-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Role of the P2X Receptor in Infectious Diseases
ATP is an extracellular signal for the immune system, particularly during an inflammatory response. It is sensed by the P2X7 receptor, the expression of which is upregulated by pro-inflammatory cytokines. Activation of the P2X7 receptor opens a cation-specific channel that alters the ionic environment of the cell, activating several pathways, including (i) the inflammasome, leading to production of IL-1β and IL-18; (ii) the stress-activated protein kinase pathway, resulting in apoptosis; (iii) the mitogen-activated protein kinase pathway, leading to generation of reactive oxygen and nitrogen intermediates; and (iv) phospholipase D, stimulating phagosome-lysosome fusion. The P2X7 receptor can initiate host mechanisms to remove pathogens, most particularly those that parasitise macrophages. At the same time, the P2X7 receptor may be subverted by pathogens to modulate host responses. Moreover, recent genetic studies have demonstrated significant associations between susceptibility or resistance to parasites and bacteria, and loss-of-function or gain-of-function polymorphisms in the P2X7 receptor, underscoring its importance in infectious disease.
Published in the journal: . PLoS Pathog 7(11): e32767. doi:10.1371/journal.ppat.1002212
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1002212Summary
ATP is an extracellular signal for the immune system, particularly during an inflammatory response. It is sensed by the P2X7 receptor, the expression of which is upregulated by pro-inflammatory cytokines. Activation of the P2X7 receptor opens a cation-specific channel that alters the ionic environment of the cell, activating several pathways, including (i) the inflammasome, leading to production of IL-1β and IL-18; (ii) the stress-activated protein kinase pathway, resulting in apoptosis; (iii) the mitogen-activated protein kinase pathway, leading to generation of reactive oxygen and nitrogen intermediates; and (iv) phospholipase D, stimulating phagosome-lysosome fusion. The P2X7 receptor can initiate host mechanisms to remove pathogens, most particularly those that parasitise macrophages. At the same time, the P2X7 receptor may be subverted by pathogens to modulate host responses. Moreover, recent genetic studies have demonstrated significant associations between susceptibility or resistance to parasites and bacteria, and loss-of-function or gain-of-function polymorphisms in the P2X7 receptor, underscoring its importance in infectious disease.
Introduction
In addition to its role in cellular metabolism, the purine nucleotide ATP acts as an important extracellular messenger in a range of physiological processes, including synaptic transmissions, taste, bone formation/resorption, male fertility, blood pressure regulation, and inflammation [1]–[3]. Its effects are mediated through activation of purinergic receptors such as the P1 adenosine and the P2 nucleotide receptors [4]. Purinergic receptors are found on all types of cells in mammalian tissues, with many cells expressing multiple P1 and P2 subtypes [5].
P2 receptors are classified into two subfamilies—the P2X ligand-gated ion channels and the P2Y G protein–coupled receptors [4]. To date, seven P2X subunits (P2X1–P2X7) and eight P2Y subunits (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14) have been identified [5]. P2X receptors are fast acting and open a cation-selective channel within milliseconds of ATP binding, whereas P2Y receptors are slower acting because their activation proceeds through second-messenger pathways, in the form of G proteins. Most P2X receptors have a low affinity for ATP, usually within the µM–mM range, while P2Y receptors are responsive to nM concentrations of ATP [6].
The P2X Family
There are seven mammalian P2X subunits that assemble as homo - or heterotrimers to form functional receptors [7]. Each subunit consists of intracellular N and C termini and two membrane-spanning segments, separated by an extracellular loop containing ten conserved cysteine residues, thought to form disulfide bonds, and lysine and phenylalanine residues involved in activation by ATP [7]. The C termini of the various P2X subunits vary in length from 25 amino acids in the P2X6 receptor to 240 amino acids in the P2X7 receptor and are associated with the functional properties specific to each receptor [2], [8]. Each subunit is able to bind a molecule of ATP although the sensitivity to ATP binding varies widely within the family, with the P2X1 receptor requiring nM levels for activation, whereas the P2X7 receptor requires mM concentrations [3]. Brief exposure of all P2X receptors to ATP opens up a channel that renders the cell permeable to Na+, K+, and Ca2+, causing an increase in intracellular Ca2+ and Na+ concentrations and a decrease in intracellular K+. This leads to depolarisation of the cell membrane and initiation of downstream Ca2+ signalling pathways [3]. Prolonged exposure of the P2X1 and P2X3 receptors to ATP results in desensitisation and closure of the pore; however, prolonged exposure of the P2X2, P2X4, P2X5, and P2X7 receptors to ATP results in sustained membrane depolarisation and the opening of large transmembrane pores [2] that are permeable to hydrophilic molecules between 314 Da and 900 Da, depending on the cell type studied [9], [10].
The P2X7 Receptor
The P2X7 receptor is highly expressed by cells of the haemopoietic lineage and can mediate cell death, killing of infectious organisms, and regulation of the inflammatory response [7], [11], [12]. The receptor is constitutively expressed. Under normal physiological conditions, activity of the receptor is kept at a low level by the extracellular concentration of divalent cations such as Ca2+ and Mg2+, which appear to alter the affinity of ATP binding in an allosteric manner, and this is believed to prevent unnecessary cell permeability and pore formation [7], [13]. Under pathophysiological conditions, for example at sites of inflammation or infection, expression is up-regulated by inflammatory cytokines [14]. Extracellular concentrations of Ca2+ and Mg2+ decrease as a consequence of dead and damaged cells releasing their cytosolic contents. This “dilution”, combined with an increase in ATP (also released from lysing cells), enhances P2X7 receptor activation [7], [13].
The alteration in the ionic environment of the cell, resulting from P2X7 receptor activation, triggers a number of cellular pathways, depending on cell type (Figure 1), including: (i) the inflammasome, leading to production of IL-1β and IL-18 [15]–[17]; (ii) the stress-activated protein kinase pathway, resulting in apoptosis [18]; (iii) the mitogen-activated protein kinase pathway, leading to generation of reactive oxygen and nitrogen intermediates [11], [19]–[21]; and (iv) phospholipase D, stimulating phagosome–lysosome fusion [22]. The involvement of the P2X7 receptor in these pathways suggests that it functions as a major regulator of inflammation. Indeed, absence of the P2X7 receptor alters immune cell function with the effect seen dependent on context. For example, in a murine model of arthritis, P2X7 receptor–deficient animals showed a reduction in the incidence and severity of the disease compared with wild-type animals [23]; however, in a study of autoimmune encephalomyelitis, P2X7 receptor–deficient animals had exacerbated neuroinflammation compared with wild-type animals [24]. Moreover, a number of change-of-function polymorphisms, most of which render the receptor inactive or with reduced function, have also been noted in the human population (Table 1) [25]–[32], and genetic association studies have uncovered links between some of these polymorphisms and resistance/susceptibility to mood disorders, bone diseases, and, most particularly, infectious disease [26]. It should be noted, however, that immune cells also express P2Y receptors, such as P2Y2, which are also activated by inflammatory cytokines and, so, may also contribute to the regulation of inflammation [5].
Fig. 1. Intracellular pathways in immune cells stimulated by P2X7 receptor activation. 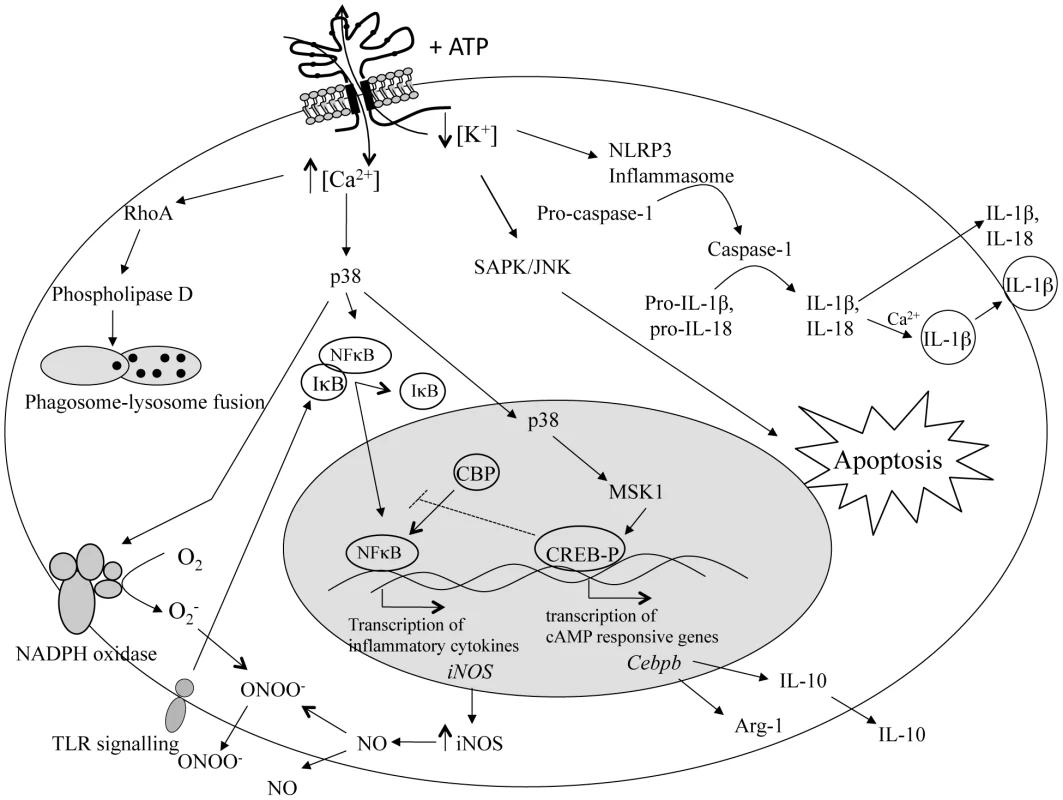
Activation of the P2X7 receptor with extracellular ATP opens a cation-specific ion channel that results in the influx of Ca2+ and Na+ and the efflux of K+. Prolonged exposure to ATP creates a pore in the cell membrane that further increases the intracellular Ca2+ concentration as well as allowing passage of larger molecules. This alteration in the ionic environment of the cell triggers a number of cellular pathways. Efflux of K+ stimulates the formation of the inflammasome, resulting in the activation of caspase-1. Caspase-1 then cleaves pro-IL-1β and pro-IL-18 to produce IL-1β and IL-18, which are then secreted from the cell as part of the inflammatory response. The efflux of K+ and influx of Na+ also activates the stress-activated protein kinase (SAPK)/c-Jun N-terminal kinases (JNK) pathway, resulting in the induction of apoptosis. The influx of Ca2+ activates phospholipase D via RhoA, leading to phagosome/lysosome fusion and the killing of intracellular pathogens. Influx of Ca2+ can also activate the mitogen-activated protein kinase p38, stimulating a number of downstream effects. Phosphorylation of p38 leads to the assembly of NADPH oxidase at the plasma membrane, and the subsequent production of superoxide (O2−), enhances nuclear factor kappa B (NFκB) activation via toll-like receptor (TLR) signalling and the subsequent transcription of inducible nitric oxide synthase (iNOS) and production of nitric oxide (NO), as well as production of tumour necrosis factor (TNF) and IL-6. It can also lead to the phosphorylation of cAMP response elements binding protein (CREB) via mitogen- and stress-activated kinase 1 (MSK1). Phosphorylated CREB (CREB-P) sequesters CREB binding protein (CBP), a co-transcription factor required for NFκB-mediated gene transcription, and inhibits transcription of NFκB-controlled genes. Phosphorylated CREB/CBP also stimulates the production of cAMP-responsive genes such as Cebpb that act to modulate the inflammatory response through the production of arginase-1 (Arg-1) and IL-10. Tab. 1. Single nucleotide polymorphisms identified in the human P2X<sub>7</sub> receptor. 
The P2X7 Receptor and Intracellular Bacteria
Mycobacteria, such as Mycobacterium tuberculosis, the causative agent of human tuberculosis, are able to survive and replicate in phagosomes within macrophages by inhibiting phagosomal fusion with lysosomes [33]. Treatment of infected macrophages with ATP, however, can overcome the phagosome–lysosome fusion block, leading to the killing of intracellular bacilli [34]. The process appears to be mediated by P2X7 receptors; bactericidal activity is markedly reduced in P2X7 receptor–deficient macrophages [34]. It involves activation of phospholipase D; thus, phospholipase D blockers inhibit killing of intracellular mycobacteria following ATP treatment [34]. Blocking macrophage phospholipase D activity, however, does not inhibit macrophage apoptotic death, demonstrating that, while ATP stimulation leads to macrophage apoptosis and mycobacterial death, these processes can be uncoupled [34]. More recently, autophagy has been shown to have a role in the control of mycobacterial infections. ATP treatment rapidly induces autophagy and mycobacterial killing in a process dependent on P2X7 receptor activation and Ca2+ influx [35].
Evidence for P2X7 receptor involvement in mycobacterial killing comes from studies showing that loss-of-function polymorphisms in the human P2X7 receptor gene lead to increased susceptibility to M. tuberculosis. Fernando et al. [36] investigated the prevalence of the 1513 A>C polymorphism in two independent Southeast Asian cohorts, and found a strong association with the 1513 A>C polymorphism and extrapulmonary tuberculosis. Subsequent studies have also found that the 1513C allele is a risk factor in the development of extrapulmonary and pulmonary tuberculosis in numerous ethnic populations, including Mexican [37], Russian Slavic [38], and a North Indian Punjabi population [39]. Recently, an association with the 1513C allele and the development of extrapulmonary tuberculosis in Turkish children was also identified [40]. Additional studies have shown associations between tuberculosis and alleles other than 1513C. Li et al. [41] studied various P2X7 receptor polymorphisms in a Gambian population. They found that a protective effect against tuberculosis was associated with a P2X7 receptor promoter polymorphism at position −762, but the 1513 A>C polymorphism discussed above did not show any significant association. Interestingly, Sambasivan et al. [42] found an association between development of clinical tuberculosis and the presence of the −762C or 1729T allele but not the 1513C allele in an Indian cohort, while Xiao et al. [43] did not find an association between either the 1513C allele or the −762C allele and the development of pulmonary tuberculosis in a Chinese Han cohort. Overall, though, a recent meta-analysis of P2X7 receptor gene polymorphism association studies revealed a strong association between the 1513 A>C polymorphism and susceptibility to tuberculosis [44].
Data from in vitro studies provide evidence for potential mechanistic explanations for the association of polymorphisms in the human P2X7 receptor gene with susceptibility to tuberculosis. Saunders et al. [45] activated P2X7 receptors on BCG-infected macrophages from wild-type and homozygous 1513C donors and showed that P2X7 receptor–activated wild-type macrophages undergo apoptosis and kill intracellular bacteria; however, macrophages homozygous for the 1513 A>C loss-of-function polymorphism fail to undergo apoptosis upon exposure to ATP, resulting in mycobacterial survival. The effect of other P2X7 receptor polymorphisms was further assessed by Fernando et al. [46] and Shemon et al. [25], who showed that several P2X7 receptor polymorphisms (946 G>A, 1729 T>A, and 155+1 g>t) result in reduced macrophage apoptosis and mycobacterial killing. This effect was augmented in compound heterozygous donors (donors with a heterozygous loss of function polymorphism at more than one position on the P2X7 receptor gene).
The P2X7 receptor has also been implicated in the innate response to obligate intracellular bacteria of the Chlamydia genus. The macrophage is an important reservoir and source of dissemination of Chlamydia [47], [48]. Like mycobacteria, Chlamydia within macrophages are susceptible to ATP treatment, which results in the death of ∼70%–90% of all intracellular bacteria [49]. This appears to be the result of P2X7 receptor–dependent stimulation of phospholipase D activity and subsequent phagolysosome fusion. Macrophages from P2X7 receptor gene knockout mice show no phospholipase D activation after treatment with ATP and are completely unable to induce ATP-dependent chlamydial death [50]. Furthermore, inhibiting phospholipase D activation in normal murine macrophages, by the addition of butan-1-ol, restores the levels of infection to ∼50%, indicating that phospholipase D is at least partly responsible for chlamydial death [50]. Moreover, treatment of epithelial cells (the preferred target cell for chlamydiae) with P2X7 receptor agonists reduces the infectiveness of the bacteria, again associated with activation of phospholipase D [51].
Although Chlamydia is clearly vulnerable to P2X7 receptor–dependent killing, it has developed some resistance to this killing. Chlamydia-infected J774 murine macrophages are resistant to apoptosis following treatment with ATP, whereas uninfected cells undergo apoptosis via P2X7 receptor–dependent pathways [49]. Infection results in markedly reduced activation of the P2X7 receptor; the mechanism by which this is achieved remains to be elucidated, but it is known that live, growing organisms are required [49].
The P2X7 Receptor and Intracellular Parasites
The P2X7 receptor may also play a role in clearance of intracellular parasites of the Leishmania genus. Murine macrophages and cells cultured from cutaneous lesions of mice infected with Leishmania amazonensis are more sensitive to P2X7 receptor–mediated pore formation, inhibiting growth of the intracellular parasite [52]. Killing of L. amazonensis via the P2X7 receptor is independent of nitric oxide; instead, it is associated with host cell apoptosis [52]. However, when P2X7 receptor gene knockout mice and parental C57BL/6J mice are infected intradermally with Leishmania major, no difference in resolution of lesions is observed, suggesting that, in the absence of P2X7 receptors, other anti-parasitic defence mechanisms compensate to control infection in vivo (C. Miller, A. Zakrzewski, M. Katrib, N. Smith, unpublished observations).
Recently, the P2X7 receptor has been implicated in the immune response to another intracellular protozoan, Toxoplasma gondii, a parasite that is able to infect and survive in cells of the monocyte/macrophage lineage. However, the role of the P2X7 receptor in the response to T. gondii is an intriguing and complex one. Activation of “wild-type” human or murine macrophages with ATP induces killing of tachyzoites of both virulent and avirulent strains of the parasite, but the same treatment of macrophages from humans with the 1513 A>C loss-of-function polymorphism or macrophages from P2X7 receptor knockout mice has no effect on parasite viability [53], [54]. Interestingly, P2X7 receptor–mediated killing of T. gondii is not linked to generation of nitric oxide but is, rather, associated with either phagolysosome formation accompanied by production of free oxygen radicals [53] or host cell apoptosis [54]. Thus, P2X7 receptor–mediated killing of T. gondii has much in common with similarly mediated killing of Mycobacteria, Chlamydia, and Leishmania.
However, susceptibility to either congenital or ocular toxoplasmosis in humans is not associated significantly with the 1513 A>C loss-of-function polymorphism [55]. Intriguingly, though, resistance to both congenital and ocular toxoplasmosis, in the United States and Brazil, respectively, is associated positively with the 1068 T>C polymorphism [55]. This polymorphism has recently been revealed to code for a gain-of-function phenotype in P2X7 receptor activity [27] and may be the true ancestral primate sequence [55]. Thus, the American and Brazilian association studies could be interpreted to indicate that reduced inflammatory disease of the foetus or the eye is a consequence of efficient control of parasite load in the presence of a fully functional, ancestral P2X7 receptor [55]. Larger scale association studies may be needed to truly resolve the association, or otherwise, of various polymorphisms in the P2X7 receptor and congenital or acquired toxoplasmosis.
In vivo murine evidence for a significant role of the P2X7 receptor in controlling T. gondii is also inconsistent. In one study, where mice were infected intraperitoneally with tachyzoites of the type 2, avirulent ME49 strain of T. gondii, splenic parasite burdens in different mouse strains were in proportions consistent with their relative P2X7 receptor activity [54]; thus, P2X7 receptor knockout mice harboured more parasites than the parental C57BL/6J mice, whereas, in another study, using the same parasites and route of infection, no difference in parasite burden between receptor knockout mice and the parental strain was observed in vivo [56]. C57BL/6J mice are known to possess a proline-to-leucine polymorphism at amino acid 453 in their P2X7 receptor that affects some [22], [57]–[59], but not all [22], [60], [61], functions of the receptor and so, C57BL/6J mice, in turn, had higher parasite burdens than resistant BALB/c mice with fully functioning P2X7 receptors. It should be noted, however, that BALB/c and C57BL/6J mice display differing susceptibilities to T. gondii infection and multiple genes are associated with this [62]. This may help explain the discrepancy seen in the in vivo studies.
The apparent inconsistency between P2X7 receptor–mediated killing of T. gondii in vitro versus in vivo may simply reflect the fact that type 2 strains of T. gondii, like ME49, induce a wide variety of pro-inflammatory responses that can control the reproduction of this parasite [63]. Put another way, P2X7 receptor–mediated killing is just one means of control of T. gondii, and others remain operative in knockout mice so that little effect on parasite burden is observed in vivo. This does, however, indicate that the P2X7 receptor plays a contributory rather than major role in controlling T. gondii, which is underscored by the fact that production of several cytokines known to be important in the control of T. gondii, including gamma-interferon and IL-12, are not inhibited in T. gondii–infected P2X7 receptor knockout mice [56].
Most intriguingly, P2X7 receptor knockout mice are very susceptible to acute toxoplasmosis, losing weight faster and more dramatically than either C57BL/6J or BALB/c mice [56]. This susceptibility is not related to relative parasite burdens or a general alteration in cytokine responses but is associated with an inability of the knockout mice to limit nitric oxide production [56], a well-known cause of immunopathology in parasitic infections, including T. gondii [63]. P2X7 receptor knockout mice infected with T. gondii also display a delayed—but not abrogated—production of IL-10 [56], which may partially explain their inability to control nitric oxide production. However, it must be stressed that these observations are correlative rather than definitive evidence that the susceptibility to toxoplasmosis apparent in P2X7 receptor–deficient mice is due to an over-exuberant inflammatory response and, as this immunoregulatory role for the P2X7 receptor has not been noted previously, it does require in-depth follow-up.
It is tempting to speculate that extracellular ATP is an effective danger signal for the immune system, produced as a result of damage caused by factors like nitric oxide. Since nitric oxide is potentially so damaging, its production is tightly controlled, possibly through a negative feedback mechanism. In this scenario, perhaps cells lacking the P2X7 receptor to sense extracellular ATP continue producing nitric oxide, whereas cells with functional receptors are able to regulate nitric oxide production. Figure 1 presents a possible molecular explanation. CREB [64] is a transcription factor that has been linked to the suppression of inducible nitric oxide synthase. Extracellular ATP induces activation of CREB as well as suppressing LPS-induced nitric oxide production [65]. Although the exact mechanism has not been elucidated, it is thought that activated CREB sequesters a transcriptional co-activator, CBP, preventing it from interacting with the nuclear factor kappa B (NFκB) subunit p65, thus inhibiting expression of inducible nitric oxide synthase [65]. Hence, during infection with T. gondii, extracellular ATP, operating through the P2X7 receptor, may activate CREB and down-regulate nitric oxide production. Cells lacking the P2X7 receptor would, therefore, be less responsive to any build-up in extracellular ATP. The downstream effect of this would be a lack of phosphorylation, allowing CBP to remain bound to NFκB, and inducible nitric oxide synthase transcription and nitric oxide production to continue indefinitely. Pathology would result.
Conclusion
Clearly, the P2X7 receptor comprises an important part of the host arsenal against invading pathogens. However, there is growing evidence of bacteria and parasites subverting the P2X7 receptor pathways for their own advantage, be that through P2X7 receptor–mediated apoptosis, phospholipase D production, or inhibition of these, as highlighted above.
We speculate that many other pathogens also modulate the host P2X7 receptor. For example, intracellular parasites of various genera—including Theileria [66], Leishmania [67], [68], Cryptosporidium [69], [70], and Microsporidia [71], and bacteria such as Brucella [72], [73]—all prevent apoptosis of host cells to aid their own survival. Other pathogens, such as Plasmodium [74], [75], Tritrichomonas [76], [77], Streptococcus [78], [79], and Legionella [80], actually increase apoptosis of host cells, thereby reducing the numbers of circulating immune cells and increasing their chances for survival. Intriguingly, T. gondii is able to both induce and suppress apoptosis in a cell type–dependent manner that allows it to establish a persistent infection [81]. Could some, or all of these, be affecting host cell apotosis via the P2X7 receptor? Legionella [82], Francisella [83], [84], and Leishmania [85] prevent phagolysosome fusion—perhaps this is in a P2X7 receptor–dependent manner analogous to Chlamydia? And, although no definitive link with the P2X7 receptor exists, infection of peritoneal macrophages with Trypanosoma cruzi down-regulates expression of P2X7 receptors [86], which may reduce the immune pressure on these parasites. It would be interesting to investigate whether the P2X7 receptor is a common target in the immune evasion strategies of these diverse pathogens. It should also be noted that many of these studies have been conducted in vitro, and it will be important to confirm that the P2X7 receptor is playing a non-redundant role in controlling these pathogens in vivo using P2X7 receptor knockout mice.
Perhaps surprisingly, extracellular pathogens may also be affected by, or react to, P2X7 receptor activity. For example, Pseudomonas aeruginosa, a major pathogen in the lungs of cystic fibrosis patients, and Vibrio cholerae, the causative agent of cholera, have been shown to secrete multiple enzymes with ATP-modifying activities, such as adenylate kinase, ATPase, and 5′-nucleotidase [87]–[89]. It is believed that these enzymes are used to modulate external ATP levels and, thereby, increase P2X7 receptor function, mediating apoptosis of macrophages. Extracellular bacteria, such as Staphylococcus aureus and Escherichia coli, have been linked to P2X7 receptor dependence in a study of caspase-1 activation and subsequent IL-1β secretion [90]. It is unclear in these studies, however, how this stimulation of apoptosis affects the survival and growth of the bacteria. Thus, future work may well reveal that the P2X7 receptor has—sometimes quite unexpected—roles in modulating a variety of infectious diseases, not just those that parasitise macrophages.
Zdroje
1. CraneJKNaeherTMChoudhariSSGirouxEM 2005 Two pathways for ATP release from host cells in enteropathogenic Escherichia coli infection. Am J Physiol Gastrointest Liver Physiol 289 G407 G417
2. JarvisMFKhakhBS 2009 ATP-gated ion channels. Neuropharmacol 56 208 215
3. YoungMT 2010 P2X receptors: dawn of the post-structure era. Trends Biochem Sci 35 83 90
4. AbbracchioMPBurnstockGVerkhratskyAZimmermannH 2009 Purinergic signalling in the nervous system: an overview. Trends Neurosci 32 19 29
5. BurnstockG 2007 Purine and pyrimidine receptors. Cell Mol Life Sci 64 1471 1483
6. NorthRABarnardEA 1997 Nucleotide receptors. Curr Opin Neurobiol 7 346 357
7. JiangL-H 2009 Inhibition of P2X7 receptors by divalent cations: old action and new insight. Eur Biophys J 38 339 346
8. KhakhBSNorthRA 2006 P2X receptors as cell-surface ATP sensors in health and disease. Nature 442 527 532
9. WileyJSGargettCEZhangWSnookMBJamiesonGP 1998 Partial agonists and antagonists reveal a second permeability state of human P2Z/P2X7 channel. Am J Physiol 275 C1224 C1231
10. FariaRXCascabulhoCMReisRAAlvesLA 2010 Large-conductance channel formation mediated by P2X(7) receptor activation is regulated through distinct intracellular signaling pathways in peritoneal macrophages and 2BH4 cells. Naunyn Schmiedebergs Arch Pharmacol 382 73 87
11. GavalaMLPfeifferZABerticsPJ 2008 The nucleotide receptor P2RX7 mediates ATP-induced CREB activation in human and murine monocytic cells. J Leukoc Biol 84 1159 1171
12. BulanovaEBudagianVOrinskaZKoch-NolteFHaagF 2009 ATP induces P2X7 receptor-independent cytokine and chemokine expression through P2X1 and P2X3 receptors in murine mast cells. J Leukoc Biol 85 692 702
13. GudipatyLHumphreysBDBuellGDubyakGR 2001 Regulation of P2X7 nucleotide receptor function in human monocytes by extracellular ions and receptor density. Am J Physiol Cell Physiol 280 943 953
14. HumphreysBDDubyakGR 1998 Modulation of P2X7 nucleotide receptor expression by pro - and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol 64 256 273
15. KannegantiTDLamkanfiMKimYGChenGParkJH 2007 Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 26 433 443
16. PétrilliVPapinSDostertCMayorAMartinonF 2007 Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ 14 1583 1589
17. YuHBFinlayBB 2008 The caspase-1 inflammasome: A pilot of innate immune responses. Cell Host and Microbe 4 198 208
18. HumphreysBDRiceJKertseySBDubyakGR 2000 Stress-activated protein kinase/JNK activation and apoptotic induction by the macrophage P2X7 nucleotide receptor. J Biol Chem 275 26791 26798
19. GuerraANGavalaMLChungHSBerticsPJ 2007 Nucleotide receptor signalling and the generation of reactive oxygen species. Purinergic Signalling 3 39 51
20. PfeifferZAGuerraANHillLMGavalaMLPrabhuU 2007 Nucleotide receptor signalling in murine macrophages is linked to reactive oxygen species generation. Free Radic Biol Med 42 1506 1516
21. RuffellDMourkiotiFGambardellaAKirstetterPLopezRG 2009 A CREB-C/EBPβ cascade induces M2 macrophage specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci U S A 106 17475 17480
22. Le StunffHAugerRKanellopoulosJRaymondM-N 2004 The Pro-451 to Leu polymorphism within the C-terminal tail of the P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. J Biol Chem 279 16918 16926
23. LabasiJMPetrushovaNDonovanCMcCurdySLiraP 2002 Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol 168 6436 6445
24. ChenLBrosnanCF 2006 Exacerbation of experimental autoimmune encephalomyelitis in P2X7R-/ - mice: evidence for loss of apoptotic activity in lymphocytes. J Immunol 176 3115 3126
25. ShemonANSluyterRFernandoSLClarkeALDao-UngLP 2006 A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem 81 2079 2086
26. FullerSJStokesLSkarrattKKGuBJWileyJS 2009 Genetics of the P2X7 receptor and human disease. Purinergic Signalling 5 257 262
27. StokesLFullerSJSluyterRSkarrattKKGuBJ 2010 Two haplotypes of the P2X7 receptor containing the Ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1β secretion. FASEB J 24 2916 2927
28. DenlingerLCSorknessCAChinchilliVMLemanske RFJr 2006 Human P2X7 pore function predicts allele linkage disequilibrium. Clin Chem 52 995 1004
29. CabriniGFalzoniSForchapSLPellegattiPBalboniA 2005 A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemia lymphocytes. J Immunol 175 82 89
30. GuBJSluyterRSkarrattKKShemonANDao-UngLP 2004 An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J Biol Chem 23 31287 95
31. GuBJZhangWWorthingtonRASlutyerRDao-UngP 2001 A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem 276 11135 11142
32. WileyJSDao-UngLPLiCShemonANGuBJ 2003 An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem 278 17108 17113
33. VergneIChuaJLeeHHLucasMBelisleJDereticV 2005 Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 102 4033 4038
34. FairbairnIPStoberCBKumararatneDSLammasDA 2001 ATP-mediated killing of intracellular mycobacteria is a P2X7-dependent process inducing bacterial death by phagosome-lysosome fusion. J Immunol 167 3300 3307
35. BiswasDQureshiOSLeeWYCroudaceJEMuraM 2008 ATP-induced autophagy is associated with rapid killing of intracellular mycobacteria within human monocytes/macrophages. BMC Immunology 9 35
36. FernandoSLSaundersBMSluyterRSkarrattKKWileyJS 2007 A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Resp Crit Care Med 175 360 366
37. Niño-MorenoPPortales-PérezDHernández-CastroBPortales-CervantesLFlores-MerazV 2007 P2X7 and NRAMP1/SLC11 A1 gene polymorphisms in Mexican mestizo subjects with pulmonary tuberculosis. Clin Exp Immunol 148 469 477
38. MokrousovISapozhnikovaNNarvskayaO 2008 Mycobacterium tuberculosis co-existence with humans: making an imprint on the macrophage P2X7 receptor gene? J Med Microbiol 57 581 584
39. SharmaSKumarVKhoslaRKajalNSarinB 2010 Association of P2X7 receptor +1513 (A>C) polymorphism with tuberculosis in a Punjabi population. Int J Tuberc Lung Dis 14 1159 1163
40. TekinDKayaaltiZDalgicNCakirESoylemezogluT 2010 Polymorphism in the p2x7 gene increases susceptibility to extrapulmonary tuberculosis in Turkish children. Pediatr Infect Dis 29 779 782
41. LiCMCampbellSJKumararatneDSBellamyRRuwendeC 2002 Association of a polymorphism in the P2X7 gene with tuberculosis in a Gambian population. J Infect Dis 186 1458 1462
42. SambasivanVMurthyKJReddyRVijayakshimiVHasanQ 2010 P2X7 gene polymorphisms and risk assessment for pulmonary tuberculosis in Asian Indian. Dis Markers 28 43 48
43. XiaoJSunLJiaoWLiZZhaoS 2009 Lack of association between polymorphisms in the P2X7 gene and tuberculosis in a Chinese Han population. FEMS Immunol Med Microbiol 55 107 111
44. XiaoJSunLYanHJiaoWMiaoQ 2010 Metaanalysis of P2X7 gene polymorphisms and tuberculosis susceptibility. FEMS Immunol Med Microbiol 60 165 170
45. SaundersBMFernandoSLSluyterRBrittonWJWileyJS 2003 A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J Immunol 171 5442 5446
46. FernandoSLSaundersBMSluyterRSkarrattKKWileyJS 2005 Gene dosage determines the negative effects of polymorphic alleles of the P2X7 receptor on adenosine triphosphate-mediated killing of mycobacteria by human macrophages. J Infect Dis 192 149 155
47. KoehlerLNettelnbrekerEHudsonAPOttNGérardHC 1997 Ultrastructural and molecular analysis of the persistence of Chlamydia trachomatis (serovar K) in human monocytes. Microb Pathog 22 133 142
48. GérardHCBraniganPJSchumacher HRJrHudsonAP 1998 Synovial Chlamydia trachomatis in patients with reactive arthritis/Reiters's syndrome are viable but show aberrant gene expression. J Rheumatol 25 734 742
49. Coutinho-SilvaRPerfettiniJLPersechiniPMDautry-VarsatAOjciusDM 2001 Modulation of P2Z/P2X7 receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol Cell Physiol 280 C81 C89
50. Coutinho-SilvaRStahlLRaymondMNJungasTVerbekeP 2003 Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity 19 403 412
51. DarvilleTWelter-StahlLCruzCSaterAAAndrewsCWJr 2007 Effect of the purinergic receptor P2X7 on Chlamydia infection in cervical epithelial cells and vaginally infected mice. J Immunol 179 3707 3714
52. ChavesSPTorres-SantosECMarquesCFigliuoloVRPersechiniPM 2009 Modulation of P2X7 purinergic receptor in macrophages by Leishmania amazonensis and its role in parasite elimination. Microbes Infect 11 842 849
53. CorreaGda Silva CMMoreira-SouzaACVommaroRCCoutinho-SilvaR 2010 Activation of the P2X7 receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect 12 497 504
54. LeesMPFullerSJMcLeodRBoulterNRMillerCM 2010 P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii by human and mouse macrophages. J Immunol 184 7040 7046
55. JamiesonSEPeixoto-RangelALHargraveACRoubaixL-AMuiEJ 2010 Evidence for associations between the purinergic receptor P2X7 (P2RX7) and toxoplasmosis. Genes and Immunity 11 374 383
56. MillerCMZakrzewskiAMIkinRJBoulterNRKatribM 2011 Dysregulation of the inflammatory response to the parasite Toxoplasma gondii in P2X7 receptor-deficient mice. Int J Parasitol 41 301 308
57. AdriouchSDoxCWelgeVSemanMKoch-NobleF 2002 A natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol 169 4108 4112
58. YipLWoehrleTCorridenRHirshMChenY 2009 Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J 23 1685 1693
59. SuadicaniSOIglesiasRSprayDCScemesE 2009 Point mutation in the mouse P2X7 receptor affects intercellular calcium waves in astrocytes. ASN Neuro Apr 14 1 1 doi:10.1042/AN20090001 pii: e00005
60. AswardFDennertG 2006 P2X7 receptor expression levels determine lethal effects of a purine based danger signal in T lymphocytes. Cell Immunol 243 58 65
61. HongSSchwarzNBrassASemanMHaagF 2009 Differential regulation of P2X7 receptor activation by extracellular nicotinamide adenine dinucleotide and ecto-ADP-ribosyltransferases in murine macrophages and T cells. J Immunol 183 578 592
62. SuzukiYSherAYapGParkDNeyerLE 2000 IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. J Immunol 164 5375 5382
63. MillerCMBoulterNRIkinRJSmithNC 2009 The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol 39 23 39
64. WenAYSakamotoKMMillerLS 2010 The role of the transcription factor CREB in immune function. J Immunol 185 6413 6419
65. BrautigamVMFrasierCNikodemovaMWattersJJ 2005 Purinergic receptor modulation of BV-2 microglial cell activity: Potential involvement of p38 MAP kinase and CREB. J Neuroimmunol 166 113 125
66. HeusslerVTMachadoJJrFernandezPCBotteronCChenCG 1999 The intracellular parasite Theileria parva protects infected T cells from apoptosis. Proc Natl Acad Sci USA 96 7312 7317
67. MooreKJMatlashewskiG 1994 Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J Immunol 152 2930 2937
68. AgaEKatschinskiDMvan ZandbergenGLaufsHHansenB 2002 Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J Immunol 169 898 905
69. ChenXMLevineSASplinterPLTietzPSGanongAL 2001 Cryptosporidium parvum activates nuclear factor kappa B in biliary epithelia preventing epithelial cell apoptosis. Gastroenterology 120 1774 1783
70. LiuJEnomotoSLanctoCAAbrahamsenMSRutherfordMS 2008 Inhibition of apoptosis in Cryptosporidium parvum-infected intestinal epithelial cells is dependent on surviving. Infect Immun 76 3784 3792
71. del AguilaCIzquierdoFGranjaAGHurtadoCFenoyS 2006 Encephalitozoon microsporidia modulates p53-mediated apoptosis in infected cells. Int J Parasitol 36 869 876
72. EskraLMathisonASplitterG 2003 Microarray analysis of mRNA levels from RAW 264.7 macrophages infected with Brucella abortus. Infect Immun 71 1125 1133
73. GrossATerrazaAOuahrani-BettacheSLiautardJPDornandJ 2000 In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect Immun 68 342 351
74. Toure-BaldeASarthouJLAribotGMichelPTrapeJF 1996 Plasmodium falciparum induces apoptosis in human mononuclear cells. Infect Immun 64 744 750
75. PinoPVouldoukisIKolbJPMahmoudiNDesportes-LivageI 2003 Plasmodium falciparum-infected erythrocyte adhesion induces caspase activation and apoptosis in human endothelial cells. J Infect Dis 187 1283 1290
76. SinghBNLucasJJHayesGRKumarIBeachDH 2004 Tritrichomonas foetus induces apoptotic cell death in bovine vaginal epithelial cells. Infect Immun 72 4151 4158
77. LucasJJHayesGRKalsiHKGilbertROChoeY 2008 Characterization of a cysteine protease from Tritrichomonas foetus that induces host-cell apoptosis. Arch Biochem Biophys 477 239 243
78. BraunJSNovakRMurrayPJEischenCMSusinSA 2001 Apoptosis-inducing factor mediates microglial and neuronal apoptosis caused by Pneumococcus. J Infect Dis 184 1300 1309
79. ColinoJSnapperCM 2003 Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. J Immunol 171 2354 2365
80. TakamatsuRTakeshimaEIshikawaCYamamotoKTeruyaH 2010 Inhibition of Akt/GSK3beta signalling pathway by Legionella pneumophila is involved in induction of T-cell apoptosis. Biochem J 427 57 67
81. LüderCGGrossU 2005 Apoptosis and its modulation during infection with Toxoplasma gondii: molecular mechanisms and role in pathogenesis. Curr Top Microbiol Immunol 289 219 237
82. RoyCRBergerKHIsbergRR 1998 Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol 28 663 674
83. ClemensDLLeeBYHorwitzMA 2004 Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect Immun 72 3204 3217
84. ClemensDLLeeBYHorwitzMA 2009 Francisella tularensis phagosomal escape does not require acidification of the phagosome. Infect Immun 77 1757 1773
85. DesjardinsMDescoteauxA 1997 Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. J Exp Med 185 2061 2068
86. CasabulhoCMMenna-BarretoRFCoutinho-SilvaRPersechiniPMHenriques-PonsA 2008 P2X7 modulatory web in Trypanosoma cruzi infection. Parasitol Res 103 829 838
87. ZaborinaOMisraNKostalJKamathSKapatralV 1999 P2Z-independent and P2Z receptor-mediated macrophage killing by Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect Immun 67 5231 5242
88. ZaborinaODhimanNLing ChenMKostalJHolderIA 2000 Secreted products of a nonmucoid Pseudomonas aeruginosa strain induce two modes of macrophage killing: external-ATP-dependent, P2Z-receptor-mediated necrosis and ATP-independent, caspase-mediated apoptosis. Microbiol 146 2521 2530
89. PunjVZaborinaODhimanNFalzariKBagdasarianM 2000 Phagocytic cell killing mediated by secreted cytotoxic factors of Vibrio cholerae. Infect Immun 68 4930 4937
90. FranchiLKannegantiTDDubyakGRNúñezG 2007 Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem 282 18810 18818
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other SpeciesČlánek Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome ofČlánek The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other Species
- Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive—Is a “Treat-to-Test” Strategy More Realistic?
- Ultra-Efficient PrP Amplification Highlights Potentialities and Pitfalls of PMCA Technology
- Assessing Predicted HIV-1 Replicative Capacity in a Clinical Setting
- Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment
- Anti-filarial Activity of Antibiotic Therapy Is Due to Extensive Apoptosis after Depletion from Filarial Nematodes
- West Nile Virus Experimental Evolution and the Trade-off Hypothesis
- Shedding Light on the Elusive Role of Endothelial Cells in Cytomegalovirus Dissemination
- Galactosaminogalactan, a New Immunosuppressive Polysaccharide of
- Fatal Prion Disease in a Mouse Model of Genetic E200K Creutzfeldt-Jakob Disease
- BST2/Tetherin Enhances Entry of Human Cytomegalovirus
- Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus
- Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection
- Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in
- A Molecular Mechanism for Bacterial Susceptibility to Zinc
- Genomic Transition to Pathogenicity in Chytrid Fungi
- Evolution of Multidrug Resistance during Infection Involves Mutation of the Essential Two Component Regulator WalKR
- ChemR23 Dampens Lung Inflammation and Enhances Anti-viral Immunity in a Mouse Model of Acute Viral Pneumonia
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
- A Gammaherpesvirus Cooperates with Interferon-alpha/beta-Induced IRF2 to Halt Viral Replication, Control Reactivation, and Minimize Host Lethality
- Early Secreted Antigen ESAT-6 of Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner
- CD4 T Cell Immunity Is Critical for the Control of Simian Varicella Virus Infection in a Nonhuman Primate Model of VZV Infection
- The Role of the P2X Receptor in Infectious Diseases
- Down-Regulation of Shadoo in Prion Infections Traces a Pre-Clinical Event Inversely Related to PrP Accumulation
- Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates
- Single Molecule Analysis of Replicated DNA Reveals the Usage of Multiple KSHV Genome Regions for Latent Replication
- The Critical Role of Notch Ligand Delta-like 1 in the Pathogenesis of Influenza A Virus (H1N1) Infection
- Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
- Murine Gamma Herpesvirus 68 Hijacks MAVS and IKKβ to Abrogate NFκB Activation and Antiviral Cytokine Production
- EBV Tegument Protein BNRF1 Disrupts DAXX-ATRX to Activate Viral Early Gene Transcription
- SAG101 Forms a Ternary Complex with EDS1 and PAD4 and Is Required for Resistance Signaling against Turnip Crinkle Virus
- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- Rab7A Is Required for Efficient Production of Infectious HIV-1
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- Novel Anti-bacterial Activities of β-defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation
- CD11b, Ly6G Cells Produce Type I Interferon and Exhibit Tissue Protective Properties Following Peripheral Virus Infection
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A Kinase Chaperones Hepatitis B Virus Capsid Assembly and Captures Capsid Dynamics
- Towards a Structural Comprehension of Bacterial Type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Pathovar
- Indirect DNA Readout by an H-NS Related Protein: Structure of the DNA Complex of the C-Terminal Domain of Ler
- The Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of into Human Hepatocytes
- The Human Herpesvirus-7 (HHV-7) U21 Immunoevasin Subverts NK-Mediated Cytoxicity through Modulation of MICA and MICB
- Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity
- Murid Herpesvirus-4 Exploits Dendritic Cells to Infect B Cells
- Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection
- Evolution of a Species-Specific Determinant within Human CRM1 that Regulates the Post-transcriptional Phases of HIV-1 Replication
- Transcriptome Analysis of Transgenic Mosquitoes with Altered Immunity
- Antibody Evasion by a Gammaherpesvirus O-Glycan Shield
- UDP-glucose 4, 6-dehydratase Activity Plays an Important Role in Maintaining Cell Wall Integrity and Virulence of
- Protease-Resistant Prions Selectively Decrease Shadoo Protein
- A LysM and SH3-Domain Containing Region of the p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells
- Deletion of AIF Ortholog Promotes Chromosome Aneuploidy and Fluconazole-Resistance in a Metacaspase-Independent Manner
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



