-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMurine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
Human gammaherpesviruses are associated with the development of lymphoproliferative diseases and B cell lymphomas, particularly in immunosuppressed hosts. Understanding the molecular mechanisms by which human gammaherpesviruses cause disease is hampered by the lack of convenient small animal models to study them. However, infection of laboratory strains of mice with the rodent virus murine gammaherpesvirus 68 (MHV68) has been useful in gaining insights into how gammaherpesviruses contribute to the genesis and progression of lymphoproliferative lesions. In this report we make the novel observation that MHV68 infection of murine day 15 fetal liver cells results in their immortalization and differentiation into B plasmablasts that can be propagated indefinitely in vitro, and can establish metastasizing lymphomas in mice lacking normal immune competence. The phenotype of the MHV68 immortalized B cell lines is similar to that observed in lymphomas caused by KSHV and resembles the favored phenotype observed during MHV68 infection in vivo. All established cell lines maintained the MHV68 genome, with limited viral gene expression and little or no detectable virus production - although virus reactivation could be induced upon crosslinking surface Ig. Notably, transcription of the genes encoding the MHV68 viral cyclin D homolog (v-cyclin) and the homolog of the KSHV latency-associated nuclear antigen (LANA), both of which are conserved among characterized γ2-herpesviruses, could consistently be detected in the established B cell lines. Furthermore, we show that the v-cyclin and LANA homologs are required for MHV68 immortalization of murine B cells. In contrast the M2 gene, which is unique to MHV68 and plays a role in latency and virus reactivation in vivo, was dispensable for B cell immortalization. This new model of gammaherpesvirus-driven B cell immortalization and differentiation in a small animal model establishes an experimental system for detailed investigation of the role of gammaherpesvirus gene products and host responses in the genesis and progression of gammaherpesvirus-associated lymphomas, and presents a convenient system to evaluate therapeutic modalities.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002220
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002220Summary
Human gammaherpesviruses are associated with the development of lymphoproliferative diseases and B cell lymphomas, particularly in immunosuppressed hosts. Understanding the molecular mechanisms by which human gammaherpesviruses cause disease is hampered by the lack of convenient small animal models to study them. However, infection of laboratory strains of mice with the rodent virus murine gammaherpesvirus 68 (MHV68) has been useful in gaining insights into how gammaherpesviruses contribute to the genesis and progression of lymphoproliferative lesions. In this report we make the novel observation that MHV68 infection of murine day 15 fetal liver cells results in their immortalization and differentiation into B plasmablasts that can be propagated indefinitely in vitro, and can establish metastasizing lymphomas in mice lacking normal immune competence. The phenotype of the MHV68 immortalized B cell lines is similar to that observed in lymphomas caused by KSHV and resembles the favored phenotype observed during MHV68 infection in vivo. All established cell lines maintained the MHV68 genome, with limited viral gene expression and little or no detectable virus production - although virus reactivation could be induced upon crosslinking surface Ig. Notably, transcription of the genes encoding the MHV68 viral cyclin D homolog (v-cyclin) and the homolog of the KSHV latency-associated nuclear antigen (LANA), both of which are conserved among characterized γ2-herpesviruses, could consistently be detected in the established B cell lines. Furthermore, we show that the v-cyclin and LANA homologs are required for MHV68 immortalization of murine B cells. In contrast the M2 gene, which is unique to MHV68 and plays a role in latency and virus reactivation in vivo, was dispensable for B cell immortalization. This new model of gammaherpesvirus-driven B cell immortalization and differentiation in a small animal model establishes an experimental system for detailed investigation of the role of gammaherpesvirus gene products and host responses in the genesis and progression of gammaherpesvirus-associated lymphomas, and presents a convenient system to evaluate therapeutic modalities.
Introduction
The human gammaherpesviruses EBV and KSHV are characterized by their ability to establish latent infections and their close association with a wide variety of malignancies [1]. EBV is the etiologic agent of infectious mononucleosis, and is tightly associated with development of endemic Burkitt's lymphoma, 30–40% of Hodgkin's lymphoma and nearly half of the lymphomas that arise in immunosuppressed patients [2], [3]. KSHV is present in all cases of Kaposi's sarcomas, and is also associated with the development of the rare primary effusion lymphomas (PELs) that occur in some AIDS patients [4], [5]. In addition, KSHV is the etiologic agent of the lymphoproliferative disorder Multicentric Castelman's disease [6]. Both EBV - and KSHV-associated lymphoproliferative diseases and B cell lymphomas mostly occur in immunodeficient individuals, such as arising in HIV infected patients and following organ transplantation. However, because of the narrow tropism of the human gammaherpesviruses, no tractable small animal model is available for dissecting the genesis of EBV - and KSHV-associated lymphomas.
Gammaherpesviruses have been divided into 2 subfamilies – the γ1-herpesviruses (lymphocryptoviruses), of which EBV is a member, and the γ2-herpesviruses (rhadinoviruses), which include KSHV and MHV68. All characterized lymphocryptoviruses share the property of being able to growth transform primary B cells in tissue culture. However, only the T lymphotropic rhadinoviruses (herpesvirus saimiri and herpesvirus ateles) have been shown to transform lymphocyte targets [7], [8]. It has thus been generally assumed that the B lymphotropic rhadinoviruses are non-transforming, and this has significantly impeded studies into the role of KSHV genes involved in the genesis of lymphomas and lymphoproliferative disease. However, in the case of both KSHV and MHV68, the failure to immortalize B cells in culture may be due to their inability to efficiently infect primary B cells in tissue culture [9] and/or the absence of appropriate culture conditions. Here we explore this possibility by targeting virus infection of B cell progenitor populations using fetal liver cells isolated from day 15 embryos cultured in the presence of IL-7 to drive B cell differentiation.
Results
Generation of MHV68 immortalized B cell lines
Fetal liver is the major site of B lymphopoiesis during embryonic development, prior to the initiation of B cell development in the bone marrow [10]. The early progenitor cells from murine fetal liver can differentiate into immature B cells upon co-culture with IL-7 producing stromal cells in vitro [11], [12] or into mature B cells upon transfer into severe combined immunodeficient (SCID) mice [13], [14]. Using a recombinant MHV68 harboring a YFP expression cassette, pilot experiments demonstrated that MHV68 can infect fetal liver cultures derived from day 15 mouse embryos (data not shown). This led to the development of a two-stage culture strategy in which MHV68 infected fetal liver cells were initially cultured on IL-7 expressing T220 fibroblasts [15] for 4 to 5 days to drive B lineage development, followed by transfer of the infected fetal liver cells onto mouse embryo fibroblasts (MEFs) (Fig. 1A). The T220 fibroblast and MEF monolayers are permissive for MHV68 replication, and thus were destroyed after several days of co-culture with the infected fetal liver cells. The infected fetal liver cells were maintained as a bulk culture for 3-4 weeks to generate a sufficient population of infected YFP+ cells that could then purified by flow cytometry (Fig. 1B). Cell lines were generated from sorted YFP+ cells by either single cell sorting or limiting dilution cell culture. Cell lines derived by this approach displayed lymphoblastoid morphology with ovoid or slightly elongated shape and the presence of villipodia projecting from the cell surface (Fig. 1C), similar to the appearance of EBV immortalized lymphoblastoid cell lines [16].
Fig. 1. Generation of MHV68 immortalized fetal liver-derived B cell lines. 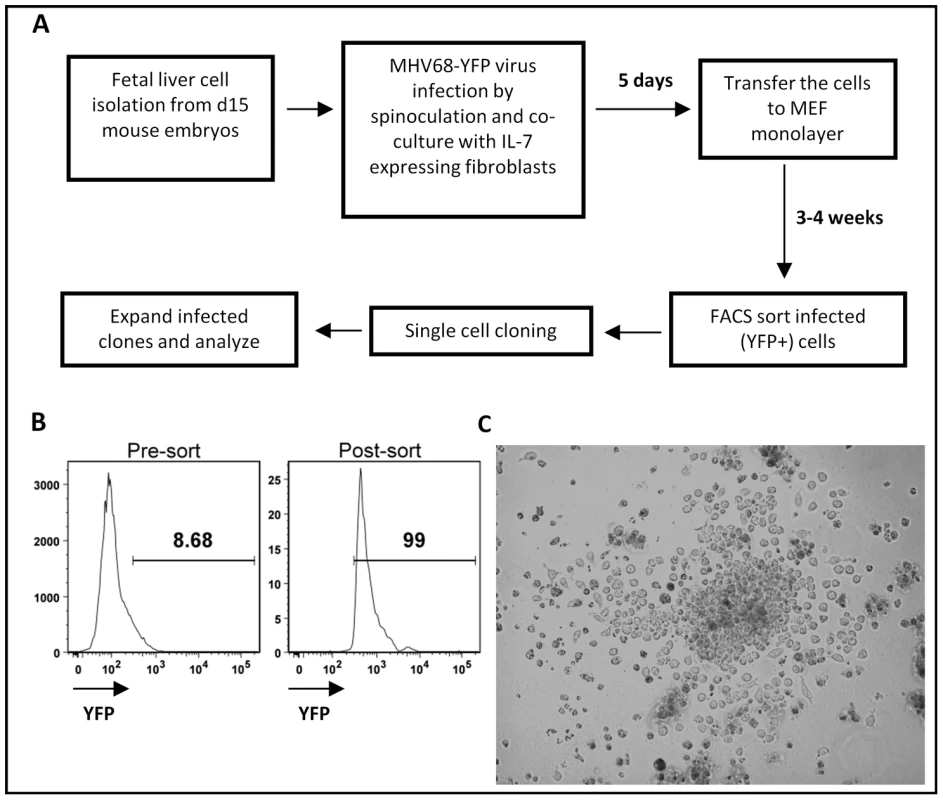
(A) Two stages in vitro culture system for establishing MHV68 infected fetal liver-derived B cell lines. (B) Purification of MHV68-YFP infected fetal liver cells by flow cytometry. Shown are representative pre- and post-sort analyses of YFP-expression. (C) Morphology of a representative MHV68-YFP infected culture at 2.5 weeks post-infection, showing extensive clumping of cells and lymphoblastoid phenotype. Surface staining demonstrated that all the cell lines established bore cell surface markers characteristic of B lymphocytes (B220+/CD19+/c-kit-/CD43-). Surprisingly, all cell lines were sIgD-/sIgM-, suggesting that they either were arrested at an early stage of development or had undergone isotype switching (Fig. 2 and data not shown). Subsequent staining with a panel of isotype specific antibodies revealed that these cell lines expressed surface immunoglobulin, having undergone class switch recombination to IgG2a (Fig. 2 and data not shown). Staining for light chain usage revealed that all the cell lines were Igκ+ and Igλ - (Fig. 2). Notably, even though these cell lines arose from fetal liver, all were CD5 - (Fig. 2). In addition, they were I-Ab intermediate or low and CD138 (syndecan) intermediate or high (Fig. 2), suggesting that these cell lines have differentiated toward a plasmablast phenotype. Consistent with this interpretation, ELISA analyses of the culture supernatants demonstrated significant levels of secreted IgG2a and kappa light chain (Fig. S1). Furthermore, cytoplamic expression of IgG2a was detected in MHV68 immortalized cell lines by immunofluorescent staining (Fig. S2). We also examined the expression of germline transcripts directed by the I promoter of each isotype (Fig. S3A), which revealed the presence of IgG2a germline transcripts and low levels of IgG3 germ line transcripts (Fig. S3A). After immunoglobulin class switch recombination (CSR), post-switch transcripts containing the Iµ exon spliced to the 5′ exon of the relevant rearranged isotype (directed by constitutive active Iµ promoter) are generated [17], [18]. Correspondingly, we readily detected the expression of IgG2a post-switch transcripts (Fig. S3A), and not any of the other immunoglobulin isotypes (data not shown). The IgG2a post-switch transcripts were further confirmed by cloning and sequencing the amplified PCR product (Fig. S3B). Finally, consistent with the observation of class switch recombination, RT-PCR analyses detected the expression of AID in these cell lines (data not shown). Taken together, these data support the conclusion that MHV68 infection of a fetal liver B cell progenitor population(s) not only leads to their immortalization, but also directs these cells to differentiate into IgG2a-expressing plasmablasts.
Fig. 2. Surface phenotyping MHV68 infected cell lines derived from infection of day 15 fetal liver cells. 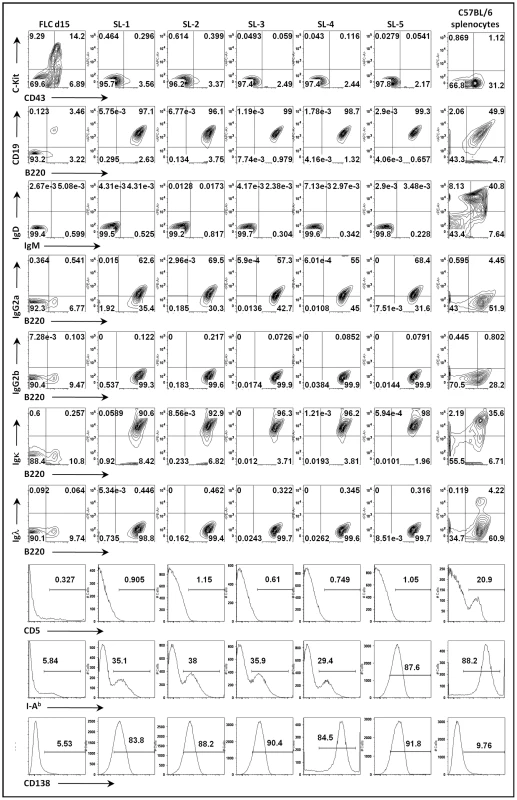
Cell surface expression was analyzed for individual cell lines derived from single cell cloning by flow cytometry. Day 15 fetal liver cells (FLC d15) and splenocytes harvested from naive C57BL/6 mice (BL6 splenocytes) were used as staining controls. Next, we determined the clonality of B cell lines transformed by MHV68. Transformation of B lymphocytes by murine retroviruses leads to outgrowth of transformed lines that are oligo or pauciclonal in nature [19]. To determine the clonality of the B cell clones we used primer sets capable of amplifying >98% of rearranged Ig gene segments. We amplified and sequenced Ig H chain gene segments from 2 of the established MHV68-transformed B cell lines (Fig. S4). Surprisingly, we found that these B cell lines exhibited high clonal diversity. Of the 16 H chain gene segment sequences obtained, junction analyses indicated that there were 10 unique junctions (Table S1). Further analysis of JH usage, as well as V-region nucleotide sequence comparison, revealed an additional 2 sequences which were determined to be unique. Thus, 12 out of 16 (75%) heavy chain sequences were unique. This data suggests that, unlike murine leukemia virus, MHV68 transformation yields a polyclonal population of immortalized B cells. This observed polyclonality could be due to one of the two following reasons: (a) MHV68 targets and immortalizes a variety of mature B cells; or (b) the virus immortalizes a progenitor cell, prior to VDJ rearrangement. The latter seems likely since multiple junction sequences were obtained within each of the 2 cloned cell lines analyzed, although it is possible that only those wells that were seeded with more than one MHV68 infected B cell grew out as cell lines. We also analyzed VH segment usage from transformed B cells and all of them were confined to the VH5 family (Table S2). This skewing most likely reflects the predominant rearrangement of the D-proximal VH5 family V gene segments in fetal liver, rather than MHV68 transformation being restricted to select BCR-bearing B cells.
Retention of viral genome and ability to trigger virus reactivation in MHV68 immortalized B cell lines
Since mock infected day 15 fetal liver cell cultures did not survive and expand following transfer of the cultures to mouse embryo fibroblast feeder layers, we conclude that virus infection is required for outgrowth of fetal liver-derived B cell lines using the assay described above (Fig. 1A). To determine whether there is an ongoing requirement for virus infection, we assessed the frequency of cells harboring viral genome using a limiting-dilution PCR analysis (Fig. 3A). These analyses demonstrated that the viral genome is faithfully maintained in all the cell lines generated, even after serial passage for more than 6 months in tissue culture (Fig. 3A). To assess viral gene expression in these B cell lines, semi-quantitative RT-PCR analyses were performed for a panel of candidate latency-associated and known replication-associated viral genes (Fig. 3B). Tetradecanoyl-phorbol-13-acetate (TPA)-treated A20-HE cells [20] (generated by MHV68 infection of the murine A20 B cell line) served as a positive control for expression of replication cycle-associated viral transcripts in the setting of virus reactivation from B cells (Fig. 3B). Expression of the replication-associated genes encoding the viral DNA polymerase (pol), major capsid protein (MCP) and ORF25 were only detected at low levels (Fig. 3B) in the MHV68 transformed B cell lines, consistent with the majority of the infected cells being latently infected. This was further underscored by detection of transcripts for the known latency-associated genes M2, ORF 73 encoding the latency-associated nuclear antigen (mLANA), and the viral bcl-2 homolog (M11) (Fig. 3B). In addition, expression of viral genes that may play a role in both latency and virus replication (v-cyclin, M8 and K3) were also detected at significant levels in most of the transformed cell lines.
Fig. 3. Analyses of viral genome frequency, viral gene expression and induction of virus reactivation from MHV68 immortalized B cell lines. 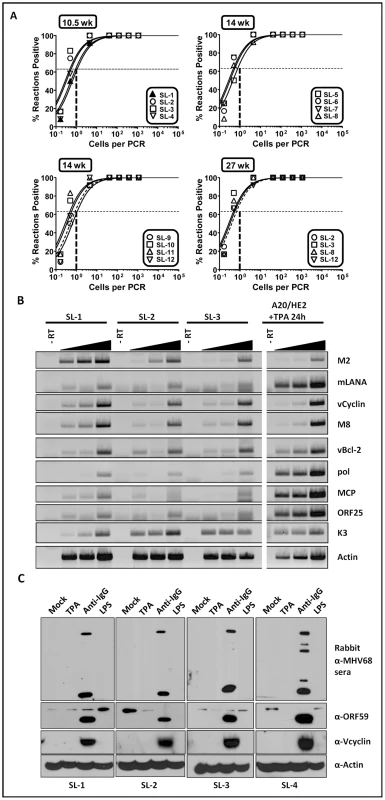
(A) The MHV68 genome is stably maintained in the established fetal-liver derived B cell lines. Shown was limiting dilution PCR detection of the MHV68 genome. The intersection of the horizontal and vertical dashed lines depicts the predicted point, based on a Poisson distribution, where every cell harbors viral genome. (B) Semi-quantitative RT-PCR of a panel of viral genes known to be associated with either virus replication and/or latency. cDNA synthesized from total RNA isolated from individual cell lines was serially diluted 1∶1, 1∶5, or 1∶25 prior to PCR amplification. (C) Immunoblot analyses of viral lytic gene expression after treating cells with candidate reactivation stimuli. The cells were stimulated with the indicated reagents for 72 hr, followed by immunoblot detection with either a rabbit anti-MHV68 antiserum, a chicken anti-ORF59 antibody, a rabbit anti-vCyclin antibody or a mouse anti-actin antibody. A hallmark of herpesvirus latency is the ability to enter the viral replication program (reactivation) given the appropriate stimulus [21]. We assessed a panel of MHV68 transformed B cells lines for induction of lytic antigen expression following treatment with either TPA, crosslinking surface immunoglobulin, or addition of lipopolysaccharide (LPS) to the culture medium. Somewhat surprisingly, TPA did not trigger virus reactivation (Fig. 3C). However, anti-Ig treatment was able to induce virus reactivation in all cell lines tested - although output virus titers were low (Fig. 3C and data not shown). This is reminiscent of the difficulty in triggering EBV reactivation from LCLs generated using umbilical cord lymphocytes compared to those generated from adult peripheral B cells [22].
Viral cyclin and mLANA are required for MHV68 immortalization of murine day 15 fetal liver-derived B cells
To begin to assess the viral requirements for B cell immortalization, we considered candidate latency-associated viral genes for which we consistently detected transcripts in the MHV68 immortalized B cell lines (Fig. 3) and whose functions would contribute to B cell immortalization. As such, we chose three viral genes to analyze - two genes that are conserved among all known rhadinoviruses (v-cyclin and LANA) and one gene that is unique to Old World rodent rhadinoviruses (M2). The v-cyclin gene is an obvious candidate to examine since we have previously shown that it is capable of inducing lymphomas when expression was targeted to T cells in transgenic mice [23]. Similarly, mLANA would be expected to play a central role in B cell immortalization based on the role of KSHV LANA in maintenance of the viral episome during latency, as well as its role in regulating lytic gene expression. The other candidate gene we examined is the M2 gene, a novel MHV68 gene product that we have shown can enhance survival and proliferation of primary murine B cells [24]. Day 15 fetal liver cells were infected with either wild type virus, the v-cyclin null virus (v-cyclin,Stop), mLANA null virus (mLANA.Stop) or the M2 null virus (M2.Stop), and cultured as described above. Notably, while wild type virus infected B cells formed many colonies which were easily detected by light microscopy, the v-cyclin.Stop virus infected B cells formed a small number of disorganized colonies that were sparse in cell number and failed to expand (Fig. 4 and Table 1). Fetal liver-derived B cells infected with the mLANA.Stop virus also failed to generate any infected foci, and YFP+ cells were rapidly lost in these cultures (Table 1). In contrast, M2.Stop virus infected fetal liver-derived B cells formed colonies from which YFP+ cells were isolated and immortalized cell lines established (Table 1 and data not shown). It is perhaps notable that the generation of immortalized cell lines from the M2.Stop cultures appeared less efficient than that observed with wild type MHV68 – however, this interpretation should be taken with some caution because the assay used was not designed to be quantitative.
Fig. 4. The MHV68 cyclin D homolog is required for immortalization of fetal liver-derived B cells. 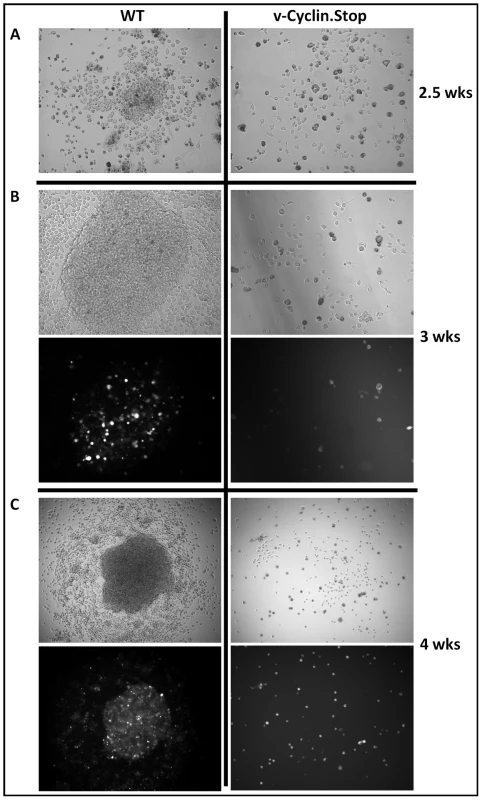
Fetal liver (FL) cells, isolated from embryos at day 15 of gestation, were infected with wild-type (WT) MHV68-YFP or v-Cyclin.Stop-YFP viruses at an MOI of 10 by spinoculation. (A) Colonies were readily apparent in WT infected FL cell cultures by 2.5 weeks post-infection, but not in v-Cyclin.Stop-infected FL cell cultures. (B and C) The colonies derived from WT-infected FL cells grew rapidly and contained numerous YFP bright cells. In contrast, there was little evidence of cellular proliferation or colony formation in the v-Cyclin.Stop infected FL cell cultures. The images were taken at magnification 200X (A) or 100X (B and C), and both brightfield (top panels) and YFP-fluorescence images (bottom panels) are shown. Tab. 1. Immortalization of FL-derived B cells. 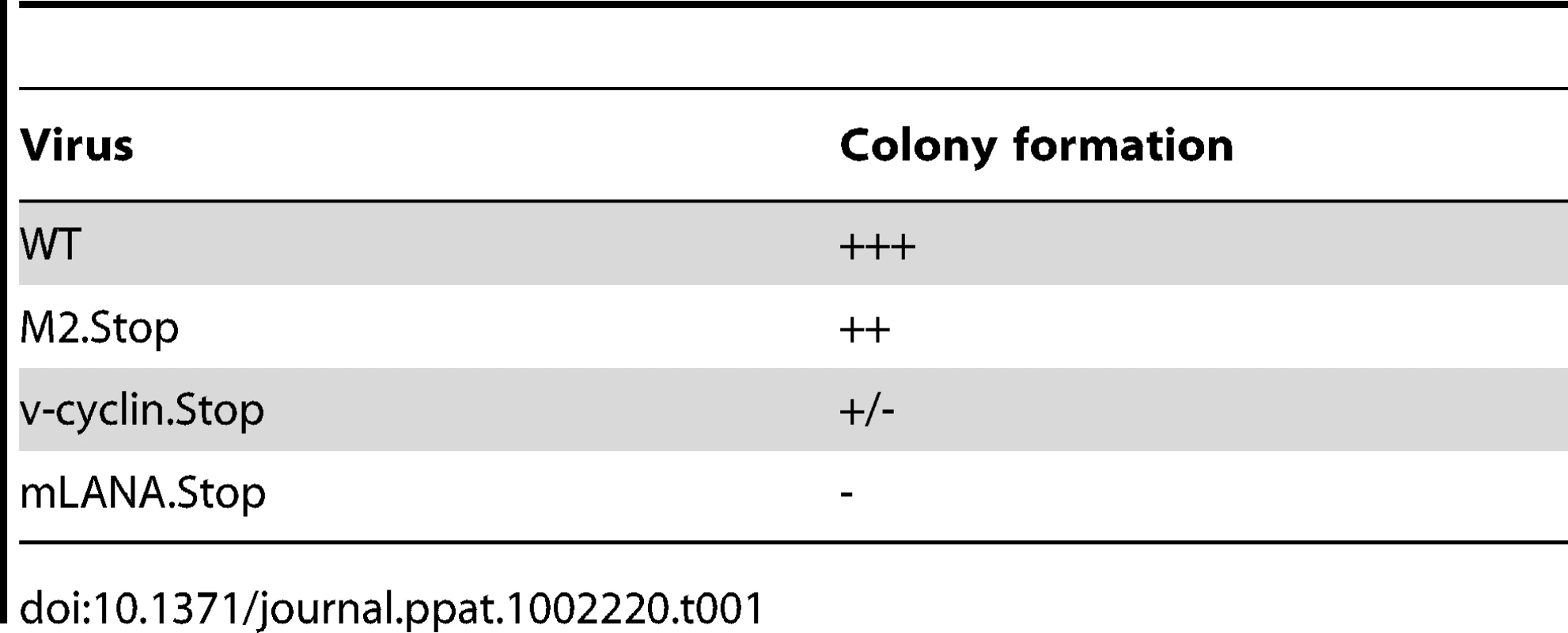
Induction of lymphomas upon adoptive transfer of MHV68 immortalized B cell lines into T cell-deficient mice
During EBV infection in immunocompetent individuals, outgrowth of EBV growth transformed B cells is tightly controlled by a vigorous CD8+ cytotoxic T lymphocyte (CTL) response which targets viral antigens expressed in these B cells. Immune suppression leading to diminished EBV-specific CTL responses thus appears to be central in the progression of EBV-associated lymphoproliferative disease and the genesis of lymphomas in AIDS patients and organ transplant recipients. To determine whether the MHV68 growth transformed B cell lines recapitulate features of EBV in immuncompromised individuals, we assessed the ability of the cloned cell lines to form tumors when injected either subcutaneously or intraperitoneally into immunocompromised mice (athymic nude mice or RAG2-/ - mice) (Fig. 5). Importantly, as expected, no tumors were observed when MHV68 infected fetal liver-derived B cell lines were injected into either B cell-deficient mice (µMT) or wild-type C57BL/6 mice (Fig. 5C) – demonstrating that an intact host immune system is able to control outgrowth of MHV68 transformed B cell lines. This is consistent with the induction of T cell-mediated control of gammaherpesvirus transformed B cells, as observed following EBV infection of immunocompetent individuals. However, as expected, all of the MHV68 infected B cell lines tested were tumorigenic in both athymic nude (T cell-deficient) and RAG2-deficient (B and T cell-deficient) mice, with the appearance of subcutaneous tumors or tumors in lymph nodes appearing 14 to 28 days after injection of cells (Fig. 5). All tumors showed characteristics of lymphomas histopathologically, and all inoculated mice (following either subcutaneous or intraperitoneal injection of tumor cells) showed metastasis of these lymphomas to the spleen (Fig. 5B). The presence of B cells in these lesions was verified by immunohistochemical staining of tumor tissue sections. All tumor tissue sections were homogeneously infiltrated with B220-expressing B cells (Fig. S5). A low level of CD8+ T cells were apparent in the spleens of mock infected nude mice, but not in spleens recovered from mice inoculated with the MHV68-infected fetal liver-derived B cell lines (Fig. S5). Explanted tumors could readily be cultured ex vivo, were viral genome positive, and exhibited a similar pattern of viral gene and cell surface marker to the parental cloned cell lines (Fig. S6 and data not shown). However, it is notable that all the explanted tumor cell lines had upregulated expression of CD5 and expressed higher levels of MHC II than the parent cell lines (Fig. S6B). The upregulation in CD5 expression resonates with the ability of fetal liver-derived B cell progenitors to effectively reconstitute CD5+ B cells in vivo [25], [26].
Fig. 5. Induction of lymphomas by MHV68 immortalized FL cell lines in athymic nude and Rag 2-deficient mice. 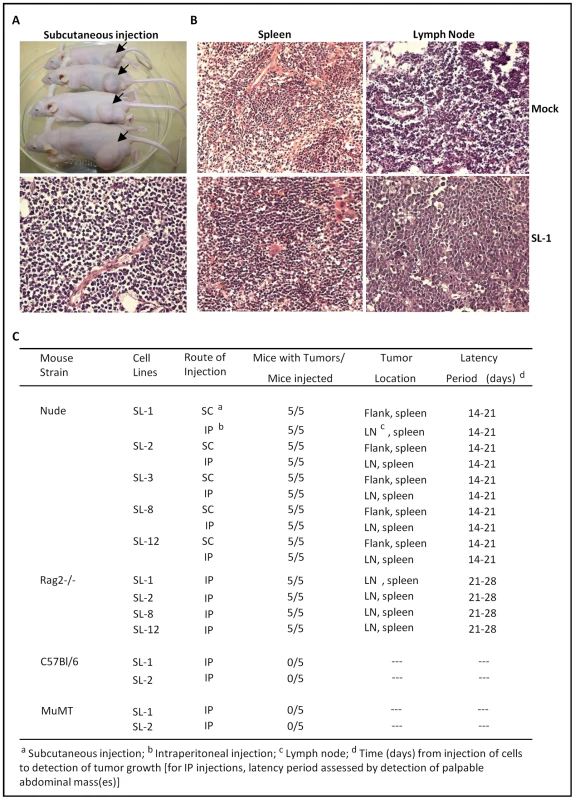
(A) Subcutaneous tumors at the site of injection on the rear flank of Nu/J mice (upper panel). Also shown in a representative H&E stained tumor section (lower panel). (B) Representative H&E stained tumor sections of either mock treated or Nu/J mice inoculated intraperitoneally with the SL-1 MHV68 transformed B cell line. Lymphomas were apparent in both the spleen and lymph node in Nu/J mice injected with MHV68 immortalized FL cell lines. (C) Summary of lymphoma induction using the indiciated MHV68 immortalized B cell lines inoculated into athymic nude, Rag 2-deficient, C57Bl/6 or MuMT (B cell-deficient) mice. Discussion
The ability to study the pathogenesis of EBV and KSHV in the infected host has been significantly hampered by the lack of robust small animal models. Although significant progress has been made in the generation of humanized mice, most notably using NOD/SCID/IF2Rγnull mice as recipients of human hematopoietic stem cells, the T cell responses that are generated are clearly not normal and, at least in the case of EBV infection, are ultimately insufficient to control virus infection [27], [28], [29]. Similarly, while a number of important insights have been gained from the generation of various transgenic mice (e.g., those harboring the KSHV LANA gene and associated regulatory elements) [30], [31], [32], [33], [34], this approach cannot assess the contribution that host immune control and selection play in the genesis of gammaherpesvirus-associated lymphoproliferative diseases. Non-human primate models offer the possibility to fully explore the pathogenesis of gammaherpesviruses that are very closely related to the human pathogens (rhesus lymphocryptovirus and rhesus rhadinovirus), but to date only limited studies have been carried out using these models - presumably due to the substantial expense associated with breeding gammaherpesvirus-free colonies of Rhesus macaques. Thus, the MHV68-mouse model stands alone as the only tractable small animal model that has been extensively studied.
A detailed analysis of viral gene expression in established MHV68 immortalized B cell lines will be required before all the viral genes required for immortalization can be determined. However, our initial characterization of viral transcripts detected in these B cell lines identified several viral genes whose products are candidates for being involved in driving B cell survival and/or growth (v-cyclin, mLANA, vBcl-2 and M2). Furthermore, we have shown here that both the v-cyclin and mLANA are required for immortalization of fetal liver-derived B cells. The latency genes K-cyclin and LANA, homologs of MHV68 v-cyclin and mLANA, are expressed in all KSHV-associated maglinancies, Kaposi's sarcomas (KS), primary effusion lymphomas (PEL) and multicentric Castleman's diseases (MCD) [35], [36], [37], [38]. Furthermore K-cyclin, which modulates the cell-cycle by phosphorylation of p27 in PEL cells, has been implicated in the development of KS tumors and the induction of lymphomas by cooperating with p53 loss [39], [40], [41]. The MHV68 v-cyclin has also been shown to be an oncogene [23]; mice harboring a v-cyclin transgene under the control of the lck proximal promoter (active during early T cell development) develop high grade lymphoblastic lymphomas. Here we detected the expression of v-cyclin mRNA prior to the induction of reactivation and expression of v-cyclin protein after anti-Ig cross-linking to induce reactivation in MHV68 transformed FL cells. Furthermore, v-cyclin null virus induced abortive transformation of fetal liver-derived B cells, indicating that v-cyclin is essential for MHV68 growth transformation of murine B cells. In this light, the v-cyclin gene has been shown to promote MHV68-infected primary endothelial cell survival [42]. The mechanism(s) by which v-cyclin contributes to B cell immortalization is currently under investigation, but likely involves a cdk-dependent activity.
LANA, a conserved rhadinovirus gene, has been shown to be expressed in every KSHV-associated malignancy examined. Thus, LANA has a strong likelihood of being critically involved in the formation of rhadinovirus tumors, but to date, this fact has not been shown in the context of virus infection. Our data demonstrate a requirement for mLANA in MHV68 growth transformation of murine B cells, suggesting the conserved function to KSHV LANA homolog. KSHV LANA exhibits transforming properties in primary rat fibroblasts [43]. Similar to v-cyclin-expressing transgenic mice, the KSHV LANA homolog has been shown to be a potent inducer of B cell tumors when expressed in transgenic mice [35]. Further, LANA was shown to give a significant survival advantage to B cells responding to antigen [44]. The mechanisms responsible have not yet been clearly identified, but LANA has been observed interacting with many proteins involved in cell growth pathways, inhibiting p53-mediated apoptosis [45] and regulating the cell cycle [43], [46], [47]. During primary infection of fibroblasts, mLANA has been shown to be required for efficient replication of the virus, and in the absence of mLANA, the virus shows dysregulated expression of viral genes and an early hyperlytic phenotype, resulting in a lower output of virus overall [48]. These data are consistent with the observation in KSHV that LANA may interact directly with and downregulate transcription from the immediate-early ORF50 promoter [49]. These data, taken together argue strongly for the requirement of mLANA in the efficient development of MHV68-derived tumors. mLANA likely sets the stage for transformation, by interacting with and altering pro-growth and anti-apoptotic pathways, stabilizing the cell and by turning off lytic genes, preventing the infected FL cells from succumbing to lytic infection. This is not to say LANA is a strict requirement for rhadinovirus transformation--MHV68 can establish latency in the absence of mLANA [50] and EBV can establish LCLs in the absence of the major latency-associated protein EBNA-1, though the process is less efficient by several thousandfold [51]. Further investigation of the role of mLANA in MHV68 transformation is planned.
Gammaherpesviruses encode homologs of cellular antiapoptotic BCL-2 proteins (vBCL-2), inhibiting apoptosis in response to diverse stimuli. EBV-encoded BHRF1 and BALF1, two vBCL-2 homologs, KSHV-encoded vBCL2, as well as MHV68-encoded vBCL-2 exhibit antiapoptotic functions which protect the infected cells from apoptosis induced by the host anti-viral immune responses [52]. MHV68 vBCL-2 has been shown to be important during MHV68 chronic infection and disease, but not acute infection [53]. Recently, it has also been shown to antagonize the host autophagy during MHV68 persistent infection [54]. The oncogenic potential of vBCL-2 is an obvious candidate for analysis in B cell immortalization.
There are also strong parallels between the observed phenotype of the MHV68 transformed B cell lines and the previously characterized impact of M2 protein expression on B cell growth and differentiation [24], [55]. We have previously shown that expression of M2 in primary murine B cells enhances survival and proliferation, both of which are dependent on M2 induction of cellular IL-10 expression [24]. M2 expression also drives B cell differentiation – including CSR, downregulation of MHC II and B220 surface expression and upregulation in surface syndecan expression and secreted IgG, consistent with differentiation toward a plasmablast phenotype [24]. Furthermore, the observation that the 12 cell lines characterized had isotype switched to IgG2a recapitulates a bias observed during MHV68 infection in vivo, where nearly half of the infected B cells detected in the spleen at the peak of latency are IgG2a+. Importantly, this phenotype in vivo is dependent on a functional M2 gene product. Finally, we have previously shown that the presence of MHV68 in plasma cells during virus infection in mice is dependent on the presence in the virus of a functional M2 gene [55]. Studies are underway to define how viral genes regulate MHV68 immortalization of fetal liver-derived B cells.
In summary, we have demonstrated that infection of fetal liver progenitor B cells overcomes a barrier previously encountered in studies attempting to generate MHV68 transformed B cell lines using mature B lymphocytes [9]. This may have implications to studies of KSHV, which to date have failed to show any B cell transforming activity. The ability to transform murine B cells with MHV68 opens the door to developing the mouse model of gammaherpesvirus infection to dissect the mechanisms through which gammaherpesviruses manipulate B cell biology and contribute to B cell lymphomagenesis. Importantly, the ability of these cell lines to generate tumors in athymic nude mice and Rag2-/ - mice, but not in either C57Bl/6 or B cell-deficient mice, underscores the critical role of T cells in controlling the outgrowth of MHV68 latently infected B cells. The adoptive transfer of MHV68 immortalized B cell lines has the added advantage that there appears to be little or no reactivation disease apparent in these animals – an issue that complicates studies of MHV68 infected immunocompromised mice where a number of cellular reservoirs (e.g., infected macrophages) ultimately contribute significantly in the setting of immunocompromise to disease associated with MHV68 replication [56]. The latter likely obscures, at least in some settings, the detection and/or development of MHV68-associated lymphomas since this may lag behind the development of end-stage reactivation disease. Finally, we anticipate that this new model will also facilitate studies to identify cellular factors contributing to the generation of gammaherpesvirus-associated B cell lymphomas.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Emory University Institutional Animal Care and Use Committee, and in accordance with established guidelines and policies at Emory University School of Medicine (Protocol Number: 046-2010).
Viruses, cell culture, mice
Murine gammaherpesvirus 68-YFP (MHV68-YFP), M2.Stop-YFP, mLANA.Stop-YFP, and v-Cyclin.Stop-YFP viruses were prepared as previously described [55]. C57Bl/6 mice, Nu/J nude and RAG-2-/ - mice were sterile housed, treated and bred with the approval of the Emory University Institutional Animal Care and Use Committee, and in accordance with established guidelines and policies at Emory University School of Medicine (Atlanta, GA). IL-7-expressing T220 fibroblasts [15] and mouse embryo fibroblasts (MEF) were cultured in DMEM medium with 10% FCS, 100 U/ml penicillin and 100 µg/ml streptomycin.
Generation of MHV68 immortalized fetal liver cell lines
Fetal liver (FL) cells were obtained at day 15 of gestation. The dispersed cells were washed with PBS and resuspended in RPMI 1640 medium with 5% FBS, 100 U/ml penicillin and100 µg/ml streptomycin. Fetal liver cells were infected at an MOI = 10 with either wild type or specific MHV68 mutants. All viruses used harbored a yellow fluorescent protein transgene expression cassette introduced between orf 29a and orf 27 in the viral genome, as previously described [57]. Fetal liver cells were mixed with virus in the presence of polybrene (5 µg/ml), and plated into 6-well plates seeded with IL-7 expressing fibroblasts (either the T220 or LTK cell lines). Spinoculation was carried out by centrifugation at 1800rpm for 1 hr at room temperature, followed by incubation at 37°C for 5 days. At day 5 post-infection, live cells were transferred onto MEF monolayers for 3 to 4 days, followed by culturing for 2 to 4 weeks to obtain sufficient cell numbers to allow MHV68 infected YFP-positive cells to be recovered by flow cytometry. During this expansion the cultures were fed weekly. The sorted YFP positive cells were subcloned in 96-well plates, either by limiting dilution cloning or single cell sorting by flow cytometry. Cell lines were recovered and further cultured in RPMI 1640 medium with 5% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin.
Flow cytometry and fluorescence activated cell sorting (FACS)
Flow cytometric analyses were done as previously described [55]. Briefly, single-cell suspensions were incubated with PE-, PerCP-, APC, APC-Cy7, and/or PacBlue-conjugated mAb on ice for 15 min, and then washed with 0.5% FBS/PBS. The stained cells were subjected to analyses on a LSRII flow cytometer (BD Biosciences). The antibodies used for staining were purchased from BD Biosciences except where noted, including: APC-anti-CD117 (C-kit) , PE-anti-CD43, APC-anti-CD19, APC-Cy7-anti - B220, PerCP-anti-IgM, PE-anti-IgD, PerCP-anti-CD5, PE-anti-IAb, PE-anit-CD138, PE-anti-IgG2a (Southern Biotech), PE-anti-IgG2b (Southern Biotech), PE-anti-Igκ (Southern Biotech), anti-Igλ (Southern Biotech). For YFP-positive cell sorting, the single cell suspensions were resuspended with 0.5%FBS/PBS and directly subjected to separation on a FACSAria™ II flow cytometer (BD Biosciences).
Analysis of immunoglobulin gene rearrangements
RNA was prepared from two representative MHV68 transformed B cell lines, and RT-PCR performed to amplify VH gene segments. Primers for immunoglobulin heavy chain variable regions were made by creating consensus primers from VH sequences obtained from the international ImMunoGeneTics Information system (www.imgt.org). Consensus primers were designed for each VH family and specific primers were made for all four Jh segments. PCR amplification for heavy chains were run using pooled VH and JH primers and high-fidelity Taq polymerase. PCR products were cloned and sequenced. Heavy chain sequences were input into the IMGT program V-QUEST for analysis.
Immunoglobulin Isotyping
1-2×106 cells of representative MHV68 immortalized FL-derived cell lines were plated in 6-well plates, supernatants were collected after culturing cells for 5 days and subjected to antibody isotyping using an ELISA mouse mAb isotyping kit (Thermo scientific), based on manufacturer's instructions. Each cell line was analyzed in triplicate.
Immunofluorescence staining, immunohistopathology and immunohistochemistry
For histopathological analyses, tissues were fixed in formalin, dehydrated, and embedded in paraffin. The embedded tissues were cut at about 5 µm of thickness prior to staining with hematoxylin and eosin for histopathological analyses. For immunohistochemical analyses, the sections were deparaffinized, rehydrated and incubated with citrate buffer (pH 6) for antigen retrieval before the sections were subjected to immunofluorescence staining. The stained slides were dehydrated in 95%, 100% ethanol and xylene. Anti-fade mounting medium (Invitrogen) with DAPI counterstaining (Invitrogen, USA) were used prior to examining tissue sections on a LSM510 META confocal microscope (Zeiss). The reagents used for the tissue section staining included: biotin anti-mouse CD8α (BD Biosciences), purified rat anti-mouse CD45R (B220) (BD Biosciences), Alexa fluor 647-conjugated streptavidin and Alexa fluor 555-conjugated secondary antibodies (Invitrogen).
Limiting dilution PCR analyses
The frequency of MHV68 genome-positive cells was determined using a previously described nested PCR assay (LD-PCR) [58]. Briefly, cells were counted, resuspended in an isotonic solution, and diluted into a background of 104 uninfected NIH 3T12 cells. Following cell lysis with proteinase K, two rounds of nested PCR were performed on each sample to detect the presence of the MHV68 ORF50. To ensure sufficient sensitivity of the nested PCR reaction, 10, 1, or 0.1 copies of a gene 50 containing plasmid (pBamH I N) were diluted into a background of 104 uninfected cells and analyzed in parallel with the experimental sample.
RT-PCR and semi-quantitative RT-PCR
Total RNA was extracted from the cells using TRIzol reagent (Invitrogen) according to manufacturer's instructions. 2 µg RNA were used for first-strand cDNA synthesis (Invitrogen) prior to PCR analysis. For semi-quantitative RT-PCR analyses of viral gene expression, viral transcripts were amplified from serial dilutions of cDNA (1∶1, 1∶5 and 1∶25) in 35 cycles of PCR using primers previously described [20]. cDNA from the reaction without reverse transcriptase was used as a negative control for the PCR reaction. RNA derived from HE2 cells, treated with TPA to trigger virus reactivation, served as a positive control for viral gene expression. For PCR of viral gene expression from explant-derived tumor cells, viral gene transcripts were amplified from cDNA directly without dilution.
Germline immunoglobulin gene and postswitch transcripts were amplified by PCR as previously described [18]. For PCR of B cell-associated genes, the following primers were used to obtain the indicated products: AID-F: 5′-ACATCTCAGACTGGGACCTG-3′, AID-R: 5′-TCAAAATCCCAACATACGAAATG-3′; PAX5-F: 5′-AACTTGCCCATCAAGGTGTC-3′, PAX5-R: 5′-CTGATCTCCCAGGCAAACAT-3′; PU.1-F: 5′ CCCTCCATCGGATGACTTGGTTAC-3′, PU.1-R: 5′-GCTTCTCCATCAGACACCTCCAGG-3′; CD79a-F: 5′ - GTGAAAACAATGGCAGGAA-3′, CD79a-R: 5′ - AGGTTCAGGCCCTCATAGAG-3′; CD79b-F: 5′-TCTCAGAAGAGGGACGCATT-3′, CD79b-R: 5′ - AATGTTCAAGCCCTCATAGG-3′; J chain-F: 5′-ATGAAGACCCACCTGCTT CTC-3′, J chain-R: 5′-GTCAGGGTAGCAAGAATCG GG-3′; GAPDH-F: 5′-CCATCACCATCTT CCAGGAG-3′, GAPDH-R: 5′-CCTGCTTCACCACC TTCTTG-3′.
Reactivation stimulation and immunoblots
MHV68 immortalized FL cell lines were treated with 20 ng/ml 12-O-tetradecanoyl-phorbol-13-acetate (TPA), F(ab′)2 anti-mouse IgG (5 µg/ml), LPS (10 µg/ml), trichostatin A (5 µM), sodium butyrate (2 mM), or 5-azacytidine (5 µM) for 48 hr prior to the harvest. Immunoblot analyses were performed as previously described [20] to detect lytic viral antigen expression.
Lymphoma induction in immunodeficient mice
The tumorigenic ability of MHV68 immortalized cell lines was tested in 6 to 8 week-old female Nu/J nude mice which were purchased from The Jackson Laboratory, or RAG2-/ - mice which were bred at Emory University. Cells were suspended in PBS and injected subcutaneously or intraperitoneally at a concentration of 2.5-5×106 cells in a volume of 0.2 ml. Control mice were injected with PBS. The animals were euthanized by carbon dioxide inhalation when visible tumors reached a diameter of 1-2 cm. All animals were autopsied and examined for metastases. Tumors were excised under sterile conditions and divided into two fragments, one of which was fixed in 10% neutral buffered formalin (Sigma) and processed for histopathology and immunohistochemistry and the other fragment was explanted into cell culture in RPMI 1640 medium with 10% FCS, 100 U/ml penicillin and100 µg/ml streptomycin.
Supporting Information
Zdroje
1. SpeckSHGanemD 2010 Viral latency and its regulation: lessons from the gamma-herpesviruses. Cell Host Microbe 8 100 115
2. YoungLSRickinsonAB 2004 Epstein-Barr virus: 40 years on. Nat Rev Cancer 4 757 768
3. Thorley-LawsonDAAlldayMJ 2008 The curious case of the tumour virus: 50 years of Burkitt's lymphoma. Nat Rev Microbiol 6 913 924
4. ChangYCesarmanEPessinMSLeeFCulpepperJ 1994 Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266 1865 1869
5. CesarmanEChangYMoorePSSaidJWKnowlesDM 1995 Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 332 1186 1191
6. CarboneACesarmanESpinaMGloghiniASchulzTF 2009 HIV-associated lymphomas and gamma-herpesviruses. Blood 113 1213 1224
7. JohnsonDRJondalM 1981 Herpesvirus-transformed cytotoxic T-cell lines. Nature 291 81 83
8. BiesingerBMuller-FleckensteinISimmerBLangGWittmannS 1992 Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci U S A 89 3116 3119
9. JarousseNChandranBCoscoyL 2008 Lack of heparan sulfate expression in B-cell lines: implications for Kaposi's sarcoma-associated herpesvirus and murine gammaherpesvirus 68 infections. J Virol 82 12591 12597
10. RaffMCMegsonMOwenJJCooperMD 1976 Early production of intracellular IgM by B-lymphocyte precursors in mouse. Nature 259 224 226
11. DenisKADorshkindKWitteON 1987 Regulated progression of B lymphocyte differentiation from cultured fetal liver. J Exp Med 166 391 403
12. GunjiYSudoTSudaJYamaguchiYNakauchiH 1991 Support of early B-cell differentiation in mouse fetal liver by stromal cells and interleukin-7. Blood 77 2612 2617
13. GodinIEGarcia-PorreroJACoutinhoADieterlen-LievreFMarcosMA 1993 Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature 364 67 70
14. MartinezMJMinguetSGonzaloPSoroPGde AndresB 2001 Long-lived polyclonal B-cell lines derived from midgestation mouse embryo lymphohematopoietic progenitors reconstitute adult immunodeficient mice. Blood 98 1862 1871
15. LinQTaniuchiIKitamuraDWangJKearneyJF 1998 T and B cell development in BP-1/6C3/aminopeptidase A-deficient mice. J Immunol 160 4681 4687
16. RoweDT 1999 Epstein-Barr virus immortalization and latency. Front Biosci 4 D346 371
17. LiSCRothmanPBZhangJChanCHirshD 1994 Expression of I mu-C gamma hybrid germline transcripts subsequent to immunoglobulin heavy chain class switching. Int Immunol 6 491 497
18. MuramatsuMKinoshitaKFagarasanSYamadaSShinkaiY 2000 Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102 553 563
19. WhitlockCAZieglerSFTreimanLJStaffordJIWitteON 1983 Differentiation of cloned populations of immature B cells after transformation with Abelson murine leukemia virus. Cell 32 903 911
20. ForrestJCSpeckSH 2008 Establishment of B-cell lines latently infected with reactivation-competent murine gammaherpesvirus 68 provides evidence for viral alteration of a DNA damage-signaling cascade. J Virol 82 7688 7699
21. MillerG 1990 The switch between latency and replication of Epstein-Barr virus. J Infect Dis 161 833 844
22. KingWVan SantenVKieffE 1981 Epstein-Barr virus RNA. VI. Viral RNA in restringently and abortively infected Raji cells. J Virol 38 649 660
23. van DykLFHessJLKatzJDJacobyMSpeckSH 1999 The murine gammaherpesvirus 68 v-cyclin gene is an oncogene that promotes cell cycle progression in primary lymphocytes. J Virol 73 5110 5122
24. SiegelAMHerskowitzJHSpeckSH 2008 The MHV68 M2 protein drives IL-10 dependent B cell proliferation and differentiation. PLoS Pathog 4 e1000039
25. HardyRRHayakawaK 1991 A developmental switch in B lymphopoiesis. Proc Natl Acad Sci U S A 88 11550 11554
26. HayakawaKHardyRRHerzenbergLAHerzenbergLA 1985 Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med 161 1554 1568
27. MelkusMWEstesJDPadgett-ThomasAGatlinJDentonPW 2006 Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med 12 1316 1322
28. ShultzLDIshikawaFGreinerDL 2007 Humanized mice in translational biomedical research. Nat Rev Immunol 7 118 130
29. YajimaMImadomeKNakagawaAWatanabeSTerashimaK 2008 A new humanized mouse model of Epstein-Barr virus infection that reproduces persistent infection, lymphoproliferative disorder, and cell-mediated and humoral immune responses. J Infect Dis 198 673 682
30. GuoHGSadowskaMReidWTschachlerEHaywardG 2003 Kaposi's sarcoma-like tumors in a human herpesvirus 8 ORF74 transgenic mouse. J Virol 77 2631 2639
31. FakhariFDJeongJHKananYDittmerDP 2006 The latency-associated nuclear antigen of Kaposi sarcoma-associated herpesvirus induces B cell hyperplasia and lymphoma. J Clin Invest 116 735 742
32. PrakashOTangZYPengXColemanRGillJ 2002 Tumorigenesis and aberrant signaling in transgenic mice expressing the human herpesvirus-8 K1 gene. J Natl Cancer Inst 94 926 935
33. CaldwellRGWilsonJBAndersonSJLongneckerR 1998 Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 9 405 411
34. StunzLLBuschLKMunroeMESigmundCDTygrettLT 2004 Expression of the cytoplasmic tail of LMP1 in mice induces hyperactivation of B lymphocytes and disordered lymphoid architecture. Immunity 21 255 266
35. FakhariFDDittmerDP 2002 Charting latency transcripts in Kaposi's sarcoma-associated herpesvirus by whole-genome real-time quantitative PCR. J Virol 76 6213 6223
36. JennerRGAlbaMMBoshoffCKellamP 2001 Kaposi's sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J Virol 75 891 902
37. DittmerDP 2003 Transcription profile of Kaposi's sarcoma-associated herpesvirus in primary Kaposi's sarcoma lesions as determined by real-time PCR arrays. Cancer Res 63 2010 2015
38. Paulose-MurphyMHaNKXiangCChenYGillimL 2001 Transcription program of human herpesvirus 8 (kaposi's sarcoma-associated herpesvirus). J Virol 75 4843 4853
39. SugayaMWatanabeTYangAStarostMFKobayashiH 2005 Lymphatic dysfunction in transgenic mice expressing KSHV k-cyclin under the control of the VEGFR-3 promoter. Blood 105 2356 2363
40. VerschurenEWKlefstromJEvanGIJonesN 2002 The oncogenic potential of Kaposi's sarcoma-associated herpesvirus cyclin is exposed by p53 loss in vitro and in vivo. Cancer Cell 2 229 241
41. SarekGJarviluomaAOjalaPM 2006 KSHV viral cyclin inactivates p27KIP1 through Ser10 and Thr187 phosphorylation in proliferating primary effusion lymphomas. Blood 107 725 732
42. SuarezALvan DykLF 2008 Endothelial cells support persistent gammaherpesvirus 68 infection. PLoS Pathog 4 e1000152
43. RadkovSAKellamPBoshoffC 2000 The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat Med 6 1121 1127
44. SinSHFakhariFDDittmerDP 2010 The viral latency-associated nuclear antigen augments the B-cell response to antigen in vivo. J Virol 84 10653 10660
45. FriborgJJrKongWHottigerMONabelGJ 1999 p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402 889 894
46. FujimuroMWuFYApRhysCKajumbulaHYoungDB 2003 A novel viral mechanism for dysregulation of beta-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat Med 9 300 306
47. AnFQCompitelloNHorwitzESramkoskiMKnudsenES 2005 The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from p16 INK4A-induced cell cycle arrest. J Biol Chem 280 3862 3874
48. ForrestJCPadenCRAllenRD3rdCollinsJSpeckSH 2007 ORF73-null murine gammaherpesvirus 68 reveals roles for mLANA and p53 in virus replication. J Virol 81 11957 11971
49. LuFDayLGaoSJLiebermanPM 2006 Acetylation of the latency-associated nuclear antigen regulates repression of Kaposi's sarcoma-associated herpesvirus lytic transcription. J Virol 80 5273 5282
50. PadenCRForrestJCMoormanNJSpeckSH 2010 Murine gammaherpesvirus 68 LANA is essential for virus reactivation from splenocytes but not long-term carriage of viral genome. J Virol 84 7214 7224
51. HummeSReisbachGFeederleRDelecluseHJBoussetK 2003 The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proc Natl Acad Sci U S A 100 10989 10994
52. CuconatiAWhiteE 2002 Viral homologs of BCL-2: role of apoptosis in the regulation of virus infection. Genes Dev 16 2465 2478
53. GangappaSvan DykLFJewettTJSpeckSHVirginHWt 2002 Identification of the in vivo role of a viral bcl-2. J Exp Med 195 931 940
54. EXHwangSOhSLeeJSJeongJH 2009 Viral Bcl-2-mediated evasion of autophagy aids chronic infection of gammaherpesvirus 68. PLoS Pathog 5 e1000609
55. LiangXCollinsCMMendelJBIwakoshiNNSpeckSH 2009 Gammaherpesvirus-driven plasma cell differentiation regulates virus reactivation from latently infected B lymphocytes. PLoS Pathog 5 e1000677
56. WeckKEDal CantoAJGouldJDO′GuinAKRothKA 1997 Murine gamma-herpesvirus 68 causes severe large-vessel arteritis in mice lacking interferon-gamma responsiveness: a new model for virus-induced vascular disease. Nat Med 3 1346 1353
57. CollinsCMBossJMSpeckSH 2009 Identification of infected B-cell populations by using a recombinant murine gammaherpesvirus 68 expressing a fluorescent protein. J Virol 83 6484 6493
58. WeckKEKimSSVirginHISpeckSH 1999 B cells regulate murine gammaherpesvirus 68 latency. J Virol 73 4651 4661
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



