-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
One of the earliest and most crucial events in animal development is the establishment of the embryonic dorsal axis. In amphibians and fish, this event depends on the transport of so-called “dorsal determinants” from one region of the egg, at the pole opposite from the site where the oocyte nucleus lies, towards the site of axis induction. There, the dorsal determinant activates the Wnt signaling pathway, which in turn triggers dorsal gene expression. Dorsal determinant transport is mediated by the reorganization of a cellular network composed of microtubules. We determine that hecate, a zebrafish gene active during egg formation that is essential for embryonic axis induction, is required for an early step in this microtubule reorganization. We find that hecate corresponds to glutamate receptor interacting protein 2a, which participates in other animal systems in Wnt-based pathways. We also show that the microtubule reorganization dependent on hecate results in a subtle symmetry-breaking event that subsequently becomes amplified by a more general transport process independent of hecate function. Our data reveal new links between glutamate receptor interacting protein 2a, Wnt signaling and axis induction, and highlights basic mechanisms by which small changes early in development translate into global changes in the embryo.
Published in the journal: . PLoS Genet 10(6): e32767. doi:10.1371/journal.pgen.1004422
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004422Summary
One of the earliest and most crucial events in animal development is the establishment of the embryonic dorsal axis. In amphibians and fish, this event depends on the transport of so-called “dorsal determinants” from one region of the egg, at the pole opposite from the site where the oocyte nucleus lies, towards the site of axis induction. There, the dorsal determinant activates the Wnt signaling pathway, which in turn triggers dorsal gene expression. Dorsal determinant transport is mediated by the reorganization of a cellular network composed of microtubules. We determine that hecate, a zebrafish gene active during egg formation that is essential for embryonic axis induction, is required for an early step in this microtubule reorganization. We find that hecate corresponds to glutamate receptor interacting protein 2a, which participates in other animal systems in Wnt-based pathways. We also show that the microtubule reorganization dependent on hecate results in a subtle symmetry-breaking event that subsequently becomes amplified by a more general transport process independent of hecate function. Our data reveal new links between glutamate receptor interacting protein 2a, Wnt signaling and axis induction, and highlights basic mechanisms by which small changes early in development translate into global changes in the embryo.
Introduction
Dorsoventral axis formation is a fundamental process in early vertebrate embryogenesis. In many vertebrates such as amphibians and teleosts, evidence indicates that maternally-supplied dorsal determinants trigger the formation of the future dorsal organizer. Embryological manipulations have indicated that the dorsal determinants are initially localized to the vegetal pole and subsequently translocate animally to the prospective dorsal side in a microtubule-dependent process in both amphibians (reviewed in [1]) and teleosts [2]–[4]. In amphibians, translocation of the signal from the vegetal pole to the dorsal side is initiated by cortical rotation, the microtubule-dependent movement of the egg cortex with respect to its core that is triggered by fertilization and is implemented by transport along microtubule tracks (reviewed in [1]).
Although not readily apparent in the zebrafish embryo, detailed analysis has indicated the existence of processes that share similarities with the amphibian cortical rotation. Early studies showed that fluorescent polystyrene beads injected at the vegetal pole were transported animally along microtubule-based cortical tracks in a microtubule dependent manner [2], and that this movement had temporal dynamics and functional requirements similar to that of the movement of putative dorsal determinants as defined by embryological manipulations [3], [4]. Subsequent work showed that maternal factors such as Syntabulin and Wnt8a, involved in axis induction, localized to the vegetal pole of the egg and upon fertilization and egg activation shifted animally towards the presumed prospective dorsal region of the embryo [5]–[7]. Further studies of microtubule rearrangements in live embryos confirmed that the tracks of bundled microtubules that form at the zebrafish vegetal pole upon egg activation become aligned in the direction of the future dorsal side of the embryo, and showed bulk cortical particle movement analogous to a cortical rotation [8].
The movement of the dorsal determinant results in the activation of the Wnt/βcatenin signaling pathway and the activation of β-catenin-dependent targets [1]. This well-known pathway is characterized by the activation of Frizzled receptors by the Wnt ligand, and an intracellular cascade involving the activation of Dishevelled and the downregulation of a β-catenin degradation complex that includes GSK3, Axin and Adenomatous polyposis coli (APC), leading to the accumulation of β-catenin in the nucleus [9]. Nuclear β-catenin in turn interacts with transcription factors of the Tcf family to activate transcription of target genes. Wnt/βcatenin pathway components and/or nuclear accumulation of β-catenin have been shown to be involved in embryonic axis determination in diverse deuterostomes such as fish, amphibians, mammals and amphioxus, as well as in lineages as basal as echinoderms, Cnidarians and planaria (reviewed in [10]) implying that the pathway was recruited for axis determination very early in animal evolution.
Although the involvement of Wnt/βcatenin activation across species is well documented, the identity of the molecules that activate the pathway in the early embryo, often referred to as dorsal determinants, remains unknown in most cases. In Xenopus, wnt11 mRNA is first located at the vegetal pole and becomes enriched at the future dorsal side after fertilization, and depletion of wnt11 mRNA results in embryos defective in dorsal axis induction [11]. Thus, Wnt11, together with ubiquitously present Wnt5 [12], [13] has been proposed to be the dorsal determinant in this amphibian species. Studies in the zebrafish exclude a function for Wnt11 or Wnt 5 in axis induction but suggest a role for Wnt8a in this process [7]. Maternal zebrafish wnt8a mRNA is localized during oogenesis to the vegetal pole of the oocyte and, upon fertilization, wnt8a mRNA experiences a shift from its original location at the vegetal pole to an off-center region thought to correspond to the dorsal side [7]. These studies suggest that, while Wnt/β-catenin pathway activation may be highly conserved in axis induction across the animal kingdom, maternally-based mechanisms that lead to the activation of the pathway vary.
Efforts from several laboratories have used forward genetics approaches to identify maternal factors essential for various aspects of early embryonic development in the zebrafish [5], [14]–[18]. Several reports have documented maternal-effect mutations affecting zebrafish dorsal axis induction [5], [15], [17], [18]. Mutations in three maternal-effect genes, ichabod, tokkaebi and hecate, cause specific ventralized phenotypes, consistent with a role for these genes in axis induction. Overexpression of Wnt signaling pathway components in ichabod mutant embryos indicate that this mutation acts within the Wnt/β-catenin signaling pathway at a level downstream of the β-catenin degradation complex [15]. These and other results show that ichabod corresponds to a β-catenin-2 gene expressed maternally and involved in axis induction [19].
Similar overexpression analysis has shown that tokkaebi and hecate, in contrast to ichabod, act upstream of the β-catenin-degradation machinery [5], [20]. Specifically, overexpression of components that activate the pathway at multiple points can rescue the hecate and tokkaebi mutant phenotypes. Rescue by an exogenous Wnt ligand suggests that the Wnt/β-catenin pathway is intact in tokkaebi and hecate mutant embryos, and suggests that, rather than being required for an integral component of Wnt/β-catenin signaling, these genes are required to regulate an endogenous signal that activates this pathway. Consistent with such a proposed role, positional cloning reveals that tokkaebi corresponds to the syntabulin (sybu) gene, which codes for a linker of the kinesin I motor protein involved in cargo transport along microtubules [6]. Both sybu mRNA and protein are localized to the vegetal pole of the egg, and Sybu protein exhibits a slight off-center shift upon egg activation [6]. These data suggest a role for Sybu in the microtubule-dependent transport of vegetally localized dorsal determinants.
Here, we present the molecular characterization of the zebrafish maternal-effect gene hecate (hec). Maternal homozygosity for three independent mutant hec alleles results in embryos with reduced expression of dorsal organizer genes and defects in the formation of dorsoanterior structures ([20]; this report). Positional cloning reveals that hec encodes the Glutamate receptor interacting protein 2a (Grip2a). We find that grip2a mRNA, like wnt8a mRNA and Sybu protein, is localized to the vegetal pole of the oocyte and early embryo, where during egg activation it is shifted off-center corresponding to the previously proposed teleost cortical rotation [8]. The Drosophila Grip homologue has recently been shown to potentiate Wnt signaling at the neuromuscular junction by interacting with the Frizzled receptor on the cytoplasmic side of endocytosing membrane vesicles [21], [22], suggesting a potential mechanism of action for zebrafish Grip2a in axis induction at the level of Frizzled receptor regulation. Unexpectedly, however, we find that hec mutants show defects in the alignment and bundling of microtubules at the vegetal cortex, which result in corresponding defects in the asymmetric movement of wnt8a mRNA and are sufficient to explain the observed axis induction defects.
The short-range shift in vegetally localized factors such as grip2a mRNA also led us to re-examine the functional significance of the previously observed animally-directed cortical transport on axis induction. We find that, although short-range shifts in vegetal signals are affected in hec mutant embryos, these mutants do not exhibit a defect in the long-range, animally directed translocation of cortically injected dorsal beads that occurs in lateral regions of the yolk cortex. Furthermore, we show that, contrary to our expectations, such movements are not restricted to a single arc corresponding to the prospective dorsal region, but occur in multiple meridional arcs even in opposite regions of the embryo. Together, our results propose a role for hec function in the reorganization and bundling of microtubules at the vegetal cortex to mediate a symmetry-breaking event likely corresponding to the teleost cortical rotation. This asymmetry is subsequently amplified by a cortical animally-directed transport mechanism that is neither dependent on hec function nor restricted to the prospective dorsal axis.
Results
Maternal-effect mutations in hecate affect dorsoanterior development
Embryos from mothers homozygous for the mutant hec allele, for simplicity referred to here as hec mutant embryos, display a range of ventralized phenotypes [17], [20], [23] (Figure 1A–E; Table 1). In the most severe cases, 24-hour mutant embryos exhibit a severe radial ventralization and lack all dorsoanterior-derived structures (V4 class, according to criteria in [24]; Figure 1A). More moderate phenotypes can also be observed, such as embryos that lack all anterior structures as well as the notochord (a dorsally-derived structure), and display an expansion of posterior somites (V3 class; Figure 1B). More weakly ventralized embryos are also observed that lack the anterior-most head structures and the notochord and exhibit expanded posterior somites (V2 class; Figure 1C), and embryos with reduced eyes and some notochord defects (class V1; Figure 1D). In many clutches, a fraction of embryos from mutant mothers are indistinguishable from wild-type embryos (Figure 1E). Such variability in phenotypes can be observed in maternal-effect mutants (some examples can be found in [17], [25], [26]), in particular those exhibiting axis induction defects [5], [15], [27], possibly by inherent variation in the maternal composition of individual eggs coupled to gene or pathway redundancy (see also Discussion). In mutant clutches with a weak expression of the phenotype, a small and variable fraction of embryos exhibits axis duplication phenotypes (Figure 1F) instead of axis formation defects (see Discussion).
Fig. 1. Axis induction defects in embryos from mothers homozygous for three different hecate mutant alleles. 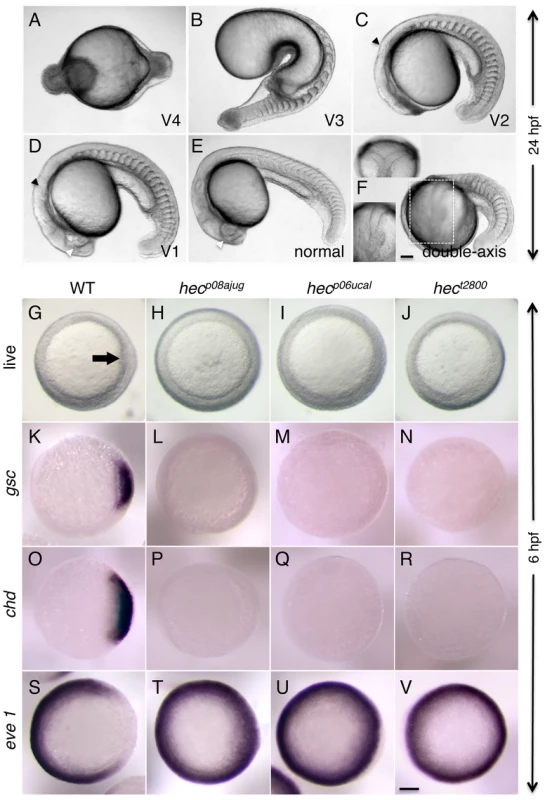
A–F) Side views (except as indicated in (F)) of live embryos at 24 hpf showing a range of phenotypes from most severe (A) to normal (E) and double-axis embryos (F). A) Class V4 embryo exhibiting complete radial symmetry and lacking all anterior (e.g. head structures) or dorsalmost (e.g. notochord) structures. B) Class V3 embryo exhibiting a rudimentary axis but lacking all anterior and dorsalmost structures. C) Class V2 embryo lacking anteriormost structures such as the eyes, but showing formation of more posterior head structures such as the otic vesicles (black arrowhead, also indicated in D). D) Class V1 embryo with a relatively complete axis but reduced anterior structures, such as the eyes (white arrowhead, also labeled in E), and lacking a properly formed notochord. E) Normal embryo derived from hec mutant females indistinguishable from wild-type. Note somites in (E) are chevron-shaped, while they are blocky in (B–D) indicative of defects in notochord formation, or encircling the entire embryo in (A). F) A double-axis embryo found in hec mutant clutches exhibiting weak expressivity. Insert in the lower left shows the left axis indicated by the dashed rectangle, out of focus in the main image. Insert in the upper left panel shows a dorsal view, showing the bifurcated axis. Note the lack of defined anterior structures in both axes, as well as the lack of a notochord along the trunk, also reflected by block-shaped somites in this region. Images in A–F are side views, except for upper left insert in (F). G–I) Animal views of live wild-type embryos (G) and embryos from females homozygous for each of the three hec mutant alleles. The dorsal thickening or shield (arrow) is absent in mutant embryos. K–V) In situ hybridization analysis to detect expression of dorsally-expressed genes (gsc, chd) and ventrally-expressed genes (eve 1). The expression domains of gsc and cho is reduced in hec mutant embryos, while the expression domain of eve is expanded in these embryos. (K–V) are animal view of embryos, dorsal to the right when identifiable, at the shield stage (6 hpf). Magnification bars in (F) and (V) correspond to 100 µm for panels sets (A–F) and (G–V), respectively. Dorsal view insert in panel (F, upper left) has been reduced to 75% size. Tab. 1. Phenotypic strength of hecate alleles. 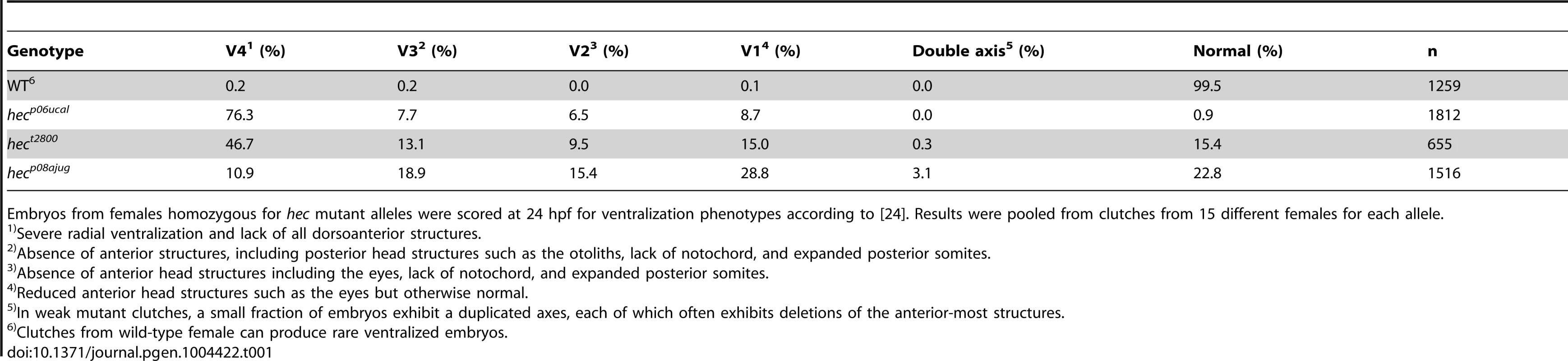
Embryos from females homozygous for hec mutant alleles were scored at 24 hpf for ventralization phenotypes according to [24]. Results were pooled from clutches from 15 different females for each allele. The hect2800 allele was originally isolated in an early-pressure-based screen for recessive maternal-effect mutations [14], [17] and its effects described previously [20], [23]. Two additional alleles, hecp08ajug and hecp06ucal were identified in another maternal-effect mutant screen, based on a four-generation scheme [16], [18]. Using DNA markers linked to the hect2800 allele [20], we determined that the hecp08ajug and hecp06ucal alleles were linked to the same SSLP markers, z59658 and z24511 on chromosome 8. In addition, we carried out pair-wise crosses between individuals carrying the three mutant alleles to test for non-complementation. All crosses resulted in females that exhibited the hec mutant phenotype in their offspring in the expected proportions, i.e. Mendelian for F1 females (approximately 50% in crosses between homozygous males and heterozygous females of all allelic combinations) and maternal-dependent (near 100%) for F2 embryos (Table S1), indicating that all three mutations are part of the same complementation group.
A comparison of the phenotypes for the three hec alleles suggests that they fall within an allelic series. Embryos from mutant females carrying each of the three alleles were classified and scored at 24 hours post-fertilization (hpf; Table 1). The hecp06ucal allele shows the strongest average phenotype among the three alleles, where most embryos (76.3% from 3-month females) exhibit the strongest (V4) phenotype, while hect2800 mutants exhibit intermediate phenotype (46.7% V4 class) and hecp08ajug mutants show the weakest phenotype (10.9% V4 class). Double-axis embryos are observed only in offspring derived from females mutant for the two weaker alleles hecp08ajug and hect2800, and only in clutches with weak penetrance and expressivity (Table 1 and data not shown). In the case of ichabod and tokkaebi mutations [5], [15] axis induction defects have been reported to vary with maternal age, with younger females exhibiting stronger phenotypes. We tested embryos from 3 month old and 12 month old mutant females and find a similar trend for the effects of the three hec alleles on axis induction (Figure S1).
Previous studies have shown that the ventralized phenotype of hec mutant embryos is associated with a reduction in the embryonic shield and changes in patterns of gene expression in dorsal - and ventral-specific genes in the late blastula embryo. Expression of gene markers of the various germ layers, such as bmp2 and gata2 (ectoderm), no tail (mesendodermal precursors), and foxa2 (endoderm) was unaffected, other than regional differences due to predicted changes in dorsoventral specification [20]. As expected, embryos from females mutant for the newly isolated hec alleles, hecp06ucal and hecp08ajug, similar to the hect2800 allele [20], exhibit a reduction in the shield region corresponding to the dorsal organizer at the incipient dorsal region (Figure 1G–J), as well as a reduction in dorsal-specific gene expression (goosecoid, Figure 1K–N; chordin, Figure 1O–R) and a concomitant expansion of a ventrally-expressed gene (eve 1) (Figure 1S–V).
Together, phenotypic, linkage and complementation analysis indicate that these three mutations are alleles of the same gene.
hecate encodes the zebrafish Glutamate receptor interacting protein 2a (Grip2a)
To better understand the function of the hec gene, we determined its molecular identity using a positional cloning approach. We initially identified linkage of the hec locus between SSLP markers z59658 and z24511 on chromosome 8 through mapping of the hect2800 allele [20]. Homozygous mutant males were crossed to heterozygous females to generate large numbers of fish for fine mapping. Fine mapping analysis of 1762 meioses with newly identified RFLP markers further narrowed the critical region containing hec to a genomic region between gpd1a-1 and the zC150E8y RFLP in the Ensembl database, corresponding to an interval of 383 Kb (Figure 2A). Five overlapping BAC clones were identified and aligned as a contig covering the whole critical region (Figure 2B). Within this critical region, there are 11 predicted genes according to the Ensembl database of the zebrafish genome and the GENSCAN program. Sequencing of cDNA products from wild-type and mutant alleles revealed the presence of mutations in all three hec alleles in the gene glutamate receptor interacting protein 2a (grip2a, NP_001116760.1), one of two grip2 genes in the zebrafish genome [28].
Fig. 2. Molecular identification of the hecate locus. 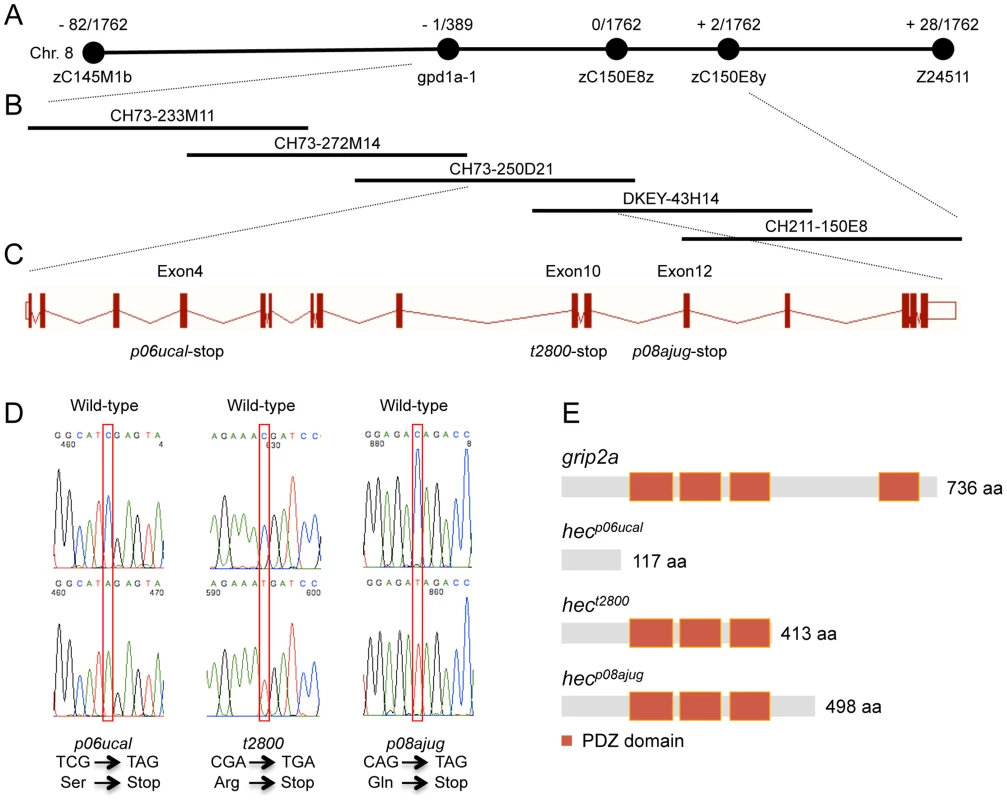
A) Linkage map of the hec locus. The number of recombinants over the total number of analyzed meiosis is indicated. hec linkage was initially identified between SSLP markers z59658 and z24511 on chromosome 8. Fine mapping analysis with newly identified RFLP markers further narrowed the region between the gene gpd1a-1 and the RFLP zC150E8y. B) Contig of five BAC clones covering the hec critical region. CH73-233M11, CH73-272M14, CH73-250D21, DKEY-43H14 and CH211-150E8 are five sequenced and overlapping BAC clones in this interval. C) Exon-intron structure of the hec/grip2a gene, which contains 16 exons. The hecp06ucal, hect2800 and hecp08ajug alleles each cause a premature stop-codon in exon 4, exon 10 and exon 12, respectively. D) Sequence traces of the cDNA products from wild-type and the three mutant hec alleles. Nucleotide substitutions are indicated by the red box. Mutant cDNAs show a C-A transversion in codon 118 (hecp06ucal), a C-T transversion in codon 414 (hect2800), or a C-T transversion in codon 499 (hecp08ajug), all creating premature STOP codons. E) Schematic diagram showing the protein domain structures of Grip2a in the wild-type and mutant alleles. Red boxes represent conserved PDZ domains. The zebrafish grip2a gene has 16 exons, which produces a 3,124 bp transcript and a 736 amino acid protein (Figure 2C). The three mutant alleles result in truncated forms of the Grip2a protein: hecp06ucal allele has a C-A transversion in codon 118 in exon 4, hect2800 has a C-T transversion in codon 414 in exon 10, and hecp08ajug has a C-T transversion in codon 499 in exon 12, and these three mutations generate premature nonsense (stop) codons (Figure 2D). A search in the Conserved Domain Database (CDD) in NCBI [29] indicates that Grip2a protein has four PDZ domains. The premature stop-codons for these different alleles delete the most C-terminal PDZ domain in the hect2800 and hecp08ajug alleles, and all four PDZ domains in the hecp06ucal allele (Figure 2E). The retention of PDZ domains and size of the predicted truncated proteins roughly correlates with the observed phenotypic strength in the various mutant allele backgrounds (Table 1, Figure S1), although we have not determined expression levels for the mutant proteins to confirm their relative activities. The identification of mutations in these three independently isolated alleles indicates that hec encodes Grip2a. This is further substantiated by the localization of grip2a mRNA in the region of the embryo affected by the hec mutation (see below).
Using BLAST searches on Ensembl and NCBI genome databases, homologous grip1 and grip2 genes were found for all vertebrate species, such as fish, amphibians, birds, and mammals. In invertebrate lineages, a distantly related Grip gene was identified only in Drosophila. Grip1 and Grip2 protein sequences among eight representative species were used to construct a phylogenetic tree using ClustalW (Figure S2). grip2 occurs as a single copy in amphibians, birds and mammals but is duplicated in the zebrafish and other fish species such as fugu and medaka, likely a consequence of an extra round of whole genome duplication in the ray-finned fish lineage [30], [31]. Drosophila and all vertebrate Grip1 and Grip2 proteins contain PDZ domains but zebrafish Hec/Grip2a and Fugu Grip2b contain 4 predicted PDZ domains instead of the 7 PDZ domains predicted in other members of this family (Figure S2 and not shown).
grip2a mRNA is localized to the vegetal region of the oocyte, developing an early asymmetry upon egg activation
Quantitative RT-PCR analysis of mRNA from wild-type embryos spanning early development indicates highest levels of grip2a mRNA in the 1-cell stage embryo, gradually declining to negligible levels at 50% epiboly (5.25 hpf) and thereafter (Figure S3). In adults, expression can be detected in wild-type females and isolated ovaries, but not in males or female carcasses where the ovaries have been removed (Figure S3). Thus, at our level of analysis, hec/grip2a is specifically expressed in ovaries as a maternal-specific transcript, which is consistent with the strict maternal effect observed in females homozygous for the three hec mutant alleles.
We examined the spatial expression pattern of hec/grip2a at various developmental stages during embryogenesis using whole mount in situ hybridization (Figure 3). grip2a mRNA is detected in the vegetal pole region of the yolk in early zygotes and cleavage-stage embryos (Figure 3A–E). Similar to the case of wnt8a mRNA [7] and Sybu protein [6], the grip2a mRNA localization domain is not precisely aligned with the vegetal pole in activated eggs or early embryos. Instead, grip2a mRNA is consistently located slightly off-center in the post-activation stages examined, from the early 1-cell stage embryo 10 minutes post-fertilization (mpf) until late cleavage stages (Figure 3A–D). This off-center shift is not observed in manually extruded mature, inactive eggs, where the grip2a mRNA localization domain is instead located at the vegetal pole in a radially symmetric manner (data not shown). In early embryos the extent of asymmetry of the grip2a mRNA localization domain, appears similar throughout the cleavage and blastula stages until mRNA levels become markedly reduced in the late blastula embryo (sphere stage; 4 hpf; Figure 3E). Localized grip2a mRNA can no longer be detected starting at the onset of epiboly (30% epiboly; 4.66 hpf; not shown). In contrast to its Xenopus homologue [32], zebrafish grip2a mRNA does not localize to the zebrafish germ plasm (Figure 3C and data not shown), present at the furrows corresponding to the first and second blastomeric divisions [33]–[37], nor does it become incorporated into the primordial germ cells (Figure 3D,E and data not shown), which form four cell clusters during the late cleavage stages ([33], [35], [38]; see below).
Fig. 3. Whole mount in situ hybridization analysis of the expression of zebrafish grip2a mRNA in early embryos. 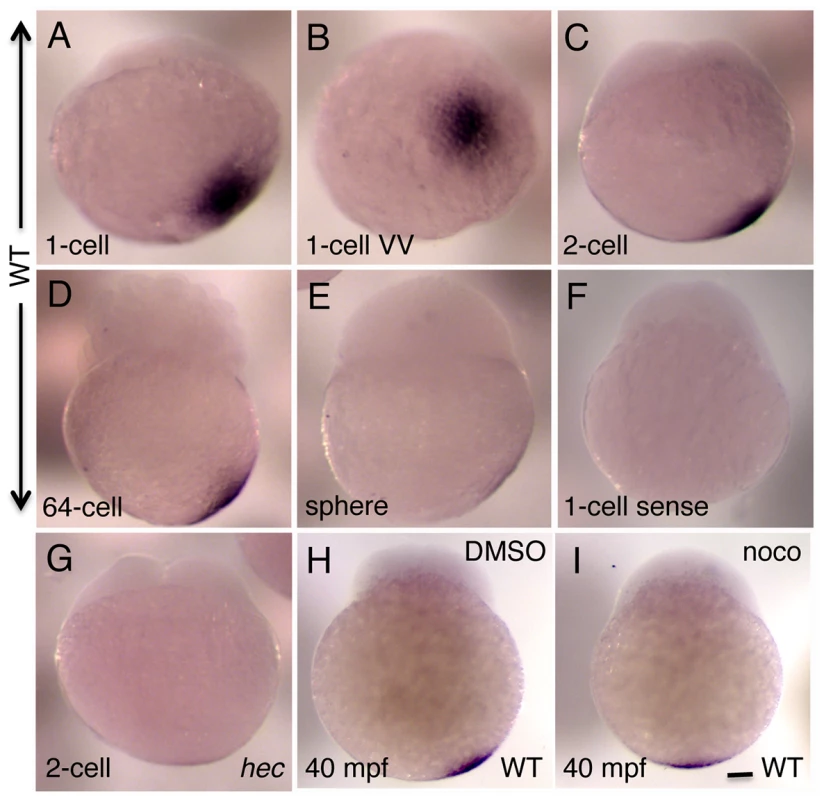
A–E) grip2a mRNA is localized in a slightly off-center position at the vegetal pole of early embryos, and remains in that position until the sphere stage when it becomes undetectable. F) Control sense probe. G) The domain of grip2a mRNA localization in hec mutants is reduced in intensity and appears to lack an off-center shift. H,I) Representative control (DMSO)- and nocodazole-treated embryos showing the lack of off-center shift in nocodazole-treated embryos (see Figure S4 for details). All panels are side views (except for (B), which is a vegetal view of the embryo in (A)) at the following stages: A,B: 1-cell (20 mpf), C,F,G: 2-cell (45 mpf), D: 64-cell (2 hpf), E: sphere (4 hpf). (H,I) are at 40 mpf, which approximately corresponds to the 2-cell stage (C) in untreated embryos. Magnification bar in (I) corresponds to 100 µm for all panels. To further confirm the slight, off-center shift in the grip2a mRNA localization domain, and to determine whether this shift, like that of Sybu protein and wnt8a mRNA, depends on an intact microtubule network, we tested the effect of early nocodazole treatment on the grip2a mRNA localization pattern in wild-type embryos. Embryos were treated at 5 mpf and fixed at 30 and 40 mpf for in situ hybridization. For both time points, control (solvent-treated) embryos show an off-center shift in the domain of grip2a mRNA localization so that it is located within an arc at 0–20° from the true vegetal pole of the embryo, while nocodazole-treated embryos do not exhibit a discernable shift in mRNA localization (Figure 3H, 3I; Figure S4). Thus, grip2a mRNA is located at the vegetal pole of the embryo in mature oocytes, but upon egg activation (typically coupled to fertilization) this mRNA exhibits a short-range, off-center translocation within the vegetal region of the embryo. Once in an off-center position grip2a mRNA remains static until its degradation at late cleavage stages.
Whole mount in situ hybridization of embryos from mutant female mothers homozygous for each of the three hec alleles show significantly reduced levels of localized grip2a mRNA during the early cleavage stages, ranging from reduced to nearly undetectable levels (Figure 3G and data not shown). In those embryos where grip2a mRNA localization can be discerned, the domain of localization appears centered at the vegetal pole, consistent with the absence of an off-center shift for vegetally localized products in hec mutants (see below). Quantitation of total grip2a mRNA levels in mutant embryos at the 2-cell stage indicates that, for all alleles, grip2a mRNA abundance is drastically reduced to approximately 15–25% that in wild-type embryos (Figure S3). It is possible that hec/grip2a function is required for the localization of its own mRNA, which when not localized is unstable. Alternatively, the reduction in apparent grip2a mRNA localization in hec mutant embryos might be a consequence of a decrease in grip2a mRNA abundance in these embryos, possibly by non-sense mediated mRNA decay as has been proposed for other maternal transcripts [39], [40].
Vegetal localization of grip2a mRNA is initiated during oogenesis and depends on oocyte polarity genes
To visualize how the pattern of grip2a mRNA localization is established during oogenesis, we carried out in situ hybridization analysis of wild-type oocytes at various stages using a grip2a antisense probe (Figure 4). In early stage I oocytes, grip2a transcripts appear sharply localized to a compact, spherical region at one region of the oocyte (Figure 4A, 4D). By late stage I, grip2a mRNA acquires a more spread, subcortical localization pattern still centered in one region of the oocyte (Figure 4A, 4E). This cortical localization is maintained until the end of oogenesis (Figure 4A, 4F and data not shown).
Fig. 4. Localization of grip2a mRNA in wild-type and mutant oocytes. 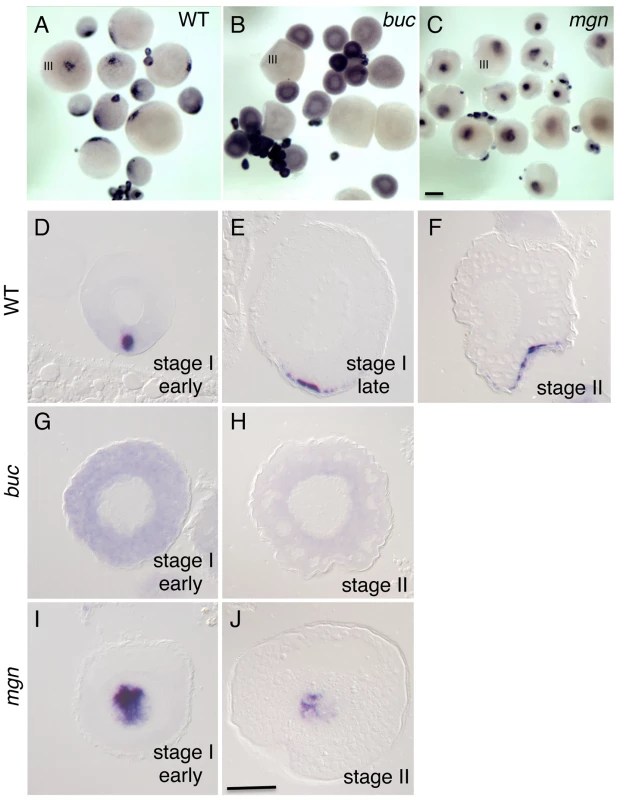
A–C) Whole mount in situ hybridization of dissected ovaries from wild-type (A), bucky ball (buc, B) and magellan (mgn, C) mutant females. In (A–C) oocytes at stage III of development are indicated. Smaller oocytes are at stages I and II, which are difficult to differentiate in whole mounts at this magnification. The grip2a mRNA localization domain is observed in an asymmetric cortical position in wild-type oocytes (A) but is unlocalized and diffuse in buc oocytes (B) and internally-located in mgn mutants (C). D–J) Sections of wild-type and mutant oocytes at the indicated stages after labeling to detect grip2a mRNA. Stages are as indicated in the panel and were determined by size and oocyte morphology according to [101]. D–F) Wild-type oocytes showing localization to the presumptive Balbiani Body (D) and subsequent localization to a cortical domain of the oocyte corresponding to the presumptive vegetal pole (E–F). G,H) buc mutant oocytes lack the Balbiani body [39], [43] and the grip2a mRNA subcellular localization domain in stage I and II oocytes. I) mgn mutant stage I oocytes exhibit an enlarged Balbiani body [40] and displayed an enlarged grip2a mRNA localization domain. J) Stage II mgn mutant oocytes fail to localize transcripts to the vegetal pole which instead persist in an internal domain [40], as observed also for grip2a mRNA. Number of oocytes examined were as follows: wild-type: early stage I: 13, stage II: 12; buc: early stage I: 35, stage II: 26; mgn: early stage I: 23, stage II: 18. Magnification bar in (C) corresponds to 250 µm for panels (A–C), and in (J) to 50 µm for panels (D–J). The localization pattern of grip2a mRNA in stage I oocytes is reminiscent of the zebrafish Balbiani body, a conserved aggregate of organelles present in animal oocytes shown to anchor subcellularly localized oocyte mRNAs [41]–[43]. We therefore tested whether grip2a mRNA localization is dependent on Balbiani body formation, using zebrafish mutants that affect this structure. Oocytes mutant for the gene bucky ball lack the Balbiani body [39], [43], and we found that bucky ball mutant oocytes lack grip2a mRNA subcellular localization during oogenesis (Figure 4B, 4G, 4H). Moreover, oocytes mutant for the cytoskeletal linker protein magellan (macf1), which exhibit an enlarged Balbiani body with an abnormal location [40], exhibit grip2a mRNA mislocalization (Figure 4C, 4I, 4J) similar to other Balbiani-localized transcripts. These results indicate that grip2a mRNA becomes localized to the vegetal cortex during oogenesis by a Balbiani body-dependent mechanism.
hecate/grip2a is required for microtubule rearrangements at the vegetal pole
The rescue of hec mutant embryos by overexpression of Wnt pathway components has suggested that hec likely activates signaling at an upstream step of the pathway [20]. Given that an early event in the pathway leading to Wnt signaling activation is the reorganization of microtubules at the vegetal pole required for the transport of local determinants, we tested whether this reorganization is affected in hec mutant embryos (Fig. 5; Figure S5). Consistent with previous studies [2], [8], [27], we find that microtubules at the vegetal cortex in wild-type appear as parallel tracks of bundled microtubules at 20 mpf (Figure 5A). In hec mutant embryos, such parallel tracks of bundled microtubules are not observed (Figure 5B). In these mutants, unbundled microtubules typically appear to radially emanate from one or more aster-like structures at the vegetal pole region (Figure 5C–F). Exposure to the microtubule-stabilizing drug taxol [44] during the first cell cycle (5 to 35 mpf) does not influence the degree of residual axis induction in hec mutants (Figure S6), suggesting that the observed defects may not be simple consequence of altered microtubule dynamics. Labeling of the F-actin cortical network in the vegetal cortex region shows a similar appearance in wild-type and mutant embryos (Figure S7), including the presence of F-actin rich protrusions as previously described [45].
Fig. 5. Microtubule reorganization at the vegetal cortex is affected in hecate mutants. 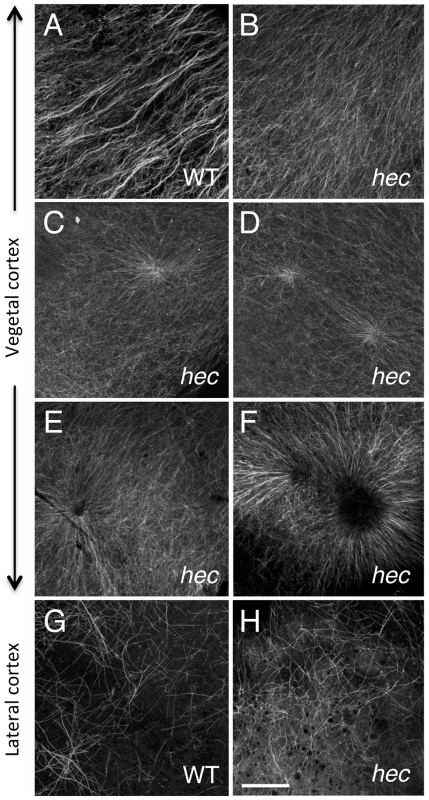
A–F) Cortical microtubule network at the vegetal pole in wild-type (A) and hec mutant (B–F) embryos at 20 mpf. Microtubules appear oriented in the same direction and bundled in wild-type embryos (A). The extent of co-orientation and bundling is greatly reduced in hec mutant embryos (B), where microtubules form multiple aster-like structures which can have a well focused-center (C,D) or can exhibit a central microtubule-free zone (E,F) and often overlap (F) or interdigitate (D). The relatively unbundled microtubule arrangement shown in (B) also corresponds to a sector of a large aster-like structure emanating from a not shown central core. Up to 6 aster-like structures were observed in the vegetal cortex of a single embryo. G,H) Cortex in mediolateral regions shows a loose and apparently random network of microtubules which appears similar in both wild-type (G) and hec mutant (H) embryos (n = 8 for wild-type and mutants). All images are z-axis projections of confocal image stages. The phenotype was fully (100%) penetrant according to the two main categories (wild-type, aligned and bundled microtubules; mutant, radialy oriented and unbundled microtubules, with 10 wt and 25 hec mutant embryos imaged. Magnification bar in (H) corresponds to 40 µm for all panels. Short-range symmetry breaking and anchoring of vegetally localized factors are affected in hec mutant embryos
Since vegetal cortical microtubules have been proposed to mediate the off-center shift of factors involved in axis induction that are initially localized to the vegetal pole, such as grip2a mRNA (this report), wnt8a mRNA ([7]; Figure 6A, 6C) and Sybu protein ([6]; Figure 6E, 6G), we tested whether these shifts were affected in hec mutant embryos. As noted above the grip2a mRNA localization domain in hec mutants, when still detectable, fails to undergo an off-center shift (Figure 3G). In situ hybridization analysis to detect wnt8a mRNA shows this mRNA also fails to undergo a noticeable off-center shift in one-cell (30 mpf) hec mutant embryos (Figure 6B). At the 4-cell stage (60 mpf), wnt8a mRNA localization at the vegetal pole is significantly reduced or undetectable (Figure 6D), although this defect is associated with an overall decrease in the relative expression of wnt8a mRNA (Figure S8). In the case of Sybu protein, wild-type embryos show localization centered at the vegetal pole until 20 mpf (Figure 6E) and an off-center shift of protein localization by 30 mpf (Figure 6G), as previously reported [6]. In hec mutants, the Sybu protein localization domain can be initially detected centered at the vegetal pole of hec mutants (Figure 6F). However, Sybu protein is no longer detectable by 30 mpf (Figure 6H), precluding testing an effect on Sybu protein off-center movement. These data indicate that hec/grip2a is essential for the short-range, symmetry-breaking transport of vegetally-localized factors, and are consistent with hec function being essential for microtubule reorganization in this region. The reduction in vegetally localized wnt8a mRNA and Sybu protein in hec mutants contrasts with the perduring vegetal localization of these factors in embryos with a perturbed microtubule network ([6], [7]; Figure 3I, 6I).
Fig. 6. Defects in the vegetal localization of wnt8a mRNA and Sybu protein. 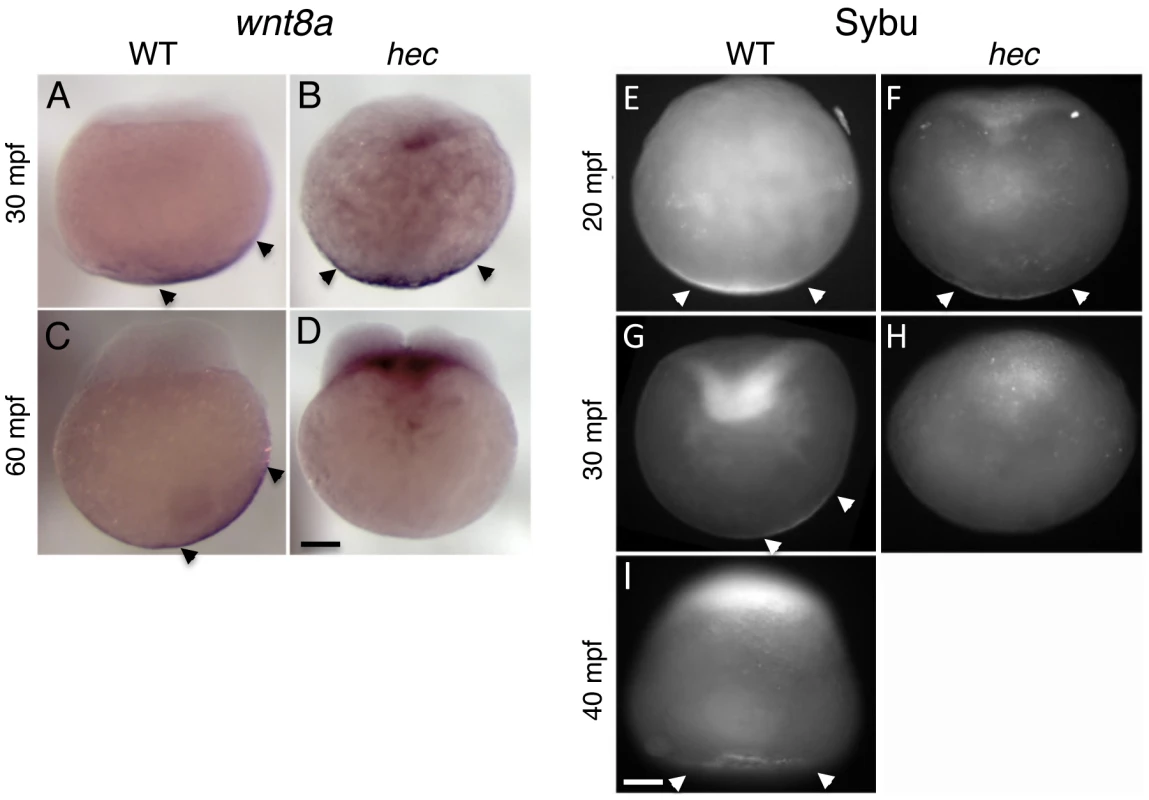
A–D) Off-center shift of wnt8a mRNA is affected in hec mutants. Whole mount in situ hybridization of wild-type embryos (A,C) and hec mutant embryos (B,D) at the 1- (A,B, 30 mpf) and 4- (C,D, 60 mpf) cell stages. Images show representative embryos. A majority of wild-type embryos showed a clear off-center shift (85%, n = 27 at 30 mpf and 74%, n = 47 at 60 mpf). A majority of hec mutant embryos showed vegetal localization without a shift at 30 mpf (79%, n = 33, remaining embryos show no localization) and absence of localization at 60 mpf (89%, n = 38, remaining embryos show reduced vegetal localization without a shift). The apparent label at the base of the blastodisc is observed in a majority of mutant embryos (71%, n = 38) but not in wild-type (C) or control embryos labeled with other probes (not shown) and may reflect remaining wnt8a mRNA that has lost anchoring at the vegetal pole and has moved animally through the action of axial streamers [83]. E–I) Localization of Sybu protein is affected in hec mutants. Whole mount immunofluorescence to detect Sybu protein of untreated wild-type (E,G) and hec mutant (F,H,) embryos and nocodazole-treated wild-type embryos (I) at the indicated stages. In wild-type embryos, an off-center shift in Sybu protein can be observed starting at 30 mpf (G). In hec mutants, Sybu protein becomes undetectable levels by this same time point (H). Patterns of localization of Sybu protein at 10 mpf and 20 mpf time points (combined n: 32 WT, 19 mutant for 10–20 mpf), and 30 mpf and 40 mpf time points were similar and have been combined. 59% (n = 32) of wild-type and 63% (n = 19) of hec mutant embryos showed centered vegetal localization during 10–20 mpf. At 30–40 mpf, the percent of embryos that showed vegetal localization, now with an off-center shift, was reduced to 25% (n = 28) in wild-type, and 0% (n = 25) of hec mutants showed any localization at these time points. Treatment of wild-type embryos with nocodazole inhibits the shift but does not result in delocalization from the vegetal cortex (I, embryo at 40 mpf), as previously shown [6]. Magnification bars in (D) and (I) correspond to 100 µm for panels sets (A–D) and (E–I), respectively. Long-range animally-directed transport is independent of hecate/grip2a function and not restricted to the prospective dorsal region
The short-range off-center shift observed in the case of Sybu protein and grip2a mRNA, which occurs within the confines of the vegetal region, contrasts with the long-range transport thought to be involved in transporting a putative dorsal signal to blastomeres at the animal region [2]. wnt8a mRNA has been observed to reach the base of the blastomeres by the 16-cell stage (1.5 hpf; [7]), although in our experiments this RNA exhibits a relatively static off-center shift throughout the first 60 mpf (Figure 6A, 6C), similar to the short-range movement of Sybu protein and grip2a mRNA. The animally-directed translocation of a putative dorsal signal is thought to be reflected in the microtubule-dependent, animally-oriented movement of small (0.2 µm) polystyrene fluorescent beads during the first several cell cycles. When injected into the vegetal region, these beads reach the base of the blastomeres at the animal region by traveling through cortical paths [2].
Using the transport of microinjected fluorescent beads as an assay for this long-range transport mechanism, we tested whether long-range vegetal-to-animal movement along the cortex might be affected in hec mutant embryos. As previously reported [2], in wild-type embryos bead movement from the vegetal region is observed along a meridional arc along the cortex reaching the base of the blastomeres at the animal pole (41% (n = 87); Figure 7A,A′). In hec mutant embryos, beads appear to be transported to a similar extent as in wild-type, also reaching the base of the blastodisc (Figure 7B,B′) and at a similar observed frequency (39% (n = 51)). Thus, hec function does not appear to be required for long-range animally-directed transport along the lateral cortex. The apparently normal movement of animally-directed beads in hec mutants appears to conflict with the role of this gene in early microtubule reorganization but is consistent with the observed presence of multiple microtubule populations [2], [8]: aligned bundles of short microtubules at the vegetal region, which we find to be dependent on hec function, and a more dispersed and randomly oriented network in more medial regions, which appears unaffected in hec mutants (Figure 5G, 5H and data not shown).
Fig. 7. Long-range animally-directed transport is not affected in hecate mutants. 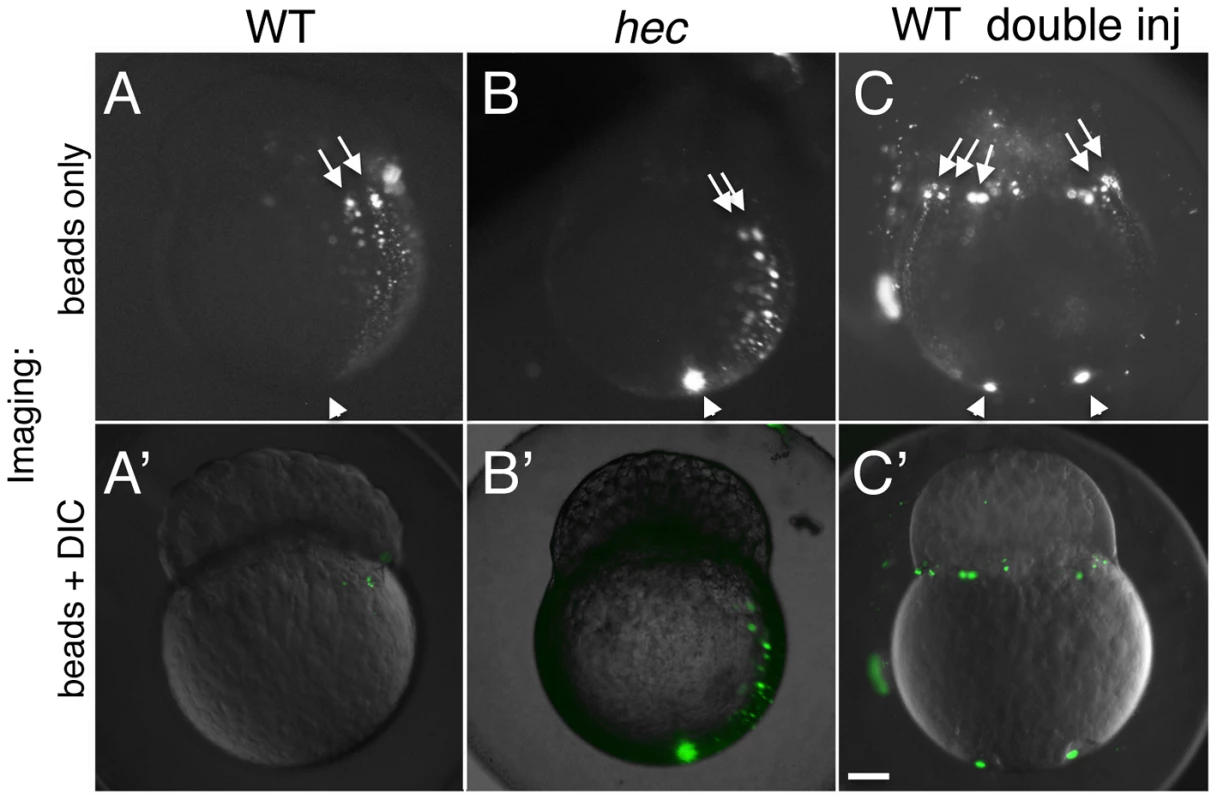
A–C) Paths of injected 0.2 um fluorescent beads after injection into the vegetal pole with a single (A,B) or double (C) injection in wild-type (A,C) or hec mutant (B) embryos. (A′–C′) show merged imaged including the fluorescent channel (shown in A–C) and corresponding DIC optics at low intensity. The extent and frequency of bead transport appeared similar in wild-type and mutant embryos (A,B, see text). Injections into two opposite sides of the vegetal pole results in multiple animally-directed paths, indicating that the entirety of the mediolateral cortex is competent for bead movement. Arrowheads and arrows in (A–C) indicate site of injection in the vegetal region and animally-directed paths along mediolateral regions, respectively. Magnification bar in (C′) corresponds to 100 µm for all panels. The direction of aligned short microtubule bundles at the vegetal pole has been shown to correlate with the dorsal axis but only occurs in a limited area within the vegetal half of the early embryo [8]. Given that directionality of a long-range movement might depend on an earlier oriented symmetry-breaking event, we wondered whether the long-range animally directed movement was specific to the putative dorsal region, or whether the entirety of the cortex was competent to support such transport. To test whether long-range animally-directed transport was specific to the prospective dorsal region of the embryo or was a general property of the lateral cortex, we carried out two slightly off-center fluorescent bead injections on opposite sides of wild-type embryos. Such doubly-injected embryos show animally-directed bead transport along meridional arches in opposite regions of the embryo (Figure 7C,C′), an observation inconsistent with only the prospective dorsal region mediating long range vegetal-to-animal cortical transport. Instead, our data suggest that the entirety of the cortex can mediate long-range animally-directed movement.
The apparently normal long-range transport of beads in hec mutant embryos, in the presumed absence of an early short-range symmetry-breaking process, may reflect asymmetries in the location of the beads during injection, followed by the action of this hec-independent long-range transport mechanism. Our data suggest that the transport of dorsal determinants to the prospective site of dorsal induction depends on two sequential processes: (i) an initial short-range transport dependent on hec-mediated formation of short aligned microtubule bundles that results in determinant asymmetry at the vegetal region of the embryo, and (ii) a subsequent long-range transport through lateral cortical regions, which is independent of hec function and not specific to the prospective dorsal region.
Divergence of grip function in zebrafish and Xenopus
In Xenopus, the homologous gene grip2 (previously referred to as grip2.1), like zebrafish hec/grip2a, is expressed maternally and its mRNA is localized to the vegetal pole of the egg and early embryo [32]. Following this vegetal localization, Xenopus grip2 mRNA becomes incorporated into primordial germ cells (PGCs) where it plays a role in their migration and survival [32]. In contrast, although localized to the vegetal pole, zebrafish grip2a mRNA does not localize to the zebrafish germ plasm or PGCs (Figure 3 and data not shown). We used whole mount in situ hybridization to test whether germ plasm localization or PGC development may be affected in hec mutant embryos (Figure 8). The localization patterns of dazl mRNA, a germ plasm component initially localized to the vegetal pole of the egg ([36], [46], [47]; Figure 8A), is not affected in these mutants (Figure 8D). During the first two cell cycles, dazl mRNA localizes normally to the furrows in hec mutants (Figure 8B, 8E), as does vasa mRNA (Figure 8C, 8F), an animal germ plasm component already present in the animal cortical region during egg activation [33], [36], [38], [48]. During embryonic development, although PGC migration is abnormal in strong hec mutants due to their radially symmetric, ventroposteriorized morphology, the average number of induced PGCs (as determined by cells expressing vasa in the 10.5 hpf embryo [33], [38], [48]) is similar to that in wild-type embryos (Figure 8G, 8H; Figure S9). Thus, as opposed to the case of Xenopus grip2, our observations do not support a role for zebrafish hec/grip2a in PGC development.
Fig. 8. Germ plasm recruitment and PCG determination appears unaffected in hecate mutants. 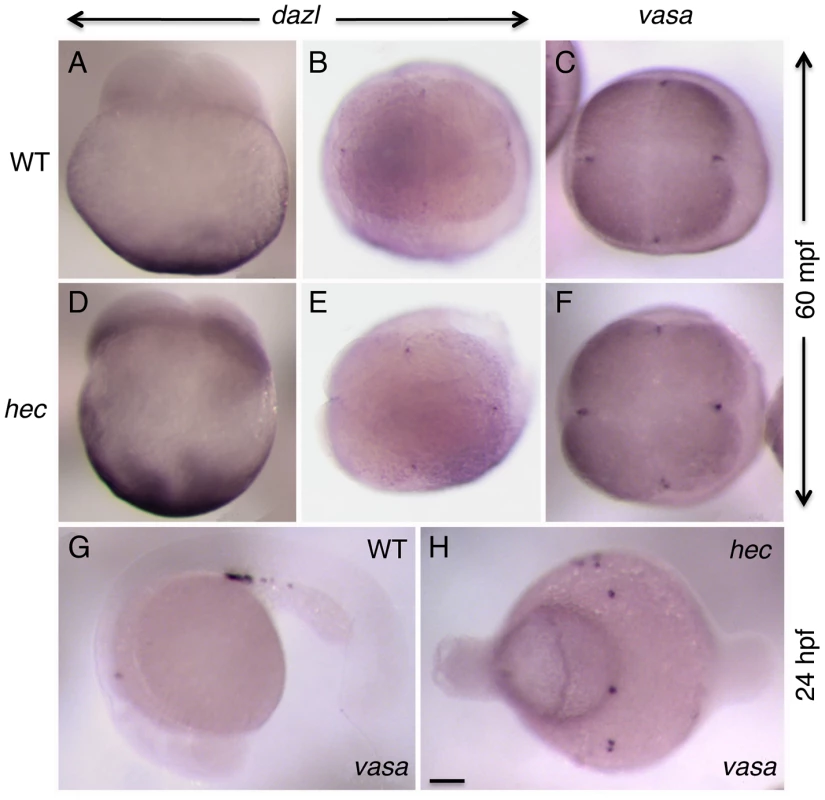
A–F) Whole mount in situ hybridization of 4-cell stage (60 mpf) embryos to label the germ line-specific genes dazl1 (A,B,D,E), and vasa (C,F) in wild-type (A–C) and hec mutant (D–F) embryos. Early localization of dazl mRNA to the vegetal pole (A,D, side views), and dazl mRNA and vasa mRNA to the germ plasm as it becomes recruited to the furrows of the first and second cleavage cycles (B,C,E,F, animal views). Localization of dazl domains of recruitment at the furrow is not detected in the side views as the levels of mRNA in these domains are relatively low and the focal plane is not optimal for their visualization. G,H) PGC determination, as determined by vasa expression in 24-hour embryos (G), appears unaffected in hec mutants (H), although the PGCs in mutant embryos do not become clustered as in wild-type due to the aberrant cell specification in these mutants. Quantification of the number of vasa-expressing cells is presented in Figure S9. Magnification bar in (H) corresponds to 100 µm for all panels. Two other zebrafish grip-related genes, grip1 and grip2b, exhibit maternal expression as assayed by RT-PCR of RNA from 1–4 cell embryos but do not show localization to the vegetal pole or germ plasm at these early stages, or in PGCs in the 24-hour embryo ([28]; our own data). Together, these data indicate that, in spite of similar mRNA localization patterns at the vegetal pole of the egg, grip homologues in Xenopus and zebrafish have divergent roles in PGC development and axis induction, respectively.
Discussion
A gynogenesis-based forward genetic screen in the zebrafish led to the isolation of a mutation in hec, which results in axis induction defects [17], [20], [23]. Here, we report the isolation of two additional alleles of hec, identified through an F3 inbreeding screen, and the molecular identification of this gene as encoding Glutamate receptor interacting protein 2a. This molecular assignment, as well as the localization pattern of its products, suggests that vegetally localized grip2a mRNA in zebrafish acts at the vegetal pole in the early events of dorsal axis induction. Accordingly, we find that hec functions to reorganize and align microtubule bundles that are involved in the symmetry-breaking transport of factors localized to the vegetal cortex, which is essential for axis induction.
Role of hecate/grip2a in cortical microtubule rearrangements and axis induction
In both teleosts and amphibians, dorsal determinants are initially localized at the vegetal pole and then translocate to the prospective dorsal side in a microtubule-dependent manner to initiate the dorsal cell fate program (reviewed in [1], [49], [50]). Genetic and molecular searches have identified factors localized to the vegetal cortex with a role in axis induction in the zebrafish, such as the kinesin-1 linker Syntabulin [5], [6] and the wnt8a mRNA [7]. We show that the mRNA product for zebrafish hec/grip2a is another zebrafish maternal factor involved in axis induction, whose product is also localized to the vegetal pole of the oocyte and early embryo.
Zebrafish hec/grip2a is expressed solely during oogenesis as a maternal transcript, which is consistent with the identification of multiple maternal-effect mutant alleles of this gene, all of which lack associated zygotic defects. In particular, fish homozygous for the hecp06ucal allele, predicted to lack all four PDZ domains present in the wild-type protein and therefore likely a null, exhibit a highly penetrant maternal-effect phenotype yet are themselves viable. These observations indicate that hec/grip2a has a dedicated function in early axis determination. While we cannot rule out that hec/grip2a is expressed in older embryos or adults at levels below the sensitivity of our assays, other related genes such as grip1 or grip2b are expressed at later stages of development ([28]; our own data) when they may provide essential zygotic functions.
Mechanisms inducing microtubule bundling and alignment, essential for the establishment of the primary body axis, remain incompletely understood. In Xenopus, cortical rotation and microtubule reorganization are dependent on both kinesin and dynein motor activity [51], [52] and other factors such as Trim36, a ubiquitin ligase whose mRNA is localized to the vegetal egg cortex [53], Dead end, an RNA binding factor needed for trim36 mRNA vegetal cortex localization [54] and the lipid droplet component Perilipin 2, whose mRNA is also localized to the Xenopus vegetal pole [55], [56]. We find that zebrafish hec/grip2a function is required for the reorganization of vegetal cortex microtubules into bundles normally directed towards the prospective dorsal axis [2], [8]. The lack of microtubule network alignment in hec mutants and associated defects in the transport of putative dorsal determinants such as wnt8a mRNA likely result in the axis induction defects observed in these mutants.
Grip was originally identified as a factor interacting with AMPA-type glutamate receptors [57] and its multiple PDZ domains are thought to facilitate protein-protein interactions within large macromolecular complexes, including the surface presentation and trafficking of transmembrane proteins [58]–[60]. grip genes have been implicated in epithelial development in both mouse and zebrafish embryos ([28], [61], reviewed in [62]). Other studies have implicated Drosophila Grip as a mediator of Wnt ligand activity in the postsynaptic terminal of the neuromuscular junction [21], [22].
Our findings suggest parallels between subcellular transport at the vegetal pole of the zebrafish zygote and transport of neurotransmitter receptors in neurons. In the zebrafish zygote, transport of wnt8a mRNA depends on microtubules and occurs concomitantly with the movement of the kinesin adaptor Syntabulin [6], [7]. Similarly, in dendrites Glutamate receptors associated with mammalian GRIP1 are driven by kinesin along microtubules [63], and Syntabulin has been shown to be required for axonal transport [64]–[66]. In neurons, glutamate receptors and GRIP associate with membrane vesicles [67], [68]. Although membrane vesicles have not been reported to be associated with dorsal determinants in zebrafish, studies in Xenopus implicate membrane vesicles in the transport of dorsal determinants [69]–[72]. Further studies will be required to determine mechanisms driving the reorganization of vegetal cortex microtubules, the precise role of Grip2a in this process and whether Grip factors have an analogous cytoskeletal restructuring function in other systems.
Is Grip2a required for downstream events in Wnt signaling involved in axis induction?
Our previous studies have shown that manipulations to activate Wnt signaling, including the overexpression of wnt8 mRNA, can rescue the hec mutant phenotype, which suggests that hec acts in an upstream event required for Wnt signaling activation during axis induction [20]. Our identification of a role for Grip2a on cytoskeletal events needed for the relocation of dorsal determinants is consistent with such an upstream role. However, our studies do not rule out a more direct role for Grip2a as a regulator of Wnt pathway components. We note that the off-center shift of vegetally localized grip2a mRNA upon egg activation could provide an asymmetric source of Grip2a protein to influence Wnt signaling activity at the prospective dorsal region. Drosophila Grip is known to interact with the Wnt receptor Frizzled-2, promoting the trafficking of a Frizzled-2 C-terminal fragment to the nucleus to activate target genes [21], [22], [73], [74], and it is possible that some of these interactions are conserved in the zebrafish embryo. It is also possible that Grip2a regulates non-canonical Wnt signaling, such as Wnt/calcium signaling, which in turn influences axis induction. hec mutants exhibit an increased frequency of intracellular calcium transients in blastula stage embryos (2.00–3.33 hpf; [20]), and the resulting intracellular calcium increase has been proposed to attenuate Wnt/β-catenin signaling pathway activity [75]–[79]. Further studies will be necessary to determine whether hec/grip2a, in addition to functioning in cytoskeletal organization in the early zebrafish embryo, has a direct role in the regulation of Wnt/β-catenin signaling and axis induction.
A multiple-step mechanism for the transport of dorsal determinants in zebrafish
Multiple studies in Xenopus have indicated the formation of long tracks of cortical microtubules associated with the cortical rotation [80]–[82]. While in this organism the cortical rotation involves the concerted movement of the cortex along a distance corresponding to a 30° arc, microtubule tracks and so-called fast transport of subcellular components, such as membrane organelles and specific factors, encompass a longer distance corresponding to a 60–90° arc (reviewed in [1]). Thus, in Xenopus both the cortical rotation and fast transport may participate in the relocation of dorsal determinants. In zebrafish embryos, a cortical rotation-like process results in the displacement of granules along a 20° arc from the vegetal pole of the embryo [8], with mediolateral regions of the cortex exhibiting a loose meshwork of microtubules independent of dorsoventral position [2], [8]. The restriction of microtubule bundling and alignment to the vegetal region of the zebrafish embryo raises the question of how long-range transport of dorsal determinants to the animal pole is achieved.
We found that injected beads reach the base of blastomeres in hec mutant embryos, which lack a cortical rotation-like movement, with a frequency similar to that observed in wild-type embryos. This suggests that transport of beads along the mediolateral cortex is independent of hec/grip2a function and aligned vegetal microtubules. Our finding that beads are able to move animally along opposite sides in multiply injected embryos, further suggests that the entirety of the mediolateral cortex, not just the prospective dorsal region, is competent for long-range vegetal-to-animal transport. Previous studies have identified animally-directed transport movement of cytoplasmic particles along cortical “meridional” streamers, hypothesized to mediate transport of vegetally-injected fluorescent beads [83]. This meridional transport along the mediolateral cortex may depend on various possible structures or processes, such as perpendicular bundles aligned along the animal-vegetal axis in deep regions of the cortex [8], incipient yolk cytoplasmic layer (YCL) microtubules emanating from marginal blastomeres into the yolk [84], an emerging property of the loose network of cortical microtubules found in this region of the embryo [2], [8], or other insofar unidentified cytoskeletal networks. Further analysis of the dynamic aspects of cytoskeletal networks in this region will be required to understand the mechanistic basis of this meridional transport system and its role in axis induction. In addition, our results do not exclude the possibility that diffusion of a translated protein such as Wnt8a may also contribute to long-range transport, as previously suggested [8].
Together, these data indicate that the transport of the putative dorsal determinant in the early zebrafish embryo involves at least two separate mechanisms: a short-range transport dependent on hec/grip2a function and aligned microtubule bundles, and a subsequent long range transport relying on the mediolateral cytoskeletal network (Figure 9A). We hypothesize that the former generates an off-center, symmetry-breaking shift in initially symmetrically localized putative dorsal determinants, while the latter acts as a more general conduit that amplifies the early asymmetry. In hec mutants, the initial symmetry-breaking event is affected, so that even with a functional long-range animal transport mechanism, in most embryos an insufficient amount of dorsal determinants reaches the blastomeres at the animal pole (Figure 9B). A dual mechanism of dorsal determinant transport may also explain how a fraction of embryos from females homozygous for the presumptive null allele, hecp06ucal, which lacks all 4 conserved PDZ domains, can develop a normal dorsal axis. In such embryos small fluctuations may occur in the position of the vegetally localized dorsal determinant, which could be amplified by the mediolateral transport system that is unaffected in these mutants. The presence of an embryo-wide pathway directing long-range transport towards the animal pole may also explain the appearance of double-axis embryos observed only in the weakest hec mutant clutches. In these cases, an aberrantly organized vegetal microtubule network may result in the off-center vegetal shift of dorsal determinants in more than one direction, leading to their animally-directed transport along multiple paths and resulting in supernumerary or expanded regions of axis induction. A wider distribution of dorsal determinants in weak hec mutants would result in their reduced concentration in animal regions and reduced Wnt pathway activation, consistent with the observed lack of anterior-most structures in the resulting double-axis embryos. This dual transport model suggests mechanisms by which small, directed changes, like the specific early short range symmetry-breaking event, can be amplified during early development by an embryo-wide mechanism to result in large differences in cell fate specification.
Fig. 9. Amplification of hecate/grip2a-dependent symmetry breaking event by a general animal-directed long-range transport system. 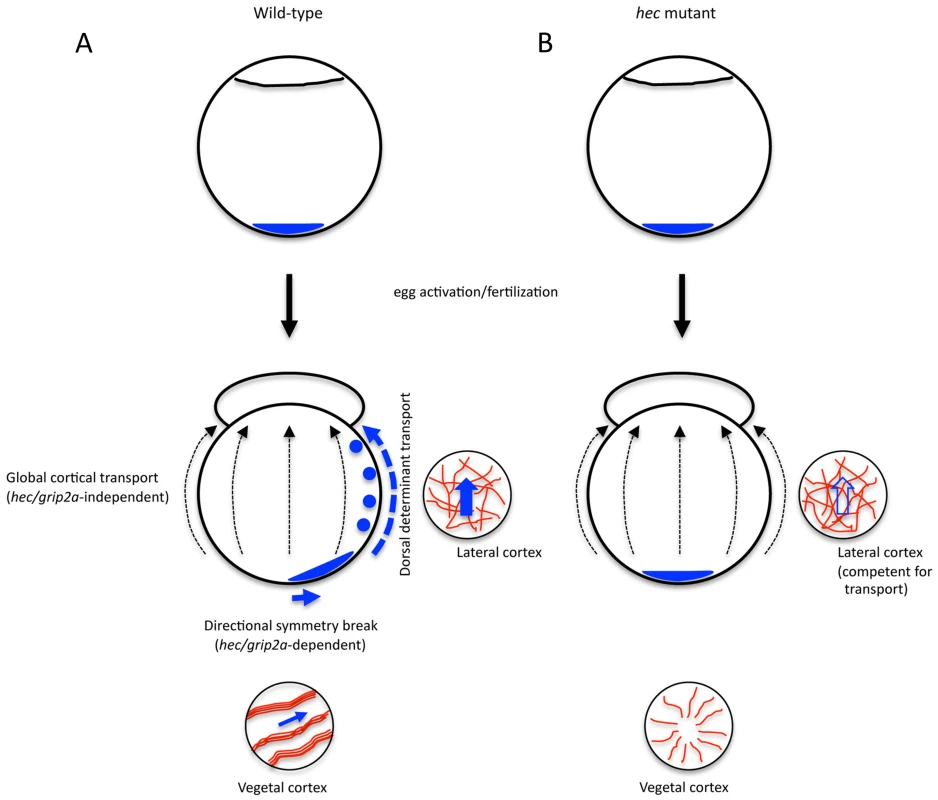
A) Cortical shifts of various vegetally localized components, including wnt8a mRNA, Sybu protein and grip2a mRNA ([6], [7], [8]; this report) are short-range and dependent on microtubule bundling and alignment, itself dependent on hec function. In wild-type embryos, such a short-range shift generates a symmetry breaking event that is subsequently amplified by long-range, animally-directed transport mechanism independent of hec function and not restricted to the prospective dorsal axis. B) In hec mutant embryos, neither reorganization of vegetal microtubules into aligned bundles nor a short-range shift occur, so that, even though long-range transport remains intact, vegetal determinant transport to the animal pole is affected. The mechanistic basis for the long-range transport, occurring in the region of a loosely organized mediolateral microtubule cortical network remains to be determined (see Discussion). hecate may represent a gene duplication adapted for axis induction
Using BLAST searches on Ensembl and NCBI genome databases, homologous grip1 and grip2 genes were found for all vertebrate species. In Drosophila, there is an ancient single Grip gene. All other species containing recognizable grip genes are vertebrates. In amphibians, birds and mammals, there is one grip1 gene and one grip2 gene. On the other hand, zebrafish have one grip1 gene and two grip2 genes, likely due to an ancestral genome duplication in the teleost lineage [30], [31]. Following published nomenclature [28], we refer to the grip2 gene corresponding to hec, located in chromosome 8, as grip2a, and the copy located in chromosome 22, as grip2b. Phylogenetic analysis indicates that zebrafish grip2a is only present in fish species (zebrafish, fugu and medaka). Drosophila Grip and all vertebrate Grip1 and Grip2 proteins (including zebrafish Grip2b) contain seven conserved PDZ domains, while zebrafish Grip2a contains only 4 PDZ domains.
The amphibian grip2 homolog was identified as a novel vegetally-localized mRNA in Xenopus oocytes that is present in the mitochondrial cloud (Balbiani body) and subsequently in the germ plasm throughout oogenesis and early embryogenesis [32], [85], [86]. In Xenopus grip2 morphants, PGC numbers are significantly reduced, and PGCs are also present at ectopic locations along the anteroposterior axis in tailbud stage embryos [32], [86]. However, unlike the case of Xenopus grip2, zebrafish grip2a mRNA does not localize to the germ plasm or PGCs, and the number of PGCs is unaffected in strongly ventralized mutants (i.e. radially symmetric ventralized embryos). These observations suggest that in zebrafish PGCs are determined independently of hec/grip2a function. Therefore, it appears that Xenopus grip2 and zebrafish grip2a mRNA, in spite of a similar localization at the vegetal pole of the oocyte, have distinct functions in early embryogenesis: Xenopus grip2 is involved in PGC development, while zebrafish grip2a appears to be devoted to dorsal axis formation.
Despite this divergent function, the fact that zebrafish hec/grip2a and Xenopus grip2 are maternally expressed and localized to the vegetal pole during oogenesis, the site of localization of both germ plasm components and dorsal determinants in these two lineages, suggests that an ancestral grip gene may have functioned in both axis induction and PGC development. There is precedent for a relationship between these two processes, notably in Drosophila [87] but also in systems as basal as planaria [88], [89] and annelids [90]. This relationship is also supported by the recent finding that the germ cell-specific factor Dead end is required for microtubule rearrangements and cortical rotation in Xenopus [54]. The presence of a single localization system involved in both the induction of the primary embryonic axis and the separation between germ cell and soma may constitute a simple mechanism for the species patterning and propagation. Studies of the role of grip genes in other organisms may shed light on the relationship between axis induction and PGC determination during evolution.
Materials and Methods
Ethics statement
All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies, and all animal work was approved by the appropriate committee (University of Wisconsin – Madison assurance number A3368-01).
Fish maintenance and genetic lines
Fish stocks were raised and maintained under standard conditions at 28.5° [91]. The hect2800 allele was originally isolated in an early-pressure-based screen for recessive maternal-effect mutations [14], [17], while the hecp06ucal and hecp08ajug alleles were found in a four-generation scheme based on natural crosses ([16], [18], see also [92]). The hect2800 allele was induced in an AB/Tübingen hybrid background [14], [17], which was further hybridized with the WIK line during linkage mapping. The hecp08ajug and hecp06ucal alleles were induced in a Tübingen background, which was hybridized to an AB line in an F4 genetic screen coordinated with linkage mapping [16], [18]. Homozygous mutant hec fish were identified by genotyping the flanking SSLP markers z59658 and z24511, which are 1.2 cM apart on linkage group 8 and both of which were polymorphic for all three alleles. Mutant embryos were obtained by crossing homozygous hec females to AB males. Embryos from females homozygous mutant for the hecp06ucal allele were used unless otherwise specified. Wild-type control embryos were derived from either the AB line or heterozygous sibling females. Clutches were synchronized through 5-minute collections during natural spawning. Oocytes were collected from wild-type, bucky ballp106re [39], [43] and magellanp6cv [40] mutants. Embryos were collected and developed in E3 embryonic medium [14] and were staged according to the age and morphological standards described in [93].
For complementation tests of hect2800, hecp08ajug and hecp06ucal mutants, homozygous mutant males of one allele were crossed with heterozygous females of another allele to produce offspring, which were raised to adulthood. Female adult fish were crossed with wild-type males and phenotyped as wild-type or ventralized mutant by examining the resulting clutches at 24 hpf. Only those clutches producing more than 50 embryos were scored and non-complementation was indicated by the presence of ventralization phenotypes similar in expressivity and penetrance to those in clutches from mutant females from the original three mutant alleles on their own.
Isolation and genotyping of genomic DNA
Fish were anesthetized with MESAB (0.014%) and the tail fin was clipped using a razor blade and placed into 100 ul DNA lysis buffer (10 mM Tris, pH 8.0; 10 mM EDTA, pH 8.0; 200 mM NaCl; 1% Triton X-100) containing 5 µg 10 mg/ml Proteinase K. Tissue lysates were incubated overnight at 55°C, and were incubated at 94°C for 10 minutes to inactivate Proteinase K. Lysates were diluted 1∶6 with water, and 2.5 µl of this genomic DNA diluted lysate was used per 10 µl PCR reaction. For a 10 µl PCR reaction, 2 µl of GoTaq green Buffer, 0.2 µl of dNTPs, 0.05 µl of GoTaq DNA polymerase (Promega), 2.5 ul genomic DNA diluted lysate, and 1 µl each of 10 µM forward and reverse primers were used. For SSLP markers, PCR products were analyzed on a 2% high-resolution agarose gel right after the PCR reaction. For RFLP markers, PCR products were used for FastDigest Restriction Enzyme (Fermentas) digestion for 30 min, and then analyzed on a 1.5% regular agarose gel.
Positional cloning and sequence analysis
Initial linkage was identified by bulk segregant analysis with SSLP markers. Once initial linkage of the mutation was obtained, genotypically identified homozygous mutant males were crossed to heterozygous females to generate large numbers of fish for fine mapping [92].
Chromosome walking was conducted by screening the CHORI-211 BAC library using marker z67047 and zC150E8z (0 recombination/1762 genomes). PCR-based screening of the primary pool and secondary pools identified 2 positive BAC clones. Individual BAC clones were ordered from BACPAC resources center (http://bacpac.chori.org).
To find the mutation in hec/grip2a, 5 fragments of hec/grip2a cDNA from mutant and wild-type 1-cell embryo cDNA were amplified by RT-PCR with 5 primer pairs, which cover the entire hec/grip2a coding region:
1. 5′-ATGTCCTGCATCTTGCTTCCAGAG-3′ and 5′-CCTCAGTGGGAATCCCATTAATGG-3′
2. 5′-TGGAGTGTTACAAGTTGGCGACAG-3′ and 5′-TGAATGGCTTCGCTCAGAGGTTTG-3′
3. 5′-TTCATATCGGTGACCGAGTTTTGG-3′ and 5′-GACATTATTGTAGCCTCAAGCTCG-3′
4. 5′-GAGACCTGCGGTCAGTCAGAAATC-3′ and 5′-GTGCTCTGTGTTTCTCATTTGTGG-3′
5. 5′-AGGACACTTCCCAACAGTCTGCAC-3′ and 5′-ACCTGATCACTTCTAACCCAACAG-3′
All PCR products were cloned into pGEM-T easy vector and sequenced.
For Phylogenetic analysis, homologous grip1 and grip2 genes were found using BLAST searches on Ensembl and NCBI genome databases. A phylogenetic tree was constructed and drawn using ClustalW in the MegAlign program from Lasergen. PDZ domains were identified using CD-search in the Conserved Domain Database (CDD) in NCBI [29]. Schematic diagram of the protein domain structures for each gene were drawn using DomainDraw [94].
RT-PCR and quantitative RT-PCR
Total RNA was isolated from whole embryos using TRIzol reagent (Invitrogen). cDNA was synthesized using random primers (Invitrogen) and AMV Reverse Transcriptase (Promega). RT-PCR reactions were performed with primer pairs derived from hec/grip2a and ef1α, using 30 cycles at an annealing temperature of 58°C (in the semi-quantitative range). Absence of genomic contamination was verified by a negative control RT reaction without the Reverse Transcriptase. The following primers were used for the amplifications:
hec/grip2a, 5′-GAGACCTGCGGTCAGTCAGAAATC-3′ and 5′-TATGAAGCTCTAGAGGCACTGACG-3′,
wnt8a, 5′-CGGAAAAATGGGTGGTCGTG-3′ and 5′-AGTCGACCAGCTTCGTTGTT-3′,
ef1α, 5′-ACCGGCCATCTGATCTACAA-3′ and 5′-CAATGGTGATACCACGCTCA-3′.
Quantitative (q) RT-PCR was performed on an iCycler machine (Bio-Rad) using iQ SYBR Green Supermix (Bio-Rad). The thermal profile used for amplification is: 95°C for 3 min, 40 cycles of 94°C for 30 s, 58°C for 30 s and 72°C for 30 s. The relative mRNA level was quantified and normalized to ef1α.
In situ hybridizations and antibody labeling
In situ hybridizations of embryos were carried out as described previously [95]. Probes for in situ included goosecoid [96], chordin [97], even skipped 1 [98], vasa [33], dazl [46] and wnt8a [7], [99]. For the grip2a in situ probe, a fragment of grip2a cDNA was cloned into pGEM-T easy vector as described in the positional cloning section. Five different probes were tested against 5 different cDNA sequences, all of which showed the same expression pattern. Subsequently, all the expression data was acquired using one of the probes. Antisense digoxygenin probe was generated by linearizing and transcribing with SacII and SP6 RNA polymerase, while sense probe control was generated by linearization with SpeI and transcription with T7 RNA polymerase. Images were acquired with a Leica-FLIII microscope and a color camera (Diagnostic Instruments Spot Insight).
Ovaries were dissected from euthanized females and fixed overnight at 4°C in 4% paraformaldehyde. Fixed ovaries were then dehydrated in MeOH.
Whole mount in situ hybridization of oocytes was performed as previously described [100]. Following staining, oocytes were embedded in JB-4 Plus Plastic resin and 7 micron sections were cut using a microtome. Stained sections were coated with Permount (Fisher) prior to addition of a coverslip.
Antibody labeling of microtubules was as previously described [36]. Prior to the labeling procedure, embryos were dissected using fine dissecting forceps to generate two halves. To image cortical microtubules, bisections were carried out along an equatorial plane, the vegetal halves were labeled and mounted in 50% glycerol with DABCO reagent to prevent bleaching, with the vegetal cortex facing the coverslip. For imaging of mediolateral microtubules, bisections were carried out along a meridional plane and each mediolateral halves were labeled and mounted as above with the mediolateral cortex facing the coverslip. Images were acquired using an upright Zeiss LSM510 confocal microscope using an oil immersion 63× objective and collected as single 1.5 µm optical sections with a pinhole diameter of about 1 Airy unit, a low scan speed (preset 6) and noise filtering through a 4-pass line mode average. The resulting images were analyzed with Fiji software.
Antibody labeling of embryos to detect Sybu protein was carried in whole mount embryos using whole embryos as described previously [6], and images were acquired using a Zeiss Axioplan2 fluorescent microscope and OpenLab software.
Nocodazole treatment was carried out through exposure by 10 mpf of dechorionated embryos to a final concentration of 4 µg/ml nocodazole in E3 (diluted from a 5 mg/ml solution in DMSO), followed by fixation at the indicated periods.
Phalloidin labeling was carried out as in [36], with the exception that dechorionated embryos were labeled whole and equatorially bisected prior to mounting with the vegetal pole facing the coverslip.
Fluorescent beads injection
Fluorescent beads injection experiments were carried as previously described [2]. A suspension of 0.2 mm fluorescent polystyrene beads (1 ul; Polysciences) was diluted in 23 ml water and colored with trace amounts of phenol red (0.05%). The injection solution is microinjected into the embryos near the vegetal pole using a Phemtojet microinjector (Eppendorf). Embryos had been injected at the 2-cell stage and imaged at 2 hours later. Embryos were mounted in methyl cellulose and imaged with an upright fluorescence microscope (Zeiss, Axioplan II) and a black and white digital camera (Zeiss, Axiocam).
Supporting Information
Zdroje
1. HoustonDW (2012) Cortical rotation and messenger RNA localization in Xenopus axis formation. WIREs Dev Biol 1 : 371–388.
2. JesuthasanS, SträhleU (1997) Dynamic microtubules and specification of the zebrafish embryonic axis. Curr Biol 7 : 31–42.
3. MizunoT, YamahaE, KuroiwaA, TakedaH (1999) Removal of vegetal yolk causes dorsal deficiencies and impairs dorsal-inducing ability of the yolk cell in zebrafish. Mech Dev 81 : 51–63.
4. OberEA, Schulte-MerkerS (1999) Signals from the yolk cell induce mesoderm, neuroectoderm, the trunk organizer, and the notochord in zebrafish. Dev Biol 215 : 167–181.
5. NojimaH, ShimizuT, KimC-H, YabeT, BaeY-K, et al. (2004) Genetic evidence for involvement of maternally derived Wnt canonical signaling in dorsal determination in zebrafish. Mech Dev 121 : 371–386.
6. NojimaH, RothhämelS, ShimizuT, KimC-H, YonemuraS, et al. (2010) Syntabulin, a motor protein linker, controls dorsal determination. Development 137 : 923–933.
7. LuF-I, ThisseC, ThisseB (2011) Identification and mechanism of regulation of the zebrafish dorsal determinant. Proc Natl Acad Sci USA 108 : 15876–15880.
8. TranLD, HinoH, QuachH, LimS, ShindoA, et al. (2012) Dynamic microtubules at the vegetal cortex predict the embryonic axis in zebrafish. Development 139 : 3644–3652.
9. GordonMD, NusseR (2006) Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 281 : 22429–22433.
10. PetersenCP, ReddienPW (2009) Wnt signaling and the polarity of the primary body axis. Cell 139 : 1056–1058.
11. TaoQ, YokotaC, PuckH, KofronM, BirsoyB, et al. (2005) Maternal Wnt11 activates the canonical Wnt signaling pathway required for axis formation in Xenopus embryos. Cell 120 : 857–871.
12. ChaS-W, TadjuidjeE, TaoQ, WylieC, HeasmanJ (2008) Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development 135 : 3719–3729.
13. ChaS-W, TadjuidjeE, WhiteJG, WellsJ, MayhewC, et al. (2009) Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr Biol 19 : 1573–1580.
14. PelegriF, Schulte-MerkerS (1999) A gynogenesis-based screen for maternal-effect genes in the zebrafish,. Meth Cell Biol 60 : 1–20.
15. KellyC, ChinAJ, LeathermanJL, KozlowskiDJ, WeinbergES (2000) Maternally controlled β-catenin-mediated signaling is required for organizer formation in the zebrafish. Development 127 : 3899–3911.
16. DoschR, WagnerDS, MintzerKA, RunkeG, WiemeltAP, et al. (2004) Maternal control of vertebrate development before the midblastula transition: mutants from the zebrafish I. Dev Cell 6 : 771–780.
17. PelegriF, DekensMPS, Schulte-MerkerS, MaischeinH-M, WeilerC, et al. (2004) Identification of recessive maternal-effect mutations in the zebrafish using a gynogenesis-based method. Dev Dyn 231 : 325–336.
18. WagnerDS, DoschR, MintzerKA, WiemeltAP, MullinsMC (2004) Maternal control of development at the midblastula transition and beyond: mutants from the zebrafish II. Dev Cell 6 : 781–790.
19. BellipanniG, VargaM, MaegawaS, ImaiY, KellyC, et al. (2006) Essential and opposing roles of zebrafish β-catenins in the formation of dorsal axial structures and neuroectoderm. Development 133 : 1299–1309.
20. Lyman-GingerichJ, WestfallTA, SlusarskiDC, PelegriF (2005) hecate, a zebrafish maternal effect gene, affects dorsal organizer induction and intracellular calcium transient frequency. Dev Biol 286 : 427–439.
21. AtamanB, AshleyJ, GorczycaD, GorczycaM, MathewD, et al. (2006) Nuclear trafficking of Drosophila Frizzled-2 during synapse development requires the PDZ protein dGRIP. Proc Natl Acad Sci USA 103 : 7841–7846.
22. KorkutC, AtamanB, RamachandranP, AshleyJ, BarriaR, et al. (2009) Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 139 : 393–404.
23. Lyman-GingerichJ, LindemanR, PutiriE, StolzmannK, PelegriF (2006) The analysis of axis induction mutant embryos reveals morphogenetic events associated with zebrafish yolk extension formation. Dev Dyn 235 : 2749–2760.
24. KishimotoY, LeeK-H, ZonL, HammerschmidtM, Schulte-MerkerS (1997) The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development 124 : 4457–4466.
25. MohlerJ, WieschausEF (1986) Dominant maternal effect mutations of Drosophila melanogaster causing the production of double-abdomen embryos. Genetics 112 : 808–822.
26. LehmannR, Nüsslein-VolhardC (1991) The maternal gene nanos has a central role in posterior pattern formation of the Drosophila embryo. Development 112 : 679–691.
27. MeiW, LeeKW, MarlowFL, MilerAL, MullinsMC (2009) hnRNP I is required to generate the Ca2+ signal that causes egg activation in zebrafish. Development 136 : 3007–3017.
28. CarneyTJ, FeitosaNM, SonntagC, SlanchevK, KlugerJ, et al. (2010) Genetic analysis of fin development in zebrafish identifies Furin and Hemicentin1 as potential novel Fraser Syndrome disease genes. PLoS Genet 6: e1000907.
29. Marchler-BauerA, BryantSH (2004) CD-Search: protein domain annotations on the fly. Nuc Acids Res 32: W327–331.
30. ChristoffelsA, KohEG, ChiaJM, BrennerS, AparicioS, et al. (2004) Fugu genome analysis provides evidence for a whole-genome duplication early during the evolution of ray-finned fishes. Mol Biol Evol 21 : 1146–1151.
31. SémonM, WolfeKH (2007) Reciprocal gene loss between Tetraodon and zebrafish after whole genome duplication in their ancestor. Trends Genet 23 : 108–112.
32. TarbashevichK, KoebernickK, PielerT (2007) XGRIP2.1 is encoded by a vegetally localizing, maternal mRNA and functions in germ cell development and anteroposterior PGC positioning in Xenopus laevis. Dev Biol 311 : 554–565.
33. YoonC, KawakamiK, HopkinsN (1997) Zebrafish vasa homologue RNA is localized to the cleavage planes of 2 - and 4-cell-stage embryos and is expressed in the primordial germ cells. Development 124 : 3157–3165.
34. PelegriF, KnautH, MaischeinH-M, Schulte-MerkerS, Nüsslein-VolhardC (1999) A mutation in the zebrafish maternal-effect gene nebel affects furrow formation and vasa RNA localization. Curr Biol 9 : 1431–1440.
35. KnautH, PelegriF, BohmannK, SchwarzH, Nüsslein-VolhardC (2000) Zebrafish vasa RNA but not its protein is a component of the germ plasm and segregates asymmetrically prior to germ line specification. J Cell Biol 149 : 875–888.
36. TheuschEV, BrownKJ, PelegriF (2006) Separate pathways of RNA recruitment lead to the compartmentalization of the zebrafish germ plasm. Dev Biol 292 : 129–141.
37. EnoC, PelegriF (2013) Gradual recruitment and selective clearing generate germ plasm aggregates in the zebrafish embryo. Bioarchitecture 3 : 125–132.
38. BraatAK, ZandbergenT, van de WaterS, GoosHJT, ZivkovicD (1999) Characterization of zebrafish primordial germ cells: morphology and early distribution of vasa RNA. Dev Dyn 216 : 153–167.
39. BontemsF, SteinA, MarlowF, LyauteyJ, GuptaT, et al. (2009) Bucky ball organizes germ plasm assembly in zebrafish. Curr Biol 19 : 414–422.
40. GuptaT, MarlowFL, FerriolaD, MackiewiczK, DapprichJ, et al. (2010) Microtubule actin crosslinking factor 1 regulates the balbiani body and animal-vegetal polarity of the zebrafish oocyte. PLoS Genet 6: e1001073.
41. CoxRT, SpradlingAC (2003) A Balbiani body and the fusome mediate mitochondrial inheritance during Drosophila oogenesis. Development 130 : 1579–1590.
42. PeplingME, WilhelmJE, O'HaraAL, GephartGW, SpradlingAC (2007) Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc Natl Acad Sci USA 104 : 187–192.
43. MarlowFL, MullinsMC (2008) Bucky ball functions in Balbiani body assembly and animal-vegetal polarity in the oocyte and follicle cell layer in zebrafish. Dev Biol 321 : 40–50.
44. De BrabanderM, GeuensG, NuydensR, WillebrordsR, De MeyJ (1961) Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc Natl Acad Sci USA 78 : 5608–5612.
45. DonovanMJ, HartNH (1986) Cortical granule exocytosis is coupled with membrane retrieval in the egg of Brachydanio. J Exp Zool 237 : 391–405.
46. MaegawaS, YasudaK, InoueK (1999) Maternal mRNA localization of zebrafish DAZ-like gene. Mech Dev 81 : 223–226.
47. HashimotoY, MaegawaS, NagaiT, YamahaE, SuzukiH, et al. (2004) Localized maternal factors are required for zebrafish germ cell formation. Dev Biol 268 : 152–161.
48. OlsenLC, AaslandR, FjoseA (1997) A vasa-like gene in zebrafish identifies putative primordial germ cells. Mech Dev 66 : 95–105.
49. De RobertisEM, KurodaH (2004) Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol 20 : 285–308.
50. PelegriF (2003) Maternal factors in zebrafish development. Dev Dyn 228 : 535–554.
51. MarrariY, TerasakiM, ArrowsmithV, HoulistonE (2000) Local inhibition of cortical rotation in Xenopus eggs by an anti-KRP antibody. Dev Biol 224 : 250–262.
52. MarrariY, RouviereC, HoulistonE (2004) Complementary roles for dynein and kinesins in the Xenopus egg cortical rotation. Dev Biol 271 : 38–48.
53. CuykendallTN, HoustonDW (2009) Vegetally localized Xenopus trim36 regulates cortical rotation and dorsal axis formation. Development 136 : 3057–3065.
54. MeiW, JinZ, LaiF, SchwendT, HoustonDW, et al. (2013) Maternal Dead-End1 is required for vegetal cortical microtubule assembly during Xenopus axis specification. Development 140 : 2334–2344.
55. ChanAP, KlocM, EtkinLD (1999) fatvg encodes a new localized RNA that uses a 25-nucleotide element (FVLE1) to localize to the vegetal cortex of Xenopus oocytes. Development 126 : 4943–4953.
56. ChanAP, KlocM, LarabellCA, LeGrossM, EtkinLD (2007) The maternally localized RNA fatvg is required for cortical rotation and germ cell formation. Mech Dev 124 : 350–363.
57. DongH, ZhangP, SongI, PetraliaRS, LiaoD, et al. (1999) Characterization of the Glutamate receptor-interacting proteins GRIP1 and GRIP2. J Neurosci 19 : 6930–6941.
58. LeeHJ, ZhengJJ (2010) PDZ domains and their binding partners: structure, specificity, and modification. Cell Commun Signal 8 : 8.
59. BardL, GrocL (2011) Glutamate receptor dynamics and protein interaction: lessons from the NMDA receptor. Mol Cell Neurosci 48 : 298–307.
60. IvarssonY (2012) Plasticity of PDZ domains in ligand recognition and signaling. FEBS Lett 586 : 2638–2647.
61. TakamiyaK, KostourouV, AdamsS, JadejaS, ChalepakisG, et al. (2004) A direct functional link betwen the multi-PDZ domain protein GRIP1 and the Fraser sydrome protein Fras1. Nat Genet 36 : 172–177.
62. SugiuraT, ShimizuT, KijimaA, MinakataS, KatoY (2011) PDZ adaptors: their regulation of epithelial transporters and involvement in human diseases. J Pharm Sci 100 : 3620–3635.
63. SetouM, SeogDH, TanakaY, KanaiY, TakeiY, et al. (2002) Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature 417 : 83–87.
64. SuQ, CaiQ, GerwinC, SmithCL, ShengZH (2004) Syntabulin is a microtubule-associated protein implicated in syntaxin tranport in neurons. Nat Cell Biol 6 : 941–953.
65. CaiQ, GerwinC, ShengZH (2005) Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J Cell Biol 170 : 959–969.
66. CaiQ, PenPY, ShengZH (2007) Syntabulin-kinesin-1 family member 5B-mediated axonal tranport contributes to activity-dependent presynaptic assembly. J Neurosci 27 : 7284–7296.
67. FagniL, AngoF, PeroyJ, BockaertJ (2004) Identification and functional roles of metabotropic glutamate receptor-interacting proteins. Sem Cell Dev Biol 15 : 289–298.
68. FurukawaJ (2012) Structure and function of glutamate receptor amino terminal domains. J Physiol 590 : 63–72.
69. LarabellCA, TorresM, RowningBA, YostC, MillerJR, et al. (1997) Establishment of the dorsoventral axis in Xenopus embryos is presaged by early asymmetries in β-catenin that are modulated by the Wnt signalling pathway. J Cell Biol 136 : 1123–1134.
70. RowningBA, WellsJ, WuM, GerhartJC, MoonRT, et al. (1997) Microtubule-mediated transport of organelles and localization of β–catenin to the future dorsal side of Xenopus eggs. Proc Natl Acad Sci USA 94 : 1224–1229.
71. MillerJR, RowningBA, LarabellCA, Yang-SnyderJA, BatesR, et al. (1999) Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J Cell Biol 146 : 427–437.
72. WeaverC, FarGHIII, PanW, RowningBA, WangJ, et al. (2003) GBP binds kinesin light chain and translocates during cortical rotation in Xenopus eggs. Development 130 : 5425–5436.
73. SwanLE, WichmannC, PrangeU, SchmidA, SchmidtM, et al. (2004) A glutamate receptor-interacting protein homolog organizes muscle guidance in Drosophila. Genes Dev 18 : 223–237.
74. SwanLE, SchmidtM, SchwarzT, PonimaskinE, PrangeU, et al. (2006) Complex interaction of Drosophila GRIP PDZ domains and Echinoid during muscle morphogenesis. EMBO J 25 : 3640–3651.
75. KumeS, InoueT, MikoshibaK (2000) Gαs family G proteins activate IP3-Ca2+ signaling via Gβγ and transduce ventralizing signals in Xenopus. Dev Biol 226 : 88–103.
76. SaneyoshiT, KumeS, AmasakiY, MikoshibaK (2002) The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature 417 : 295–299.
77. WestfallTA, BrimeyerR, TwedtJ, GladonJ, OlberdingA, et al. (2003) Wnt5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/β-catenin activity. J Cell Biol 162 : 889–898.
78. WestfallTA, HjertosB, SlusarskiDC (2003) Requirement for intracellular calcium modulation in zebrafish dorsal-ventral patterning. Dev Biol 259 : 380–391.
79. WuS-Y, ShinJ, SepichDS, Solnica-KrezelL (2012) Chemokine GPCR signaling inhibits β-catenin during zebrafish axis formation. PLoS Biol 10: e1001403.
80. ElinsonRP, RowningB (1988) Transient array of parallel microtubules in frog eggs: potential tracks for a cytoplasmic rotation that specifies the dorso-ventral axis. Dev Biol 128 : 185–197.
81. HoulistonE, ElinsonRP (1991) Patterns of microtubule polymerization relating to cortical rotation in Xenopus laevis eggs. Development 112 : 107–117.
82. SchroederMM, GardDL (1992) Organization and regulation of cortical microtubules during the first cell cycle of Xenopus eggs. Development 114 : 699–709.
83. FuentesR, FernándezJ (2010) Ooplasmic segregation in the zebrafish zygote and early embryo: pattern of ooplasmic movements and transport pathways. Dev Dyn 239 : 2172–2189.
84. Solnica-KrezelL, DrieverW (1994) Microtubule arrays of the zebrafish yolk cell: organization and function during epiboly. Development 120 : 2443–2455.
85. KaneshiroK, MiyauchiM, TanigawaY, IkenishiK, KomiyaT (2007) The mRNA coding for Xenopus glutamate receptor interacting protein 2 (XGRIP2) is maternally transcribed, transported through the late pathway and localized to the germ plasm. Biochem Biophys Res Commun 355 : 902–906.
86. KirilenkoP, WeierudFK, ZornAM, WoodlandHR (2008) The efficiency of Xenopus primordial germ cell migration depends on the germplasm mRNA encoding the PDZ domain protein Grip2. Differentiation 76 : 392–403.
87. St. JohnstonD, Nüsslein-VolhardC (1992) The origin of pattern and polarity in the Drosophila embryo. Cell 68 : 201–219.
88. RebscherN, Zelada-GonzálezF, BanischTU, RaibleF, ArendtD (2007) Vasa unveils a common origin of germ cells and of somatic stem cells from the posterior growth zone in the polychaete Platynereis dumerilii. Dev Biol 306 : 599–611.
89. WangY, ZayasRM, GuoT, NewmarkPA (2007) nanos function is essential for development and regeneration of planarian germ cells. Proc Natl Acad Sci USA 104 : 5901–5906.
90. GazaveE, BéhagueJ, LaplaneL, GuillouA, PréauL, et al. (2013) Posterior elongation in the annelid Platynereis dumerilii involves stem cells molecularely related to primordial germ cells. Dev Biol 382 : 246–267.
91. Brand M, Granato M, Nüsslein-Volhard C (2002) Keeping and raising zebrafish. In: Nüsslein-Volhard C, Dahm R, editors. Zebrafish - A Practical Approach. Oxford: Oxford University Press. 7–37 p.
92. PelegriF, MullinsM (2011) Genetic screens for mutations affecting adult traits and parental-effect genes. Meth Cell Biol 104 : 83–120.
93. KimmelC, BallardWW, KimmelSR, UllmannB, SchillingTF (1995) Stages of embryonic development in the zebrafish. Dev Dyn 203 : 253–310.
94. FinkJL, HamiltonN (2007) DomainDraw: a macromolecular feature drawing program. In Silico Biol 7 : 145–150.
95. PelegriF, MaischeinH-M (1998) Function of zebrafish β-catenin and TCF-3 in dorsoventral patterning. Mech Dev 77 : 63–74.
96. Schulte-MerkerS, HammerschmidtM, BeuchleD, ChoK, DeRobertisEM, et al. (1994) Expression of the zebrafish goosecoid and no tail gene products in wild-type and mutant ntl embryos. Development 120 : 843–852.
97. Schulte-MerkerS, LeeKJ, McMahonAP, HammerschmidtM (1997) The zebrafish organizer requires chordino. Nature 387 : 862–863.
98. JolyJ-S, JolyC, Schulte-MerkerS, BoulkebacheH, CondamineH (1993) The ventral and posterior expression of the homeobox gene eve1 is perturbed in dorsalized and mutant embryos. Development 119 : 1261–1275.
99. LekvenAC, ThorpeCJ, WaxmanJS, MoonRT (2001) Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neuroectoderm pattterning. Dev Cell 1 : 103–114.
100. ThisseC, ThisseB, SchillingTF, PostlethwaitJH (1993) Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development 119 : 1203–1215.
101. SelmanK, WallaceRA, SarkaA, QiX (1993) Stages of oocyte development in the zebrafish, Brachydanio rerio. J Morphol 218 : 203–224.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 6- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Akutní intermitentní porfyrie
- Příjem alkoholu a menstruační cyklus
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
- Transfer zmraženého embrya zlepšuje výsledky IVF
-
Všechny články tohoto čísla
- Inflammation: Gone with Translation
- Recombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
- Caspase Inhibition in Select Olfactory Neurons Restores Innate Attraction Behavior in Aged
- Accurate, Model-Based Tuning of Synthetic Gene Expression Using Introns in
- A Novel Peptidoglycan Binding Protein Crucial for PBP1A-Mediated Cell Wall Biogenesis in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
- Introgression from Domestic Goat Generated Variation at the Major Histocompatibility Complex of Alpine Ibex
- Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in
- Coordination of Wing and Whole-Body Development at Developmental Milestones Ensures Robustness against Environmental and Physiological Perturbations
- Phenotypic Dissection of Bone Mineral Density Reveals Skeletal Site Specificity and Facilitates the Identification of Novel Loci in the Genetic Regulation of Bone Mass Attainment
- Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of -Factors in Two Independent Origins of C Photosynthesis
- Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity
- Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation
- Rosa26-GFP Direct Repeat (RaDR-GFP) Mice Reveal Tissue- and Age-Dependence of Homologous Recombination in Mammals
- Abnormal Type I Collagen Post-translational Modification and Crosslinking in a Cyclophilin B KO Mouse Model of Recessive Osteogenesis Imperfecta
- : Clonal Reinforcement Drives Evolution of a Simple Microbial Community
- Reviving the Dead: History and Reactivation of an Extinct L1
- Defective iA37 Modification of Mitochondrial and Cytosolic tRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate tRNA
- Early Back-to-Africa Migration into the Horn of Africa
- Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency
- Microbial Succession in the Gut: Directional Trends of Taxonomic and Functional Change in a Birth Cohort of Spanish Infants
- Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia
- Genetic Determinants of Long-Term Changes in Blood Lipid Concentrations: 10-Year Follow-Up of the GLACIER Study
- Palaeosymbiosis Revealed by Genomic Fossils of in a Strongyloidean Nematode
- Early Embryogenesis-Specific Expression of the Rice Transposon Enhances Amplification of the MITE
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
- Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
- N-WASP Is Required for Structural Integrity of the Blood-Testis Barrier
- The Transcription Factor TFII-I Promotes DNA Translesion Synthesis and Genomic Stability
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
- Digital Genotyping of Macrosatellites and Multicopy Genes Reveals Novel Biological Functions Associated with Copy Number Variation of Large Tandem Repeats
- ATRA-Induced Cellular Differentiation and CD38 Expression Inhibits Acquisition of BCR-ABL Mutations for CML Acquired Resistance
- The EJC Binding and Dissociating Activity of PYM Is Regulated in
- JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling
- Mouse Y-Linked and Are Expressed during the Male-Specific Interphase between Meiosis I and Meiosis II and Promote the 2 Meiotic Division
- Rasa3 Controls Megakaryocyte Rap1 Activation, Integrin Signaling and Differentiation into Proplatelet
- Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
- Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis
- The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
- The First Endogenous Herpesvirus, Identified in the Tarsier Genome, and Novel Sequences from Primate Rhadinoviruses and Lymphocryptoviruses
- Sequence of a Complete Chicken BG Haplotype Shows Dynamic Expansion and Contraction of Two Gene Lineages with Particular Expression Patterns
- Background Selection as Baseline for Nucleotide Variation across the Genome
- CPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
- The Effects of Codon Context on Translation Speed
- Glycogen Synthase Kinase (GSK) 3β Phosphorylates and Protects Nuclear Myosin 1c from Proteasome-Mediated Degradation to Activate rDNA Transcription in Early G1 Cells
- Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
- Muscle Structure Influences Utrophin Expression in Mice
- BLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
- Identification of Late Larval Stage Developmental Checkpoints in Regulated by Insulin/IGF and Steroid Hormone Signaling Pathways
- Transport of Magnesium by a Bacterial Nramp-Related Gene
- Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
- The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription
- The Rim15-Endosulfine-PP2A Signalling Module Regulates Entry into Gametogenesis and Quiescence Distinct Mechanisms in Budding Yeast
- Regulation of Hfq by the RNA CrcZ in Carbon Catabolite Repression
- Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions
- Positive Feedback of Expression Ensures Irreversible Meiotic Commitment in Budding Yeast
- Hecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
- Regulatory Mechanisms That Prevent Re-initiation of DNA Replication Can Be Locally Modulated at Origins by Nearby Sequence Elements
- Speciation and Introgression between and
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Early Back-to-Africa Migration into the Horn of Africa
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



