-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Chronic dissecting aneurysm of ascending aorta with a large intramural thrombus and isolated aortic defects
Chronická disekujúca aneuryzma vzostupnej časti aorty s rozsiahlym intramurálnym trombom a izolovanými defektami v stene aorty
V popisovanom prípade autori dokumentujú makroskopický a mikroskopický pitevný nález chronickej disekujúcej aneuryzmy vzostupnej časti aorty a aortálneho oblúka s izolovanými trhlinami v stene aorty bez jej prasknutia u 71-ročnej ženy s minimálnou klinickou symtomatológiou. Aneuryzmatické rozšírenie priesvitu aorty bolo podmienené nielen vyklenutím cievnej steny na základe prítomnosti organizovaného intramurálneho trombu, ale aj v dôsledku prítomnosti plošného defektu intimy a medie aorty so zachovaním celistvosti obvodu fibroticky zmenenou adventíciou. Histologicky bola v torakálnej aorte zistená cystická degenerácia medie s minimálnymi prejavmi aterosklerózy.
Klíčová slova:
disekujúca aneuryzma aorty – disekcia aorty – intramurálny trombus aorty – degenerácia medie – akumulácia mukoidnej extracelulárnej hmoty
Authors: Silvia Farkašová Iannaccone 1; Dorota Sopková 1; Marián Švajdler jr. 2; Daniel Farkaš 3; Lucia Mistríková 4; Roman Mezencev 5
Authors place of work: Department of Forensic Medicine, Faculty of Medicine, Pavol Jozef Šafárik University, Košice, Slovak Republic 1; Šikl’s Department of Pathology, Charles University, Medical Faculty Hospital, Pilsen, Czech Republic 2; Medico-Legal and Pathological-Anatomical Department of Health Care Surveillance Authority, Košice, Slovak Republic 3; Department of Heart Surgery, East Slovak Institute for Cardiovascular Diseases, Košice, Slovak Republic 4; School of Biological Sciences, Georgia Institute of Technology, Atlanta, GA, USA 5
Published in the journal: Čes.-slov. Patol., 55, 2019, No. 2, p. 115-119
Category: Původní práce
Summary
We present macroscopic and microscopic findings in a case of chronic dissecting aneurysm of ascending aorta and aortic arch associated with isolated tears of aortic wall without its rupture in a 71-year-old female presenting with minimal clinical symptomatology. Aneurysmal dilation of the aorta was caused not only by the bridging of the vascular wall based on the presence of an organizing intramural thrombus in the false lumen between the separated layers, but also by a wide flat defect in the aortic intima and media with the preservation of the aortic wall integrity due to fibrotical alteration of tunica adventicia. Histologic examination of the thoracic aorta detected cystic medial degeneration with mild atherosclerosis.
Keywords:
dissecting aortic aneurysm – aortic dissection – aortic intramural thrombus – medial degeneration – mucoid extracellular matrix accumulation
Aneurysm (bulge) is a localized widening (dilation) of the artery that occurs when the arterial wall is significantly weakened (1). More precisely, the aneurysm is defined as an enlargement of the vessel that increases its normal size by more than 50 % (2). Based on their localization, aortic aneurysms can be classified as thoracic, abdominal, or thoracoabdominal (3). Aortic dissection represents a condition, in which the blood from the lumen penetrates aortic media through a defect in the inner layer (intima) and spreads to a various distance in the aortic wall (1,3,4). The term “intramural hematoma” is sometimes used specifically for cases of aortic wall dissection without detected intimal tear and without flow in the false lumen, or more loosely for cases with thrombosed false lumen regardless intimal defects (5). Major factor in the etiology of aortic dissection is arterial hypertension, and the most common histological pathology in the aortic wall is medial degeneration (6).
Here we present a case of a 71-year-old woman with a medical history of arterial hypertension and chronic ischemic heart disease, who was admitted to the hospital for exertional dyspnea and complaints of chest tightness. CT scan upon her admission revealed an aortic arch aneurysm; nevertheless, the patient died due to cardiac arrest right before the surgery was about to be performed. This case report presents a macroscopic and microscopic autopsy finding of a chronic dissecting aneurysm with isolated aortal tears and a flat fibrous defect without the evident rupture of the aortic wall.
CASE REPORT
The case report presents a case of a 71-year-old woman, who was admitted to the Internal Medicine Clinic due to exertional dyspnea accompanied by the chest tightness, which led to the assumption of an acute coronary syndrome. Her medical history revealed that she was treated for arterial hypertension and ischemic heart disease, other than that it contained information on nephrectomy performed 9 years prior to her death for renal cell carcinoma associated with paraneoplastic hypercoagulability and lung embolism. In the morning of the second day of
hospitalization, the patient experienced a pre-collapse state with immeasurable blood pressure, without a loss of consciousness. CT scan revealed an aortic arch aneurysm with the compression of mediastinal structures. For the purpose of emergency surgery, the patient was transported to the specialized hospital. Performed echocardiography showed an enlargement of the aortic arch with cranially localized intramural hematoma affecting two thirds of the circumeference of the aortic wall. Compression of the right atrium with signs of moderate pulmonary hypertension was detected, but the function of the heart was not affected. The patient died in the operating room due to cardiac arrest just before the surgical procedure was initiated.Postmortem external examination of the dead body of the lenght of 155 cm and the weight of 80 kg showed obesity with BMI being 33.3 kg/m2. Autopsy revealed a significant enlargement of the ascending aorta and the aortic arch, which was bent forward above the anterior surface of the heart (Fig. 1). Outer surface of the aorta showed no damage to its wall integrity. After cutting the thoracic aorta, an aneurysmal enlargement was detected, with the presence of a multi-layered intramural thrombus between the separated layers of the aortic wall. The aneurysm was located 4.5 cm above the aortic valve, and it ended immediately beyond the left subclavian artery (Fig. 2). The maximum outer circumference of the aorta was 26 cm, and the largest luminal diameter was 8.5 cm, while the diameter of the lumen of ascending aorta at its origin was 3.5 cm. Proximal 3-cm segment of the intramural thrombus was covered by endothelium and a portion of the split tunica media. The intial third of this intramural lesion appeared as an organized, relatively homogeneous and rigid thrombus, while its distal parts included fresh and fragile mural thrombus. The total length of the intramural and mural thrombus (in the direction of the blood flow) was 17 cm, and it affected approximately two thirds of the aortic circumference. The thickness of the intramural and mural thrombus combined was 4-5 cm. Two pseudocanals passing throught the mural thrombus were detected. The first pseudocanal of the width of 0.4-0.7 cm was connecting aortic lumen with the origin of the brachiocephalic trunk and a portion of mural thrombus was entering the trunk’s lumen in the form of centrally located intraluminal thrombus of the total length of 3 cm without obturation of the lumen (Fig. 3). Second similar pseudocanal of the width of 0.3 cm connected aortic lumen with the origin of the left common carotid artery. Three separate defects were detected in the lumen of the aortic arch, all of them without rupture. The first defect (defect no. 1) was located in front of the intramural thrombus, and presented as intimal tear with an intermittent “zipper-like” shape, positioned perpendicular to the direction of the blood flow with the length of 3.5 cm. This defect displayed a focal loss of the typical yellow endothelial coloration, and it somewhat resembled the condition after surgical suturing (stapler). Second isolated defect (defect no. 2), an intimal tear perpendicular to the direction of the blood flow with the length of 3.5 cm, was present above the “zipper-like” lesion and under the origin of brachiocephalic trunk. The last defect (defect no. 3) in the aortic arch lumen was located left to the previous ones. This defect had the shape of irregular triangle with base of 4 cm perpendicular to the direction of the blood flow and located proximally, and the length of 9 cm with apex pointing to the distal direction. On the cut surface of this defect no intima and media were macroscopically visible; it was formed by fibrous (granulation) tissue partially covered by a blood clot (Fig. 4). Ascending aorta and aortich arch showed mild to moderate atherosclerosis. The origin of the aorta, and the aortic valve were normal, as well as the course of both coronary arteries, their lumen was not narrowed by the enlarged aortic arch. Other major macroscopic findings included: (i) severe coronary atherosclerosis, (ii) concentric left ventricular hypertrophy (560 g), (iii) moderate to severe dispersive myofibrosis, (iv) chronic venous congestion in the liver, (v) pulmonary edema with recent pneumorrhagias, and (vi) splenomegaly (430 g).
Fig. 1. The front view of the heart and aneurysmal enlargement of the aorta. 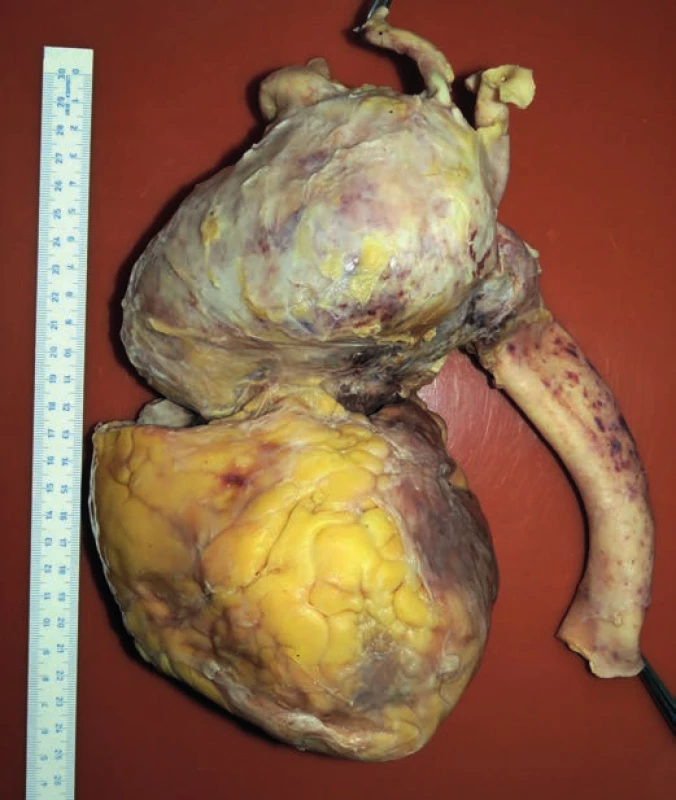
Fig. 2. The view of the left ventricular outflow tract, ascending aorta and aortic arch showing chronic dissection with intramural hematoma and isolated defects in the aorta’s wall. Defect no. 1 (arrows pointing upwards). Defect no. 2 (arrows pointing downwards). Defect no. 3 (arrows pointing to the left). 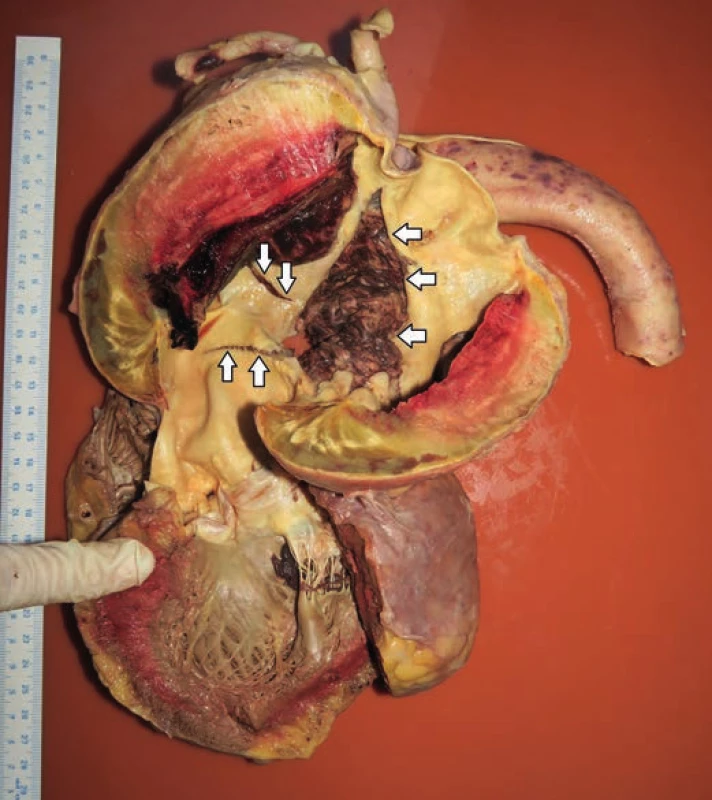
Fig. 3. Transverse cut through brachiocephalic trunk and aortic intramural thrombus. Yellow lining is showing the course of pseudocanal connecting lumen of the aorta with brachiocephalic trunk. 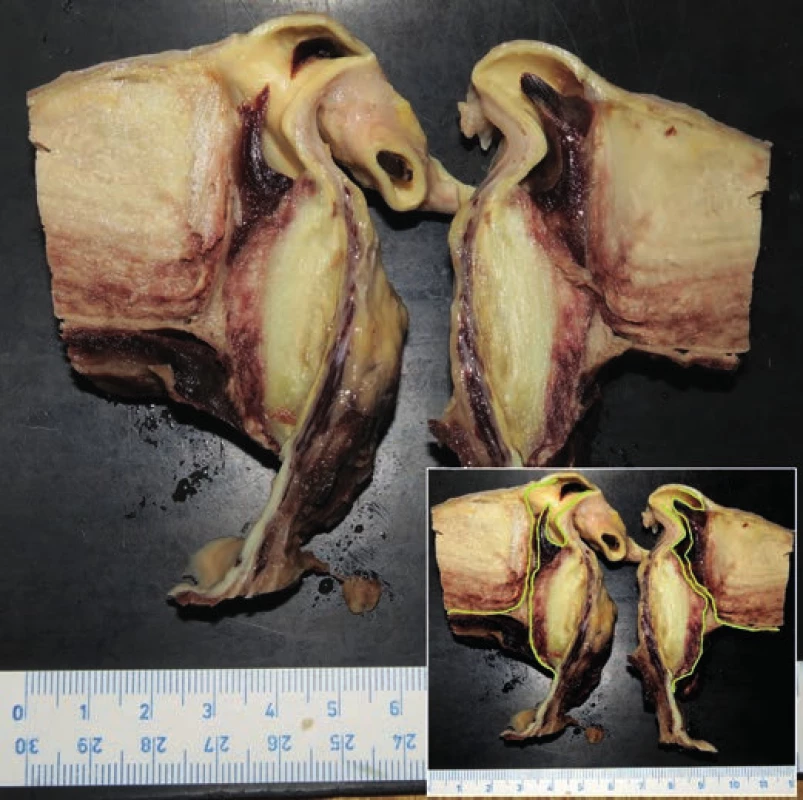
Fig. 4. Transverse cut through the lumen of the aortic arch intersecting the defect no. 3 (arrows). 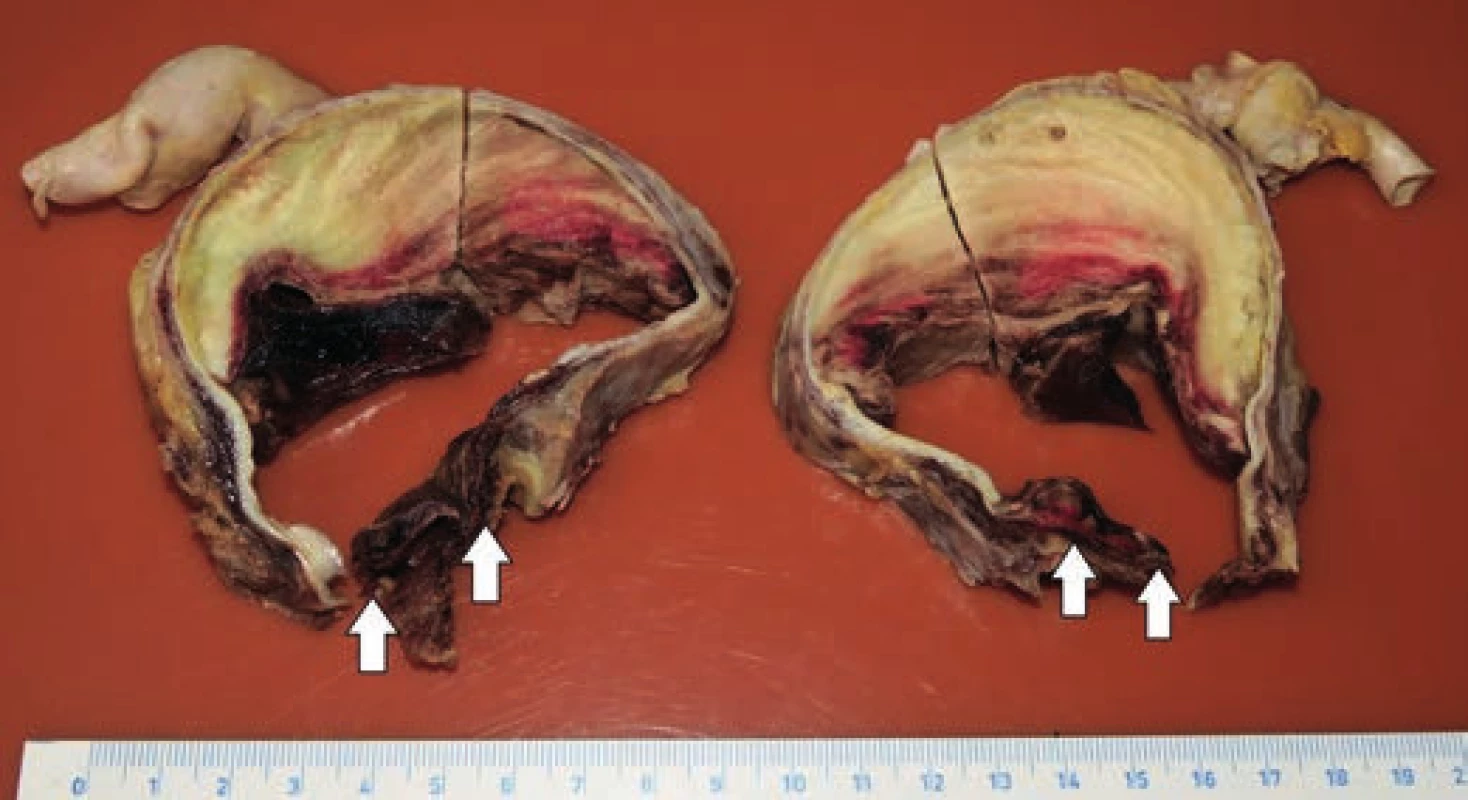
Histologic examination of the undamaged segments of aorta (ascending aorta just above the aortic valve, descending aorta), and all three defects in aortic arch showed mild intimal atherosclerotic changes. Adventitia displayed no visible inflammatory changes, and no changes were detected in the vasa vasorum likewise. The examination of the aortic media in all the excisions revealed changes characteristic for moderate medial degeneration, such as multifocal mucoid extracellular matrix accumulation, frequent band-like loss of smooth muscle cells nuclei (Fig. 5) and multifocal fragmentation of elastic fibers (Fig. 6). In the marginal parts of the triangular defect of the aortic arch, we detected a loss of intima and the inner third of the aortic media (with the presence of superficial thrombus), while the media was partially replaced by a nonspecific granulation tissue with areas of a recent bleeding. In the central part of this defect, media was completely replaced by a connective tissue attached to the adventitia, with combined thickness of 0.6 cm.
Fig. 5. Moderate medial degeneration of aortic arch (H&E x40). 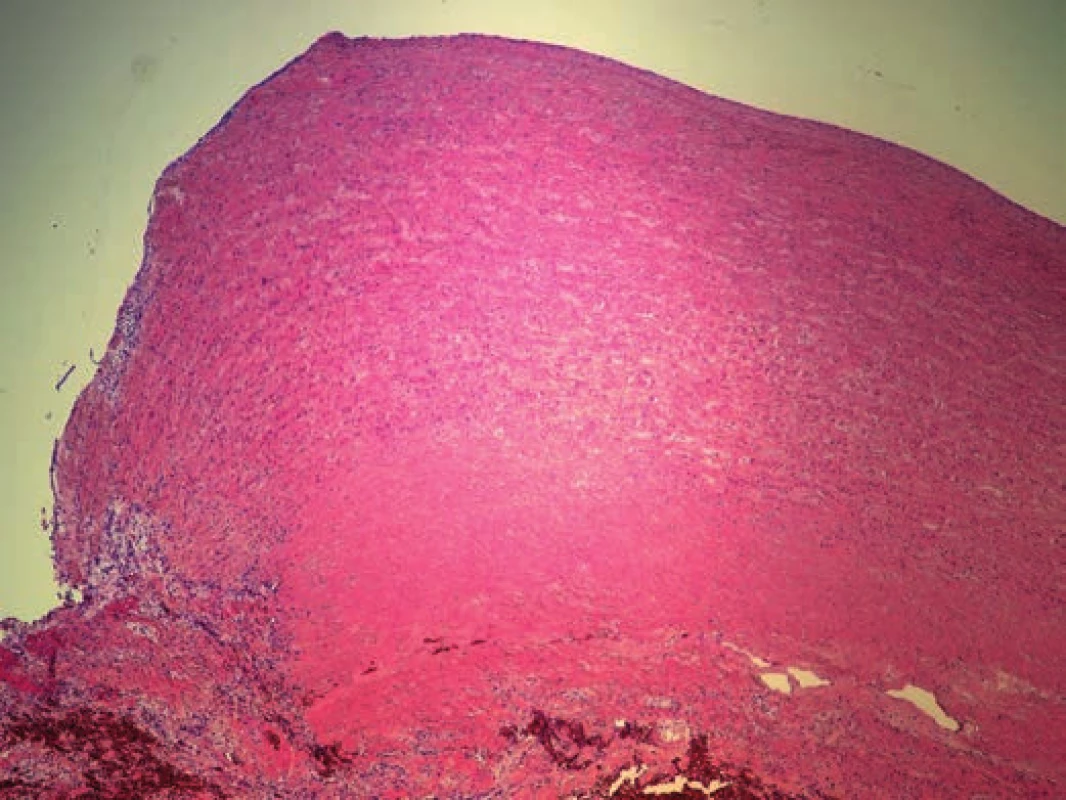
Fig. 6. Multifocal fragmentation of elastic fibers (EVG x100). 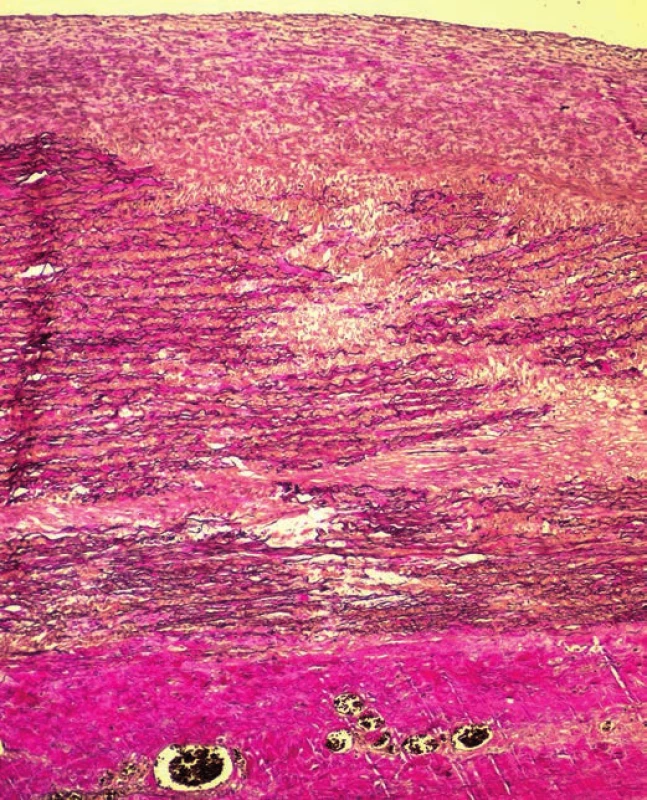
The cause of death in the presented case of a 71-year-old woman was an acute heart failure with underlying hypertension and generalized atherosclerosis affecting mostly coronary arteries.
DISCUSSION
Aorta, as the largest artery in the human body, is divided into four sections based on its anatomical course and histological structure (6). In addition to that, each section has a different embryological origin. Aortic root originates from lateral plate mesoderm, while ascending aorta and aortic arch are neural crest derived structures, and the descending aorta is derived from paraxial mesoderm (7).
In this report we discuss the case of dissecting aortic aneurysm that presented as 2.4-fold higher diameter of aortic arch relative to the diameter of the ascending aorta. Considering criteria for aneurysm defined as enlargement of vessel by at least 50 %, our case displays an aortic aneurysm with considerable dilation of the aortic arch.
Aortic aneurysms can be classified based on different criteria as (i) thoracic, abdominal, or thoracoabdominal, (ii) acute or chronic, (iii) true or false, and (iv) with or without aortic dissection (3). Abdominal aneurysms are considerably more common than the thoracic ones, and these epidemiological differences, together with genetic, structural, biochemical and clinical differences, suggest that abdominal and thoracic aneurysms represent distinct disease entities (8).
Thoracic aneurysms are often associated with genetic syndromes such as Marfan syndrome, Loeys-Dietz syndrome, and Ehlers-Danlos syndrome of vascular type (all with autosomal dominant pattern of inheritance) (8,9). Abdominal aneurysms, which were previously considered to be a consequence of advanced atherosclerosis, are now seen as a separate vascular disease associated with proteolysis, inflammation, smooth muscle apoptosis, polygenic inheritance and major etiologic role of environmental factors (10,11).
The term “dissecting aneurysm” was first introduced in 1826 by René Laennec (1781-1826) (4). Aortic dissection is now defined as a condition, where through a defect in the aortic inner layer blood penetrates from the lumen to the aortic wall and dissects media, either in the direction of the blood flow (anterograde), or in the opposite direction (retrograde) towards aortic root (1,3,4). Defects (tears) of the aortic intima are mostly transverse or oblique with a sharp or serrated edges, usually 1-5 cm in length (6). It is not clear why these intimo-medial tears occur, since they appear in apparently normal intima (1). Based on the observation of 40 patients, in whom an axial displacement of the aortic root up to 1.4 cm was detected, a possible mechanism of the dissection of ascending aorta was suggested. The displacement was increased in patients with aortic insufficiency and reduced in patients with hypokinesis of the left ventricle. The place of the highest stress induced by the aortic root displacement was located in the right lateral region of the aortic wall, approximately 2 cm above the sinotubular junction, which can explain more frequent occurrence of the dissection observed in this region, as well as transverse shape of the intimal tears. Similar effects were observed in connection with an increase of blood pressure to 180 mm Hg without any axial displacement (16).
According to the Stanford classification, the thoracic aortic dissection involving ascending aorta is considered as type A, and the dissection limited only to the descending thoracic aorta as type B (3,4). Type A is reportedly more prevalent than type B, with respective prevalences 62 % and 38 % (17).
The most common genetic syndrome associated with thoracic aortic dissection is Marfan syndrome, where mutations in the fibrillin-1 gene located on chromosome 15q15–31 lead to abnormal aortic wall architecture (12). Fibrillin-1 represents a major component of extracellular matrix providing elasticity to the specialized tissues that are repeatedly subjected to mechanical stress (13). Other predisposing conditions leading to the thoracic aortic dissection include arterial hypertension, dilation of the ascending aorta (annuloaortic ectasia), bicuspid aortic valve, and coarctation of the aorta (4). Exogenous risk factors include cocaine and amphetamine abuse with transient hypertension (3,14,15). Predisposing factor in the case presented in this report was arterial hypertension. There was no mention of the aortic insufficiency or any inflammatory or systemic connective tissue diseases in the medical record of the patient.
Degenerative changes of aortic media represent the major histopathological finding in aortic dissection (3,6). The histological depiction of typical dystrophic changes within the media of aorta with dissecting aneurysm was first described in 1928 by Otto Gsell. He described necrosis of the muscle cells in the wall of aorta, followed by elastic tissue degeneration and production of collagen. This ultimately leads to the disruption of the arterial wall, creating tears that are subsequently filled with mucoid substances. One year later, Jakob Erdheim named these changes as “medionecrosis aortae idiopathica cystica”, emphasizing predominant role of mucoid alterations within the media (3).
Presented case displayed true aneurysmal enlargement of the ascending aorta and aortic arch, as well as an aortic dissection of type A according to the Stanford classification. Aneurysmal enlargement was primarily caused by chronic dissecting aneurysm. In contrast to the majority of cases of aortic dissection, in which the aneurysmal enlargement is mostly undetected, the described case represents a truly rare finding. Thus, although some reports correctly point to the inappropriateness of the use of the term “dissecting aneurysm” (1,4,13), our case appears to be a rare finding, which by virtue of its morphological features meets the current criteria for aneurysm, and fits the definition of aortic dissection as well. As a result, it represents a true case of dissecting aortic aneurysm. The presented case is unusual also due to the fact that the aneurysmal enlargement was caused not only by the presence of chronic dissection, but also due to the widening of the lumen caused by a wide flat defect in aortic intima and media. This flat defect occured opposite to intramural hematoma and we hypothesize that it had developed by widening a simple intimo-medial tear due to increased shear stress caused by narrowing of true aortic lumen while maintaining blood flow rate (18). We also conclude, that the development of this tear was accompanied by a formation of fibrous tissue arising from the edges of the defect, where aortic media and adventitia were exposed. This mechanism corresponds to the absence of aortic media in the central part of the defect with connective tissue attached to the adventitia from the aortal luminal surface, which prevented the blood leaking through the arterial wall. Formation of fibrous tissue accompanied by a persistent high blood pressure resulted in widening of the defect and further contributed to the dilation of aorta.
As a result, this finding does not represent a “typical” false aneurysm (pseudoaneurysm) that usually occurs as a result of the arterial wall rupture. In pseudoaneurysms, blood leaking through the wall is spacially contained due to local anatomical arrangements (1), while in our case the aneurysm was not formed by the periarterial bleeding through damaged aortic wall.
In most cases of aortic dissection, no evident defect of the aortic wall is found (6). Histologic examination of the aorta in the presented case revealed a mild atherosclerosis with no significant changes in adventitia or vasa vasorum. We detected multifocal mucoid extracellular matrix accumulation, frequent band-like loss of smooth muscle cells nuclei and multifocal fragmentation of elastic fibers in the media. All these are consistent with definition of moderate medial degeneration, as previously reported (13,19). This condition can also explain development of thoracic aneurysm (6). Differential diagnosis of histological findings needs to include isolated thoracic aortitis, which presents changes in the media identical to those in medial degeneration. These pathologic entities can be distinguished by lymphoplasmacellular infiltrate observed in aortic media and adjacent adventicia in thoracic aortitis (20).
Aneurysms usually present with no clinical symptoms until the rupture of the arterial wall occurs; therefore, they are most often detected incidentally during the imaging examination performed for some other reasons. Growth rate of aneurysm is unpredictable, and can increase steadily or exponentially with the time and the age of an individual (3). Aortic rupture and dissection are often accompanied by a sharp and severe pain (3,4,13). The presence of the thoracic aneurysm can be indicated by various respiratory and gastrointestinal problems due to the compression of airways and esophagus, persistent cough, or pain (bone erosions) (6). Other complications also include thromboembolism (3). Medical record of our patient included the information on exertional dyspnea and chest tightness. Despite the presence of aortic dissection with intramural and mural thrombus, no evident signs of peripheral embolism were detected during the autopsy, nor there were any reported clinical signs that would indicate embolism during the hospitalization. Finding of partial continuation of the aortic thrombus into the lumen of brachiocephalic trunk was also unusual, and this finding considerably increased the risk of fatal complication of planned reconstruction of the aorta. In patients with genetic disorders or positive family history of aortic dissection, aneurysms of 4 to 4.5 cm in diameter are surgically repaired. For patients without any genetic disorder, 5-cm diameter represents a threshold for preventive surgical treatment, and only conservative treatment with regular observations is applied if the diameter of aneurysm is less than 5 cm (3).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
Correspondence address:
Dorota Sopková, M.D.
Department of Forensic Medicine
Faculty of Medicine, Pavol Jozef Šafárik University
Trieda SNP 1, 040 11 Košice
Slovak Republic
tel: +421948898087
fax: +421556224275
e-mail: d.sopkova@gmail.com
Zdroje
-
Povýšil C, Šteiner I, et al. Speciální patologie (2nd ed). Praha: Galén Karolinum; 2007 : 4–11.
-
Gott VL, Cameron DE, Alejo DE, Greene PS, Shake JG, Caparrelli DJ, Dietz HC. Aortic root replacement in 271 Marfan patients: a 24-year experience. Ann Thorac Surg 2002; 73(2): 438-443.
-
Lang F. Encyclopedia of Molecular Mechanisms of Disease. Berlin: Springer-Verlag Berlin Heidelberg; 2009 : 87–121.
-
Šteiner I. Kardiopatologie pro patology i kardiology. Praha: Galén; 2010 : 99–101.
-
Mukohara N. Intramural hematoma - contradiction to the theory of rupture of the vasa vasorum at onset. Ann Thorac Cardiovasc Surg 2014; 20(6): 949-950.
-
Buja LM, Butany J. Cardiovascular Pathology (4th ed). London: Academic Press; 2016 : 111–112.
-
Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol 2012; 30(2): 165-173.
-
Ruddy JM, Jones JA, Spinale FG, Ikonomidis JS. Regional heterogeneity within the aorta: relevance to aneurysm disease. J Thorac Cardiovasc Surg 2008; 136(5): 1123-1130.
-
Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 2011; 473(7347): 308-316.
-
Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol 2011; 8(2): 92-102.
-
Toghill BJ, Saratzis A, Bown MJ. Abdominal aortic aneurysm-an independent disease to atherosclerosis? Cardiovasc Pathol 2017; 27 : 71-75.
-
Dietz HC, Cutting GR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991; 352(6333): 337–339.
-
Kumar V, Abbas AK, Aster JC. Robbins Basic Pathology (10th ed). Philadelphia: Elsevier; 2017.
-
Dettmeyer RB. Forensic Histopathology. Berlin: Springer; 2011 : 176.
-
Spigset O. Drug-induced aortic aneurysms, ruptures and dissections. In: Grundmann RT, ed. Etiology, Pathogenesis and Pathophysiology of Aortic Aneurysms and Aneurysm Rupture. Rijeka: InTech; 2011 : 159–170.
-
Beller CJ, Labrosse MR, Thubrikar MJ, Robicsek F. Role of aortic root motion in the pathogenesis of aortic dissection. Circulation 2004; 109(6): 763-769.
-
Ince H, Nienaber CA. Diagnosis and management of patients with aortic dissection. Heart 2007; 93(2): 266-270.
-
Sakariassen KS, Orning L, Turitto VT. The impact of blood shear rate on arterial thrombus formation. Future Sci OA 2015; 1(4): FSO30.
-
Halushka MK, Angelini A, Bartoloni G, et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association For European Cardiovascular Pathology: II. Noninflammatory degenerative diseases — nomenclature and diagnostic criteria. Cardiovasc Pathol 2016; 25(3): 247-257.
-
Laco J, Šteiner I, Holubec T, Dominik J, Holubcova Z, Vojacek J. Isolated thoracic aortitis: clinicopathological and immunohistochemical study of 11 cases. Cardiovasc Pathol 2011; 20(6): 352-360.
Štítky
Patologie Soudní lékařství Toxikologie
Článek Cytology of Ovarian cystsČlánek Cytopatologie - díl 3.Článek Cytology of synovial fluidČlánek Hormonal cytologyČlánek Jaká je vaše diagnóza?
Článek vyšel v časopiseČesko-slovenská patologie

2019 Číslo 2-
Všechny články tohoto čísla
- Cytology of Ovarian cysts
- Synovial metaplasia of the endometrium: report of two new cases
- Moje osobné stretnutie s nositeľom Nobelovej ceny Dr. Danielom Carletonom Gajdusekom v Martine v roku 1990
- Chronic dissecting aneurysm of ascending aorta with a large intramural thrombus and isolated aortic defects
- Jaká je vaše diagnóza?
- Potential use of immunohistochemistry for diagnosis precision in historic paraffin blocks
- Odpověď: Fibrooseálny pseudotumor prstov (fibro-osseal pseudotumor of digits)
- Cytopatologie - díl 3.
- Na liberecké patologii je práce široce multidisciplinární
- Monitor aneb nemělo by vám uniknout, že...
- Cytology of synovial fluid
- Cytopathology of soft tissue tumors
- Hormonal cytology
- Česko-slovenská patologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Hormonal cytology
- Cytology of synovial fluid
- Cytology of Ovarian cysts
- Cytopathology of soft tissue tumors
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



