-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
The relationship between epigenetic marks on chromatin and the regulation of DNA replication is poorly understood. Mutations of the H3K27 methyltransferase genes, ARABIDOPSIS TRITHORAX-RELATED PROTEIN5 (ATXR5) and ATXR6, result in re-replication (repeated origin firing within the same cell cycle). Here we show that mutations that reduce DNA methylation act to suppress the re-replication phenotype of atxr5 atxr6 mutants. This suggests that DNA methylation, a mark enriched at the same heterochromatic regions that re-replicate in atxr5/6 mutants, is required for aberrant re-replication. In contrast, RNA sequencing analyses suggest that ATXR5/6 and DNA methylation cooperatively transcriptionally silence transposable elements (TEs). Hence our results suggest a complex relationship between ATXR5/6 and DNA methylation in the regulation of DNA replication and transcription of TEs.
Published in the journal: . PLoS Genet 8(7): e32767. doi:10.1371/journal.pgen.1002808
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002808Summary
The relationship between epigenetic marks on chromatin and the regulation of DNA replication is poorly understood. Mutations of the H3K27 methyltransferase genes, ARABIDOPSIS TRITHORAX-RELATED PROTEIN5 (ATXR5) and ATXR6, result in re-replication (repeated origin firing within the same cell cycle). Here we show that mutations that reduce DNA methylation act to suppress the re-replication phenotype of atxr5 atxr6 mutants. This suggests that DNA methylation, a mark enriched at the same heterochromatic regions that re-replicate in atxr5/6 mutants, is required for aberrant re-replication. In contrast, RNA sequencing analyses suggest that ATXR5/6 and DNA methylation cooperatively transcriptionally silence transposable elements (TEs). Hence our results suggest a complex relationship between ATXR5/6 and DNA methylation in the regulation of DNA replication and transcription of TEs.
Introduction
Faithful DNA replication requires that each origin of replication fire only once per cell cycle. Re-replication has recently been suggested to be an inducer of gene copy number changes and hence threatens genome stability [1]. Multiple mechanisms that prevent re-replication are known [2], but the regulation of DNA replication at the level of chromatin remains elusive. Especially poorly understood is DNA replication of heterochromatin, which is late replicating in both plants and animals [3], [4], [5]. In Arabidopsis, heterochromatin is primarily pericentromeric and is enriched in repetitive elements such as transposons that are transcriptionally silenced by epigenetic modifications such as DNA methylation (in both CG and non-CG sequence contexts), H3 lysine 9 dimethylation (H3K9me2) and H3K27me1 [6]. The ATXR5 and ATXR6 methyltransferases catalyze H3K27 monomethylation and function redundantly to suppress over-replication of heterochromatin, likely by inhibiting re-replication [7].
Given the close correlation between sites of re-replication and sites enriched with DNA methylation, we investigated the role of DNA methylation in atxr5 atxr6-induced re-replication. We found that loss of DNA methylation suppressed the re-replication phenotype of atxr5 atxr6 mutants, suggesting a role for DNA methylation in re-replication. We also profiled the transcriptome in different mutants by RNA sequencing (RNA-seq), and found that many TEs are cooperatively silenced by ATXR5/6 and DNA methylation.
Results/Discussion
Re-replication in atxr5 atxr6 mutants is closely confined to heterochromatin
We previously found that most re-replicated sites overlapped with heterochromatin [7], but the extent of the overlap has not been examined. We utilized Illumina sequencing to examine the DNA contents of atxr5 atxr6 double mutants at the boundaries of previously defined heterochromatic patches in the arms of chromosomes [8]. Interestingly we found that DNA content in atxr5 atxr6 decreased sharply at the boundaries of the heterochromatic patches (Figure 1). Moreover, the sizes of re-replicated regions closely tracked the sizes of defined heterochromatin (Figure 1), suggesting that re-replication is closely confined to heterochromatin and is unable to spread into flanking euchromatin. The close correlation of re-replication with heterochromatin led us to hypothesize that certain marks of heterochromatin, such as DNA methylation, may be required for the occurrence of aberrant replication.
Fig. 1. Heterochromatin is specifically re-replicated in atxr5 atxr6 double mutants. 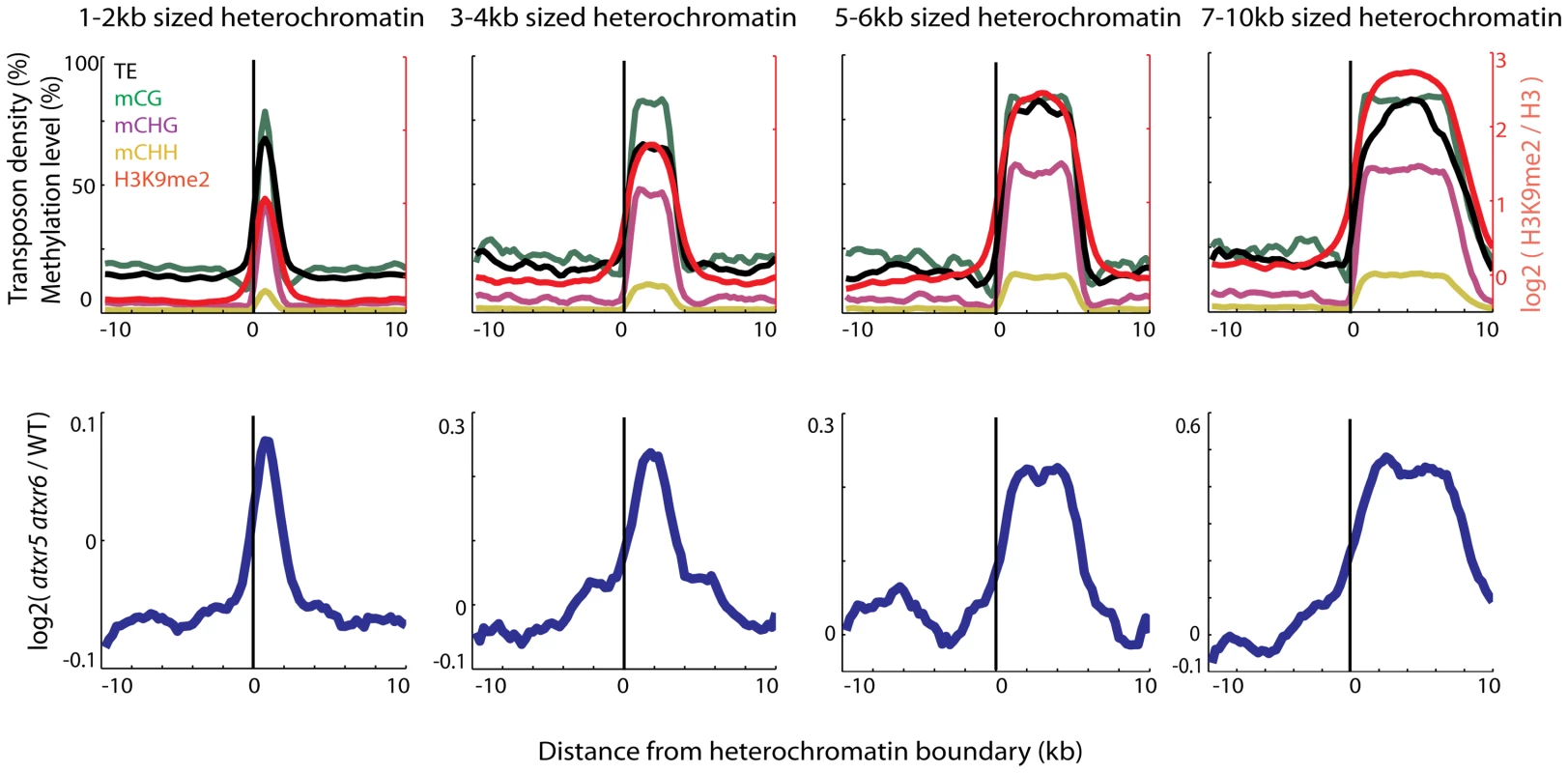
Top panels show enrichment of DNA methylation (CG, CHG, CHH, where H = A,T or C) [11], transposable element (TE) densities (TE per base-pair) and H3K9me2 [8] over the boundaries of heterochromatic regions of indicated sizes. Values were plotted +/−10 kilobase from the boundary of heterochromatin in 500 bp bins. x = 0 is the heterochromatin boundary, x<0 is outside the region, and x>0 is into the region. Bottom panels show the distribution of DNA contents from atxr5 atxr6 mutants relative to wild-type (log2 ratios) [7]. Heterochromatic regions were defined using previously characterized H3K9me2 regions [8]. Plots in both top and bottom panels were smoothed by taking the moving average over +/−1 bins and +/−3 bins, respectively. Loss of DNA methylation suppresses the re-replication defect in atxr5 atxr6 mutants
To test the role of DNA methylation in re-replication, we crossed atxr5 atxr6 mutants to met1, cmt3 and ddm1 mutants, to reduce CG, non-CG, and both CG and non-CG methylations, respectively. Previous studies indicated that atxr5 atxr6 mutants themselves show no reduction of DNA methylation [9] and loss of DNA methylation does not affect H3K27me1 levels [10], suggesting that DNA methylation and H3K27me1 are independent of each other. To confirm that DNA methylation levels were decreased in ddm1 atxr5 atxr6 triple mutants we performed whole genome bisulfite sequencing (BS-seq) on atxr5 atxr6 and ddm1 atxr5 atxr6 mutants. We observed significant loss of DNA methylation in ddm1 atxr5 atxr6 backgrounds (Figure S1).
To compare genomic DNA contents between similar cell types, we sorted and collected 8C nuclei from leaves and sequenced the DNA. We chose 8C nuclei because we previously showed high levels of heterochromatin re-replication in nuclei of this ploidy level as compared to 2C or 4C nuclei (Figure S2A) [7]. Strikingly, by examining the distribution of sequenced reads across the chromosomes we observed suppression of heterochromatic re-replication in all the mutants compared to atxr5 atxr6 mutants (Figure 2A–2D, 2F). This suggested that factors involved in DNA methylation maintenance are required for re-replication in atxr5 atxr6 mutants. Consistently, ddm1 and met1, which show the most dramatic losses of DNA methylation [11], most significantly suppressed re-replication (Figure 2B, 2C, 2F). The relatively weak but reproducible suppression in cmt3 atxr5 atxr6 mutants could be explained by the fact that non-CG sites are relatively lowly methylated (<7%) in Arabidopsis [11].
Fig. 2. Relationship between ATXR5/6 and DNA methylation in regulating DNA replication in heterochromatin. 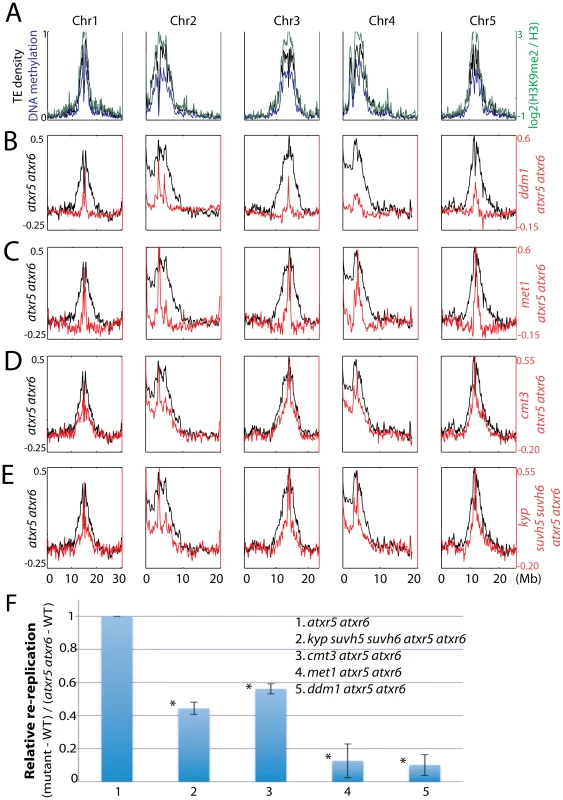
A. Chromosomal distribution of transposable element (TE) density, DNA methylation [20] and H3K9me2 [8] data are presented to mark the locations of pericentromeric heterochromatin. B. Chromosomal views of the log2 ratio of genomic DNA reads of atxr5 atxr6 mutants to wild type (WT) are shown in black, and the log2 ratio of ddm1 atxr5 atxr6 mutants to WT are shown in red. C. met1 atxr5 atxr6 mutants, D. cmt3 atxr5 atxr6 mutants, and E. kyp suvh5 suvh6 atxr5 atxr6 mutants. F. Quantitation of reads in heterochromatin in mutants. Fraction of reads falling into previously defined pericentromeric heterochromatin [8] was calculated. *P<10−5 relative to atxr5 atxr6 mutants. We next tested the role of H3K9me2 in regulating re-replication by generating kyp suvh5 suvh6 atxr5 atxr6 quintuple mutants. Because kyp suvh5 suvh6 mutants are depleted in both H3K9me2 and non-CG methylation, we reasoned that if H3K9me2 played a dominant role in regulating re-replication, kyp suvh5 suvh6 atxr5 atxr6 mutants should exhibit a stronger degree of suppression of re-replication than do cmt3 atxr5 atxr6 mutants, which lose non-CG methylation but retain a significant amount of H3K9me2 [12]. However, we observed a very similar degree of suppression of re-replication in kyp suvh5 suvh6 atxr5 atxr6 mutants compared to cmt3 atxr5 atxr6 mutants (Figure 2E, 2F; Figure S3). Hence the reduction of DNA re-replication observed in kyp suvh5 suvh6 atxr5 atxr6 mutants is likely due to losses of DNA methylation rather than losses of H3K9me2. We also crossed atxr5 atxr6 to drm1 drm2 double mutants. DRM1 and DRM2 maintain asymmetric methylation at a subset of cytosines. Consistent with the fact that drm1 drm2 mutants show only limited reductions in DNA methylation genome-wide [11], we did not observe significant suppression in drm1 drm2 atxr5 atxr6 quadruple mutants (Figure S4).
Finally, we crossed atxr5 atxr6 to mom1, which exhibits transcriptional derepression of TEs without altering DNA methylation [13]. We did not observe significant suppression of re-replication in mom1 atxr5 atxr6 mutants (Figure S5), further supporting our hypothesis that it is the loss of DNA methylation that causes suppression of re-replication. Flow cytometry analyses on multiple biological replicates of all the mutants confirmed the results obtained by DNA sequencing (Figure S2B). It should be noted that DNA methylation single mutants in wild type ATXR5 ATXR6 backgrounds did not cause significant changes in DNA content (Figure S2C). In sum, these results suggest that DNA methylation plays a role in the induction of re-replication in atxr5 atxr6, consistent with the hypothesis that DNA methylation promotes DNA replication in heterochromatin.
Relationship between transcriptional derepression and re-replication in atxr5 atxr6 mutants
Analyses at a few loci have shown that atxr5 atxr6 mutants exhibit transcriptional derepression of certain TEs [9]. A possible explanation for the TE reactivation could be that more permissive chromatin assembled on additional DNA copies of TEs resulting from re-replication might allow for transcription to occur. Conversely, it is also possible that the transcriptional derepression in atxr5 atxr6 mutants may in some way be causing the re-replication defect. To examine the relationship between heterochromatin re-replication and transposon derepression in atxr5 atxr6 mutants, we analyzed the transcriptome of atxr5 atxr6 mutants by performing RNA-seq on cotyledons, which we found show significant re-replication (Figure S6). We defined 100 TEs that were consistently upregulated in biological replicates by applying stringent thresholds (see Materials and Methods) (Table S1). These TEs were highly methylated in wild type, and did not lose DNA methylation in atxr5 atxr6 (Figure S7). In addition we sequenced genomic DNA from cotyledons of atxr5 atxr6 mutants, and defined re-replicated TEs (see Materials and Methods). Importantly, a subset of TEs that were transcriptionally derepressed was not re-replicated (Figure 3A, Figure S8). The presence of TEs derepressed in atxr5 atxr6 mutants that were not overlapping with re-replicated regions suggests that TE reactivation is not due to increased DNA copy numbers at these loci. Re-replicated TEs were much more numerous than those transcriptionally derepressed in atxr5 atxr6 mutants, where only 1.4% of TEs that showed re-replication also showed derepression. These results suggest that heterochromatin re-replication and transposon derepression are likely two separate phenomena in atxr5 atxr6 mutants, which is consistent with the observations that DNA methyltransferase mutants do not act as enhancers of the DNA replication defects in atxr5 atxr6 mutants, as would be expected if there were a simple relationship between transcriptional derepression and DNA replication.
Fig. 3. Relationship between ATXR5/6 and DNA methylation in transcriptionally silencing TEs. 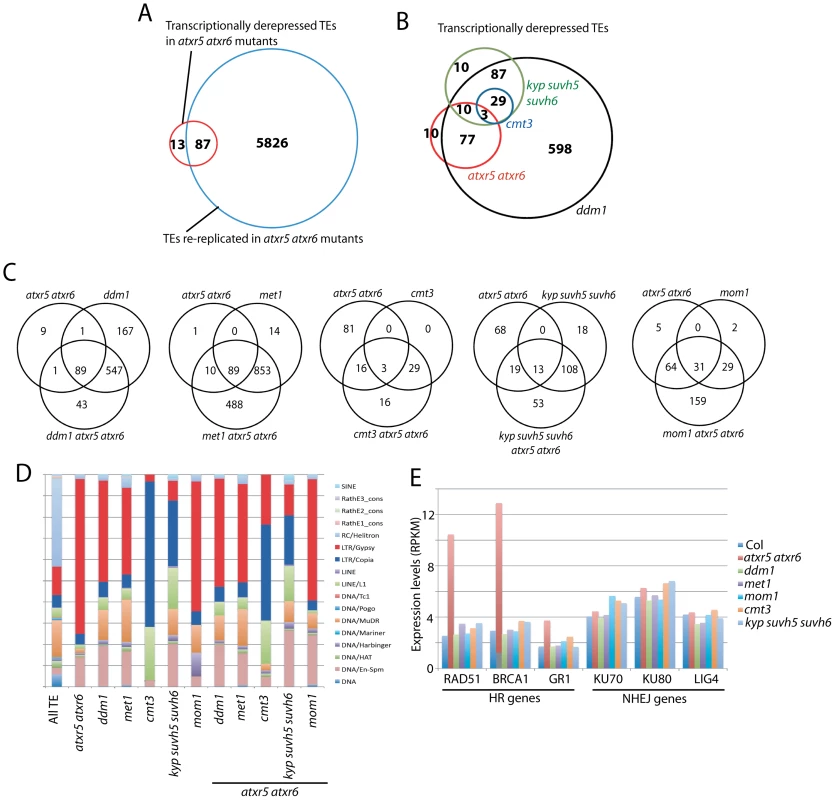
A. Overlap of re-replicated TEs in atxr5 atxr6 mutants, with transcriptionally reactivated TEs in atxr5 atxr6 mutants. B. Overlap of TEs transcriptionally derepressed in atxr5 atxr6 mutants and DNA methylation mutants. C. Overlap of TEs derepressed in indicated mutants. D. TE families of derepressed TEs in indicated mutants. E. Normalized expression levels of key genes in homologous recombination (HR) and non-homologous end-joining (NHEJ) DNA repair pathways. Comparison of TEs regulated by ATXR5/6 and DNA methylation
We next examined the relationship between TEs derepressed in atxr5 atxr6 mutants and DNA methylation mutants. Again, TEs were defined to be derepressed using stringent thresholds. We found significant overlap between TEs derepressed in ddm1 and met1 mutants (Figure S9A). And consistent with the dependence of non-CG methylation on H3K9 methylation, we found that all TEs derepressed in cmt3 mutants overlapped with those derepressed in kyp suvh5 suvh6 mutants (Figure 3B). Most TEs derepressed in atxr5 atxr6 mutants overlapped with those derepressed in ddm1 and met1 mutants (Figure 3B, Figures S9B and S10), however there was little overlap with those derepressed in cmt3 and kyp suvh5 suvh6 mutants (Figure 3B). Hence ATXR5/6 and H3K9me2/non-CG methylation generally regulate different TEs at a transcriptional level.
TEs regulated by ATXR5/6 were over-represented by LTR/Gypsy type TEs, whereas TEs regulated by KYP SUVH5 SUVH6 and CMT3 were over-represented by LTR/Copia and LINE/L1 type TEs (Figure 3D). This was not necessarily predicted based on methylation levels since it was not the case that certain types of TEs were preferentially CG or non-CG methylated (Figure S11). Hence different silencing pathways tend to regulate specific types of TEs. Notably, a large subset of TEs became reactivated only when combining atxr5 atxr6 mutants with DNA methylation mutants (Figure 3C, Figure S9D). Hence, a relatively large proportion of TEs are cooperatively silenced by ATXR5/6 and DNA methylation. It is not clear why ddm1 atxr5 atxr6 mutants did not show as many additional TEs derepressed as met1 atxr5 atxr6 mutants. We also found that the combination of mom1 with atxr5 atxr6 mutants caused activation of many additional TEs (Figure 3C and Figure S9C).
Over-expression of DNA repair genes in atxr5 atxr6 mutants
An additional insight from the transcriptome of atxr5 atxr6 mutants was that genes in the homologous recombination (HR) DNA repair pathway were over-expressed (Figure 3E). This was confirmed by quantitative RT-PCR analyses (Figure S12). None of the other tested mutants showed this effect (Figure 3E), suggesting that induction of these HR genes is likely due to re-replication. Furthermore, the ddm1 mutant which caused the most significant suppression of re-replication in the atxr5 atxr6 background, also caused a significant suppression of the expression of these HR genes (Figure S13), further supporting the hypothesis that these HR genes are up-regulated in response to DNA damage caused by re-replication.
In summary, our results show that DNA methylation is required for the heterochromatic re-replication defect in atxr5 atxr6, suggesting that DNA methylation positively regulates DNA replication in heterochromatin. We further find that DNA methylation and ATXR5/6 act synergistically in the transcriptional suppression of transposons. The molecular mechanisms causing these relationships are unclear, and it may be that losing DNA methylation causes mobilization of unknown pathways that result in suppression of re-replication. Our findings suggest a complex interplay between epigenetic marks in regulating DNA replication and transcriptional silencing in heterochromatin.
Materials and Methods
Plant material
All mutant lines in this study were in the Columbia background. Previously characterized mutant alleles were used for crosses: cmt3–11, ddm1–2, met1–3, mom1–2, atxr5 atxr6 [9] and kyp suvh5 suvh6 [14]. met1–3 mutants in both wild-type and atxr5 atxr6 mutant backgrounds used in this study were second generation homozygous lines. ddm1–2 mutants in both wild-type and atxr5 atxr6 mutant backgrounds were sixth generation homozygous lines. Plants were grown under continuous light.
Flow cytometry
For sorting nuclei: One gram of mature rosette leaves were collected from 3–4-week-old plants, chopped in 0.5 ml of filtered Galbraith buffer, and stained with propidium iodide. A BD FACS Aria II in the UCLA Jonsson Comprehensive Cancer Center (JCCC) Flow Cytometry Core Facility was used to sort the nuclei. For sequencing, 7,000–9,000 8C nuclei of each sample were collected, and purified DNA with Picopure purification kit (Arcturus) following manufacturer instructions.
For generating flow cytometry profiles: Three mature rosette leaves were pooled from separate 4-week-old plants, chopped in 2 ml of filtered Galbraith buffer, and stained with a solution of propidium iodide and RNAse A. A BD FACScan flow cytometer in the UCLA JCCC Flow Cytometry Core Facility was used to generate the FACS profiles. For each sample at least 10,000 nuclei were analyzed, and widths of peaks (coefficient of variation values) [7] were calculated using Cyflogic analysis software (http://www.cyflogic.com).
Illumina genomic library preparation
Genomic DNA was sonicated to 200 bp with a Covaris S2, and Illumina libraries were generated following manufacturer instructions. The libraries were sequenced using Illumina Genome Analyzer II following manufacturer instructions.
Illumina mRNA–seq library preparation
RNA–seq experiments were performed in two biological replicates for each genotype. 0.1 g of tissue was ground in Trizol. Total RNA were treated with DNaseI (Roche), and cleaned up with phenol-chlorophorm and precipitated with ethanol. Libraries were generated and sequenced following manufacturer instructions (Illumina). For verification experiments, the same RNA extraction protocol was used. Single stranded cDNA was synthesized using polyA primers and Superscript II (Invitrogen). For quantitative PCR analysis, cDNA were amplified with iQ SYBR Green Supermix (Biorad) using primers previously described [15] and ACTIN gene was used as an internal control. Primers are listed in Table S2.
Whole-genome bisulfite sequencing (BS-seq) library generation
0.5∼1 ug of genomic DNA was used to generate BS-seq libraries. Libraries were generated as previously described [11].
Illumina read alignment and analysis
Genomic DNA sequenced reads were base-called using the standard Illumina software. We used Bowtie [16] to uniquely align the reads to the Arabidopsis thaliana genome (TAIR8), allowing up to 2 mismatches. For all genomic libraries, reads mapping to identical positions were collapsed into single reads.
Method for defining re-replicated regions: Genome was tiled into 100 bp bins, and scores of reads for each bin were computed before the log2 ratio of atxr5 atxr6 to wild-type was taken. Score = (# reads+c)/0.1 kb/(# million mapping reads), where c is a pseudocount defined by (# million mapping reads)/10. In this case, cWT = 6.8 and catxr5atxr6 = 5.3. Pseudocounts were used to avoid divisions by zero. Next, the genome was tiled into 1 kb bins (500 bp overlap), and Z-scores of log2(atxr5 atxr6/wild-type) were computed. A Z>2 cutoff was applied and regions within 500 bp were merged. Transposable elements (TEs) overlapping with these regions by 1 bp were considered to be re-replicating in atxr5 atxr6 double mutants.
Both gene and TE expression in the RNA-seq data was measured by calculating reads per kilobase per million mapped reads (RPKM) [17]. P-values to detect differential expression were calculated by Fisher's exact test and Benjamini-Hochberg corrected [18] for multiple testing. TEs upregulated in wild-type and mutants were defined by mutant/wild-type>4 and P<0.01. To avoid divisions by zero, TEs with expression levels of zero were assigned the lowest non-zero TE expression value in each sample. Only TEs defined as upregulated in all biological replicates were considered as being derepressed. TEs defined to be upregulated are listed in Table S1.
BS-seq data was mapped to the TAIR8 genome by BS Seeker [19] by allowing up to 2 mismatches. Methylation levels were computed by calculating #C/(#C+#T).
All sequencing data have been deposited at Gene Expression Omnibus (GEO) (accession number GSE38286).
Supporting Information
Zdroje
1. GreenBMFinnKJLiJJ 2010 Loss of DNA replication control is a potent inducer of gene amplification. Science 329 943 946
2. AriasEEWalterJC 2007 Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells. Genes Dev 21 497 518
3. LeeTJPascuzziPESettlageSBShultzRWTanurdzicM 2010 Arabidopsis thaliana chromosome 4 replicates in two phases that correlate with chromatin state. PLoS Genet 6 e1000982 doi:10.1371/journal.pgen.1000982
4. WhiteEJEmanuelssonOScalzoDRoyceTKosakS 2004 DNA replication-timing analysis of human chromosome 22 at high resolution and different developmental states. Proc Natl Acad Sci U S A 101 17771 17776
5. BirneyEStamatoyannopoulosJADuttaAGuigoRGingerasTR 2007 Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 447 799 816
6. LawJAJacobsenSE 2010 Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet 11 204 220
7. JacobYStroudHLeblancCFengSZhuoL 2010 Regulation of heterochromatic DNA replication by histone H3 lysine 27 methyltransferases. Nature 466 987 991
8. BernatavichuteYVZhangXCokusSPellegriniMJacobsenSE 2008 Genome-wide association of histone H3 lysine nine methylation with CHG DNA methylation in Arabidopsis thaliana. PLoS ONE 3 e3156 doi:10.1371/journal.pone.0003156
9. JacobYFengSLeBlancCABernatavichuteYVStroudH 2009 ATXR5 and ATXR6 are H3K27 monomethyltransferases required for chromatin structure and gene silencing. Nat Struct Mol Biol 16 763 768
10. MathieuOProbstAVPaszkowskiJ 2005 Distinct regulation of histone H3 methylation at lysines 27 and 9 by CpG methylation in Arabidopsis. Embo J 24 2783 2791
11. CokusSJFengSZhangXChenZMerrimanB 2008 Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 452 215 219
12. InagakiSMiura-KamioANakamuraYLuFCuiX 2010 Autocatalytic differentiation of epigenetic modifications within the Arabidopsis genome. Embo J 29 3496 3506
13. AmedeoPHabuYAfsarKMittelsten ScheidOPaszkowskiJ 2000 Disruption of the plant gene MOM releases transcriptional silencing of methylated genes. Nature 405 203 206
14. EbbsMLBenderJ 2006 Locus-specific control of DNA methylation by the Arabidopsis SUVH5 histone methyltransferase. Plant Cell 18 1166 1176
15. Ramirez-ParraEGutierrezC 2007 E2F regulates FASCIATA1, a chromatin assembly gene whose loss switches on the endocycle and activates gene expression by changing the epigenetic status. Plant Physiol 144 105 120
16. LangmeadBTrapnellCPopMSalzbergSL 2009 Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10 R25
17. MortazaviAWilliamsBAMcCueKSchaefferLWoldB 2008 Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5 621 628
18. BenjaminiYHochbergY 1995 Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B 57 12
19. ChenPYCokusSJPellegriniM 2010 BS Seeker: precise mapping for bisulfite sequencing. BMC Bioinformatics 11 203
20. ZhangXYazakiJSundaresanACokusSChanSW 2006 Genome-wide high-resolution mapping and functional analysis of DNA methylation in arabidopsis. Cell 126 1189 1201
Štítky
Genetika Reprodukční medicína
Článek Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil MicronutrientČlánek The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective RachisČlánek A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 7- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Akutní intermitentní porfyrie
- Příjem alkoholu a menstruační cyklus
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
- Transfer zmraženého embrya zlepšuje výsledky IVF
-
Všechny články tohoto čísla
- Functional Evolution of Mammalian Odorant Receptors
- Oocyte Family Trees: Old Branches or New Stems?
- Allelic Heterogeneity and Trade-Off Shape Natural Variation for Response to Soil Micronutrient
- Guidelines for Genome-Wide Association Studies
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
- DNA Methyltransferases Are Required to Induce Heterochromatic Re-Replication in Arabidopsis
- Genomic Data Reveal a Complex Making of Humans
- Let-7b/c Enhance the Stability of a Tissue-Specific mRNA during Mammalian Organogenesis as Part of a Feedback Loop Involving KSRP
- The Secreted Immunoglobulin Domain Proteins ZIG-5 and ZIG-8 Cooperate with L1CAM/SAX-7 to Maintain Nervous System Integrity
- RsfA (YbeB) Proteins Are Conserved Ribosomal Silencing Factors
- Gene Conversion Occurs within the Mating-Type Locus of during Sexual Reproduction
- The Chicken Frizzle Feather Is Due to an α-Keratin () Mutation That Causes a Defective Rachis
- Meta-Analysis of Genome-Wide Scans for Total Body BMD in Children and Adults Reveals Allelic Heterogeneity and Age-Specific Effects at the Locus
- Balancing Selection at the Tomato Guardee Gene Family Maintains Variation in Strength of Pathogen Defense
- Large-Scale Introgression Shapes the Evolution of the Mating-Type Chromosomes of the Filamentous Ascomycete
- OSD1 Promotes Meiotic Progression via APC/C Inhibition and Forms a Regulatory Network with TDM and CYCA1;2/TAM
- Intact p53-Dependent Responses in miR-34–Deficient Mice
- FANCJ/BACH1 Acetylation at Lysine 1249 Regulates the DNA Damage Response
- CED-10/Rac1 Regulates Endocytic Recycling through the RAB-5 GAP TBC-2
- Histone H2A Mono-Ubiquitination Is a Crucial Step to Mediate PRC1-Dependent Repression of Developmental Genes to Maintain ES Cell Identity
- F-Box Protein Specificity for G1 Cyclins Is Dictated by Subcellular Localization
- The Gene Encodes a Nuclear Protein That Affects Alternative Splicing
- A Key Role for Chd1 in Histone H3 Dynamics at the 3′ Ends of Long Genes in Yeast
- Genome-Wide Association Analysis in Asthma Subjects Identifies as a Novel Bronchodilator Response Gene
- GRHL3/GET1 and Trithorax Group Members Collaborate to Activate the Epidermal Progenitor Differentiation Program
- Brain-Specific Rescue of Reveals System-Driven Transcriptional Rhythms in Peripheral Tissue
- Recent Loss of Self-Incompatibility by Degradation of the Male Component in Allotetraploid
- Pregnancy-Induced Noncoding RNA () Associates with Polycomb Repressive Complex 2 and Regulates Mammary Epithelial Differentiation
- The HEI10 Is a New ZMM Protein Related to Zip3
- The SCF Ubiquitin E3 Ligase Ubiquitylates Sir4 and Functions in Transcriptional Silencing
- Induction of Cytoprotective Pathways Is Central to the Extension of Lifespan Conferred by Multiple Longevity Pathways
- Role of Architecture in the Function and Specificity of Two Notch-Regulated Transcriptional Enhancer Modules
- Loss of ATRX, Genome Instability, and an Altered DNA Damage Response Are Hallmarks of the Alternative Lengthening of Telomeres Pathway
- A Regulatory Loop Involving PAX6, MITF, and WNT Signaling Controls Retinal Pigment Epithelium Development
- The Three Faces of Riboviral Spontaneous Mutation: Spectrum, Mode of Genome Replication, and Mutation Rate
- Unmet Expectations: miR-34 Plays No Role in p53-Mediated Tumor Suppression In Vivo
- A Genome-Wide Association Meta-Analysis of Circulating Sex Hormone–Binding Globulin Reveals Multiple Loci Implicated in Sex Steroid Hormone Regulation
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- A Trans-Species Missense SNP in Is Associated with Sex Determination in the Tiger Pufferfish, (Fugu)
- Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk
- Evidence of Inbreeding Depression on Human Height
- Comparative Genomics of Plant-Associated spp.: Insights into Diversity and Inheritance of Traits Involved in Multitrophic Interactions
- Detecting Individual Sites Subject to Episodic Diversifying Selection
- Regulates Rhodopsin-1 Metabolism and Is Required for Photoreceptor Neuron Survival
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- Three Dopamine Pathways Induce Aversive Odor Memories with Different Stability
- TDP-1/TDP-43 Regulates Stress Signaling and Age-Dependent Proteotoxicity in
- Rapid Turnover of Long Noncoding RNAs and the Evolution of Gene Expression
- The Yeast Rab GTPase Ypt1 Modulates Unfolded Protein Response Dynamics by Regulating the Stability of RNA
- Histone H2B Monoubiquitination Facilitates the Rapid Modulation of Gene Expression during Arabidopsis Photomorphogenesis
- Cellular Variability of RpoS Expression Underlies Subpopulation Activation of an Integrative and Conjugative Element
- Genetic Variants in , , and Influence Male Recombination in Cattle
- Differential Impact of the HEN1 Homolog HENN-1 on 21U and 26G RNAs in the Germline of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Guidelines for Genome-Wide Association Studies
- The Role of Rice HEI10 in the Formation of Meiotic Crossovers
- Identification of Chromatin-Associated Regulators of MSL Complex Targeting in Dosage Compensation
- GWAS Identifies Novel Susceptibility Loci on 6p21.32 and 21q21.3 for Hepatocellular Carcinoma in Chronic Hepatitis B Virus Carriers
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



