples of mosquito CRVPs include an An. stephensi peptide annotated as salivary-secreted serine protease inhibitor [48] and a putative cysteine-rich protease inhibitor found in the sialotranscriptome of adult female Culex quinquefasciatus [49]. The specific role of these proteins in mosquitoes remains unknown [46,47].
Here, we describe a requirement for CRVP379 during DENV infection in mosquito cells and live mosquitoes, including a direct correlation between the amount of CRVP379 expressed and the level of DENV infection. We demonstrate the importance of an interaction between CRVP379 and prohibitin, a putative DENV receptor protein in mosquitoes. We also assess the tissue-specific expression of CRVP379 during DENV infection. Finally, we use both RNAi and specific antibody to demonstrate that blocking CRVP379 results in inhibition of DENV infection in Ae. aegypti. These results further our understanding of DENV pathogenesis in the mosquito vector and highlight a potential target protein for the creation of a DENV TBV to break the host-vector transmission cycle.
Results
Silencing virally up-regulated genes alters DENV infection in mosquito cells
We previously used microarray analysis to identify a number of Ae. aegypti genes that were significantly up-regulated during infection with DENV and other selected flaviviruses [35]. These genes are likely required for flaviviral infection of Ae. aegypti or are part of the mosquito immune response to viral infection. To elucidate the role of these genes and their corresponding proteins, we reduced gene expression through RNAi knockdown and analyzed the effect on viral infection. We designed siRNA against 100 genes that were significantly up-regulated during DENV infection of Ae. aegypti (S1 Fig). The siRNA was used to silence these genes in an Ae. aegypti cell line, Aag2, and the resulting effects on DENV infection were examined. Cells were infected with DENV 72h after siRNA transfection and analyzed for infection using qRT-PCR 24h post-infection. We found that gene silencing both increased and decreased DENV infection, as expected (S2 Fig). The silencing of 9 genes caused cytotoxicity beyond our ability to accurately measure infection levels. Silencing approximately 55 individual genes decreased DENV infection of the cells to below 60% of control infection (Fig 1A), which is greater than 40% inhibition of infection. A number of these genes encode hypothetical proteins for which the function is not known. Several of our target genes do have putative known functions, including a cytochrome P450 (AAEL009762), histone H3 (AAEL003685) and a cysteine-rich venom protein (AAEL000379).
Fig. 1. Silencing select virally-up-regulated genes reduces DENV infection in mosquito cells. 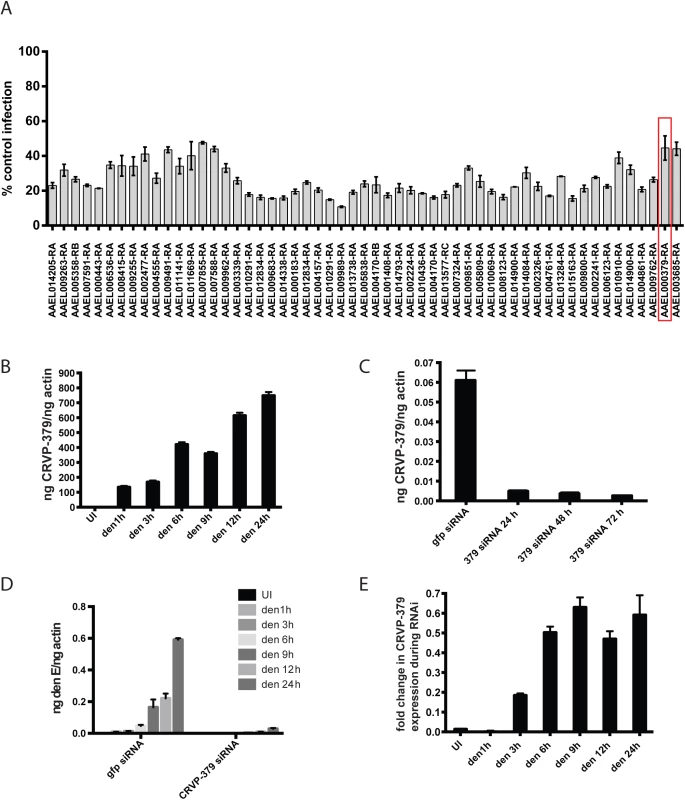
A. The mosquito genes listed in S1 Fig were knocked down in Aag2 cells using RNAi and the effects on DENV infection were analyzed. The genes that reduced infection below 60% of control are shown. Aag2 cells were infected with DENV (MOI of 1.0) 72 h post-knockdown and analyzed for infection by qRT-PCR 24h post-infection. Data is displayed as percent control infection (using scrambled siRNA). Both DENV infection and qRT-PCR analysis were done in triplicate, data is pooled and error bars indicate standard deviation. B. DENV infection increases CRVP379 in Aag2 cells over time. Aag2 cells were infected with DENV (MOI of 1.0) and infection was measured using qRT-PCR analysis at the timepoints indicated. P<0.01. C. Expression of CRVP379 during RNAi knockdown. CRVP379 siRNA was transfected into Aag2 cells and gene expression was analyzed by qRT-PCR. Samples were taken at 24, 48 and 72h post-knockdown. Expression after transfection of GFP control siRNA is also indicated. D. Reduction of CRVP379 reduces DENV infection over time. Either siRNA against CRVP379 or GFP was transfected into Aag2 cells and cells were infected with DENV (MOI of 1.0) at 72 h post-knockdown. Cells were analyzed for infection by qRT-PCR at the timepoints indicated. E. DENV infection increases CRVP379 expression during siRNA knockdown. Either siRNA against CRVP379 or GFP was transfected into Aag2 cells and cells were infected with DENV at 72 h post-knockdown. Gene expression was analyzed by qRT-PCR at the timepoints indicated. Data is expressed as the fold change in CRVP379 expression in cells with GFP siRNA versus cells with CRVP379 siRNA during DENV infection. B-E. Data is pooled from 6 separate experiments, error bars indicate standard deviation. The cysteine-rich venom protein (CRVP), which we will call CRVP379, was a particularly interesting target. CRVP proteins are known to contain a trypsin inhibitor-like (TIL) domain, which indicates that the CRVP379 protein could be a serine protease inhibitor. Both serine proteases and their inhibitors are known to be involved in DENV infection and pathogenesis in the mosquito vector as well as in mammals [50–55]. The expression levels of 19 mosquito CRVP genes were examined during flavivirus infection with the previous microarray analysis. CRVP379 was the only CRVP significantly up-regulated in Ae. aegypti during infection with any of the 3 prototypic flavivirus infections, including DENV, West Nile virus (WNV) and Yellow Fever virus (YFV), at all timepoints tested (Table 1) [35]. In addition, the 19 CRVP genes that we looked at in our microarray actually have very low sequence identify at the amino acid level (S3 Fig). This indicates that they may not have identical or even similar functions, though they are grouped in a protein family due to the presence of multiple cysteines and a TIL domain. Therefore, we decided to assess the role of CRVP379 in DENV infection of the mosquito vector in greater detail.
Tab. 1. Expression of CRVPs in Ae. aegypti during flavivirus infection. 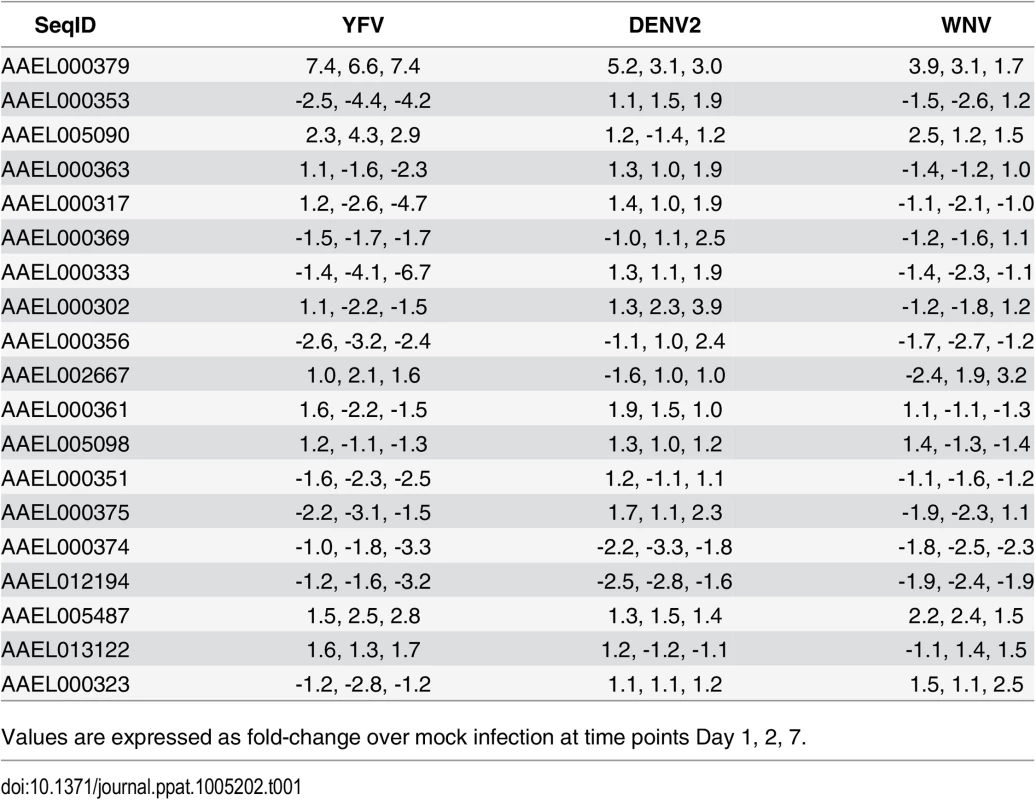
Values are expressed as fold-change over mock infection at time points Day 1, 2, 7. DENV infection requires CRVP379 in mosquito cells
Looking at gene expression over time in DENV-infected Aag2 cells, we found that CRVP379 expression increased more than 800-fold when compared to mock-infected cells (Fig 1B, P<0.01). Since the gene was upregulated in mosquito cells during DENV infection, we wanted to examine the phenotype during DENV infection with loss of function experiments. We used RNA interference (RNAi) with siRNA to reduce CRVP379 gene expression. To confirm gene knockdown, levels of CRVP379 were measured after siRNA transfection over time (Fig 1C), and the expression levels remained below 10% at 72h post-transfection. To determine how the reduction of CRVP379 altered DENV infection, we examined infection levels in Aag2 cells at various timepoints from 1 to 24h post-infection during siRNA knockdown. Silencing CRVP379 reduced DENV infection at all timepoints measured, as compared to infection in control cells transfected with siRNA against GFP (Fig 1D). Interestingly, we noticed that levels of CRVP379 were slightly elevated during DENV infection even during siRNA knockdown, when compared to uninfected cells. This can be seen by looking at the fold change in CRVP379 expression in GFP siRNA-transfected cells as compared to CRVP379 siRNA-transfected cells during DENV infection over time (Fig 1E). Together, these results indicated that the silencing effects of RNAi on CRVP379 are slightly overcome by the gene upregulation during DENV infection, but that infection levels still remained quite low when compared to cells with no CRVP379 silencing.
Endogenous CRVP379 is sufficient for optimal DENV infection
Since we found that the presence of CRVP379 is required for DENV infection, we wanted to test whether increasing CRVP379 levels would enhance infection levels. To do this, we cloned the CRVP379 coding region into the insect expression vector pAc5.1/V5-His (Life Technologies, CA), resulting in pAcCRVP379 vector. This expression vector was transfected into an Ae. aegypti mosquito cell line, Aag2, and the cells were subsequently infected with DENV. A vector expressing GFP was transfected as a control into a separate group of DENV-infected cells. Transfection levels as measured by GFP transfection are over 50%, which will give meaningful results when looking at gene expression and effects on DENV infection (S4 Fig). At 48h post-infection, levels of CRVP379 expression were measured by qRT-PCR. The expression levels of CRVP379 were over 1000 times higher in the cells that were transfected with the CRVP379 plasmid (Fig 2A). We next infected the transfected cells with DENV at 48h post-transfection. At 24 hpi, RNA was isolated and qRT-PCR done to measure DENV infection in the cells. We found that the overexpression of CRVP379 did not increase DENV infection levels in the cells (Fig 2B). This indicated that the endogenous levels of CRVP379 protein are sufficient and that the virus has likely evolved to require only those amounts for optimum infection.
Fig. 2. DENV infection optimally enhances CRVP379 expression. 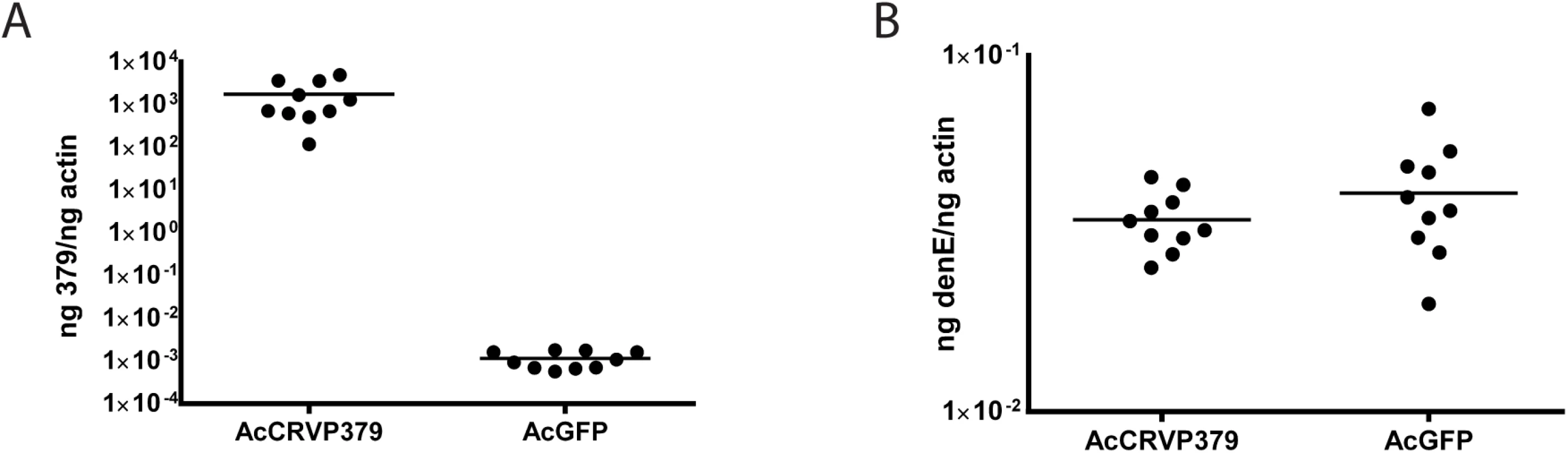
Aag2 cells were transfected with an insect expression vector encoding CRVP379 (AcCRVP379) or GFP (AcGFP) and A. CRVP379 expression was measured by qRT-PCR at 48 h post-transfection. B. Cells were infected with DENV (MOI of 1.0) at 48 h post-transfection and infection levels were measure by qRT-PCR at 24 hpi. Acquisition of DENV requires CRVP379 in live mosquitoes
Since we found that CRVP379 was required for DENV infection of mosquito cells, we decided to next look at the requirements for infection in live Ae. aegypti. To do this, we designed dsRNA against the CRVP379 coding region and inoculated mosquitoes via intra-thoracic injection. At days 2, 4 and 8 post-injection, we dissected out midgut tissues and measured levels of CRVP379 expression by qRT-PCR analysis. Although knockdown was not achieved in all tissues tested, a near-complete reduction of CRVP379 expression (over 95%) in midguts was seen 70% of the time by day 8 (Fig 3-all panels). We next examined the effects of silencing CRVP379 on DENV acquisition in the mosquito midgut. Mosquitoes were again injected with dsRNA against CRVP379 or a control dsRNA against GFP protein. At day 4 post-injection, mosquitoes were infected with DENV by blood feeding using a hemotek apparatus. At day 4 post-infection, midgut tissues were dissected out and analyzed for both CRVP379 expression and DENV infection by qRT-PCR. Since not all midgut tissues had reduction in CRVP379 expression, we analyzed each midgut individually in order to examine the effects on DENV infection in midguts that did have reduced CRVP379. The levels of CRVP379 in the selected midguts are shown in Fig 3A. In the midguts that had reduced CRVP379 expression, DENV infection was almost completely inhibited, as compared to infection in control mosquito midguts (Fig 3B, P<0.01). We also analyzed the data after adding back in the midguts that did not have sufficient gene knock down and looked at levels of DENV infection. Fig 3C shows the levels of CRVP379 in these midguts. Interestingly, in midgut tissues where CRVP379 was not knocked down, DENV infection was comparable to levels in the GFP dsRNA-injected mosquitoes (Fig 3D-squares). Plotting the data points as level of DENV versus level of CRVP379, there is a correlation between expression of CRVP379 in the mosquito midgut and level of DENV infection in that same midgut (Fig 3E, r = 0.6442, P<0.0001). This indicates that CRVP379 levels are directly related to levels of DENV infection in the mosquito midgut. Finally, we repeated the RNAi experiment and allowed the DENV infection to disseminate for 7 days. At day 7 post-infection, whole mosquitoes were homogenized and analyzed for both CRVP379 expression and DENV infection by qRT-PCR (Fig 3F). The mosquitoes that received the dsRNA against CRVP379 had a significant reduction in DENV infection levels as compared to the control mosquitoes. This indicates that the reduction of CRVP379 blocks DENV infection in the whole mosquito.
Fig. 3. Silencing CRVP379 inhibits DENV acquisition in live mosquitoes. 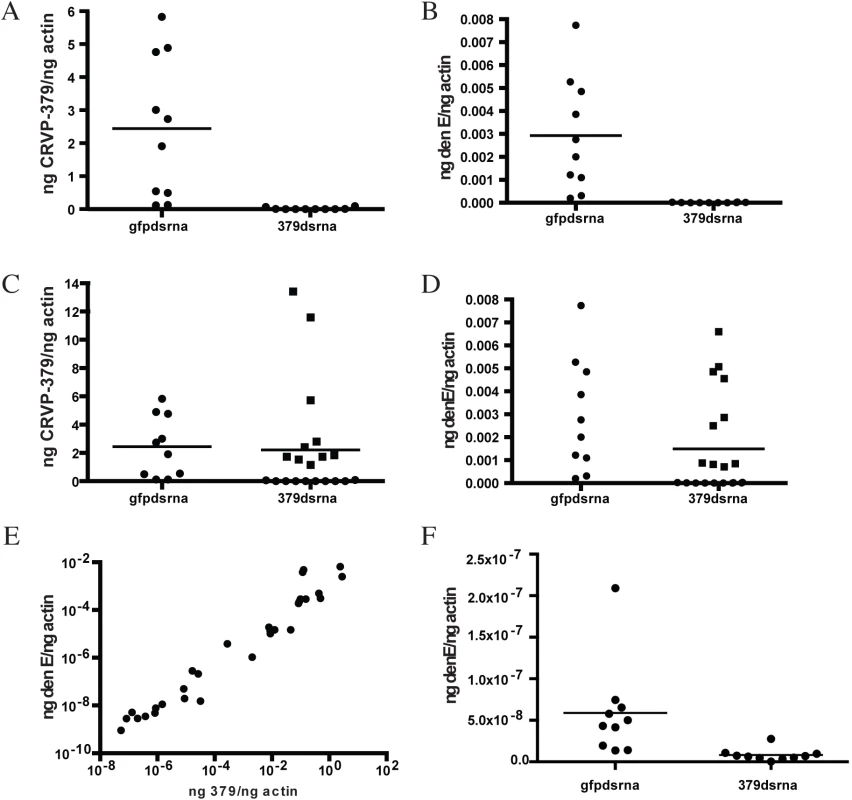
A-E. Mosquitoes were intra-thoracically injected with either dsRNA against the coding region of CRVP379 or dsRNA against GFP as control. At 4 dpmi, mosquitoes were infected with DENV through blood feeding. At 4 dpi, midgut tissues were dissected and individually analyzed for gene expression with qRT-PCR analysis. Each data point represents one mosquito midgut. A. Levels of CRVP379 in select midguts where RNAi was successful, as compared to levels in control mosquitoes. P<0.01. B. Levels of DENV infection in select midguts where RNAi was successful, as compared to levels in control mosquitoes. P<0.01. Infection rates in midguts where CRVP379 RNAi was successful ranged from to .000000765–.0000315 ng DENV E/ng actin. C. Levels of CRVP379 in midguts where RNAi was both successful and unsuccessful, as compared to levels in control mosquitoes. D. Levels of DENV infection in midguts where RNAi was both successful and unsuccessful, as compared to levels in control mosquitoes. C-D. Squares represent midguts where RNAi did not knock down CRVP379 successfully, cirlces represent midguts where RNAi did knock down CRVP379 successfully. E. Levels of DENV infection correspond to levels of CRVP379 expression. Both midguts where RNAi did and did not knock down CRVP379 were analyzed for both CRVP379 expression and DENV infection by qRT-PCR. Data is plotted as ngs of DENV E versus levels of CRVP379, normalized to mosquito actin. Data correlated with Pearson, r = 0.6442, P<0.0001 F. Silencing CRVP379 reduces DENV infection in whole mosquitoes. Mosquitoes were intra-thoracically injected with either dsRNA against the coding region of CRVP379 or dsRNA against GFP as control. At 4 dpmi, mosquitoes were infected with DENV through blood feeding. At 7 dpi, homogenized whole mosquitoes were individually analyzed for gene expression with qRT-PCR analysis. During DENV infection, CRVP379 interacts with mosquito prohibitin, a putative DENV receptor
After establishing that DENV requires CRVP379 in both mosquito cells and live Ae. aegypti, we next wanted to investigate the mechanistic role that CRVP379 plays during infection. To do this, we used the tandem affinity purification (TAP) assay to identify putative mosquito proteins that bind CRVP379 during DENV infection. We cloned the coding region of CRVP379 into the NTAP vector (Stratagene, CA), which fuses the gene to purification tags, and transfected this plasmid into Aag2 mosquito cells. Cells were infected with DENV 24h post-transfection and lysed 24h post-infection. The cell lysate was processed and CRVP379 was purified using the expressed tags, along with interacting mosquito proteins. The resulting solution was sent for LC/MS-MS analysis to determine which proteins were pulled out of the mosquito cell lysate by CRVP379 during DENV infection. A separate set of cells was transfected with the NTAP vector expressing GFP as control. Table 2 lists the mosquito proteins that putatively bound CRVP379 and were not identified during control experiments. One of the proteins identified, prohibitin, was previously characterized as binding to DENV in Aedes A7 cells [56] and has also been suggested as a putative DENV receptor in mosquito cells [57]. Since we found that prohibitin binds CRVP379 during DENV infection using the TAP assay, this may indicate that the proteins act together to facilitate DENV entry into mosquito cells.
Tab. 2. CRVP379 tandem affinity purification assay results. 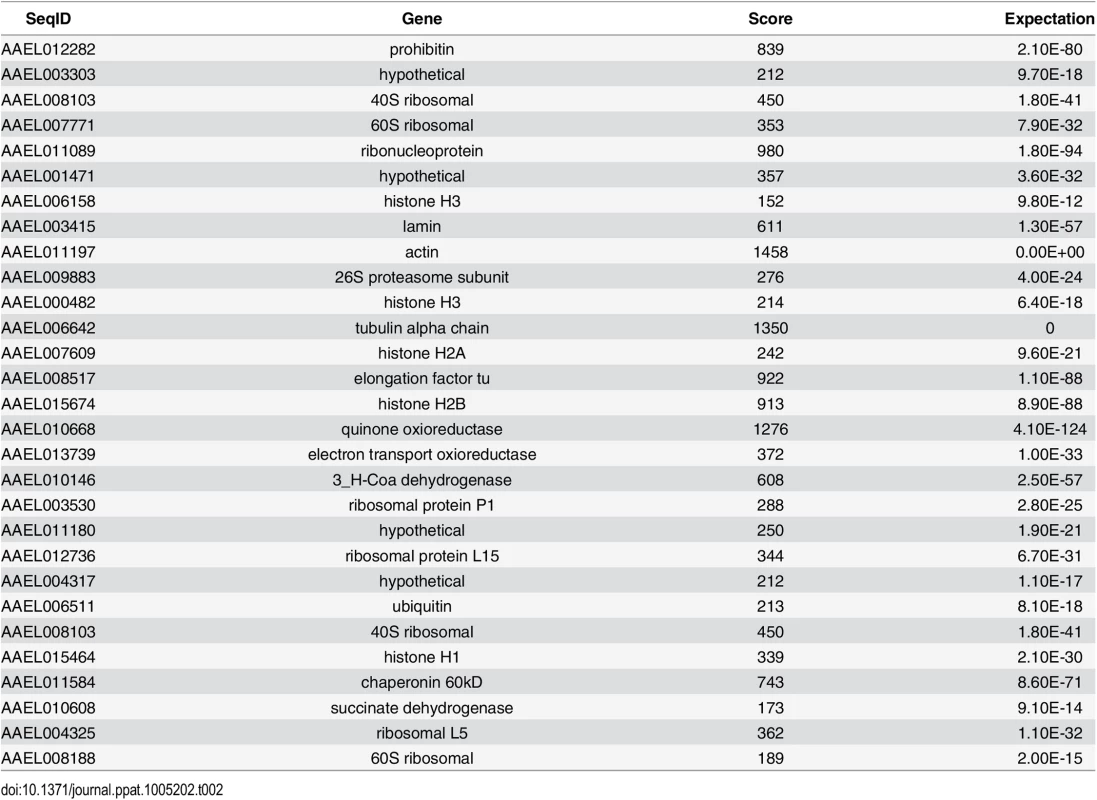
In establishing prohibitin as a putative receptor in mosquito cells, Kuadkitkan et al used siRNA against the mosquito prohibitin gene to inhibit protein production and saw a significant decrease in DENV infection in mosquito cells [57]. To confirm that prohibitin is required for DENV infection in mosquitoes, we designed dsRNA against the mosquito prohibitin gene and examined the impact of silencing prohibitin on DENV infection. Ae. aegypti were intra-thoracically inoculated with the dsRNA and at 4 days post micro-injection (dpmi), mosquitoes were infected with DENV via blood feeding. At 4 days post-infection (dpi), mosquito midguts (MG) were dissected and analyzed for infection by qRT-PCR analysis. Our results show that DENV infections levels were greatly inhibited by prohibitin silencing as compared to the control mosquitoes (Fig 4A), confirming a requirement for prohibitin in DENV mosquito infection. We next performed co-immunoprecipitation to confirm the protein interaction between CRVP379 and prohibitin. Aag2 cells were transfected with an expression vector coding for His-tagged CRVP379 protein and/or infected with DENV. Cells were then lysed and antibody against the His tag was used to pull down His-CRVP379 from the cell lysate. Western blot analysis identified prohibitin protein in the immunoprecipitate (Fig 4B), demonstrating that His-tagged CRVP379 bound prohibitin during DENV infection in the mosquito cells. We then used immunoflourescent imaging to visualize the putative prohibitin-CRVP379 protein interaction. Aag2 cells were transfected with the His-tagged CRVP379 expression construct and infected with DENV 48 hours post-transfection. At 24 hours post-infection, cells were fixed and stained with antibodies against the His tag and prohibitin protein. Fig 4C shows that the two proteins were highly colocalized during DENV infection. We next wondered whether prohibitin overexpression could rescue the mosquito cells that were resistant to DENV infection due to reduced CRVP379 expression. To investigate this, we transfected CRVP379 siRNA into Aag2 cells and then overexpressed mosquito prohitibin before infecting the cells with DENV. We found that the overexpression of prohibitin did not significantly increase DENV infection in cells with reduced CRVP379, though there was a slight enhancement (S5 Fig). This indicates that, though the proteins may act together to facilitate DENV infection in mosquito cells, prohibitin cannot replace the function of CRVP379 protein. Interestingly, the overexpression of prohibition in control Aag2 cells (with siRNA against GFP) did increase DENV infection (S5 Fig).
Fig. 4. CRVP379 interacts with mosquito prohibitin during DENV infection. 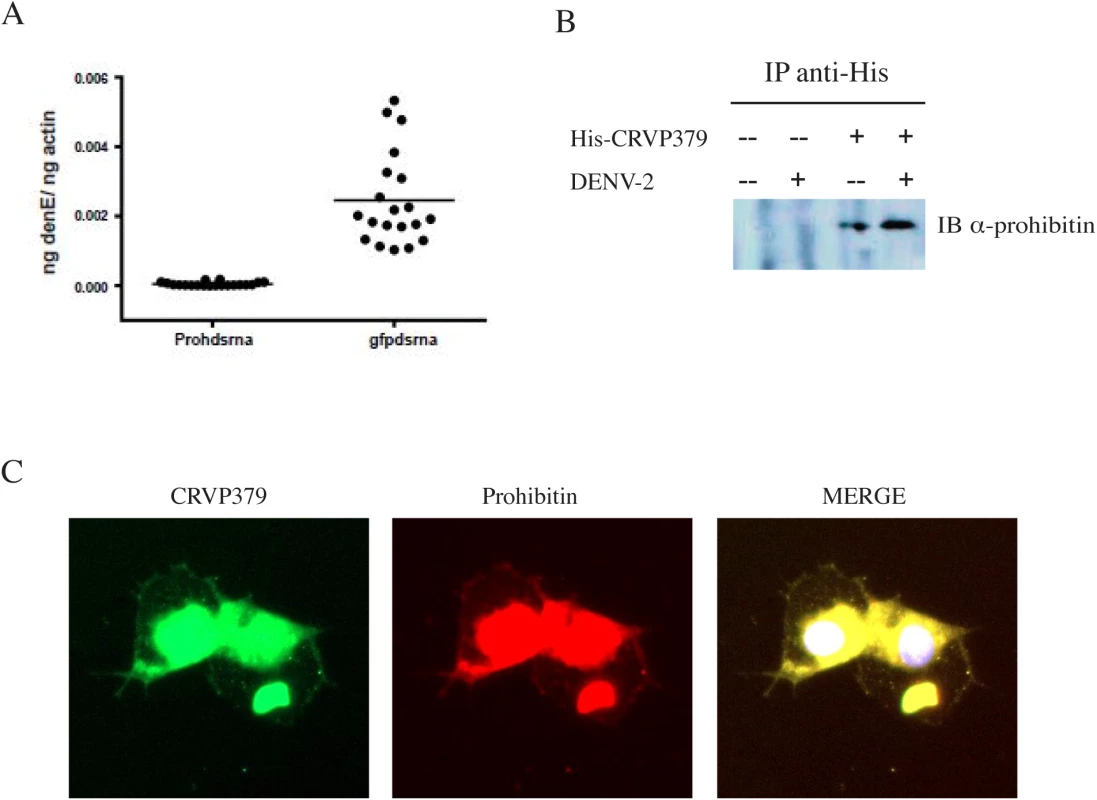
A. Prohibitin is required for DENV infection of Aedes aegypti. Mosquitoes were intra-thoracically injected with either dsRNA against the coding region of prohibitin (Prohdsrna) or dsRNA against GFP (gfpdsrna) as control. At 4 dpmi, mosquitoes were infected with DENV through blood feeding. At 4 dpi, midgut tissues were dissected and individually analyzed for gene expression with qRT-PCR analysis. Each data point represents one mosquito midgut. P<0.01. B. Aag2 cells were transfected with His-tagged CRVP379 and/or infected with DENV. At 48h post-infection, antibodies against the His tag were used to pull the His-tagged CRVP379 protein out of cell lysates. The precipitated solution was run on SDS-PAGE gel and Western blot analysis was performed using antibody against prohibitin. C. CRVP379 and prohibitin co-localize in mosquito cells during DENV infection. Aag2 cells were transfected with His-tagged CRVP379 and infected with DENV 48h post-transfection. Cells were fixed in 4% paraformaldehyde 24h post-infection and stained with antibodies against the His-tag (green) and against prohibitin (red). DAPI was used to localize the nucleus. Representative images are shown. Antisera against CRVP379 inhibits DENV infection in mosquitoes
Since we found that inhibiting CRVP379 gene expression using RNA interference reduced DENV in Ae. aegypti, we next wanted to try and inhibit protein function with antibody and examine the effects on DENV infection. To do this, recombinant protein consisting of residues 21–128 of CRVP379 was expressed in E. coli along with a GST tag for purification. To generate polyclonal antiserum, rabbits were immunized with the recombinant CRVP379 (rCRVP379). We used the antisera in Western blot analysis to confirm that antibodies would bind the recombinant protein (Fig 5A). We next ensured that the polyclonal antisera contained antibodies that recognized endogenous CRVP379 protein in the mosquito. To test this, we used the antisera to stain Aag2 cells and found that there was a strong reaction between the CRVP379 antisera and protein in the cells (Fig 5B). We then used the antisera to probe mosquito midgut tissue for endogenous protein. Fig 5C demonstrates that the CRVP379 antisera, but not the pre-immune control sera, recognized protein in the dissected mosquito MG tissue. We also used the antisera to probe MG tissue with reduced CRVP379 expression due to RNAi (S6 Fig). To confirm that the antisera did recognize the CRVP379 protein in the mosquito, we ectopically expressed a His-tagged CRVP379 protein in Aag2 cells and used antibody against the His tag along with the CRVP379 antisera. Staining with the CRVP379 antisera colocalized with the anti-His staining, indicating that the antisera recognized the CRVP379 protein (Fig 5D). We then looked at tissue-specific expression of CRVP379 and found that levels are increased in both the salivary glands (SG) and midguts (MG) of DENV-infected mosquitoes, as compared to uninfected mosquito tissues, at all timepoints examined (Fig 5E). We also used ELISA analysis with the CRVP379 antisera using both Aag2 cell lysate, Ae. aegypti salivary gland tissue and Ae. aegypti saliva to confirm that the antisera bound endogenous CRVP379 protein (Fig 5F). Next, we tested the effects of the antisera on DENV infection in Aag2 cells. We used two experimental protocols; in one, the antisera was incubated with the cells for 2h at RT and then infected with DENV (pretreatment group), in the second, antisera and DENV were incubated for 1h at RT and then added to cells (simultaneous group). We used pre-immune sera for a control and also did the same experiment in the Huh-7 human liver cell line as an additional control, as antisera against a mosquito protein should not have an effect on DENV infection in mammalian cells. Infection was analyzed by qRT-PCR analysis at 24 hpi. We found that the antisera against CRVP379 inhibited DENV infection in Aag2 cells at dilutions up to 1/100 (Fig 6A). We also found that incubating the antisera with the cells before DENV infection resulted in a slightly larger reduction in infection levels (Fig 6A). We did not see any reduction in DENV infection in either experimental group using Huh-7 cells (Fig 6B). We then tested the effects of the antisera against CRVP379 on DENV infection in Ae. aegypti. Mosquitoes were fed a mixture of human blood, DENV and either CRVP379 antisera or preimmune sera at 1/10 and 1/100 dilutions. We also used control antisera against two unrelated, GST-tagged mosquito proteins MMP (AAEL003012) and PC (AAEL011045). On 3 dpi, mosquito MG were dissected and qRT-PCR was done to analyze DENV infection. The antisera against CRVP379 significantly reduced the DENV infection in the mosquitoes at both 1/10 and 1/100 dilution as compared to mosquitoes which fed on the preimmune sera (Fig 6C). The antisera against the control GST-tagged proteins did not reduce DENV acquisition in the mosquito MG tested (Fig 6D).
Fig. 5. Antibodies against CRVP379 recognize native protein in Ae. aegypti. 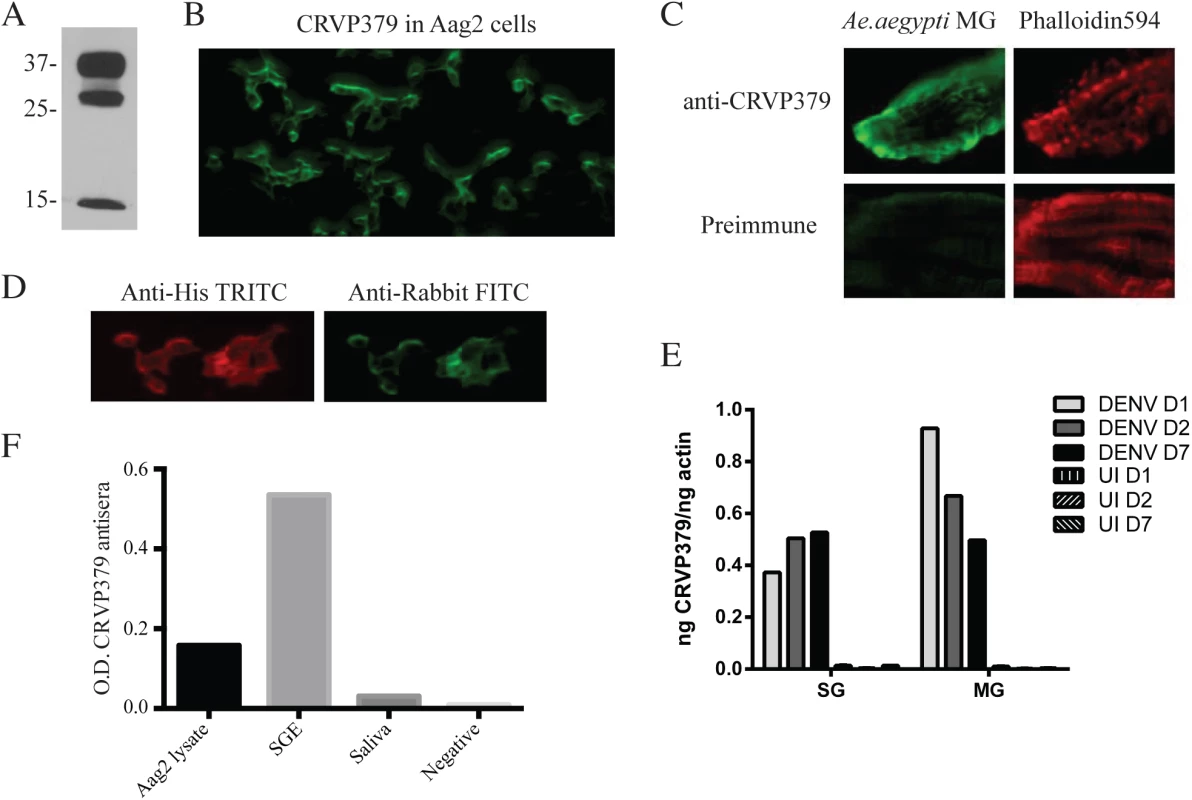
A. CRVP379 antisera binds recombinant protein. An SDS-PAGE gel was run using rCRVP379 and Western blot analysis was done using the CRVP379 antisera. B. Antibodies were made against mosquito CRVP379 and used to bind endogenous protein in the Aag2 cell line. Aag2 cells were infected with DENV and cell fixed 24 hpi for staining. A representative image is shown at 20X. C. CRVP379 antibodies bind endogenous CRVP379 in mosquito midguts. Ae. aegypti MG were dissected and fixed in 4% paraformaldehyde before staining. Both preimmune sera and sera from CRVP-379 injected mice were used for staining (green). Phalloidin594 was used to highlight midgut structure (red). D. Antisera against CRVP379 recognizes the CRVP379 protein in mosquito cells. Aag2 cells were transfected with an expression construct encoding His-tagged CRVP379 protein. Cells were fixed 48h post-transfection and stained with both CRVP379 antisera and antibody against the His tag. Secondary antibodies were used as indicated. E. CRVP379 is increased in both MG and SG of DENV-infected mosquitoes. Mosquitoes were either infected with DENV or mock solution and organs dissected at 1, 2 and 7 dpi. CRVP379 gene expression was analyzed by qRT-PCR analysis and is shown as ng CRVP379 normalized to actin. DENV was used at 105 PFU/mL for infection in mosquitoes. F. CRVP379 antisera binds mosquito cells and tissues. ELISA analysis was done with Aag2 cell lysate, Aedes aegypti salivary gland extract (SGE) and Aedes aegypti extracted saliva. O.D. values at 450nm are presented on the graph. Fig. 6. CRVP379 antisera blocks infection of DENV in mosquitoes. 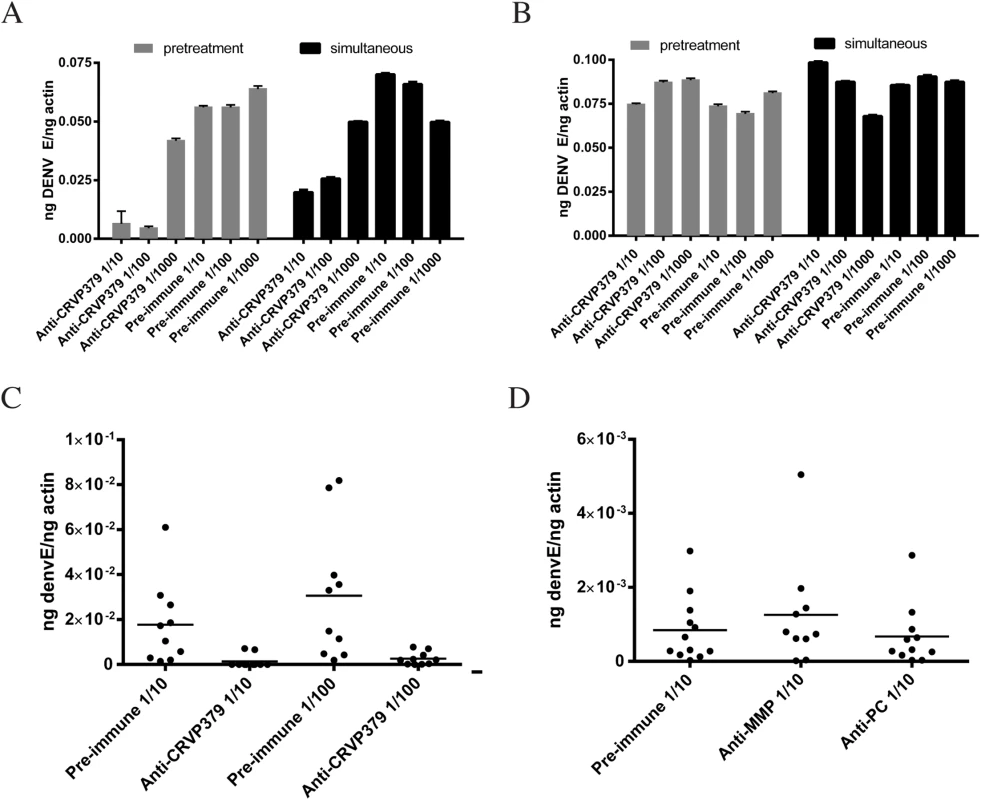
A/B. CRVP379 antisera inhibits DENV infection in mosquito cells. Aag2 (A) or Huh7 (B) cells were either incubated with antisera against CRVP379 or control preimmune sera for 2h at RT and then infected with DENV (pretreatment group) or antisera against CRVP379 or control preimmune sera was incubated with DENV for 1h at RT and then added to cells (simultaneous group). Infection was analyzed by qRT-PCR at 24 hpi. C/D. CRVP379 antisera inhibits DENV infection in mosquitoes. Ae. aegypti were fed a mixture of blood, DENV and either CRVP379 antisera or preimmune sera as indicated. Antisera were used at dilutions of 1/10 or 1/100 (C). Infection rates in midguts with CRVP379 antisera ranged from to .00008785–.07833 ng DENV E/ng actin. A separate group of mosquitoes was fed antisera against control mosquito proteins, MMP and PC (D). At 3 dpi, mosquito MG were dissected and qRT-PCR analysis done to quantify DENV infection. Results are shown as ng DENV E normalized to mosquito actin. Each data point represents one MG. Discussion
Flaviviruses are known to modify gene expression in their mosquito transmission vectors during infection. Our previous results showed that infection of Ae. aegypti with either DENV, WNV or YFV, modifies expression levels of at least 405 genes [35]. The study of mosquito genes modified during flavivirus infection may lead to the identification of key vector antiviral mechanisms as well as key factors for interruption of the viral life cycle. One of the genes that we identified as being significantly upregulated during DENV infection was the CRVP379 gene. Cysteine-rich venom protein (CRVPs) are members of a large family of cysteine-rich secretory proteins (CRISPs), predominantly found in mammalian males and reptile venom [58]. CRISPs contain characteristic cysteine rich C-terminal domains thought to act as ion channel regulators [58] and are also characterized by their role in proteolytic and defense mechanisms [59]. CRISPs and CRVPs have been described in a broad spectrum of insects and higher vertebrates [59–62].
Recently, Bonizzoni et al found several CRVP genes to have differentially regulated expression during DENV infection of Ae. aegypti, including CRVP379, which was shown to be upregulated on day 1 and day 14 in the mosquito MG during infection [42]. Here, we found that CRVP379 was the only CRVP significantly upregulated in mosquitoes after infection with DENV. Furthermore, knockdown of CRVP379 protein both in vivo and in vitro was able to reduce viral infection, and we found a significant positive association between the level of CRVP and DENV infection. This indicates that CRVP379 is specifically required for DENV infection of Ae. aegypti, at least in our current studies. The genetic variations between both DENV and mosquito strains likely contributes to differences among various studies, and consistent reporting of these discrepancies warrants further research into these variations along with additional transcriptomic analysis of the impact of DENV infection on mosquitoes.
Recent work has shown that another mosquito venom protein, a member of the antigen-5 family (Ag5), is upregulated in the salivary glands of Ae. aegypti during Chikungunya virus infection [63]. This protein is present in the saliva of several insects and is associated with platelet aggregation inhibition in blood sucking arthropods [46,64]. More investigation is needed to determine whether mosquito CRVP proteins and the Ag5 proteins are related or have similar functions during virus infection. Another group of CRVP proteins, the Cysteine-rich secretory proteins, Antigen 5, and Pathogenesis-related 1 proteins (CAP) superfamily, has been descried in the sialotranscriptome of Ae. Aegypti as well as in Culex [49,65,66]. Previous reports indicate that these genes could be preferentially expressed in the salivary glands of female mosquitos, perhaps suggesting an important role during blood feeding. Our study showed that up-regulation of CRVP379 occurs in both midgut and salivary glands of mosquitoes, and expression in salivary glands increased from day 1 to day 7 during DENV infection. This suggests that CRVP379 may be found in the saliva of DENV-infected mosquitoes. We have previously found that Ae. aegypti and certain Anopheles saliva have the ability to induce an antibody response in humans that can be correlated with the level of exposure to mosquito bites and disease status [67,68]. Several insect proteins from the CAP superfamily have also been reported to stimulate mammalian immune responses [69]. Given that CRVP379 has no homologous proteins in humans, we suspect that it will be a potent immunogen if used as a TBV.
Many CRVP proteins contain trypsin inhibitor-like (TIL) domains found in members of the serine protease inhibitor family [70] and functional sequence analysis confirmed that CRVP379 does contain a TIL domain from amino acids 23–79. Serine proteases and their inhibitors are known to have very specific interactions, and they play central roles in many cellular processes [71–73]. In addition, both serine proteases and their inhibitors have been shown to have an impact on DENV infectivity in both mammals and mosquitoes [50,55,74]. As such, we sought to identify the serine protease that CRVP379 potentially bound by using the TAP assay to investigate which mosquito proteins CRVP379 bound during DENV infection. We discovered that CRVP379 interacted with a number of mosquito genes during infection, including histones, ubiquitin and prohibitin.
Previous research has suggested that prohibitin may be a receptor for DENV in mosquitoes, as expression levels of this protein correlate with the susceptibility of DENV infection in both Ae. aegypti and Ae. albopictus cell lines [57]. Prohibitin is a protein pervasive expressed and highly conserved in eukaryotic cells [75] and has been previously described as an inhibitor of cell proliferation [76]. Prohibitin is found in several cellular compartments including nucleus, mitochondria and cytoplasm [57]. Furthermore, a recent report shows that Cry4B, one of the major insecticidal toxins produced by Bacillus thuringiensis israelensis, co-precipitates and co-localizes with prohibitin in Ae. aegypti larva midgut, and this interaction is able to reduce DENV infection under physiological conditions [77]. These findings suggest that the inhibition of proteins that interact with viral receptors may potentially block mosquito infection. Additionally, several proteins have been reported to bind prohibitin conferring resistance to bacteria phagocytosis [78] as well as cell surface expressed binding protein [79]. In DENV infection, it has been suggested that prohibitin interacts directly at the cell surface with the viral envelope protein. Our current work shows that CRVP379 is able to interact with several other mosquito proteins including prohibitin, suggesting that CRVP379 may be involved in virus cell entry along with other putative roles.
In spite of decades of effort, there are currently no approved DENV vaccines available. A recent study with a live-attentuated tetravalent DENV vaccine developed by Sanofi Pasteur has demonstrated partial protection against DENV [22] and shows promising results, though efforts continue to develop vaccines that will confer full protection. A vector-based vaccine would nicely complement these efforts at traditional vaccine development and could contribute as an additional strategy to combat the increasing global spread of DENV. The development of vaccines targeting either a pathogen or vector protein to prevent transmission to human hosts is considered essential to the eradication of many emerging tropical diseases, including malaria. Transmission-blocking vaccines (TBVs) are currently being developed and have been shown to be successful at preventing malaria infection of Anopheles mosquitoes (12–15). One of these, a TBV developed against the Plasmodium protein Pfs25, was able to prevent the transmission of malaria from infected mice to naïve mosquitoes (12,13). Another group found that vaccinating mice with the mosquito protein serpin-2 prevented the transmission of Plasmodium berghei to a naïve group of mosquitoes (16). In addition, an arthropod-specific TBV based on the outer surface protein A (OspA) of Borrelia burgdorferi, the causative agent of Lyme disease, has been shown to protect mice from spirochete infection (17). Proteins of the sand fly have also been used successfully as TBVs to prevent the transmission of Leishmania (18).
Dengue virus is transmitted to humans in saliva during mosquito probing and blood feeding. During this process, mosquitoes take in host factors contained in the blood including host antibodies, complement proteins and immune cells that remain active for several hours post-feeding [80,81]. Previous studies have shown that the presence of antibodies against mosquito proteins are able to disrupt mosquito infection and transmission of pathogens [82,83]. This type of TBV has several advantages over a TBV targeting pathogen antigens, including the ability to target a conserved molecule among vector genera and that the targeted genes may also affect mosquito survival in nature. Recently, Cheng et al demonstrated that antibodies against mosquito C-type lectin proteins were able to block DENV infection in Aedes mosquitoes [34]. Their data strongly suggested that a TBV targeting DENV acquisition in mosquitoes is possible and may be close at hand. Our results inhibiting DENV in Aedes using antisera targeting CRVP379 protein showed a similar reduction in viral infection and suggests that CRVP379 may also be a viable target for the development of a TBV. Importantly, CRVP379 has no homolog in humans and extremely low sequence identity to any protein in the human proteome. This means there should not be any off-target immune reaction targeting self if CRVP379 were to be used to stimulate antibody production in humans.
In the present study, we identified a mosquito protein that was required for DENV infection in mosquito cells, CRVP379. We showed a correlation between this protein and DENV infection levels in vitro and in vivo. The interaction between CRVP379 and prohitibin, a putative viral receptor in mosquitoes, may be the mechanism behind the requirement for infection. In addition, antiserum against CRVP379 protein was able to significantly inhibit DENV acquisition in Ae. aegypti. Given that the CRVP379 protein was also upregulated in the mosquito during infection with WNV and YFV, it stands to reason that a TBV developed against this protein may act to block acquisition and transmission of multiple, globally important flaviviruses. We have also been able to detect an antibody response against CRVP379 in human serum samples, indicating that the protein is immunogenic. We are currently designing studies to correlate levels of these antibodies with putative protection against dengue virus infection and disease severity upon infection.
Materials and Methods
Cell culture and virus growth
The Aag2 Ae. aegypti cell line (ATCC, VA) was used for transfection and infection studies. The cells were grown at 30°C and 5% CO2 in DMEM supplemented with 10% heat-inactivated fetal bovine serum (Gemini, CA), 1% penicillin-streptomycin and 1% tryptose phosphate broth (Sigma, MO). Dengue virus stock was grown in C6/36 Ae. albopictus cell line using the same media. The dengue strain used was DENV-2 New Guinea C. Cells were infected at an m.o.i. of 1.0, virus was allowed to propagate for 6–8 days, supernatant was removed, spun down and virus stock was stored at -80°C until use.
RNAi
S1 Fig provides a complete list of the siRNA molecules used in our in vitro knock-down studies. Dharmafect4 reagent (Dharmacon,) was used to transfect the siRNA into the Aag2 cells according to manufacturer’s instrucitons. For gene knockdown in live mosquitoes, dsRNA was produced from 500bp coding regions of either Ae. aegypti CRVP379, Ae. aegypti prohibitin or GFP. Briefly, PCR was used to produce a DNA template with T7 overhangs that was then used with the Ambion Megascript kit to produce the dsRNA molecules. The dsRNA was transfected into mosquitoes as described.
Mosquito infections
The Rockefeller strain of Ae. aegypti were infected by blood-feeding, using 400 μL of DENV-infected C6/36 cell supernatant added to 1 mL serum-inactivated human donor blood (The Blood Center, New Orleans, LA). Mosquitoes were fed for 20 minutes at room temperature using a hemotek feeder and maintained in groups of 10 at 30°C, 80% humidity. Mosquitoes were supplied sucrose water as a source of dietary sugar. At the conclusion of experiments, mosquitoes were briefly washed in 70% ethanol and then rinsed in sterile PBS. Organs were dissected in sterile PBS and transferred to Eppendorf tubes separately. Mosquito organs were stored in PBS with protease inhibitors for protein assays and homogenized in RLT buffer (Qiagen, CA) for gene expression assays.
qRT-PCR analysis
RNA was isolated from infected Ae. aegypti mosquitoes on Days 1, 2, 7 and purified using RNeasy kit (Qiagen, CA) according to manufacturer’s instructions. The quantitative RT-PCR (qRT-PCR) analysis was done using the QuantiFast kit according to manufacturer’s instructions (Qiagen, CA). Oligos for the qRT-PCR reactions were:
DENV envelope: F: 5’-CATTCCAAGTGAGAATCTCTTTGTCA-3’
R: 5’-CAGATCTCTGATGAATAACCAACG-3’; Ae. aegypti Actin: F: 5’-GAACACCCAGTCCTGCTGACA-3’, R: 5’-TGCGTCATCTTCTCACGGTTAG-3’
Mosquito transfection
DNA plasmids were injected according to our published whole-body transfection method [84]. Briefly, Cellfectin II (Invitrogen, CA) was mixed with S2 Schneider’s medium at a 1 : 1 ratio and then keep at RT for 10 min. Plasmid DNA was combined with this mixture and incubated at RT for 30 minutes before thoracic microinjection into Aedes aegypti. Mosquitoes were injected with 500ng plasmid/300 nL solution. The dsRNA was injected as previously described and as indicated in the figure legends.
Immunofluorescence analysis
Aag2 Ae. aegypti cells were infected with DENV at an MOI of 0.1. At 24 hours post-infection, infected cells and control cells were fixed in 4% paraformaldehyde for 20 min at RT, washed with PBS(-) and then stained for infection using antibodies against CRVP379 (L2 Diagnostics, CT), DENV envelope gene (Millipore, MA) and/or prohibitin (Abcam, MA). The antibodies were diluted in 1% BSA at 1/250 and cells were incubated for 20 minutes at RT. Any secondary antibodies used were standard (anti-mouse or anti-rabbit TRITC and FITC, DAPI and phalloidin), and were diluted according to manufacturer’s instructions. Infection was visualized using fluorescent microscopy, equipment and specifics can be found in figure legends.
TAP expression plasmid constructs
All plasmids were prepared using Qiagen miniprep kits (Valencia, CA) after standard transformation into DH5α competent bacterial cells. The tagged virus protein nTAP expression plasmids were made by cloning the coding regions for each viral protein into the N-terminal TAP plasmid (Stratagene, CA).
Western blots
Solutions were run on a 4–12% SDS-PAGE gel for 1.5 h at 15 milliamps per gel (unless figure legend indicates otherwise). The proteins were then transferred to PVDF membrane. The membrane was blocked with 5% milk in 1% TBST for 1h at RT and then incubated with the appropriate primary antibody overnight at 4°C. The membrane was washed and then incubated with the appropriate horseradish peroxidase secondary antibody for 1h at RT. The protein blots were incubated with ECL substrates (Amersham, NJ) for 5 min at RT and then detected on Kodak film. Antisera production is described below and prohibitin antibody was purchased from Santa Cruz.
Transfection of plasmids (cells)
The expression plasmids were made from pAc5.1/V5-His A vector (Invitrogen, CA) and cloning was done using PCR along with gene-specific primers as previously described [85]. We used the Qiagen mini-prep kit to isolate DNA from bacterial cultures after transforming DH5-alpha cells. Plasmids were transfected into cells using Effectene (Qiagen, CA) according to manufacturer’s instructions. Briefly, for a 10 cm2 plate, 10 μg of DNA was mixed with 500 μL buffer EC and 32 μL enhancer was added. This was allowed to incubate for 5 min on the benchtop. Then, 30 μL Effectene reagent was added and the solution vortexed briefly. After 10 min incubation, the solution was added to the cells.
TAP assay
The TAP assay was used to identify mosquito cell proteins that interacted with CRVP379 protein. All steps were done at 4°C to maintain the protein interactions. The cell or tissue lysates were applied to streptavidin resin, incubated at 4°C for 2 h, washed, and bound proteins eluted off. A second purification step was done with calmodulin resin and the proteins were boiled off into PBS(-). The eluted proteins were analyzed at the Yale University W.M. Keck Foundation core facility. The eluate was subjected to trypsin digestion followed by LC/MS-MS (liquid chromatography and mass spectometry) for peptide sequencing and identification using the recently completed Aedes aegypti mosquito genome [47]. Putative mosquito proteins were identified via amino acid sequence identity to both known mosquito proteins and their mammalian counterparts using the BLAST software on the NCBI website. Mosquito proteins found to bind the tags alone as well as proteins found to bind tagged green fluorescent protein were eliminated as putative interacting partners.
Protein and antisera production
A recombinant protein consisting of residues 21–128 of CRVP379 was synthetically cloned into the pGEX-6p-1 expression vector (GE Life Sciences) into the BamH1 and Xho1 sites. The recombinant plasmid was transformed into Rosetta DE3 pLys2 E. coli cells. GST-CRVP379 protein was purifed from the bacteria cells as inclusion bodies by passing the E coli cells through a cell disruptor at 20 psi of pressure. Inclusion bodies were used to immunize rabbits to generate polycolonal antisera (CoCalico Biologicals, Reamstown, PA). Prior to immunization, rabbits were bled to obtain pre-immune control sera.
ELISA analysis
Serum samples were coated onto a 96-well ELISA plate (Thermo Fisher Sci, MA) and incubated overnight at 4°C. The plate was blocked with 1% BSA in PBS(-) and incubated with recombinant CRVP for an hour at RT. The proteins were washed off, antibodies were added for 30 min at RT, washed off and secondary-HRP was added for 30 min at RT, washed off and TMB substrate was added for 20 min at RT. Stop solution was added and the O.D. of the wells was read at 450 nm.
Supporting Information
Zdroje
1. Kao CL, King CC, Chao DY, Wu HL, Chang GJ (2005) Laboratory diagnosis of dengue virus infection: current and future perspectives in clinical diagnosis and public health. J Microbiol Immunol Infect 38 : 5–16. 15692621
2. Overgaard HJ, Alexander N, Matiz MI, Jaramillo JF, Olano VA, et al. (2012) Diarrhea and dengue control in rural primary schools in Colombia: study protocol for a randomized controlled trial. Trials 13 : 182. doi: 10.1186/1745-6215-13-182 23034084
3. Gubler DJ (2002) Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol 10 : 100–103. 11827812
4. Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, et al. (2010) Dengue: a continuing global threat. Nat Rev Microbiol 8: S7–16. doi: 10.1038/nrmicro2460 21079655
5. Hales S, de Wet N, Maindonald J, Woodward A (2002) Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet 360 : 830–834. 12243917
6. Kyle JL, Harris E (2008) Global spread and persistence of dengue. Annu Rev Microbiol 62 : 71–92. doi: 10.1146/annurev.micro.62.081307.163005 18429680
7. Gubler DJ (1998) Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11 : 480–496. 9665979
8. Gubler DJ, Clark GG (1995) Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerg Infect Dis 1 : 55–57. 8903160
9. Guzman MG, Alvarez M, Halstead SB (2013) Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Arch Virol 158 : 1445–1459. doi: 10.1007/s00705-013-1645-3 23471635
10. van den Berg H, Velayudhan R, Ebol A, Catbagan BH Jr., Turingan R, et al. (2012) Operational efficiency and sustainability of vector control of malaria and dengue: descriptive case studies from the Philippines. Malar J 11 : 269. doi: 10.1186/1475-2875-11-269 22873707
11. Jansen CC, Beebe NW (2010) The dengue vector Aedes aegypti: what comes next. Microbes Infect 12 : 272–279. doi: 10.1016/j.micinf.2009.12.011 20096802
12. Miller M BS, Henderson DA. Control and Eradication. (2006) Disease Control Priorities in Developing Countries. In: Jamison DT BJ, Measham AR, et al., editors., editor. 2nd edition ed. Washington (DC): World Bank; 2006. Chapter 62.: Washington (DC): World Bank; 2006. Chapter 62.
13. Pinheiro FP, Corber SJ (1997) Global situation of dengue and dengue haemorrhagic fever, and its emergence in the Americas. World Health Stat Q 50 : 161–169. 9477544
14. Wilder-Smith A, Macary P (2014) Dengue: challenges for policy makers and vaccine developers. Curr Infect Dis Rep 16 : 404. doi: 10.1007/s11908-014-0404-2 24781826
15. Scott RM, Nisalak A, Eckels KH, Tingpalapong M, Harrison VR, et al. (1980) Dengue-2 vaccine: viremia and immune responses in rhesus monkeys. Infect Immun 27 : 181–186. 6766903
16. Miller BR, Beaty BJ, Aitken TH, Eckels KH, Russell PK (1982) Dengue-2 vaccine: oral infection, transmission, and lack of evidence for reversion in the mosquito, Aedes aegypti. Am J Trop Med Hyg 31 : 1232–1237. 7149108
17. Kochel TJ, Raviprakash K, Hayes CG, Watts DM, Russell KL, et al. (2000) A dengue virus serotype-1 DNA vaccine induces virus neutralizing antibodies and provides protection from viral challenge in Aotus monkeys. Vaccine 18 : 3166–3173. 10856796
18. Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, et al. (1038) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476 : 454–457. doi: 10.1038/nature10356 21866160
19. Johnson AJ, Roehrig JT (1999) New mouse model for dengue virus vaccine testing. J Virol 73 : 783–786. 9847388
20. Guirakhoo F, Weltzin R, Chambers TJ, Zhang ZX, Soike K, et al. (2000) Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J Virol 74 : 5477–5485. 10823852
21. Fink K, Shi PY (2014) Live attenuated vaccine: the first clinically approved dengue vaccine? Expert Rev Vaccines 13 : 185–188. doi: 10.1586/14760584.2014.870888 24350687
22. Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, et al. (2012) Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380 : 1559–1567. doi: 10.1016/S0140-6736(12)61428-7 22975340
23. Guzman MG, Hermida L, Bernardo L, Ramirez R, Guillen G Domain III of the envelope protein as a dengue vaccine target. Expert Rev Vaccines 9 : 137–147.
24. Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS (1989) Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg 40 : 444–451. 2712199
25. Nikin-Beers R, Ciupe SM (2015) The role of antibody in enhancing dengue virus infection. Math Biosci 263 : 83–92. doi: 10.1016/j.mbs.2015.02.004 25707916
26. Flipse J, Wilschut J, Smit JM (2013) Molecular mechanisms involved in antibody-dependent enhancement of dengue virus infection in humans. Traffic 14 : 25–35. doi: 10.1111/tra.12012 22998156
27. Burke DS, Kliks S (2006) Antibody-dependent enhancement in dengue virus infections. J Infect Dis 193 : 601–603; author reply 603–604.
28. Halstead SB (2003) Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60 : 421–467. 14689700
29. Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, et al. (2015) Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 372 : 113–123. doi: 10.1056/NEJMoa1411037 25365753
30. Kay BH, Kemp DH (1994) Vaccines against arthropods. Am J Trop Med Hyg 50 : 87–96. 8024089
31. Carter R (2001) Transmission blocking malaria vaccines. Vaccine 19 : 2309–2314. 11257353
32. Coutinho-Abreu IV, Ramalho-Ortigao M (2010) Transmission blocking vaccines to control insect-borne diseases: a review. Mem Inst Oswaldo Cruz 105 : 1–12. 20209323
33. Wang P, Conrad JT, Shahabuddin M (2001) Localization of midgut-specific protein antigens from Aedes aegypti (Diptera: Culicidae) using monoclonal antibodies. J Med Entomol 38 : 223–230. 11296827
34. Liu Y, Zhang F, Liu J, Xiao X, Zhang S, et al. (2014) Transmission-blocking antibodies against mosquito C-type lectins for dengue prevention. PLoS Pathog 10: e1003931. doi: 10.1371/journal.ppat.1003931 24550728
35. Colpitts TM, Cox J, Vanlandingham DL, Feitosa FM, Cheng G, et al. (2011) Alterations in the Aedes aegypti transcriptome during infection with West Nile, dengue and yellow fever viruses. PLoS Pathog 7: e1002189. doi: 10.1371/journal.ppat.1002189 21909258
36. Chisenhall DM, Londono BL, Christofferson RC, McCracken MK, Mores CN (2014) Effect of dengue-2 virus infection on protein expression in the salivary glands of Aedes aegypti mosquitoes. Am J Trop Med Hyg 90 : 431–437. doi: 10.4269/ajtmh.13-0412 24445208
37. Chisenhall DM, Christofferson RC, McCracken MK, Johnson AM, Londono-Renteria B, et al. (2014) Infection with dengue-2 virus alters proteins in naturally expectorated saliva of Aedes aegypti mosquitoes. Parasit Vectors 7 : 252. doi: 10.1186/1756-3305-7-252 24886023
38. Baron OL, Ursic-Bedoya RJ, Lowenberger CA, Ocampo CB (2010) Differential gene expression from midguts of refractory and susceptible lines of the mosquito, Aedes aegypti, infected with Dengue-2 virus. J Insect Sci 10 : 41. doi: 10.1673/031.010.4101 20572793
39. Behura SK, Gomez-Machorro C, deBruyn B, Lovin DD, Harker BW, et al. (2014) Influence of mosquito genotype on transcriptional response to dengue virus infection. Funct Integr Genomics 14 : 581–589. doi: 10.1007/s10142-014-0376-1 24798794
40. Campbell CL, Harrison T, Hess AM, Ebel GD (2014) MicroRNA levels are modulated in Aedes aegypti after exposure to Dengue-2. Insect Mol Biol 23 : 132–139. doi: 10.1111/imb.12070 24237456
41. Zhang M, Zheng X, Wu Y, Gan M, He A, et al. (2013) Differential proteomics of Aedes albopictus salivary gland, midgut and C6/36 cell induced by dengue virus infection. Virology 444 : 109–118. doi: 10.1016/j.virol.2013.06.001 23816433
42. Bonizzoni M, Dunn WA, Campbell CL, Olson KE, Marinotti O, et al. (2012) Complex modulation of the Aedes aegypti transcriptome in response to dengue virus infection. PLoS One 7: e50512. doi: 10.1371/journal.pone.0050512 23209765
43. Behura SK, Severson DW (2012) Intrinsic features of Aedes aegypti genes affect transcriptional responsiveness of mosquito genes to dengue virus infection. Infect Genet Evol 12 : 1413–1418. doi: 10.1016/j.meegid.2012.04.027 22579482
44. Sim S, Ramirez JL, Dimopoulos G (2012) Dengue virus infection of the Aedes aegypti salivary gland and chemosensory apparatus induces genes that modulate infection and blood-feeding behavior. PLoS Pathog 8: e1002631. doi: 10.1371/journal.ppat.1002631 22479185
45. Dai J, Narasimhan S, Zhang L, Liu L, Wang P, et al. (2010) Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the lyme disease agent. PLoS Pathog 6: e1001205. doi: 10.1371/journal.ppat.1001205 21124826
46. Arca B, Lombardo F, Francischetti IM, Pham VM, Mestres-Simon M, et al. (2007) An insight into the sialome of the adult female mosquito Aedes albopictus. Insect Biochem Mol Biol 37 : 107–127. 17244540
47. Nene V, Wortman JR, Lawson D, Haas B, Kodira C, et al. (2007) Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316 : 1718–1723. 17510324
48. Calvo E, Pham VM, Lombardo F, Arca B, Ribeiro JM (2006) The sialotranscriptome of adult male Anopheles gambiae mosquitoes. Insect Biochem Mol Biol 36 : 570–575. 16835022
49. Ribeiro JM, Charlab R, Pham VM, Garfield M, Valenzuela JG (2004) An insight into the salivary transcriptome and proteome of the adult female mosquito Culex pipiens quinquefasciatus. Insect Biochem Mol Biol 34 : 543–563. 15147756
50. Conway MJ, Watson AM, Colpitts TM, Dragovic SM, Li Z, et al. (2014) Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J Virol 88 : 164–175. doi: 10.1128/JVI.02235-13 24131723
51. Furuta T, Murao LA, Lan NT, Huy NT, Huong VT, et al. (2012) Association of mast cell-derived VEGF and proteases in Dengue shock syndrome. PLoS Negl Trop Dis 6: e1505. doi: 10.1371/journal.pntd.0001505 22363824
52. Bonizzoni M, Britton M, Marinotti O, Dunn WA, Fass J, et al. (2013) Probing functional polymorphisms in the dengue vector, Aedes aegypti. BMC Genomics 14 : 739. doi: 10.1186/1471-2164-14-739 24168143
53. Gulley MM, Zhang X, Michel K (2013) The roles of serpins in mosquito immunology and physiology. J Insect Physiol 59 : 138–147. doi: 10.1016/j.jinsphys.2012.08.015 22960307
54. Xi Z, Ramirez JL, Dimopoulos G (2008) The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog 4: e1000098. doi: 10.1371/journal.ppat.1000098 18604274
55. Brackney DE, Foy BD, Olson KE (2008) The effects of midgut serine proteases on dengue virus type 2 infectivity of Aedes aegypti. Am J Trop Med Hyg 79 : 267–274. 18689635
56. Paingankar MS, Gokhale MD, Deobagkar DN (2010) Dengue-2-virus-interacting polypeptides involved in mosquito cell infection. Arch Virol 155 : 1453–1461. doi: 10.1007/s00705-010-0728-7 20571839
57. Kuadkitkan A, Wikan N, Fongsaran C, Smith DR (2010) Identification and characterization of prohibitin as a receptor protein mediating DENV-2 entry into insect cells. Virology 406 : 149–161. doi: 10.1016/j.virol.2010.07.015 20674955
58. Gibbs GM, O'Bryan MK (2007) Cysteine rich secretory proteins in reproduction and venom. Soc Reprod Fertil Suppl 65 : 261–267. 17644967
59. Udby L, Calafat J, Sorensen OE, Borregaard N, Kjeldsen L (2002) Identification of human cysteine-rich secretory protein 3 (CRISP-3) as a matrix protein in a subset of peroxidase-negative granules of neutrophils and in the granules of eosinophils. J Leukoc Biol 72 : 462–469. 12223513
60. Parkinson NM, Conyers C, Keen J, MacNicoll A, Smith I, et al. (2004) Towards a comprehensive view of the primary structure of venom proteins from the parasitoid wasp Pimpla hypochondriaca. Insect Biochem Mol Biol 34 : 565–571. 15147757
61. Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, et al. (2010) Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science 330 : 86–88. doi: 10.1126/science.1191864 20929810
62. Schambony A, Hefele JA, Gentzel M, Wilm M, Wedlich D (2003) A homologue of cysteine-rich secretory proteins induces premature degradation of vitelline envelopes and hatching of Xenopus laevis embryos. Mech Dev 120 : 937–948. 12963113
63. Tchankouo-Nguetcheu S, Bourguet E, Lenormand P, Rousselle JC, Namane A, et al. (2012) Infection by chikungunya virus modulates the expression of several proteins in Aedes aegypti salivary glands. Parasit Vectors 5 : 264. doi: 10.1186/1756-3305-5-264 23153178
64. Ribeiro JM, Arca B, Lombardo F, Calvo E, Phan VM, et al. (2007) An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genomics 8 : 6. 17204158
65. Gibbs GM, Roelants K, O'Bryan MK (2008) The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins—roles in reproduction, cancer, and immune defense. Endocr Rev 29 : 865–897. doi: 10.1210/er.2008-0032 18824526
66. Ribeiro JM, Arca B, Lombardo F, Calvo E, Phan VM, et al. (2007) An annotated catalogue of salivary gland transcripts in the adult female mosquito, Aedes aegypti. BMC Genomics 8 : 6. 17204158
67. Londono-Renteria B, Cardenas JC, Cardenas LD, Christofferson RC, Chisenhall DM, et al. (2013) Use of anti-Aedes aegypti salivary extract antibody concentration to correlate risk of vector exposure and dengue transmission risk in Colombia. PLoS One 8: e81211. doi: 10.1371/journal.pone.0081211 24312537
68. Londono-Renteria BL, Eisele TP, Keating J, James MA, Wesson DM (2010) Antibody response against Anopheles albimanus (Diptera: Culicidae) salivary protein as a measure of mosquito bite exposure in Haiti. J Med Entomol 47 : 1156–1163. 21175067
69. Ameri M, Wang X, Wilkerson MJ, Kanost MR, Broce AB (2008) An immunoglobulin binding protein (antigen 5) of the stable fly (Diptera: Muscidae) salivary gland stimulates bovine immune responses. J Med Entomol 45 : 94–101. 18283948
70. Suzuki M, Itoh T, Anuruddhe BM, Bandaranayake IK, Shirani Ranasinghe JG, et al. (2010) Molecular diversity in venom proteins of the Russell's viper (Daboia russellii russellii) and the Indian cobra (Naja naja) in Sri Lanka. Biomed Res 31 : 71–81. 20203422
71. Plotnick MI, Mayne L, Schechter NM, Rubin H (1996) Distortion of the active site of chymotrypsin complexed with a serpin. Biochemistry 35 : 7586–7590. 8652540
72. Wei A, Rubin H, Cooperman BS, Christianson DW (1994) Crystal structure of an uncleaved serpin reveals the conformation of an inhibitory reactive loop. Nat Struct Biol 1 : 251–258. 7656054
73. Han J, Zhang H, Min G, Kemler D, Hashimoto C (2000) A novel Drosophila serpin that inhibits serine proteases. FEBS Lett 468 : 194–198. 10692585
74. Rizvi N, Chaturvedi UC, Mathur A (1991) Inhibition of the presentation of dengue virus antigen by macrophages to B cells by serine-protease inhibitors. Int J Exp Pathol 72 : 23–29. 1888663
75. Morrow IC, Parton RG (2005) Flotillins and the PHB domain protein family: rafts, worms and anaesthetics. Traffic 6 : 725–740. 16101677
76. McClung JK, Danner DB, Stewart DA, Smith JR, Schneider EL, et al. (1989) Isolation of a cDNA that hybrid selects antiproliferative mRNA from rat liver. Biochem Biophys Res Commun 164 : 1316–1322. 2480116
77. Kuadkitkan A, Smith DR, Berry C (2012) Investigation of the Cry4B-prohibitin interaction in Aedes aegypti cells. Curr Microbiol 65 : 446–454. doi: 10.1007/s00284-012-0178-4 22767320
78. Sharma A, Qadri A (2004) Vi polysaccharide of Salmonella typhi targets the prohibitin family of molecules in intestinal epithelial cells and suppresses early inflammatory responses. Proc Natl Acad Sci U S A 101 : 17492–17497. 15576509
79. Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W (2004) Reversal of obesity by targeted ablation of adipose tissue. Nat Med 10 : 625–632. 15133506
80. Grotendorst CA, Carter R (1987) Complement effects of the infectivity of Plasmodium gallinaceum to Aedes aegypti mosquitoes. II. Changes in sensitivity to complement-like factors during zygote development. J Parasitol 73 : 980–984. 3116195
81. Margos G, Navarette S, Butcher G, Davies A, Willers C, et al. (2001) Interaction between host complement and mosquito-midgut-stage Plasmodium berghei. Infect Immun 69 : 5064–5071. 11447187
82. Chugh M, Adak T, Sehrawat N, Gakhar SK (2011) Effect of anti-mosquito midgut antibodies on development of malaria parasite, Plasmodium vivax and fecundity in vector mosquito Anopheles culicifacies (Diptera: culicidae). Indian J Exp Biol 49 : 245–253. 21614887
83. Ramasamy MS, Sands M, Kay BH, Fanning ID, Lawrence GW, et al. (1990) Anti-mosquito antibodies reduce the susceptibility of Aedes aegypti to arbovirus infection. Med Vet Entomol 4 : 49–55. 1966777
84. Cheng G, Liu L, Wang P, Zhang Y, Zhao YO, et al. (2011) An in vivo transfection approach elucidates a role for Aedes aegypti thioester-containing proteins in flaviviral infection. PloS one 6: e22786. doi: 10.1371/journal.pone.0022786 21818390
85. Colpitts TM, Cox J, Nguyen A, Feitosa F, Krishnan MN, et al. (2011) Use of a tandem affinity purification assay to detect interactions between West Nile and dengue viral proteins and proteins of the mosquito vector. Virology 417 : 179–187. doi: 10.1016/j.virol.2011.06.002 21700306
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized VirusČlánek Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell ProliferationČlánek Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite InterfaceČlánek Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Expression of Concern: Misregulation of Underlies the Developmental Abnormalities Caused by Three Distinct Viral Silencing Suppressors in Arabidopsis
- Preparing for the Next Epidemic with Basic Virology
- Effectively Communicating the Uncertainties Surrounding Ebola Virus Transmission
- Translating Basic Research into Clinical Applications: Malaria Research at an NIH Lab
- A Gut Odyssey: The Impact of the Microbiota on Spore Formation and Germination
- Papillomavirus E6 Oncoproteins Take Common Structural Approaches to Solve Different Biological Problems
- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Dimensions of Horizontal Gene Transfer in Eukaryotic Microbial Pathogens
- Addressing the Complications of Ebola and Other Viral Hemorrhagic Fever Infections: Using Insights from Bacterial and Fungal Sepsis
- Time for Chocolate: Current Understanding and New Perspectives on Cacao Witches’ Broom Disease Research
- Ganglioside and Non-ganglioside Mediated Host Responses to the Mouse Polyomavirus
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Structure Elucidation of Coxsackievirus A16 in Complex with GPP3 Informs a Systematic Review of Highly Potent Capsid Binders to Enteroviruses
- CD39 Expression Identifies Terminally Exhausted CD8 T Cells
- Abiotic Stresses Antagonize the Rice Defence Pathway through the Tyrosine-Dephosphorylation of OsMPK6
- Dissociation of Tissue Destruction and Bacterial Expansion during Bubonic Plague
- Interferon-γ: The Jekyll and Hyde of Malaria
- CCR2 Inflammatory Dendritic Cells and Translocation of Antigen by Type III Secretion Are Required for the Exceptionally Large CD8 T Cell Response to the Protective YopE Epitope during Infection
- A New Glycan-Dependent CD4-Binding Site Neutralizing Antibody Exerts Pressure on HIV-1
- The Suramin Derivative NF449 Interacts with the 5-fold Vertex of the Enterovirus A71 Capsid to Prevent Virus Attachment to PSGL-1 and Heparan Sulfate
- Trans-generational Immune Priming Protects the Eggs Only against Gram-Positive Bacteria in the Mealworm Beetle
- Peripheral Vγ9Vδ2 T Cells Are a Novel Reservoir of Latent HIV Infection
- Respiratory Syncytial Virus Disease Is Mediated by Age-Variable IL-33
- TRIM21 Promotes cGAS and RIG-I Sensing of Viral Genomes during Infection by Antibody-Opsonized Virus
- Modeling the Effects of Vorinostat Reveals both Transient and Delayed HIV Transcriptional Activation and Minimal Killing of Latently Infected Cells
- Identification of a Novel Lipoprotein Regulator of Spore Germination
- Calcium Regulation of Hemorrhagic Fever Virus Budding: Mechanistic Implications for Host-Oriented Therapeutic Intervention
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
- Comparative Life Cycle Transcriptomics Revises Genome Annotation and Links a Chromosome Duplication with Parasitism of Vertebrates
- The Autophagy Receptor TAX1BP1 and the Molecular Motor Myosin VI Are Required for Clearance of Salmonella Typhimurium by Autophagy
- Carcinogenic Parasite Secretes Growth Factor That Accelerates Wound Healing and Potentially Promotes Neoplasia
- Effector OspB Activates mTORC1 in a Manner That Depends on IQGAP1 and Promotes Cell Proliferation
- Dengue Virus Infection of Requires a Putative Cysteine Rich Venom Protein
- Distinct Viral and Mutational Spectrum of Endemic Burkitt Lymphoma
- Fundamental Roles of the Golgi-Associated Aspartyl Protease, ASP5, at the Host-Parasite Interface
- Phenotypic and Functional Alterations in Circulating Memory CD8 T Cells with Time after Primary Infection
- Systematic Identification of Cyclic-di-GMP Binding Proteins in Reveals a Novel Class of Cyclic-di-GMP-Binding ATPases Associated with Type II Secretion Systems
- Influenza Transmission in the Mother-Infant Dyad Leads to Severe Disease, Mammary Gland Infection, and Pathogenesis by Regulating Host Responses
- Myeloid Cell Arg1 Inhibits Control of Arthritogenic Alphavirus Infection by Suppressing Antiviral T Cells
- The White-Nose Syndrome Transcriptome: Activation of Anti-fungal Host Responses in Wing Tissue of Hibernating Little Brown Myotis
- Influenza Virus Reassortment Is Enhanced by Semi-infectious Particles but Can Be Suppressed by Defective Interfering Particles
- Identification of the Mechanisms Causing Reversion to Virulence in an Attenuated SARS-CoV for the Design of a Genetically Stable Vaccine
- Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells
- The Histone Acetyltransferase Hat1 Regulates Stress Resistance and Virulence via Distinct Chromatin Assembly Pathways
- C-di-GMP Regulates Motile to Sessile Transition by Modulating MshA Pili Biogenesis and Near-Surface Motility Behavior in
- Modulation of the Surface Proteome through Multiple Ubiquitylation Pathways in African Trypanosomes
- Crystal Structure of the Human Cytomegalovirus Glycoprotein B
- Depletion of . GlmU from Infected Murine Lungs Effects the Clearance of the Pathogen
- Immunologic Control of Papillomavirus Type 1
- Requires Host Rab1b for Survival in Macrophages
- Structure Analysis Uncovers a Highly Diverse but Structurally Conserved Effector Family in Phytopathogenic Fungi
- PD-L1 Expression on Retrovirus-Infected Cells Mediates Immune Escape from CD8 T Cell Killing
- Phospho-dependent Regulation of SAMHD1 Oligomerisation Couples Catalysis and Restriction
- IL-4 Induced Innate CD8 T Cells Control Persistent Viral Infection
- Crystal Structures of a Piscine Betanodavirus: Mechanisms of Capsid Assembly and Viral Infection
- BCG Skin Infection Triggers IL-1R-MyD88-Dependent Migration of EpCAM CD11b Skin Dendritic cells to Draining Lymph Node During CD4+ T-Cell Priming
- Antigenic Characterization of the HCMV gH/gL/gO and Pentamer Cell Entry Complexes Reveals Binding Sites for Potently Neutralizing Human Antibodies
- Rescue of a Plant Negative-Strand RNA Virus from Cloned cDNA: Insights into Enveloped Plant Virus Movement and Morphogenesis
- Geminivirus Activates to Accelerate Cytoplasmic DCP2-Mediated mRNA Turnover and Weakens RNA Silencing in
- Disruption of Sphingolipid Biosynthesis Blocks Phagocytosis of
- The Fungal Exopolysaccharide Galactosaminogalactan Mediates Virulence by Enhancing Resistance to Neutrophil Extracellular Traps
- The Timing of Stimulation and IL-2 Signaling Regulate Secondary CD8 T Cell Responses
- Structural and Functional Analysis of Murine Polyomavirus Capsid Proteins Establish the Determinants of Ligand Recognition and Pathogenicity
- The Dual Role of an ESCRT-0 Component HGS in HBV Transcription and Naked Capsid Secretion
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Chronobiomics: The Biological Clock as a New Principle in Host–Microbial Interactions
- Interferon-γ: The Jekyll and Hyde of Malaria
- Crosslinking of a Peritrophic Matrix Protein Protects Gut Epithelia from Bacterial Exotoxins
- Antigen-Specific Th17 Cells Are Primed by Distinct and Complementary Dendritic Cell Subsets in Oropharyngeal Candidiasis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



