-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaDetection of Antibodies to Chlamydophila pneumoniae by Immunoblotting in Patients with Cardiovascular Diseases
Detection of Antibodies to Chlamydophila pneumoniae by Immunoblotting in Patients with Cardiovascular Diseases
Chlamydophila pneumoniae, one of the most prevalent human pathogens worldwide, is not only a significant cause of pneumonia, but may also be associated with cardiovascular diseases (CVD) as suggested by multiple studies. A total of 228 sera from CVD patients with hypertension, ischemic heart disease or previous reconstructive vascular surgery were screened for the presence of anti-C. pneumoniae IgG and IgA antibodies by ELISA. Out of 150 positive serum samples, 80 with similar IgG and IgA levels were investigated by immunoblot (IB). IgG antibodies were directed predominantly against the 35 kDa and 39 kDa proteins as well as 50‑54 and 56-60 kDa proteins of C. pneumoniae. IgA antibodies reacted most frequently with the 50-54 and 56-60 kDa proteins.
Key words:
cardiovascular diseases – Chlamydophila pneumoniae antibodies – immunoblotting.
Autoři: E. Kováčová 1; M. Mongiellová 2; J. Tomka 3; R. Slyško 3; J. Kazár 2
Působiště autorů: Department of Animal Physiology and Ethology, Faculty of natural Sciences, Commenius University, Bratislava 1; Research Base of the Slovak Medical University, Bratislava 2; National Instutute of Cardiovascular Diseases, Bratislava 3
Vyšlo v časopise: Epidemiol. Mikrobiol. Imunol. 58, 2009, č. 1, s. 15-18
Souhrn
Chlamydophila pneumoniae patrí k najrozšírenejším ľudským patogénnom na celom svete. Je nielen významnou príčinou pneumónií, ale mnohé štúdie naznačujú možný vzťah medzi infekciou C. pneumoniae a kardiovaskulárnymi chorobami (KVCH). Celkove sme vyšetrili 228 pacientov s vybranými KVCH (hypertenzia, ischemická choroba srdca a rekonštrukčný chirurgický zákrok na cievach) na prítomnosť IgG a IgA protilátok proti C. pneumoniae metódou ELISA. Zo 150 pozitívne reagujúcich vzoriek sér sme 80, ktoré mali podobné hladiny IgG a IgA protilátok, ďalej vyšetrili metódou imunoblotu. IgG protilátky boli namierené predovšetkým voči 35kDa a 39kDa proteínom, ako aj voči 50-54 a 56-60 kDa proteínom C. pneumoniae. Pokiaľ ide o IgA protilátky, tieto reagovali najčastejšie s 50-54 a 56-60 kDa proteínmi.
Kľúčové slová:
Kardiovaskulárne choroby, protilátky proti Chlamydophila pneumoniae, metóda imunoblotu.C. pneumoniae is one of the most frequent pathogens related to respiratory tract infections [8]. It is believed to cause about 10% of the cases of community-acquired pneumonia. It was described also as a risk factor for atherosclerosis based on the antibody detection against this pathogen and the presence of C. pneumoniae in atherosclerotic lesions in CVD patients, respectively [3, 15].
Though C. pneumoniae infection can be demonstrated by direct detection of the agent or by PCR-based detection of specific nucleotide sequences [19], routine laboratory diagnosis and seroepidemiological studies are performed usually by serological examination [1]. Microimmunofluorescence (MIF) assay considered formerly as a “gold standard” has some disadvantages such as differences in the antigen preparation, experience of the individual investigators, and difficulties in interpretation of the test resulting in interlaboratory variations in reporting the IF titers [14]. This evoked the necessity for development of alternative methods, e.g. ELISA and IB, respectively. Of several studies performed to recognize the species-specific antibodies appearing during C. pneumoniae infection and thus to improve exact serological diagnosis, IB was the most frequently used [2, 6, 9].
In our previous study we found an association of Chlamydia pneumoniae antibodies and markers of inflammation in sera of patients with cardiovascular diseases [11]. The purpose of this study was not only to analyze the anti-C. pneumoniae antibodies according to their immunoglobulin (Ig) classes, but also to detect the protein antigens to which the Ig antibody classes were directed.
Material and Methods
A total of 228 sera of the patients (62-years-old on the average) with selected CVD, namely coronary heart disease (CHD), hypertension (blood pressure ≥140/90 mm Hg) and those who underwent reconstructive vascular surgery (RVS) – endoarterectomy with removal of intimomedial complex of carotids and excision of the wall of abdominal aorta, respectively, were tested for the presence of anti-C. pneumoniae IgG and IgA antibodies by SeroCP-IgG and Sero CP-IgA ELISA kits (Savyon Diagnostics Ltd, Israel) with absorbance (A450) ≥1.1 nm as a cut off value. ELISA was performed and calculated according to the manufacturer instructions. Of 150 positively reacting sera with similar absorbance values, 80 specimens (60 from males and 20 from females) were further tested by .IgG + IgM + IgA IB kit of C. pneumoniae (AID GmbH, Germany) to detect Ig classes of antibodies directed to different protein antigens of C. pneumoniae. Sera of 15 persons with no evidence of CVD and with the absence or presence of chlamydial antibodies were used as controls.
Results
Analysis of the Ig antibody response to immunodominant protein antigens of C. pneumoniae by IB revealed that tested sera reacted with a broad range of antigens (Table 1). As to the IgG, predominant specific antibody activity was directed to the 35 kDa (44 samples), 39 kDa (35 samples), 50-54 kDa proteins (50 samples) and 56-60 kDa (25 samples) proteins, 39 kDa corresponding to the major outer membrane protein (MOMP) and 56-60 kDa to the heat-shock protein (HSP). Of the proteins with high molecular mass, reactivity was observed only in one sample reacting with 90 kDa protein. Most of serum samples bound also in the low molecular range, but the value of this binding, except for lipopolysaccharide (LPS), is not known and clear.
Tab. 1. The reactivities of sera of CVD patients with C. pneumoniae antigens in IB. 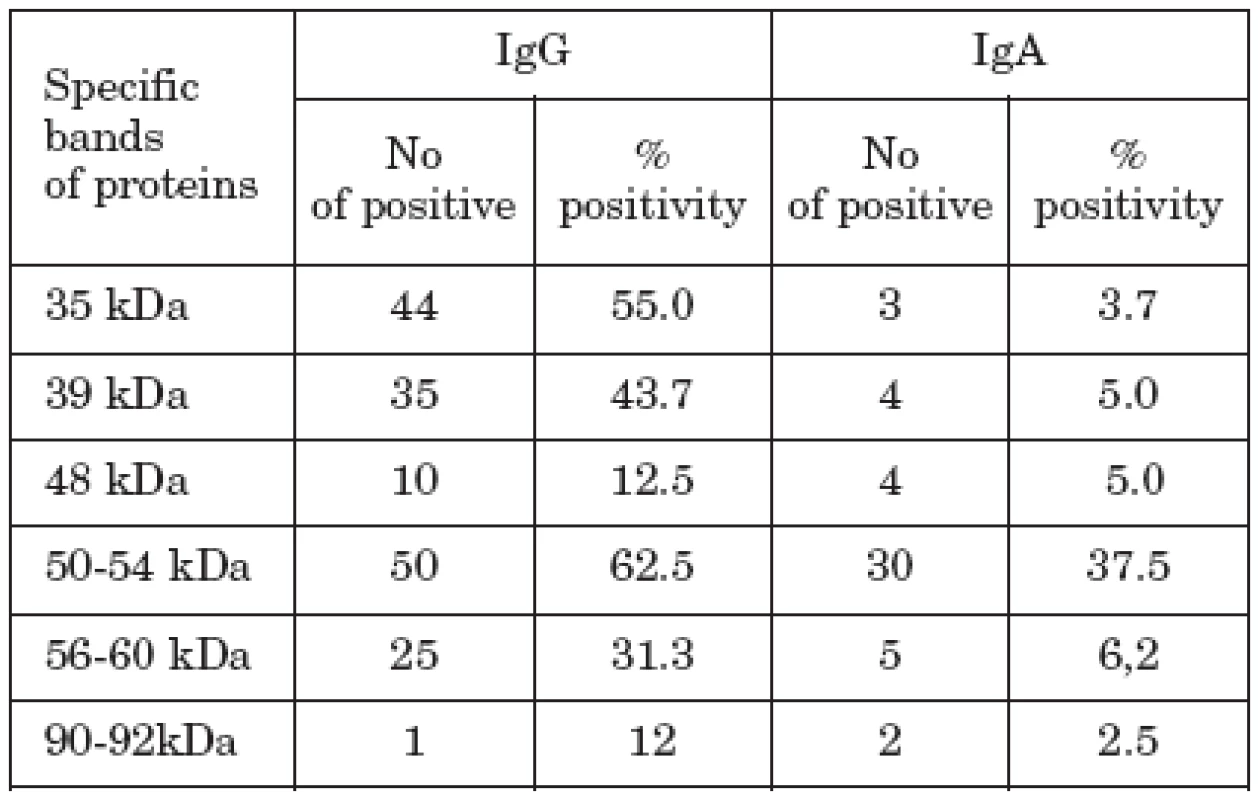
IgA antibodies reacted most frequently with low molecular weight proteins, often found in LPS region and 50-54 kDa proteins (30 samples), followed by 30 kDa (3 samples), 39 kDa (4 samples), and 56-60 kDa proteins (5 samples). Two samples reacted also with 90-92 kDa proteins. Both IgG and IgA reacting protein bands were evenly distributed irrespective of the clinical status of the patients.
IgM antibodies reaction patterns (not included in Table 1) occurred particularly between 54-60 kDa proteins (7 samples), whereas 39 kDa (MOMP band) and high molecular 98 kDa proteins were recognized only in one the same sample.
Of 15 serum samples of healthy persons, in which the presence of IgG and/or IgA antibodies was detected in 4 by previous ELISA testing, we found with IgG discrete bands in region of 50-54 kDa protein and HSP region, respectively, but only in one sample. As to IgA, we found reactivity with 50-54 kDa protein again in one sample.
As follows from Figs. 1 and 2, presenting the IB results with selected sera, IgG and IgA recognition patterns were similar, but the bands were more intensive in case of IgG.
Fig. 1. Typical IB protein profiles with C. pneumoniae IgA and IgG, respectively, in sera of CVD patients (lanes No 1-16 correspond to the sera of cardiovascular patients, lanes 17-19 to control sera). 
Discussion
Many studies analyzed the proteins and their reactivity in IB but the importance of individual proteins is not clear. When testing sera from C. pneumoniae ELISA positive patients by IB, significant antigenic differences among the strains of this agent were observed [18]. It is possible that distinct clinical disease syndrome such as respiratory tract infections or atherosclerosis are associated with different C. pneumoniae serovars [10].
In C. pneumoniae-infected patients, of the immunodominant proteins, a 39-kDa MOMP was genus specific and 53, 46, and 43 kDa proteins exhibited species specificity [9]. In another study, 160, 97 to 99, 60-62, 40, 27, and 15 kDa protein antigens were found by IB in patients with a current C. pneumoniae infection [6]. Two proteins with molecular masses of 43 and 53 kDa have been recognized frequently during human C. pneumoniae infection and the 53 kDa protein has potential for its serological diagnosis [4].
As to the patients with cardiovascular diseases, IgG serological responses were directed mostly to 40, 54, 60, 75, and 98 kDa proteins, so that cardiovascular risk from chlamydial infection could be linked to certain antigens [13]. On the other hand, the difference in prevalence of antibodies between the CHD patients and the healthy controls was significant when Chlamydia lipopolysaccharide antibodies were measured but not when antibodies to MOMP were tested [16]. In another study, as important antigens were found a 60 kDa HSP and outer membrane protein 2 [5].
In our study we found C. pneumoniae IgG antibodies directed mostly to 35 kDa (in 55.0%) and 50-54 kDa (in 62.5%) proteins (Table 1), followed by their reactivity with the MOMP (in 43.3%) and HSP (in 31.3%). IgA antibodies reacted the most frequently with 50-54 kDa protein (in 37.5%). From our results follows that apart from proteins of MOMP and HSP regions, the detection of Ig reactivity with 50-54 kDa proteins can be also of some importance in detecting C. pneumoniae infection. Whether it can be used for differentiation of possible participation of C. pneumoniae in CVD and other clinical manifestations caused by this agent remains to be elucidated by further studies.
Another problem is the significance of detection of anti-C. pneumoniae antibodies in CVD patients. In our recent study [12], the presence of anti-C. pneumoniae antibodies in 66.2% of 228 CVD patients corresponded with the presence of inflammatory markers, namely C-reactive protein (in 69.7%) and interleukin-6 (81.1%). Correlation of the chlamydial HSP antibodies and CRP with C. pneumoniae in atherosclerotic plaques was described also by Fong et al. [7].
IB certainly contributes to serological diagnosis of chlamydial infections, but detecting antibodies alone without appropriate clinical symptoms is not sufficient for antibiotic treatment of infection with C. pneumoniae [17]. Moreover, the spectrum of possible immundominant protein antigens of C. pneumoniae is wide and further studies are necessary to determine their importance not only in chlamydial infections in all, but namely in patients with CVD. To determine the role of serological tests, IB including, in the possible relationship between C. pneumoniae infection and atherosclerosis, their strict description and evaluation is mandatory [16].
Acknowledgement
This study was supported by the Science and Technology Assistance Agency under contract No 21-035702.
Do redakce došlo 16. 6. 2008
MUDr. E. Kováčová,
Slovenská zdravotnícka univerzita
Limbová 12
833 03 Bratislava
Slovenská republika
e-mail: jan.kazar@szu.sk
Zdroje
1. Bas, S., Muzzin, P., Ninet, B., Bornard, J.E. et al. Chlamydial serology: comparative diagnostic value of immunoblotting, microimmunofluorescence test, and immunoassays using different recombinant proteins as antigens. J Clin Microbiol, 2001, 39, 1368-1377.
2. Biendo, M., Eb, F., Lefebvre, J.F., Orfila, J. Limits of the microimmunofluorescence test and advantages of immunoblotting in the diagnosis of chlamydiosis. Clin Diagn Lab Immunol, 1996, 3, 706-709.
3. Blasi, C. The role of the infectious agents in the pathogenesis and evolution of arteriosclerosis. Ann Ital Med Int, 2004, 19, 249-261.
4. Campbell, L.A., Roberts, S., Inoue, S., Kong, L., Kuo, C.-C. Evaluation of Chlamydia pneumoniae 43 and 53 kilodalton recombinant proteins for serodiagnosis by Western Blot. Clin Diagn Lab Immunol, 2001, 8, 1231-1233.
5. Ciervo, A., Vica, P., Petrucca, A., Biasucci, L.M. et al. Antibodies to 60-kilodalton heat shock protein and outer membrane protein 2 of Chlamydia pneumoniae in patients with coronary heart diseases. Clin Diagn Lab Immunol, 2002, 9, 68-74.
6. Essig A., Simnacher U., Susa M., Marre R. Analysis of the humoral immune response to Chlamydia pneumoniae by immunoblotting and immunoprecipitation. Clin Diagn Lab Immunol, 1999, 6, 819-825.
7. Fong, I.V., Chiu, B., Viira, E.R., Tucker, W., Peeling, R.W.. Chlamydia heat shock protein (CHSP-60) antibody and reactive protein (CRP) correlation with Chlamydia pneumoniae in atherosclerotic plaques. J Infect Dis 2002, 186, 1469-1473.
8. Grayston, J.T., Campbell, L.A., Kuo, C.-C., Mordhorst, P. et al. A new respiratory pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis, 1990, 161, 618-625.
9. Iijima Y., Miyashita N., Kishimoto T., Kanamoto Y. et al. Characterization of Chlamydia pneumoniae species-specific proteins immunodominant in humans. J Clin Microbiol, 1994, 32, 583-588.
10. Jantos, C.A., Heck, S., Roggendorf, R., Sen-Gupta, M., Hegemann, H. Antigenic and molecular analyses of different Chlamydia pneumoniae strains. J Clin Microbiol, 1997, 34, 620-623.
11. Kazár, J., Kovacova E., Koncova K., Cvachova S. et al. Chlamydia pneumoniae antibodies and markers of inflammation in patients with cardiovascular diseases. Bratisl Lek Listy, 2005, 106, 341-344.
12. Kazar, J., Kovacova, E., Mongiellova, V., Gajdos, M. et al. Anti-cytomegalovirus antibodies and other atherosclerosis risk factors in patients with cardiovascular diseases. J Ger Cardiol, 2007, 4, 131-134.
13. Maass M., Giefers J. Cardiovascular disease risk from prior Chlamydia pneumoniae infection can be related to certain antigens recognized in the immunoblot profile. J Infect, 1997, 35, 171-176.
14. Peeling, R.W., Wang, S.-P., Grayston, J.T., Blasi, F. et al. Chlamydia pneumoniae serology: interlaboratory variations in microimmunofluorescence assay results. J Infect Dis, 2000, 181, S426-S429.
15. Saikku, P., Leinonen, M., Mattila, K., Ekman, M.R. et al. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet, 1988, ii: 983-986.
16. Schumacher, A., Lerkerod, A.B., Seljeflot, I., Sommervoll, L. et al. Chlamydia pneumoniae serology: importance of methodology in patients with coronary heart disease and healthy individuals. J Clin Microbiol, 2001, 39, 1859-1864.
17. Teislerová, D., Zampachová, E. Value of western blotting in serodiagnosis of chlamydial infections (in Czech). Klin Microbiol Infect Lek, 2007, 13, 21-25.
18. Wagels, A.G., Rasmussen, S., Timms, P. Comparison of Chlamydia pneumoniae isolates by western blot (immunoblot) analysis and DNA sequencing of the omp 2 gene. J Clin Microbiol, 1994, 32, 2820-2823.
19. Zeman, K., Pospíšil, L., Čenderle, J., Štroblová, H. et al. Direct and indirect evidence of Chlamydia pneumoniae in patients with significant stenosis of a carotis of atherosclerotic origin. Scripta medica (Brno), 2004, 77, 173-180.
Štítky
Hygiena a epidemiologie Infekční lékařství Mikrobiologie
Článek vyšel v časopiseEpidemiologie, mikrobiologie, imunologie
Nejčtenější tento týden
2009 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
-
Všechny články tohoto čísla
- Detection of Antibodies to Chlamydophila pneumoniae by Immunoblotting in Patients with Cardiovascular Diseases
- Onychocola canadensis: prvé izoláty z onychomykóz na Slovensku
- Molekulárno-biologická a fenotypová charakterizácia humánnych izolátov Salmonella enterica serovar Paratyphi B dT+, alebo Salmonella Java
- Systémový lupus erythematosus – súčasný pohľad na genetickú determináciu, imunopatogenézu a liečbu
- Výskyt a charakteristika salmonel ve vybraných lokalitách České republiky – porovnání epidemiologických a laboratorních dat
- Srovnání citlivosti spor Bacillus subtilis a spor českých kmenů Clostridium difficile vůči dezinfekčním prostředkům
- Zpráva z Celostátního sjezdu mikrobiologie a epidemiologie 2008
- Dovětek výboru Společnosti pro epidemiologii a mikrobiologii ČLS JEP
- Plán akcí Společnosti pro epidemiologii a mikrobiologii v roce 2009
- TEST
- Epidemiologie, mikrobiologie, imunologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Systémový lupus erythematosus – súčasný pohľad na genetickú determináciu, imunopatogenézu a liečbu
- Výskyt a charakteristika salmonel ve vybraných lokalitách České republiky – porovnání epidemiologických a laboratorních dat
- Srovnání citlivosti spor Bacillus subtilis a spor českých kmenů Clostridium difficile vůči dezinfekčním prostředkům
- Molekulárno-biologická a fenotypová charakterizácia humánnych izolátov Salmonella enterica serovar Paratyphi B dT+, alebo Salmonella Java
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



