-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Worse Comes to Worst: Bananas and Panama Disease—When Plant and Pathogen Clones Meet
article has not abstract
Published in the journal: . PLoS Pathog 11(11): e32767. doi:10.1371/journal.ppat.1005197
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1005197Summary
article has not abstract
Bananas: Their Origin and Global Rollout
The banana is the most popular fruit in the world and ranks among the top ten food commodities for Southeast Asia, Africa, and Latin America [1]. Notably, the crop is largely produced by small-holder farmers, with around 85% of the global production destined for local markets and only 15% entering international trade [1]. Bananas evolved in the Indo-Malayan archipelago thousands of years ago. The majority of all edible varieties developed from specific (inter - and intra-) hybridizations of two seeded diploid Musa species (M. acuminata and M. balbisiana) and subsequent selection of diploid and triploid seedless clones [2,3]. Despite rich genetic and phenotypic diversity [4], only a few clones developed, over time, into global commodities—either as dessert bananas, such as the triploid “Cavendish” clones, or as important staple foods such as cooking bananas and plantains [4,5]. Currently, bananas are widely grown in the (sub)tropics and are consumed in nearly all countries around the world, providing crucial nutrition for millions of people. Edible bananas reproduce asexually through rhizomes, but since the early 1970s, tissue culture has enabled mass production of cultivars [6]. This facilitates the rapid rollout of genetically identical plants, which have consumer-preferred traits and outstanding agronomical performance, onto vast acreages around the world. However, the typical vulnerability of monocultures to diseases has taken its toll on banana production over the last century. In 1876, a wilting disease of banana was reported in Australia [7], and in 1890, it was observed in the “Gros Michel” plantation crops of Costa Rica and Panama [8,9]. There it developed major epidemics in the 1900s that are among the worst in agricultural history [10], linking its most prone geographical area to its colloquial name: Panama disease. It was only in 1910 that the soil-borne fungus Fusarium oxysporum f.sp. cubense (Foc) was identified as the causal agent in Cuba, from which the name of the forma specialis was derived [10].
Genetic Diversity of Fusarium oxysporum f.sp. cubense, the Causal Agent of Panama Disease
Foc belongs to the F. oxysporum species complex: a suite of asexual, morphologically similar, pathogenic and non-pathogenic strains affecting a wide variety of crops [11]. Foc likely co-evolved with its host species Musa in its center of origin [12–15]. Traditionally, phenotyping has identified three Foc races (1, 2, and 4) that cause disease in different subsets of banana and plantain cultivars [5,8]. However, Foc race designations are cumbersome and hence other methods unveiling genetic diversity were developed. Vegetative compatibility group (VCG) analyses largely divide Foc into 24 unique VCGs (VCG0120 through VCG0126 and VCG0128 through VCG01224) [5,13,16]. Later, DNA markers revealed the polyphyletic origin of Foc, as some VCGs are taxonomically closer to other F. oxysporum formae speciales than to other Foc VCGs [12,14,17]. Moreover, strains belonging to diverse VCGs infect particular banana cultivars and, hence, were grouped in the same race, suggesting that pathogenicity towards a specific cultivar evolved either convergently [5,12,14] or resulted from horizontal gene transfer among members of the F. oxysporum complex [18]. Overall, Foc lineages show a remarkable dichotomy, referred to as types or clades [12–14,19–22]. High-resolution genotyping-by-sequencing analyses using DArTseq—which generates short sequence reads after a genome-wide complexity reduction through restriction enzyme digestion [23]—validate and extend these findings (Fig 1). Based on genome-wide DArTseq markers, 24 Foc strains (representing all hitherto known VCGs) split into two groups. These largely corroborate the aforementioned clades, except for VCG0123 [13,14,20,22], VCG01210 [19], VCG01212 [20], and VCG01214 [21], which were occasionally reported in opposite clades, and VCGs 01221 to 01224, which were never classified before but now clearly belong to clade 2 (Fig 1).
Fig. 1. Genetic diversity of the banana pathogen F. oxysporum f. sp. cubense. 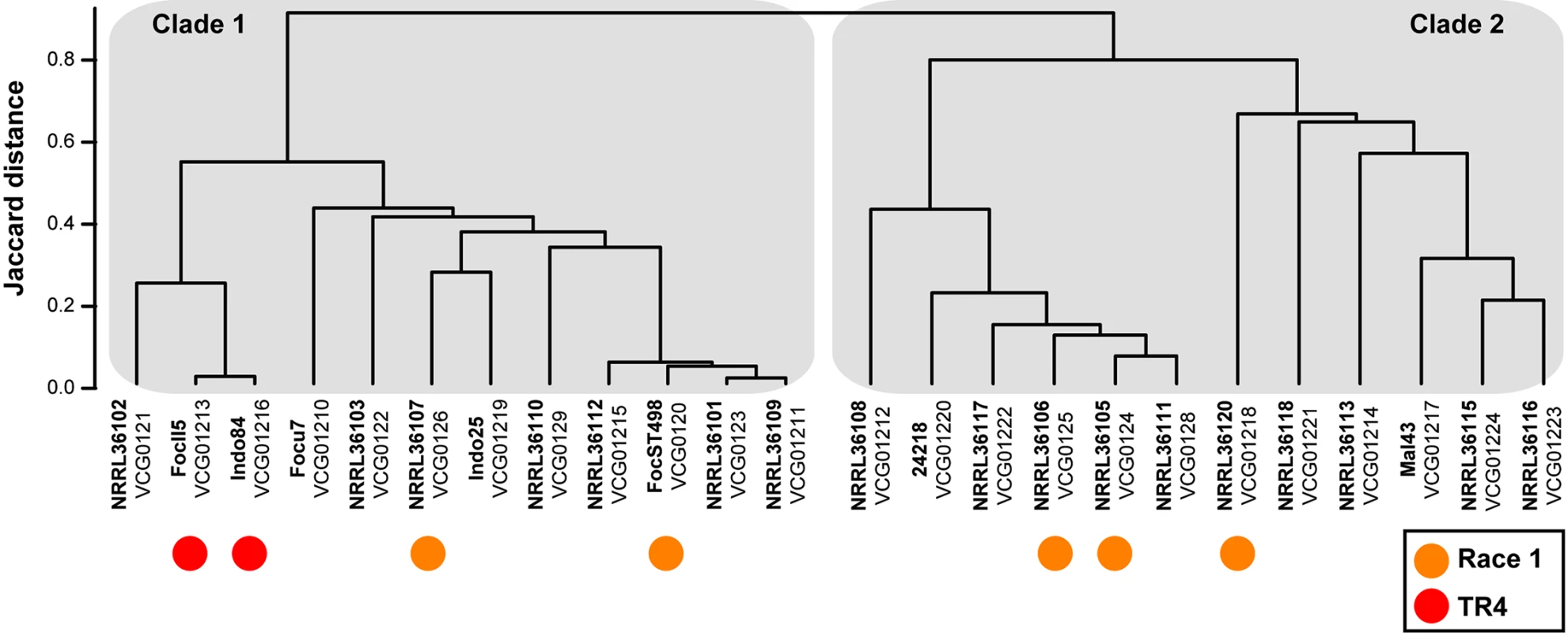
Genotyping-by-sequencing analyses of the hitherto identified 24 vegetative compatibility groups (VCG) in F. oxysporum f. sp. cubense resulted in 12,978 DArTseq markers that divide Foc into two distinct clades—clade 1 and clade 2. VCG01216 is considered the same as VCG01213 [13]. The labels for race 1 isolates are based on personal communications with I. Buddenhagen and M. Dita. Although VCG01213 contains all TR4 isolates that cause the current Panama disease epidemic in Cavendish bananas, VCG0120—which has also been considered as race 4 [5]—and VCG0124 [36] have also been recovered from symptomatic Cavendish plants. Unfortunately, it is not well known which VCGs (the so-called Foc race 1 strains) caused the Panama disease epidemic in “Gros Michel” and, hence, their geographical dissemination is still unclear (I. Buddenhagen and M. Dita, personal communications). The current epidemic in Cavendish bananas, however, is caused by VCG01213 [5], colloquially called Tropical Race 4 (TR4).
Panama Disease: History Repeats Itself
Large railway projects in Central America in the late 1800s facilitated industrial banana production and trade [10], which was entirely based on “Gros Michel” bananas [8]. The unparalleled vulnerability of “Gros Michel” to race 1 strains drove aggressive land-claiming policies in order to continue banana production. However, this did not stop the epidemic as Panama disease was easily entering these new areas through infected planting material. Hence, by the 1960s, the epidemic reached a tipping point with the total collapse of “Gros Michel” [9]. Fortunately, there was a remedy: Cavendish bananas—maintained as interesting specimens in botanical gardens in the United Kingdom and in the United Fruit Company collection in Honduras—were identified as resistant substitutes for “Gros Michel.” A new clone was “born” that, along with the new tissue culture techniques, helped save and globalize banana production [5,8,9].
However, in the late 1960s, Panama disease emerged in Cavendish bananas in Taiwan, but TR4 was only identified as its cause in 1994 [9,24,25]. Surprisingly, this initial outbreak did not awaken the banana industry and awareness levels remained low, despite the lack of any Cavendish replacement that met market demands and the susceptibility of many local banana cultivars to TR4 [5] (see also http://panamadisease.org/en/news/26). Thus, TR4 threatens not only the export trade but also regional food provision and local economies.
Tropical Race 4, a Single Pathogen Clone, Threatens Global Banana Production
Ever since TR4 destroyed the Cavendish-based banana industry in Taiwan, its trail in Southeast Asia seems unstoppable with incursions and expansions in the Chinese provinces of Guangdong, Fujian, Guangxi, and Yunnan as well as on the island of Hainan. Since the 1990s, TR4 has also wiped out Cavendish plantations in Indonesia and Malaysia; between 1997 and 1999, it significantly reduced the banana industry near Darwin in the Northern Territory of Australia. It was first observed in the early 2000s in a newly planted Cavendish banana farm in Davao (on island of Mindanao, Philippines), where it currently threatens the entire banana export trade [26]. Since 2013, incursions outside Southeast Asia were reported in Jordan [27], Pakistan, and Lebanon [28], informally announced in Mozambique and Oman, and just recently noted in the Tully region of Northern Queensland, Australia. By now, TR4 may have affected up to approximately 100,000 hectares, and it is likely that it will disseminate further—either through infected plant material, contaminated soil, tools, or footwear, or due to flooding and inappropriate sanitation measures [5,29]. Clearly, the current expansion of the Panama disease epidemic is particularly destructive due to the massive monoculture of susceptible Cavendish bananas.
Foc is a haploid asexual pathogen [8] and is therefore expected to have a predominantly clonal population structure [13,14,19–22]. Comparison of re-sequencing data of TR4 isolates from Jordan, Lebanon, Pakistan, and the Philippines—with the publicly available reference genome sequence of Foc TR4 strain II-5 (http://www.broadinstitute.org/)—indeed shows a very low level of single nucleotide polymorphisms (SNPs) (about 0.01%). This, together with a highly similar set of DArTseq markers, suggests that the temporal and spatial dispersal of TR4 is due to a single clone (Fig 2). This finding underscores the need for global awareness and quarantine campaigns in order to protect banana production from another pandemic that particularly affects vulnerable, small-holder farmers.
Fig. 2. Phylogeography of F. oxysporum f. sp. cubense Tropical Race 4 (TR4). 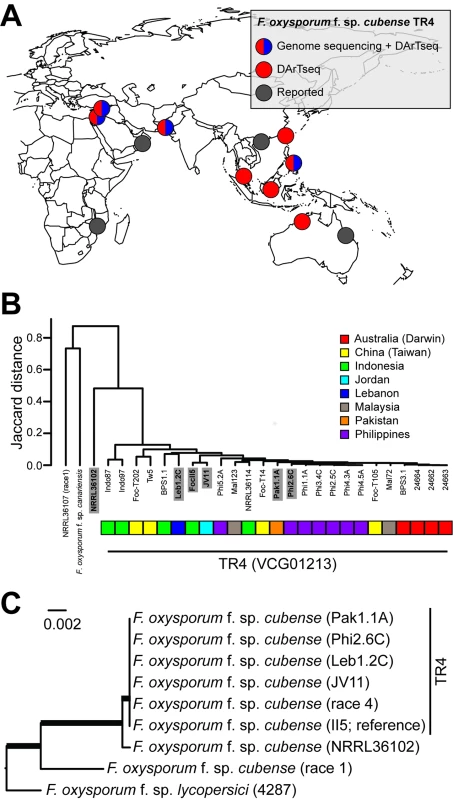
(A) Geographical locations of proclaimed TR4 incursions in Southeast Asia, Australia, Africa, the Middle East, and the Indian subcontinent. Different colors indicate if and how the genetic diversity of collected isolates was assessed. (B) Limited genetic diversity between multiple Foc TR4 isolates from distinct geographical locations revealed by hierarchical clustering, based on 4,298 DArTseq markers. Countries of origin for each of the TR4 isolates are indicated by different colors. (C) Phylogenetic analysis of selected Foc TR4 isolates (highlighted in bold in panel B) and related F. oxysporum species, based on whole-genome re-sequencing data. Phylogenetic tree analysis was performed using REALPHY [37], applying the PhyML algorithm for tree constructing (Foc II5 reference genome). The F. oxysporum f.sp. lycopersici and the F. oxysporum f. sp. cubense II5 genomes, as well as Foc race 4 and race 1 genomes, are publicly available at GenBank (http://www.ncbi.nlm.nih.gov/genome/genomes/707). Robustness of the grouping was assessed by 500 bootstrap replicates, and thick branches indicate maximum support. Strategies for Sustainable Panama Disease Management
Any disease management eventually fails in a highly susceptible monoculture. Managing Panama disease with its soil-borne nature, long latency period, and persistence once established is, therefore, impossible without drastic strategy changes. Evidently, exclusion is the primary measure to protect banana production, which requires accurate diagnosis based not only on visual inspection, as this overlooks important aspects of its genetic diversity and epidemiology. New molecular-based diagnostics rapidly detect TR4 in (pre)symptomatic plants [30], soil, and water and, hence, can be used for surveillance and containment, which are key to avoiding an encounter of TR4 with Cavendish monocultures. Additionally, a thorough understanding of Foc epidemiology and pathology is urgently required, as this facilitates developing effective methods to destroy infected plants and (biological) soil treatments, thus reducing the inoculum quantity. Furthermore, we showed that high-throughput genome analyses unveil Foc population diversity (Figs 1 and 2), rather than lengthy and cumbersome VCG analyses, which enables resistance deployment strategies. Finally, effective disease management cannot be achieved without adequate disease resistance levels. “Cavendish”-based somaclones [31] do not satisfy local or international industry demands (apart from the epidemiological risks), as this germplasm is, at most, only partially resistant to TR4 [32]. Instead, the substantial genetic diversity for TR4 resistance in (wild) banana germplasm, such as accessions of Musa acuminata ssp. malaccensis [4], can be exploited in breeding programs and/or along with various transformation techniques [33–35] to develop a new generation of banana cultivars in conformity with consumer preferences. Developing new banana cultivars, however, requires major investments in research and development and the recognition of the banana as a global staple and cash crop (rather than an orphan crop) that supports the livelihoods of millions of small-holder farmers. Until new, commercially viable, and resistant banana cultivars reach markets, any potential disease management option needs to be scrutinized, thereby lengthening the commercial lifespan of contemporary banana accessions. The current TR4 epidemic and inherent global attention should be the wake-up call for these much needed strategy changes.
Supporting Information
Zdroje
1. FAOSTAT (2013) FAO statistical database. http://faostat3.fao.org/home/E.
2. Simmonds NW, Shepherd K (1955) Taxonomy and origins of cultivated bananas. J Linn Soc Bot 55 : 302–312.
3. Perrier X, De Langhe E, Donohue M, Lentfer C, Vrydaghs L, et al. (2011) Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc Natl Acad Sci U S A 108 : 11311–11318. doi: 10.1073/pnas.1102001108 21730145
4. D'Hont A, Denoeud F, Aury JM, Baurens FC, Carreel F, et al. (2012) The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 488 : 213–217. doi: 10.1038/nature11241 22801500
5. Ploetz RC (2006) Panama disease: An old nemesis rears its ugly head. Part2. The Cavendish era and beyond. Plant Health Progress. St. Paul USA: Plant Management Network.
6. Gowen S, Israeli Y, Lahav E, Reuveni O (1995) In vitro culture of bananas. Bananas and Plantains: Springer Netherlands. pp. 147–178.
7. Bancroft J (1876) Report of the board appointed to enquire into the cause of disease affecting livestock and plants. Votes and Proceedings 1877 3 : 1011–1038.
8. Stover RH (1962) Fusarial wilt (Panama disease) of bananas and other Musa species. UK: Commonwealth Mycological Institute 117 p.
9. Ploetz RC (1994) Panama disease: Return of the first banana menace. International Journal of Pest Management 40 : 326–336.
10. Ploetz RC (2005) Panama Disease: An old nemesis rears its ugly head. Part1: The beginnings of the banana export trades. Plant Health Progress. St. Paul USA: Plant Management Network.
11. O'Donnell K, Gueidan C, Sink S, Johnston PR, Crous PW, et al. (2009) A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet Biol 46 : 936–948. doi: 10.1016/j.fgb.2009.08.006 19715767
12. O'Donnell K, Kistler HC, Cigelnik E, Ploetz R (1998) Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc Natl Acad Sci U S A 95 : 2044–2049. 9482835
13. Bentley S, Pegg KG, Moore NY, Davis RD, Buddenhagen I (1998) Genetic variation among vegetative compatibility groups of Fusarium oxysporum f.sp. cubense analyzed by DNA fingerprinting. Phytopathology 88 : 1283–1293. doi: 10.1094/PHYTO.1998.88.12.1283 18944830
14. Fourie G, Steenkamp ET, Gordon TR, Viljoen A (2009) Evolutionary relationships among the Fusarium oxysporum f. sp. cubense vegetative compatibility groups. Appl Environ Microbiol 75 : 4770–4781. doi: 10.1128/AEM.00370-09 19482953
15. Ploetz R, Pegg K (1997) Fusarium wilt of banana and Wallace's line: Was the disease originally restricted to his Indo-Malayan region? Australas Plant Path 26 : 239–249.
16. Kistler HC, Alabouvette C, Baayen RP, Bentley S, Brayford D, et al. (1998) Systematic numbering of vegetative compatibility groups in the plant pathogenic fungus Fusarium oxysporum. Phytopathology 88 : 30–32. doi: 10.1094/PHYTO.1998.88.1.30 18944995
17. Baayen RP, O'Donnell K, Bonants PJM, Cigelnik E, Kroon LPNM, et al. (2000) Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing wilt and rot disease. Phytopathology 90 : 891–900. doi: 10.1094/PHYTO.2000.90.8.891 18944511
18. Ma LJ, van der Does HC, Borkovich KA, Coleman JJ, Daboussi MJ, et al. (2010) Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 464 : 367–373. doi: 10.1038/nature08850 20237561
19. Boehm EWA, Ploetz R, Kistler HC (1994) Statistical analysis of electrophoretic karyotype variation among vegetative compatibility groups of Fusarium oxysporum f.sp. cubense. MPMI 7 : 196–207.
20. Bentley S, Pegg KG, Dale JL (1995) Genetic variation among a world-wide collection of isolates of Fusarium oxysporum f.sp. cubense analysed by RAPD-PCR fingerprinting. Mycol Res 99 : 1378–1384.
21. Koenig RL, Ploetz R, Kistler HC (1997) Fusarium oxysporum f.sp. cubense consists of a small number of divergent and globally distributed clonal lineages. Phytopathology 87 : 915–923. doi: 10.1094/PHYTO.1997.87.9.915 18945062
22. Groenewald S, Van Den Berg N, Marasas WF, Viljoen A (2006) The application of high-throughput AFLP's in assessing genetic diversity in Fusarium oxysporum f. sp. cubense. Mycol Res 110 : 297–305. 16483757
23. Cruz VM, Kilian A, Dierig DA (2013) Development of DArT marker platforms and genetic diversity assessment of the U.S. collection of the new oilseed crop lesquerella and related species. PLoS One 8 : 1–13.
24. Buddenhagen I (2009) Understanding strain diversity in Fusarium oxysporum f.sp. cubense and history of introduction of Tropical Race 4 to better manage banana production. Proc. IS on Banana Crop Prot., Sust. Prod. & Impr. Livelihoods. Eds.: Jones D. and van den Bergh I.. Acta Hort. 828, ISHS 2009.
25. Pegg KG, Moore NY, Sorensen S. Fusarium wilt in the Asian Pacific region. In: Valmayor R.V. SCH, Ploetz R.C., Lee S.W. and Roa V.N., editor; 1993 14–18 December, 1992; Pingtun, Taiwan (Los Banos, Laguna, Philippines: TBRI, ASPNET, INIBAP),. pp. 314 pp.
26. Molina AB, Fabregar E, Sinohin VG, Yi G, Viljoen A (2009) Recent occurrence of Fusarium oxysporum f.sp. cubense tropical race 4 in Asia. Acta Hort 828 : 109–115.
27. García-Bastidas F, Ordóñez N, Konkol J, Al-Qasim M, Naser Z, et al. (2013) First report of Fusarium oxysporum f. sp. cubense Tropical Race 4 associated with Panama Disease of banana outside Southeast Asia. Plant Dis 98 : 694–694.
28. Ordonez N, Garcia FA, Laghari H, Akkary M, Harfouche EN, et al. (2015) First report of Fusarium oxysporum f. sp. cubense tropical race 4 causing Panama disease in Cavendish bananas in Pakistan and Lebanon. Plant Dis 99 : 1448.
29. Ploetz RC, Pegg KG (2000) Fusarium wilt. In: Diseases of Banana, Abaca and Enset.; Jones DR, editor. Wallingford, UK: CABI Publishing.
30. Dita MA, Waalwijk C, Buddenhagen IW, Souza MT Jr, Kema GHJ (2010) A molecular diagnostic for tropical race 4 of the banana fusarium wilt pathogen. Plant Pathol 59 : 348–357.
31. Hwang SC, Ko WH (2004) Cavendish banana cultivars resistant to Fusarium wilt acquired through somaclonal variation in Taiwan somaclonal variation in Taiwan. Plant Dis 88 : 580–588.
32. Ploetz RC (2015) Management of Fusarium wilt of banana: A review with special reference to tropical race 4. Crop Prot 73 : 7–15.
33. Ghag SB, Shekhawat UK, Ganapathi TR (2014) Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnol J 12 : 541–553. doi: 10.1111/pbi.12158 24476152
34. Paul JY, Becker DK, Dickman MB, Harding RM, Khanna HK, et al. (2011) Apoptosis-related genes confer resistance to Fusarium wilt in transgenic 'Lady Finger' bananas. Plant Biotechnol J 9 : 1141–1148. doi: 10.1111/j.1467-7652.2011.00639.x 21819535
35. Ghag SB, Shekhawat UK, Ganapathi TR (2014) Native cell-death genes as candidates for developing wilt resistance in transgenic banana plants. AoB Plants 6: plu1037.
36. Thangavelu R, Mustaffa MM (2010) First report on the occurrence of a virulent strain of Fusarium wilt pathogen (Race-1) infecting Cavendish (AAA) group of Bananas in India. Plant Dis 94 : 1379–1379.
37. Bertels F, Silander OK, Pachkov M, Rainey PB, van Nimwegen E (2014) Automated reconstruction of whole-genome phylogenies from short-sequence reads. Mol Biol Evol 31 : 1077–1088. doi: 10.1093/molbev/msu088 24600054
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin InfectionČlánek Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Parasite Glycobiology: A Bittersweet Symphony
- On the Discovery of TOR As the Target of Rapamycin
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
- PML/TRIM19-Dependent Inhibition of Retroviral Reverse-Transcription by Daxx
- Cleavage of a Neuroinvasive Human Respiratory Virus Spike Glycoprotein by Proprotein Convertases Modulates Neurovirulence and Virus Spread within the Central Nervous System
- Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) Induces the Oncogenic miR-17-92 Cluster and Down-Regulates TGF-β Signaling
- Interferon-α Subtypes in an Model of Acute HIV-1 Infection: Expression, Potency and Effector Mechanisms
- Perivascular Arrest of CD8 T Cells Is a Signature of Experimental Cerebral Malaria
- Targeting HIV Reservoir in Infected CD4 T Cells by Dual-Affinity Re-targeting Molecules (DARTs) that Bind HIV Envelope and Recruit Cytotoxic T Cells
- Evolution and Emergence of Enteroviruses through Intra- and Inter-species Recombination: Plasticity and Phenotypic Impact of Modular Genetic Exchanges in the 5’ Untranslated Region
- Interferon-γ Inhibits Ebola Virus Infection
- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- P-Type Cyclin CYC3 Modulates Endomitotic Growth during Oocyst Development in Mosquitoes
- Diversity of across Evolutionary Scales
- 50 Years of Disease in Humans: The Dramatic Emergence of a Cluster of Novel Fungal Pathogens
- Worse Comes to Worst: Bananas and Panama Disease—When Plant and Pathogen Clones Meet
- Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection
- Infection-Induced Retrotransposon-Derived Noncoding RNAs Enhance Herpesviral Gene Expression via the NF-κB Pathway
- Structural Insight into Archaic and Alternative Chaperone-Usher Pathways Reveals a Novel Mechanism of Pilus Biogenesis
- Increased Susceptibility of Humanized NSG Mice to Panton-Valentine Leukocidin and Skin Infection
- Global Analysis of the Fungal Microbiome in Cystic Fibrosis Patients Reveals Loss of Function of the Transcriptional Repressor Nrg1 as a Mechanism of Pathogen Adaptation
- The Transcription and Translation Landscapes during Human Cytomegalovirus Infection Reveal Novel Host-Pathogen Interactions
- The N-terminal Helical Region of the Hepatitis C Virus p7 Ion Channel Protein Is Critical for Infectious Virus Production
- Activation of Type I and III Interferon Response by Mitochondrial and Peroxisomal MAVS and Inhibition by Hepatitis C Virus
- Hsp70 Isoforms Are Essential for the Formation of Kaposi’s Sarcoma-Associated Herpesvirus Replication and Transcription Compartments
- Distinct Upstream Role of Type I IFN Signaling in Hematopoietic Stem Cell-Derived and Epithelial Resident Cells for Concerted Recruitment of Ly-6C Monocytes and NK Cells via CCL2-CCL3 Cascade
- and Bats: Story of an Emerging Friendship
- Emergence of Pathogenicity in Lagoviruses: Evolution from Pre-existing Nonpathogenic Strains or through a Species Jump?
- Ebolavirus Evolution: Past and Present
- Host and Symbiont Jointly Control Gut Microbiota during Complete Metamorphosis
- Non-Human Primates Harbor Diverse Mammalian and Avian Astroviruses Including Those Associated with Human Infections
- Lactate Dehydrogenase Is Associated with the Parasitophorous Vacuole Membrane and Is a Potential Target for Developing Therapeutics
- Five Questions about Mycoviruses
- Phosphorylation of a Myosin Motor by TgCDPK3 Facilitates Rapid Initiation of Motility during egress
- Ethanolamine Signaling Promotes Niche Recognition and Adaptation during Infection
- Cross-Species Transmission and Differential Fate of an Endogenous Retrovirus in Three Mammal Lineages
- Memory Th1 Cells Are Protective in Invasive Infection
- Transcription Factor SomA Is Required for Adhesion, Development and Virulence of the Human Pathogen
- An -Methyltransferase Is Required for Infection of Tick Cells by
- RNA-seq Brings New Insights to the Intra-Macrophage Transcriptome of Typhimurium
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins
- On the Discovery of TOR As the Target of Rapamycin
- Parasite Glycobiology: A Bittersweet Symphony
- Broadening of Virus-Specific CD8 T-Cell Responses Is Indicative of Residual Viral Replication in Aviremic SIV Controllers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



