-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Intravascular fasciitis leading to an aortic dissection. A case report
Intravaskulární fasciitida vedoucí k disekci aorty. Kazuistika
Předkládáme kazuistiku 61leté pacientky s náhodným nálezem intravaskulární fasciitidy (IVF) postihující ascendentní aortu, zastižené v resekátu aorty získaném během výkonu pro akutní aortální disekci. Makroskopicky nebyl v aortě či v okolních měkkých tkáních patrný žádný infiltrativní proces. Mikroskopicky byla léze neostře ohraničená a sestávala z dužnatých vřetenitých či oválných buněk, uložených v hojném myxoidním stromatu. Imunohistochemicky byla pozitivní cytoplazmatická exprese β –cateninu a přibližně 25 % buněk vykazovalo pozitivní průkaz calponinu. Ki-67 index byl přibližně 25 % (přičemž v hot spot oblastech tento index dosahoval až 50 %). V ojedinělých izolovaných buňkách byl také pozitivní průkaz alfa aktinu. Ostatní vyšetřované markery zahrnující hladkosvalový aktin, desmin, h-caldesmon, D2-40, DOG1, S100 protein, CD34, CD31, ERG, melan A, HMB45, IgG, IgG4, ALK, cytokeratin AE1/AE3 a LCA byly v nádorových buňkách negativní. Intravaskulární fasciitida je benigní myofibroblastická proliferace, která se nejčastěji vyskytuje v podkožních tkáních a svým vzhledem může napodobovat malignitu. Prezentujeme teprve druhý popsaný případ IVF postihující aortu.
Klíčová slova:
intravaskulární fasciitida – nodulární fasciitida – měkkotkáňové léze – myofibroblasty – aorta
Authors: Michaela Bártů 1; Pavel Dundr 1; Kristýna Němejcová 1
; Petra Prokopová 2; Iva Zambo 3; Štěpán Černý 4
Authors place of work: Department of Pathology, First Faculty of Medicine and General University Hospital, Charles University in Prague, Czech Republic 1; Department of Pathology, Na Homolce Hospital, Czech Republic 2; 1st Department of Pathological Anatomy, St. Anne’s University Hospital Brno, Czech Republic 3; Department of Cardiac Surgery, Head of Hybrid Operating Room, Na Homolce Hospital, Czech Republic 4
Published in the journal: Čes.-slov. Patol., 63, 2018, No. 4, p. 196-199
Category: Původní práce
Summary
Here we report on the case of a 61-year-old female with an accidental finding of intravascular fasciitis (IVF) affecting the ascending aorta in a specimen resected due to an acute aortic dissection. No infiltrative process of the aorta or surrounding soft tissues was grossly apparent. Microscopically, the lesion had poorly defined margins and was composed of plump spindle - and oval-shaped cells set in an abundant myxoid stroma. Immunohistochemically, cytoplasmic positivity for β-catenin was observed and about 25% of cells were positive for calponin. The Ki-67 index was approximately 25% (increasing to about 50% in hot spot areas). Occasional isolated cells also showed positivity for alpha actin. Other markers were all negative in the tumor cells including; smooth muscle actin, desmin, h-caldesmon, D2-40, DOG1, S100 protein, CD34, CD31, ERG, melan A, HMB45, IgG, IgG4, ALK, cytokeratin AE1/AE3, and LCA. Intravascular fasciitis is a benign myofibroblastic proliferation which most commonly occurs in subcutaneous tissues and may mimic malignancy. To the best of our knowledge this is only the 2nd ever reported case of IVF affecting the aorta.
Keywords:
intravascular fasciitis – nodular fasciitis – soft tissue lesions – myofibroblasts – aorta
Intravascular fasciitis is a distinctive variant of nodular fasciitis involving muscular arteries or veins and was first described in literature by Patchefsky and Enzinger in 1981 (1). While nodular fasciitis is a relatively common benign reactive soft tissue proliferation, its intravascular subset is still considered particularly rare. Since its first description in 1981 only about 35 cases have been reported to date (2).
The typical intravascular involvement can mimic malignant spread and can lead to misdiagnosis of a sarcoma infiltrating the vascular network (3). This diagnostic confusion is further promoted by some of the common histological features of intravascular fasciitis which include a widely varied morphologic pattern, high cellularity and mitotic activity (4).
A case of intravascular fasciitis involving the aorta and leading to an aortic dissection requiring emergency surgery is presented in this report and its clinical, histopathologic and immunohistochemical features are discussed. To the best of our knowledge only one case of IVF affecting the aorta has, as yet, ever been described (5).
CLINICAL HISTORY
A 61-year-old female with no significant previous medical history presented with a sudden-onset chest pain and was admitted for a suspected acute coronary syndrome (ACS). The examination ruled out ACS and a thoracic CT (computed tomography) angiogram revealed a Stanford type A aortic dissection and a dilatation of the ascending aorta. During the operation the ascending aorta and aortic arch, along with the initial segments of the brachiocephalic artery, left common carotid artery and left subclavian artery, were resected and replaced with prosthetic grafts. Perioperative examination of the descending aorta showed an intramural hematoma and a partially dissected large atherosclerotic plaque, so a stent was placed into the lumen in order to provide further support. No complications were encountered during the procedure and the resected ascending aorta was sent for a histologic evaluation. Postoperative recovery was uneventful, the patient’s condition continued to improve, and a CT angiogram confirmed that the replacement was properly positioned and showing no signs of leakage. One month later the patient remained clinically well and was recovering successfully, which is the last follow up information available.
MATERIALS AND METHODS
The obtained specimen was processed into seven formalin-fixed paraffin-embedded tissue blocks which were then stained with hematoxylin-eosin. Selected sections were also stained with alcian blue, and other sections were analyzed immunohistochemically using the avidin-biotin complex method with antibodies against the following antigens: cytokeratin AE1/AE3 (1 : 50, Dako, Glostrup, Denmark), Ki-67 (clone MIB-1, 1 : 50, Dako), S-100 protein (1 : 1600, Dako), desmin (clone D33, 1 : 200, Dako), muscle specific actin (clone HHF35, 1 : 400, Dako), CD45/LCA (clone 2B11+PD7/26, 1 : 100, Dako), α-actin (clone 1A4, 1 : 400, Dako), β-catenin (clone β-catenin-1, 1 : 400, Dako), calponin (clone CALP, 1 : 200, Dako), h-caldesmon (clone h-CD, 1 : 50, Dako), D2-40 (1 : 100, Dako), DOG1 (clone SP31, 1 : 800, Cell Marque, Sierra College Blvd., Rocklin, CA), CD34 (clone QBEnd 10, 1 : 50, Dako), CD31 (clone JC70A, 1 : 25, Dako), ERG (clone EP111, 1 : 200, Dako), melan A (clone A103, 1 : 25, Novocastra Laboratories Ltd., Newcastle Upon Tyne, United Kingdom), HMB45 (1 : 50, Dako), IgG (1 : 1600, Dako), IgG4 (clone MRQ-44, 1 : 50, Zytomed Systems, Berlin, Germany) and ALK (clone 5A4, 1 : 10, Zytomed Systems).
RESULTS
The pathologic specimen was composed of the ascending aorta which was 85 mm long and 57 mm wide, with intramural yellowish plaques and a 30 mm long rupture. The adjacent soft tissues formed a poorly circumscribed mass of 65x53x15 mm dimensions. There were no gross signs of clear pathologic tissue infiltration or mass of any kind.
Microscopic examination revealed the wall of the aorta from the area of dissection with a partially organized mural thrombus, focal hemorrhage into the surrounding soft tissues and an unexpected myofibroblastic proliferation affecting all the layers of the vessel from the subendothelium outward and spreading out onto the adjacent extravascular soft tissue (Fig. 1, 2).
Fig. 1. Myofibroplastic proliferation within the aortic wall (arrow) affecting all the histological layers of the aorta (H&E, original magnification 40x). 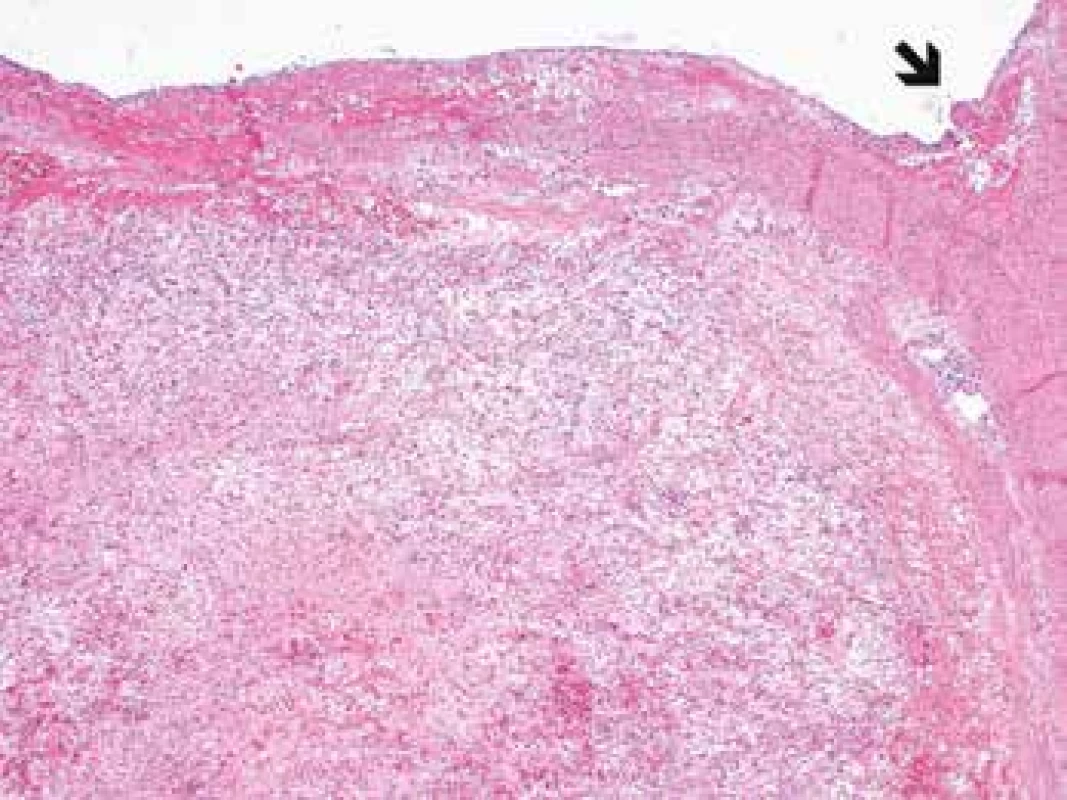
Fig. 2. Myofibroplastic proliferation within the aortic wall affecting all the histological layers of the aorta (H&E, original magnification 100x). 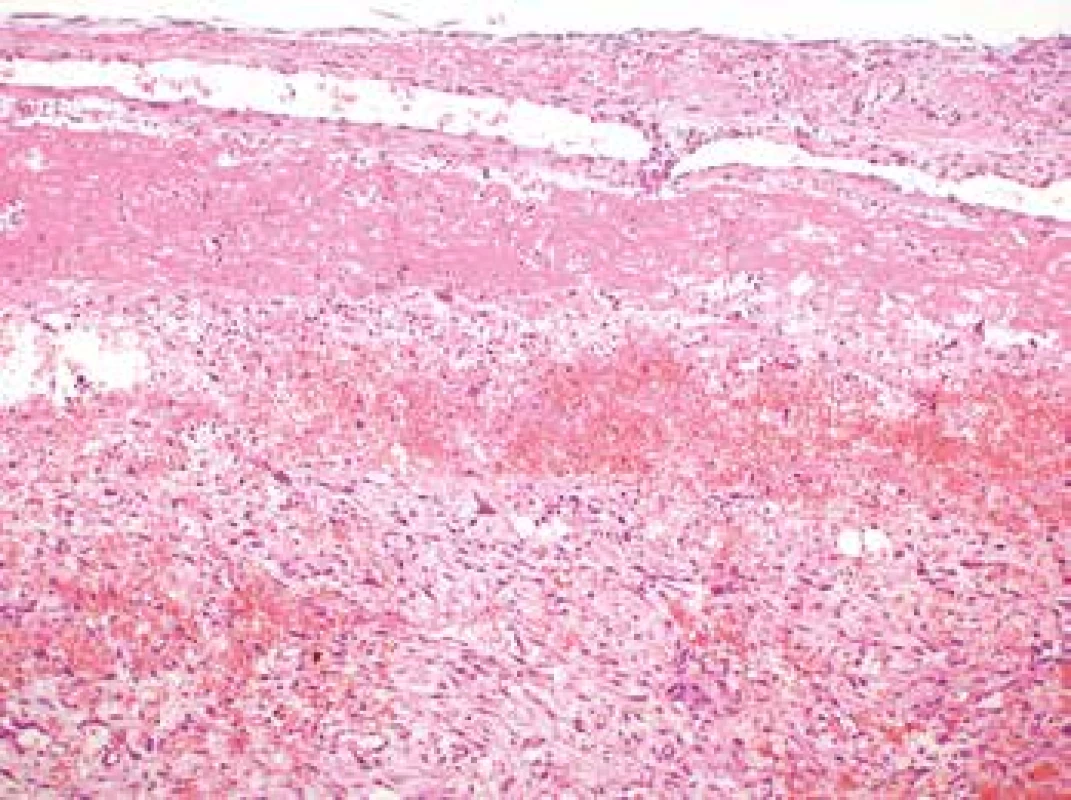
The lesion had poorly defined margins and showed infiltrative growth into the adjacent fat tissue where it grew around the nerves present. It was composed of plump spindle or stellate cells arranged in a subtle fascicular pattern (Fig. 3). The nuclei were partially spindle - and partially oval-shaped with certain irregularities, finely granular chromatin, occasional prominent nucleoli and frequent, but typical mitotic figures (11 mitoses per 10 high power fields). The lesional cells were set in an abundant myxoid (which when stained with alcian blue showed focal increase in acidic mucopolysacharides) or oedematous stroma. Scattered lymphocyte infiltration with an admixture of isolated plasma and mastocyte cells was present throughout the lesion, accompanied by focal extravasations of erythrocytes. Occasional large multinucleated cells were present. The surrounding fat tissue contained occasional lymphoepitelial structures of thymic residue character.
Fig. 3. The lesion composed of plump spindle or stellate cells arranged in a subtle fascicular pattern (H&E, original magnification 400x). 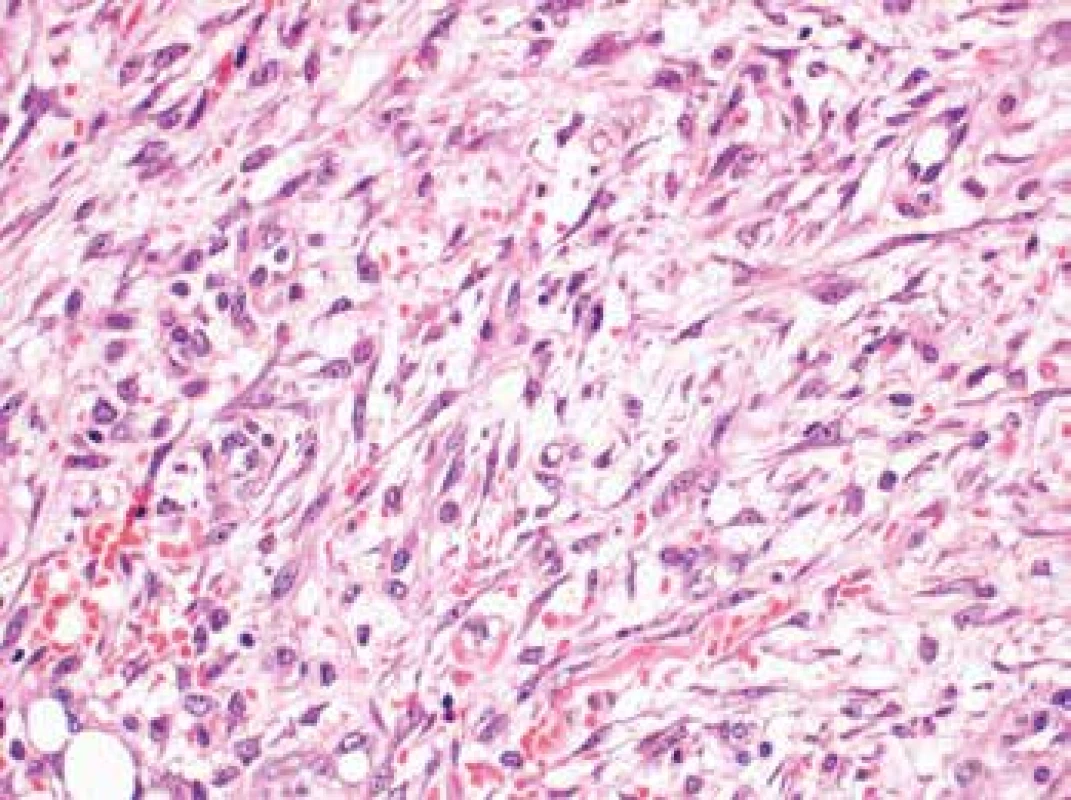
Immunohistochemically, the lesional cells expressed β-catenin (cytoplasmic positivity), calponin (c. 25 % of cells) with occasional isolated positivity for alpha actin (Fig. 4). The proliferation fraction (Ki-67 index) was c. 25 % (increasing to 50 % in hot spot areas). Infrequent lymphocytes present in the interstitial tissue tested positive for LCA. Cytokeratin AE1/AE3 was not expressed in the lesional cells but showed positivity in the residual thymic cells. Other markers including muscle specific actin, desmin, h-caldesmon, D2-40, DOG1, S100 protein, CD34, CD31, ERG, melan A, HMB45, IgG, IgG4, and ALK were all negative.
Fig. 4. Lesional cells expressing occasional isolated positivity for alpha actin (original magnification 200x). 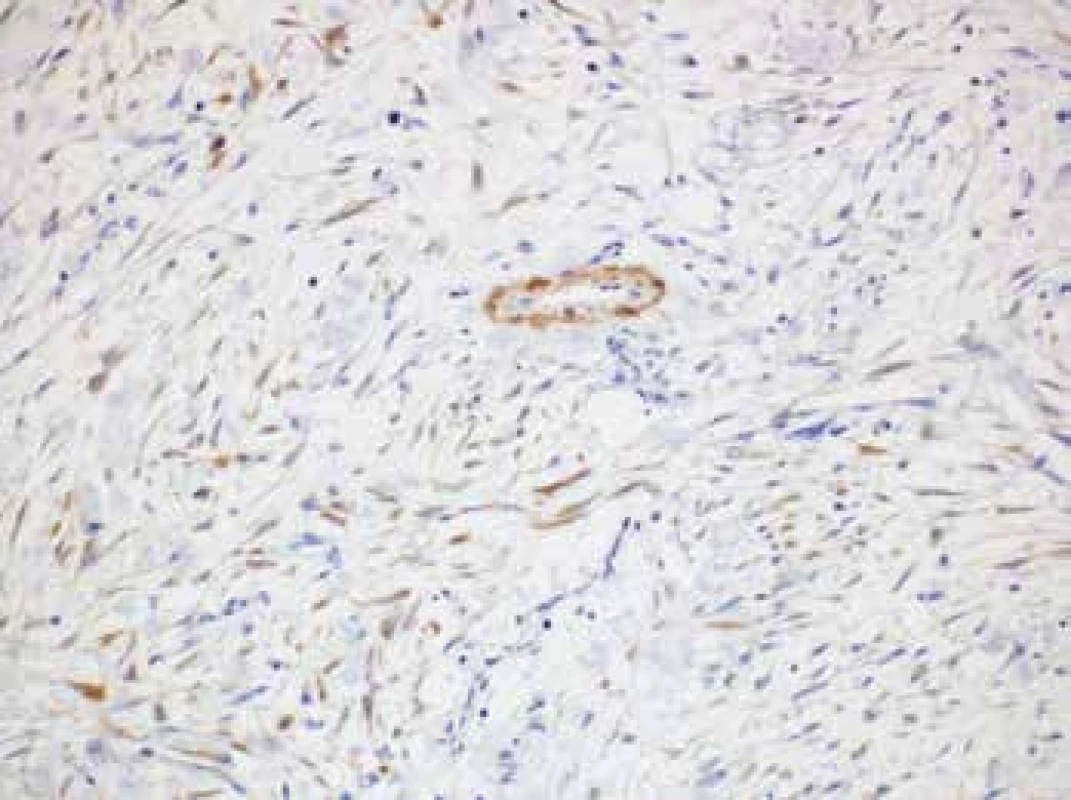
A diagnosis of intravascular nodular fasciitis with intramural involvement of the aorta was made based on the clinical and pathological findings.
DISCUSSION
Intravascular fasciitis is a rare variant of nodular fasciitis (NF) with a unique intravascular growth pattern (6), characterized by a reactive proliferation of myofibroblasts in the superficial or deep fascia with involvement of vessels (7). The affected vessels can display intraluminal, intramural or extramural pattern of involvement, with possible spread into the surrounding soft tissues (which was also observed in our case). Other specific subtypes of NF which have thus far been described include cranial fasciitis, ossifying fasciitis and dermal fasciitis (6).
The most common sites of involvement include the upper extremity (8), head and neck (4), but also oral soft tissues (9-11). This distribution is in accordance with the original report published by Patchefsky, where the most commonly involved site was the upper extremity (41 %), the head and neck (29 %), lower extremity (18 %) and finally the trunk (12 %). Most of the reported cases of IVF describing the involved vessels are limited to small to medium-sized veins and arteries. Until now, the largest reported case dealt with an IVF affecting the ascending aorta of a 26-year-old male weight lifter and also presented as aortic dissection (5). A review of literature shows two distinct forms of clinical presentation; either as a painless, asymptomatic slowly growing mass or as a rapidly growing and progressing lesion accompanied by slight aching pain (1). While there seems to be no predilection for gender, the majority of the reported patients were under 30 years of age (4,12). The mean age of patients included in the original Patchefsky’s series was 20.5.
The gross appearance of IVF is described variably as either a non-encapsulated nodular mass with a firm-to-soft or gelatinous consistency or as a poorly circumscribed infiltrative mass (4). If intravascular growth is predominant, the lesion takes on a characteristic serpentine or plexiform configuration. The size of most reported lesions ranges between 2.0 cm and 5.0 cm (4,12).
The etiology of both nodular and intravascular fasciitis remains unclear, although several theories have been put forward. In their original report Patchefsky and Enzinger suggested a “field effect” implying that the stimulation and proliferation of myofibroblasts occurs as a reactive response to local injury (1). Other possible causes include pre-existing trauma and thrombosis formation. Viral etiology was also mentioned as a potential causative factor due to the presence of a lymphocytic infiltration, but according to Samaratunga et al. (13) no viral cytopathic effects have been recorded.
Histologically, IVF shares its main characteristics with nodular fasciitis, the key difference between these two conditions being the intimate association with blood vessels. The vascular involvement is typically longitudinal, leading to a characteristic serpentine or multinodular growth pattern. Any one of the structural layers of the vascular wall can be involved (intima, media and adventitia), including the perivascular soft tissues. An extensive review of literature shows that all reported cases share the same characteristic features: that the lesions are composed of mostly bland plump spindle fibroblasts or myofibroblasts set in a loose stroma with frequent extravasations of erythrocytes and scattered scarce lymphocyte infiltration (12). The architectural patterns identified in reported cases are highly variable and most commonly include storiform patterns, interlacing fascicles or non-specific, haphazard arrangement of lesional cells (6). The nuclei are large, vesicular and typically bland. Mitoses can be frequent, but not in their atypical forms. About a third of cases of IVF are said to contain multinucleated giant cells, resembling a benign fibrous histiocytoma or a giant cell soft tissue tumor, which were however not present in our case (2,12). Perivascular soft tissue involvement is also frequently seen, and in a number of cases this is much more prominent than the vascular component itself (13).
The most problematic aspect of establishing a correct diagnosis remains the distinction between a malignant tumor, such as fibrosarcoma, angiosarcoma, vascular leiomyosarcoma, myxofibrosarcoma, low-grade fibromyxoid sarcoma, and IVF. Nodular fasciitis is probably the most common benign mesenchymal lesion misdiagnosed as a sarcoma. Diagnostic difficulties are usually due to the condition’s rarity combined with rich cellularity, mitotic activity and variant morphologic patterns (6,13). The growth pattern together with vascular involvement may suggest the possibility of malignant behavior which is well evidenced by the fact that in Patchefsky’s original report almost a third of described cases was originally diagnosed as a malignant lesion, namely sarcoma (1). The distinction from sarcoma is usually based upon the growth pattern (for example herring-bone pattern in fibrosarcoma), cellularity, and the presence of nuclear atypia.
Moreover, the differential diagnosis of IVF includes intravascular fibroangiomatous proliferations such as intravascular papillary endothelial hyperplasia (Masson’s tumor), intravascular pyogenic granuloma, angioleiomyoma, or intravascular myopericytoma. The distinction was not problematic in our case as these conditions could be ruled out based upon the absence of their typical histopathological features (12). The myofibroblastic origin of the lesional cells can be further confirmed by their positive smooth muscle actin and negative CD31 and CD34 staining (which were positive only in the vascular component).
According to the literature, IVF was previously reported almost exclusively to be located in small to medium-sized muscular arteries or veins (4). To the best of our knowledge this is only the 2nd case of IVF affecting the aorta and the first case in which the aorta was infiltrated to such extent – the only other reported instance described a process limited to the adventitia (3).
In conclusion it is important to be aware of the existence of this rare variant of nodular fasciitis and to not let its specific growth pattern be misinterpreted as a vascular invasion by a sarcoma which would expose the patient to all the negatives of an unnecessarily overaggressive treatment.
ACKNOWLEDGEMENTS
This work was supported by Ministry of Health, Czech Republic (Conceptual development of research organization 64165, General University Hospital in Prague), by Charles University (Project Progress Q28/LF1 and SVV 260367), and by OPPK (Research Laboratory of Tumor Diseases, CZ.2.16/3.1.00/24509).
The authors extend their thanks to Zachary H.K. Kendall, B.A. for English language corrections.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
Correspondence address:
Michaela Bártů, MD
Department of Pathology,
First Faculty of Medicine Charles University
and General University Hospital in Prague
Studničkova 2, Prague 2, 12800, Czech Republic
tel.: +420224968711
email: michaela.bartu@vfn.cz
Zdroje
1. Patchefsky AS, Enzinger FM. Intravascular fasciitis: a report of 17 cases. Am J Surg Pathol 1981; 5(1): 29-36.
2. Lee HG, Pyo JY, Park YW, Ro JY. Intravascular fasciitis of the common femoral vein. Vasa 2015; 44(5): 395-398.
3. Sticha RS, Deacon JS, Wertheimer SJ, Danforth RD, Jr. Intravascular fasciitis in the foot. J Foot Ankle Surg 1997; 36(2): 95-99.
4. Samaratunga H, Searle J, O’Loughlin B. Nodular fasciitis and related pseudosarcomatous lesions of soft tissues. Aust N Z J Surg 1996; 66(1): 22-25.
5. Gwan-Nulla DN, Davidson WR, Jr., Grenko RT, Damiano RJ, Jr. Aortic dissection in a weight lifter with nodular fasciitis of the aorta. Ann Thorac Surg 2000; 69(6): 1931-1932.
6. Reiser V, Alterman M, Shlomi B, Issakov J, Dagan Y, Kleinman S, et al. Oral intravascular fasciitis: a rare maxillofacial lesion. Oral Surg Oral Med Oral Pathol Oral Radiol 2012; 114(2): e40-44.
7. Zheng Y, George M, Chen F. Intravascular fasciitis involving the flank of a 21-year-old female: a case report and review of the literature. BMC Res Notes 2014; 7 : 118.
8. Ito M, Matsunaga K, Sano K, Sakaguchi N, Hotchi M. Intravascular fasciitis of the forearm vein: a case report with immunohistochemical characterization. Pathol Int 1999; 49(2): 175-179.
9. Chi AC, Dunlap WS, Richardson MS, Neville BW. Intravascular fasciitis: report of an intraoral case and review of the literature. Head Neck Pathol 2012; 6(1): 140-145.
10. Kahn MA, Weathers DR, Johnson DM. Intravascular fasciitis: a case report of an intraoral location. J Oral Pathol 1987; 16(6): 303-306.
11. Kuklani R, Robbins JL, Chalk EC, Pringle G. Intravascular fasciitis: report of two intraoral cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol 2016; 121(1): e19-25.
12. Hsiao Y-H, Chiu C-S. Intravascular fasciitis of the scalp: A case report. Dermatologica Sinica 2013; 31(1): 35-37.
13. Samaratunga H, Searle J, O’Loughlin B. Intravascular fasciitis: a case report and review of the literature. Pathology 1996; 28(1): 8-11.
Štítky
Patologie Soudní lékařství Toxikologie
Článek vyšel v časopiseČesko-slovenská patologie

2018 Číslo 4-
Všechny články tohoto čísla
-
Cytopathologist is the best…
… to do more with even less - Cytopatologie - ze škaredého kačátka se stává labuť
- Monitor aneb nemělo by vám uniknout, že...
- Moderní cytopatologie
- Negynekologická cytologie – návod na přežití
- Carcinoma of the uterine cervix in Czech Republic and possibilities of its prevence
- Molekulárně genetické metody ve skríningu karcinomu děložního hrdla
- MUDr. Jiří Kudrmann
- Cytology of effusions in the coelom cavities
- Hyalinizing trabecular tumor of the thyroid gland with transcapsular invasion: a case report
- Jaká je vaše diagnóza?
- Intravascular fasciitis leading to an aortic dissection. A case report
- Monitor aneb nemělo by vám uniknout, že...
- Setkání profesora Antonína Fingerlanda s budoucím nositelem Nobelovy ceny Dr. Danielem Carletonem Gajduskem v Antverpách v roce 1959
- Jaká je vaše diagnóza? Odpověď: Dysplastický cerebelární gangliocytom (onemocnění Lhermitte-Duclos)
- Monitor aneb nemělo by vám uniknout, že...
-
Cytopathologist is the best…
- Česko-slovenská patologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Cytology of effusions in the coelom cavities
- Carcinoma of the uterine cervix in Czech Republic and possibilities of its prevence
- Hyalinizing trabecular tumor of the thyroid gland with transcapsular invasion: a case report
- Molekulárně genetické metody ve skríningu karcinomu děložního hrdla
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



