-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Breaking the Stereotype: Virulence Factor–Mediated Protection of Host Cells in Bacterial Pathogenesis
article has not abstract
Published in the journal: . PLoS Pathog 6(9): e32767. doi:10.1371/journal.ppat.1001057
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1001057Summary
article has not abstract
Bacterial pathogens have evolved extraordinary mechanisms to efficiently infect host organisms. A majority of these pathogens do so by delivering virulence factors into host cells, which act to dampen host defenses or utilize the host as a niche for replication. Although regulation of virulence factor expression by bacterial pathogens is a well known pathogenic mechanism [1], the concept of host-protective virulence factors is emerging. Recently, several strategies by which pathogens appear to be attenuating their own lethality towards host cells have been documented, suggesting that increased hostility and damage of host cells is not necessarily beneficial to the pathogen. Virulence is often defined as the ability of a pathogen to inflict damage on host cells, and the following discussion addresses the concept that increased virulence is not always beneficial to the pathogen, and moderating it to preserve host cells is a mechanism several pathogens use as part of their overall pathogenic strategy. This strategy is well known for obligate intracellular pathogens, but has become an emerging theme in extracellular and facultative intracellular bacteria.
Yersinia spp., Shigella flexneri, Helicobacter pylori, and diarrheagenic Escherichia coli are well known for their ability to kill host cells. For Yersinia, death of infected macrophages dampens cytokine release and enables the pathogen to propagate with minimal challenges from the immune system [2]. Two recent studies suggest that cytotoxicity caused by Yersinia species is tightly regulated. Yersinia pestis, the etiologic agent of plague, and gastroenteritis-inducing Yersinia pseudotuberculosis and Yersinia enterocolitica all encode a cytotoxic virulence factor called YopJ/P (YopJ in the two former species and YopP in the latter), which are translocated into infected cells via a type III secretion system (T3SS) [2], [3]. Altering the cytotoxicity of Y. pseudotuberculosis affects its virulence. Decreased secretion of YopJ was shown to enhance Y. pseudotuberculosis pathogenesis in vivo [4]. Similarly for Y. pestis, enhanced cytotoxicity results in decreased incidence of pneumonic plague in vivo [5]. Tight regulation of cytotoxicity by pathogenic Yersinia is an efficient virulence strategy. Increased apoptosis of infected immune cells decreases production of proinflammatory cytokines; however, some inflammation at the early stages of infection is thought to facilitate tissue damage necessary for movement of bacteria and infected cells to other sites of replication within the host [4].
Enteropathogenic Escherichia coli, enterohaemorrhagic E. coli (EPEC and EHEC, respectively), and Citrobacter rodentium are attaching and effacing (A/E) pathogens that cause severe diarrheagenic disease [6]. The ability of A/E pathogens to kill intestinal epithelial cells has been well documented [7]–[14]. The type III secreted (T3S) effector EspF has a role in host cell death by causing mitochondrial-dependent apoptosis [11], [15]. We recently found that the T3S effector EspZ modulates cytotoxicity towards host cells. An EPEC espZ mutant (ΔespZ) caused enhanced cytotoxicity in host cells when compared to the wild-type strain [16], which was surprising since the ΔespZ strain is severely attenuated for virulence in vivo [17]. EspZ acts in part through the host transmembrane glycoprotein CD98 to activate focal adhesion kinase (FAK)-based survival pathways (Figure 1) [16]. Others found that the T3S effector NleH also dampens apoptosis of EPEC-infected cells, but via interaction with a Bcl-2-related protein involved in the mitochondrial death pathway (Figure 1) [18]. Unlike EspZ, NleH is not essential for EPEC colonization and only moderately impacts on A/E pathogen disease in vivo [19], [20]; however, there are likely other host-protective virulence factors that act redundantly to NleH during EPEC infection.
Fig. 1. Strategies evolved by bacterial pathogens to restrain virulence. 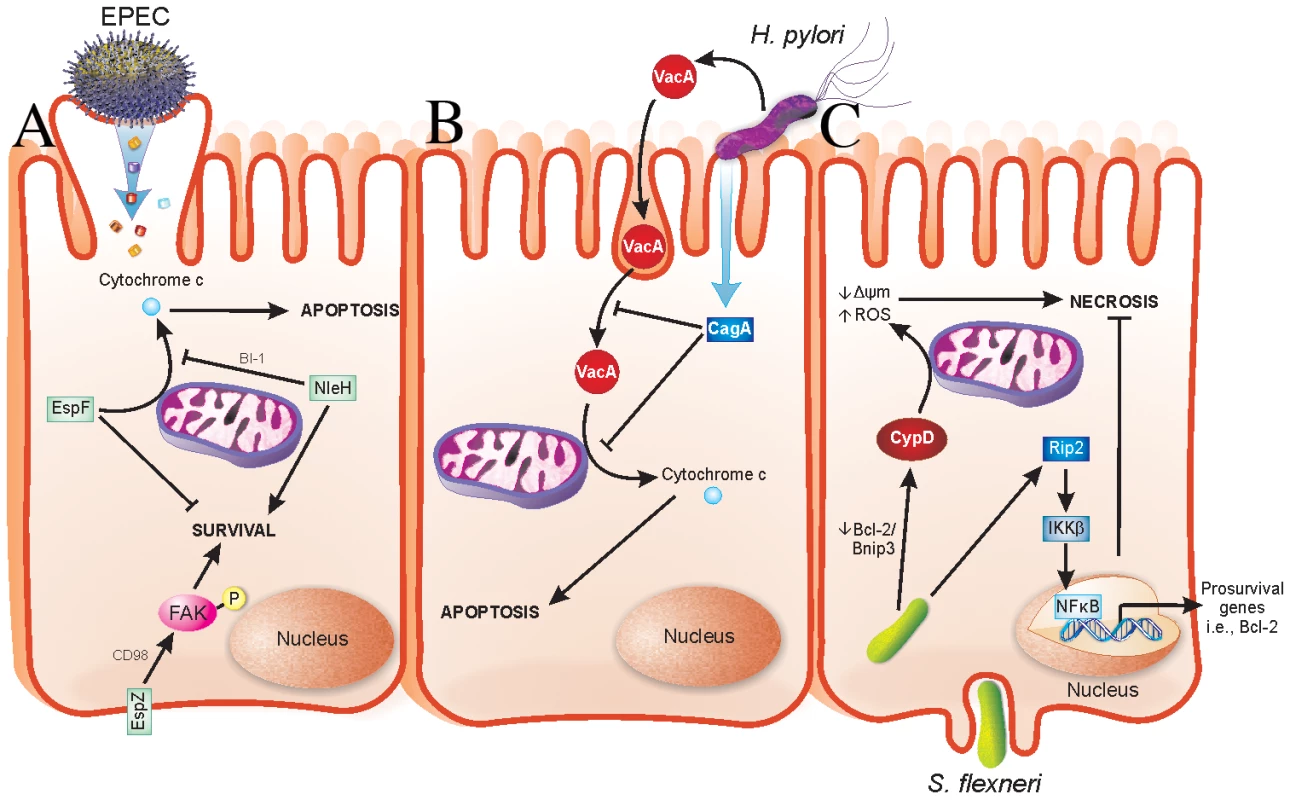
(A) EPEC injects effector proteins into intestinal epithelial cells (IECs) via a T3SS. EspF localizes to mitochondria and causes release of cytochrome c into the host cell cytosol, which results in apoptotic death of the host cells. NleH interacts with Bax inhibitor-1 (BI-1), which inhibits release of cytochrome c from mitochondria. EspZ interacts with CD98, which then stimulates phosphorylation of focal adhesion kinase (FAK) to promote survival. Localization of NleH and EspZ in host cells during early stages of EPEC infection is unclear and has been portrayed as shown for simplicity. (B) H. pylori injects virulence factors into gastric epithelial cells via a type IV secretion system in addition to secreting soluble toxins. VacA is an H. pylori–secreted toxin that enters cells by pinocytosis and penetrates intracellular endosome trafficking pathways. VacA causes release of cytochrome c from mitochondria of infected cells, thus mediating host cell apoptosis. CagA is a T4S virulence factor, which prevents both pinocytosis/trafficking and cytochrome c release by VacA. Functions of CagA are dependent on its phosphorylation state, not depicted here. (C) S. flexneri enters IECs from their basolateral surface and then resides in the cell cytoplasm. Prosurvival signaling is initiated by Nod1 activation of Rip2 signaling, which terminates in expression of pro-survival genes, including Bcl-2, via NFκB activation and nuclear translocation. Conversely, S. flexneri facilitates a decrease in the Bcl-2/Bnip3 ratio, which leads to CypD-mediated disruption of mitochondria and oxidative stress-induced necrotic cell death. H. pylori causes apoptosis of infected gastric epithelial cells [21]. Apoptosis induction by H. pylori has been linked to a secreted toxin called VacA, which induces cytochrome c release from mitochondria (Figure 1) [22]. Recently, it was determined that VacA-mediated apoptosis is counteracted by a type IV secreted (T4S) protein called CagA by both blocking pinocytosis of VacA and inhibiting VacA-mediated cytochrome c release from mitochondria [23] (Figure 1). Interestingly, loss of CagA in a VacA+ H. pylori strain decreases bacterial colonization and the incidence of gastric hyperplasia, adenocarcinoma, and inflammation [24]. Similar to the aforementioned pathogens, H. pylori has evolved a delicate interplay between host-protective and -detrimental virulence factors that are able to fine-tune virulence while promoting their propagation.
S. flexneri, the etiologic agent of bacilliary dysentery, causes death of infected macrophages and epithelial cells [25]. Despite this, several host-protective strategies are employed by S. flexneri. The T3S effector OspE was recently found to enhance adhesion of infected host cells to the underlying extracellular matrix [26]. Whether OspE activates host cell survival pathways directly is unknown; however, its interaction with integrin-linked kinase inhibits sloughing of infected cells into the intestinal lumen [26], consequently preventing anoikis of Shigella-infected cells. An ospE mutant does not colonize as efficiently as wild-type S. flexneri in vivo; thus, OspE may enhance colonization by preventing premature release of infected cells [26]. Epithelial cells succumb to S. flexneri infection via necrotic cell death, which functions to release intracellular bacteria and enhance inflammation [25]. Interestingly, survival pathways involving Rip2/IKKβ/NFκB are activated early during infection, followed by mitochondrial dysfunction and necrotic cell death (Figure 1) [25]. The early expression of pro-survival genes may enable S. flexneri to postpone cell death in a similar manner to EPEC, thus ensuring greater bacterial load prior to dissemination. The mechanism(s) by which S. flexneri enhances NFκB-mediated pro-survival signals are unknown.
All of the above pathogens have evolved strategies to attenuate their own host-damaging virulence factors. In many of these scenarios, removal of host-protective mediators actually reduces pathogenicity of the bacteria. The observation that EPEC encodes a host-protective virulence factor that is essential for its pathogenesis suggests that protecting host cells may be a key to the pathogenic strategies of other bacterial pathogens. The concept of host-protective virulence factors is only just emerging, and we believe host-protective virulence factors will become more apparent in other pathogenic strategies and may become interesting targets to combat bacterial disease. Importantly, virulence phenotypes that appear counterintuitive should not be ignored. Future studies into pathogenic mechanisms of virulent bacteria will likely reveal important roles for effectors or regulatory mechanisms that help the host cell and promote bacterial pathogenesis.
Zdroje
1. MekalanosJJ
1992
Environmental signals controlling expression of virulence determinants in bacteria.
J Bacteriol
174
1
7
2. AepfelbacherM
TrasakC
RuckdeschelK
2007
Effector functions of pathogenic Yersinia species.
Thromb Haemost
98
521
529
3. MillsSD
BolandA
SoryMP
van der SmissenP
KerbourchC
1997
Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein.
Proc Natl Acad Sci U S A
94
12638
12643
4. BrodskyIE
MedzhitovR
2008
Reduced secretion of YopJ by Yersinia limits in vivo cell death but enhances bacterial virulence.
PLoS Pathog
4
e1000067
doi:10.1371/journal.ppat.1000067
5. ZaubermanA
TidharA
LevyY
Bar-HaimE
HalperinG
2009
Yersinia pestis endowed with increased cytotoxicity is avirulent in a bubonic plague model and induces rapid protection against pneumonic plague.
PLoS ONE
4
e5938
doi:10.1371/journal.pone.0005938
6. CroxenMA
FinlayBB
2010
Molecular mechanisms of Escherichia coli pathogenicity.
Nat Rev Microbiol
8
26
38
7. VallanceBA
DengW
JacobsonK
FinlayBB
2003
Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium.
Infect Immun
71
3443
3453
8. VallanceBA
DengW
KnodlerLA
FinlayBB
2002
Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection.
Infect Immun
70
2070
2081
9. Abul-MilhM
WuY
LauB
LingwoodCA
Barnett FosterD
2001
Induction of epithelial cell death including apoptosis by enteropathogenic Escherichia coli expressing bundle-forming pili.
Infect Immun
69
7356
7364
10. CraneJK
VezinaCM
2005
Externalization of host cell protein kinase C during enteropathogenic Escherichia coli infection.
Cell Death Differ
12
115
127
11. CraneJK
McNamaraBP
DonnenbergMS
2001
Role of EspF in host cell death induced by enteropathogenic Escherichia coli.
Cell Microbiol
3
197
211
12. FlynnAN
BuretAG
2008
Caspases-3, -8, and -9 are required for induction of epithelial cell apoptosis by enteropathogenic E. coli but are dispensable for increased paracellular permeability.
Microb Pathog
44
311
319
13. Barnett FosterD
Abul-MilhM
HuescaM
LingwoodCA
2000
Enterohemorrhagic Escherichia coli induces apoptosis which augments bacterial binding and phosphatidylethanolamine exposure on the plasma membrane outer leaflet.
Infect Immun
68
3108
3115
14. CraneJK
MajumdarS
PickhardtDF3rd
1999
Host cell death due to enteropathogenic Escherichia coli has features of apoptosis.
Infect Immun
67
2575
2584
15. NougayredeJP
DonnenbergMS
2004
Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway.
Cell Microbiol
6
1097
1111
16. ShamesSR
DengW
GuttmanJA
de HoogCL
LiY
2010
The pathogenic E. coli type III effector EspZ interacts with host CD98 and facilitates host cell prosurvival signaling.
Cell Microbiol
12
1322
1339
17. DengW
PuenteJL
GruenheidS
LiY
VallanceBA
2004
Dissecting virulence: systematic and functional analyses of a pathogenicity island.
Proc Natl Acad Sci U S A
101
3597
3602
18. HemrajaniC
BergerCN
RobinsonKS
MarchesO
MousnierA
2010
NleH effectors interact with Bax inhibitor-1 to block apoptosis during enteropathogenic Escherichia coli infection.
Proc Natl Acad Sci U S A
107
3129
3134
19. Garcia-AnguloVA
DengW
ThomasNA
FinlayBB
PuenteJL
2008
Regulation of expression and secretion of NleH, a new non-locus of enterocyte effacement-encoded effector in Citrobacter rodentium.
J Bacteriol
190
2388
2399
20. HemrajaniC
MarchesO
WilesS
GirardF
DennisA
2008
Role of NleH, a type III secreted effector from attaching and effacing pathogens, in colonization of the bovine, ovine, and murine gut.
Infect Immun
76
4804
4813
21. MossSF
CalamJ
AgarwalB
WangS
HoltPR
1996
Induction of gastric epithelial apoptosis by Helicobacter pylori.
Gut
38
498
501
22. GalmicheA
RassowJ
DoyeA
CagnolS
ChambardJC
2000
The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release.
EMBO J
19
6361
6370
23. OldaniA
CormontM
HofmanV
ChiozziV
OregioniO
2009
Helicobacter pylori counteracts the apoptotic action of its VacA toxin by injecting the CagA protein into gastric epithelial cells.
PLoS Pathog
5
e1000603
doi:10.1371/journal.ppat.1000603
24. FrancoAT
JohnstonE
KrishnaU
YamaokaY
IsraelDA
2008
Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors.
Cancer Res
68
379
387
25. CarneiroLA
TravassosLH
SoaresF
TattoliI
MagalhaesJG
2009
Shigella induces mitochondrial dysfunction and cell death in nonmyleoid cells.
Cell Host Microbe
5
123
136
26. KimM
OgawaM
FujitaY
YoshikawaY
NagaiT
2009
Bacteria hijack integrin-linked kinase to stabilize focal adhesions and block cell detachment.
Nature
459
578
582
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent AutoimmunityČlánek Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on VirulenceČlánek Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral LoadČlánek A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Azole Drugs Are Imported By Facilitated Diffusion in and Other Pathogenic Fungi
- Two Genes on A/J Chromosome 18 Are Associated with Susceptibility to Infection by Combined Microarray and QTL Analyses
- Impact of Simian Immunodeficiency Virus Infection on Chimpanzee Population Dynamics
- Breaking the Stereotype: Virulence Factor–Mediated Protection of Host Cells in Bacterial Pathogenesis
- The Canine Papillomavirus and Gamma HPV E7 Proteins Use an Alternative Domain to Bind and Destabilize the Retinoblastoma Protein
- Rescue of HIV-1 Release by Targeting Widely Divergent NEDD4-Type Ubiquitin Ligases and Isolated Catalytic HECT Domains to Gag
- Steric Shielding of Surface Epitopes and Impaired Immune Recognition Induced by the Ebola Virus Glycoprotein
- Dynamics of the Multiplicity of Cellular Infection in a Plant Virus
- HLA Class I Binding of HBZ Determines Outcome in HTLV-1 Infection
- Pathogenic Bacteria Target NEDD8-Conjugated Cullins to Hijack Host-Cell Signaling Pathways
- The HA and NS Genes of Human H5N1 Influenza A Virus Contribute to High Virulence in Ferrets
- SRFR1 Negatively Regulates Plant NB-LRR Resistance Protein Accumulation to Prevent Autoimmunity
- Cyclin-Dependent Kinase Activity Controls the Onset of the HCMV Lytic Cycle
- The N-Terminal Domain of the Arenavirus L Protein Is an RNA Endonuclease Essential in mRNA Transcription
- Generation of Neutralizing Antibodies and Divergence of SIVmac239 in Cynomolgus Macaques Following Short-Term Early Antiretroviral Therapy
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- Intracellular Proton Conductance of the Hepatitis C Virus p7 Protein and Its Contribution to Infectious Virus Production
- The Transcriptome of the Human Pathogen at Single-Nucleotide Resolution
- The Epidermal Growth Factor Receptor (EGFR) Promotes Uptake of Influenza A Viruses (IAV) into Host Cells
- Surface Co-Expression of Two Different PfEMP1 Antigens on Single -Infected Erythrocytes Facilitates Binding to ICAM1 and PECAM1
- Sequestration and Tissue Accumulation of Human Malaria Parasites: Can We Learn Anything from Rodent Models of Malaria?
- Phylogenomics of Ligand-Gated Ion Channels Predicts Monepantel Effect
- Generation of Covalently Closed Circular DNA of Hepatitis B Viruses via Intracellular Recycling Is Regulated in a Virus Specific Manner
- CpG-Methylation Regulates a Class of Epstein-Barr Virus Promoters
- Molecular and Evolutionary Bases of Within-Patient Genotypic and Phenotypic Diversity in Extraintestinal Infections
- A Bistable Switch and Anatomical Site Control Virulence Gene Expression in the Intestine
- Are Members of the Fungal Genus (a) Commensals; (b) Opportunists; (c) Pathogens; or (d) All of the Above?
- Structures of Receptor Complexes of a North American H7N2 Influenza Hemagglutinin with a Loop Deletion in the Receptor Binding Site
- Phylogenetic Approach Reveals That Virus Genotype Largely Determines HIV Set-Point Viral Load
- The Coevolution of Virulence: Tolerance in Perspective
- Involvement of the Cytokine MIF in the Snail Host Immune Response to the Parasite
- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins
- High Content Phenotypic Cell-Based Visual Screen Identifies Acyltrehalose-Containing Glycolipids Involved in Phagosome Remodeling
- A Novel Small Molecule Inhibitor of Hepatitis C Virus Entry
- The Microbiota Mediates Pathogen Clearance from the Gut Lumen after Non-Typhoidal Diarrhea
- RNA Polymerases (L-Protein) Have an N-Terminal, Influenza-Like Endonuclease Domain, Essential for Viral Cap-Dependent Transcription
- Pathogen Specific, IRF3-Dependent Signaling and Innate Resistance to Human Kidney Infection
- Cellular Entry of Ebola Virus Involves Uptake by a Macropinocytosis-Like Mechanism and Subsequent Trafficking through Early and Late Endosomes
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Formation of Mobile Chromatin-Associated Nuclear Foci Containing HIV-1 Vpr and VPRBP Is Critical for the Induction of G2 Cell Cycle Arrest
- Association of Tat with Promoters of PTEN and PP2A Subunits Is Key to Transcriptional Activation of Apoptotic Pathways in HIV-Infected CD4+ T Cells
- Metal Hyperaccumulation Armors Plants against Disease
- Cyclin-Dependent Kinase-Like Function Is Shared by the Beta- and Gamma- Subset of the Conserved Herpesvirus Protein Kinases
- Role of Acetyl-Phosphate in Activation of the Rrp2-RpoN-RpoS Pathway in
- Ebolavirus Is Internalized into Host Cells Macropinocytosis in a Viral Glycoprotein-Dependent Manner
- A Novel Family of IMC Proteins Displays a Hierarchical Organization and Functions in Coordinating Parasite Division
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Structure of the Extracellular Portion of CD46 Provides Insights into Its Interactions with Complement Proteins and Pathogens
- The Length of Vesicular Stomatitis Virus Particles Dictates a Need for Actin Assembly during Clathrin-Dependent Endocytosis
- Inhibition of TIR Domain Signaling by TcpC: MyD88-Dependent and Independent Effects on Virulence
- The Coevolution of Virulence: Tolerance in Perspective
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



