-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
and Bats: Story of an Emerging Friendship
article has not abstract
Published in the journal: . PLoS Pathog 11(11): e32767. doi:10.1371/journal.ppat.1005176
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1005176Summary
article has not abstract
A growing number of recent studies have highlighted bats as a reservoir for Leptospira bacteria, pointing out the potential role of bats in the epidemiology of the most widespread zoonotic disease in the world [1]. Because leptospirosis is a largely neglected disease, a number of unanswered questions remain about the ecology and evolution of Leptospira, especially those associated with bats. Here we summarize what has been recently learned about this emerging but enigmatic host–pathogen association. We show how this system can provide exciting new opportunities to obtain insights into the evolutionary ecology of bat-borne pathogens and propose future directions to disentangle the role of bats in human leptospirosis.
What Do We Know, Briefly, about Leptospirosis and Leptospira?
Leptospirosis is a bacterial disease of humans and animals caused by pathogenic spirochetes of the genus Leptospira. In humans, leptospirosis is an important (re-)emerging zoonosis of global public health concern [1], although tropical regions display the highest human incidence [2]. Over 500,000 human cases of severe leptospirosis are thought to occur each year worldwide, with a mortality rate of over 10%. Asymptomatic or subclinical human infections are common, making leptospirosis likely far more prevalent than currently diagnosed or recognized [3].
Leptospira are a complex of highly diversified bacteria comprising 22 species that include pathogenic (Leptospira interrogans, L. kirschneri, L. borgpetersenii, L. mayottensis, L. santarosai, L. noguchii, L. weilii, L. alexanderi, L. kmetyi, and L. alstonii), intermediate (i.e., species of unclear pathogenicity: L. broomii, L. fainei, L. inadai, L. licerasiae, L wolffii), and saprophytic (i.e., free-living and generally considered not to cause disease: L. biflexa, L. idonii, L. meyeri, L. terpstrae, L. vanthielli, L. wolbachii, L. yanagawae) species [4]. Alongside genetic characterization, serological classification (based on bacterial cell surface antigens) differentiates nearly 300 Leptospira serovars, of which more than 200 are considered pathogenic [5]. However, serovars are not indicative of the taxonomic relation among strains because one serovar may belong to more than one species (e.g., L. interrogans serovar Hardjo and L. borgpetersenii serovar Hardjo) and multiple serovars occur within the same species [6]. A wide variety of mammals can be infected, but rodents are recognized as significant reservoir hosts. Pathogenic and intermediate leptospires reside in the kidneys of infected animals and are spread through the excretion of urine into the environment [1]. Thus, contaminated soil or water as well as direct contact with infected animals are the main sources of leptospirosis.
To What Extent Are Bats Infected with Leptospira?
Growing scientific interest in bats as reservoirs of pathogens and the global importance of human leptospirosis have led to the emergence of investigations on the presence of Leptospira in wild bats during the last few years (Fig 1). Different techniques such as dark-field microscopy, serology by Microscopic Agglutination Test (MAT), PCR detection, and bacterial culture have been used. To date, Leptospira infection has been evidenced in over 50 bat species belonging to 8 of the 9 investigated bat families, encompassing various geographical regions in the tropics and subtropics [7–30] as well as Europe, although to a limited extent (Fig 2) [31,32]. Leptospira prevalence and seroprevalence in bat populations vary according to bat species and location. Given that bat sampling is often opportunistic, small sample sizes may account for the bias observed in the results. Moreover, a recent study revealed that the prevalence of Leptospira excretion in bat urine is highly variable over time, ranging from 6% to 45% within the same colony over a five-month period [20]. Thus, infection dynamics leading to variations in Leptospira shedding should be taken into account when bat populations are monitored for prevalence.
Fig. 1. Cumulative number of publications investigating <i>Leptospira</i> infection in bats over the past 75 years. 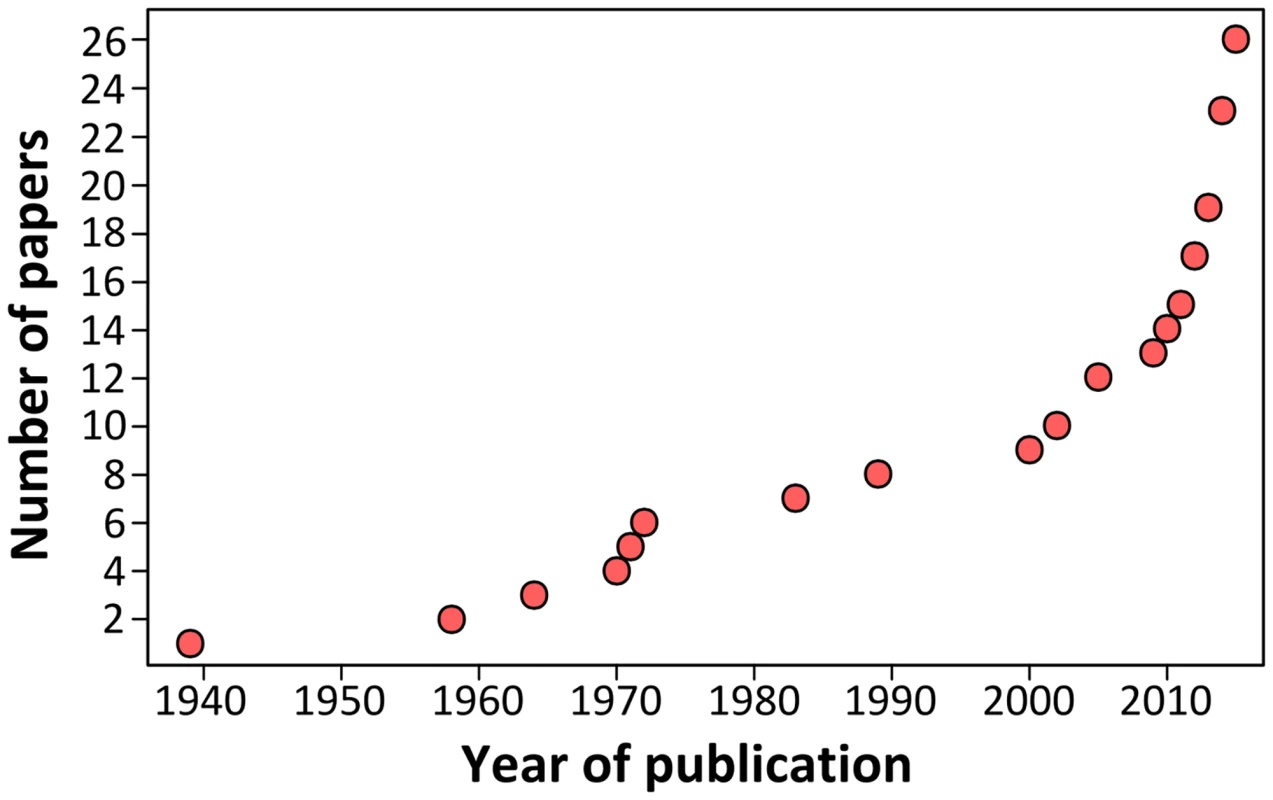
Fig. 2. Geographic distribution and diversity of Leptospira in bats. 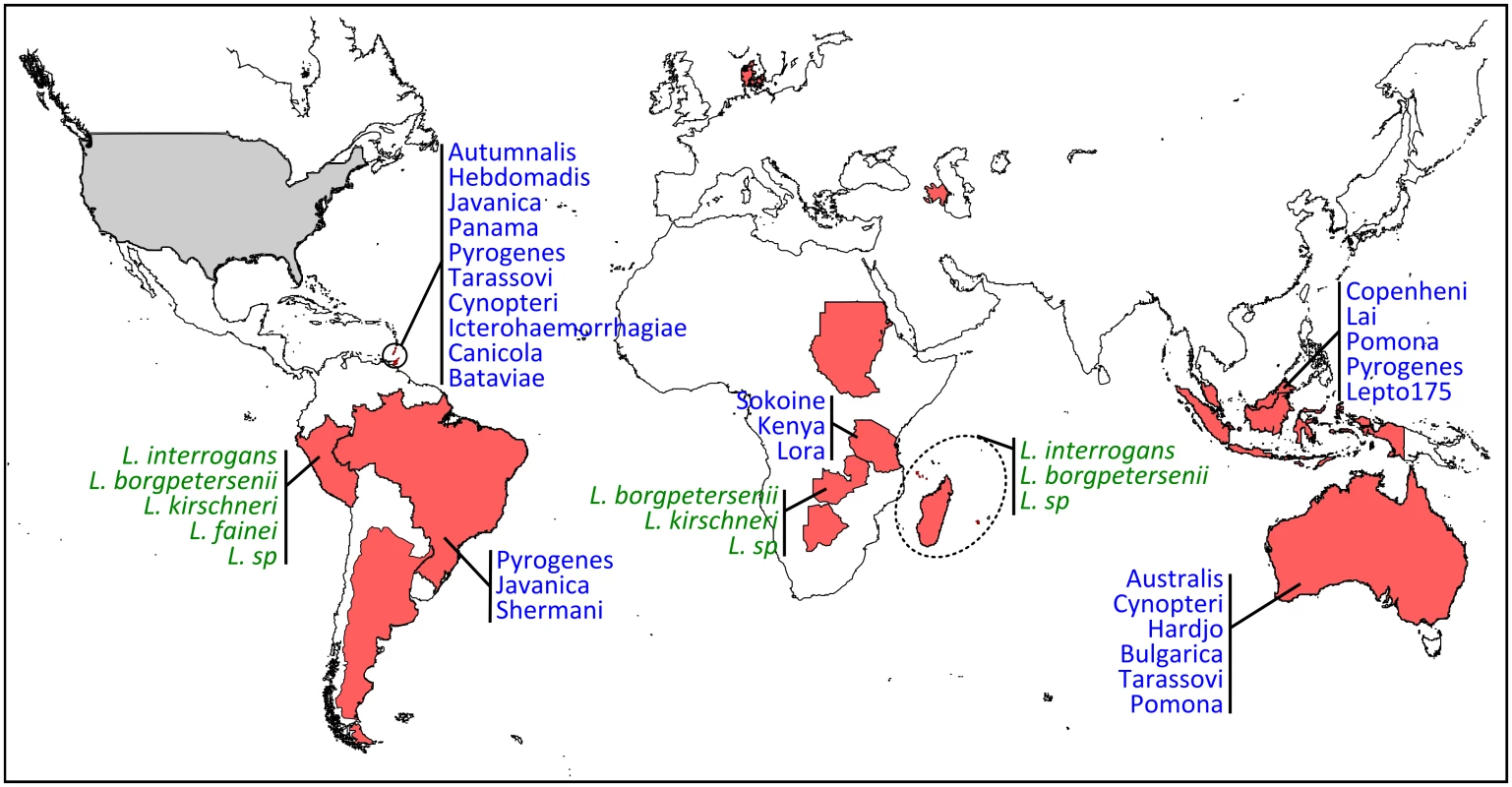
Countries are highlighted in red when PCR and/or serology were found positive (corresponding to the following bat families: Phyllostomidae, Mollosidae, Vespertillionidae, Hipposideridae, Miniopteridae, Nycteridae, Mormoopidae, Pteropodidae) and in grey for negative results (Thyropteridae). When available, Leptospira diversity is shown in green when genetic data have been used for species identification and in blue when serological analyses have been performed for serovar determination. Which Leptospira Infect Bats, and When?
There is increasing evidence that bats are infected by highly diverse leptospires, especially in tropical regions with high bat species richness [8,16,19]. Based on genetic identification, bats are infected by at least four species, i.e., L. interrogans, L. borgpetersenii, L. kirschneri, L. fainei, and likely yet-undescribed genetic clades (Fig 2) [8,19,28]. The use of multilocus sequence analysis has largely improved our view of Leptospira diversity in bats and has shown strong host specificity [19] as well as coinfection with multiple Leptospira [16,28]. The evolution of bat-borne Leptospira diversity and host specificity is probably linked to both cospeciation and host-switching events [33] but also to ecological features such as colony density, feeding behavior, and migration [34]. According to whole-genome [35] and field-based studies [36], which suggest that different Leptospira species have evolved towards different modes of transmission, bat species roosting in high-density colonies may be, for example, primarily infected by Leptospira dependent on host-to-host transmission, such as hypothesized for L. borgpetersenii [35].
Dynamics of Leptospira infection in bat populations remains largely overlooked. Bat roosting behavior is thought to favor Leptospira transmission via urine [20]. Indeed, the reproduction and aggregation behavior of bats within their roosts have been shown to be linked to active Leptospira transmission, leading to high rates of infection in maternity colonies [20]. As demonstrated for RNA viruses, increased prevalence during seasonal bat reproduction may thus be associated with higher risk of spillover [37–40]. However, based on current research, there is very little evidence to disentangle whether bat-borne Leptospira persist within the host and/or in the environment, as well as whether they are maintained in nature by perpetuation within and between bat colonies [38]. Field monitoring of Leptospira excretion in natural bat populations suggests that bats may develop an immune response after acute infection and then stop excreting Leptospira [20]. In contrast, observation of natural Leptospira-infected bats in Denmark showed that leptospires are able to colonize the renal tubules of bats followed by continuous excretion in urine up to five months. This would indicate that chronic infection may occur in bats [31], as already characterized in chronic asymptomatic animal carriers such as rats [41].
What Is the Public Health Risk of Bat-Borne Leptospira?
Because of their abundance and spatial distribution, bats may contribute to the global maintenance and dissemination of pathogenic leptospires. However, the role of bats as carriers of strains of leptospires associated with human leptospirosis remains uncertain. Direct transmission of bat-borne Leptospira to humans has already been suggested, but never evidenced, following a case of serologically confirmed human leptospirosis after bat exposure [42]. Contact with urine and contaminated water is the main form of disease transmission. Human encroachment into bat habitats as well as increasing urbanization, which facilitates bat roosting in artificial structures, are likely to increase the opportunity for bat-borne Leptospira spillover [34]. Indeed, evidence of leptospiral infection of kidneys has already been reported in bats roosting in schools and houses [10,16].
Indirect transmission of bat-borne Leptospira to humans may also occur through spillover between bat-borne Leptospira and other animal hosts, in particular ground-dwelling species such as rodents that reside or forage under bat roosts [8,13,15]. Such transmission between bats and rodents has already been suggested, as L. interrogans, a typical rodent-borne Leptospira species, has been evidenced in insectivorous and frugivorous bats [8,16]. Elucidating the ecological conditions that may favor bat-borne Leptospira transmission thus represents a major challenge for public health.
What Are Future Directions for Research into Bat-Borne Leptospira?
The widespread pattern and enigmatic features of Leptospira infection in bats represent a challenging opportunity to study the evolutionary ecology of bat-borne infectious agents of possible importance for public health. While other studies mostly focus on viruses, the study of transmission cycles involving bats and bacterial pathogens in particular will provide an original system to understand general patterns of bat-borne pathogen epidemiology.
As a model system, continued research on the ecology of host and bacteria is necessary. It has been recently shown that Leptospira excretion in bats can be highly dynamic [20], but ecological factors that drive spatial and temporal variations of infection remain uncertain. For example, what are the roles of environmental factors such as weather seasonal patterns in the transmission dynamics of Leptospira in bat populations? Is Leptospira infection in bats maintained through epidemic episodes during the bat reproductive season in maternity colonies, or does it persist endemically within any single local population? Do males play a particular role in dispersing Leptospira among colonies compared to phylopatric females? Some of these questions can be addressed using long-term data sets by monitoring bat population dynamics, Leptospira excretion, and immune response in bat colonies. Noninvasive urine sampling should be preferred, as it allows the collection of a high number of samples while limiting the disturbance of colonies [20]. This will require the validation of urine shedding as a good proxy of renal infection, as recently demonstrated in rats [43]. Parallel investigation of rodent populations in the vicinity of bat colonies would be necessary to assess potential exchanges between these two animal hosts, as already shown for other infectious agents such as paramyxoviruses [44,45].
Improvement of bacterial culture from noninvasive bat samples (such as urine) would be a crucial step for understanding Leptospira–bat associations. First of all, it would improve genetic characterization of bat-borne strains and thus provide a more comprehensive picture of Leptospira evolution in bats. Secondly, bacterial isolates would allow experimental studies to investigate chronic manifestations in bats, as already demonstrated for rodents [4], as well as the assessment of the survival of bat-borne Leptospira in soil and water, in order to determine the role of environment as a source of infection. Animal models would further enable assessment of host specificity and virulence of bat-borne strains and the potential for possible spillovers. Finally, the development of serological diagnostic tests, designed to express a narrow specificity towards bat-borne strains, will allow us to assess the potential exposure of rodent and domestic animal populations to bat-borne Leptospira and to determine the burden of acute and asymptomatic Leptospira infection in humans from bat origin.
Zdroje
1. Adler B (2015) Leptospira and Leptospirosis. Curr Topics Microbiol 387 : 1–293.
2. Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N (2008) The globalization of leptospirosis: worldwide incidence trends. Int J Infect Dis 12 : 351–357. 18055245
3. Ganoza CA, Matthias MA, Saito M, Cespedes M, Gotuzzo E, et al. (2010) Asymptomatic renal colonization of humans in the peruvian Amazon by Leptospira. PLoS Neglect Trop 4: e612.
4. Ko AI, Goarant C, Picardeau M (2009) Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol 7 : 736–747. doi: 10.1038/nrmicro2208 19756012
5. Cerqueira GM, Picardeau M (2009) A century of Leptospira strain typing. Infect Genet Evol 9 : 760–768. doi: 10.1016/j.meegid.2009.06.009 19540362
6. Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, et al. (2003) Leptospirosis : a zoonotic disease of global importance. Lancet Infect Dis 3 : 757–771. 14652202
7. Bunnell JE, Hice CL, Watts DM, Montrueil V, Tesh RB, et al. (2000) Detection of pathogenic Leptospira spp. infections among mammals captured in the Peruvian Amazon basin region. Am J Trop Med Hyg 63 : 255–258. 11421373
8. Matthias MA, Díaz MM, Campos KJ, Calderon M, Willig MR, et al. (2005) Diversity of bat-associated Leptospira in the Peruvian Amazon inferred by bayesian phylogenetic analysis of 16S ribosomal DNA sequences. Am J Trop Med Hyg 73 : 964–974. 16282313
9. Zetun C, Hoffmann J, Silva R, Souza L, Langoni H (2009) Leptospira spp. and Toxoplasma gondii antibodies in Vampire bats (Desmodus rotundus) in Botucatu region, Brazil. J Venom Anim Toxins incl Trop Dis 15 : 546–552.
10. Bessa TAF, Spichler A, Chapola EGB, Husch AC, de Almeida MF, et al. (2010) The contribution of bats to leptospirosis transmission in Sao Paulo City, Brazil. Am J Trop Med Hyg 82 : 315–317. doi: 10.4269/ajtmh.2010.09-0227 20134010
11. Ramirez NN, Alegre EA, De Biasio MB, Bastiani CE (2014) Detecciósn de leptospiras patógenas en tejido renal de murciélagos de Corrientes, Argentina. Rev Vet 25 : 16–20.
12. Emanuel ML, Mackerras IM, Smith DJW (1964) The epidemiology of leptospirosis in North Queensland. J Hyg 62 : 451–484. 14244080
13. Smythe LD, Field HE, Barnett L, Smith CS, Sohnt MF, et al. (2002) Leptospiral antibodies in flying foxes in Australia. J Wildlife Dis 38 : 182–186.
14. Cox TE, Smythe LD, Leung LK-P (2005) Flying foxes as carriers of pathogenic Leptospira species. J Wildlife Dis 41 : 753–757.
15. Tulsiani SM, Cobbold RN, Graham GC, Dohnt MF, Burns M, et al. (2011) The role of fruit bats in the transmission of pathogenic leptospires in Australia. Ann Trop Med Parasitol 105 : 71–84. doi: 10.1179/136485911X12899838413501 21294951
16. Lagadec E, Gomard Y, Guernier V, Dietrich M, Pascalis H, et al. (2012) Pathogenic Leptospira spp. in bats, Madagascar and Union of the Comoros. Emerg Infect Dis 18 : 1696–1698. doi: 10.3201/eid1810.111898 23017768
17. Desvars A, Naze F, Benneveau A, Cardinale E, Michault A (2013) Endemicity of leptospirosis in domestic and wild animal species from Reunion Island (Indian Ocean). Epidemiol Infect 141 : 1154–1165. doi: 10.1017/S0950268812002075 22998941
18. Desvars A, Naze F, Vourc’h G, Cardinale E, Picardeau M, et al. (2012) Similarities in Leptospira serogroup and species distribution in animals and humans in the Indian ocean island of Mayotte. Am J Trop Med Hyg 87 : 134–140. doi: 10.4269/ajtmh.2012.12-0102 22764304
19. Dietrich M, Wilkinson DA, Soarimalala V, Goodman SM, Dellagi K, et al. (2014) Diversification of an emerging pathogen in a biodiversity hotspot: Leptospira in endemic small mammals of Madagascar. Mol Ecol 23 : 2783–2796. doi: 10.1111/mec.12777 24784171
20. Dietrich M, Wilkinson DA, Benlali A, Lagadec E, Ramasindrazana B, et al. (2015) Leptospira and Paramyxovirus infection dynamics in a bat maternity enlightens pathogen maintenance in wildlife. Environ Microbiol: in press.
21. Collier WA, Mochtar A (1939) Een serologisch afwijkende leptospira-stam uit der nier eener vleermuis. Gen Tschr Ned Ind 79 : 226–231.
22. Alston JM, Broom JC (1958) Leptospirosis in Man and Animals. E. & S. Livingstone. Edinburgh, UK.
23. Van Peenen PFD, Light RH, Sulianti Saroso J (1971) Leptospirosis in wild mammals of Indonesia—Recent surveys. Se Asian J Trop Med 2 : 496–502.
24. Thayaparan S, Robertson IAN, Amraan F, Ut LSU, Abdullah MT (2013) Serological Prevalence of Leptospiral Infection in Wildlife in Sarawak, Malaysia. Borneo J Res Sc Technol 2 : 71–74.
25. Mgode GF, Mbugi HA, Mhamphi GG, Ndanga D, Nkwama EL (2014) Seroprevalence of Leptospira infection in bats roosting in human settlements in Morogoro municipality in Tanzania. Tanz J Health Res 16 : 1–7.
26. Everard CR, Fraser-Chanpong GM, Bhagwandin LJ, Race MW, James AC (1983) Leptospires in wildlife from Trinidad and Grenada. J Wildlife Dis 19 : 192–199.
27. Sebek Z, Sixl W, Reinthaler F, Valova M, Scneeweiss W, et al. (1989) Results of serological examination for leptospirosis of domestic and wild animals in the Upper Nile province (Sudan). J Hyg Epid Microb Im 33 : 337–345.
28. Ogawa H, Koizumi N, Ohnuma A, Mutemwa A, Hang BM, et al. (2015) Molecular epidemiology of pathogenic Leptospira spp. in the straw-colored fruit bat (Eidolon helvum) migrating to Zambia from the Democratic Republic of Congo. Infect Genet Evol 32 : 143–147. doi: 10.1016/j.meegid.2015.03.013 25791930
29. Harkin KR, Hays M, Davis R, Moore M (2014) Use of PCR to Identify Leptospira in Kidneys of Big Brown Bats (Eptesicus fuscus) in Kansas and Nebraska, USA. J Wildlife Dis 50 : 651–654.
30. Jobbins SE, Alexander KA (2015) Evidence of Leptospira sp. infection among a diversity of African wildlife species : beyond the usual suspects. Trans R Soc Trop Med Hyg: 349–351. doi: 10.1093/trstmh/trv007 25669841
31. Fennestad KL, Borg-Petersen C (1972) Leptospirosis in danish wild mammals. J Wildlife Dis 8 : 343–351.
32. Tagi-Zade TA, Mardanly AS, Akhmedov IB, Alekperov FP, Gasanov SN (1970) Examination of bats for leptospirosis in the territory of Azerbaijan SSR. Zhurnal Mikrobiol Epidemiol i Immunobiol 9 : 118–121.
33. Lei BR, Olival KJ (2014) Contrasting patterns in mammal-bacteria coevolution: Bartonella and Leptospira in bats and rodents. PLoS Neglect Trop 8: e2738.
34. Hayman DTS, Bowen RA, Cryan PM, McCracken GF, O’Shea TJ, et al. (2013) Ecology of zoonotic infectious diseases in bats: current knowledge and future directions. Zoonoses Public Hlth 60 : 2–21.
35. Bulach DM, Zuerner RL, Wilson P, Seemann T, McGrath A, et al. (2006) Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. P Natl Acad Sci USA 103 : 14560–14565.
36. Cosson J-F, Picardeau M, Mielcarek M, Tatard C, Chaval Y, et al. (2014) Epidemiology of Leptospira Transmitted by Rodents in Southeast Asia. PLoS Neglect Trop 8: e2902.
37. Amman BR, Carroll SA, Reed ZD, Sealy TK, Balinandi S, et al. (2012) Seasonal Pulses of Marburg Virus Circulation in Juvenile Rousettus aegyptiacus Bats Coincide with Periods of Increased Risk of Human Infection. PLoS Pathog 8: e1002877. doi: 10.1371/journal.ppat.1002877 23055920
38. Plowright RK, Eby P, Hudson PJ, Smith IL, Westcott D, et al. (2015) Ecological dynamics of emerging bat virus spillover. P Roy Soc B-Biol Sci 282.
39. Field H, Crameri G, Kung NY, Wang LF (2012) Ecological aspects of hendra virus. Curr Topics Microbiol 359 : 11–23.
40. Khan SU, Islam MA, Rahman MZ, Island A, Sazzad HMS, et al. (2013) Nipah virus shedding among Pteropus bats in the context of human outbreak in Bangladesh, 2012. ASTMH 62nd Annual Meeting, 13–17 November, Washington, DC.
41. Monahan AM, Callanan JJ, Nally JE (2009) Review paper: Host-pathogen interactions in the kidney during chronic leptospirosis. Vet Pathol 46 : 792–799. doi: 10.1354/vp.08-VP-0265-N-REV 19429975
42. Vashi NA, Reddy P, Wayne DB, Sabin B (2010) Bat-associated leptospirosis. J Gen Intern Med 25 : 162–164. doi: 10.1007/s11606-009-1210-7 20012224
43. Costa F, Wunder EA, Oliveira D De, Bisht V (2015) Patterns in Leptospira Shedding in Norway Rats (Rattus norvegicus) from Brazilian Slum Communities at High Risk of Disease Transmission. PLoS Neglect Trop 9: e0003819.
44. Wilkinson DA, Mélade J, Dietrich M, Ramasindrazana B, Soarimalala V, et al. (2014) Highly Diverse Morbillivirus-Related Paramyxoviruses in Wild Fauna of the Southwestern Indian Ocean Islands: Evidence of Exchange between Introduced and Endemic Small Mammals. J Virol 88 : 8268–8277. doi: 10.1128/JVI.01211-14 24829336
45. Drexler JF, Corman VM, Müller MA, Maganga GD, Vallo P, et al. (2012) Bats host major mammalian paramyxoviruses. Nat Comm 3 : 796.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin InfectionČlánek Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Parasite Glycobiology: A Bittersweet Symphony
- On the Discovery of TOR As the Target of Rapamycin
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
- PML/TRIM19-Dependent Inhibition of Retroviral Reverse-Transcription by Daxx
- Cleavage of a Neuroinvasive Human Respiratory Virus Spike Glycoprotein by Proprotein Convertases Modulates Neurovirulence and Virus Spread within the Central Nervous System
- Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Induces the Oncogenic miR-17-92 Cluster and Down-Regulates TGF-β Signaling
- Interferon-α Subtypes in an Model of Acute HIV-1 Infection: Expression, Potency and Effector Mechanisms
- Perivascular Arrest of CD8 T Cells Is a Signature of Experimental Cerebral Malaria
- Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells
- Evolution and Emergence of Enteroviruses through Intra- and Inter-species Recombination: Plasticity and Phenotypic Impact of Modular Genetic Exchanges in the 5’ Untranslated Region
- Interferon-γ Inhibits Ebola Virus Infection
- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- P-Type Cyclin CYC3 Modulates Endomitotic Growth during Oocyst Development in Mosquitoes
- Diversity of across Evolutionary Scales
- 50 Years of Disease in Humans: The Dramatic Emergence of a Cluster of Novel Fungal Pathogens
- Worse Comes to Worst: Bananas and Panama Disease—When Plant and Pathogen Clones Meet
- Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection
- Infection-Induced Retrotransposon-Derived Noncoding RNAs Enhance Herpesviral Gene Expression via the NF-κB Pathway
- Structural Insight into Archaic and Alternative Chaperone-Usher Pathways Reveals a Novel Mechanism of Pilus Biogenesis
- Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin Infection
- Global Analysis of the Fungal Microbiome in Cystic Fibrosis Patients Reveals Loss of Function of the Transcriptional Repressor Nrg1 as a Mechanism of Pathogen Adaptation
- The Transcription and Translation Landscapes during Human Cytomegalovirus Infection Reveal Novel Host-Pathogen Interactions
- The N-terminal Helical Region of the Hepatitis C Virus p7 Ion Channel Protein Is Critical for Infectious Virus Production
- Activation of Type I and III Interferon Response by Mitochondrial and Peroxisomal MAVS and Inhibition by Hepatitis C Virus
- Hsp70 Isoforms Are Essential for the Formation of Kaposi’s Sarcoma-Associated Herpesvirus Replication and Transcription Compartments
- Distinct Upstream Role of Type I IFN Signaling in Hematopoietic Stem Cell-Derived and Epithelial Resident Cells for Concerted Recruitment of Ly-6C Monocytes and NK Cells via CCL2-CCL3 Cascade
- and Bats: Story of an Emerging Friendship
- Emergence of Pathogenicity in Lagoviruses: Evolution from Pre-existing Nonpathogenic Strains or through a Species Jump?
- Ebolavirus Evolution: Past and Present
- Host and Symbiont Jointly Control Gut Microbiota during Complete Metamorphosis
- Non-Human Primates Harbor Diverse Mammalian and Avian Astroviruses Including Those Associated with Human Infections
- Lactate Dehydrogenase Is Associated with the Parasitophorous Vacuole Membrane and Is a Potential Target for Developing Therapeutics
- Five Questions about Mycoviruses
- Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
- Ethanolamine Signaling Promotes Niche Recognition and Adaptation during Infection
- Cross-Species Transmission and Differential Fate of an Endogenous Retrovirus in Three Mammal Lineages
- Memory Th1 Cells Are Protective in Invasive Infection
- Transcription Factor SomA Is Required for Adhesion, Development and Virulence of the Human Pathogen
- An -Methyltransferase Is Required for Infection of Tick Cells by
- RNA-seq Brings New Insights to the Intra-Macrophage Transcriptome of Typhimurium
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- On the Discovery of TOR As the Target of Rapamycin
- Parasite Glycobiology: A Bittersweet Symphony
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



