-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
The YbeB (DUF143) family of uncharacterized proteins is encoded by almost all bacterial and eukaryotic genomes but not archaea. While they have been shown to be associated with ribosomes, their molecular function remains unclear. Here we show that YbeB is a ribosomal silencing factor (RsfA) in the stationary growth phase and during the transition from rich to poor media. A knock-out of the rsfA gene shows two strong phenotypes: (i) the viability of the mutant cells are sharply impaired during stationary phase (as shown by viability competition assays), and (ii) during transition from rich to poor media the mutant cells adapt slowly and show a growth block of more than 10 hours (as shown by growth competition assays). RsfA silences translation by binding to the L14 protein of the large ribosomal subunit and, as a consequence, impairs subunit joining (as shown by molecular modeling, reporter gene analysis, in vitro translation assays, and sucrose gradient analysis). This particular interaction is conserved in all species tested, including Escherichia coli, Treponema pallidum, Streptococcus pneumoniae, Synechocystis PCC 6803, as well as human mitochondria and maize chloroplasts (as demonstrated by yeast two-hybrid tests, pull-downs, and mutagenesis). RsfA is unrelated to the eukaryotic ribosomal anti-association/60S-assembly factor eIF6, which also binds to L14, and is the first such factor in bacteria and organelles. RsfA helps cells to adapt to slow-growth/stationary phase conditions by down-regulating protein synthesis, one of the most energy-consuming processes in both bacterial and eukaryotic cells.
Published in the journal: . PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002815
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002815Summary
The YbeB (DUF143) family of uncharacterized proteins is encoded by almost all bacterial and eukaryotic genomes but not archaea. While they have been shown to be associated with ribosomes, their molecular function remains unclear. Here we show that YbeB is a ribosomal silencing factor (RsfA) in the stationary growth phase and during the transition from rich to poor media. A knock-out of the rsfA gene shows two strong phenotypes: (i) the viability of the mutant cells are sharply impaired during stationary phase (as shown by viability competition assays), and (ii) during transition from rich to poor media the mutant cells adapt slowly and show a growth block of more than 10 hours (as shown by growth competition assays). RsfA silences translation by binding to the L14 protein of the large ribosomal subunit and, as a consequence, impairs subunit joining (as shown by molecular modeling, reporter gene analysis, in vitro translation assays, and sucrose gradient analysis). This particular interaction is conserved in all species tested, including Escherichia coli, Treponema pallidum, Streptococcus pneumoniae, Synechocystis PCC 6803, as well as human mitochondria and maize chloroplasts (as demonstrated by yeast two-hybrid tests, pull-downs, and mutagenesis). RsfA is unrelated to the eukaryotic ribosomal anti-association/60S-assembly factor eIF6, which also binds to L14, and is the first such factor in bacteria and organelles. RsfA helps cells to adapt to slow-growth/stationary phase conditions by down-regulating protein synthesis, one of the most energy-consuming processes in both bacterial and eukaryotic cells.
Introduction
Escherichia coli harbors a core set of about 190 genes that are conserved in more than 90% of all completely sequenced genomes [1]. Most of them encode well-understood proteins involved in metabolism, transcription, translation, or replication. However, a few of these highly conserved proteins remain functionally uncharacterized and thus enigmatic. One of these mysterious proteins is YbeB. In 2004 it was proposed by Galperin and Koonin as one of 10 top targets of conserved hypothetical proteins for experimental characterization [2]. In recent interactome studies, we and others found this protein to interact with various proteins, including several ribosomal components [3], [4], [5], [6]. Moreover, YbeB was shown to co-sediment with the large ribosomal subunit (LRS) [7], suggesting that it functions in protein translation. Recently it has been suggested that its mitochondrial homologue, C7orf30, is involved in ribosome biogenesis and/or translation [5], [8] although these studies were not able to explain their observations mechanistically. In this work we characterize YbeB's molecular function by identifying its binding site on the LRS and reveal a molecular mechanism of YbeB action: it is down-regulating protein synthesis under nutrient shortage by binding to protein L14 of the LRS, acting as a ribosomal silencing factor (“RsfA”) by blocking ribosome subunit joining. Thus, we will use the term “RsfA” below.
Results
RsfA homologues are conserved from bacteria to humans and interact with the ribosomal protein L14
In the Pfam database (V26.0) RsfA sequence homologues are known for at least 2,928 species, including nearly all bacteria as well as almost all eukaryotic species (Pfam entry PF02410, Interpro IPR004394). However, the RsfA protein family is conspicuously absent in archaea (Figure 1A). In the STRING 9.0 database [9] RsfA is clustered with the orthologous protein group “COG0799”, consisting of 932 RsfA homologues in 920 different species, indicating that there is usually one rsfA gene per genome. A multiple sequence alignment of ten representative RsfA orthologues, however, exhibits only limited conservation when compared to ribosomal protein L14 (Figure S1).
Fig. 1. RsfA and L14 and their interaction are conserved in bacteria and eukaryotic organelles. 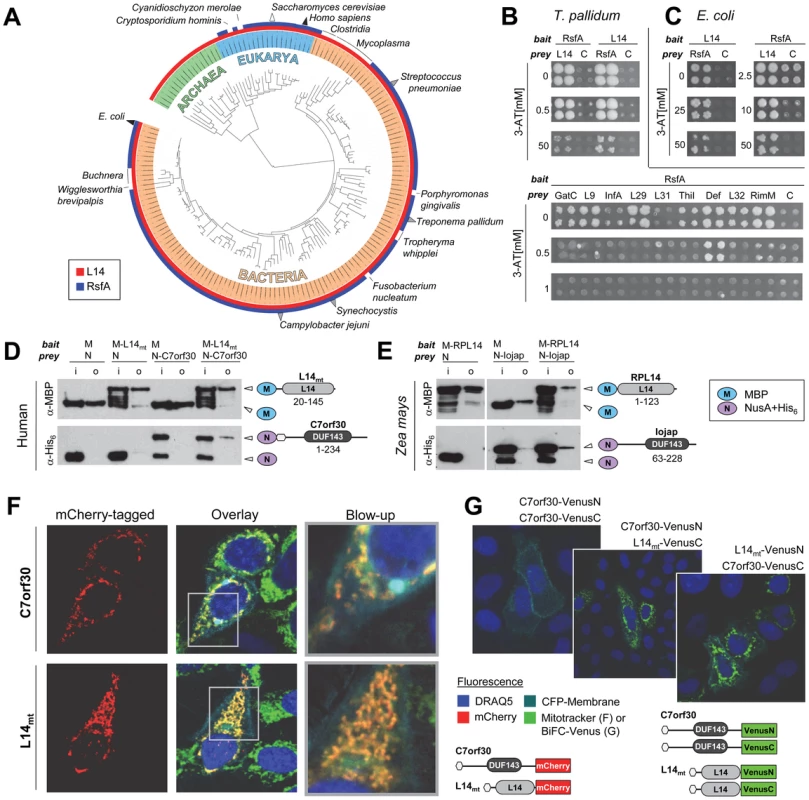
(A) Phylogenetic distribution of RsfA (Interpro entry IPR004394 [DUF143]) and ribosomal protein L14 (IPR000218) on the iTOL tree of life [59]. Triangles indicate species in which the RsfA-L14 interaction was detected by binary detection assays (grey), co-purification with the LRS (white) or both (black). Known RsfA-L14/LRS interactions are listed in Table S1. (B) T. pallidum RsfA (TP0738) interacts strongly with L14 (TP0199) and very weakly with other proteins involved in translation [6] in yeast-two-hybrid assays. C, control (with empty prey vector to measure self-activation of the bait). This interaction is also conserved in E. coli (C). (D, E) RsfA and L14 homologues from human and maize interact in pull down experiments. RsfA homologues were tagged with NusA-His6 (N) and L14 homologues with maltose binding protein (M) (human mtRsfA = C7orf30, mitochondrial ribosomal protein L14 = L14mt; maize RsfA = Iojap, maize chloroplastic L14 = RPL14); i = input samples, o = output samples. Constructs with the corresponding Interpro signatures and the range of cloned codons are illustrated on the right. (F) Human mitochondrial C7orf30 (mtRsfA) co-localizes with L14mt exclusively into mitochondria as visualized by MitoTracker Green. Nuclei visualized by DRAQ5 (blue) and membranes by eCFP-membrane (cyan). Co-localization of both mtRsfA (C7orf30) and L14mt in mitochondria is indicated in yellow. (G) Bi-molecular fluorescence complementation (BiFC) reveals the interaction of mtRsfA (C7orf30) and L14mt in mitochondria. Overlay images represent DRAQ5 (blue), CFP-membrane (cyan) and BiFC stained cells. Green fluorescence indicates interaction-dependent regeneration of the Venus protein. Constructs are shown below. Here, the hexagons symbolize the native N-termini including mitochondrial localization sequences. Interestingly, more than 80% of all eukaryotic RsfA orthologues are predicted to localize to mitochondria or chloroplasts according to the WoLF PSort program [10]. For the yeast orthologue ATP25, the mitochondrial localization has been experimentally confirmed [11] and the Zea mays homologue, Iojap, was found in chloroplast fractions [12]. This strongly suggests that RsfA functions in a strictly conserved process of bacterial origin. Previously, Butland and colleagues reported L14, L19, L4, L7/L12 and others as interaction partners of RsfA based on protein complex data [3]. Similarly, we found that several interactors of RsfA's Treponema pallidum orthologue TP0738 were involved in protein synthesis [6]. Although these observations provided the first experimental hint that RsfA might function in translation, this has never been functionally demonstrated. Since previous studies have revealed RsfA's association with the large ribosomal subunit (LRS) which offers multiple binding sites, we re-tested all previously detected interactions of T. pallidum RsfA that are involved in protein translation. As expected, several proteins indeed tested positive (Figure 1B). However, the interaction of RsfA with L14 was by far the strongest as determined by using increasing concentrations of 3-amino-triazole (3-AT), a competitive inhibitor of the yeast two-hybrid reporter gene HIS3. In fact, only the interaction with L14 was detectable at more than 1 mM 3-AT. Furthermore, the L14-RsfA interaction was the only one that was detectable in a reciprocal screen, i.e. with RsfA used as both bait and prey.
Given the conservation of RsfA, we wanted to establish to which extent the interactions of RsfA of T. pallidum are conserved in other species. To this end, we first retested whether the interactions of T. pallidum RsfA are conserved in E. coli. We also included eight putative interaction partners that have been identified in a protein complex together with E. coli RsfA and L14 [3] and four interologous pairs detected by Y2H in Campylobacter jejuni [13]. Surprisingly, only the interaction with L14 was conserved in E. coli as a strong (up to 50 mM 3-AT) and reciprocal interaction (Figure 1C, all tested interactions and reference sets are listed in Table S2 and the complete Y2H assays are shown in Figure S2). Moreover, we confirm the interaction of RsfA with L14 from E. coli independently in a pull-down experiment (Figure S3A).
Thus, we conclude that L14 is the primary and specific binding target of RsfA on the LRS and that all other interactions are species specific or even artifacts.
Next we tested whether this particular interaction is conserved in other bacteria. Notably, we could verify the interaction in all tested species, including gram-positive Streptococcus pneumoniae and the cyanobacterium Synechocystis PCC 6803 (Figure S3B and S3C). In addition, we confirmed the interaction between the corresponding orthologues of RsfA/L14 of both human (C7orf30/mitochondrial L14) and Zea mays (Iojap/chloroplastic RPL14) as shown in Figure 1D and 1E, respectively.
In HeLa cells human C7orf30 co-localized with L14mt exclusively to mitochondria (Figure 1F). This supports the hypothesis that eukaryotic RsfA orthologues are functionally active only in organelles. Finally, we verified the human protein interaction in vivo by a bimolecular fluorescence complementation assay using C-terminally tagged Split-Venus constructs (Figure 1G). In summary, these results strongly suggest that the interaction of RsfA and L14 is universally conserved in all species that encode RsfA homologues and that in fact their specific binding site at the LRS is in the ribosomal protein L14.
RsfA binds to critical residues of ribosomal protein L14 at the ribosomal subunit interface
In order to map the exact binding site of RsfA we used the LRS 3D structure (PDB id: 2AWB [14]: first, we identified amino acids of L14 that (i) are highly conserved (Figure 2A(a) and 2A(b)) and that (ii) are located on the surface exposed towards the 30S small subunit interface. These criteria identified T97, R98, K114, and S117. (Figure 2A(b,c)). In fact, docking a homology model of RsfA and a crystal structure of L14 predicted these residues to be at their interaction interface (Figure 2B). In order to test whether the identified residues of L14 are indeed essential for the L14-RsfA interaction, we substituted T97, R98, K114, and S117 with a single alanine each and tested these L14 constructs if they still bound RsfA by another Y2H experiment (Figure 2C): the K114A and T97A mutants lost the interaction with RsfA already in the presence of 0 to 1 mM 3-AT, while in R98A the interaction was lost at 10 mM and higher concentrations. S117A did not appear to affect the interaction. Several control mutations including moderately conserved amino acids (D80A, F100A, E121A) and none-conserved ones (R49A, K51A) did not show any difference in the Y2H assay compared to the assayed wild type L14 (Figure 2A, 2C).
Fig. 2. Mapping the RsfA binding site on ribosomal protein L14. 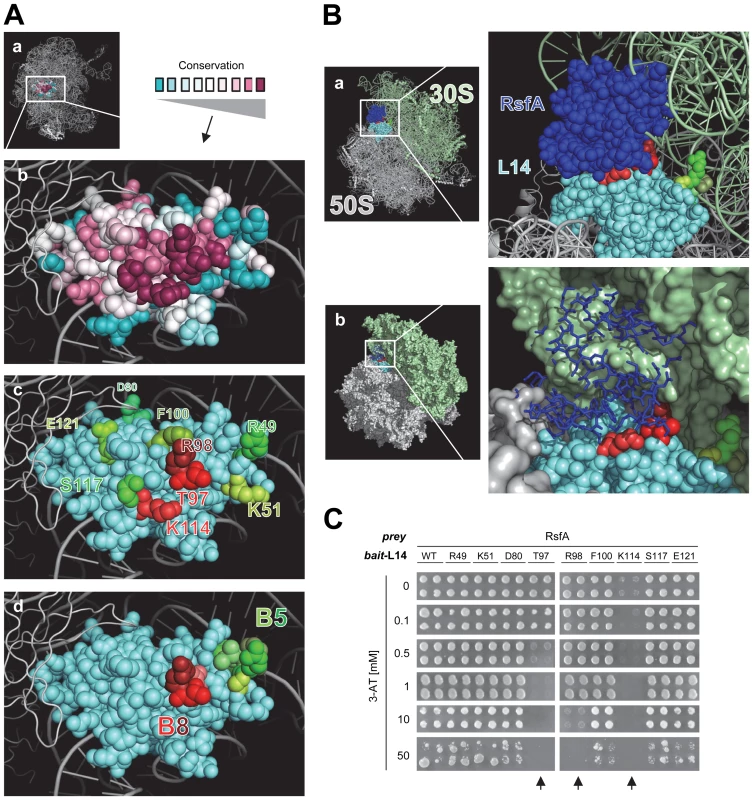
(A) L14 in the context of the 3D structure of the 50S ribosomal subunit (a) (PDB: 2AWB) [14]. (b) Conserved residues of L14: magenta (highly conserved), grey (moderately conserved), turquoise (little or no conservation). (c) Mutated residues for interaction epitope mapping (red or green); residues involved in (red colors) and not involved (green colors) in RsfA-binding based on results from subfigure (C). (d) Residues of L14 highlighted that are involved in formation of intersubunit bridges with the 16S rRNA of the 30S subunit (bridge B5 (green colors), bridge B8 (red colors)) [15]. (B) A docking model of L14 on the E. coli 50S subunit with bound RsfA. Critical L14 residues that mediate RsfA interaction (or that contact 16S rRNA) are colored in red according to A(c) and A(d). When RsfA is bound to L14 on a 50S subunit, 30S subunit joining is sterically blocked, clearly visible in B(b) as shown by the structural overlap of RsfA (dark blue) and the 30S subunit. A model of the ribosome with bound RsfA is available as Dataset S1. (C) L14 interaction epitope mapping. Amino acids (see Figure 2A(c)) were mutated to alanine and the constructs tested by Y2H experiments. WT, wild type L14 construct; mutated residues and their positions are indicated. In the experiment, all bait constructs were simultaneously tested for reporter gene self-activation. No construct resulted in self-activation (data not shown). T97A, R98A, or K114A mutations (highlighted by arrows) abolished or weakened RsfA binding as indicated by 3-AT titrations; all other tested L14 mutation constructs are comparable to wild type L14. In summary, the interaction epitope assay confirms that the docking model (Figure 2B) is largely correct. The RsfA-interaction epitope of L14 involves the highly conserved residues K114, T97, and R98 (but not S117) while K114 and T97 are the most critical ones. Notably, T97 and R98 are involved in bridge B8 (Figure 2A(d)) that contacts the small ribosomal subunit [15]. The docking model predicts that binding of RsfA to these residues, as a consequence, would sterically interfere with ribosome subunit joining (Figure 2B(b)) and thus might block translation.
RsfA confers a strong selective advantage under natural growth conditions
Although RsfA is phylogenetically highly conserved, its gene deletion has been reported not to result in any obvious growth disadvantage in E. coli [7], [16]. We designed a sensitive growth experiment, which compares the WT and the rsfA deletion strain under competitive growth conditions: we mixed equal amounts of both cell types and monitored the populations at constant time intervals under log-phase conditions. Figure 3A demonstrates that the amounts of mutant cells decreased continuously. In other words, WT cells in rich medium steadily overgrew the mutant cells leaving only about 10 to 25% of mutant cells after 35 generations. This modest effect reveals that RsfA mutant cells suffer from a disadvantage when competing with WT cells. Strikingly, a much stronger difference was observed, when cells grown in rich medium were diluted in minimal medium: the WT strain overgrew the mutant ΔrsfA strain within only five generations. The opposite growth transition (poor→rich media) is better tolerated by the mutant strain. The addition of amino acids to the minimal medium completely rescues this striking growth defect of the rsfA mutant in the rich→poor media transition (see Discussion).
Fig. 3. RsfA inhibits translation during both stationary phase and the transition from rich to poor media. 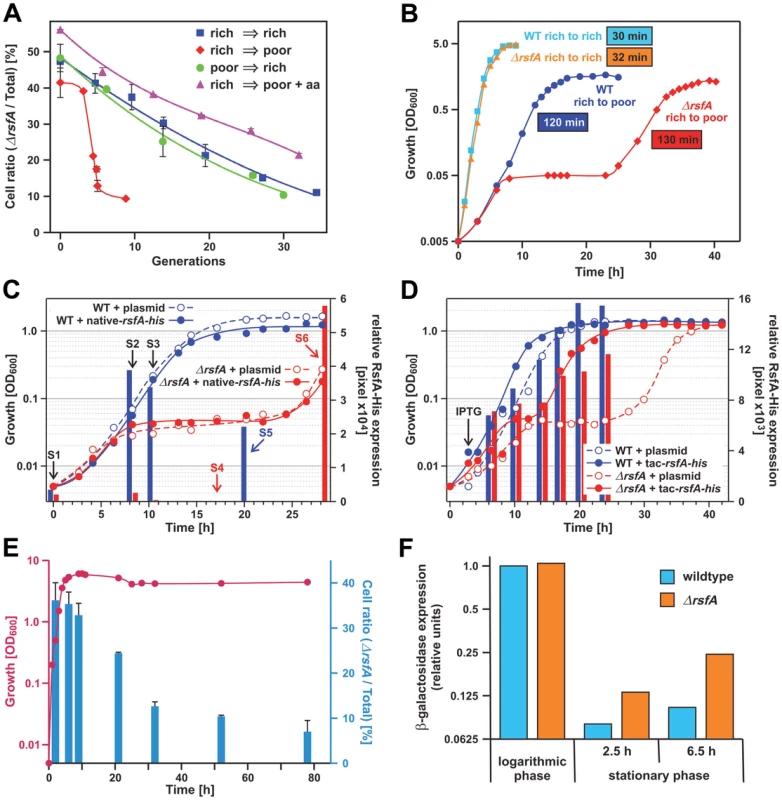
(A) Growth competition experiment: equal numbers of E. coli wild type and ΔrsfA cells derived from an overnight LB-culture were mixed and grown in rich medium (LB, rich→rich), poor medium (M9, rich→poor) and poor medium plus 2% casamino acids as indicated (rich→poor+aa). Growth was maintained in log phase conditions by regular dilutions in the corresponding media. Shown is the fraction of viable ΔrsfA mutant cells in the total cell population. (B) Wild type and mutant strains were grown overnight in rich medium (LB) and then diluted in rich (rich→rich) or poor M9 medium (rich→poor). The generation time was derived from the slopes of the regression lines made of the points indicating the logarithmic phase. The errors of the generation-time determinations are below ±5%, i.e. generation times of 30 and 32 min are not significantly different. (C) Wild type and mutant strains transformed with a plasmid harboring the gene for RsfA fused with a His-tag under control of the native promoter or the corresponding empty plasmid were grown overnight in rich medium (LB) and then diluted in poor M9 medium. At certain times samples were withdrawn (S1–S6) and the relative amount of RsfA was quantified by Western-blot (represented with bars). S1–S3: samples were analyzed from both strains. S4–S6: samples were analyzed only from wild type (blue) or mutant strain (red). (D) Same as (C) but using a plasmid with a His-tagged RsfA gene under a tac promoter. After ∼3 h incubation in M9 medium 0.2 mM IPTG (final concentration) was added to all strains in order to induce expression from the tac promoter. (E) Viability competition similar to the growth competition described under (A) but in a batch culture without dilution. Red, growth of the mixture of ΔrsfA and WT strains; blue, the fraction (in %) of the mutant strain. (F) Expression of β-galactosidase as reporter to test translational activity of logarithmic and stationary phase cells in WT and ΔrsfA cells induced by 2% arabinose. Induction time was 3 h in logarithmic and 2.5 and 6.5 h in stationary phase. The expression level was derived from the band-intensity on a gel (Coomassie-stained SDS-PAGE). These strong defects seen with the ΔrsfA strain in minimal medium rather than in rich medium should be evident also in a direct determination of the doubling times of wild type versus mutant in separate cultures. In rich medium the generation times of WT and mutant strains were not significantly different (30 and 32 min, respectively; Figure 3B). However, a change from rich to poor medium revealed a dramatic difference: initially the ΔrsfA mutant strain showed a growth like the WT strain for about 7 h, but then growth was abrogated for about 14 h before it resumes almost with the same doubling time as the WT strain (130 versus 120 min). The growth block for many hours demonstrates that the lack of the rsfA gene poses a serious adaptation problem on the cells after a transition from rich to poor medium.
It has been reported that the rsfA (formerly ybeB) knock-out can cause a defect in cell separation in a distinct genetic background, and this defect can be complemented with genes of the rsfA operon downstream of the rsfA gene indicating a polarity effect of the rsfA deletion [17]. Therefore, we tested whether we can complement the strong mutant phenotype observed in Figure 3A and 3B by introducing a plasmid carrying the rsfA gene. If so, it would prove that the mutant phenotype is caused by the absence of the RsfA factor. To this end, we removed the kanamycin cassette in place of the chromosomal rsfA gene and introduced a plasmid with the rsfA gene under the native promoter; the expressed RsfA carried a His-tag at the C-terminus to monitor the expression by anti-His antibodies. Figure 3C demonstrates that the mutant phenotype could not be cured probably due to the fact that after the shift to the poor medium RsfA was not sufficiently expressed, whereas taking up growth after 30 h was accompanied by a strong RsfA expression (see red bars in Figure 3C). Therefore, we performed the same experiment but now with the rsfA gene under a tac promoter. The forced RsfA expression could heal the mutant phenotype (Figure 3D; red closed circles). We conclude that (i) the RsfA expression is regulated in a way we do not yet understand, and (ii) that the lack of RsfA is responsible for the mutant phenotype.
Figure 3A and 3B demonstrate that mutant and WT strains showed almost the same growth behavior under log-phase conditions in rich medium (LB). But what happens in a batch culture, when a mixture of both strains reaches the stationary phase in rich medium and protein synthesis has to be down regulated? This was tested in the next experiment. The stationary phase is reached after about 7 h (red line in Figure 3E). At various time points aliquots were taken and the fraction of ΔrsfA mutant strains were determined (blue bars). Until reaching the stationary phase the fraction of mutant cells remains constant at about 35%, but thereafter the fraction of mutant cells sharply declined to less than 10%. This viability competition assay indicates that the mutant cells have serious problems to form stable stationary-phase cells.
The experiments shown in Figure 3A–3E disclose two strong phenotypes caused by the lack of RsfA: (i) The cells adapt poorly after the transition from rich to poor media, and (ii) the viability of cells is dramatically impaired during the stationary phase, eventually causing cell death.
RsfA acts as a negative modulator of protein translation in vivo
Given RsfA's physical association with the large ribosomal subunit/L14, we wondered whether RsfA has an effect on protein synthesis. To this end we expressed β-galactosidase (as an L-arabinose inducible reporter) in an E. coli gene deletion strain (ΔrsfA) and wild type (WT) cells. At stationary phase the β-galactosidase expression was strongly repressed in wild type cells as expected (Figure 3F). In striking contrast, the ΔrsfA mutant exhibited a significant accumulation of β-galactosidase in the stationary phase. These results demonstrate that RsfA acts as a negative modulator of protein translation in vivo in the stationary phase. Together with the viability assay (Figure 3E) these results suggest that silencing protein synthesis plays an important role for reorganization of the metabolic conversion on the way to the stationary phase.
RsfA is a ribosomal silencing factor that interferes with the association of ribosomal subunits
Next we tested whether RsfA interferes with ribosomal elongation in vitro using a highly resolved E. coli system just containing purified elongation factors EF-Tu, EF-Ts, EF-G, purified precharged [14C]Phe-tRNA, poly(U) programmed ribosomes and GTP as energy source. We added 30S subunits to an excess of 50S subunits in order to facilitate association to 70S ribosomes. Purified RsfA suppressed the translational activity dramatically down to about 20%, when RsfA was added to the 50S subunits before the oligo(Phe) synthesis (Figure 4A, left panel). To test whether RsfA blocks ribosomal activities via interfering with association of the subunits as suggested by our protein docking model (Figure 2B), we subjected an aliquot to a sucrose-gradient analysis before incubating for oligo(Phe) synthesis (Figure 4B). The gradients demonstrate that in the absence of RsfA clearly more 70S ribosomes are formed on the cost of ribosomal subunits. However, when RsfA was added to programed 70S ribosomes carrying an AcPhe-tRNA at the ribosomal P site, no inhibition was observed indicating that RsfA does not interfere with ribosomal functions during the elongation phase (Figure 4A, right panel). We conclude that RsfA blocks association of the ribosomal subunits to functional 70S ribosomes.
Fig. 4. RsfA inhibits translation by blocking ribosomal subunit joining. 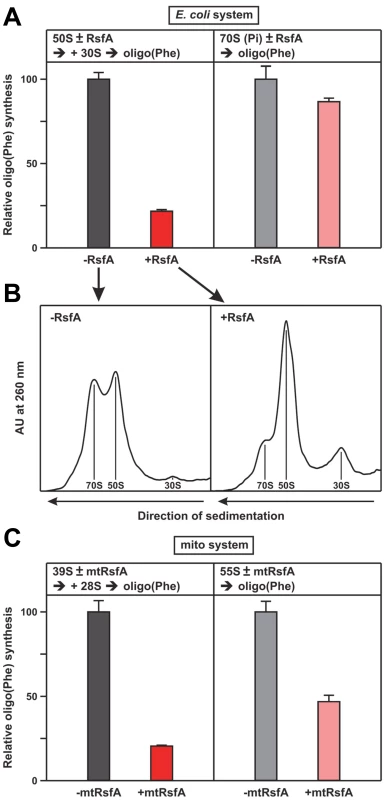
(A) Oligo(Phe) synthesis in a pure system containing pre-charged Phe-tRNAs (ten times over ribosomes), 30S and 50S subunits and the purified factors EF-Tu, EF-Ts and EF-G plus/minus RsfA from E. coli, 100% corresponds to 7 Phe incorporated per ribosome. Left panel, when indicated RsfA was added to the 50S subunits, before 30S subunits were added starting oligo(Phe) synthesis. Right panel, AcPhe-tRNA was bound to 70S ribosomes in the presence of poly(U) before the addition of RsfA. (B) Sister-aliquots from the same samples shown in (A) were analyzed on a sucrose gradient before oligo(Phe) synthesis. The presence of RsfA significantly reduces the fraction of 70S ribosomes. (C) Oligo(Phe)-synthesis as in (A) but with purified mitochondrial components (pig liver) and human mtRsfA (C7orf30). 39S and 28S indicate the large and small ribosomal subunits, 55S the associated mitochondrial ribosomes. For details see Experimental Procedures. Corresponding experiments with the translational elements of mitochondrial ribosomes from mammalian cells (pig liver) confirmed these results. In the presence of purified mitochondrial factors mtEF-Tu, mtEF-Ts, mtEF-G1, poly(U) and [14C]Phe-tRNA oligo(Phe) synthesis was severely reduced upon addition of the mitochondrial RsfA orthologue C7orf30 (mtRsfA; Figure 4C). The results suggest that the function of RsfA is conserved from bacteria to eukaryotic mitochondria.
Discussion
The cellular synthesis machinery runs at high speed in the exponential (logarithmic) phase of bacterial growth. The growth rate slows in semi-log phase and finally comes to a halt at higher cell density in the stationary phase, usually caused by nutrient depletion. Several bacterial factors bind to ribosomes and thus support the dormant state of the ribosomes in the stationary phase, such as the ribosome modulation factor (RMF), hibernation promoting factor (HPF) or stationary-phase-induced ribosome-associated protein (SRA) [18], [19], [20], [21]. RMF (homologues exist only in the γ-proteobacteria) alone or together with the more broadly distributed HPF are essential for the formation of 70S dimers in the stationary phase, so called 100S particles; an inactivation of the RMF gene causes a viability defect at prolonged periods in stationary phase [22], [23]. Phenotypical effects of knock-out strains concerning the other factors have not been reported.
A first analysis of RsfA-binding partners identified a group of proteins including a number of ribosomal proteins [6]. Similarly, other groups suggested various ribosomal proteins as binding partners [3], [4], [5], the common denominator being that all proteins were derived from the large subunit. Thorough analyses presented here identified the ribosomal protein L14 as the docking station (Figure 1B–1G, Figure 2), and mutation of conserved amino acid residues of L14 at the surface of this protein abolished RsfA binding, clearly demonstrating L14 as the binding protein (Figure 2). Interestingly, the three most conserved residues of RsfA as shown by the multiple sequence alignment (Figure S1A) are located at the interface with L14 predicted by docking. The three residues are W120, D124 and R140 (alignment numbers), corresponding to residue numbers W77, D81 and R95 in E. coli RsfA. D81 is predicted to be in direct contact with R98 of L14 that was shown to disrupt the interaction when mutated. Another such critical residue, K114 of L14, is predicted to be in contact with a fairly conserved residue with RsfA L103 (position 148 in the alignment).
The only other known protein that like RsfA also docks to the ribosomal protein L14 of eukaryotic ribosomes is the so-called initiation factor eIF6, which is not a homologue to RsfA and is thought to block ribosome association in archaea and in eukaryotes from yeast to man [24], [25], [26], [27], [28], [29]. However, in eukaryotes eIF6 is rather a 60S assembly factor and plays an essential role in the late pre-25S rRNA processing and the export of the 60S subunit from the nucleolus to the cytoplasm [30]. Depletion of eIF6 is eventually lethal, in contrast to RsfA. Interestingly, eIF6 is restricted to the eukaryotic nucleus/cytoplasm and to archaea [27], while RsfA is present in almost all bacteria and their descendent eukaryotic organelles (Figure 1A).
Studies with the human mitochondrial homologue of RsfA, C7orf30, have recently suggested that this protein is involved in ribosomal assembly and/or translation [5], [8]. Our results do not indicate any assembly defects as deletion strains of rsfA appear to have perfectly assembled ribosomes (sucrose gradients not shown) and actually translate as well as wild type strains at logarithmic phase (Figure 3F). In addition, we could show that C7orf30 inhibits translation by mitochondrial ribosomes (Figure 4C). It remains possible that C7orf30 has multiple roles in mitochondria or that its role in ribosome assembly is indirect.
In rich medium bacterial cells produce proteins at maximum rates to sustain cell division. Furthermore, bacterial cells take up many metabolic precursors such as amino acids and thus block corresponding synthesis pathways. In contrast, in poor/minimal medium protein synthesis must be down-regulated in a concerted fashion in order to save energy and resources, and at the same time many synthesis pathways such as those for the synthesis of amino acids have to be switched on [31], [32]. The results presented here suggest that RsfA plays a prominent role in this down-regulation by silencing ribosome activities. We observe two strong phenotypes with the ΔrsfA strain: (i) the viability is strongly impaired in the stationary phase (Figure 3E) and (ii) after a transition from rich to poor media the adaptation phase lasts more than 10 hours before resuming growth again in striking contrast to WT cells (Figure 3B), which overgrow the mutant strain in a few generations. Just adding casamino acids to the minimal medium relieves the strong growth defects of the ΔrsfA strain (Figure 3A). Adding amino acids will switch off most of the amino-acid synthesis pathways similar to the situation during the logarithmic phase in the presence of rich medium, when the silencing effect of RsfA is not strictly required. In contrast, during starvation and in the absence of ribosomal silencing (ΔrsfA), energy would be wasted affecting the conversion of the metabolic network, eventually causing deleterious growth defects. Accordingly, protein synthesis is seriously attenuated in the stationary phase, when RsfA is present (i.e. wild type cells) in contrast to protein synthesis in the ΔrsfA strain (Figure 3F). Attenuation of protein synthesis by RsfA seems to be of utmost importance for reorganization the metabolic state on the way to the stationary phase, since the absence of this factor threatens seriously the viability in the stationary phase (Figure 3E), and it explains the well-known effect that ribosomes are much less active, when derived from the stationary rather than from log-phase cells [33].
When RsfA is added to ribosomal subunits it blocks 70S formation and thus protein synthesis (Figure 4A and 4B), whereas the factor does not interfere with the elongation phase of protein synthesis when added to ribosomes that have passed the initiation phase (Figure 4A, right panel). We conclude that RsfA, as a ribosomal silencing factor, is damping the translational activity under restricted energy (stationary phase) or nutrient conditions (growth in poor medium) thus harmonizing translation with the general metabolic state, i.e. RsfA works in line with the stringent response [34] and thus plays a key role in the physiology of the stationary phase and the translational adaptation during the transition from rich to poor medium.
Our experiments suggest a direct silencing effect of RsfA sketched in Figure 5: when the ribosomal activity should be silenced, RsfA binds to the ribosomal protein L14 at the interface of the large subunit and by impairing association of the ribosomal subunits translation is hampered. We demonstrated that RsfA damps the ribosomal elongation in bacterial and mammalian mitochondrial systems (Figure 4A and 4C). The importance of RsfA in eukaryotic organelles is indicated by the fact that a mutation in the gene of the RsfA orthologue Iojap in Zea mays leads to irregular albino patterns on maize leafs and germless seeds due to failure of proplastids to differentiate into chloroplasts [35], [36], [37], [38]. Photosynthesis and respiration can vary enormously in plastids and mitochondria, respectively, and as suggested by the experiment shown in Figure 4C, the RsfA orthologue might accordingly regulate protein synthesis in these organelles using the mechanism suggested here.
Fig. 5. A model of RsfA action. 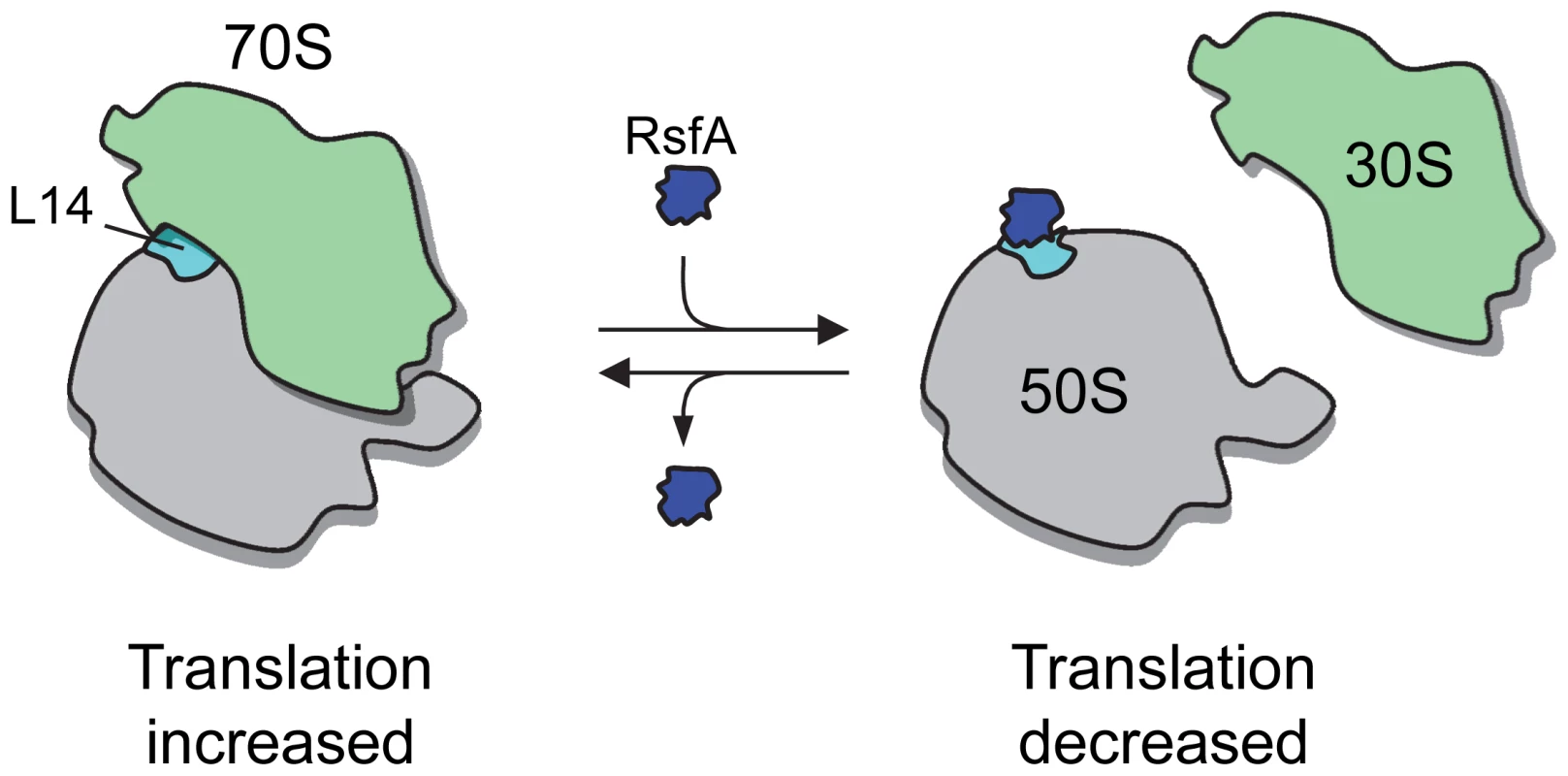
In rich medium and during exponential growth, RsfA is either not present or not active, so that protein synthesis is fully active. In starving cells, RsfA binds to ribosomal L14 and, as a consequence, blocks ribosomal subunit joining and thus protein synthesis. Materials and Methods
Cloning
ORFs were cloned into pDONR207 by using the Gateway Technology (Invitrogen). Zea mays cDNA was kindly provided by F. Hochholdinger (Tübingen, Germany), HeLa cDNA by O. Kassel (Karlsruhe, Germany), S. pneumoniae TIGR4 DNA by D. Nelson (UMBI, MD, USA), T. pallidum DNA by T. Palzkill (Houston, USA), and Synechocystis PCC 6803 DNA by T. Lamparter (Karlsruhe, Germany). All ORFs were cloned with a stop codon at the 3′-ends. Entry plasmids were sequenced, shuttled into expression vectors (see below), and finally verified by PCR reactions. For the interologous tests E. coli ORFs were kindly provided as pENTR/Zeo clones by S.V. Rajagopala [39] except for RsfA and L14 which have been cloned in this study.
E. coli L14 (b3310) alanine substitutions were directionally introduced by performing standard fusion PCR reactions using mutagenic primers. For cloning PrimeStar HS DNA Polymerase was used (Takara Bio Inc.).
Yeast two-hybrid assays
Entry plasmids were recombined with the bait and prey vector pGBKT7g and pGADT7g (Clontech) [40]. These were individually transformed into the haploid yeast strains AH109 and Y187 [41], [42]. After mating the haploids and enrichment of diploids, yeast growth was observed on solid starvation medium lacking Leucine, Tryptophan, and Histidine. The medium contained various concentrations of 3-AT (0 to 100 mM). Detailed procedures were done as described elsewhere [43].
In case of the L14-interaction epitope mapping experiment bait and prey plasmids were sequentially cotransformed into haploid yeast strain CG-1945 (Clontech) and then assayed as described above.
Pull down assays
ORFs were shuttled from entry plasmids into pNusA (Santhera, Liestal, Switzerland), pETG-40A, or pETG-30A (EMBL, Heidelberg, Germany) and transformed or co-transformed into E. coli BL21(DE3) (combinations, see main text, Figure 1D and 1E and Figure S3A). Proteins were expressed following standard protocols. Cell pellets were lysed in 500 µl buffer (50 mM Tris-HCL pH 8.0, 100 mM NaCl, 50 µg/ml chicken egg white lysozyme, 50 µM PMSF, Sarcosyl/Triton-X 100 0.1%, each) and then sonicated and centrifuged. The supernatants were used for pull-down experiments: for E. coli RsfA and L14 corresponding volumes of 50 µg soluble protein fractions of co-expressed proteins were applied to beads and aliquots saved as input controls. For human and Zea mays proteins 25 µg soluble fractions were mixed and then applied to the beads. MBP fusions were co-purified with their GST baits on 20 µl glutathione beads and NusA-tagged preys with their MBP fusions on 20 µl amylose beads under buffer conditions indicated above but w/o lysozyme. Binding occurred at room temperature for 30 min. Then, the beads were washed and finally boiled in 50 µl Laemmli buffer. 10 µl of output (∧ = 10 µg protein input) and 10 µg input samples were separated by SDS PAGE using 12% gels. Proteins were transferred onto a polyvinylidene fluoride membrane by semi-dry Western blotting. The recombinant bait and prey proteins were labeled by standard immunodetection procedure and then analyzed by enhanced chemiluminescence.
In vivo localization and BiFC assays
Human C7orf30 (mtRsfA) and L14mt full-length ORFs were cloned into pcDNA3.1-HA-mCherry [44], pcDNA3.1(+)-HA-VN, and pcDNA3.1(+)-HA-VC [45] (Note: an N-terminal HA tag from the vector backbones was removed under consideration that the native mitochondrial localization peptides of mtRsfA ( = C7orf30) and L14mt are N-terminally exposed).
For localization studies, Hela cells were transfected (100 ng, each plasmid) with mCherry-tagged C7orf30 or L14mt using Promofectin (Promokine, Germany). 100 ng pECFP-Mem (Clontech) was co-transfected to stain cell membranes. 24 h later, MitoTracker Green FM (100 nM f.c., Invitrogen) was added. After washing, DRAQ5 (1∶2,000, Biostatus) was added fur nuclear staining.
For BiFC assays [46], Hela cells were prepared correspondingly. Exceptions: Mitotracker staining was not done and instead of localization constructs, cells were co-transfected with BiFC plasmid constructs (50 ng, each) in combinations as given in Figure 1G.
30 min post DRAQ5 administration cells were analyzed by fluorescence microscopy using a Zeiss LSM 510 Meta confocal laser scanning microscope.
Conservation of L14 residues
Multiple alignments were generated using ClustalW [47] with the L14 amino acid sequences from E. coli, T. pallidum, S. pneumoniae, Synechocystis PCC 6803, C. jejuni, H. sapiens, Zea mays, Chromobacterium violaceum, Bacillus halodurans, and S. cerevisiae using default parameters. Based on that alignment the conservation scores were calculated with the ConSurf Server [48]. 3D images (Figure 2A) were presented using PyMol 1.5 (http://pymol.org).
Protein docking
Structures of unbound proteins: the E. coli L14 structure was taken from 2AWB PDB entry, chain K [14]. Because the crystal structure of E.coli RsfA is not available, we used I-TASSER server [49] to build a model of that protein. The server built a single model using as templates 2ID1_A and 2O5A_A. The server has estimated the accuracy of the model as 0.90±0.06 (TM-score) and 1.6±1.4 Å (RMSD).
An unconstrained rigid body docking was performed of individual L14 and RsfA structures with GRAMM-X [50]. We then used the coordinates of L14 to superimpose 100 top scored docking models onto the entire 70S unit (2AWB and 2AW7 PDB IDs). Then, each model was evaluated for the backbone clashes between the predicted RsfA position and the rest of the 50S subunit. We defined a clash as having less than 2 Å distance between backbone atoms in order to tolerate some degree of unknown conformational re-arrangement of the 50S components that were not used in docking. Model #17 was the first one in order of the docking score where RsfA had no clashes with other parts of 50S (parts not seen by the docking procedure). Model #17 contained certain surface exposed amino acid residues of L14 that are highly conserved (Figure 2B). To test whether these are involved in mediating the interaction with RsfA they were subjected to alanine substitution constructs (see above and Figure 2A) and analyzed in Y2H experiments (Figure 2C). The interface contacts were defined as having less than 4.6 Å distance between any heavy atoms of the docking subunits. We used PyMol 1.5 (http://pymol.org) for the post-docking analysis and graphics.
β-galactosidase expression in logarithmic and stationary phase
ΔrsfA (b0637) [16] and wild type (BW25113) were transformed with a β-galactosidase reporter plasmid, pBAD24-lacZ-HA (based on pBAD24HA) [51], [52] and selected on LB agar containing 50 µg/ml ampicillin. Both were grown overnight in LB in the presence of 50 µg/ml ampicillin and 0.4% glucose as inhibitor of leaky expression. For stationary phase expression cultures were centrifuged at 5,000 rpm (15 min) and pellets were resuspended in the cell-free supernatant of an LB overnight culture (BW25113/ΔrsfA, no plasmid) lacking glucose. β-galactosidase expression was induced with 2% arabinose; the resuspension was adjusted to the same cell density as the previous stationary-phase culture. For logarithmic phase expression overnight cultures were centrifuged at 5,000 rpm for 15 min and pellets were resuspended in fresh LB medium (no glucose) with 50 µg/ml ampicillin for both strains. Cultures were then diluted to OD600 = 0.05 and grown for 2 h. β-galactosidase expression was induced by adding 2% arabinose to the medium. The cultures were shaken at 37°C. Every hour 300 µl suspension was withdrawn, 100 µl from it was loaded into a well of a 96-well plate (flat bottom) and the growth was followed by monitoring the extinction at 600 nm (ELISA spectrophotometer). The rest of aliquots were centrifuged at 12,000 rpm for 5 min and pellets were resuspended in 20 µl loading buffer (2×) Tris-glycine SDS and incubated at 95°C for 5 min to denature proteins. Samples were loaded on SDS-polyacrylamide gel (10%) and the β-galactosidase amount was quantified as relative protein-band intensity using ImageJ 1.45.
Growth/viability competition assay
For growth competition assays (Figure 3A) the same amount of cells from overnight cultures of wild type and ΔrsfA strains were mixed, yielding a final OD600 of 0.01 in a volume of 5 ml, and incubated with mild shaking either in LB (rich) or M9 medium with 0.4% glucose (poor). Aliquots were withdrawn every 3 h or 6 h or 24 h (depending on the growth rate) and OD600 was measured. Simultaneously, dilutions to approximately 5,000 cells/ml (according to the assumption that 1 OD600 corresponds roughly to 109 cells) were made and 100 µl of each was plated in duplicates on either LB plates or LB plates containing 25 µg/ml kanamycin. The number of colonies (ΔrsfA contained a kanR-cassette, WT not) was counted after incubation at 37°C for overnight. For viability competition experiment in stationary phase (LB medium; Figure 3F) ΔrsfA mutant and wild type strain were separately grown overnight. Subsequently two cultures were diluted to OD600 = 0.005 and incubated with shaking till 0.5 OD600. Then two cultures were mixed and the fitness of ΔrsfA was monitored as numbers of colonies on LB plates (mutant and wild type colonies) and LB plates containing kanamycin (only mutant colonies) after 2, 6, 9, 21, 32, 52, 78 hours of incubation at 37°C.
Romoval of the kanR-cassette in the ΔrsfA strain
The kanamycin resistance gene that substituted the rsfA was removed by introducing a flippase-encoding plasmid pCP20 as described elsewhere [53]. The successful flip-out was verified by a genotyping PCR.
Media shift rich to poor
For the media shift (Figure 3B) wild type and ΔrsfA strains were grown overnight in LB medium (rich) and then diluted in either LB (rich) or M9 medium (poor) yielding a start OD600 = 0.005. Cultures were incubated at 37°C with shaking (200 rpm) and growth was monitored measuring the OD600 over a time of up to 40 hours.
For curing the phenotype of the ΔrsfA strain during the transition from rich to poor (Figure 3C and 3D) ΔrsfA cells lacking the kanamycin resistance gene and wild type cells were transformed with a plasmid harbouring the gene coding for RsfA fused with a C-terminal His-tag under control of either the native promoter or the IPTG inducible tac-promoter and with the corresponding empty plasmid.
The transformed strains were grown overnight in rich (LB) medium at 37°C and then diluted in poor M9 medium yielding a start OD600 = 0.005 and incubated like described above. At several time points samples were withdrawn and the expression of RsfA was analysed after SDS-PAGE and Western-blot using an antibody directed against the His-tag. The intensity of the RsfA-His bands was quantified using ImageQuant 5.2 and normalized for correction of the input to a non-altered protein band of the Coomassie stained gel.
Expression and purification of E. coli RsfA and human mtRsfA
The gene coding for E. coli RsfA (b0637) was expressed as an N-terminal His6 tag fusion in E. coli BL21(DE3). Expression was induced at OD600 = 0.4 with 0.1 mM IPTG and carried out for 2 h at 30°C to decrease the formation of inclusion bodies. The soluble protein was purified via nickel-nitrilotriacetic-acid-agarose (Qiagen, according to the manufacturer's manual) and anion exchange chromatography (Source 15Q, GE Healthcare). The purified protein was dialyzed against 20 mM Hepes, 6 mM Mg-acetate, 150 mM K-acetate, 4 mM β-mercaptoethanol, pH 7.6 at 0°C.
The gene coding for the mature human mitochondrial RsfA (C7orf30; amino acids 23–234) was expressed and the protein purified like the E. coli RsfA orthologue.
Both proteins were expressed using the Gateway System-compatible plasmid pHGWA [54].
Isolation of ribosomal components
Ribosomes and ribosomal subunits were prepared from E. coli strains CAN20-12E [55] as described [56]. Preparation of mammalian mitochondrial ribosomes and ribosomal subunits (pig liver) followed [57] with minor modifications. Hepes-buffer and TCEP were utilized instead of Tris-buffer and 2-mercaptoethanol, respectively. Isolation of mitochondrial factors are described in [58].
Poly(U)-dependent oligo(Phe) synthesis with precharged Phe-tRNA and sucrose gradient analysis
18 pmol 50S ribosomes were incubated with 180 µg poly(U) with or without 360 pmol RsfA in 90 µl for 10 min at 37°C in binding buffer (20 mM Hepes, pH 7.6 at 0° C, 4.5 mM Mg-acetate, 150 mM K-acetate, 4 mM β-mercaptoethanol, 2 mM spermidine, 0.05 mM spermine, H20M4.5K150SH4Spd2Spm0.05). Reaction was further incubated with 10 pmol 30S ribosomes for 10 min at 37°C and then analyzed in poly(U) dependent oligo(Phe) synthesis and sucrose gradient centrifugation.
15 µl of the reaction was used for oligo(Phe) synthesis. 2.4 pmol EF-G together with the ternary complex mix were added yielding 30 µl in binding buffer H20M4.5K150SH4Spd2Spm0.05. The ternary complex mix contained in 15 µl 30 pmol [14C]Phe-tRNAPhe, 45 pmol EF-Tu, 45 pmol EF-Ts, 3 mM GTP and was preincubated 5 min at 37°C. Incubation was at 30°C for 2 min and 12.5 µl aliquots were precipitated with TCA, incubated at 90°C in the presence of 2 drops of 1% (w/v) BSA and filtered through glass filters and counted.
60 µl of the reaction was mixed with 40 µl H20M4.5K150SH4Spd2Spm0.05 and loaded onto a 10–30% sucrose gradient prepared in the same buffer. Centrifugation was carried out at 42,000 rpm for 4 h in an SW60 rotor. The gradient was pumped out from bottom to top and the A260 was measured to obtain the ribosome profile.
The corresponding assay with mitochondrial components from pig liver was performed in H20M4.5K150SH4Spd2Sp0.05 pH7.5 (at 0°C). mtRsfA was pre-incubated with 2.5 pmol large subunit 39S in 80 molar excess over ribosomes, before the same amount of 28S subunits were added; likewise 2.5 pmol 55S ribosomes were incubated with the same amount of RsfA. EF-G1 was added in a 0.8-fold excess over ribosomes. 37.5 pmol of [14C]Phe-tRNA were present and the mitochondrial factors mtEF-Tu and mtEF-Ts, were added both in an excess of 1.5 over Phe-tRNA. The total volume was 100 µl, the main incubation 20 min at 30°C. The following processing was as described above.
The oligo(Phe) synthesis with reassociated 70S ribosomes (Figure 4A, right panel) was performed in the following way: 3 pmol 70 S ribosomes were incubated with 30 µg poly(U) and 6 pmol Ac-Phe-tRNA for 10 min at 37°C. When indicated 60 pmol RsfA was added and the oligo(Phe) synthesis performed as described above. The total volume was 20 µl, the mixture was incubated for 5 min at 37°C.
Supporting Information
Zdroje
1. YamadaTBorkP 2009 Evolution of biomolecular networks: lessons from metabolic and protein interactions. Nat Rev Mol Cell Biol 10 791 803
2. GalperinMYKooninEV 2004 ‘Conserved hypothetical’ proteins: prioritization of targets for experimental study. Nucleic Acids Res 32 5452 5463
3. ButlandGPeregrin-AlvarezJMLiJYangWYangX 2005 Interaction network containing conserved and essential protein complexes in Escherichia coli. Nature 433 531 537
4. GavinACAloyPGrandiPKrauseRBoescheM 2006 Proteome survey reveals modularity of the yeast cell machinery. Nature 440 631 636
5. WanschersBFSzklarczykRPajakAvan den BrandMAGloerichJ 2012 C7orf30 specifically associates with the large subunit of the mitochondrial ribosome and is involved in translation. Nucleic Acids Res 40 4040 4051
6. TitzBRajagopalaSVGollJHauserRMcKevittMT 2008 The binary protein interactome of Treponema pallidum–the syphilis spirochete. PLoS ONE 3 e2292 doi:10.1371/journal.pone.0002292
7. JiangMSullivanSMWalkerAKStrahlerJRAndrewsPC 2007 Identification of novel Escherichia coli ribosome-associated proteins using isobaric tags and multidimensional protein identification techniques. J Bacteriol 189 3434 3444
8. RorbachJGammagePAMinczukM 2012 C7orf30 is necessary for biogenesis of the large subunit of the mitochondrial ribosome. Nucleic Acids Res 40 4097 4109
9. SzklarczykDFranceschiniAKuhnMSimonovicMRothA 2011 The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res 39 D561 568
10. HortonPParkKJObayashiTFujitaNHaradaH 2007 WoLF PSORT: protein localization predictor. Nucleic Acids Res 35 W585 587
11. ZengXBarrosMHShulmanTTzagoloffA 2008 ATP25, a new nuclear gene of Saccharomyces cerevisiae required for expression and assembly of the Atp9p subunit of mitochondrial ATPase. Mol Biol Cell 19 1366 1377
12. HanC-dMartienssenRA 1995 The Iojap (Ij) protein is associated with 50S chloroplast ribosomal subunits. MNL 69 32 34
13. ParrishJRYuJLiuGHinesJAChanJE 2007 A proteome-wide protein interaction map for Campylobacter jejuni. Genome Biol 8 R130
14. SchuwirthBSBorovinskayaMAHauCWZhangWVila-SanjurjoA 2005 Structures of the bacterial ribosome at 3.5 A resolution. Science 310 827 834
15. GaoHSenguptaJValleMKorostelevAEswarN 2003 Study of the structural dynamics of the E coli 70S ribosome using real-space refinement. Cell 113 789 801
16. BabaTAraTHasegawaMTakaiYOkumuraY 2006 Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2 2006 0008
17. BernhardtTGde BoerPA 2004 Screening for synthetic lethal mutants in Escherichia coli and identification of EnvC (YibP) as a periplasmic septal ring factor with murein hydrolase activity. Mol Microbiol 52 1255 1269
18. WilsonDNNierhausKH 2007 The weird and wonderful world of bacterial ribosome regulation. Crit Rev Biochem Mol Biol 42 187 219
19. UetaMOhniwaRLYoshidaHMakiYWadaC 2008 Role of HPF (hibernation promoting factor) in translational activity in Escherichia coli. J Biochem 143 425 433
20. YoshidaHYamamotoHUchiumiTWadaA 2004 RMF inactivates ribosomes by covering the peptidyl transferase centre and entrance of peptide exit tunnel. Genes Cells 9 271 278
21. IzutsuKWadaAWadaC 2001 Expression of ribosome modulation factor (RMF) in Escherichia coli requires ppGpp. Genes Cells 6 665 676
22. WadaAMikkolaRKurlandCGIshihamaA 2000 Growth phase-coupled changes of the ribosome profile in natural isolates and laboratory strains of Escherichia coli. J Bacteriol 182 2893 2899
23. UetaMWadaCWadaA 2010 Formation of 100S ribosomes in Staphylococcus aureus by the hibernation promoting factor homolog SaHPF. Genes Cells 15 43 58
24. KlingeSVoigts-HoffmannFLeibundgutMArpagausSBanN 2011 Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science 334 941 948
25. GreberBJBoehringerDGodinic-MikulcicVCrnkovicAIbbaM 2012 Cryo-EM Structure of the Archaeal 50S Ribosomal Subunit in Complex with Initiation Factor 6 and Implications for Ribosome Evolution. J Mol Biol 418 145 160
26. PechMNierhausKH 2012 Identical Binding Sites-Nonidentical Functions in Eukarya and Archaea: The Complex of aeIF6 with the Large Ribosomal Subunit. J Mol Biol 418 131 133
27. BenelliDMarziSManconeCAlonziTla TeanaA 2009 Function and ribosomal localization of aIF6, a translational regulator shared by archaea and eukarya. Nucleic Acids Res 37 256 267
28. GartmannMBlauMArmacheJPMielkeTTopfM 2010 Mechanism of eIF6-mediated inhibition of ribosomal subunit joining. J Biol Chem 285 14848 14851
29. GuoJJinZYangXLiJFChenJG 2011 Eukaryotic initiation factor 6, an evolutionarily conserved regulator of ribosome biogenesis and protein translation. Plant Signal Behav 6 766 771
30. BiswasAMukherjeeSDasSShieldsDChowCW 2011 Opposing action of casein kinase 1 and calcineurin in nucleo-cytoplasmic shuttling of mammalian translation initiation factor eIF6. J Biol Chem 286 3129 3138
31. AnderssonSKurlandCG 1990 Codon Preferences in Free-Living Microorganisms. Microbiological Reviews 54 198 210
32. DongHJNilssonLKurlandCG 1996 Co-variation of tRNA abundance and codon usage in Escherichia coli at different growth rates. J Mol Biol 260 649 663
33. SchepsRWaxRRevelM 1971 Reactivation in vitro of inactive ribosomes from stationary phase Escherichia coli. Biochim Biophys Acta 232 140 150
34. EnglishBPHauryliukVSanamradATankovSDekkerNH 2011 Single-molecule investigations of the stringent response machinery in living bacterial cells. Proc Natl Acad Sci U S A 108 E365 373
35. RhoadesMM 1943 Genic Induction of an Inherited Cytoplasmic Difference. Proc Natl Acad Sci U S A 29 327 329
36. ShumwayLKWeierTE 1967 The Chloroplast Structure of Iojap Maize. American Journal of Botany 54 773 780
37. ThompsonDWalbotVCoeEH 1983 Plastid Development in Iojap - and Chloroplast Mutator-Affected Maize Plants. American Journal of Botany 70 940 950
38. JenkinsMT 1924 Heritable characters of maize. XX. Iojap-striping, a chlorophyll defect. J Hered 15 467 472
39. RajagopalaSVYamamotoNZweifelAENakamichiTHuangHK 2010 The Escherichia coli K-12 ORFeome: a resource for comparative molecular microbiology. BMC Genomics 11 470
40. UetzPDongYAZeretzkeCAtzlerCBaikerA 2006 Herpesviral protein networks and their interaction with the human proteome. Science 311 239 242
41. HarperJWAdamiGRWeiNKeyomarsiKElledgeSJ 1993 The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75 805 816
42. JamesPHalladayJCraigEA 1996 Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144 1425 1436
43. CagneyGUetzPFieldsS 2000 High-throughput screening for protein-protein interactions using two-hybrid assay. Methods Enzymol 328 3 14
44. DiefenbacherMSekulaSHeilbockCMaierJVLitfinM 2008 Restriction to Fos family members of Trip6-dependent coactivation and glucocorticoid receptor-dependent trans-repression of activator protein-1. Mol Endocrinol 22 1767 1780
45. RoderIVChoiKRReischlMPetersenYDiefenbacherME 2010 Myosin Va cooperates with PKA RIalpha to mediate maintenance of the endplate in vivo. Proc Natl Acad Sci U S A 107 2031 2036
46. HuCDChinenovYKerppolaTK 2002 Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell 9 789 798
47. LarkinMABlackshieldsGBrownNPChennaRMcGettiganPA 2007 Clustal W and Clustal X version 2.0. Bioinformatics 23 2947 2948
48. LandauMMayroseIRosenbergYGlaserFMartzE 2005 ConSurf 2005: the projection of evolutionary conservation scores of residues on protein structures. Nucleic Acids Res 33 W299 302
49. RoyAKucukuralAZhangY 2010 I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5 725 738
50. TovchigrechkoAVakserIA 2006 GRAMM-X public web server for protein-protein docking. Nucleic Acids Res 34 W310 314
51. GuzmanLMBelinDCarsonMJBeckwithJ 1995 Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177 4121 4130
52. TitzBHauserREngelbrecherAUetzP 2007 The Escherichia coli protein YjjG is a house-cleaning nucleotidase in vivo. FEMS Microbiol Lett 270 49 57
53. CherepanovPPWackernagelW 1995 Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158 9 14
54. BussoDDelagoutte-BussoBMorasD 2005 Construction of a set Gateway-based destination vectors for high-throughput cloning and expression screening in Escherichia coli. Anal Biochem 343 313 321
55. ZaniewskiRPetkaitesEDeutscherMP 1984 A multiple mutant of Escherichia coli lacking the exoribonucleases RNase II, RNase D, and RNase BN. J Biol Chem 259 11651 11653
56. BlahaGStelzlUSpahnCMTAgrawalRKFrankJ 2000 Preparation of functional ribosomal complexes and the effect of buffer conditions on tRNA positions observed by cryoelectron microscopy. Methods Enzymol 317 292 309
57. SuzukiTTerasakiMTakemoto-HoriCHanadaTUedaT 2001 Structural compensation for the deficit of rRNA with proteins in the mammalian mitochondrial ribosome. Systematic analysis of protein components of the large ribosomal subunit from mammalian mitochondria. J Biol Chem 276 21724 21736
58. TsuboiMMoritaHNozakiYAkamaKUedaT 2009 EF-G2mt is an exclusive recycling factor in mammalian mitochondrial protein synthesis. Mol Cell 35 502 510
59. LetunicIBorkP 2007 Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23 127 128
60. CrooksGEHonGChandoniaJMBrennerSE 2004 WebLogo: a sequence logo generator. Genome Res 14 1188 1190
61. UchiyamaIHiguchiTKawaiM 2010 MBGD update 2010: toward a comprehensive resource for exploring microbial genome diversity. Nucleic Acids Res 38 D361 365
Štítky
Genetika Reprodukční medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 7- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Vztah užívání alkoholu a mužské fertility
- Transfer zmraženého embrya zlepšuje výsledky IVF
- Velké děti po kryoembryotransferu
-
Všechny články tohoto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání


