-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenome-Wide Joint Meta-Analysis of SNP and SNP-by-Smoking Interaction Identifies Novel Loci for Pulmonary Function
Genome-wide association studies have identified numerous genetic loci for spirometic measures of pulmonary function, forced expiratory volume in one second (FEV1), and its ratio to forced vital capacity (FEV1/FVC). Given that cigarette smoking adversely affects pulmonary function, we conducted genome-wide joint meta-analyses (JMA) of single nucleotide polymorphism (SNP) and SNP-by-smoking (ever-smoking or pack-years) associations on FEV1 and FEV1/FVC across 19 studies (total N = 50,047). We identified three novel loci not previously associated with pulmonary function. SNPs in or near DNER (smallest PJMA = 5.00×10−11), HLA-DQB1 and HLA-DQA2 (smallest PJMA = 4.35×10−9), and KCNJ2 and SOX9 (smallest PJMA = 1.28×10−8) were associated with FEV1/FVC or FEV1 in meta-analysis models including SNP main effects, smoking main effects, and SNP-by-smoking (ever-smoking or pack-years) interaction. The HLA region has been widely implicated for autoimmune and lung phenotypes, unlike the other novel loci, which have not been widely implicated. We evaluated DNER, KCNJ2, and SOX9 and found them to be expressed in human lung tissue. DNER and SOX9 further showed evidence of differential expression in human airway epithelium in smokers compared to non-smokers. Our findings demonstrated that joint testing of SNP and SNP-by-environment interaction identified novel loci associated with complex traits that are missed when considering only the genetic main effects.
Published in the journal: . PLoS Genet 8(12): e32767. doi:10.1371/journal.pgen.1003098
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003098Summary
Genome-wide association studies have identified numerous genetic loci for spirometic measures of pulmonary function, forced expiratory volume in one second (FEV1), and its ratio to forced vital capacity (FEV1/FVC). Given that cigarette smoking adversely affects pulmonary function, we conducted genome-wide joint meta-analyses (JMA) of single nucleotide polymorphism (SNP) and SNP-by-smoking (ever-smoking or pack-years) associations on FEV1 and FEV1/FVC across 19 studies (total N = 50,047). We identified three novel loci not previously associated with pulmonary function. SNPs in or near DNER (smallest PJMA = 5.00×10−11), HLA-DQB1 and HLA-DQA2 (smallest PJMA = 4.35×10−9), and KCNJ2 and SOX9 (smallest PJMA = 1.28×10−8) were associated with FEV1/FVC or FEV1 in meta-analysis models including SNP main effects, smoking main effects, and SNP-by-smoking (ever-smoking or pack-years) interaction. The HLA region has been widely implicated for autoimmune and lung phenotypes, unlike the other novel loci, which have not been widely implicated. We evaluated DNER, KCNJ2, and SOX9 and found them to be expressed in human lung tissue. DNER and SOX9 further showed evidence of differential expression in human airway epithelium in smokers compared to non-smokers. Our findings demonstrated that joint testing of SNP and SNP-by-environment interaction identified novel loci associated with complex traits that are missed when considering only the genetic main effects.
Introduction
Spirometric measures of pulmonary function, particularly forced expiratory volume in one second (FEV1) and its ratio to forced vital capacity (FEV1/FVC), are important clinical tools for diagnosing pulmonary disease, classifying its severity, and evaluating its progression over time. These measures also predict other morbidities and mortality in the general population [1]–[3]. Genetic factors likely play a prominent role in determining the maximal level of pulmonary function in early adulthood and its subsequent decline with age [4], [5]. A relatively uncommon deficiency of α-1 antitrypsin, due to homozygous mutations of the SERPINA1 gene, is a well-established genetic risk factor for accelerated decline in pulmonary function, but it accounts for little of the population variability in pulmonary function.
Genome-wide association studies (GWAS) have identified many common genetic variants underlying pulmonary function. The first GWAS of pulmonary function implicated HHIP for FEV1/FVC [6], [7]. GWAS meta-analyses for FEV1/FVC and FEV1 from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) and SpiroMeta Consortia have together identified 26 additional novel loci in or near the following genes: ADAM19, AGER-PPT2, ARMC2, C10orf11, CCDC38, CDC123, CFDP1, FAM13A, GPR126, HDAC4, HTR4, INTS12-GSTCD-NPNT, KCNE2, LRP1, MECOM (EVI1), MFAP2, MMP15, NCR3, PID1, PTCH1, RARB, SPATA9, TGFB2, THSD4, TNS1, and ZKSCAN3 [8]–[10].
Inhaled pollutants, especially cigarette smoking, can have important adverse effects on pulmonary function. Candidate gene studies have not consistently identified interactions with cigarette smoking in relation to pulmonary function. Despite the importance of smoking and other environmental factors in the etiology of many complex human diseases and traits, few GWAS have incorporated gene-by-environment interactions [11]–[14]. Meta-analyses are generally necessary to provide sufficient sample size to detect moderate effects, and methods for joint testing of single nucleotide polymorphism (SNP) main effects and SNP-by-environment interactions in the meta-analysis setting have only recently been developed [15], [16]. This strategy has the potential to identify novel loci that would not emerge from analyses based on the SNP main or interactive effects alone [15]–[17]. The well-documented and consistent deleterious effect of cigarette smoking on pulmonary function [18] makes it a good candidate for such an approach, since genetic factors may have heterogeneous effects on pulmonary function depending on smoking exposure. We conducted genome-wide joint meta-analyses (JMA) of SNP and SNP-by-smoking interaction (ever-smoking or pack-years) associations with cross-sectional pulmonary function measures (FEV1/FVC and FEV1) in 50,047 study participants of European ancestry.
Results
Table S1 presents characteristics of the 50,047 participants from 19 studies contributing to our analyses. As expected, mean FEV1 and FVC values were lower in studies with the oldest participants. Standardized residuals of FEV1 and FEV1/FVC (see Methods) were used as the phenotypes for the JMA, in order to maximize comparability with our recent GWAS meta-analysis from the CHARGE and SpiroMeta Consortia [10]. Our original GWAS meta-analyses, conducted separately in CHARGE and SpiroMeta, showed that we were able to identify replicable genetic loci whether using actual pulmonary function measures [8] or their standardized residuals [9]. The standardized residual approach was similarly taken in GWAS of other complex quantitative traits, such as height and body mass index from the Genetic Investigation of ANthropometric Traits (GIANT) Consortium [19], [20].
In each of the 19 studies, four regression models with differing SNP-by-smoking interaction terms were run: (1) SNP-by-ever-smoking for standardized FEV1/FVC residuals, (2) SNP-by-pack-years for standardized FEV1/FVC residuals, (3) SNP-by-ever-smoking for standardized FEV1 residuals, and (4) SNP-by-pack-years for standardized FEV1 residuals. Study-specific genomic inflation factors (λgc) were calculated for the 1 degree-of-freedom (d.f.) SNP-by-smoking interaction term, to ensure that there was no substantial inflation due to the main effect of smoking being misspecified [21]. All study-specific results had 1 d.f. λgc≤1.09 (Table S2), which is of comparable magnitude to other studies with large sample sizes [10], [19], [22], [23].
The study-specific regression coefficients from each of the four models were then combined in JMA, and the resulting λgc values from the 2 d.f. JMA, calculated across all SNPs, ranged from 1.056 to 1.064. The quantile-quantile plots (Figure S1) show substantial deviation from expectation for SNPs having low P values from the JMA (PJMA). The JMA results corresponding to the top SNP from each previously implicated locus [8]–[10] are presented in Table S3. To identify novel loci among the genome-wide significant loci implicated by our JMA models, the genomic regions surrounding the most significant SNP from each of the 27 previously implicated loci [8]–[10] (500 kb upstream to 500 kb downstream of each SNP) were removed from consideration (Table S3). Following the removal of all previously implicated loci [8]–[10], the quantile-quantile plots show that some deviation remained between observed and expected P values for high-signal SNPs suggesting the presence of novel signals.
In the JMA of SNP and SNP-by-smoking in relation to FEV1/FVC, we observed two novel loci containing several significant SNP associations at the standard genome-wide Bonferroni-corrected threshold of PJMA<5×10−8, when considering interaction with ever-smoking (Figure 1A) or pack-years (Figure 1B). The SNP associations from both loci also exceeded the more conservative genome-wide significance threshold of PJMA<1.25×10−8, based on additional Bonferroni correction for the four JMA models.
Fig. 1. Genome-wide joint meta-analysis (JMA) of SNP and SNP-by-smoking interaction in relation to pulmonary function. 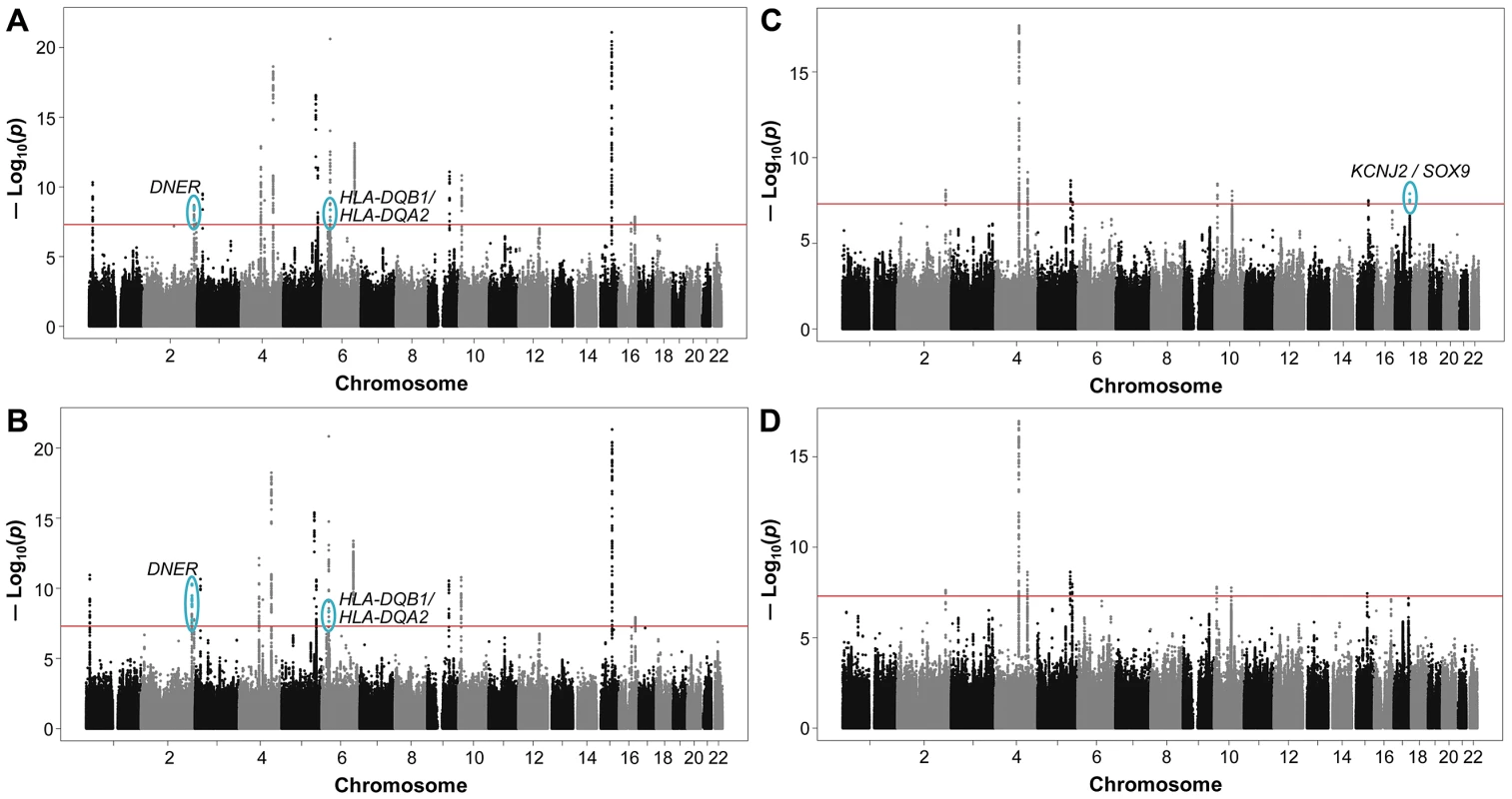
The Manhattan plots show the chromosomal position of SNPs in comparison to their −log10 PJMA values. JMA results are shown for models with (A) SNP-by-ever-smoking interaction term in relation to FEV1/FVC, (B) SNP-by-pack-years interaction term in relation to FEV1/FVC, (C) SNP-by-ever-smoking interaction term in relation to FEV1, and (D) SNP-by-pack-years interaction term in relation to FEV1. SNPs located within previously implicated loci are shown, but these loci were not considered when identifying novel loci from the joint modeling of SNP main effects and smoking interactive effects. Novel loci on chromosomes 2, 6, and 17 (shown in blue and circled) were identified as those having SNPs with genome-wide significant P values at the standard threshold (P<5×10−8 as indicated by the solid red line). Names of the novel gene (or closest genes) are provided. The most statistically significant result was for rs7594321, an intronic SNP located in DNER (delta/notch-like EGF-related receptor) on chromosome 2, which gave PJMA = 2.64×10−9 (corresponding PINT = 0.27) in the ever-smoking model and PJMA = 5.00×10−11 (corresponding PINT = 0.0069) in the pack-years model (Table 1). For the ever/never-smoking interaction model, the observed level of significance for the JMA is plausible in the presence of a nominally significant SNP main effect and a nonsignificant interactive effect, as detailed in Text S1. The rs7594321 T allele had a positive β coefficient for the genetic main association and a negative β coefficient for the interaction (Table 1, Table S4 for study-specific results). The regression coefficients correspond to a per allele change of 0.049 (95% CI: 0.030, 0.068) in never-smokers and 0.035 (95% CI: 0.016, 0.053) in ever-smokers. A conserved binding site for the Zic1 transcription factor is located 115 base pairs away from rs7594321. Further, rs7594321 is located upstream of the previously implicated PID1 gene (Figure 2A), but it is 713 kb away from the previously implicated SNP (rs1435867), which is located downstream of PID1. There is no linkage disequilibrium (LD) between rs7594321 and rs1435867 (r2 = 0, D′ = 0).
Fig. 2. Regional association plots of novel loci implicated for pulmonary function. 
Three novel loci contained SNPs associated with FEV1/FVC or FEV1 at the standard genome-wide significance threshold (P<5×10−8) in joint meta-analyses of SNP and SNP-by-smoking interaction. SNPs are shown within 500 kb of the most significant SNPs on chromosomes (A) 2q36.3 associated with FEV1/FVC, (B) 6p21.32 associated with FEV1/FVC, and (C) 17q24.3 associated with FEV1. Pairwise r2 values were based on the HapMap CEU population, and progressively darker shades of red indicate higher r2 values. Estimated recombination rates from HapMap are shown as background lines. Tab. 1. Genome-wide significant SNPs from the joint meta-analysis (JMA) of SNP and SNP-by-smoking (ever-smoking or pack-years) interaction in relation to pulmonary function. 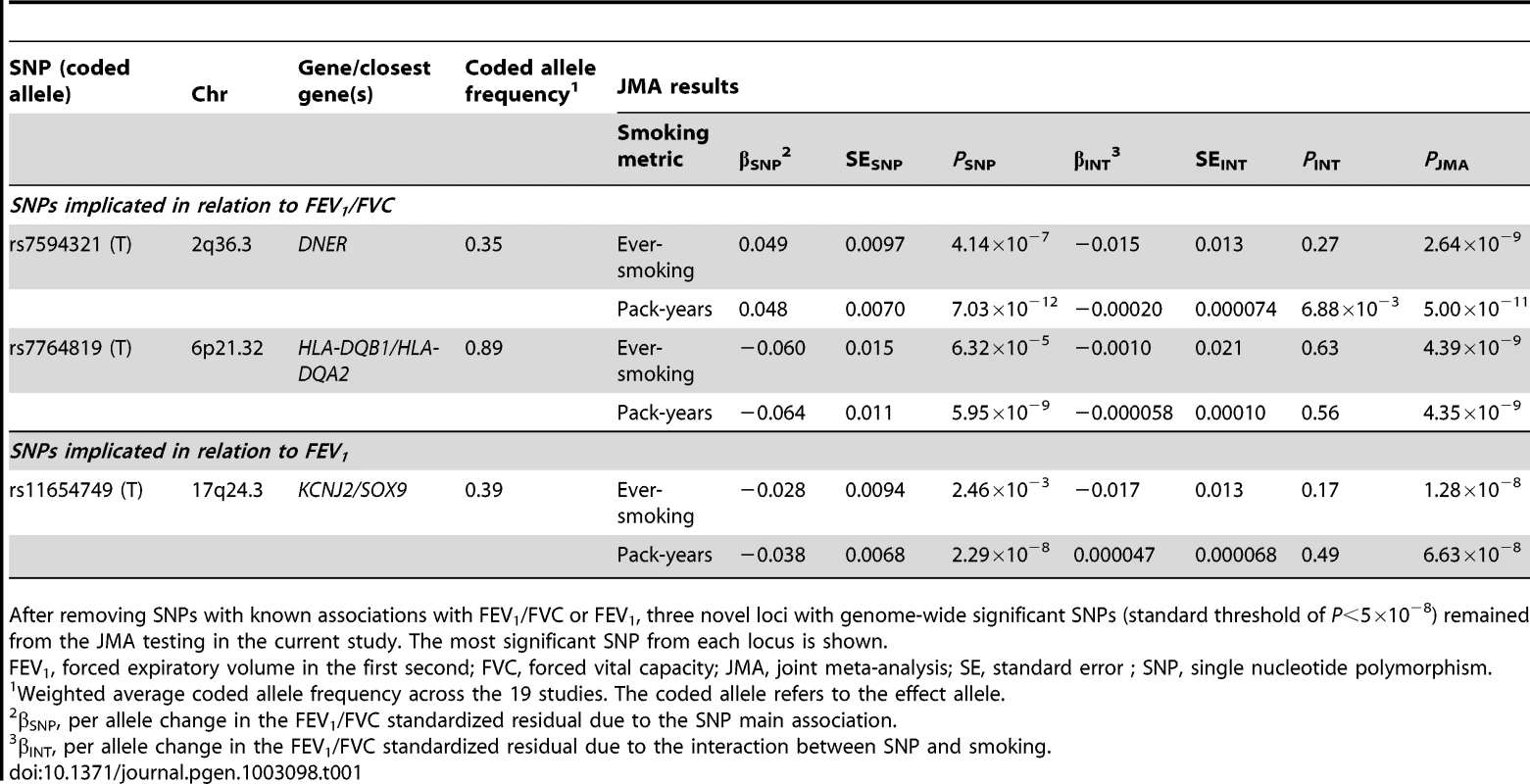
After removing SNPs with known associations with FEV1/FVC or FEV1, three novel loci with genome-wide significant SNPs (standard threshold of P<5×10−8) remained from the JMA testing in the current study. The most significant SNP from each locus is shown. Our next most statistically significant SNP (rs7764819) is intergenic between two human leukocyte antigen (HLA) genes, HLA-DQB1 and HLA-DQA2, on chromosome 6 (Figure 2B). The HLA-DQ region is highly variable, and the association signal in this region is largely driven by two SNPs that are in high LD with one another (rs7764819 and rs7765379, r2 = 1) but only low to moderate LD with all other genotyped and imputed SNPs. A GWAS meta-analysis of asthma implicating the HLA-DQ region similarly found highly significant associations with only a few SNPs [24]. Our top SNP rs7764819 gave PJMA = 4.39×10−9 in the ever-smoking model and PJMA = 4.35×10−9 in the pack-years model for FEV1/FVC (Table 1). The corresponding PINT values were >0.05 (see Text S1). The rs7764819 T allele had negative β coefficients for both the main association and interaction (Table 1, Table S5 for study-specific results), which correspond to a SNP effect of −0.060 (95% CI: −0.09, −0.031) in never-smokers and −0.070 (95% CI: −0.10, −0.042) in ever-smokers. Although rs7764819 is located 529 kb away from a previously implicated AGER SNP (rs2070600), there is some LD between the two SNPs (r2 = 0.29, D′ = 0.81). Conserved binding sites for two transcription factors, HTF and Lmo2, are located within 100 kb of rs7764819.
Besides the DNER and HLA-DQB1/HLA-DQA2 loci, SNPs from 12 other chromosomal regions having PJMA values between 5×10−8 and 1×10−6 from either smoking model in relation to FEV1/FVC are presented in Table S6. Secondary meta-analyses of the interaction product terms alone identified no SNP-by-smoking (ever-smoking or pack-years) interactions at genome-wide statistical significance with FEV1/FVC. SNPs from two chromosomal regions had PINT values between 5×10−8 and 1×10−6 in relation to FEV1/FVC, as shown in Table S7.
For FEV1, the JMA of SNP and SNP-by-smoking gave genome-wide significant associations (PJMA<5×10−8) in the ever-smoking model for four SNPs on chromosome 17 (Figure 1C). However, these SNP associations did not exceed the more conservative significance threshold of PJMA<1.25×10−8. No novel loci reached genome-wide significance level in the pack-years model in relation to FEV1 (Figure 1D).
The most significant SNP (rs11654749) from both smoking models is intergenic between KCNJ2 (a potassium inwardly-rectifying channel also known as KIR2.1) and SOX9 (sex determining region Y-box 9) (Figure 2C). Conserved binding sites for four transcription factors (HNF-1, CP2, Cdc5, and FOXF2) are located within 100 kb upstream or downstream of rs11654749. The rs11654749 SNP gave PJMA = 1.28×10−8 in the ever-smoking model and PJMA = 6.63×10−8 in the pack-years model (Table 1). The corresponding PINT values were >0.05 (see Text S1). The rs11654749 T allele had negative β coefficients for both the main association and interaction (Table 1, Table S8 for study-specific results). These estimates correspond to a SNP effect of −0.028 (95% CI: −0.047, −0.010) in never-smokers and −0.046 (95% CI: −0.063, −0.029) in ever-smokers. To better understand the magnitude of these β estimates, we compared our results with those observed in one of our previous GWAS meta-analyses of SNP main effects [9], where standardized residuals of the pulmonary function measures were similarly computed. For a SNP with MAF around 40%, an absolute β value of 0.028 would be equivalent to 19 mL per copy of the risk allele (comparable to a year of FEV1 decline in healthy never-smokers), and an absolute β value of 0.046 would be equivalent to 31 mL per copy of the risk allele (comparable to a year and a half of FEV1 decline in healthy never-smokers) [25].
Besides this KCNJ2/SOX9 locus, SNPs from five other chromosomal regions have PJMA values between 5×10−8 and 1×10−6 from either smoking model in relation to FEV1 as shown in Table S6. In secondary meta-analyses of the interaction product terms, there were no SNP-by-smoking (ever-smoking or pack-years) interactions implicated at genome-wide statistical significance with FEV1. SNPs from four chromosomal regions had PINT values between 5×10−8 and 1×10−6 in relation to FEV1, as shown in Table S7.
None of the most significant SNPs from the three novel loci we identified by the JMA were associated with FEV1/FVC or FEV1 at or near genome-wide significance in our previous GWAS meta-analysis of 48,201 participants from the CHARGE and SpiroMeta Consortia. In fact, the lowest P value observed for these SNPs was 1.04×10−5 (Table 2) [10].
Tab. 2. Look-up evaluation of SNP main associations with FEV1/FVC and FEV1 using data generated by our previous genome-wide association study meta-analysis (N = 48,201), for the most significant SNP from each of the three novel loci implicated at genome-wide significance in the joint meta-analysis. 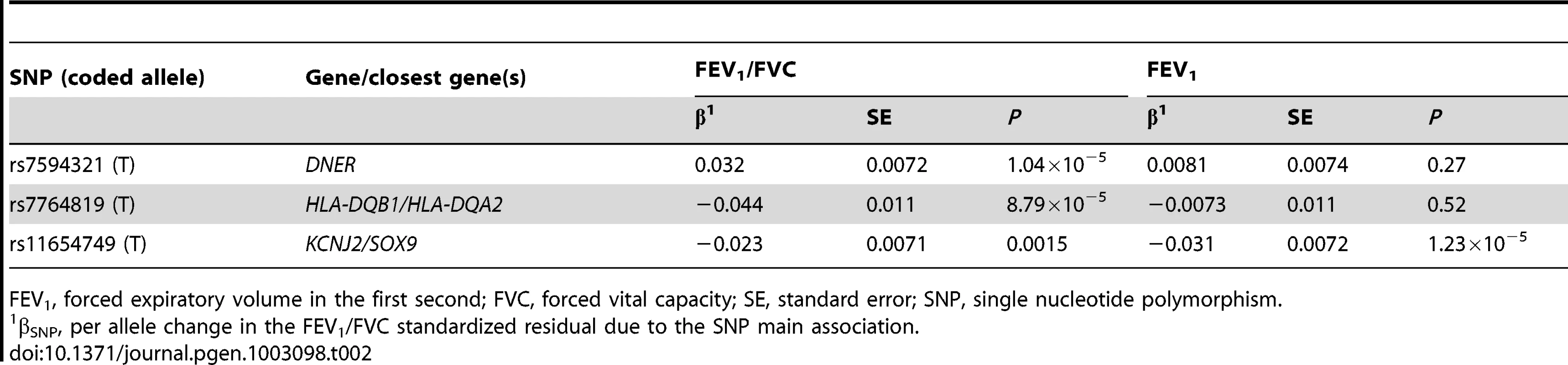
FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; SE, standard error; SNP, single nucleotide polymorphism. To evaluate whether the three novel loci identified by the JMA were related to smoking, we evaluated their SNP associations with ever-smoking and cigarettes per day using GWAS meta-analysis results from the Oxford-GlaxoSmithKline (Ox-GSK) Consortium (N = 41,150) [26]. None of our implicated SNPs were associated with these smoking phenotypes at P<0.05 (Table S9), adding confidence that our JMA-implicated SNP associations were not simply reflective of smoking main effects.
Expression analyses
Three genes (DNER, KCNJ2, and SOX9) harboring or flanking novel genome-wide significant SNPs were selected for follow-up mRNA expression profiling in human lung tissue and a series of primary cells. Transcripts of all three genes were found in lung tissue, airway smooth muscle, and bronchial epithelial cells; DNER and KCNJ2 transcripts were also found in peripheral blood cells (Table S10).
In a separate line of investigation, using the publically available Gene Expression Omnibus repository [27], [28], we found that the expression profiling of DNER and SOX9 showed differential expression in human airway epithelium of smokers compared to non-smokers (Figure S2A and S2B) [29]. Expression profiling of KCNJ2 did not show statistically significant differential expression by smoking status (Figure S2C) [29]. We also identified novel genome-wide significant SNPs in the HLA-DQ region, but we did not examine HLA-DQ expression given the known expression of class II MHC antigens on a range of airway cell types [30], [31]. However, the lead SNP in this region (rs7764819) was associated with statistically significant effects on HLA-DQB1 expression (P = 1.2×10−14), according to an eQTL analysis database of lymphoblastoid cell lines [32].
Discussion
Few GWAS have accounted for potential interaction with environmental risk factors. To identify novel genetic risk factors that are missed when considering only genetic main effects [33], we used the newly available JMA method [15] to simultaneously summarize regression coefficients for the main SNP and SNP-by-smoking interactive effects in 50,047 participants from 19 studies, based on models that were fully saturated for the main effect of smoking. This study represents the most comprehensive analysis to date of gene-by-smoking interaction in relation to pulmonary function. We identified two novel loci (DNER and HLA-DQB1/HLA-DQA2) having highly significant evidence for association with FEV1/FVC. A third novel locus (KCNJ2/SOX9) was associated with FEV1. For the most significant SNPs at each of these three loci, there was no evidence for heterogeneity across the studies (smallest heterogeneity P = 0.59), indicating that the associations were not driven by one or a few studies and thus reflect accumulation of evidence across the studies. None of these three loci had previously been associated with pulmonary function. The comparison of results with our prior GWAS meta-analysis of SNP main effects [10], using a comparable sample size, suggested that the SNP associations for our top SNPs were weaker in our previous analyses that examined only genetic main effects. However, our analyses and those of Manning et al. [14] suggest that some of the benefit of using the joint test for some findings comes from the careful adjustment for the environmental main effect. Thus, future studies aimed at replicating these findings may wish to jointly test the SNP main and interactive effects [15], [16], [33] instead of implementing a standard test of only the SNP main effects. If there is no evidence for interaction at a given locus, the saturation of the main effect of the environmental factor may be important. The joint testing is applicable for both candidate gene [15] and genome-wide [14] approaches. Further, there was minimal overlap in the top SNPs associated with FEV1/FVC and FEV1, as similarly observed in our previous GWAS meta-analyses of SNP main effects [8]–[10]. Given that the biological underpinnings of these discrepant association findings remain unknown, future studies should evaluate these genetic loci in the context of the pulmonary function measure for which they were originally implicated.
Given that pulmonary function is a phenotype for which numerous genetic loci have been identified in GWAS and smoking is clearly associated with pulmonary function, it might seem surprising that none of the genome-wide significant SNPs implicated by the JMA demonstrated a substantial interaction per se. The lack of strong interactive effects does not negate the well-established harmful effects of cigarette smoking nor the need for broad public health campaigns to curb smoking. Instead, our findings demonstrate the value of applying the newly developed joint methods to uncover novel genetic risk factors that might shed light on the mechanisms leading to reduced pulmonary function.
Our pattern of SNP main and interactive results resemble the patterns seen in another recent application of the same JMA method to incorporate the interaction with body mass index (BMI) into GWAS of type 2 diabetes traits (fasting insulin and blood glucose) [14]. In that study with a sample size of 96,453, nearly double that of ours, the top JMA finding had a corresponding interaction P value of 1.6×10−4 [14]. In our study, the smallest interaction P value for our top JMA finding was 6.9×10−3. In both our GWAS of smoking and pulmonary function and the recent GWAS of BMI and diabetes traits [14], the SNPs newly implicated by the JMA had marginally significant associations with the trait under study in models with no interaction term, but they became genome-wide significant when accounting for the environmental factor (cigarette smoking or BMI) and the SNP-by-environment interaction. Our JMA included careful modeling of the environmental factor to saturate the environmental main effects along with the interaction testing. In the GWAS of diabetes traits [14], the careful modeling of the environmental factor appeared to account for some of the novel findings from the JMA, consistent with the modest evidence for interaction [14]. Although our previous GWAS meta-analysis was conducted in ever/never-smoking strata, the regression models were not adjusted for smoking status or pack-years [10]. Some of our novel JMA findings compared with our previous GWAS findings may reflect, in part, the saturated modeling of the smoking main effect rather than the interaction per se.
The current analysis of 50,047 participants included only 1,846 more participants than our previous GWAS meta-analysis of SNP main effects [10]. To evaluate the likelihood that this 3.8% increase in sample size above that in our previous meta-analysis of pulmonary function was sufficient to explain our identification of these three novel loci at genome-wide statistical significance in the current JMA, we calculated the statistical power to detect genetic main associations (QUANTO [34]) with minor allele frequency (MAF) and β estimates comparable to the three genome-wide significant SNPs presented in Table 1. The current study (total N = 50,047 participants) had only 0.7% to 4.2% more statistical power than our previous GWAS meta-analysis (total N = 48,201 participants) [10], suggesting that the JMA-implicated SNPs are not merely reflective of increased power to detect genetic main effects. Instead, our novel JMA findings demonstrate an advantage of the method used to jointly test the SNP and SNP-by-smoking interactive effects, including the benefit of the saturated modeling of the smoking main effect.
SNPs located in the DNER gene were significantly associated with FEV1/FVC, even at the more conservative P value threshold of 1.25×10−8. The JMA results for DNER SNPs were driven by both smoking-adjusted main effects and interaction with quantitative smoking history. The DNER protein product is a ligand of the Notch signaling pathway that has been implicated in neuronal differentiation and maturation [35], [36], adipogenesis [37], and hair-cell development [38]. The Notch pathway is a critical controller of cellular differentiation in multiple organs including the lung [39], [40]. Interestingly, the expression levels of many members of the Notch signaling cascade are significantly altered in airway epithelial cells of smokers [41]. We confirmed the expression of DNER transcripts in lung and peripheral cells, and by mining publicly available transcriptional profiling databases [29], we found that DNER is expressed in bronchial epithelial cells of non-smoking adults and, importantly, its expression is significantly higher in smokers (Figure S2A). Collectively, these results suggest that DNER plays a role in cigarette smoke-induced airflow obstruction and further corroborate the importance of the Notch signaling circuitry in the pathogenesis of obstructive lung disease.
Also in relation to FEV1/FVC, intergenic SNPs between HLA-DQB1 and HLA-DQA2 exceeded the more conservative genome-wide significance threshold. The eQTL analyses indicated that the lead SNP is associated with expression of HLA-DQB1 specifically. However, the major histocompatibility complex region is highly polymorphic with complex LD patterns, and a few specific functional SNPs might explain the observed associations [42]. Genetic variations within this region have been associated with several autoimmune disorders [43] and asthma [24], [44], [45], and an interaction between HLA variants and cigarette smoking has been previously implicated [46]. We found little evidence for interaction with smoking at this locus, suggesting that the JMA results were primarily driven by smoking-adjusted genetic main effects. It is most likely that this locus was not identified in our previous GWAS meta-analysis, because the genetic main associations were not evaluated with careful adjustment for smoking status and pack-years. Adjustment for smoking in the current analysis may have removed residual variance in the outcome that is not attributable to genetic variation [14], thus making the identification of the newly associated SNPs possible.
Intergenic SNPs between KCNJ2 and SOX9 were significantly associated with FEV1 at the standard P value threshold, but not the more conservative threshold. Similar to the HLA region, it appears that the JMA results for the KCNJ2/SOX9 region were primarily driven by smoking-adjusted genetic main effects. This region is enriched for long-range regulatory elements for SOX9, although the possibility of this region containing KCNJ2 regulatory elements cannot be discounted [47]. KCNJ2 is a member of the inwardly-rectifying potassium channel family, which regulates membrane potential and cell excitability and is expressed in many tissues including myocardium, neurons, and vasculature. This potassium channel also affects human bronchial smooth muscle tone and airflow limitation [48]. Dominant negative mutations in KCNJ2 cause the Andersen syndrome, characterized by ventricular arrhythmias, periodic paralysis, and a number of skeletal and cardiac abnormalities [49]. SOX9 is a transcription factor that is essential for cartilage formation, [50] but it is also abundantly expressed in other tissues including the respiratory epithelium during development [51]. Sox9−/− and Sox9+/− mice have multiple skeletal anomalies and severe tracheal cartilage malformations and die prematurely from respiratory insufficiency [50], [52]. Mutations in SOX9 cause campomelic dysplasia characterized by skeletal defects and autosomal sex reversal [53]. These individuals develop respiratory distress due to chest wall abnormalities, narrowed airways resulting from tracheobronchial defects and hypoplastic lungs [54]. We confirmed that KCNJ2 and SOX9 transcripts were present in human lung tissue and peripheral cells. Using publicly available microarray data [29], we established that SOX9 is expressed in human airway epithelial cells and its expression is significantly down-regulated in smokers relative to non-smoking adults (Figure S2B). Taken together, these results suggest that SOX9 may be involved in cigarette smoke-induced airflow obstruction, but further investigation is required to elucidate putative mechanisms.
Most of the previously implicated SNPs had genome-wide significant (or nearly significant) associations with pulmonary function in the JMA, but some were associated with pulmonary function at P values that did not approach the genome-wide statistical significance threshold in the JMA analysis. This pattern has two possible explanations. First, the identification of these SNPs at genome-wide statistical significance in our most recent analysis [10] required a sample size of nearly 95,000 individuals, which was obtained by combining discovery and replication cohorts, including additional genotyping on thousands of participants from studies without GWAS data. In the current analysis, the sample size is greatly reduced because of the need for detailed quantitative smoking data and because we were unable to perform additional genotyping in studies without GWAS data. Second, Manning et al.[15] showed that a meta-analysis of main SNP effects has slightly greater power than the JMA under the scenario of no interaction, so it is not surprising that a few of the prior SNP findings had varying levels of significance between our prior GWAS meta-analyses [8]–[10] and the current JMA study. While our sample size of over 50,000 study participants is large, and the study of Manning et al. [14] examining SNP-by-BMI interaction in relation to fasting insulin is nearly twice as large, identification of interactions is challenging from a statistical power perspective. Given the multiple testing issues in genome interaction testing, even larger sample sizes will likely be needed to identify gene-by-environment interactions with rare variants or with the modest effect sizes that we generally expect. Nonetheless, our findings exemplify the greater power achieved by using the joint methods, such as those reported by Manning et al. [15] and Kraft et al. [16], [33], to incorporate interaction with a clearly associated environmental risk factor. The novel genetic loci identified here for pulmonary function would have remained unknown using standard GWAS approaches.
Methods
Ethics statement
Nineteen independent studies contributed to our analyses. All study protocols were approved by the respective local Institutional Review Boards, and written informed consent for genetic studies was obtained from all participants included in our analyses.
Cohort studies
Of the 19 studies contributing to our analyses, 18 studies came from the CHARGE [8], [55] or SpiroMeta [9] Consortium: Age, Gene, Environment, Susceptibility (AGES) – Reykjavik Study [56]; Atherosclerosis Risk in Communities (ARIC) Study [57]; British 1958 Birth Cohort (B58C) [58]; Coronary Artery Risk Development in Young Adults (CARDIA) [59], [60]; Cardiovascular Health Study (CHS) [61]; European Community Respiratory Health Survey (ECRHS) [62]; European Prospective Investigation into Cancer and Nutrition (EPIC, obese cases and population-based subsets) [63]; Framingham Heart Study (FHS) [64], [65]; Health, Aging, and Body Composition (Health ABC) Study [66]; Northern Finland Birth Cohort of 1966 (NFBC1966) [67], [68]; Multi-Ethnic Study of Atherosclerosis (MESA) [69], [70]; Rotterdam Study (RS-I, RS-II, and RS-III) [71]; Swiss Study on Air Pollution and Lung Diseases in Adults (SAPALDIA) [72]; Study of Health in Pomerania (SHIP) [73]; and TwinsUK [74]. We reached out to other population-based studies with GWAS genotyping and data available on cigarette smoking and pulmonary function, resulting in the inclusion of LifeLines [75]. Given the greater power needed to detect novel genetic loci with subtle gene-environment interaction regardless of the statistical method used [16], we chose to maximize statistical power to discover novel genetic loci by combining all available participants and to use the regression coefficients across the many different component studies as evidence for consistency. This approach was similarly taken by another large-scale GWAS consortium for discovering SNP main effects [24].
Pulmonary function measurements and smoking information
All studies were included in our previous GWAS meta-analysis of pulmonary function or the follow-up replication analyses, wherein their pulmonary function testing protocols were described [10]. For studies with spirometry at a single visit (B58C, LifeLines, MESA, NFBC1966, SHIP, RS-I, RS-II, and RS-III), we analyzed FEV1/FVC and FEV1 measured at that visit. For studies with spirometry at more than one visit, we analyzed measurements from the baseline visit (AGES, ARIC, CARDIA, CHS, ECRHS, EPIC obese cases, EPIC population-based, Health ABC, and SAPALDIA) or the most recent examination with spirometry data (FHS and TwinsUK).
Smoking history (current-, past-, and never-smoking) was ascertained by questionnaire at the time of pulmonary function testing. Pack-years of smoking were calculated for current and past smokers by multiplying smoking amount (packs/day) and duration (years smoked). Table S11 presents the specific questions used to ascertain smoking history and pack-years in each of the 19 studies.
Genotyping, quality control, and imputation
Study participants were genotyped on various genotyping platforms, and standard quality control filters for call rate, Hardy-Weinberg equilibrium p-value, MAF, and other measures were applied to the genotyped SNPs (Table S12). To generate a common set of SNPs for meta-analysis, imputation was conducted with reference haplotype panels from HapMap phase II subjects of European ancestry (CEU) (Table S12) [76]. Imputed genotype dosage values (estimated reference allele count with a fractional value ranging from 0 to 2.0) were generated for approximately 2.5 million autosomal SNPs. Among participants with genome-wide SNP genotyping data, exclusions were made due to standard quality control metrics (call rate, discordance with prior genotyping, and genotypic and phenotypic sex mismatch among others), missing pulmonary function data, or missing covariate data (Table S13).
Statistical analysis
Our analyses included 50,047 participants from 19 studies who passed their study-specific quality control and had complete data on pulmonary function and smoking. Each study transformed the pulmonary function measures to residuals using linear regression of FEV1/FVC (%) and FEV1 (mL) on age, age2, sex, and standing height as predictors. Principal component eigenvectors and recruitment site were also included as covariates to adjust for population stratification (if applicable). The residuals were converted to z scores (henceforth referred to as standardized residuals). We confirmed that smoking was inversely associated with the FEV1/FVC and FEV1 standardized residuals in all 19 studies (meta-analysis β = −0.0030 and corresponding P<1×10−6 for pack-years of smoking).
The FEV1/FVC and FEV1 standardized residuals were used as the phenotypes for genome-wide association testing with linear regression models, which included the following predictor variables: imputed SNP genotype dosages, smoking history (dichotomous variable, 0 = never-smokers and 1 = ever-smokers), smoking status (dichotomous variable, 0 = never - and past-smokers and 1 = current-smokers), pack-years of smoking (continuous variable), and a SNP-by-smoking interaction product term. Two of the 19 studies (FHS and TwinsUK) had much relatedness among participants, and we took appropriate account of relatedness in the association testing (Table S12). Four regression models with interaction terms for ever-smoking or pack-years were specified in relation to standardized residuals for FEV1/FVC or FEV1. As it has long been advised in studying interactions, the regression models were designed to fully saturate the main smoking effect on pulmonary function, so that the interaction terms do not capture residual main effects [77]. In each of the 19 studies, the genome-wide analyses were implemented with robust variance estimation using the software packages indicated in Table S12.
Our analyses were aimed at finding novel loci associated with pulmonary function when considering an interaction with cigarette smoking, so we chose to implement JMA of SNP main and interactive SNP-by-smoking effects (two d.f. test of the null hypothesis βSNP = 0 and βINT = 0) [15]. Manning et al. previously compared the joint methods, such as JMA, with other methods that incorporate gene-environment interaction (such as screening by main effects [78] or conducting a 1 d.f. meta-analysis of the interaction product term), and they found that the joint methods offer optimal statistical power over a range of scenarios for SNP main and interactive effects [15], [33]. Therefore, our analyses centered on the JMA method, which simultaneously estimates regression coefficients for the SNP and SNP-by-smoking interaction terms, while accounting for their covariance, to generate a joint test of significance [15]. It also accounts for the unequal variances from studies of different sample sizes. Secondarily, we implemented meta-analyses of just the β coefficient from the interaction term for comparison with the JMA results. Of note, the two-step gene-environment interaction study designs by Murcray et al. [79], [80] and Gauderman et al. [81] are applicable to case-control or case-parent trio studies, respectively, and were thus not considered for our population-based studies of continuous traits.
The JMA was conducted with fixed effects on approximately 2.5 million SNPs using METAL software (version 2010-02-08) [82] and patch source code provided by Manning et al. [82]. Genomic control correction was applied by computing λgc as the ratio of the observed and expected (2 d.f.) median chi-square statistics and dividing the observed chi-square statistics by λgc. SNPs having PJMA<5×10−8 (the standard Bonferroni-adjusted P value) were considered statistically significant [83]. Further correction for the four different (albeit related) JMA models yielded a conservative PJMA threshold of 1.25×10−8. In addition to reporting the PJMA for the most significant SNP from each novel locus, we used the β and standard error (SE) estimates from the JMA results to calculate the P values corresponding to the SNP main association (PSNP) and the SNP-by-ever-smoking interaction (PINT) [15].
Bioinformatics analysis
Gene annotation was performed using the gene prediction tracks “UCSC Genes” and “RefSeq Genes” in the UCSC browser (http://genome.ucsc.edu). The “sno/miRNA” track from the USCS browser was used to search for any microRNA within 100 kb upstream or downstream of each SNP, and the “TFBS Conserved” track was used to search for conserved transcription factor binding sites (TFBSs) at or near the most significant SNPs. The SNAP program [84] was used to infer LD patterns, based on the HapMap phase II CEU population.
Expression analyses
We used separate types of expression analyses to confirm the biologic plausibility of our findings. First, we carried out mRNA expression profiling to show whether or not the implicated genes are expressed in human tissues relevant to pulmonary function. The mRNA expression profiles of implicated genes were determined using reverse transcription polymerase chain reaction (RT-PCR). RNA was sourced from lung (Ambion/ABI), human bronchial epithelial cells (Clonetics) [85], and peripheral blood mononuclear cells (3H Biomedica). RNA from human airway smooth muscle cells, cultured as previously described from tissue obtained at thoracotomy [86], was extracted using a commercially available kit (Qiagen). Ethical approval for the use of primary cells was obtained from the local ethics committees. cDNA was generated using 1 µg of RNA template using random hexamers and a SuperScript kit (Invitrogen) as directed by the manufacturer. PCR assays were designed to cross intron-exon boundaries, where possible and where splice variation was known, in order to detect all variants. The GAPDH gene was used as a positive control for the cDNA quality, and water was used as a negative control. Primer sequences for the genes of interest are given in Table S14. All PCR were done using Platinum Taq High Fidelity (Invitrogen) with 100 ng of cDNA template in a 25 µL reaction. Cycling conditions were as follows: 94°C for 2 minutes, 35 cycles of 94°C for 45 seconds, 55°C for 30 seconds, and 68°C for 90 seconds. Following PCR, gel bands were directly sequenced to confirm the presence of the gene's transcript.
Second, we used another publically available data repository to investigate whether any of the implicated genes showed evidence for differential expression depending on smoking history. The gene expression profiles of human airway epithelium from healthy smokers (N = 10) and nonsmokers (N = 12) were obtained from the Gene Expression Omnibus site (http://www.ncbi.nlm.nih.gov/geo/) [27], [28], based on robust multichip average processing of probe intensities from Affymetrix HG-U133 Plus 2.0 microarrays (GEO dataset number GSE4498) [29]. Mean expression levels of genes around our genome-wide significant findings from the JMA were compared between smokers versus nonsmokers. The P value for the difference in means between smokers and nonsmokers was calculated using the nonparametric Mann-Whitney test.
Third, our genome-wide significant SNPs from novel loci were searched against an expression quantitative trait loci (eQTL) data repository based on lymphoblastoid cell lines [32], to investigate whether any of the implicated SNP variants might influence the expression of the nearby genes. P<5×10−8 was used to designate statistically significant eQTL associations.
Supporting Information
Zdroje
1. HoleDJ, WattGC, Davey-SmithG, HartCL, GillisCR, et al. (1996) Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ 313 : 711–716.
2. SchunemannHJ, DornJ, GrantBJ, WinkelsteinWJr, TrevisanM (2000) Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest 118 : 656–664.
3. MyintPK, LubenRN, SurteesPG, WainwrightNW, WelchAA, et al. (2005) Respiratory function and self-reported functional health: EPIC-Norfolk population study. Eur Respir J 26 : 494–502.
4. RedlineS, TishlerPV, LewitterFI, TagerIB, MunozA, et al. (1987) Assessment of genetic and nongenetic influences on pulmonary function. A twin study. Am Rev Respir Dis 135 : 217–222.
5. HubertHB, FabsitzRR, FeinleibM, GwinnC (1982) Genetic and environmental influences on pulmonary function in adult twins. Am Rev Respir Dis 125 : 409–415.
6. WilkJB, ChenTH, GottliebDJ, WalterRE, NagleMW, et al. (2009) A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet 5: e1000429 doi:10.1371/journal.pgen.1000429.
7. PillaiSG, GeD, ZhuG, KongX, ShiannaKV, et al. (2009) A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet 5: e1000421 doi:10.1371/journal.pgen.1000421.
8. HancockDB, EijgelsheimM, WilkJB, GharibSA, LoehrLR, et al. (2010) Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet 42 : 45–52.
9. RepapiE, SayersI, WainLV, BurtonPR, JohnsonT, et al. (2010) Genome-wide association study identifies five loci associated with lung function. Nat Genet 42 : 36–44.
10. Soler ArtigasM, LothDW, WainLV, GharibSA, ObeidatM, et al. (2011) Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet 43 : 1082–1090.
11. BeatyTH, RuczinskiI, MurrayJC, MarazitaML, MungerRG, et al. (2011) Evidence for gene-environment interaction in a genome wide study of nonsyndromic cleft palate. Genet Epidemiol 35 : 469–478.
12. LiuY, XuH, ChenS, ChenX, ZhangZ, et al. (2011) Genome-wide interaction-based association analysis identified multiple new susceptibility Loci for common diseases. PLoS Genet 7: e1001338 doi:10.1371/journal.pgen.1001338.
13. EgeMJ, StrachanDP, CooksonWO, MoffattMF, GutI, et al. (2011) Gene-environment interaction for childhood asthma and exposure to farming in Central Europe. J Allergy Clin Immunol 127 : 138–144, 144 e131–134.
14. ManningAK, HivertMF, ScottRA, GrimsbyJL, Bouatia-NajiN, et al. (2012) A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet 44 : 659–669.
15. ManningAK, LaValleyM, LiuC-T, RiceK, AnP, et al. (2011) Meta-analysis of gene-environment interaction: joint estimation of SNP and SNPxEnvironment regression coefficients. Genet Epidemiol 35 : 11–18.
16. AschardH, HancockDB, LondonSJ, KraftP (2010) Genome-wide meta-analysis of joint tests for genetic and gene-environment interaction effects. Hum Hered 70 : 292–300.
17. HamzaTH, ChenH, Hill-BurnsEM, RhodesSL, MontimurroJ, et al. (2011) Genome-wide gene-environment study identifies glutamate receptor gene GRIN2A as a Parkinson's disease modifier gene via interaction with coffee. PLoS Genet 7: e1002237 doi:10.1371/journal.pgen.1002237.
18. (2010). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA).
19. Lango AllenH, EstradaK, LettreG, BerndtSI, WeedonMN, et al. (2010) Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature 467 : 832–838.
20. WenW, ChoYS, ZhengW, DorajooR, KatoN, et al. (2012) Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet 44 : 307–311.
21. CornelisMC, Tchetgen TchetgenEJ, LiangL, QiL, ChatterjeeN, et al. (2012) Gene-environment interactions in genome-wide association studies: a comparative study of tests applied to empirical studies of type 2 diabetes. Am J Epidemiol 175 : 191–202.
22. ElksCE, PerryJR, SulemP, ChasmanDI, FranceschiniN, et al. (2010) Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat Genet 42 : 1077–1085.
23. LindgrenCM, HeidIM, RandallJC, LaminaC, SteinthorsdottirV, et al. (2009) Genome-wide association scan meta-analysis identifies three Loci influencing adiposity and fat distribution. PLoS Genet 5: e1000508 doi:10.1371/journal.pgen.1000508.
24. MoffattMF, GutIG, DemenaisF, StrachanDP, BouzigonE, et al. (2010) A large-scale, consortium-based genomewide association study of asthma. N Engl J Med 363 : 1211–1221.
25. KohansalR, Martinez-CamblorP, AgustiA, BuistAS, ManninoDM, et al. (2009) The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med 180 : 3–10.
26. LiuJZ, TozziF, WaterworthDM, PillaiSG, MugliaP, et al. (2010) Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet 42 : 436–440.
27. EdgarR, DomrachevM, LashAE (2002) Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30 : 207–210.
28. BarrettT, TroupDB, WilhiteSE, LedouxP, EvangelistaC, et al. (2011) NCBI GEO: archive for functional genomics data sets–10 years on. Nucleic Acids Res 39: D1005–1010.
29. HarveyBG, HeguyA, LeopoldPL, CarolanBJ, FerrisB, et al. (2007) Modification of gene expression of the small airway epithelium in response to cigarette smoking. J Mol Med (Berl) 85 : 39–53.
30. CaulfieldJJ, FernandezMH, SousaAR, LaneSJ, LeeTH, et al. (1999) Regulation of major histocompatibility complex class II antigens on human alveolar macrophages by granulocyte-macrophage colony-stimulating factor in the presence of glucocorticoids. Immunology 98 : 104–110.
31. GlanvilleAR, TazelaarHD, TheodoreJ, ImotoE, RouseRV, et al. (1989) The distribution of MHC class I and II antigens on bronchial epithelium. Am Rev Respir Dis 139 : 330–334.
32. DixonAL, LiangL, MoffattMF, ChenW, HeathS, et al. (2007) A genome-wide association study of global gene expression. Nat Genet 39 : 1202–1207.
33. KraftP, YenYC, StramDO, MorrisonJ, GaudermanWJ (2007) Exploiting gene-environment interaction to detect genetic associations. Hum Hered 63 : 111–119.
34. Gauderman WJ, Morrison JM (2006) QUANTO 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies.
35. TohgoA, EirakuM, MiyazakiT, MiuraE, KawaguchiSY, et al. (2006) Impaired cerebellar functions in mutant mice lacking DNER. Mol Cell Neurosci 31 : 326–333.
36. FukazawaN, YokoyamaS, EirakuM, KengakuM, MaedaN (2008) Receptor type protein tyrosine phosphatase zeta-pleiotrophin signaling controls endocytic trafficking of DNER that regulates neuritogenesis. Mol Cell Biol 28 : 4494–4506.
37. ParkJR, JungJW, SeoMS, KangSK, LeeYS, et al. (2010) DNER modulates adipogenesis of human adipose tissue-derived mesenchymal stem cells via regulation of cell proliferation. Cell Prolif 43 : 19–28.
38. KowalikL, HudspethAJ (2011) A search for factors specifying tonotopy implicates DNER in hair-cell development in the chick's cochlea. Dev Biol 354 : 221–231.
39. GusehJS, BoresSA, StangerBZ, ZhouQ, AndersonWJ, et al. (2009) Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development 136 : 1751–1759.
40. TsaoPN, VasconcelosM, IzvolskyKI, QianJ, LuJ, et al. (2009) Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136 : 2297–2307.
41. TilleyAE, HarveyBG, HeguyA, HackettNR, WangR, et al. (2009) Down-regulation of the notch pathway in human airway epithelium in association with smoking and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 179 : 457–466.
42. RaychaudhuriS, SandorC, StahlEA, FreudenbergJ, LeeHS, et al. (2012) Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet 44 : 291–296.
43. ZhernakovaA, van DiemenCC, WijmengaC (2009) Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet 10 : 43–55.
44. HirotaT, TakahashiA, KuboM, TsunodaT, TomitaK, et al. (2011) Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet 43 : 893–896.
45. TorgersonDG, AmplefordEJ, ChiuGY, GaudermanWJ, GignouxCR, et al. (2011) Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet 43 : 887–892.
46. MahdiH, FisherBA, KallbergH, PlantD, MalmstromV, et al. (2009) Specific interaction between genotype, smoking and autoimmunity to citrullinated alpha-enolase in the etiology of rheumatoid arthritis. Nat Genet 41 : 1319–1324.
47. GordonCT, TanTY, BenkoS, FitzpatrickD, LyonnetS, et al. (2009) Long-range regulation at the SOX9 locus in development and disease. J Med Genet 46 : 649–656.
48. OonumaH, IwasawaK, IidaH, NagataT, ImutaH, et al. (2002) Inward rectifier K(+) current in human bronchial smooth muscle cells: inhibition with antisense oligonucleotides targeted to Kir2.1 mRNA. Am J Respir Cell Mol Biol 26 : 371–379.
49. AndelfingerG, TapperAR, WelchRC, VanoyeCG, GeorgeALJr, et al. (2002) KCNJ2 mutation results in Andersen syndrome with sex-specific cardiac and skeletal muscle phenotypes. Am J Hum Genet 71 : 663–668.
50. BiW, DengJM, ZhangZ, BehringerRR, de CrombruggheB (1999) Sox9 is required for cartilage formation. Nat Genet 22 : 85–89.
51. LiuY, HoganBL (2002) Differential gene expression in the distal tip endoderm of the embryonic mouse lung. Gene Expr Patterns 2 : 229–233.
52. BiW, HuangW, WhitworthDJ, DengJM, ZhangZ, et al. (2001) Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci U S A 98 : 6698–6703.
53. FosterJW, Dominguez-SteglichMA, GuioliS, KwokC, WellerPA, et al. (1994) Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372 : 525–530.
54. HoustonCS, OpitzJM, SprangerJW, MacphersonRI, ReedMH, et al. (1983) The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al in 1971. Am J Med Genet 15 : 3–28.
55. PsatyBM, O'DonnellCJ, GudnasonV, LunettaKL, FolsomAR, et al. (2009) Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet 2 : 73–80.
56. HarrisTB, LaunerLJ, EiriksdottirG, KjartanssonO, JonssonPV, et al. (2007) Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 165 : 1076–1087.
57. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 129 : 687–702.
58. StrachanDP, RudnickaAR, PowerC, ShepherdP, FullerE, et al. (2007) Lifecourse influences on health among British adults: effects of region of residence in childhood and adulthood. Int J Epidemiol 36 : 522–531.
59. HughesGH, CutterG, DonahueR, FriedmanGD, HulleyS, et al. (1987) Recruitment in the Coronary Artery Disease Risk Development in Young Adults (Cardia) Study. Control Clin Trials 8 : 68S–73S.
60. FriedmanGD, CutterGR, DonahueRP, HughesGH, HulleySB, et al. (1988) CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 41 : 1105–1116.
61. FriedLP, BorhaniNO, EnrightP, FurbergCD, GardinJM, et al. (1991) The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1 : 263–276.
62. BurneyPG, LuczynskaC, ChinnS, JarvisD (1994) The European Community Respiratory Health Survey. Eur Respir J 7 : 954–960.
63. DayN, OakesS, LubenR, KhawKT, BinghamS, et al. (1999) EPIC-Norfolk: study design and characteristics of the cohort. European Prospective Investigation of Cancer. Br J Cancer 80 (Suppl 1) 95–103.
64. DawberTR, KannelWB (1966) The Framingham study. An epidemiological approach to coronary heart disease. Circulation 34 : 553–555.
65. FeinleibM, KannelWB, GarrisonRJ, McNamaraPM, CastelliWP (1975) The Framingham Offspring Study. Design and preliminary data. Prev Med 4 : 518–525.
66. YendeS, WatererGW, TolleyEA, NewmanAB, BauerDC, et al. (2006) Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax 61 : 10–16.
67. JarvelinMR, Hartikainen-SorriAL, RantakallioP (1993) Labour induction policy in hospitals of different levels of specialisation. Br J Obstet Gynaecol 100 : 310–315.
68. RantakallioP (1969) Groups at risk in low birth weight infants and perinatal mortality. Acta Paediatr Scand 193 (Suppl 193): 191+.
69. BildDE, BluemkeDA, BurkeGL, DetranoR, Diez RouxAV, et al. (2002) Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 156 : 871–881.
70. RodriguezJ, JiangR, JohnsonWC, MacKenzieBA, SmithLJ, et al. (2010) The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med 152 : 201–210.
71. HofmanA, van DuijnCM, FrancoOH, IkramMA, JanssenHL, et al. (2011) The Rotterdam Study: 2012 objectives and design update. Eur J Epidemiol 26 : 657–686.
72. MartinBW, Ackermann-LiebrichU, LeuenbergerP, KunzliN, StutzEZ, et al. (1997) SAPALDIA: methods and participation in the cross-sectional part of the Swiss Study on Air Pollution and Lung Diseases in Adults. Soz Praventivmed 42 : 67–84.
73. VolzkeH, AlteD, SchmidtCO, RadkeD, LorbeerR, et al. (2011) Cohort profile: the study of health in Pomerania. Int J Epidemiol 40 : 294–307.
74. AndrewT, HartDJ, SniederH, de LangeM, SpectorTD, et al. (2001) Are twins and singletons comparable? A study of disease-related and lifestyle characteristics in adult women. Twin Res 4 : 464–477.
75. StolkRP, RosmalenJG, PostmaDS, de BoerRA, NavisG, et al. (2008) Universal risk factors for multifactorial diseases: LifeLines: a three-generation population-based study. Eur J Epidemiol 23 : 67–74.
76. The International HapMap Project. Nature 426 : 789–796.
77. ShahaniAK (1970) A Saturated Experiment in Sequential Determination of Operating Conditions. Journal of the Royal Statistical Society Series D (The Statistician) 19 : 403–408.
78. KooperbergC, LeblancM (2008) Increasing the power of identifying gene x gene interactions in genome-wide association studies. Genet Epidemiol 32 : 255–263.
79. MurcrayCE, LewingerJP, ContiDV, ThomasDC, GaudermanWJ (2011) Sample size requirements to detect gene-environment interactions in genome-wide association studies. Genet Epidemiol 35 : 201–210.
80. MurcrayCE, LewingerJP, GaudermanWJ (2009) Gene-environment interaction in genome-wide association studies. Am J Epidemiol 169 : 219–226.
81. GaudermanWJ, ThomasDC, MurcrayCE, ContiD, LiD, et al. (2010) Efficient genome-wide association testing of gene-environment interaction in case-parent trios. Am J Epidemiol 172 : 116–122.
82. WillerCJ, LiY, AbecasisGR (2010) METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26 : 2190–2191.
83. Pe'erI, YelenskyR, AltshulerD, DalyMJ (2008) Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol 32 : 381–385.
84. JohnsonAD, HandsakerRE, PulitSL, NizzariMM, O'DonnellCJ, et al. (2008) SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics 24 : 2938–2939.
85. WadsworthSJ, NijmehHS, HallIP (2006) Glucocorticoids increase repair potential in a novel in vitro human airway epithelial wounding model. J Clin Immunol 26 : 376–387.
86. SayersI, SwanC, HallIP (2006) The effect of beta2-adrenoceptor agonists on phospholipase C (beta1) signalling in human airway smooth muscle cells. Eur J Pharmacol 531 : 9–12.
Štítky
Genetika Reprodukční medicína
Článek Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction DiseaseČlánek Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination SchemesČlánek Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 12- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Akutní intermitentní porfyrie
- Příjem alkoholu a menstruační cyklus
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
- Transfer zmraženého embrya zlepšuje výsledky IVF
-
Všechny články tohoto čísla
- A Mystery Unraveled: Essentiality of RNase III in Is Caused by Resident Prophages
- Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction Disease
- Insertion/Deletion Polymorphisms in the Promoter Are a Risk Factor for Bladder Exstrophy Epispadias Complex
- Mi2β Is Required for γ-Globin Gene Silencing: Temporal Assembly of a GATA-1-FOG-1-Mi2 Repressor Complex in β-YAC Transgenic Mice
- Dissection of a Quantitative Trait Locus for PR Interval Duration Identifies as a Novel Modulator of Cardiac Conduction
- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Construction of a Global Pain Systems Network Highlights Phospholipid Signaling as a Regulator of Heat Nociception
- Genes Contributing to Pain Sensitivity in the Normal Population: An Exome Sequencing Study
- Identification of , a Locus Controlling Dominant Resistance to Autoimmune Orchitis, as Kinesin Family Member 1C
- ATX1-Generated H3K4me3 Is Required for Efficient Elongation of Transcription, Not Initiation, at ATX1-Regulated Genes
- Dynamic and Differential Regulation of Stem Cell Factor FoxD3 in the Neural Crest Is Encrypted in the Genome
- Identification of Novel Type 2 Diabetes Candidate Genes Involved in the Crosstalk between the Mitochondrial and the Insulin Signaling Systems
- The Genetic Architecture of Adaptations to High Altitude in Ethiopia
- Population Genomics of the Endosymbiont in
- Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination Schemes
- Testicular Differentiation Occurs in Absence of R-spondin1 and Sox9 in Mouse Sex Reversals
- A Yeast GSK-3 Kinase Mck1 Promotes Cdc6 Degradation to Inhibit DNA Re-Replication
- Genetic Adaptation Associated with Genome-Doubling in Autotetraploid
- The Essential Function of RNase III Is to Silence Foreign Toxin Genes
- Long-Range Regulatory Polymorphisms Affecting a GABA Receptor Constitute a Quantitative Trait Locus (QTL) for Social Behavior in
- A New Isolation with Migration Model along Complete Genomes Infers Very Different Divergence Processes among Closely Related Great Ape Species
- Chromosome Fragile Sites in Harbor Matrix Attachment Regions That May Be Associated with Ancestral Chromosome Rearrangement Events
- Genome-Wide Association Study Implicates Testis-Sperm Specific as a Susceptibility Locus for Impaired Acrosome Reaction in Stallions
- A Mechanism of Gene Amplification Driven by Small DNA Fragments
- Base Damage within Single-Strand DNA Underlies Hypermutability Induced by a Ubiquitous Environmental Agent
- Integrative Analysis of a Cross-Loci Regulation Network Identifies as a Gene Regulating Insulin Secretion from Pancreatic Islets
- Reciprocal Signaling between the Ectoderm and a Mesendodermal Left-Right Organizer Directs Left-Right Determination in the Sea Urchin Embryo
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Genome-Wide Fine-Scale Recombination Rate Variation in
- Deciphering the Transcriptional-Regulatory Network of Flocculation in
- On Lung Function and Interactions Using Genome-Wide Data
- Genome-Wide Joint Meta-Analysis of SNP and SNP-by-Smoking Interaction Identifies Novel Loci for Pulmonary Function
- The Effective Population Size of Malaria Mosquitoes: Large Impact of Vector Control
- Recessive Mutations in Implicate β-III Spectrin in Both Cognitive and Motor Development
- An Excess of Gene Expression Divergence on the X Chromosome in Embryos: Implications for the Faster-X Hypothesis
- Reduced Life- and Healthspan in Mice Carrying a Mono-Allelic MVA Mutation
- Natural Variation at the MATE Transporter Locus Reveals Cross-Talk between Fe Homeostasis and Zn Tolerance in
- Histone Deacetylase HDA6 Is Functionally Associated with AS1 in Repression of Genes in
- A Framework for the Establishment of a Cnidarian Gene Regulatory Network for “Endomesoderm” Specification: The Inputs of ß-Catenin/TCF Signaling
- A Polycomb Group Protein Is Retained at Specific Sites on Chromatin in Mitosis
- Diapause Formation and Downregulation of Insulin-Like Signaling via DAF-16/FOXO Delays Axonal Degeneration and Neuronal Loss
- Genes That Act Downstream of Sensory Neurons to Influence Longevity, Dauer Formation, and Pathogen Responses in
- A Genome-Wide RNAi Screen Reveals MAP Kinase Phosphatases as Key ERK Pathway Regulators during Embryonic Stem Cell Differentiation
- Recurrent Targeted Genes of Hepatitis B Virus in the Liver Cancer Genomes Identified by a Next-Generation Sequencing–Based Approach
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
- Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
- Systems Genetic Analysis of Osteoblast-Lineage Cells
- Population Genomics of Inversion Polymorphisms in
- Spreading of Heterochromatin Is Limited to Specific Families of Maize Retrotransposons
- DNA Topoisomerases Maintain Promoters in a State Competent for Transcriptional Activation in
- A Histone Deacetylase Adjusts Transcription Kinetics at Coding Sequences during Morphogenesis
- Approaching the Functional Annotation of Fungal Virulence Factors Using Cross-Species Genetic Interaction Profiling
- Evidence for the Robustness of Protein Complexes to Inter-Species Hybridization
- Systematic Identification of Rhythmic Genes Reveals as a New Element in the Circadian Clockwork
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



