-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
Failure to properly condense chromosomes prior to their segregation in mitosis can lead to genome instability. The evolutionary-conserved condensin complex is key to the condensation process but the molecular mechanisms underlying its localization pattern on chromosomes remain unclear. Previous observations showed that the localization of condensin is intimately linked to regions of high transcription, although, somewhat paradoxically, its association with chromatin is disrupted by a processive polymerase activity. Here we identify several RNA processing factors as negative regulators of condensin in fission yeast. Two of these factors associate with PP1 phosphatase as an independent entity within the Cleavage and Polyadenylation Factor (CPF), a complex key for 3′ end RNA processing. Lack of this module induces only minor and context-dependent effects on gene expression. Our data suggest that this module helps maintaining the proper level of phosphatase activity within the CPF and thereby opposes the function of condensin in mitotic chromosome condensation.
Published in the journal: . PLoS Genet 10(6): e32767. doi:10.1371/journal.pgen.1004415
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004415Summary
Failure to properly condense chromosomes prior to their segregation in mitosis can lead to genome instability. The evolutionary-conserved condensin complex is key to the condensation process but the molecular mechanisms underlying its localization pattern on chromosomes remain unclear. Previous observations showed that the localization of condensin is intimately linked to regions of high transcription, although, somewhat paradoxically, its association with chromatin is disrupted by a processive polymerase activity. Here we identify several RNA processing factors as negative regulators of condensin in fission yeast. Two of these factors associate with PP1 phosphatase as an independent entity within the Cleavage and Polyadenylation Factor (CPF), a complex key for 3′ end RNA processing. Lack of this module induces only minor and context-dependent effects on gene expression. Our data suggest that this module helps maintaining the proper level of phosphatase activity within the CPF and thereby opposes the function of condensin in mitotic chromosome condensation.
Introduction
Mitotic chromosome condensation is essential for genome integrity. When defective, chromatin bridges often form in anaphase. These can lead to chromosome breaks and the irreparable loss of genetic information. A key driver of chromosome condensation is the highly conserved condensin complex (reviewed in [1]). Condensin is, with cohesin and the SMC5/6 complex, one of three highly conserved multi-subunit protein complexes containing two different proteins of the SMC (Structural Maintenance of Chromosome) family. Condensin is made of five sub-units (SMC2Cut14, SMC4Cut3, CAP-D2Cnd1, CAP-GCnd3 and CAP-HCnd2, name of the human protein followed by its name in fission yeast), which together form a protein ring big enough to entrap two chromatids. Condensin exhibits a DNA-dependent ATPase activity and a DNA supercoiling activity but how these enzymatic activities contribute to mitotic condensation remains elusive. Although condensin interacts directly with histones [2], its localization pattern along chromosomes is not uniform [3], [4]. A number of experimental evidence indicate that cis-acting elements facilitate the binding of condensin at specific loci, supporting the current view that the underlying mechanisms of condensin recruitment are, to some extent, locus-specific (reviewed in [1]).
However, Chromatin Immunoprecipitation (ChIP) studies also indicate that condensin localizes preferentially at highly expressed genes, irrespective of the transcription machinery involved (RNA Pol I, II or III) [3], [4], [5]. This observation supports the idea that a by-product of the transcription process, such as a transcription-associated chromatin structure, a specific change of topology and/or a chromatin mark, could facilitate the recruitment of condensin. It is likely that the recruitment of condensin actually results from the combination of global and locus-specific mechanisms. Our understanding of these mechanisms and their interactions remains poor.
Experimental evidence indicates that there are strong functional relationships between gene transcription and mitotic chromosome condensation. However some observations suggest that the transcription machinery plays a positive role in condensin-mediated chromosome condensation whilst other evidence indicates that gene transcription can inhibit chromosome condensation. On one hand, specific transcription-associated factors and RNA processing factors, such as the RNA Pol III component TFIIIC and the RNA helicase DDX3, have been shown to facilitate the loading of condensin [3], [6], [7],[8]; on the other hand, the stable association of RNA with chromatin maintains an open chromatin conformation [9] and an active RNA polymerase reduces the binding of condensin at repetitive sequences [10], [11]. Moreover, the transcription machinery can recruit inhibitors of condensin such as the phosphatase PP2A [12]. Finally, transcription of Pol III genes negatively correlates with their condensin-dependent clustering at centromeres [6]. In conclusion, it appears that, although the transcription machinery is required to set up the right environment for the loading of condensin, transcription, when processive, destabilizes the association of condensin with chromatin. Interestingly, a number of proteins required for the processivity of transcription leave chromatin upon mitotic entry, when condensin is loaded on chromosomes and condensation occurs [13], [14].
To gain insights into the mechanisms by which transcription influences the association of condensin with chromatin in fission yeast, we set out to identify novel regulators of chromosome condensation associated with the transcription machinery. To do this, we adopted a candidate approach and screened for deletions of non-essential components of the transcription machinery that could restore cell growth of the thermo-sensitive and condensin-defective cut3-477 mutant at high temperatures. We identified a number of suppressor mutations by this approach. Here we focus on one of them, swd2.2Δ, the deletion of a non-essential component of the Cleavage and Polyadenylation Factor (CPF), the complex responsible for the 3′end maturation of RNA Pol II transcripts in yeast (reviewed in [15]).
Results
Lack of Swd2.2 restores chromosome segregation in a condensin-deficient mutant
Chromosome condensation is defective in the conditional condensin mutant cut3-477 and mutant cells fail to grow at the restrictive temperature of 34°C [16]. We isolated several gene deletions in non-essential components of the transcription machinery which partly restored growth of cut3-477 cells at 34°C (Table S1). Here, we focused on one of the strongest of these suppressors, swd2.2Δ. At the restrictive temperature, lack of Swd2.2 (swd2.2Δ) improved the growth of cut3-477 cells (Figure 1A) and significantly decreased the percentage of anaphases displaying defective chromosome segregation (Figure 1BC). Similarly, lack of Swd2.2 partly restored growth at the restrictive temperature of the other well-characterized condensin mutant, cut14-208 [16] (Figure S1A). This suggested that Swd2.2 interferes with the formation of segregation-competent chromosomes when condensin is deficient. Alternatively, lack of Swd2.2 could activate an as yet unknown mechanism facilitating chromosome segregation in condensin-deficient cells.
Fig. 1. Swd2.2 antagonizes the association of Condensin with chromatin. 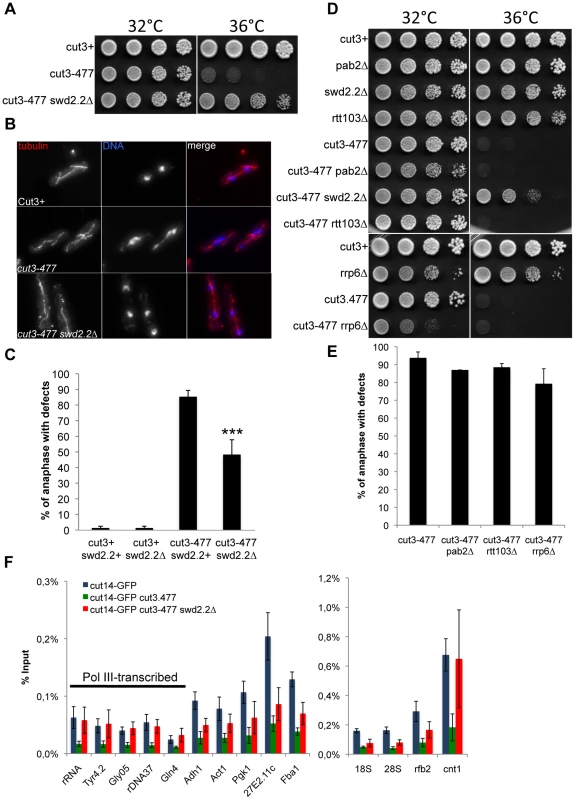
A. Serial dilutions of the indicated strains were plated on rich media at the indicated temperatures. B. Chromosome segregation in anaphase was visualized after tubulin staining in cells of the indicated genotypes grown for one generation at 34°C. C. Anaphases were scored as defective when chromatin was detected lagging between the two main DNA masses. For each genotype, a minimum of 6 independent experiments was performed in which a minimum of 100 anaphase cells was scored. ***p<0,001 Wilcoxon - Mann Whitney. D and E. Same as A and C, except that in E, 3 independent experiments were performed. F. The indicated strains were grown at 34°C for 3 hours and ChIP-qPCR was performed to analyze the amount of Cut14-GFP cross-linked to chromatin (mean ± standard deviation from 6 biological replicates). See text for details for the statistical analysis of the experiments. The suppression of cut3-477 by swd2.2Δ was remarkably specific. Deletions of other key components of the RNA processing machinery did not rescue either the growth defect or the chromosome segregation defects in cut3-477 cells (Figure 1DE), indicating that significant RNA processing defects are not sufficient to alleviate the defects caused by cut3-477. For example, lack of either Rtt103/Rhn1 [17], [18], a factor important for transcription termination, Pab2, a PolyA polymerase involved in RNA decay or the exosome sub-unit Rrp6 [19] caused no significant rescue of cut3-477 (Figure 1DE). Furthermore, lack of Swd2.2 did not rescue the rad21-K1 and smc6-74 mutations (Figure S1B), which affect respectively the condensin-related cohesin and Smc5/6 complexes, nor the two topoisomerase II mutations, top2-250 and top2-191 (Figure S1B), which also cause chromosome condensation defects [20], [21]. These observations support the idea that Swd2.2 specifically antagonizes condensin-mediated chromosome condensation.
Lack of Swd2.2 facilitates the localization of condensin in cut3-477 cells
It was reported that the amount of condensin associated with chromatin is reduced in cut3-477 cells [2]. We confirmed these observations by showing that the association with chromatin of the GFP-tagged condensin sub-unit Cut14 was drastically reduced in cut3-477 cells at the restrictive temperature of 34°C at all loci tested (p<0,01 Mann-Whitney test on 6 biological replicates, Figure 1F). Deletion of Swd2.2 improved slightly but significantly the association of Cut14 with chromatin in cut3-477 cells at 34°C (p≤0,01 Mann-Whitney test on 6 biological replicates). Surprisingly, we found that the localization of Cut14 was fully restored at kinetochores (cnt1) and RNA Pol III-transcribed genes, where its enrichment was indistinguishable in cut3+ and cut3-477 swd2.2Δ cells (p>0,30 Mann-Whitney test). Western blot analysis showed that the steady-state level of Cut14 was not affected in cells lacking Swd2.2 (Figure S2). Consistent with the observation that swd2.2Δ facilitates the localization of condensin at kinetochores in cut3-477 cells, Figure S3 shows that lack of Swd2.2 suppressed the synthetic lethal interaction between cut3-477 and mde4Δ, a cis-acting loader of condensin at kinetochores ([2]).
Taken together, these data suggest that Swd2.2 antagonizes the association of condensin with chromatin in cut3-477 cells. This probably explains why lack of Swd2.2 reduced the temperature-sensitivity of cut3-477. Note however that, in cut3+ cells, lack of Swd2.2 did not significantly affect the association of condensin with chromatin (Figure S4).
Lack of Swd2.2 did not alter Cnd2 phosphorylation or H2A.z acetylation
Previous experiments have established that the interaction between condensin and chromatin is enhanced after Aurora B-dependent phosphorylation of Cnd2 [2] and that the acetylation of H2A.z facilitates the function of condensin [22]. We wondered whether Swd2.2 might counter-act Aurora B-dependent phosphorylation of Cnd2 or H2A.z acetylation. Western blot analysis showed that neither Aurora B-dependent Cnd2 phosphorylation nor H2A.z acetylation were altered in the absence of Swd2.2 (Figure S5), suggesting that Swd2.2 acts upon condensin binding by an alternative pathway.
Lack of Swd2.2 impairs the association of the Pol III machinery with chromatin
We asked how Swd2.2 could regulate the localization of condensin at Pol III-transcribed genes in cut3-477 cells. We detected variable amounts of Swd2.2 at Pol III-transcribed loci by ChIP analysis. At some Pol III genes such as c417 or Gln4, the enrichment of Swd2.2 was comparable to its enrichment at highly transcribed Pol II genes such as act1 (Figure 2A). At all Pol III loci, the association of Swd2.2 was dependent on the PNUTS homologue Ppn1 (Figure 2A and see below). The localization of Swd2.2 at Pol III genes suggested that it could play a direct role there.
Fig. 2. Lack of Swd2.2 reduces the association of the RNA Pol III transcription machinery with chromatin. 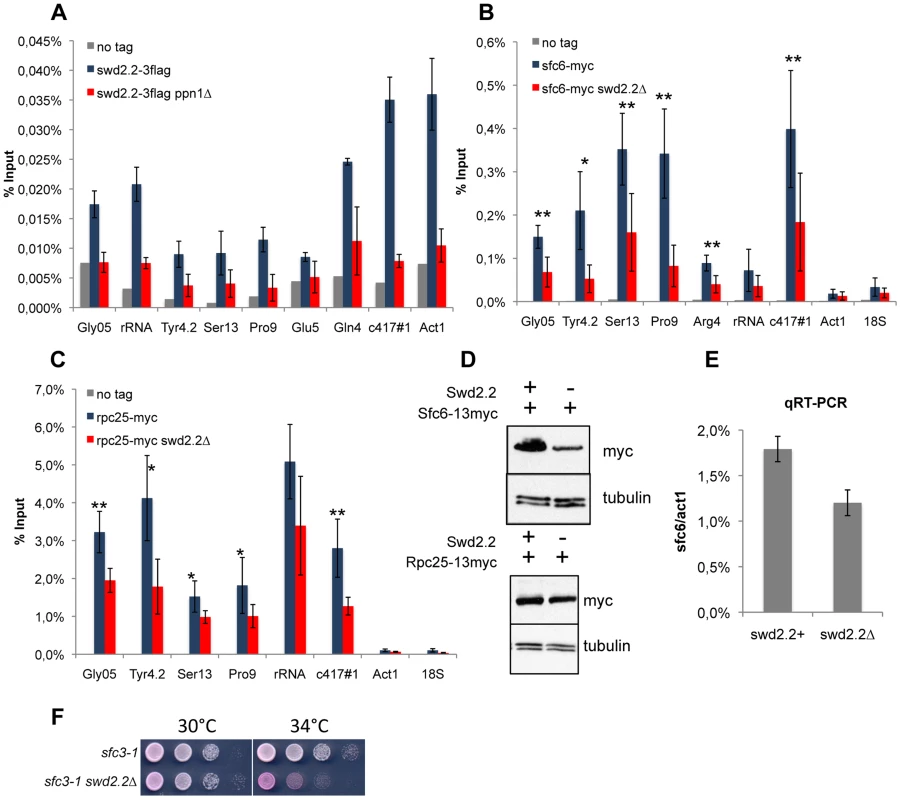
A. Asynchronous populations of the indicated strains were grown at 30°C and ChIP-qPCR was performed to analyze the amount of Swd2.2-3flag cross-linked to chromatin (mean ± standard deviation from 4 biological replicates). BC. ChIP qPCR analysis of the indicated strains grown at 30°C. Mean ± standard deviation from 5 biological replicates. *<0,05; **<0,01; Wilcoxon - Mann Whitney. D. Western blot analysis of total protein extracts of the indicated strains. Tubulin (TAT1 antibody) is used as a loading control. E. qRT-PCR analysis of sfc6 expression in swd2.2+ and swd2.2Δ cells (mean ± standard deviation from 3 biological replicates) F. Serial dilutions of the indicated strains were plated on rich media at the indicated temperatures. Observations made previously in the fission yeast TFIIIC mutant sfc3-1 [6], suggested that TFIIIC binds to condensin and facilitates its association with Pol III genes, whereas Pol III-dependent transcription opposes the function of condensin. sfc3-1, like swd2.2Δ, is a suppressor of cut3-477 and is believed to facilitate the localization of condensin at Pol III loci [2], [6]. Previous reports indicated that the association of the TFIIIC component Sfc6-13myc with chromatin was stabilized in sfc3-1 cells, whilst the binding of the RNA Pol III sub-unit Rpc25 was reduced [6]. On the contrary, we found that both the association of Sfc6-13myc and Rpc25-13myc were reduced in swd2.2Δ cells (Figure 2BC). The reduced association of Sfc6-13myc with chromatin could probably be explained in part by the fact that both gene expression and the protein stability of Sfc6 were reduced in swd2.2Δ cells (Figure 2DE). The stability of Rpc25-13myc however remained unaffected (Figure 2D). As a control, we repeated the Sfc6-13myc ChIP in sfc3-1 cells and found the opposite result to what is published [6]: in our hands, the localization of Sfc6-13myc was significantly impaired in sfc3-1 cells (Figure S6B). We confirmed however the published observation [6] that the localization of Rpc25 is indeed slightly reduced in sfc3-1 cells (Figure S6C). All our strains have been thoroughly validated (Figures S6A). We do not have an explanation for this discrepancy at this stage. We note however that lack of Swd2.2 strongly impaired the growth of sfc3-1 cells (Figure 2F), which is consistent with our observation that both swd2.2Δ and sfc3-1 destabilize the loading of TFIIIC. A common feature of both types of suppressors (sfc3-1 and swd2.2Δ) is therefore that the loading of TFIIIC and RNA Pol III is impaired at Pol III genes.
Swd2.2 facilitates the localization of the CPF but does not impact its assembly
Swd2.2 was previously shown to co-purify with the Cleavage and Polyadenylation Factor (CPF) [23], the complex responsible for the 3′end maturation of RNA Pol II transcripts in yeast (reviewed in [15]). We sought to establish the importance of Swd2.2 for CPF function. pfs2-11 is a thermo-sensitive mutation of Pfs2, an essential component of the CPF [24]. Lack of Swd2.2 enhanced the temperature-sensitivity of pfs2-11 (Figure 3A), which is consistent with the idea that Swd2.2 could facilitate CPF function. However, lack of Swd2.2 did not interfere with the interaction between Pfs2 and Yth1, another essential CPF sub-units, suggesting that Swd2.2 did not significantly impact CPF assembly (Figure 3B and see below). To establish whether lack of Swd2.2 could impact CPF localization, we analyzed the association of Pfs2 with chromatin by Chromatin Immunoprecipitation (ChIP). The enrichment of Pfs2 was mildly reduced at most loci tested in the absence of Swd2.2, suggesting that Swd2.2 facilitates the recruitment of the CPF (Figure 3C). Note that the stability of Pfs2 remained unaffected in the absence of Swd2.2 (Figure 3D).
Fig. 3. Swd2.2 facilitates the function of the CPF. 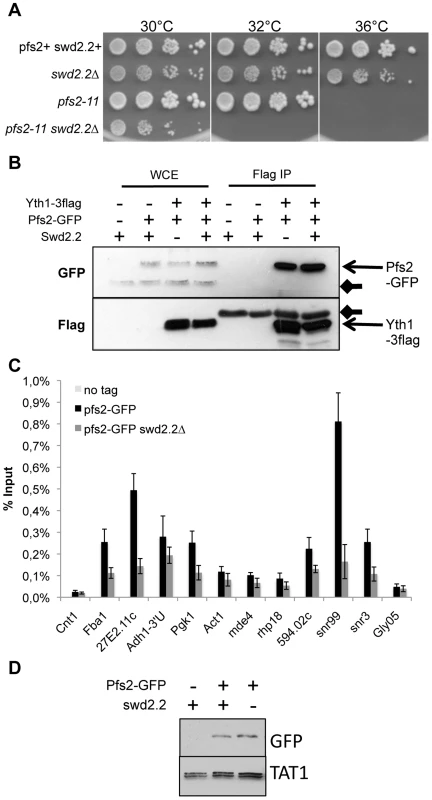
A. Serial dilutions of the indicated strains were plated on rich media at the indicated temperatures. B. The CPF component Yth1 tagged at the endogenous locus with 3flag epitopes was immuno-precipitated from cycling cells in the presence or absence of Swd2.2. Whole cell extracts (WCE) and the immuno-precipitated material (Flag IP) were analyzed by western blot. Yth1-3flag interacts with Pfs2-GFP whether or not Swd2.2 is present. Arrows indicate aspecific bands on the western blot. C. Asynchronous populations of the indicated strains were grown at 30°C and ChIP-qPCR was performed to analyze the amount of Pfs2-GFP cross-linked to chromatin (mean ± standard deviation from 4 biological replicates). D. Western blot analysis of total protein extracts of the indicated strains. Tubulin (TAT1 antibody) is used as a loading control. Lack of Swd2.2 has a minor effect on the expression of protein-coding genes
To establish the impact of Swd2.2 on gene expression, we used strand-specific tiling arrays hybridization to compare the transcriptomes of swd2.2Δ and swd2.2+ strains (raw data accessible at GEO GSE38005). Both strains were grown in triplicates for one generation at 34°C, the temperature at which lack of Swd2.2 improves chromosome segregation in cut3-477 (Figure 1A). Results showed that 47 genes were significantly under-expressed in the absence of Swd2.2 (Fold change FC≤−1,9, p<0,01), whilst 14 genes were over-expressed (FC≥+1,9, p<0,01) (Table S2). Note that of the 62 tRNA genes detected in our microarray analysis, only 6 were under-expressed (Fold change FC≤−1,9, p≤0,03). An in-depth statistical analysis established that the mis-regulated genes were all placed within a very specific genomic context (see Text S1 and Figure S7 in the supplementary data). Thus lack of Swd2.2 has only a minor impact on the expression of protein-coding transcripts (61 out of 5175 mRNA-coding genes). Importantly, no known regulator of chromosome condensation was found among the 61 mis-expressed genes.
Lack of Swd2.2 has a context-dependent impact on transcription termination
Swd2, the budding yeast homologue of Swd2.2, is part of the APT sub-complex of the CPF [25]. In the absence of APT, RNA polymerase II transcribes longer transcripts, especially at snoRNA genes [26]. We investigated the role of Swd2.2 in the termination of transcription. Our strand-specific tiling arrays suggested that transcription failed to terminate properly at about 800 genes in the absence of Swd2.2 (see Methods and Table S3). Remarkably, the vast majority of genes with transcription termination defects were convergent, overlapping genes (Figure S8), suggesting that the requirement for Swd2.2 for transcription termination is context-dependent. Note however that we did not detect a stronger enrichment of Swd2.2 at those convergent genes requiring Swd2.2 for their transcription termination (Figure S9). Our microarray analysis failed to detect significant transcription termination defects at snoRNA genes. We confirmed this using a targeted RT-PCR approach [24] and showed that the transcription profile of specific snoRNAs genes was not significantly affected in the absence of Swd2.2 (Figure S10). Taken together, these data show that the reduced association of the CPF with chromatin that results from lack of Swd2.2 had only a limited and context-dependent impact on gene expression.
Swd2.2 facilitates the association of Protein Phosphatase 1 PP1Dis2 with chromatin
In human cells, a homologue of Swd2.2, Wdr82, associates with Protein Phosphatase 1 (PP1) and its cofactor PNUTS as part of the PP1/PTW complex [27]. Blast searches highlighted significant homologies between human, xenopus PNUTS and the N-terminus of the uncharacterized fission yeast ORF SPCC74.02c (Figure S11). Interestingly, SPCC74.02c was previously shown to co-purify with Swd2.2 [23]. Based on this homology and results presented below, we propose that SPCC74.02c is the fission yeast homologue of PNUTS and we have therefore renamed it “Ppn1” for “Pombe Pnuts 1”.
Ppn1 contains three amino acids motifs known to mediate a direct interaction with PP1 [28] (Figure S11). Co-immunoprecipitation experiments showed that Ppn1 did indeed interact in vivo with fission yeast PP1Dis2. This interaction required the three PP1-binding motifs of Ppn1 (Figure 4A). Interestingly, Ppn1 did not interact with the other fission yeast PP1 isoform, PP1Sds21, even in the absence of PP1Dis2 (dis2Δ) (see below, Figure 5B). ChIP experiments indicated that PP1Dis2 and Ppn1 have largely overlapping localization patterns, except at kinetochores (cnt1), where PP1Dis2 was comparatively 5 times more abundant than Ppn1 (Figure 4B). This is consistent with previous observations that PP1Dis2 has specific, kinetochore-based loading mechanisms [29]. Lack of Ppn1 severely disrupted the nuclear localization pattern of PP1Dis2, which became enriched in the Fib1-stained nucleolus [30] (Figure 4C). Consistent with this, ChIP analysis showed that the association of PP1Dis2 with chromatin was reduced although not completely abolished in the absence of Ppn1 or when the three PP1-binding motifs of Ppn1 were mutated (p<0,01 at all sites tested except at cnt1 where p = 0,93, Mann-Whitney test on 6 biological replicates, Figure 4D). These observations are consistent with the idea that the Ppn1-PP1Dis2 complex represents a major chromatin-associated PP1 activity in fission yeast.
Fig. 4. Swd2.2 facilitates the localization of PP1 phosphatase by interacting with the PNUTS homologue Ppn1. 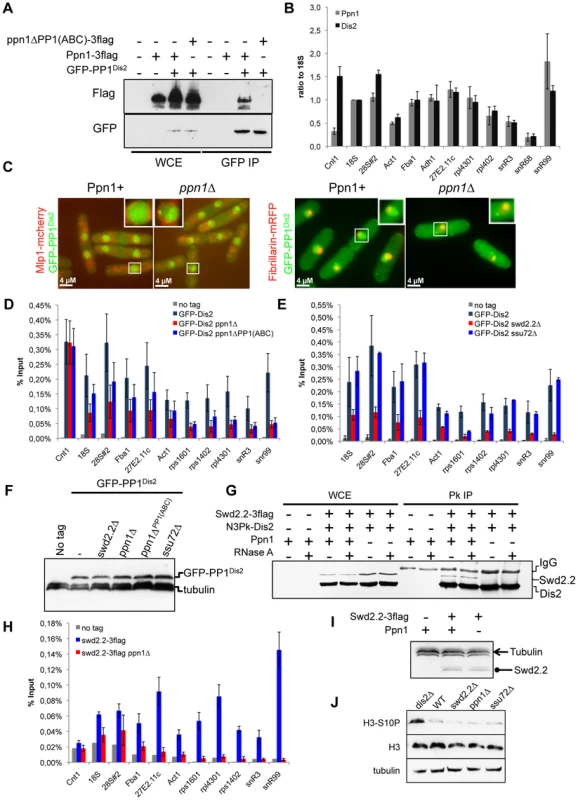
A. The interaction of Ppn1 with PP1Dis2 requires the three PP1-binding sites of Ppn1. GFP-tagged PP1Dis2 was immuno-precipitated from cycling cells in the presence of Flag-tagged Ppn1 (Ppn1-3flag) or Flag-tagged Ppn1 lacking the three PP1-binding sites (ppn1ΔPP1(ABC)-3flag). Whole cell extracts (WCE) and the immuno-precipitated material (GFP IP) were analyzed by western blot. B. Asynchronous populations of the indicated strains (Ppn1-3flag or GFP-PP1Dis2) were grown at 30°C and ChIP-qPCR was performed to analyze their enrichment at various sites along chromosomes. Enrichments were normalized to the values obtained at the RNA Polymerase I-transcribed 18S (mean ± standard deviation from 3 biological replicates). C. Lack of Ppn1 disrupts the nuclear localisation of GFP-tagged PP1Dis2. GFP-PP1Dis2 was imaged in dividing cells co-expressing (left panel) the mCherry-tagged nuclear envelope marker Mlp1 or (right panel) the mRFP-tagged nucleolar marker Fib1. DE. Asynchronous populations of the indicated strains were grown at 30°C and ChIP-qPCR was performed (mean ± standard deviation from 6 biological replicates). F. The protein stability of GFP-PP1Dis2 was assessed by western blot in the various mutant backgrounds used in DE. Tubulin was used as a loading control. G. The interaction between Flag-tagged Swd2.2 and Pk-tagged PP1Dis2 was analyzed by co-immunoprecipitation in protein extracts prepared from cycling cells in the presence or absence of Ppn1. Protein extracts were treated or not with RNase A prior to immuno-precipitation. Whole cell extracts (WCE) and the immuno-precipitated material (GFP IP) were analyzed by western blot. H. Asynchronous populations of the indicated strains were grown at 30°C and ChIP-qPCR was performed to analyze their enrichment at various sites along chromosomes. I. The protein stability of Flag-tagged Swd2.2 in the presence or absence of Ppn1 was assessed by western blot. Tubulin was used as a loading control. J. Asynchronous populations of the indicated strains were grown at 30°C. Protein extracts were prepared and analyzed by western blot using the indicated antibodies. Fig. 5. Swd2.2, Ppn1 and PP1Dis2 associate as a protein module to the CPF. 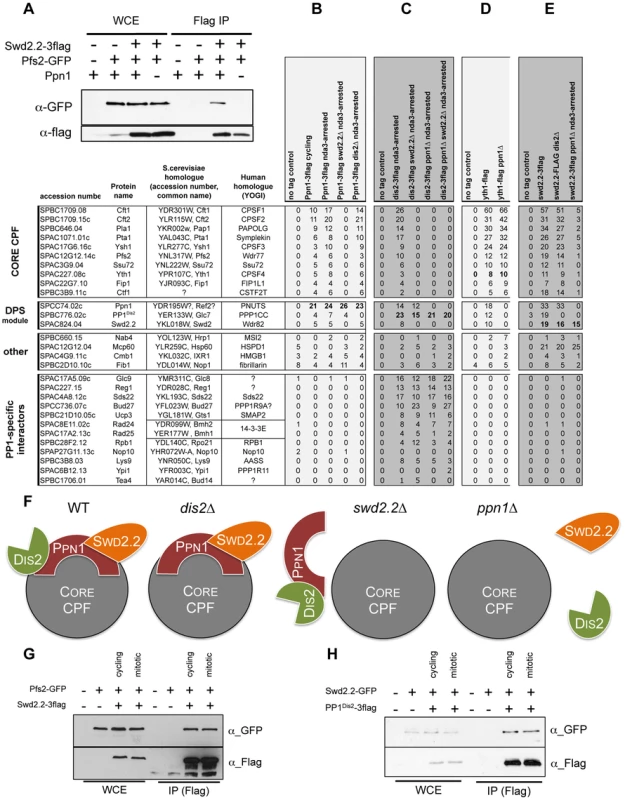
A. The interaction between Flag-tagged Swd2.2 and GFP-tagged Pfs2 was analyzed by co-immunoprecipitation in protein extracts prepared from cycling cells in the presence or absence of Ppn1. Whole cell extracts (WCE) and the immuno-precipitated material (GFP IP) were analyzed by western blot. BCDE. The proteins indicated at the top were purified by affinity and their associated partners were identified by MS/MS mass-spectrometry analysis (see Methods). The number of unique peptides recovered for each protein is indicated. F. Scheme summarizing the proteomic data. GH. Cells expressing the indicated epitope-tagged proteins were synchronized in early mitosis (mitotic) or not (cycling), using the cold-sensitive nda3KM311 mutation [33]. G. Flag-tagged Swd2.2 was immuno-precipitated to look at its interaction with the core CPF component Pfs2. H. Flag-tagged PP1Dis2 was immuno-precipitated to look at its interaction with the DPS component Swd2.2. As Ppn1 co-purified with Swd2.2 [23], we wondered whether Swd2.2 was also required for the association of PP1Dis2 with chromatin. ChIP experiments indicated that the association of PP1Dis2 with chromatin was indeed significantly reduced in the absence of Swd2.2 (p<0,01 Mann-Whitney test on 6 biological replicates) but not in the absence of the CPF-associated Ssu72 phosphatase (Figure 4E). Note that none of the mutations we tested affected the protein stability of PP1Dis2 (Figure 4F).
Co-immunoprecipitation experiments showed that Ppn1 but not RNA was required for the interaction between Swd2.2 and PP1Dis2 (Figure 4G). ChIP analysis indicated that Ppn1 was required for the association of Swd2.2 with chromatin but not for its stability (Figures 2A and 4H&I). Taken together, these observations suggested that Swd2.2, Ppn1 and PP1Dis2 form a complex in fission yeast related to the PTW/PP1 complex of vertebrates, and that this complex is important for the proper loading of PP1 phosphatase on chromosome arms.
In budding yeast, PP1Glc7 targets phospho-Ser10 on histone H3 for dephosphorylation [31] and the increased phosphorylation of Ser10 on histone H3 in some RNA processing mutants was recently shown to be associated with an increased compaction of the chromatin [32]. We wondered whether the partial rescue of the temperature sensitivity and chromosome segregation defects of cut3-477 by swd2.2Δ could stem from the failure by PP1Dis2 to dephosphorylate Ser10 on histone H3. Western blot analysis indicated that lack of PP1Dis2 (dis2Δ) resulted indeed in the increased phosphorylation of Ser10 on histone H3 (Figure 4J). However, the levels of phosphorylated Ser10 on histone H3 were not altered in the absence of Swd2.2 or Ppn1 (Figure 4J). These observations were consistent with the idea that the pool of PP1Dis2 that we still detected on chromatin in the absence of Swd2.2 or Ppn1 was sufficient to antagonize the phosphorylation of Ser10 on histone H3. Alternatively, PP1Dis2 could dephosphorylate Ser10 on histone H3 even when it is not stably associated with chromatin. Whatever the explanation, our data show that the partial rescue of cut3-477 by swd2.2Δ cannot be explained by the failure of PP1Dis2 to dephosphorylate Ser10 on histone H3.
Ppn1 also interacts with the pre-mRNA 3′ end processing machinery
The data presented above established that Swd2.2, Ppn1 and PP1Dis2 co-localize and interact functionally. It remained unclear however whether they interacted within the CPF, or as part of an independent protein complex. Co-immunoprecipitation experiments showed that Ppn1 was required for the interaction between Swd2.2 and the Pfs2 (Figure 5A and see below). This was a first indication that their function was indeed connected to the CPF.
To establish whether Ppn1 and PP1Dis2 were genuine CPF components, we first purified Ppn1 by affinity and identified its associated proteins by mass-spectrometry analysis (see Methods). This approach showed that Ppn1 co-purified with PP1Dis2 but not PP1Sds21 (Figure 5B) and that it interacted with the 13 sub-units of the CPF. This interaction was found both in interphase and in early mitotic cells, synchronized at the metaphase to anaphase transition using the cold-sensitive tubulin mutation nda3KM311 [33]. Similarly, PP1Dis2 but not PP1Sds21 co-purified with the 13 sub-units of the CPF (Figure 5C and Figure S12A). This showed that Ppn1, PP1Dis2 and Swd2.2 are genuine CPF components.
We had established that Swd2.2 was not required for the interaction between the CPF sub-units Pfs2 and Yth1 (Figure 3B). Here we sought to establish the importance of Ppn1 for CPF formation. Yth1 was affinity purified from wild-type and ppn1Δ mutants and its associated proteins identified by mass-spectrometry analysis (Figure 5D). A typical example of such purifications is shown on Figure S12B. In wild-type cells, Yth1 co-purified with all known CPF sub-units, including Swd2.2, Ppn1 and PP1Dis2. In the absence of Ppn1, Swd2.2 and PP1Dis2 dissociated from the CPF whilst the other CPF sub-units remained bound to Yth1 (Figure 5D). Similarly, lack of Swd2.2 triggered the dissociation of Ppn1 and PP1Dis2 from the CPF but of no other CPF sub-units (Figure S12A). These observations suggested that Ppn1, Swd2.2 and PP1Dis2 constitute an independent module within the CPF, whose loss does not alter CPF integrity.
To confirm this, we purified PP1Dis2, Swd2.2 and Ppn1 and identified their binding partners by mass-spectrometry analysis. PP1Dis2 bound to all CPF sub-units and various other proteins (Figure 5C). In the absence of Swd2.2, PP1Dis2 no longer bound to the CPF but remained associated with Ppn1 and its other binding partners. In contrast, when Ppn1 was missing, PP1Dis2 failed to bind to Swd2.2 and the CPF but remained associated with its other binding partners. The interaction between Swd2.2 and the CPF required Ppn1 (Figure 5A&E) and conversely, the interaction between Ppn1 and the CPF required Swd2.2 (Figure 5B). Thus Swd2.2 and Ppn1 are inter-dependent for their association with the CPF and both are necessary for the association of PP1Dis2 with the CPF. These observations are summarized on Figure 5F. Collectively, these data establish that PP1Dis2, Ppn1 and Swd2.2 form a protein module associated with the CPF whose absence does not affect significantly the composition of the core CPF. We named this module the DPS (Dis2-Ppn1-Swd2.2) module. Note that to further illustrate the quality of our affinity purifications, we provide the full list of unique peptides recovered after purifications of Yth1, Swd2.2, Ppn1 and PP1Dis2 ranked by abundance (Table S4).
Figure 5B shows that the interaction between Ppn1 and the CPF was detectable whether protein extracts were prepared from cycling or early mitotic cells. This suggested that DPS interacts with the CPF throughout the cell-cycle. We sought to confirm these observations using co-immunoprecipitation approaches. Here we show that synchronizing cells in early mitosis did not alter complex formation between Swd2.2 and the CPF sub-unit Pfs2 (Figure 5G), nor did it alter the interaction between Swd2.2 and PP1Dis2 (Figure 5H). Taken together, these data show that DPS is stable and remains associated with the CPF in early mitosis. Similarly, ChIP analysis indicated that the levels of chromatin-associated CPF remained comparable in cycling cells and in early mitotic cells (Figure S13). Taken together, these data show that DPS is present on chromatin throughout the cell-cycle. This is consistent with the idea that DPS can antagonize the action of condensin in mitosis.
DPS opposes condensin-mediated chromosome condensation
If DPS is indeed a functional module, the mutation of all its components should give similar phenotypes. To validate this prediction, we tested whether Ppn1 and PP1Dis2, like Swd2.2, could oppose condensin-mediated chromosome condensation. This is indeed what we observed. Lack of Ppn1 (ppn1Δ) significantly improved the growth of cut3-477 cells at the restrictive temperature (p<0,01, Mann-Whitney test; Figure 6A). The poor growth of cut3-477 at the restrictive temperature was also improved when Ppn1 lost its ability to bind PP1Dis2, suggesting that the negative effect of Ppn1 on cut3-477 is mediated, at least in part, by its interaction with PP1Dis2 (p<0,01, Mann-Whitney test). Note however that lack of the core CPF component Ctf1 had no effect on the growth of cut3-477 cells (Figure S14A). Similarly, lack of Ppn1 but not of Ctf1 significantly decreased the percentage of defective anaphases (Figure 6B and Figure S14B), showing that lack of Ppn1 could restore the formation of segregation-competent chromosomes in a majority of mitotic cut3-477 cells. Finally, ChIP experiments showed that lack of Ppn1, like lack of Swd2.2, also improved the localization of condensin in cut3-477 cells (p value <0,01, Mann-Whitney test on 8 biological replicates, Figure 6C).
Fig. 6. Ppn1 and Ssu72 oppose condensin-mediated chromosome condensation. 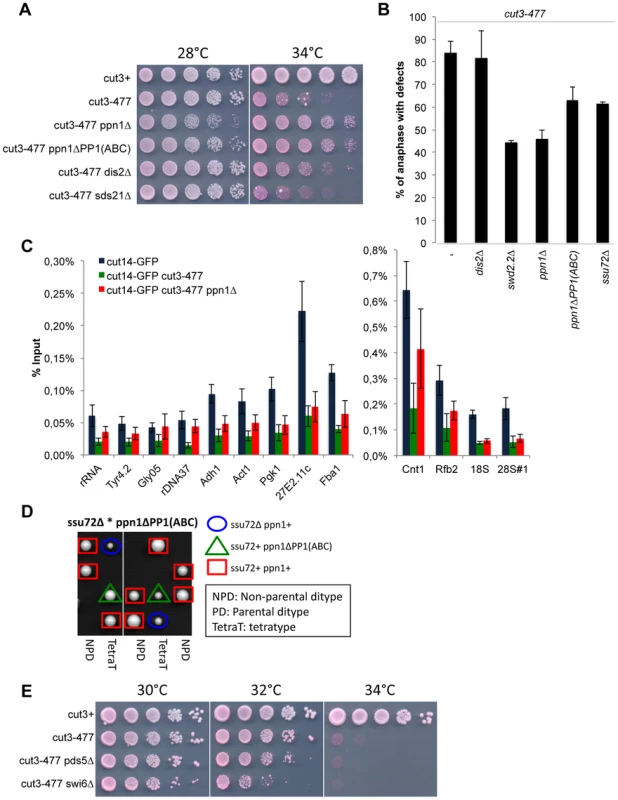
A. Serial dilutions of the indicated strains were plated on rich media at the indicated temperatures. B. Chromosome segregation in anaphase was visualized after growing cells of the indicated genotypes for one generation at 34°C. Anaphases were scored as defective when lagging chromatin was detected between the two main DNA masses. For each genotype, a minimum of 6 independent experiments was performed in which a minimum of 100 anaphase cells was scored. C. The indicated strains were grown at 34°C for 3 hours and ChIP-qPCR was performed to analyze the amount of Cut14-GFP cross-linked to chromatin (mean ± standard deviation from 8 biological replicates). See text for details for the statistical analysis of the experiments. D. Tetrad dissection was used to show that the double mutant ssu72Δ ppn1ΔPP1(ABC) is dead. E. Serial dilutions of the indicated strains were plated on rich media at the indicated temperatures. DPS is redundant with Ssu72
Lack of PP1Dis2 (dis2Δ) had a weaker suppressive effect on cut3-477 than either swd2.2Δ or ppn1Δ (Figure 6AB). To explain this observation, we speculated that another phosphatase could at least partly substitute for PP1Dis2. Here we present evidence that one such phosphatase could be the CPF-associated Ssu72 phosphatase. First, lack of Ssu72 exhibited a mild suppressive effect on cut3-477 (p = 0,05 Mann-Whitney test, Figure 6B), although it did not improve significantly the localization of condensin in cut3-477 cells (Figure S15). Secondly, we found that the double mutant ssu72Δ ppn1ΔPP1(ABC), which lacks CPF-associated phosphatases, is dead (Figure 6D). This evidence suggests that both CPF-associated phosphatases PP1Dis2 and Ssu72 regulate condensin-dependent chromosome condensation. Furthermore, they act redundantly within the CPF to perform one or more essential function(s).
We wondered what could be the substrates of CPF-associated phosphatases whose hyper-phosphorylation in the absence of DPS could facilitate the function of condensin. We have previously ruled out that histone H3 could be a relevant target (Figure 4J). Human Ssu72 was shown recently to interact with the condensin-related cohesin complex and thereby facilitate sister-chromatid cohesion [34]. This opened the possibility that a weakened cohesin function in ssu72Δ or dps mutants could facilitate the function of condensin in cut3-477 cells. To test this, we weakened chromosome cohesion by mutating two known regulators of cohesin, Swi6 (swi6Δ) and Pds5 (pds5Δ). Neither deletion was able to suppress the growth defect of cut3-477 (Figure 6E). This suggested that a weakened cohesion in ssu72 or dps mutants is not sufficient to explain their ability to suppress cut3-477.
Discussion
Here we provide evidence that the CPF sub-unit Swd2.2 is a negative regulator of condensin-mediated chromosome condensation. We show that Swd2.2 is part of a PP1-containing protein module associated with the CPF, whose absence does not alter CPF organization. We named this module the DPS module. Lack of DPS destabilized mildly the association of the CPF with chromatin but the resulting defects in gene expression remained relatively minor and context-specific. However, we show that DPS and the CPF-associated Ssu72 phosphatase oppose condensin-mediated chromosome condensation. Their relevant substrate(s) in this process is(are) still unknown, although we have shown that it is unlikely that it could be phospho-Ser10 on histone H3, cohesin or Aurora B-dependent phosphorylation of Cnd2. However at this stage, our data do not exclude the possibility that DPS and Ssu72 target other phosphorylated sites on condensin.
DPS, a conserved regulator of chromosome condensation
Human PNUTS is a scaffold for the PP1-containing PTW/PP1 complex, where it interacts directly with three proteins, Wdr82, Tox4 and PP1 [27]. Our data strongly suggest that Swd2.2 is the fission yeast functional homologue of Wdr82 and Ppn1 the homologue of PNUTS. We could not identify a homologue of Tox4 in fission yeast. Human PNUTS, like Ppn1, co-purified with the pre-mRNA 3′ processing complex [35]. As such, PTW/PP1 is reminiscent of the DPS complex we identified here.
Interestingly, we note that the other PP1 isoform in fission yeast, PP1Sds21 was not able to replace PP1Dis2 within the DPS. Furthermore, the nuclear localization pattern that PP1Dis2 adopted in the absence of Ppn1 was highly similar to the nuclear localization pattern that was reported for PP1Sds21 [36]. This shows that the ability to bind Ppn1 and the CPF is a major distinguishing feature between the two isoforms of PP1 in fission yeast. The structural features that allow PP1Dis2 but not PP1Sds21 to bind to Ppn1 remain to be elucidated.
In the human PTW/PP1 complex, the interaction between hPNUTS, Wdr82 and PP1 is likely to be relevant for chromosome condensation, as the domain of hPNUTS that mediates the interaction with both PP1 and Wdr82 is sufficient to induce chromosome decondensation in vitro [27], [37]. This suggests that the function of DPS as a negative regulator of chromosome condensation is likely to be evolutionary-conserved.
A role for DPS at Pol III-transcribed genes?
Our data show that lack of Swd2.2 had a significant impact on the association of the RNA Pol III transcription machinery with chromatin. In our hands, the effects of swd2.2Δ on the recruitment of the Pol III machinery were highly reminiscent of the effects of mutating the TFIIIC component Sfc3 [6]. Strikingly, sfc3-1 is, like swd2.2Δ, a suppressor of cut3-477 [6]. Unfortunately, we repeatedly failed to generate viable strains with a reduced expression of TFIIIC. As a consequence, we could not establish whether the reduced association of TFIIIC with chromatin observed in swd2.2Δ and sfc3-1 cells could by itself facilitate the localization of condensin. Our observations are however consistent with the idea put forward previously that the local reduction in RNA polymerase activity can facilitate the association of condensin [10].
Part of the effects of swd2.2Δ on the recruitment of the Pol III machinery is likely to be indirect, because lack of Swd2.2 had a mild impact on the expression of Sfc6, a component of TFIIIC. On the other hand, we detected a small but significant enrichment of Swd2.2 and Pfs2 at Pol III-transcribed genes, consistent with the idea that it could also play a direct role there. The fact that a factor associated with the RNA Pol II RNA processing machinery such as Swd2.2 could also play a role at Pol III genes is not unprecedented. In budding yeast in particular, the Nrd1/Nab3 complex is involved in processing both Pol II and Pol III transcripts [38] and Pcf11 in S.cerevisiae is enriched at Pol III genes [39]. Interestingly, budding yeast PP1 was shown to dephosphorylate Sen1 helicase, which associates with Nrd1/Nab3 [26] and we identified Sen1 as a suppressor of cut3-477 (Table S1). Whether or not Swd2.2 is involved in directing the activity of PP1 towards Sen1 at Pol III genes in fission yeast will require further studies.
(CTD) Phosphatases and condensation
In chicken DT40 cells, preventing the association of PP1 with chromatin was sufficient to rescue anaphase chromosome segregation when condensin was deficient [40]. Our data are consistent with these observations. Earnshaw and colleagues proposed that preventing the association of PP1 with chromatin resulted in the hyper-activation of “Regulator of Chromosome Architecture” (RCA), a hypothetical condensin-independent chromosome compacting activity [40]. Our stories differ on that point, as we show that lack of Swd2.2 and Ppn1 impacted the localization of condensin in cut3-477 cells, indicating that the target of DPS is ultimately condensin and not RCA.
Cdc14 phosphatase in budding yeast was shown to dephosphorylate the CTD domain of RNA Pol II [10]. When Cdc14 activity was impaired, RNA Pol II-dependent transcription occurred abnormally at repetitive sequences and condensin was displaced from these sequences [10]. It was concluded from these data that CTD dephosphorylation participates in the inactivation of RNA polymerase II in a way that facilitates the binding of condensin and the assembly of a condensed chromosome. Here, our work shows that PP1 and Ssu72, which, like Cdc14, have been shown to target the CTD domain for dephosphorylation [41], [42], [43], are negative regulators of condensin. Further studies are required to understand this apparent discrepancy.
We note however that, in budding yeast, lack of Ssu72 interferes with transcription elongation [44]. Interestingly, the N-terminus domain of Ppn1 shows homology to TFIIS (Figure S11), whose absence impairs transcription elongation in fission yeast [45]. This opens the possibility that Ppn1 could also modulate transcription elongation. We recently identified as a suppressor of cut3-477 a mutation in the Mediator sub-unit Nut2 [46] and our present screen also identified the deletion of the TFIIS homologue Tfs1 (tfs1Δ) as a suppressor of cut3-477 (Table S1). Taken together, these observations reinforce the idea of complex functional links between condensin and transcription and suggest that reducing the processivity of RNA Polymerase II or III could somehow facilitate the function of condensin. The mechanisms involved remain to be elucidated.
Materials and Methods
Fission yeast strains
A list of the strains used in this study is given in Table S5. Standard genetic crosses were employed to construct all strains. Yeast cells were grown according to standard procedures. Dis2-3flag, Yth1-3flag, Swd2.2-3flag, Ppn1-3flag, Yth1-GFP, Swd2.2-GFP, ppn1Δ, swd2.2Δ, rtt103Δ were generated using a standard PCR procedure. To obtain ppn1ΔPP1(ABC)-3flag, the C-terminus of Ppn1 was PCR amplified and cloned into pGEMT-easy (Promega, Madison, Wisconsin, USA). Site-directed mutagenesis was then used to mutate the three PP1-binding sites using Quickchange protocols (Stratagene). Overlapping PCR was used to add a 3xFlag tag and a cassette of resistance to nourseothricin (NatR) to the C-terminus of Ppn1. This PCR product was used to transform yeast. Proper integrants were selected by PCR and western blot and were sequenced to verify the presence of the mutations.
Immunoprecipitation
2.108 cells were frozen in liquid nitrogen and broken open in lysis buffer (50 mM HEPES [pH 7.6], 75 mM KCl, 1 mM MgCl2, 1 mM EGTA, 0.1% Triton, 1 mM sodium vanadate, microcystin, 1 µg/mL leupeptin, 1 µg/mL pepstatin, 1 µg/mL chymostatin, and 1 mM Pefabloc) using zirconium beads and a Fast-prep machine (40″, 6,5 ms−1, 3 times). Clarified extracts were then incubated for 60 min at 4°C with Protein A-coupled Dynabeads incubated previously with the proper antibody according to the manusfacturer's recommendations. The immunoprecipitated complexes were washed three times with lysis buffer and once with phosphate-buffered saline containing 0.02% Tween 20. Immunoprecipitated complexes were analyzed by immunoblot using a semi-dry transfer protocol. Cnd2 phosphorylation was analysed as described previously [2].
Protein purification
5 g of cells were broken using a Retsch MM400 mill for 180 s at 30 Hz (repeat three times). The broken cells were then resuspended in lysis buffer (50 mM Hepes-KOH [pH 7.6], 100 mM KCl, 1 mM EGTA, 10% Glycerol, 0.1% NP40, 1 mM MgCl2, 1 µg/mL leupeptin, 1 µg/mL pepstatin, 1 µg/mL chymostatin, and 1 mM Pefabloc) and then sonicated twice for 30 seconds on ice (1.2 W per mL). After centrifugation (5′, 4°C, 4000 rpm), the supernatant was filtered through a 2.7 µM then a 1.6 µM glass microfiber filter (Whatmann 25 mm GD/X). 25 µL of 50/50 slurry of anti-Flag M2 affinity agarose beads (Sigma) was added and incubated on a rotating wheel at 4°C for 30′. The beads were then washed three times in cold lysis buffer and then twice in 50 mM Hepes-KOH [pH 7.6], 50 mM KCl. Proteins were eluted by two 5′ incubations in 50 µL of 50 mM H3PO4.
Mass-spectrometry analysis
All chemicals were purchased from Sigma-Aldrich (UK) unless otherwise stated. Acetonitrile and water for LC-MS/MS were HPLC grade (Fisher, UK). Formic acid was Suprapure 98-100% (Merck, Darmstadt, Germany) and trifluoroacetic acid was 99% purity sequencing grade. All HPLC-MS connector fittings were from Upchurch Scientific or Valco (Hichrom and RESTEK, UK).
Gel-free samples were prepared and loaded onto strong cation exchange columns, reduced with DTT, alkylated with iodoacetamide, and digested with trypsin as described in [47]. Samples were dried under low pressure and reconstituted in 10 µl of 0.1% (v/v) formic acid 2.5% (v/v) acetonitrile for nano-LC-MSMS. Capillary-HPLC-MSMS data were acquired on an on-line system consisting of a micro-pump (1200 binary HPLC system, Agilent, UK) coupled to a hybrid LTQ-Orbitrap XL instrument (Thermo-Fisher, UK). The LTQ was controlled through Xcalibur 2.0.7. HPLC-MS methods have been described previously [48].
Protein identification and quantification
MS/MS data were searched using MASCOT Versions 2.3 (Matrix Science Ltd, UK) against a Schizosaccharomyces pombe database downloaded from the Sanger Institute (http://www.sanger.ac.uk/) with 5022 sequences. Variable methionine oxidation, STY phosphorylation, protein N-terminal acetylation and fixed cysteine carbamidomethylation were used in all searches. Precursor mass tolerance was set to 7 ppm and MS/MS tolerance to 0.4 amu. The significance threshold (p) was set below 0.05 (MudPIT scoring in Mascot). All LC-MS runs were combined using MaxQuant (version 1.0.13.8), assuming a false positive rate of 0.01 [49].
RNA extraction and transcription termination assay
RNA was extracted from 2.108 cells according to the procedure described in [50]. qPCR were performed a Rotorgene machine (Qiagen). Retrotranscription and qPCR were performed as described previously [51]. Briefly, cells were grown at 34°C for two hours, and total RNA extracted. Total RNA was reverse-transcribed using random hexamers and cDNAs amplified by PCR using the indicated primers. PCR products were stained with Sybr Green and quantified in gel using the FLA-5000 imaging system (Fujifilm).
Strand-specific tiling arrays
ProfilXpert (http://www.profilexpert.fr/) performed the strand-specific tiling arrays and their initial analysis. The data were established in triplicates. RNAs extracted in the lab were purified using the miRNeasy mini kit (Qiagen), dosed using a nanodrop and their quality was assessed using a Bioanalyzer 2100 (Agilent). To prepare cDNAs, 250 ng of RNA were then amplified using the WT Expression Kit (Ambion). The resulting cDNAs were then dosed (Nanodrop) and their quality assessed using a Bioanalyzer 2100 (Agilent). 5.5 µg of cDNAs were then fragmented and labelled using the GeneChIP WT Terminal Labelling Kit (Affymetrix). 5 µg of cDNAs were then hybridized on a GeneChip S.pombe Tiling 1.0 Array (Affymetrix). The data were normalized using Quantile Normalization [52] and RMA background correction [53] using the software Partek Genomics Suite 6.5. To establish which genes were deregulated in swd2.2Δ, the median value of the signals recorded for each gene in each replicate was first calculated. These median values were then averaged for the three replicates. For each gene, The Fold-change (FC) and the corresponding p-value were calculated to express variation in expression between swd2.2Δ and swd2.2+ cells. To identify genes with potential transcription termination defects, we proceeded as follows: for every gene whose 3′UTR is annotated in the fission yeast database, we calculated the ratio between the average signal recorded in the 3′UTR and the average signal recorded in the ORF in swd2.2+ and swd2.2Δ cells. We considered that genes had a transcription termination defect in cells lacking Swd2.2 when the ratio obtained for swd2.2Δ was at least 1.5 greater than the ratio obtained in swd2.2+. The data can be accessed on GEO under the accession number GSE38005.
Statistical analysis of genomic features
All statistical analysis was done using the R software (www.r-project.org). Genomic features annotations were downloaded on the 09/05/2011 from the Sanger centre (ftp://ftp.sanger.ac.uk/pub/yeast/pombe/GFF/). To test for differential expression between gDWN-1 and gDWN genes in the wild-type context, we computed the 47 logratios and tested their departure from zero using a Wilcoxon test. To test if the intergenic distance (IGR) between gDWN-1 and gDWN was particularly short or long compared to any other gene, we compared the median value of the 47 observed IGR to the distribution of median values obtained from 10,000 randomly chosen sets of 47 genes in the genome. The resulting empirical null distribution is shown on Figure S7C. The median IGR observed from the actual set of 47 gDWN genes was shorter than all simulated median IGRs. The statistical significance for claiming that these IGRs are particularly short is therefore lower than 1/10,000. Similarly, IGRterm distances observed on the set of 780 gTERM genes were computed and their median was compared to the distribution of median values from 10,000 sets of 780 genes picked at random from the genome (empirical null distribution of Figure S8C). Since no median value of the random sets was as small as the observed one, the claim for short IGRterm values of gTERM genes is supported at a significance lower than 1/10,000.
Chromatin immunoprecipitation
108 cells were treated with 1% formaldehyde (Sigma) at 18°C for 30 mn. After extensive washes with cold PBS, cells were frozen in liquid Nitrogen. Frozen cells were then broken open in cold lysis buffer (Hepes-KOH 50 mM [pH 7.5], NaCl 140 mM, EDTA 1 mM, Triton 1%, Na-deoxycholate 0.1%, PMSF 1 mM) using Acid-wash Glass beads (Sigma) and a Fast-Prep machine (6 times 1′ at 6,5 ms−1). The lysats were then sonicated at 4°C using a Diagenode sonicator. Immuno-precipitation was done overnight at (4°C) using ProtA-coupled Dynabeads previously incubated with the anti-GFP A11122 antibody (Invitrogen). Beads were washed successively with (10′ incubation on rotating wheel): Wash I buffer (20 mM Tris pH 8, 150 mM NaCl, 2 mM EDTA, 1% Triton-X100, 0.1% SDS), Wash II buffer (20 mM Tris pH 8, 500 mM NaCl, 2 mM EDTA, 1% Triton-X100, 0.1% SDS) and Wash III buffer (20 mM Tris pH 8, 1 mM EDTA, 0.5% Na-deoxycholate, 1% Igepal, 250 mM LiCl). After two additional washes in TE pH 8, the cross-links were reversed by incubation at 65°C (6 hours minimum) with elution buffer (TE pH 8, SDS 0.5%, 0.35 mg/mL Proteinase K). The immuno-precipitated DNA was then purified using the Wizard PCR purification kit (Promega) according to the manusfacturer's instructions. The DNA was then analyzed by qPCR. Primers are available on demand.
Supporting Information
Zdroje
1. PiazzaI, HaeringCH, RutkowskaA (2013) Condensin: crafting the chromosome landscape. Chromosoma 122 : 175–190.
2. TadaK, SusumuH, SakunoT, WatanabeY (2011) Condensin association with histone H2A shapes mitotic chromosomes. Nature 474 : 477–483.
3. D'AmbrosioC, SchmidtCK, KatouY, KellyG, ItohT, et al. (2008) Identification of cis-acting sites for condensin loading onto budding yeast chromosomes. Genes Dev 22 : 2215–2227.
4. KimJH, ZhangT, WongNC, DavidsonN, MaksimovicJ, et al. (2013) Condensin I associates with structural and gene regulatory regions in vertebrate chromosomes. Nat Commun 4 : 2537.
5. TanakaA, TanizawaH, SriswasdiS, IwasakiO, ChatterjeeAG, et al. (2012) Epigenetic regulation of condensin-mediated genome organization during the cell cycle and upon DNA damage through histone H3 lysine 56 acetylation. Mol Cell 48 : 532–546.
6. IwasakiO, TanakaA, TanizawaH, GrewalSI, NomaK (2010) Centromeric localization of dispersed Pol III genes in fission yeast. Mol Biol Cell 21 : 254–265.
7. PekJW, KaiT (2011) A role for vasa in regulating mitotic chromosome condensation in Drosophila. Curr Biol 21 : 39–44.
8. PekJW, KaiT (2011) DEAD-box RNA helicase Belle/DDX3 and the RNA interference pathway promote mitotic chromosome segregation. Proc Natl Acad Sci U S A 108 : 12007–12012.
9. SchubertT, PuschMC, DiermeierS, BenesV, KremmerE, et al. (2012) Df31 protein and snoRNAs maintain accessible higher-order structures of chromatin. Mol Cell 48 : 434–444.
10. Clemente-BlancoA, SenN, Mayan-SantosM, SacristanMP, GrahamB, et al. (2011) Cdc14 phosphatase promotes segregation of telomeres through repression of RNA polymerase II transcription. Nat Cell Biol 13 : 1450–1456.
11. Clemente-BlancoA, Mayan-SantosM, SchneiderDA, MachinF, JarmuzA, et al. (2009) Cdc14 inhibits transcription by RNA polymerase I during anaphase. Nature 458 : 219–222.
12. XingH, VanderfordNL, SargeKD (2008) The TBP-PP2A mitotic complex bookmarks genes by preventing condensin action. Nat Cell Biol 10 : 1318–1323.
13. ParsonsGG, SpencerCA (1997) Mitotic repression of RNA polymerase II transcription is accompanied by release of transcription elongation complexes. Mol Cell Biol 17 : 5791–5802.
14. LoomisRJ, NaoeY, ParkerJB, SavicV, BozovskyMR, et al. (2009) Chromatin binding of SRp20 and ASF/SF2 and dissociation from mitotic chromosomes is modulated by histone H3 serine 10 phosphorylation. Mol Cell 33 : 450–461.
15. RichardP, ManleyJL (2009) Transcription termination by nuclear RNA polymerases. Genes Dev 23 : 1247–1269.
16. SakaY, SutaniT, YamashitaY, SaitohS, TakeuchiM, et al. (1994) Fission yeast cut3 and cut14, members of a ubiquitous protein family, are required for chromosome condensation and segregation in mitosis. EMBO J 13 : 4938–4952.
17. KimM, KroganNJ, VasiljevaL, RandoOJ, NedeaE, et al. (2004) The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature 432 : 517–522.
18. SugiyamaT, Sugioka-SugiyamaR, HadaK, NiwaR (2012) Rhn1, a nuclear protein, is required for suppression of meiotic mRNAs in mitotically dividing fission yeast. PLoS One 7: e42962.
19. LemayJF, D'AmoursA, LemieuxC, LacknerDH, St-SauveurVG, et al. (2010) The nuclear poly(A)-binding protein interacts with the exosome to promote synthesis of noncoding small nucleolar RNAs. Mol Cell 37 : 34–45.
20. UemuraT, OhkuraH, AdachiY, MorinoK, ShiozakiK, et al. (1987) DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell 50 : 917–925.
21. PetrovaB, DehlerS, KruitwagenT, HericheJK, MiuraK, et al. (2013) Quantitative analysis of chromosome condensation in fission yeast. Mol Cell Biol 33 : 984–998.
22. KimHS, VanoosthuyseV, FillinghamJ, RoguevA, WattS, et al. (2009) An acetylated form of histone H2A.Z regulates chromosome architecture in Schizosaccharomyces pombe. Nat Struct Mol Biol 16 : 1286–1293.
23. RoguevA, ShevchenkoA, SchaftD, ThomasH, StewartAF (2004) A comparative analysis of an orthologous proteomic environment in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Mol Cell Proteomics 3 : 125–132.
24. WangSW, AsakawaK, WinTZ, TodaT, NorburyCJ (2005) Inactivation of the pre-mRNA cleavage and polyadenylation factor Pfs2 in fission yeast causes lethal cell cycle defects. Mol Cell Biol 25 : 2288–2296.
25. NedeaE, HeX, KimM, PootoolalJ, ZhongG, et al. (2003) Organization and function of APT, a subcomplex of the yeast cleavage and polyadenylation factor involved in the formation of mRNA and small nucleolar RNA 3′-ends. J Biol Chem 278 : 33000–33010.
26. NedeaE, NalbantD, XiaD, TheoharisNT, SuterB, et al. (2008) The Glc7 phosphatase subunit of the cleavage and polyadenylation factor is essential for transcription termination on snoRNA genes. Mol Cell 29 : 577–587.
27. LeeJH, YouJ, DobrotaE, SkalnikDG (2010) Identification and characterization of a novel human PP1 phosphatase complex. J Biol Chem 285 : 24466–24476.
28. HendrickxA, BeullensM, CeulemansH, Den AbtT, Van EyndeA, et al. (2009) Docking motif-guided mapping of the interactome of protein phosphatase-1. Chem Biol 16 : 365–371.
29. MeadowsJC, ShepperdLA, VanoosthuyseV, LancasterTC, SochajAM, et al. (2011) Spindle checkpoint silencing requires association of PP1 to both Spc7 and kinesin-8 motors. Dev Cell 20 : 739–750.
30. BeauregardPB, GuerinR, TurcotteC, LindquistS, RokeachLA (2009) A nucleolar protein allows viability in the absence of the essential ER-residing molecular chaperone calnexin. J Cell Sci 122 : 1342–1351.
31. HsuJY, SunZW, LiX, ReubenM, TatchellK, et al. (2000) Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell 102 : 279–291.
32. Castellano-PozoM, Santos-PereiraJM, RondonAG, BarrosoS, AndujarE, et al. (2013) R loops are linked to histone H3 S10 phosphorylation and chromatin condensation. Mol Cell 52 : 583–590.
33. TodaT, UmesonoK, HirataA, YanagidaM (1983) Cold-sensitive nuclear division arrest mutants of the fission yeast Schizosaccharomyces pombe. J Mol Biol 168 : 251–270.
34. KimHS, KimSH, ParkHY, LeeJ, YoonJH, et al. (2013) Functional interplay between Aurora B kinase and Ssu72 phosphatase regulates sister chromatid cohesion. Nat Commun 4 : 2631.
35. ShiY, Di GiammartinoDC, TaylorD, SarkeshikA, RiceWJ, et al. (2009) Molecular architecture of the human pre-mRNA 3′ processing complex. Mol Cell 33 : 365–376.
36. Alvarez-TabaresI, GrallertA, OrtizJM, HaganIM (2007) Schizosaccharomyces pombe protein phosphatase 1 in mitosis, endocytosis and a partnership with Wsh3/Tea4 to control polarised growth. J Cell Sci 120 : 3589–3601.
37. LandsverkHB, KirkhusM, BollenM, KuntzigerT, CollasP (2005) PNUTS enhances in vitro chromosome decondensation in a PP1-dependent manner. Biochem J 390 : 709–717.
38. WlotzkaW, KudlaG, GrannemanS, TollerveyD (2011) The nuclear RNA polymerase II surveillance system targets polymerase III transcripts. EMBO J 30 : 1790–1803.
39. KimH, EricksonB, LuoW, SewardD, GraberJH, et al. (2010) Gene-specific RNA polymerase II phosphorylation and the CTD code. Nat Struct Mol Biol 17 : 1279–1286.
40. VagnarelliP, HudsonDF, RibeiroSA, Trinkle-MulcahyL, SpenceJM, et al. (2006) Condensin and Repo-Man-PP1 co-operate in the regulation of chromosome architecture during mitosis. Nat Cell Biol 8 : 1133–1142.
41. KrishnamurthyS, HeX, Reyes-ReyesM, MooreC, HampseyM (2004) Ssu72 Is an RNA polymerase II CTD phosphatase. Mol Cell 14 : 387–394.
42. WashingtonK, AmmosovaT, BeullensM, JerebtsovaM, KumarA, et al. (2002) Protein phosphatase-1 dephosphorylates the C-terminal domain of RNA polymerase-II. J Biol Chem 277 : 40442–40448.
43. CiurciuA, DuncalfL, JonchereV, LansdaleN, VasievaO, et al. (2013) PNUTS/PP1 Regulates RNAPII-Mediated Gene Expression and Is Necessary for Developmental Growth. PLoS Genet 9: e1003885.
44. Reyes-ReyesM, HampseyM (2007) Role for the Ssu72 C-terminal domain phosphatase in RNA polymerase II transcription elongation. Mol Cell Biol 27 : 926–936.
45. Reyes-TurcuFE, ZhangK, ZofallM, ChenE, GrewalSI (2011) Defects in RNA quality control factors reveal RNAi-independent nucleation of heterochromatin. Nat Struct Mol Biol 18 : 1132–1138.
46. RobelletX, FauqueL, LegrosP, MollereauE, JanczarskiS, et al. (2014) A genetic screen for functional partners of condensin in fission yeast. G3 (Bethesda) 4 : 373–381.
47. Luke-GlaserS, RoyM, LarsenB, Le BihanT, MetalnikovP, et al. (2007) CIF-1, a shared subunit of the COP9/signalosome and eukaryotic initiation factor 3 complexes, regulates MEL-26 levels in the Caenorhabditis elegans embryo. Mol Cell Biol 27 : 4526–4540.
48. Le BihanT, GrimaR, MartinS, ForsterT, Le BihanY (2010) Quantitative analysis of low-abundance peptides in HeLa cell cytoplasm by targeted liquid chromatography/mass spectrometry and stable isotope dilution: emphasising the distinction between peptide detection and peptide identification. Rapid Commun Mass Spectrom 24 : 1093–1104.
49. CoxJ, MaticI, HilgerM, NagarajN, SelbachM, et al. (2009) A practical guide to the MaxQuant computational platform for SILAC-based quantitative proteomics. Nat Protoc 4 : 698–705.
50. WilhelmBT, MargueratS, WattS, SchubertF, WoodV, et al. (2008) Dynamic repertoire of a eukaryotic transcriptome surveyed at single-nucleotide resolution. Nature 453 : 1239–1243.
51. BernardP, DrogatJ, DheurS, GenierS, JaverzatJP (2010) Splicing factor Spf30 assists exosome-mediated gene silencing in fission yeast. Mol Cell Biol 30 : 1145–1157.
52. BolstadBM, IrizarryRA, AstrandM, SpeedTP (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19 : 185–193.
53. IrizarryRA, HobbsB, CollinF, Beazer-BarclayYD, AntonellisKJ, et al. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4 : 249–264.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 6- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Akutní intermitentní porfyrie
- Příjem alkoholu a menstruační cyklus
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
- Transfer zmraženého embrya zlepšuje výsledky IVF
-
Všechny články tohoto čísla
- Inflammation: Gone with Translation
- Recombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
- Caspase Inhibition in Select Olfactory Neurons Restores Innate Attraction Behavior in Aged
- Accurate, Model-Based Tuning of Synthetic Gene Expression Using Introns in
- A Novel Peptidoglycan Binding Protein Crucial for PBP1A-Mediated Cell Wall Biogenesis in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
- Introgression from Domestic Goat Generated Variation at the Major Histocompatibility Complex of Alpine Ibex
- Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in
- Coordination of Wing and Whole-Body Development at Developmental Milestones Ensures Robustness against Environmental and Physiological Perturbations
- Phenotypic Dissection of Bone Mineral Density Reveals Skeletal Site Specificity and Facilitates the Identification of Novel Loci in the Genetic Regulation of Bone Mass Attainment
- Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of -Factors in Two Independent Origins of C Photosynthesis
- Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity
- Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation
- Rosa26-GFP Direct Repeat (RaDR-GFP) Mice Reveal Tissue- and Age-Dependence of Homologous Recombination in Mammals
- Abnormal Type I Collagen Post-translational Modification and Crosslinking in a Cyclophilin B KO Mouse Model of Recessive Osteogenesis Imperfecta
- : Clonal Reinforcement Drives Evolution of a Simple Microbial Community
- Reviving the Dead: History and Reactivation of an Extinct L1
- Defective iA37 Modification of Mitochondrial and Cytosolic tRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate tRNA
- Early Back-to-Africa Migration into the Horn of Africa
- Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency
- Microbial Succession in the Gut: Directional Trends of Taxonomic and Functional Change in a Birth Cohort of Spanish Infants
- Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia
- Genetic Determinants of Long-Term Changes in Blood Lipid Concentrations: 10-Year Follow-Up of the GLACIER Study
- Palaeosymbiosis Revealed by Genomic Fossils of in a Strongyloidean Nematode
- Early Embryogenesis-Specific Expression of the Rice Transposon Enhances Amplification of the MITE
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
- Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
- N-WASP Is Required for Structural Integrity of the Blood-Testis Barrier
- The Transcription Factor TFII-I Promotes DNA Translesion Synthesis and Genomic Stability
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
- Digital Genotyping of Macrosatellites and Multicopy Genes Reveals Novel Biological Functions Associated with Copy Number Variation of Large Tandem Repeats
- ATRA-Induced Cellular Differentiation and CD38 Expression Inhibits Acquisition of BCR-ABL Mutations for CML Acquired Resistance
- The EJC Binding and Dissociating Activity of PYM Is Regulated in
- JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling
- Mouse Y-Linked and Are Expressed during the Male-Specific Interphase between Meiosis I and Meiosis II and Promote the 2 Meiotic Division
- Rasa3 Controls Megakaryocyte Rap1 Activation, Integrin Signaling and Differentiation into Proplatelet
- Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
- Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis
- The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
- The First Endogenous Herpesvirus, Identified in the Tarsier Genome, and Novel Sequences from Primate Rhadinoviruses and Lymphocryptoviruses
- Sequence of a Complete Chicken BG Haplotype Shows Dynamic Expansion and Contraction of Two Gene Lineages with Particular Expression Patterns
- Background Selection as Baseline for Nucleotide Variation across the Genome
- CPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
- The Effects of Codon Context on Translation Speed
- Glycogen Synthase Kinase (GSK) 3β Phosphorylates and Protects Nuclear Myosin 1c from Proteasome-Mediated Degradation to Activate rDNA Transcription in Early G1 Cells
- Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
- Muscle Structure Influences Utrophin Expression in Mice
- BLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
- Identification of Late Larval Stage Developmental Checkpoints in Regulated by Insulin/IGF and Steroid Hormone Signaling Pathways
- Transport of Magnesium by a Bacterial Nramp-Related Gene
- Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
- The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription
- The Rim15-Endosulfine-PP2A Signalling Module Regulates Entry into Gametogenesis and Quiescence Distinct Mechanisms in Budding Yeast
- Regulation of Hfq by the RNA CrcZ in Carbon Catabolite Repression
- Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions
- Positive Feedback of Expression Ensures Irreversible Meiotic Commitment in Budding Yeast
- Hecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
- Regulatory Mechanisms That Prevent Re-initiation of DNA Replication Can Be Locally Modulated at Origins by Nearby Sequence Elements
- Speciation and Introgression between and
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Early Back-to-Africa Migration into the Horn of Africa
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



