-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Functional Interchangeability of Late Domains, Late Domain Cofactors and Ubiquitin in Viral Budding
The membrane scission event that separates nascent enveloped virions from host cell membranes often requires the ESCRT pathway, which can be engaged through the action of peptide motifs, termed late (L-) domains, in viral proteins. Viral PTAP and YPDL-like L-domains bind directly to the ESCRT-I and ALIX components of the ESCRT pathway, while PPxY motifs bind Nedd4-like, HECT-domain containing, ubiquitin ligases (e.g. WWP1). It has been unclear precisely how ubiquitin ligase recruitment ultimately leads to particle release. Here, using a lysine-free viral Gag protein derived from the prototypic foamy virus (PFV), where attachment of ubiquitin to Gag can be controlled, we show that several different HECT domains can replace the WWP1 HECT domain in chimeric ubiquitin ligases and drive budding. Moreover, artificial recruitment of isolated HECT domains to Gag is sufficient to stimulate budding. Conversely, the HECT domain becomes dispensable if the other domains of WWP1 are directly fused to an ESCRT-1 protein. In each case where budding is driven by a HECT domain, its catalytic activity is essential, but Gag ubiquitination is dispensable, suggesting that ubiquitin ligation to trans-acting proteins drives budding. Paradoxically, however, we also demonstrate that direct fusion of a ubiquitin moiety to the C-terminus of PFV Gag can also promote budding, suggesting that ubiquitination of Gag can substitute for ubiquitination of trans-acting proteins. Depletion of Tsg101 and ALIX inhibits budding that is dependent on ubiquitin that is fused to Gag, or ligated to trans-acting proteins through the action of a PPxY motif. These studies underscore the flexibility in the ways that the ESCRT pathway can be engaged, and suggest a model in which the identity of the protein to which ubiquitin is attached is not critical for subsequent recruitment of ubiquitin-binding components of the ESCRT pathway and viral budding to proceed.
Published in the journal: . PLoS Pathog 6(10): e32767. doi:10.1371/journal.ppat.1001153
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001153Summary
The membrane scission event that separates nascent enveloped virions from host cell membranes often requires the ESCRT pathway, which can be engaged through the action of peptide motifs, termed late (L-) domains, in viral proteins. Viral PTAP and YPDL-like L-domains bind directly to the ESCRT-I and ALIX components of the ESCRT pathway, while PPxY motifs bind Nedd4-like, HECT-domain containing, ubiquitin ligases (e.g. WWP1). It has been unclear precisely how ubiquitin ligase recruitment ultimately leads to particle release. Here, using a lysine-free viral Gag protein derived from the prototypic foamy virus (PFV), where attachment of ubiquitin to Gag can be controlled, we show that several different HECT domains can replace the WWP1 HECT domain in chimeric ubiquitin ligases and drive budding. Moreover, artificial recruitment of isolated HECT domains to Gag is sufficient to stimulate budding. Conversely, the HECT domain becomes dispensable if the other domains of WWP1 are directly fused to an ESCRT-1 protein. In each case where budding is driven by a HECT domain, its catalytic activity is essential, but Gag ubiquitination is dispensable, suggesting that ubiquitin ligation to trans-acting proteins drives budding. Paradoxically, however, we also demonstrate that direct fusion of a ubiquitin moiety to the C-terminus of PFV Gag can also promote budding, suggesting that ubiquitination of Gag can substitute for ubiquitination of trans-acting proteins. Depletion of Tsg101 and ALIX inhibits budding that is dependent on ubiquitin that is fused to Gag, or ligated to trans-acting proteins through the action of a PPxY motif. These studies underscore the flexibility in the ways that the ESCRT pathway can be engaged, and suggest a model in which the identity of the protein to which ubiquitin is attached is not critical for subsequent recruitment of ubiquitin-binding components of the ESCRT pathway and viral budding to proceed.
Introduction
The membrane scission event that separates the lipid membrane of nascent enveloped virions from host cell membranes is, in many cases, an orchestrated event requiring the participation of the class E vacuolar protein sorting (VPS), or endosomal sorting complex required for transport (ESCRT) pathway. Ordinarily, the ESCRT pathway induces topologically equivalent cellular membrane scission events including the biogenesis of multivesicular bodies (MVBs) [1], [2] and the membrane abscission event at the conclusion of cell division [3], [4]. Components of the pathway can be recruited, either directly or indirectly, through the action of short peptide motifs called late (L-) domains in viral structural proteins [5], [6]. Three classes of viral L-domains and cognate cofactors have been defined thus far: PT/SAP motifs bind Tsg101 [7], [8], [9], [10], LxxLF or YPXL motifs bind ALIX [11], [12], [13], and PPxY domains bind Nedd4-like HECT ubiquitin ligases [14], [15], [16], [17], [18]. Disruption of late domain function results in the failure of membrane scission and the accumulation of assembled virions that remain tethered to the surface of the host cell by a continuous membrane.
The ESCRT machinery is composed of ∼25 proteins, many of which participate in the formation of several multiprotein complexes, known as ESCRT-0, -I, -II, -III [19], [20], [21]. ESCRT-III components are thought to drive the membrane scission event [22], [23], [24], [25] and appear to be generally required for L-domain-dependent viral budding [7], [11], [12], [13], [26]. In contrast, other components of the ESCRT-pathway appear to be required in an L-domain specific way. For example, PTAP-dependent budding is especially sensitive to ESCRT-I perturbation, while YPXL-dependent budding is especially sensitive to ALIX depletion. Since ALIX interacts directly with ESCRT-III via its Bro1 domain [11], [12], [13], [27], [28] and ESCRT-I indirectly interacts with ESCRT-III via ALIX and/or ESCRT-II, [11], [12], [13] these observations suggest that YPXL and PTAP motifs access the same core scission machinery via alternative routes.
In contrast, it has remained somewhat unclear how PPxY motifs access the scission machinery. Overexpression of certain HECT ubiquitin ligases that bind directly to PPxY or other motifs can markedly stimulate budding, and the catalytic activity of the HECT domain is essential for this activity [17], [29], [30], [31]. Indeed, overexpression of catalytically inactive or truncation mutants of the HECT ligase WWP1 inhibits PPxY-dependent budding [17], [29]. Some components of the ESCRT pathway are also required for PPxY-induced budding [7], [31], [32]. However, the precise means by which HECT ligase recruitment subsequently results in the engagement of the ESCRT machinery is not completely defined. One model invokes direct ubiquitination of Gag as the key event. This notion derives from observations that several components of the ESCRT pathway are thought to recognize ubiquitinated cargo through various low affinity ubiquitin-binding domains [7], [33], [34], [35], [36], [37] and that monoubiquitination of cellular cargos can serve as a signal for endosomal trafficking and delivery to the lysosome [21], [38], [39]. Indeed, several observations are consistent with the notion that ubiquitination of retroviral Gag promotes virus particle release. For example, studies have noted an enrichment of free ubiquitin in retrovirus particles, and ubiquitinated Gag species have also been detected therein [14], [40], [41], [42], [43]. Additionally, late budding defects have been observed in cells treated with proteasome inhibitors, perhaps due to the depletion of free ubiquitin [14], [44], [45]. Mutation of multiple ubiquitin acceptor lysine residues in Gag has been shown to inhibit particle production by retroviruses [46], [47]. Finally, direct fusion of ubiquitin to the C-terminus of Gag proteins has been shown to alleviate inhibition of particle release imposed by proteasome inhibitors, or to obviate the requirement for an L-domain in particle release [44], [48].
Other observations suggest that PPxY and ubiquitin ligase-dependent budding may involve mechanisms other than direct Gag ubiquitination. In particular, overexpression of wild-type WWP1 stimulates PPxY-dependent particle production by a lysine-free Gag protein [29] in the absence of detectable Gag ubiquitination. This finding suggests the possibility that HECT ligases may promote budding by catalyzing the ubiquitination of specific trans-acting host factors, rather than Gag. Additionally, a HECT-truncated WWP1 protein, lacking the entire HECT domain, inhibits murine leukemia virus (MLV) budding more potently than the full length WWP1 protein with a disrupted active site [17], suggesting that HECT domains may possess activities other than ubiquitin conjugation that are important for their function in viral budding. Moreover, HECT domains localize to aberrant endosomal (so called class E) compartments induced by overexpression of catalytically inactive ATPase VPS4 [17], which is required for the disassembly of ESCRT complexes after each round of budding [49], [50]. Since many VPS factors are trapped on VPS4-induced compartments, HECT domains may be recruited to these compartments by interaction with VPS proteins, either directly or through unidentified bridging factors. It has also been reported that HECT ubiquitin ligases can bind to, and/or catalyze the ligation of ubiquitin to, certain class E VPS factors [31], [32], [51]. Thus, the ubiquitin ligases might act as recruitment factors rather than, or in addition to, conjugating ubiquitin to key target proteins.
In this study we investigated the role of PPxY motifs, HECT ubiquitin ligase domains and ubiquitin in viral budding, using a lysine-free viral protein from the prototypic foamy virus (PFV), in which the attachment of ubiquitin to Gag can be rather precisely controlled. We show that the catalytic activity of a variety of HECT domains, targeted to a PPxY motif in assembling particles via a common C2/WW domain fragment of WWP1, is essential for their ability to promote PPxY-dependent VLP release. In each case, however, Gag ubiquitination is dispensable for their activity. Rather, the ability of the chimeric ubiquitin ligases to promote budding correlated broadly, albeit imperfectly, with their ability to catalyze autoubiquitination, Moreover, we show that artificial recruitment of an isolated HECT domain can also stimulate budding, while a HECT domain becomes dispensable for PPxY motif dependent budding if the C2/WW domains of WWP1 are directly linked to the C-terminal domain of Tsg101, an ESCRT-I subunit. Finally, we demonstrate that direct fusion of a single ubiquitin moiety to the C-terminus of PFV Gag is also capable of promoting budding, in a manner that recapitulates the ESCRT protein requirement for budding induced by PPxY-dependent ubiquitin ligase recruitment in the absence of ubiquitin acceptors in Gag. These results support a model in which PPxY motif-induced HECT ubiquitin ligase recruitment leads to the deposition of ubiquitin at or near the site of viral budding. However, the identity of the protein to which ubiquitin is attached, be it Gag or a bystander protein, perhaps including the HECT ubiquitin ligase itself, does not appear to be critical in order for subsequent recruitment of ubiquitin-binding class E VPS proteins and viral budding to proceed.
Results
Chimeric ubiquitin ligases encoding a panel of HECT domains stimulate PPxY-dependent budding with variable efficiency
To ascertain what properties of HECT domains are important for stimulation of virus particle release, we compared the properties of a panel of HECT domains. Nine members of the Nedd4-like HECT ubiquitin ligase family have been described in humans and these have the same domain organization as a single prototype member of this family in yeast, namely Rsp5 (reviewed in [52]). Specifically, an N-terminal C2 domain directs the protein to membranes, a central cluster of ‘WW” domains binds ligands, such as PPxY motifs, and a C-terminal HECT domain harbors the E3 ubiquitin ligase activity. Some of the intact ubiquitin ligases have been shown to vary in their ability to promote PPxY-dependent MLV virion release, due at least in part to differences in the affinities of their WW domains for the MLV L-domain [17], but whether the various the C-terminal HECT domains are equivalently able to induce particle release has not been investigated. We reasoned that variation in the ability of HECT domains to stimulate virus budding, correlated with a given property of the HECT domains, might suggest properties that are important for inducing virion release. Since WWP1 has been previously shown to be efficiently recruited by a number of PPxY-type L-domains, including that of MLV [17], we constructed a panel of chimeric ubiquitin ligases, consisting of membrane targeting and PPxY motif-binding domains (C2 and WW domains) of human WWP1, coupled to various catalytic HECT domains derived from human WWP2, Nedd4, Nedd4L, Itch, Smurf1, Bul2 or yeast Rsp5 HECT ligases (Fig. 1A).
Fig. 1. Constructs used in this study. 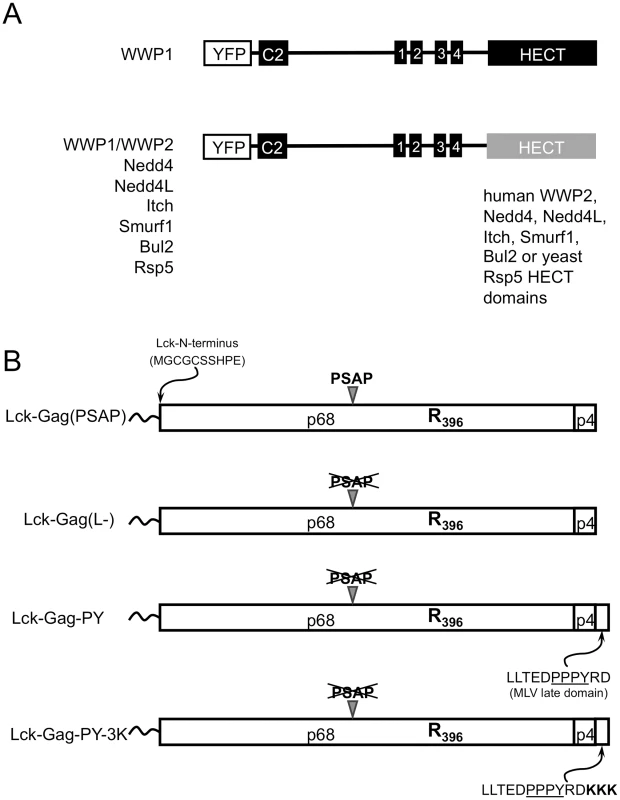
(A) Schematic representation of YFP-fused chimeric ubiquitin ligases containing the membrane targeting (C2) and late domain binding (WW) domains of WWP1 and the indicated catalytic HECT domains. Wild type and catalytically inactive mutant versions of each HECT ligase were constructed. (B) Schematic representation of the PFV Gag-derived proteins used in this study, containing a single K396R mutation, an Lck-derived membrane targeting peptide appended to the N-terminus, and the indicated late domains. To determine whether these chimeric ubiquitin ligases could support viral budding, we co-expressed each of them with a plasmid expressing a modified PFV Gag protein. Importantly, PFV Gag offers the advantage that it is naturally almost devoid of lysine resides. While PFV Gag normally requires a cognate Env protein for particle release, we have previously shown that appending a myristoylated, palmitoylated peptide from Lck at its N-terminus can overcomes this requirement by directing PFV Gag to the plasma membrane and thereby allowing the generation of extracellular particles in the absence of any other viral protein [29]. Throughout this study we used this N-terminally modified Gag protein, termed Lck-Gag, bearing a K396R mutation that renders the PFV Gag completely lysine-free. Examples of engineered variants of this Gag protein are illustrated in Fig. 1B, and include those that is otherwise unmodified and encode the natural PSAP late domain (Lck-Gag(PSAP)), a PSAP mutant that contains no known L-domain (Lck-Gag(L-)) or another variant that has a PPxY late domain derived from MLV Gag appended to its C-terminus (Lck-Gag-PY, Fig. 1B). In addition, we used an Lck-Gag-PY derivative containing three lysine residues adjacent to a PPxY late domain (Lck-Gag-PY-3K) to assess HECT ligase-induced Gag ubiquitination ([29], illustrated in Fig. 1B).
Overexpression of ubiquitin ligases encoding a variety of HECT domains (WWP1 itself, WWP1/Nedd4, WWP1/Nedd4L, WWP1/Itch, WWP1/Smurf1, or WWP1/Bul2) stimulated PPxY-dependent budding of lysine-free Lck-Gag-PY (Fig. 2A,B). Conversely, WWP1/WWP2 and WWP1/Rsp5 did not stimulate budding or had marginal activity. The strongest stimulation was observed using chimeric ligases containing the Nedd4L and Itch HECT domains. Importantly, overexpression of chimeric ligases in which the catalytic cysteine was mutated to serine, failed to stimulate PPxY-dependent particle release (Fig. 2A), indicating that the catalytic activity of each HECT domains was required, even when the viral structural proteins lack ubiquitin acceptors.
Fig. 2. Stimulation of PPxY-dependent VLP production by chimeric HECT ubiquitin ligases. 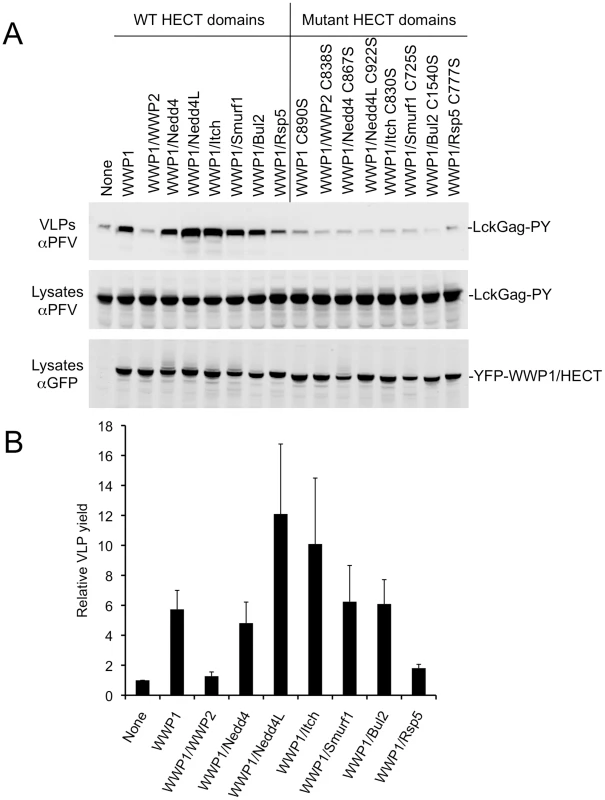
(A) Quantitative Western blot (LICOR) analysis of VLP release from 293T cells co-expressing Lck-Gag-PY and either YFP alone (None) or the indicated YFP-fused WWP1 C2/WW domains linked to the indicated HECT domains. Note that the unfused YFP is not visible in the “None” lane because it migrates to a different position on the blot. (B) Quantitation of Lck-Gag-PY protein in particles by quantitative Western blot analysis (LICOR). Values plotted are the levels of VLP associated Lck-Gag-PY protein generated in the presence of the indicated YFP-fused chimeric ubiquitin ligase, relative to that generated in the presence of YFP only (None). Data represent the mean and standard deviation of four independent experiments. Stimulation of Lck-Gag budding and HECT ubiquitin ligase autoubiquitination
To assess the relative catalytic activities of the chimeric HECT ligases, and assess whether this correlated with their differential ability to promote budding, we compared their abilities to carry out autoubiquitination and to ubiquitinate a Gag substrate encoding three lysine residues in close proximity to a PPxY late domain (Lck-Gag-PY-3K, see Fig. 1B). To accomplish this, we immunoprecipitated either Gag or HECT ubiquitin ligases from 293T cell lysates, prepared 36 hours after co-transfection with plasmids expressing Lck-Gag-PY-3K, HA-tagged ubiquitin, and each of the YFP-fused chimeric HECT ligases. Cell lysates were prepared using denaturing, detergent-rich buffer (containing 0.5% SDS) to ensure dissolution of protein complexes, and ubiquitinated species were detected by immunoprecipitation with either αPFV Gag or αGFP antibodies followed by immunoblot analysis of the precipitates with an αHA antibody (Fig. 3).
Fig. 3. Chimeric HECT ligases catalyze varying levels of Lck-Gag-PY-3K ubiquitination and autoubiquitination. 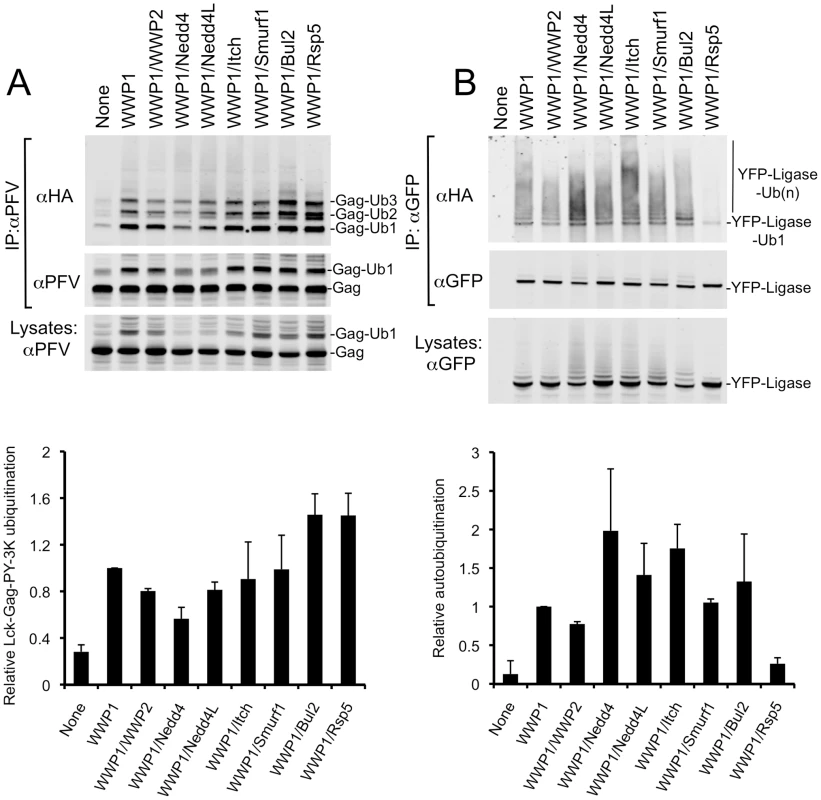
Quantitative Western blot analysis of lysine-containing Lck-Gag-PY-3K proteins (A) and chimeric HECT ligase proteins (B), immunoprecipitated from 293T cells following cotransfection with plasmids expressing Lck-Gag-PY-3K, HA-ubiquitin, and the indicated chimeric YFP-WWP1/HECT ubiquitin ligases. The αPFV Gag immunoprecipitates were probed with an αHA monoclonal antibody (A, top panel) or αPFV serum (A, middle panel). The αGFP immunoprecipitates were probed with αHA (B, top panel) and αGFP (B, middle panel) monoclonal antibodies. Alternatively, unfractionated cell lysates were probed with αPFV serum or αGFP antibody (A and B, bottom panels). Lanes marked “none” contain immunoprecipitates or lysates from cells transfected with unfused YFP in place of a YFP-fused ubiquitin ligase. Note that the unfused YFP is not visible in these lanes because is migrates to a different position on the blot. Charts below each plot show quantitation (mean+SD of two experiments) of the total signals in each lane of the blots in the upper panels that were probed for HA-ubiquitin. The levels of Gag ubiquitination and autoubiquitination are presented relative to that observed in the presence of intact WWP1, which was assigned a value of 1. Each of the chimeric HECT ubiquitin ligases was able to reasonably efficiently catalyze the addition of 1 to 3 ubiquitin moieties to the Lck-Gag-PY-3K substrate (Fig. 3A, upper panels). There was some variation in the ability of the HECT domains to catalyze the ligation of ubiquitin to Lck-Gag-PY-3K, with WWP1/Rsp5 and WWP1/Bul2 catalyzing the highest and WWP1/Nedd4 the lowest levels of ubiquitin ligation to Lck-Gag-PY-3K (Fig. 3A). However, there was no correlation between the extent to which each HECT domain stimulated Lck-Gag-PY-3K ubiquitination (Fig. 3A) and the degree to which it stimulated the release of VLPs assembled using Lck-Gag-PY or Lck-Gag-PY-3K (Fig. 2A and data not shown). For example, WWP1/Bul2 and WWP1/Nedd4, which induced the highest and lowest levels of Gag ubiquitination, respectively (Fig. 3A), stimulated budding to a similar extent (about 6-fold, Fig. 2A). Moreover, WWP1/Rsp5, which efficiently catalyzed Gag ubiquitination (Fig. 3A), enhanced particle release only marginally (Fig. 2A), much less efficiently than the WWP1/Nedd4L that induced comparatively modest levels of Gag ubiquitination (Fig. 3A).
We observed a better, albeit imperfect, correlation between the ability of the chimeric HECT ligases to catalyze autoubiquitination and to stimulate VLP production (Fig. 3B, Fig. 2B). Chimeric ligases that strongly promoted Lck-Gag-PY VLP release (e.g. WWP1/Itch and WWP1/Nedd4L) were more heavily autoubiquitinated, while those that failed or only marginally promoted VLP release (WWP1/WWP2 and WWP1/Rsp5, Fig. 2) exhibited the lowest levels of autoubiquitination (Fig. 3B). The correlation was imperfect, however, since WWP1/Nedd4, which moderately enhanced particle release (Fig. 2), was consistently highly auto-ubiquitinated (Fig. 3B). Notably, there was no correlation between the ability of the HECT ubiquitin ligases to catalyze autoubiquitination, and their ability to catalyze ubiquitin ligation to Lck-Gag-PY-3K (Fig. 3A, B). Overall, these data confirm our previous finding that direct Gag ubiquitination is dispensable for HECT ligase-dependent budding [29] and further indicates that intrinsic catalytic activity of the HECT ubiquitin ligases is critical for their ability to stimulate budding.
Reciprocal exchange of HECT ubiquitin ligase and Tsg101 domains results in functional hybrid L-domain cofactors that can promote viral budding
We next asked whether the need to recruit a HECT domain in the context of PPxY/WWP1 interaction was necessary for particle release, or whether the HECT domain could be bypassed by direct recruitment of putative downstream effectors. Additionally, we asked whether recruitment of a HECT domain in the absence of the other domains (C2 and WW) found in the Nedd4-like family of proteins was sufficient to stimulate particle budding. To accomplish this, we constructed hybrid L-domain cofactors in which the essential domains were split and linked to putatively complementing domains in another L-domain cofactor (Fig. 4A). Specifically, Tsg101 is a core component of ESCRT-I and contains two domains that are functionally important with respect to viral budding. The N-terminal ubiquitin E2 variant (UEV) domain interacts directly with P(T/S)AP peptide motifs and ubiquitin [7], while the C-terminal portion of the protein is a key structural component of ESCRT-I, interacting with other components, e.g. VPS28 and VPS37 [53], [54], [55] and is essential to support Tsg101 dependent budding. We constructed an artificial putative chimeric L-domain cofactor in which the C2/WW domains of WWP1 were linked to the C-terminal portion of Tsg101 (Tsg-C) that constitutes the core structural component of ESCRT-I (residues 157–390, Fig. 4A). Notably, overexpression of this chimeric protein, termed WWP1-Tsg-C, stimulated Lck-Gag-PY particle release in a dose-dependent manner but had no effect on particle production by the L-domain-deficient Lck-Gag(L-) protein (Fig. 4B, left and middle panels). This chimeric protein, therefore, appeared capable of recruiting a functional ESCRT-I complex to PPxY L-domains and thereby stimulating particle production. Conversely, WWP1-Tsg-C overexpression inhibited Lck-Gag(PSAP) budding in a dose-dependent manner (Fig. 4B, right panel). We surmise that since this chimeric protein lacks the domains required for interaction with PT/SAP motifs, it acts as an inhibitor of PSAP-dependent budding by sequestering endogenous components (e.g. VPS28 and VPS37) into retargeted ESCRT-I complexes that can be recruited to PPxY, but not PT/SAP, L-domains. Thus, these experiments demonstrate that the requirement for a HECT domain (and, by inference, the requirement for ubiquitin ligation) in PPxY/ubiquitin ligase dependent viral budding can be bypassed, if an alternative link to the ESCRT machinery is provided.
Fig. 4. Exchange of functional domains in L-domain cofactors. 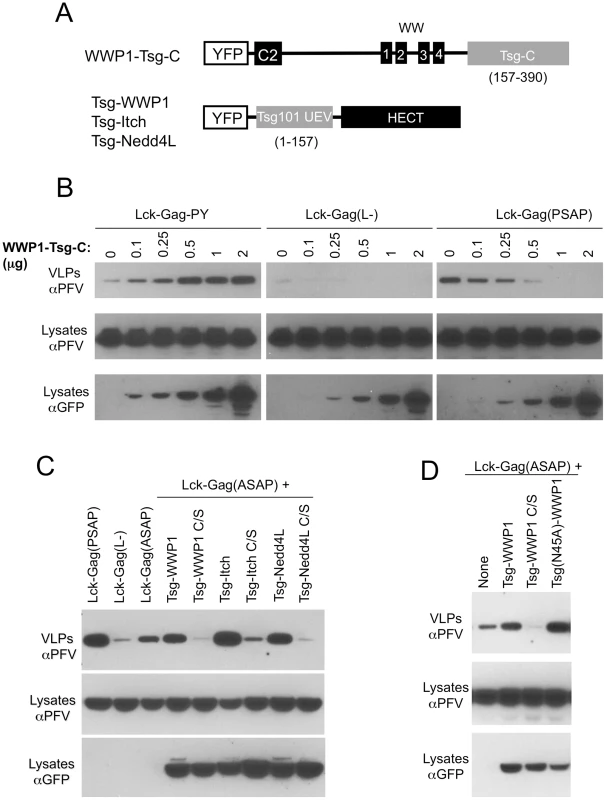
(A) Schematic representation of chimeric proteins designed to recruit ESCRT-I to PPxY motif (WWP1-Tsg-C), and isolated catalytic domain of a HECT ubiquitin ligase to a PTAP motif (Tsg-WWP1, Tsg-Itch and Tsg-Nedd4L). (B) Stimulation of PPxY-dependent budding in the absence of a HECT domain by direct recruitment of ESCRT-I. Specifically, VLP production from 293T cells expressing Lck-Gag-PY, Lck-Gag(L-) or Lck-Gag(PSAP) and increasing amounts of YFP-fused WWP1-Tsg-C (the amount of cotransfected WWP1-Tsg-C expression plasmid (in µg) is indicated) was assessed by western blotting. (C,D) VLP production from 293T cells expressing Lck-Gag containing either a wild-type (Lck-Gag(PSAP)), inactive Lck-Gag(L-), or attenuated Lck-Gag(ASAP) late domain and the indicated WT or catalytically inactive mutant (C/S) chimeric Tsg-WWP1, Tsg-Itch and Tsg-Nedd4L proteins was assessed by western blotting. The three leftmost lanes in (C) and the single leftmost lane in (D) contain lysates from cells transfected with unfused YFP as a control in place of a YFP-fused Tsg-HECT protein. Note that the unfused YFP is not visible in these lanes because it migrates to a different position on the blot. In a reciprocal experiment, we asked whether the PPxY motif and the C2/WW domains of WWP1 could be functionally replaced in the context of HECT domain/ubiquitin dependent budding. In other words, we determined whether recruitment of a HECT domain is sufficient to stimulate particle release, in the absence of the other protein domains (C2 and WW) to which it would ordinarily be linked. Specifically, we attempted to redirect P(T/S)AP-dependent particle production through a HECT domain-dependent pathway, by constructing chimeric proteins, termed Tsg-WWP1, Tsg-Itch and Tsg-Nedd4L, that contained the N-terminal UEV domain (residues 1–157) of Tsg101 linked to one of the three respective HECT domains (Fig. 4A). To test the function of these artificial putative L-domain cofactors, we also constructed an attenuated “leaky” mutant of the PT/SAP motif in the Lck-Gag(PSAP) protein, namely Lck-Gag(ASAP), by mutating the first proline residue of the PSAP motif to alanine. In the context of the HIV-1 PTAP motif, such a mutation reduces the affinity for, but does not eliminate binding to the Tsg101 UEV domain [7]. Correspondingly, the budding of Lck-Gag(ASAP), was attenuated as compared to Lck-Gag(PSAP), but the ASAP motif clearly retained some weak residual ability to stimulate budding (Fig. 4C, leftmost three lanes), suggesting that it retains some residual ability to recruit the Tsg101 UEV domain.
Overexpression of Tsg-WWP1, Tsg-Itch or Tsg-Nedd4L, respectively) resulted in clear stimulation of Lck-Gag(ASAP) budding (Fig. 4C). Tsg101-Itch was the most potent of the three Tsg101-HECT proteins tested by this approach, and its overexpression resulted in a particle yield that matched or even exceeded that observed in the presence of the intact PSAP motif (Fig. 4C). In contrast, expression of catalytically inactive versions of Tsg-WWP1, Tsg-Itch or Tsg-Nedd4L inhibited rather than enhanced Lck-Gag(ASAP) particle production (Fig. 4C). Because the Tsg101 UEV domain contains ubiquitin-binding activity that might complicate the interpretation of these results, we repeated these experiments using a mutant Tsg101 UEV domain (N45A) that is defective for ubiquitin binding, linked to a WWP1 HECT domain. The mutant Tsg(N45A)-WWP1 fusion stimulated budding at least as efficiently as did the unmanipulated Tsg-WWP1 protein (Fig. 4D).
Overall, the experiments in Fig. 4 demonstrate that the domains of the PTAP and PPxY binding cofactors can be functionally split into modular, interchangeable domains that are (i) necessary for binding to the L-domain and (ii) interface with downstream effectors that are critical for budding. Most notably, these findings suggest that simple recruitment of a HECT domain to sites of particle budding, irrespective of its mode of recruitment, and in the absence of ubiquitin acceptors on the viral protein, is sufficient to stimulate particle release and that other HECT ubiquitin ligase domains are dispensable for budding.
Direct fusion of ubiquitin to Lck-Gag promotes particle release
The aforementioned experiments demonstrated that the requirement for a catalytically active HECT domain could be obviated by direct recruitment of ESCRT-I to a viral protein (Lck-Gag-PY) whose budding would normally be dependent on such recruitment. We next asked whether the requirement for HECT domain recruitment could similarly be obviated, in the context of a nearly identical viral protein, by simply depositing ubiquitin at the site of particle assembly, in the absence of ubiquitin ligase recruitment. To mimic the deposition of ubiquitin at sites of virion assembly, in the absence of ubiquitin ligase recruitment, we expressed an Lck-Gag protein, lacking L-domains, with a single ubiquitin appended at its C-terminus (Lck-Gag-Ub, Fig. 5A). Ubiquitin is normally conjugated to proteins by an isopeptide bond between the C-terminal glycine residue of ubiquitin and the ε-amino group of a lysine residue within the substrate protein. Therefore, to avoid aberrant conjugation of our Gag-ubiquitin chimeras to other proteins we deleted two glycine residues from the C-terminus of ubiquitin (Fig. 5A).
Fig. 5. Direct fusion of ubiquitin to Lck-Gag stimulates L-domain-dependent and -independent budding. 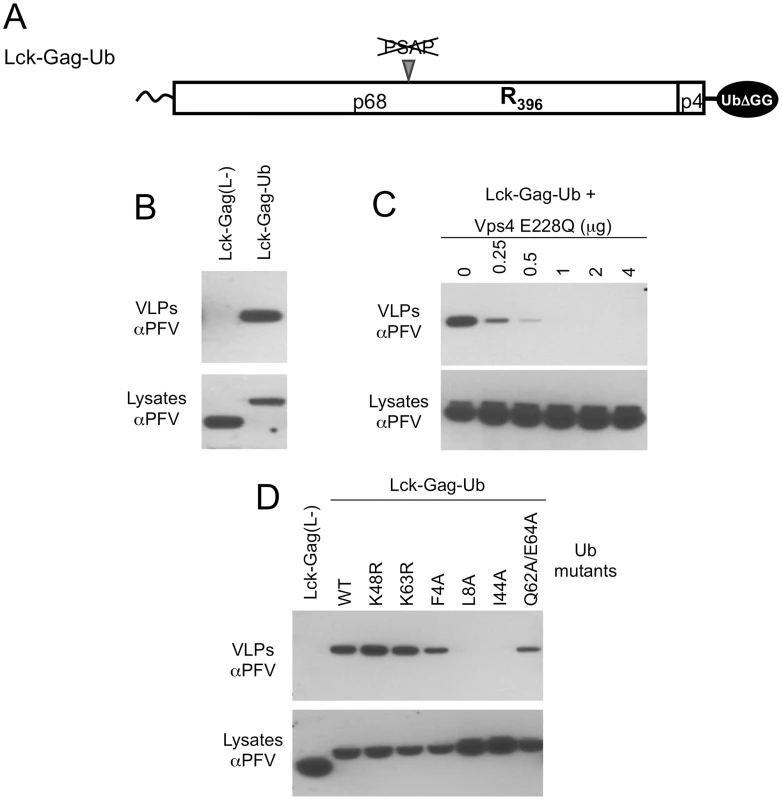
(A) Schematic representation of the Lck-Gag-Ub protein with a single ubiquitin moiety fused at the carboxyl terminus. Two glycine residues were removed from the C-terminus of ubiquitin to prevent its conjugation to other proteins. (B) VLP production from 293T cells expressing the Lck-Gag-Ub protein, or a control Lck-Gag(L-) protein was assessed by western blotting. (C) VLP production from 293T cells expressing Lck-Gag-Ub and increasing amounts of GFP-Vps4 (E228Q). (D) VLP release from 293T cells expressing Lck-Gag-Ub containing the indicated mutations in C-terminally fused ubiquitin. Cells expressing ubiquitin-fused, but L-domain-deficient Gag (Lck-Gag-Ub) generated extracellular particles while those expressing the unfused, L-domain deficient counterpart Lck-Gag(L-) protein did not (Fig. 5B). Directly fused ubiquitin-dependent particle release was strongly inhibited, in a dose dependent manner, by expression of a catalytically inactive version of the ATPase VPS4 (Fig. 5C), indicating that the ESCRT pathway was required for Lck-Gag-Ub particle release. Thus, in the context of Lck-Gag, direct ubiquitin fusion appeared capable of substituting for a PSAP or PPxY containing L-domain. These results are similar to findings made by Joshi et al. who showed that direct fusion of ubiquitin to EIAV Gag can functionally substitute for the ALIX-binding YPDL L-domain encoded therein [48]. Similarly, we also found that ubiquitin-dependent budding was dependent on the ubiquitin hydrophobic patch residues (L8 and I44) and additionally, marginally dependent on residues (Q62 and E64) that have been implicated in ubiquitin-Tsg101 UEV domain interaction (Fig. 5D). However, lysine residues (K48 and K63) that are often important for the conjugation of further ubiquitin molecules could be mutated without affecting fused ubiquitin-dependent particle release (Fig. 5D).
Ubiquitin and PSAP motifs synergize to stimulate Lck-Gag VLP release
Next we analyzed the effect of combining L-domains and ubiquitin on VLP release. To accomplish this, Lck-Gag proteins containing various combinations of the L-domains and C-terminally fused ubiquitin (Fig. 6A) were expressed. Quantitative analyses revealed that directly fused ubiquitin-dependent (Lck-Gag-Ub) particle release was at least as efficient as that driven by PSAP (Lck-Gag(PSAP)) or PPxY (Lck-Gag-PY) L-domains (Fig. 6B). Moreover, and in contrast to the previous report with EIAV Gag [48], we found that the combined presence of fused ubiquitin and a PSAP L-domain (in Lck-Gag(PSAP)-Ub) resulted in strongly synergistic effects on particle release (Fig. 6B). Specifically, Lck-Gag(PSAP)-Ub generated ∼20-fold and ∼6-fold more particles than Lck-Gag(PSAP) and Lck-Gag-Ub, respectively (Fig. 6B). No such synergy was observed when a PPxY L-domain and ubiquitin were combined in the same Gag protein. In fact, the Lck-Gag-Ub and the Lck-Gag-PY-Ub generated extracellular particles with approximately the same efficiency (Fig. 6B). Less dramatic, but nonetheless synergistic enhancement of particle release was evident when PPxY and PSAP motifs were both present (in the absence of ubiquitin fusion, Fig. 6B). In this case, the presence of the PPxY motif (in Lck-Gag(PSAP)-PY) enhanced particle release approximately ∼5-fold as compared to the situation where the PSAP motif was the only L-domain (in Lck-Gag(PSAP), Fig. 6B). Overall these results are consistent with the notion that ubiquitin behaves essentially like an L-domain, and further suggests that it functions synergistically with a PT/SAP motif, and redundantly with a PPxY motif.
Fig. 6. Synergy between a PSAP motif and ubiquitin during viral budding and effect of Gag-ubiquitin levels on particle release. 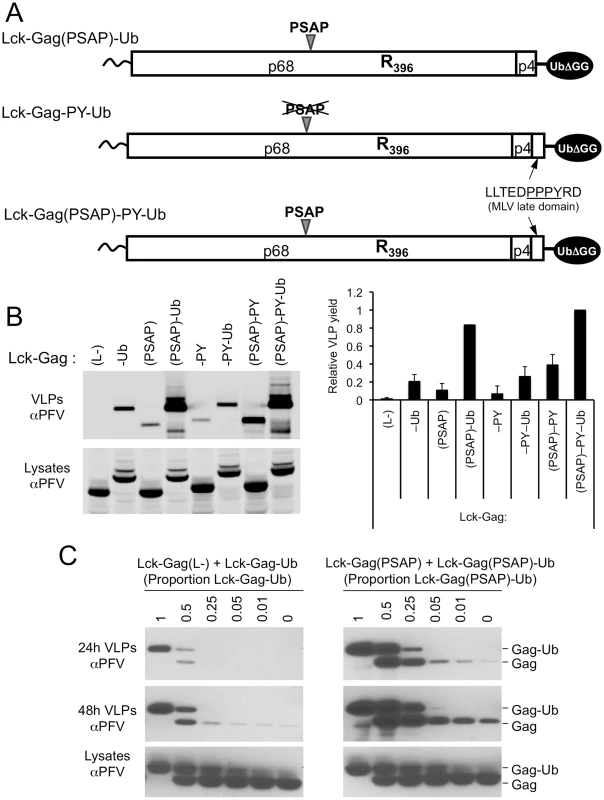
(A) Schematic representation of some examples of the Lck-Gag proteins used in these experiments, encoding PSAP and/or PPxY L-domains and a single ubiquitin moiety fused at the carboxyl-terminus. (B) VLP release from by 293T cells expressing Lck-Gag proteins containing the indicated L-domains that were present alone or in combination with each other and/or directly fused ubiquitin (see panel A), measured by quantitative western blotting. The chart to the right of the blot shows the yield of VLPs (mean+SD of 2 experiments) and values are presented relative to the VLP yield obtained in the presence of both L-domains and ubiquitin (Lck-Gag(PSAP)-PY-Ub), which was assigned a value of 1. (C) VLP production from 293T cells expressing Lck-Gag(L-) or Lck-Gag(PSAP) proteins, where the total amount of Gag protein expressed was constant, but varying proportions carried a fused ubiquitin. Cells were transfected with equal total amounts of Lck-Gag(L-)+Lck-Gag-Ub (left panels) or Lck-Gag(PSAP)+Lck-Gag(PSAP)-Ub expression plasmids, and the indicated fraction of the total transfected plasmid mixture expressed the ubiquitin fused form of the Gag protein. VLPs were harvested both at 24h and 48h after transfection and subjected to western blot analysis with αPFV antiserum. We next attempted to mimic a situation that is somewhat typical of retroviruses, where only a fraction of Gag expressed in cells carries ubiquitin. This was done by co-expressing ubiquitin-fused and unfused Lck-Gag proteins in varying proportions. When this was done in the context of a Lck-Gag proteins lacking a PSAP motif (by co-expressing Lck-Gag(L-) and Lck-Gag-Ub), particle production was most efficient when a large fraction of the total Lck-Gag protein carried ubiquitin, and no stimulation of particle production was detectable when less than 25% of the Gag protein carried fused ubiquitin (Fig. 6C, left panel). When similar experiments were done in the presence of a PSAP late domain, by co-expressing Lck-Gag(PSAP) and Lck-Gag(PSAP)-Ub, stimulation of particle release was observed when smaller fractions of Gag, as little as a few percent, carried ubiquitin (Fig. 6C, right panel). Nonetheless, larger fractions of ubiquitin fused Gag had larger stimulating effects on particle release. Thus, these experiments suggest that the greater the number of ubiquitin molecules that are present at sites of particle assembly, the more efficient is particle release; however, relatively modest amounts of ubiquitin can significantly enhance particle budding in the presence of a PSAP motif.
PPxY L-domain-dependent and directly fused ubiquitin-dependent particle release are similarly dependent on particular ubiquitin binding class E VPS factors
Several class E vacuolar protein-sorting factors have been reported to possess ubiquitin binding activity (Table 1). Although the affinity of such domains for monoubiquitin is generally quite weak (Kd>100µM), several class E factors form multiprotein complexes with several ubiquitin-binding surfaces, which could provide sufficient avidity for their retention at sites of virion assembly. Under such a scenario, efficient recruitment of ESCRT complexes might require deposition of relatively large numbers of ubiquitin molecules in the vicinity of the assembling particle, a notion that is consistent with the finding that a large fraction of Gag must carry ubiquitin to compensate for the absence of a late domain (Fig. 6C).
Tab. 1. Class E VPS factors and associated proteins encoding ubiquitin binding domains. 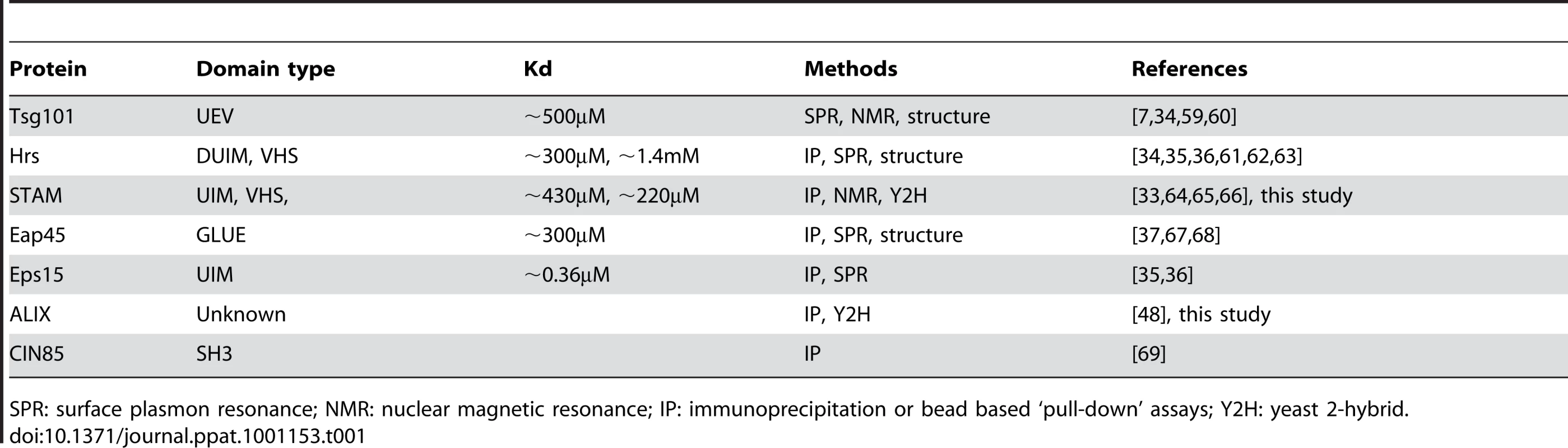
SPR: surface plasmon resonance; NMR: nuclear magnetic resonance; IP: immunoprecipitation or bead based ‘pull-down’ assays; Y2H: yeast 2-hybrid. To determine which of the mammalian ESCRT complexes and associated proteins might be most important for ubiquitin dependent budding, we performed a directed yeast two-hybrid screen in which ubiquitin binding to a range of human class E VPS factors and associated proteins was surveyed. These included components of ESCRT-0 (Hrs, HBP/STAM), ESCRT-I (Tsg101, VPS28, VPS37A,B,C, Mvb12), ESCRT-II (Eap20, Eap30, Eap45) ESCRT-III (CHMP1A, 1B, 2A, 2B, 3, 4A, 4B, 4C, 5, 6), as well as several ESCRT-associated proteins or proteins that are known to bind to components of the class E VPS pathway (ALIX, LIP5, VPS4, UBPY,CMS, CIN85). Most of these proteins, including known ubiquitin binding factors (Table 1), gave either weak or non-specific signals. Since we were testing ubiquitin binding by each protein individually and outside of its natural context and in the absence of ESCRT complex partners, it was perhaps to be expected that this assay would fail to detect ubiquitin interactions in at least some instances. Nonetheless, HBP/STAM, ALIX, and UBPY binding gave robust signals in WT ubiquitin binding assays, and binding was abolished when the ubiquitin hydrophobic patch was mutated (I44A), (Fig. 7A).
Fig. 7. Role of ubiquitin-binding class E VPS factors in ubiquitin-dependent and PPxY-dependent particle release. 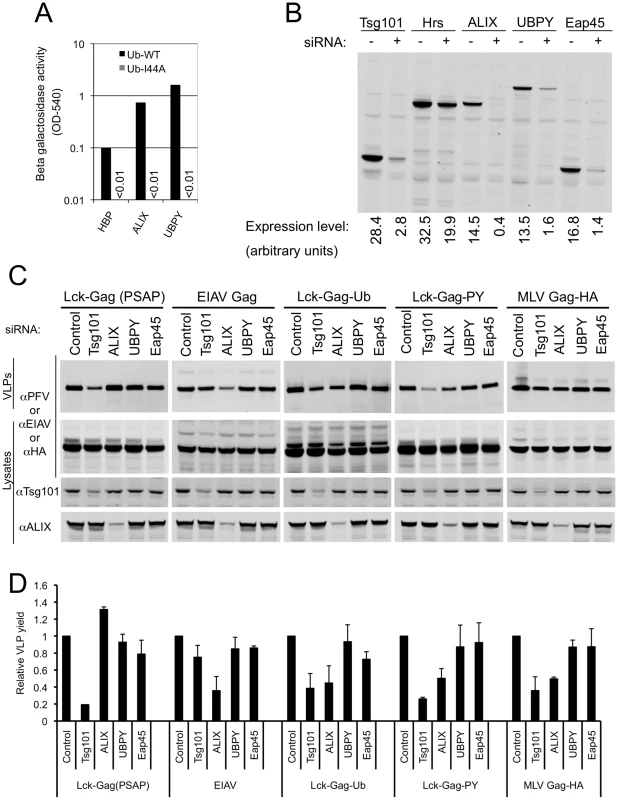
(A) Yeast two-hybrid analysis of the interaction between the indicated class E VPS factors and ubiquitin containing either an intact (Ub WT) or disrupted (Ub I44A) hydrophobic patch. β-galactosidase expression was measured (as optical density at 540nm (OD540)) in Y190 cells transformed with the indicated Gal4-DNA binding domain-ubiquitin and VP16 activation domain (-HBP, -ALIX, and -UBPY) fusion constructs. Absence of a bar indicates background levels of β-galactosidase expression. A single representative of two independent experiments is shown. (B) Validation of siRNAs targeting ubiquitin-binding ESCRT-complexes or other class E VPS factors. Lysates of 293T cells transfected with GFP-Tsg101 or YFP-Hrs, -ALIX, -UBPY, or -Eap45 expression plasmids and siRNAs targeting either luciferase (−) or the specified class E VPS factors (+) were probed with an αGFP monoclonal antibody. (C) L-domain-specific inhibition of Gag budding by class E factor depletion. Quantitative Western blot analysis of VLP production from 293T cells transfected with plasmids expressing Lck-Gag(PSAP), EIAV Gag, Lck-Gag-Ub, Lck-Gag-PY or MLV Gag-HA and siRNAs directed against the indicated class E VPS protein. Corresponding cell lysates were also probed with antibodies to PFV, EIAV, HA, Tsg101 and/or ALIX, as appropriate. (D) Quantitation of VLP release following knockdown of the indicated class E VPS proteins. Values are plotted the mean+SD of two or three independent experiments and represent the levels of particles released relative to those released from cells transfected with control luciferase siRNAs, which was assigned a value of 1. We next determined the effect of siRNA mediated disruption of known ubiquitin-binding complexes, as well the additional ESCRT-associated factors that were positive in our yeast 2-hybrid survey (ALIX and UBPY), on PPxY-dependent and fused ubiquitin-dependent Lck-Gag budding. The core components of the known ubiquitin binding ESCRT complexes (ESCRT-0, ESCRT-I and ESCRT-II) were targeted using pools of four siRNAs directed to Hrs, Tsg101 and Eap45, respectively. The potency of the siRNA pools was estimated by cotransfecting them with plasmids expressing YFP-tagged target proteins, followed by quantitative western blotting. By these criteria the Tsg101, Eap45, ALIX and UBPY siRNAs appeared effective (Fig. 7B). However, knockdown of Hrs was inefficient, so its effect on budding could not be reliably assessed. Because antibodies to Tsg101 and ALIX were available, the level of endogenous proteins could also be monitored in these siRNA experiments. Quantitative western blotting analyses (examples are shown in Fig. 7C) indicated that Tsg101 and ALIX proteins were reduced to 38±9% and 16±4% of endogenous levels, respectively. Notably, control experiments showed that Lck-Gag(PSAP) particle release was specifically inhibited (∼5-fold) by Tsg101 siRNA, but only marginally affected by EAP45, ALIX and UBPY depletion (Fig. 7C,D), while EIAV Gag particle release was specifically inhibited (∼3-fold) by ALIX depletion, but not by depletion of the other ESCRT-associated proteins (Fig. 7C,D).
Ubiquitin-dependent (Lck-Gag-Ub) budding was modestly inhibited (∼3-fold) by depletion of either Tsg101 or ALIX but was barely affected by UBPY or Eap45 siRNAs (Fig. 7C,D), suggesting that ubiquitin binding to ESCRT-I and ALIX contributes to its ability to mediate particle release. This finding mirrors a previous report using ubiquitin fused to EIAV Gag [48]. Additionally, however, we further found that Lck-Gag-PY exhibited a similar pattern sensitivity to class E factor-targeting siRNAs, in that it was modestly sensitive to Tsg101 and ALIX but not Eap45 or UBPY siRNAs (Fig. 7C,D). Similarly, the budding of an MLV Gag protein, that carries the same PPxY L-domain was also modestly sensitive to depletion of Tsg101 and ALIX Fig. 7C,D).
Because ESCRT-I and ALIX perturbation both affected ubiquitin and PPxY-dependent budding, we sought to determine whether their simultaneous depletion would exhibit a stronger inhibitory effect. Unfortunately, cotransfection of the two pools of siRNAs (or each pool together with normalizing control RNA duplexes,) rendered each somewhat less effective, perhaps due to dilution of the active siRNAs (Fig. 8A). Specifically, Tsg101 protein levels were reduced to 42±2% and 50±2% of endogenous levels, while ALIX protein levels were reduced to 30±3% and 27±2% of endogenous levels, when the Tsg101 or ALIX targeted siRNAs were cotransfected together or with normalizing control siRNAs, respectively (Fig. 8A). Thus, under these conditions, siRNAs targeting ALIX did not inhibit Lck-Gag-Ub or Lck-Gag-PY particle release (Fig. 8A,B). Nevertheless, simultaneous (albeit partial) depletion of Tsg101 and ALIX had a significantly stronger inhibitory effect on Lck-Gag-Ub, Lck-Gag-PY and MLV Gag budding (Fig. 8A, B) than did the more effective individual depletion of either Tsg101 or ALIX alone (Fig. 7C, D), suggesting that they both proteins contribute to optimal PPxY and ubiquitin-dependent budding.
Fig. 8. Effect of simultaneous ESCRT-I and ALIX disruption on ubiquitin-dependent and PPxY-dependent particle release. 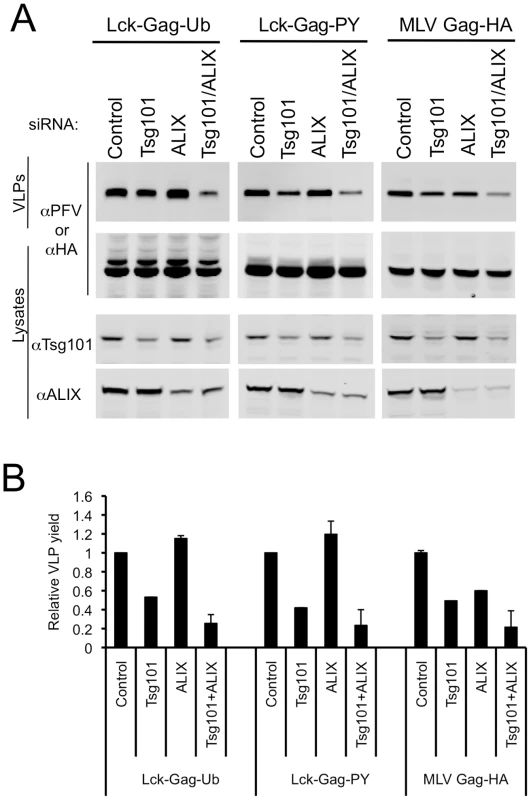
(A) Quantitative Western blot analysis of VLP production from 293T cells transfected with plasmids expressing Lck-Gag-Ub, Lck-Gag-PY or MLV Gag-HA and siRNAs targeting Tsg101, ALIX or both. Corresponding cell lysates were also probed with antibodies to PFV, HA, Tsg101 and/or ALIX, as appropriate, and as indicated. (B) Quantitation of VLP release following knockdown of Tsg101, ALIX or both. Values are plotted as the mean+SD of two or three independent experiments and represent the levels of particles released relative to those released from cells transfected with control luciferase siRNAs, which was assigned a value of 1. Discussion
The precise role of HECT ubiquitin ligases in promoting PPxY-dependent virion release has, heretofore, been somewhat unclear. Our previous studies suggest that their ubiquitin ligase activity is critical for their ability to stimulate budding [17], [29], but the functionally relevant substrate for ubiquitination has been difficult to define. Additionally, there is some evidence suggesting that HECT ubiquitin ligases may also function as adaptors for bridging factors that recruit ESCRT proteins to assembling virions [17], [32], [51].
We compared the activities of HECT domains from various Nedd4-like family HECT ubiquitin ligases by fusing them to the C2 and WW domains of WWP1. While this strategy does not illuminate which ubiquitin ligases are responsible for viral budding in the natural context, it does allow an assessment of HECT domain function in a uniform background. We found that HECT domains varied significantly in their ability to stimulate PPxY-dependent particle release in this context. This variability was evident when there were no ubiquitin acceptors in the Gag protein and correlated better with the ability of the HECT domains to drive autoubiquitination than with their ability to ubiquitinate a modified Gag substrate that contained lysines proximal to a PPxY motif. The correlation between autoubiquitination and budding was imperfect, however, and it is possible that variation among the HECT domains in their ability to catalyze different lengths and types of ubiquitin chains (e.g. K48 versus K63-linked chains), or their ability to ubiquitinate other bystander proteins, could influence their ability to stimulate viral budding. In this regard it was notable that there was no correlation between the ability of the HECT domains to undergo autoubiquitination versus their ability to catalyze ubiquitin ligation to Lck-Gag-PY-3K. It was nonetheless true that the ability of the HECT domains to stimulate budding was, in every case, absolutely dependent on their ability to catalyze the ligation of ubiquitin to a substrate. This suggests that the proposed role of HECT domains as adaptors that bind directly to downstream factors is of secondary importance in stimulating budding, or that this adaptor function requires catalytic activity. This latter scenario could, conceivably, be operative as a result of HECT autoubiquitination.
These studies underscore the remarkable flexibility in the ways that the ESCRT pathway can be engaged to achieve viral budding (Fig. 9) Using a single viral Gag protein as a model, particle budding could be achieved by: (i) conventional direct recruitment of the ESCRT pathway via PTAP binding to Tsg101, (ii) direct recruitment of the ESCRT pathway via PPxY binding to a hybrid cofactor consisting of the C2/WW domains of WWP-1 linked to the C-terminal domain of Tsg101, (iii) recruitment of a HECT ubiquitin ligase via a PPxY motif, (iv) recruitment of an isolated HECT domain to a PTAP motif using a hybrid L-domain cofactor consisting of the UEV domain of Tsg101 linked to a HECT domain or (v) direct fusion of ubiquitin to Gag. These results suggest that the cellular factors (in this case Tsg101, ubiquitin ligases and ubiquitin) that are either directly recruited or deposited at the site of viral particle budding behave as modular entities, with domains that are necessary and sufficient for their own recruitment, and distinct domains that are necessary and sufficient for the subsequent recruitment of downstream effectors of particle release (Fig. 9).
Fig. 9. Diversity of pathways that can be used to engage the ESCRT machinery. 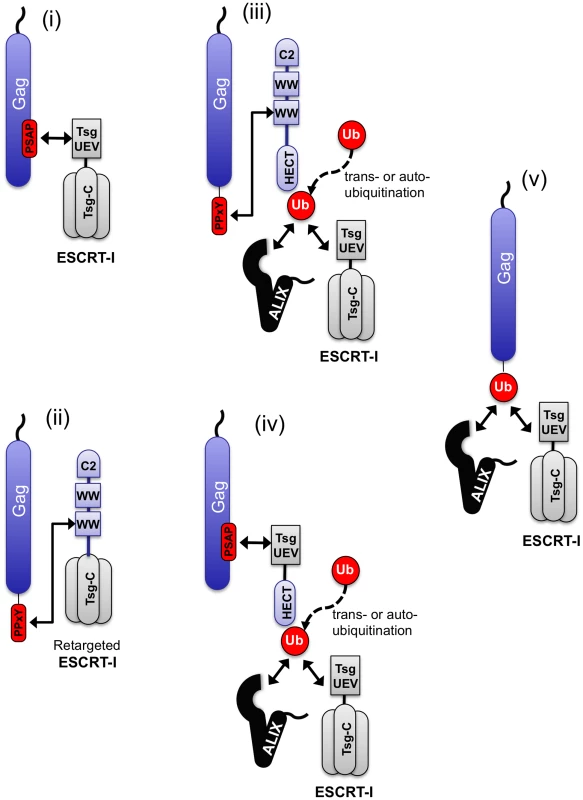
The ESCRT pathway can be engaged by Gag using a variety of natural and artificial mechanisms (i)–(v) that include ubiquitin ligation to trans-acting proteins, (perhaps including the ubiquitin ligase itself) or fusion to Gag. These studies suggest that ubiquitin functions like a transferable L-domain, recruiting class E VPS factors such as ESCRT-I and ALIX, independently of the identity if the protein to which it is attached. When HECT domains were used to promote budding, the requirement for catalytic activity was absolute, irrespective of how they were recruited to Gag and, importantly, in the absence of ubiquitin acceptors on the viral Gag protein. This finding suggests that ligation of ubiquitin to trans-acting factors, perhaps including the HECT domain itself (i.e. autoubiquitination), rather than Gag is important for viral budding. It is superficially paradoxical, therefore, that ubiquitin could promote budding of the very same Gag protein even when ubiquitin was not ligated to a trans-acting factor, but rather was directly fused to Gag. These findings suggest that the identity of the protein(s) to which ubiquitin is attached is not of critical importance, and ubiquitination substrates can, in principle, include Gag, the ubiquitin ligase itself, or other trans-acting proteins. The mere presence of ubiquitin at the site of particle assembly appears sufficient to engage the ESCRT pathway and stimulate budding.
The intrinsic manipulability of L-domains, the proteins that bind to them (specifically ESCRT-I and HECT ubiquitin ligases) and the apparent lack of importance of the identity of ubiquitination substrate suggests that each serve simply as recruitment factors to engage the downstream machinery that mediates membrane fission and particle release. Since ubiquitin binds to the very same factors (ESCRT-I and ALIX) that are bound by PT/SAP and YPXL type L-domains, and depends on them to stimulate budding, then ubiquitin itself can be conceptually viewed, in the context of viral budding, as a transferable L-domain that acts in a position-independent manner. In essence, this notion is a simple extension of the concept originally demonstrated by Parent et al, who showed that conventional L-domains function in a position independent, transferable manner [56].
A finding that is consistent with the aforementioned arguments, is that budding that was dependent either on a PPxY motif or a ubiquitin fused directly to Gag exhibited similar dependence on particular components of the ESCRT pathway. Notably, perturbation of individual segments of the pathway (ESCRT-I and ALIX) caused partial inhibition of ubiquitin-dependent Lck-Gag-PY, Lck-Gag-Ub and MLV Gag particle release. Previous work has shown that Mason-Pfizer monkey virus particle release, which is dependent on a PPxY motif, is quite strongly inhibited by depletion of Tsg101 [31] and that budding of a EIAV Gag-ubiquitin fusion protein is modestly inhibited by Tsg101 or ALIX depletion [48]. We found that simultaneous perturbation of ESCRT-I and ALIX resulted a stronger suppression of Lck-Gag-PY, Lck-Gag-Ub and MLV Gag particle release than did depletion of either protein alone, suggesting that both ESCRT-I and ALIX can contribute to optimal PPxY - and ubiquitin-dependent budding (Fig. 9). Indeed, the class E VPS pathway includes multiple ubiquitin-interacting factors, each of which could, in principle, provide parallel mechanisms for engaging the ESCRT machinery. While ESCRT-I and ALIX appeared to be most important for PPxY - and ubiquitin-dependent budding, these experiments do not exclude a contributory role for other ubiquitin binding complexes in the ESCRT pathway. A similar notion was recently demonstrated in yeast, where simultaneous disruption of ubiquitin binding by ESCRT-I, -II and Bro1 (the yeast homologue of ALIX) was necessary to block the sorting of ubiquitinated cargo to the lysosome [57]. Thus, ubiquitin has several potential entry points into the ESCRT pathway, and it appears that multiple interactions must be simultaneously inhibited in order to profoundly inhibit ubiquitin - or HECT ligase-dependent budding.
Since ubiquitin-binding class E VPS factors generally have a low affinity for individual ubiquitin molecules (Table 1), the efficiency with which they are recruited to, and retained at, sites of particle assembly is likely related to the number of ubiquitin molecules that are locally present. Indeed, in the context of direct ubiquitin fusion to Lck-Gag, particle release efficiency increased as the proportion of Gag molecules that carried a ubiquitin was increased, and directly fused ubiquitin could effectively bypass the need for a conventional L-domain only when a large fraction (>50%) of the Gag molecules were fused to ubiquitin. This approximates to ∼1000 to 2500 ubiquitin molecules per assembling virion.
Previous studies have shown that direct ubiquitin fusion to RSV or EIAV Gag can alleviate a late budding defect imposed by proteasome inhibitors or functionally replace a YPDL L-domain [44], [48]. However, this study is the first to demonstrate that ubiquitin can act synergistically with a PTAP motif, resulting in dramatically enhanced particle release when both are present. Moreover, the ability of fused ubiquitin to stimulate budding became evident at significantly lower Gag-ubiquitin abundance (5% to 25% of total Gag) when a PTAP motif was also present in Gag. Since ubiquitin could serve as an additional docking site for Tsg101, it might synergize with PTAP motifs by increasing the overall affinity of the assembling Gag lattice for individual ESCRT-I complexes. In fact, this property was predicted by previous binding studies involving Tsg101 UEV domain, PTAP containing peptides and ubiquitin [7]. Ubiquitin might also synergize with PTAP motifs by providing binding sites for distinct class E VPS factors (e.g. ALIX), thereby optimally utilizing all the available components of the ESCRT machinery. Consistent with these ideas, PTAP and PPxY L-domains behaved synergistically in driving particle release, as did PTAP and Gag-fused ubiquitin. However, a PPxY motif and Gag-fused ubiquitin behaved redundantly, consistent with the notion that that they ultimately function through the same mechanism.
Materials and Methods
Plasmid construction
pCAGGS-based expression plasmids encoding Lck-Gag(PSAP), Lck-Gag(L-), Lck-Gag-PY, and Lck-Gag-PY-3K plasmids have been described previously [29]. The Lck-Gag(ASAP) plasmid was derived from Lck-Gag(PSAP) by PCR-based site-directed mutagenesis. The Lck-Gag(PSAP)-PY plasmid was generated by replacement of a StuI/XhoI fragment from the Lck-Gag(PSAP) plasmid with the corresponding fragment from the Lck-Gag-PY plasmid. cDNAs expressing Lck-Gag-Ub (ubiquitinΔGG) fusion proteins were generated by overlap-extension PCR, using pCAGGs-Lck-Gag(PSAP), Lck-Gag(L-), and Lck-Gag(PSAP)-PY as templates for the N-terminal portions and pHA-ubiquitin as the template for the C-terminal portion. The K48R, K63R, F4A, L8A, I44A, and QE62,64AA point mutations were introduced into the Lck-Gag-Ub construct by PCR-based mutagenesis. Each cDNA was cloned into pCAGGs for expression in mammalian cells.
DNAs encoding the HECT domains from WWP1 (residues 543–922), WWP2 (491–870), Nedd4 (520–902), Nedd4L (593–975), Itch (483–862), Smurf1 (374–757), and Rsp5 (431–809) were amplified from plasmids encoding the full-length HECT ligases [17], [58]. The Bul2 HECT domain (encoding residues 1189–1572) was PCR amplified from 293T cell cDNA. The catalytically inactive WWP1 HECT domain (C890S) was amplified from a previously described full-length mutant WWP1 ligase [17]. Catalytic point mutants of the remaining HECT domains were made by PCR-based mutagenesis. Chimeric ubiquitin ligases, comprising the C2 and WW domains (residues 1–542) of WWP1 and each of the HECT domains described above were generated by overlap PCR. Likewise, plasmids expressing Tsg-WWP1, Tsg-Nedd4L and Tsg-Itch (residues 1–157 of Tsg101 fused to HECT domains of WWP1, Nedd4L, or Itch) as well as WWP1-Tsg-C (residues 1–542 of WWP1 fused to residues 157–390 of Tsg101) were constructed by overlap-extension PCR. All cDNAs encoding chimeric proteins were inserted into pCR3.1/YFP, to express proteins fused to the C-terminus of YFP, for in mammalian cells. The class E VPS factor yeast two-hybrid library and plasmids expressing Vps4 E228Q, Tsg101, Hrs, ALIX, UBPY, and Eap45 fluorescent fusion proteins in mammalian cells have been described previously [8], [12], [54]. Yeast two-hybrid plasmids encoding wild type and I44A mutant ubiquitin were constructed by PCR amplification of ubiquitinΔGG from the pHA-ubiquitin plasmid using 5′ and 3′ primers appended with EcoRI restriction sites and cloning into the pGBKT7 (Clontech) and pVP16 vectors [8].
Virus release assays
For Gag particle release assays, 5×105 293T cells in six-well plates were transfected using polyethylenimine (Polysciences) with 1 µg of pCAGGs/Gag-derived plasmids, alone or with 1 µg of pCR3.1/YFP, pCR3.1/YFP-WWP1/HECT, pCR3.1/YFP-Tsg-HECT, or pCR3.1/YFP-C2-WW-Tsg-C plasmids, or the indicated amounts of pCR3.1/YFP-Vps4 E228Q plasmid. For EIAV and MLV VLP release assays, 293T cells were transfected in the same format with 500ng of, pCR3.1/EIAVGag or pCR3.1/MLVGag-HA plasmids. VLPs were pelleted by ultracentrifugation of 2 ml of 0.22-µm-filtered culture supernatants, collected 48 hours after transfection, over a 2ml 20% sucrose cushion for 90 min at 115,000×g. VLP and cell lysates were analyzed by Western blotting.
Ubiquitination assay
293T cells (5×105) in six-well plates were cotransfected with 1 µg of pCAGGs/Lck-Gag-PY-3K, 500 ng of pHA-ubiquitin, and 1 µg of the indicated chimeric pCR3.1-WWP1-HECT ligase. At 36h after transfection, cells were thoroughly lysed at room temperature in detergent-rich RIPA buffer (50mM Tris pH 7.4, 150mM NaCl, 1mM EDTA, 1.0% glycerol, 0.5% SDS, supplemented with protease inhibitor tablets (Roche) and 5mM N-ethylmaleimide to inhibit deubiquitination) and cleared of cellular debris by microcentrifugation. The lysates were then diluted 5-fold in the same buffer containing NP-40 rather than SDS, to adjust the concentration of SDS to 0.1% and NP-40 to 1.0%, and split into two fractions. From one fraction, Gag proteins were immunoprecipitated with αPFV serum and protein G-Sepharose beads. From the other fraction, YFP-HECT ligase proteins were immunoprecipitated with αGFP monoclonal antibody and protein G-Sepharose beads. Immunoprecipitates and unfractionated cell lysates were analyzed by Western blotting.
siRNA transfections
293T cells (3×105) in six-well plates were transfected with siGENOME siRNAs targeting Luciferase, Tsg101, Hrs, Alix, UBPY, or Eap45 (Dharmacon) using Lipofectamine 2000 (Invitrogen). After 24h, cells were transfected with the same siRNAs and the indicated Gag expression plasmids. VLP and cell lysates were prepared 48 h after the second transfection. To assess knockdown efficiency, 293T cells were transfected once with YFP-Tsg101, -Hrs, -ALIX, -UBPY, or -Eap45 expression plasmids and corresponding siRNAs. GFP expression in cell lysates harvested 48 h after transfection was assayed by quantitative Western blotting.
Western blot analyses
Virion and cell lysates and immunoprecipitates were separated on polyacrylamide gels, transferred to nitrocellulose membranes, and probed with various antibodies: anti-PFV human serum, anti-HIV-1 p24CA (183-H12-5C), anti-EIAV equine serum (VMRD, Inc.), anti-GFP (Roche), and anti-HA (HA.11, Covance) anti-Tsg101 (4A10, Abcam, Cambridge, MA) or anti-ALIX rabbit serum (a gift from Wes Sundquist). Subsequently, the blots were probed with species-specific peroxidase-conjugated secondary antibodies and chemiluminescent substrate reagents. Alternatively, for quantitative Western blotting, membranes were probed with species-specific antibodies conjugated to IRDye800CW, and fluorescent signals were detected and quantified using a LICOR Odyssey scanner.
Yeast two-hybrid analyses
Yeast cells (Y190) were transformed with the pGBKT7 - and pVP16-derived plasmids described above. Transformants were selected and protein-protein interactions were assayed by β-galactosidase reporter activity as previously described [8].
Zdroje
1. HurleyJH
EmrSD
2006 The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct 35 277 298
2. RaiborgC
StenmarkH
2009 The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458 445 452
3. CarltonJG
Martin-SerranoJ
2007 Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science 316 1908 1912
4. MoritaE
SandrinV
ChungHY
MorhamSG
GygiSP
2007 Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J 26 4215 4227
5. BieniaszPD
2006 Late budding domains and host proteins in enveloped virus release. Virology 344 55 63
6. MoritaE
SundquistWI
2004 Retrovirus budding. Annu Rev Cell Dev Biol 20 395 425
7. GarrusJE
von SchwedlerUK
PornillosOW
MorhamSG
ZavitzKH
2001 Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107 55 65
8. Martin-SerranoJ
ZangT
BieniaszPD
2001 HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat Med 7 1313 1319
9. VerPlankL
BouamrF
LaGrassaTJ
AgrestaB
KikonyogoA
2001 Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55(Gag). Proc Natl Acad Sci U S A 98 7724 7729
10. DemirovDG
OnoA
OrensteinJM
FreedEO
2002 Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc Natl Acad Sci U S A 99 955 960
11. StrackB
CalistriA
CraigS
PopovaE
GottlingerHG
2003 AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114 689 699
12. Martin-SerranoJ
YarovoyA
Perez-CaballeroD
BieniaszPD
2003 Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc Natl Acad Sci U S A 100 12414 12419
13. von SchwedlerUK
StuchellM
MullerB
WardDM
ChungHY
2003 The protein network of HIV budding. Cell 114 701 713
14. StrackB
CalistriA
AccolaMA
PaluG
GottlingerHG
2000 A role for ubiquitin ligase recruitment in retrovirus release. Proc Natl Acad Sci U S A 97 13063 13068
15. HartyRN
BrownME
WangG
HuibregtseJ
HayesFP
2000 A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc Natl Acad Sci U S A 97 13871 13876
16. KikonyogoA
BouamrF
VanaML
XiangY
AiyarA
2001 Proteins related to the Nedd4 family of ubiquitin protein ligases interact with the L domain of Rous sarcoma virus and are required for gag budding from cells. Proc Natl Acad Sci U S A 98 11199 11204
17. Martin-SerranoJ
EastmanSW
ChungW
BieniaszPD
2005 HECT ubiquitin ligases link viral and cellular PPxY motifs to the vacuolar protein-sorting pathway. J Cell Biol 168 89 101
18. YasudaJ
HunterE
NakaoM
ShidaH
2002 Functional involvement of a novel Nedd4-like ubiquitin ligase on retrovirus budding. EMBO Rep 3 636 640
19. BabstM
KatzmannDJ
Estepa-SabalEJ
MeerlooT
EmrSD
2002 Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell 3 271 282
20. BabstM
KatzmannDJ
SnyderWB
WendlandB
EmrSD
2002 Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell 3 283 289
21. KatzmannDJ
BabstM
EmrSD
2001 Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106 145 155
22. WollertT
HurleyJH
Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature
23. WollertT
WunderC
Lippincott-SchwartzJ
HurleyJH
2009 Membrane scission by the ESCRT-III complex. Nature 458 172 177
24. LataS
SchoehnG
JainA
PiresR
PiehlerJ
2008 Helical structures of ESCRT-III are disassembled by VPS4. Science 321 1354 1357
25. SaksenaS
WahlmanJ
TeisD
JohnsonAE
EmrSD
2009 Functional reconstitution of ESCRT-III assembly and disassembly. Cell 136 97 109
26. ZamborliniA
UsamiY
RadoshitzkySR
PopovaE
PaluG
2006 Release of autoinhibition converts ESCRT-III components into potent inhibitors of HIV-1 budding. Proc Natl Acad Sci U S A 103 19140 19145
27. FisherRD
ChungHY
ZhaiQ
RobinsonH
SundquistWI
2007 Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell 128 841 852
28. McCulloughJ
FisherRD
WhitbyFG
SundquistWI
HillCP
2008 ALIX-CHMP4 interactions in the human ESCRT pathway. Proc Natl Acad Sci U S A 105 7687 7691
29. ZhadinaM
McClureMO
JohnsonMC
BieniaszPD
2007 Ubiquitin-dependent virus particle budding without viral protein ubiquitination. Proc Natl Acad Sci U S A 104 20031 20036
30. UsamiY
PopovS
PopovaE
GottlingerHG
2008 Efficient and specific rescue of human immunodeficiency virus type 1 budding defects by a Nedd4-like ubiquitin ligase. J Virol 82 4898 4907
31. ChungHY
MoritaE
von SchwedlerU
MullerB
KrausslichHG
2008 NEDD4L overexpression rescues the release and infectivity of human immunodeficiency virus type 1 constructs lacking PTAP and YPXL late domains. J Virol 82 4884 4897
32. MedinaG
ZhangY
TangY
GottweinE
VanaML
2005 The functionally exchangeable L domains in RSV and HIV-1 Gag direct particle release through pathways linked by Tsg101. Traffic 6 880 894
33. BacheKG
RaiborgC
MehlumA
StenmarkH
2003 STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J Biol Chem 278 12513 12521
34. BishopN
HormanA
WoodmanP
2002 Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J Cell Biol 157 91 101
35. PoloS
SigismundS
FarettaM
GuidiM
CapuaMR
2002 A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature 416 451 455
36. ShihSC
KatzmannDJ
SchnellJD
SutantoM
EmrSD
2002 Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat Cell Biol 4 389 393
37. SlagsvoldT
AaslandR
HiranoS
BacheKG
RaiborgC
2005 Eap45 in mammalian ESCRT-II binds ubiquitin via a phosphoinositide-interacting GLUE domain. J Biol Chem 280 19600 19606
38. HickeL
2001 A new ticket for entry into budding vesicles-ubiquitin. Cell 106 527 530
39. UrbanowskiJL
PiperRC
2001 Ubiquitin sorts proteins into the intralumenal degradative compartment of the late-endosome/vacuole. Traffic 2 622 630
40. PuttermanD
PepinskyRB
VogtVM
1990 Ubiquitin in avian leukosis virus particles. Virology 176 633 637
41. OttDE
CorenLV
CopelandTD
KaneBP
JohnsonDG
1998 Ubiquitin is covalently attached to the p6Gag proteins of human immunodeficiency virus type 1 and simian immunodeficiency virus and to the p12Gag protein of Moloney murine leukemia virus. J Virol 72 2962 2968
42. OttDE
CorenLV
ChertovaEN
GagliardiTD
SchubertU
2000 Ubiquitination of HIV-1 and MuLV Gag. Virology 278 111 121
43. HeideckerG
LloydPA
FoxK
NagashimaK
DerseD
2004 Late assembly motifs of human T-cell leukemia virus type 1 and their relative roles in particle release. J Virol 78 6636 6648
44. PatnaikA
ChauV
WillsJW
2000 Ubiquitin is part of the retrovirus budding machinery. Proc Natl Acad Sci U S A 97 13069 13074
45. SchubertU
OttDE
ChertovaEN
WelkerR
TessmerU
2000 Proteasome inhibition interferes with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc Natl Acad Sci U S A 97 13057 13062
46. GottweinE
JagerS
HabermannA
KrausslichHG
2006 Cumulative mutations of ubiquitin acceptor sites in human immunodeficiency virus type 1 gag cause a late budding defect. J Virol 80 6267 6275
47. SpidelJL
CravenRC
WilsonCB
PatnaikA
WangH
2004 Lysines close to the Rous sarcoma virus late domain critical for budding. J Virol 78 10606 10616
48. JoshiA
MunshiU
AblanSD
NagashimaK
FreedEO
2008 Functional replacement of a retroviral late domain by ubiquitin fusion. Traffic 9 1972 1983
49. BabstM
WendlandB
EstepaEJ
EmrSD
1998 The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J 17 2982 2993
50. BishopN
WoodmanP
2000 ATPase-defective mammalian VPS4 localizes to aberrant endosomes and impairs cholesterol trafficking. Mol Biol Cell 11 227 239
51. MarcheseA
RaiborgC
SantiniF
KeenJH
StenmarkH
2003 The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell 5 709 722
52. RotinD
KumarS
2009 Physiological functions of the HECT family of ubiquitin ligases. Nat Rev Mol Cell Biol 10 398 409
53. EastmanSW
Martin-SerranoJ
ChungW
ZangT
BieniaszPD
2005 Identification of human VPS37C, a component of endosomal sorting complex required for transport-I important for viral budding. J Biol Chem 280 628 636
54. Martin-SerranoJ
ZangT
BieniaszPD
2003 Role of ESCRT-I in retroviral budding. J Virol 77 4794 4804
55. StuchellMD
GarrusJE
MullerB
StrayKM
GhaffarianS
2004 The human endosomal sorting complex required for transport (ESCRT-I) and its role in HIV-1 budding. J Biol Chem 279 36059 36071
56. ParentLJ
BennettRP
CravenRC
NelleTD
KrishnaNK
1995 Positionally independent and exchangeable late budding functions of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J Virol 69 5455 5460
57. ShieldsSB
OestreichAJ
WinistorferS
NguyenD
PayneJA
2009 ESCRT ubiquitin-binding domains function cooperatively during MVB cargo sorting. J Cell Biol 185 213 224
58. McNattMW
McKittrickI
WestM
OdorizziG
2007 Direct binding to Rsp5 mediates ubiquitin-independent sorting of Sna3 via the multivesicular body pathway. Mol Biol Cell 18 697 706
59. PornillosO
AlamSL
RichRL
MyszkaDG
DavisDR
2002 Structure and functional interactions of the Tsg101 UEV domain. EMBO J 21 2397 2406
60. SundquistWI
SchubertHL
KellyBN
HillGC
HoltonJM
2004 Ubiquitin recognition by the human TSG101 protein. Mol Cell 13 783 789
61. HiranoS
KawasakiM
UraH
KatoR
RaiborgC
2006 Double-sided ubiquitin binding of Hrs-UIM in endosomal protein sorting. Nat Struct Mol Biol 13 272 277
62. LloydTE
AtkinsonR
WuMN
ZhouY
PennettaG
2002 Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108 261 269
63. RaiborgC
BacheKG
GilloolyDJ
MadshusIH
StangE
2002 Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol 4 394 398
64. MizunoE
KawahataK
KatoM
KitamuraN
KomadaM
2003 STAM proteins bind ubiquitinated proteins on the early endosome via the VHS domain and ubiquitin-interacting motif. Mol Biol Cell 14 3675 3689
65. HongYH
AhnHC
LimJ
KimHM
JiHY
2009 Identification of a novel ubiquitin binding site of STAM1 VHS domain by NMR spectroscopy. FEBS Lett 583 287 292
66. RenX
HurleyJH
2010 VHS domains of ESCRT-0 cooperate in high-avidity binding to polyubiquitinated cargo. EMBO J 29 1045 1054
67. HiranoS
SuzukiN
SlagsvoldT
KawasakiM
TrambaioloD
2006 Structural basis of ubiquitin recognition by mammalian Eap45 GLUE domain. Nat Struct Mol Biol 13 1031 1032
68. AlamSL
LangelierC
WhitbyFG
KoiralaS
RobinsonH
2006 Structural basis for ubiquitin recognition by the human ESCRT-II EAP45 GLUE domain. Nat Struct Mol Biol 13 1029 1030
69. StamenovaSD
FrenchME
HeY
FrancisSA
KramerZB
2007 Ubiquitin binds to and regulates a subset of SH3 domains. Mol Cell 25 273 284
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Combined Effect of Environmental and Host Factors on the Emergence of Viral RNA RecombinantsČlánek Parvovirus Minute Virus of Mice Induces a DNA Damage Response That Facilitates Viral ReplicationČlánek Phenolglycolipid-1 Expressed by Engineered BCG Modulates Early Interaction with Human Phagocytes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Social Media and Microbiology Education
- Antimicrobial Peptides: Primeval Molecules or Future Drugs?
- Phylodynamics and Human-Mediated Dispersal of a Zoonotic Virus
- Retroviral RNA Dimerization and Packaging: The What, How, When, Where, and Why
- Distinct Clones of Caused the Black Death
- Strain-Specific Differences in the Genetic Control of Two Closely Related Mycobacteria
- The Combined Effect of Environmental and Host Factors on the Emergence of Viral RNA Recombinants
- MHC Class I Bound to an Immunodominant Epitope Demonstrates Unconventional Presentation to T Cell Receptors
- Stimulates Immune Gene Expression and Inhibits Development in
- Crystal Structure of DotD: Insights into the Relationship between Type IVB and Type II/III Secretion Systems
- Cytomegalovirus microRNAs Facilitate Persistent Virus Infection in Salivary Glands
- Strategies to Avoid Killing by Human Neutrophils
- Transforming Growth Factor-β: Activation by Neuraminidase and Role in Highly Pathogenic H5N1 Influenza Pathogenesis
- Autoimmunity in Arabidopsis Is Mediated by Epigenetic Regulation of an Immune Receptor
- Functional Interchangeability of Late Domains, Late Domain Cofactors and Ubiquitin in Viral Budding
- Dengue Virus Ensures Its Fusion in Late Endosomes Using Compartment-Specific Lipids
- Nucleocapsid Promotes Localization of HIV-1 Gag to Uropods That Participate in Virological Synapses between T Cells
- Host Genetics and HIV-1: The Final Phase?
- Variations in the Hemagglutinin of the 2009 H1N1 Pandemic Virus: Potential for Strains with Altered Virulence Phenotype?
- High-Resolution Functional Mapping of the Venezuelan Equine Encephalitis Virus Genome by Insertional Mutagenesis and Massively Parallel Sequencing
- Viral Replication Rate Regulates Clinical Outcome and CD8 T Cell Responses during Highly Pathogenic H5N1 Influenza Virus Infection in Mice
- Calcineurin Inhibition at the Clinical Phase of Prion Disease Reduces Neurodegeneration, Improves Behavioral Alterations and Increases Animal Survival
- Parvovirus Minute Virus of Mice Induces a DNA Damage Response That Facilitates Viral Replication
- -Induced Inactivation of the Macrophage Transcription Factor AP-1 Is Mediated by the Parasite Metalloprotease GP63
- Epstein Barr Virus-Encoded EBNA1 Interference with MHC Class I Antigen Presentation Reveals a Close Correlation between mRNA Translation Initiation and Antigen Presentation
- Fidelity Variants of RNA Dependent RNA Polymerases Uncover an Indirect, Mutagenic Activity of Amiloride Compounds
- The Spread of Tomato Yellow Leaf Curl Virus from the Middle East to the World
- Concerted Action of Two Formins in Gliding Motility and Host Cell Invasion by
- Requirements for Receptor Engagement during Infection by Adenovirus Complexed with Blood Coagulation Factor X
- Release of Intracellular Calcium Stores Facilitates Coxsackievirus Entry into Polarized Endothelial Cells
- Gene Annotation and Drug Target Discovery in with a Tagged Transposon Mutant Collection
- Nuclear Export and Import of Human Hepatitis B Virus Capsid Protein and Particles
- Retention and Loss of RNA Interference Pathways in Trypanosomatid Protozoans
- Identification and Genome-Wide Prediction of DNA Binding Specificities for the ApiAP2 Family of Regulators from the Malaria Parasite
- Controlling Cellular P-TEFb Activity by the HIV-1 Transcriptional Transactivator Tat
- Direct Visualization of Peptide/MHC Complexes at the Surface and in the Intracellular Compartments of Cells Infected by
- Phenolglycolipid-1 Expressed by Engineered BCG Modulates Early Interaction with Human Phagocytes
- In Vitro and In Vivo Studies Identify Important Features of Dengue Virus pr-E Protein Interactions
- Inhibition of Nipah Virus Infection In Vivo: Targeting an Early Stage of Paramyxovirus Fusion Activation during Viral Entry
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Retroviral RNA Dimerization and Packaging: The What, How, When, Where, and Why
- Viral Replication Rate Regulates Clinical Outcome and CD8 T Cell Responses during Highly Pathogenic H5N1 Influenza Virus Infection in Mice
- Antimicrobial Peptides: Primeval Molecules or Future Drugs?
- Crystal Structure of DotD: Insights into the Relationship between Type IVB and Type II/III Secretion Systems
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



