-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
Tuberculosis exerts a tremendous burden on global health, with ∼9 million new infections and ∼2 million deaths annually. The Mycobacterium tuberculosis complex (MTC) was initially regarded as a highly homogeneous population; however, recent data suggest the causative agents of tuberculosis are more genetically and functionally diverse than appreciated previously. The impact of this natural variation on the virulence and clinical manifestations of the pathogen remains largely unknown. This report examines the effect of genetic diversity among MTC clinical isolates on global gene expression and survival within macrophages. We discovered lineage-specific transcription patterns in vitro and distinct intracellular growth profiles associated with specific responses to host-derived environmental cues. Strain comparisons also facilitated delineation of a core intracellular transcriptome, including genes with highly conserved regulation across the global panel of clinical isolates. This study affords new insights into the genetic information that M. tuberculosis has conserved under selective pressure during its long-term interactions with its human host.
Published in the journal: . PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1000988
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000988Summary
Tuberculosis exerts a tremendous burden on global health, with ∼9 million new infections and ∼2 million deaths annually. The Mycobacterium tuberculosis complex (MTC) was initially regarded as a highly homogeneous population; however, recent data suggest the causative agents of tuberculosis are more genetically and functionally diverse than appreciated previously. The impact of this natural variation on the virulence and clinical manifestations of the pathogen remains largely unknown. This report examines the effect of genetic diversity among MTC clinical isolates on global gene expression and survival within macrophages. We discovered lineage-specific transcription patterns in vitro and distinct intracellular growth profiles associated with specific responses to host-derived environmental cues. Strain comparisons also facilitated delineation of a core intracellular transcriptome, including genes with highly conserved regulation across the global panel of clinical isolates. This study affords new insights into the genetic information that M. tuberculosis has conserved under selective pressure during its long-term interactions with its human host.
Introduction
Mycobacterium tuberculosis represents a unique opportunity to explore the impact of evolution and genetic diversity on bacterial host adaptation and pathogenesis. Tuberculosis is an ancient disease, with evidence that the causative agent M. tuberculosis has been co-evolving with its human host since mankind emerged from Africa 50,000 years ago [1], [2]. Despite intense efforts, the disease remains a major burden on global health and a strong selective pressure on human evolution [3]. M. tuberculosis is the most prominent member of the Mycobacterium tuberculosis complex (MTC), comprised of seven closely related species with distinct host tropisms including the human pathogens M. africanum and M. canettii and the animal adapted species M. bovis (bovine), M. caprae (goats), M. pinnipedii (seals), and M. microti (rodents). Members of the MTC are considered genetically monomorphic with a high level of genomic sequence similarity (>99.95%), limited horizontal gene transfer, and a clonal population structure [4], [5]. This apparent homogeneity led to the assumption that genetic diversity among MTC strains would not be of clinical significance. However, recent data based on molecular genotyping methods (i.e. IS6110 RFLP, spoligotyping, MIRU-VNTR typing) revealed a highly diverse population structure with at least six major geographically-associated lineages that can be further subdivided into well-defined genotypes [1], [2]. Based on multilocus sequence analysis of 89 genes in 108 strains, Hershberg et al. conservatively estimated that an average pair of MTC strains would have ∼300 functional differences due to SNPs alone [1]. Thus, it is becoming apparent that the genetic diversity of the MTC has been underestimated.
There is mounting evidence that the genetic variability among clinical isolates may have dramatic consequences on the outcome of infections. For example, studies in vitro [6], [7], [8], in animal models [9], [10], [11], [12], [13], and human epidemiology studies [14] have demonstrated strain-dependent variation in key aspects of virulence such as stress survival, transmission, pathology, and lethality. It is also believed that the genetic diversity of the MTC contributes to the wide spectrum of TB clinical presentations, including acute primary TB (localized or disseminated), latent disease and reactivation [15], [16]. However, the molecular mechanisms for such variations in M. tuberculosis pathogenesis and host adaptation have been identified in only a few cases. In one example, the hypervirulence of some Beijing strains has been linked to the production of immune modulatory phenolic glycolipid (PGL), whereas a 7bp deletion in the pks 15/1 gene disables PGL synthesis in many isolates [13], [17], [18]. Genetic variation may also lead to altered metabolism and gene expression pathways important for MTC pathogenesis [19], [20], [21].
A hallmark of pathogenic mycobacteria is the ability to survive within the phagosome of professional phagocytes and persist within pulmonary granulomas for decades. This most intimate of inter-species associations has led to the evolution of strategies to resist antimicrobial effectors and subvert the immune response. Information on the “common” and “variable” responses of clinical isolates of different phylogenetic lineages to host macrophages is crucial for identification of microbial factors required for intracellular survival or involved in the adaptation to specific hosts. However, studies analyzing gene expression and virulence of MTC in infection models have largely been restricted to a limited number of individual strains [22], [23].
This study details a systematic approach to define the degree to which diversity, hard-wired into the bacterial genome, translates into functional phenotypes reflected in expression profiling and bacterial survival within macrophages. We investigated a panel of 17 strains that represent the global phylogeographic diversity of MTC: two reference strains H37Rv and CDC1551 and 3 clinical isolates each from 5 distinct genotypes. The varied survival characteristics of the clinical isolates within macrophages, in addition to the heterogeneity of the in vitro and intracellular transcriptome profiles are evidence of the powerful effect genetic variation exerts on global gene expression and host interactions. Our approach of linking well-characterized genotypes to transcriptional responses induced by conditions relevant to infection (in vitro, versus resting and activated murine macrophages) is a crucial step toward understanding the functional evolution of MTC and the varied pathologies of tuberculosis.
Results
Evolution of MTC clinical isolates evident in basal in vitro transcriptome
To determine the functional impact of the natural genetic diversity of pathogenic mycobacteria, we first studied the growth and gene expression of MTC clinical isolates from five global phylogeographical lineages in liquid culture (Figure 1A, Table 1, Supplementary Figure S1). The growth kinetics of most strains in rich medium, especially members of the Beijing, EAI, and West African 2 genotypes, were not significantly different from the reference strains CDC1551 and H37Rv. However, clinical isolates of the Haarlem and Uganda genotypes exhibited slower growth rates in vitro (Supplementary Figure S2 and S7A). Microarray profiling of log phase bacteria (OD∼0.6) yielded expression patterns that mirror the phylogenetic relationship of strains as defined by molecular typing methods (Figure 1B). Condition tree analysis of in vitro transcriptome data segregated strains into two main branches consistent with the recently described classification of “modern” clade 1 and “ancient” clade 2 strains (Figure 1B) [2]. A total of 156 genes exhibited clade specific profiles (Supplementary Figure S3, Supplementary Tables S1 and S2), highlighting the functional divergence of these two MTC lineages. Gene clusters with higher expression in clade 1 (i.e. narGHJI, nitrate reductase operon) or clade 2 (i.e. mce4 locus, implicated in cholesterol utilization [24]), confirmed by qRT-PCR analysis of narG (Supplementary Figure S10A) and mce4C (Supplementary Figure S9A) could have a significant impact on the “success” of these strains across their human host populations.
Fig. 1. Genetic and transcriptome diversity of M. tuberculosis complex (MTC) clinical isolates. 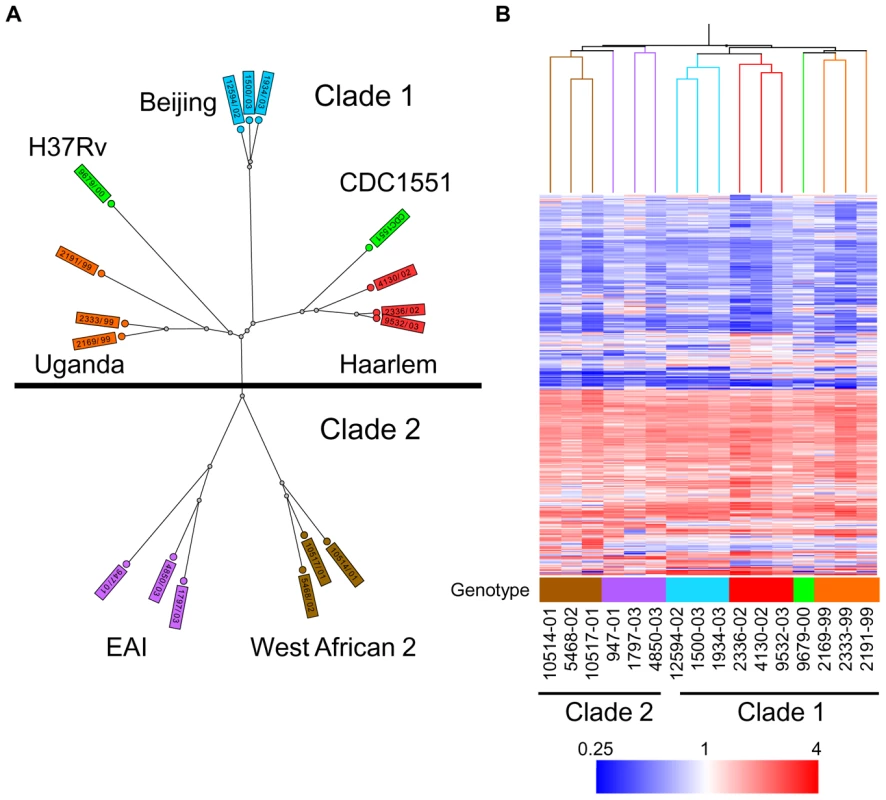
(A) Radial neighbor-joining tree based on 24 loci MIRU-VNTR and 43 spacer spoligotyping showing the phylogenetic relationship of strains in this study. Three strains each from the 5 distinct lineages pathogenic to humans plus two sequenced reference strains (H37Rv and CDC1551) were chosen to represent the global diversity of MTC. The color used to denote each genotype is maintained in all subsequent figures for clarity. (B) Condition tree of MTC clinical isolate transcription profiles in vitro during log phase growth in 7H9 medium relative to CDC1551 reference strain (three biological replicates). Expression profiles for genes passing quality filters (flagged as present in 42 of 48 samples) with differential expression in at least one strain (up or down >1.5× in at least 1 of 16) were clustered using the Spearman correlation. Each column represents the global transcription profile of a single strain. Genes were clustered vertically based on the distance measure. Red and blue indicate higher or lower gene expression than CDC1551 control, respectively. Unless otherwise indicated, the color scale for expression (4-fold up or down) was used for all subsequent figures. Tab. 1. Mycobacterium tuberculosis complex (MTC) clinical isolates investigated in this study. 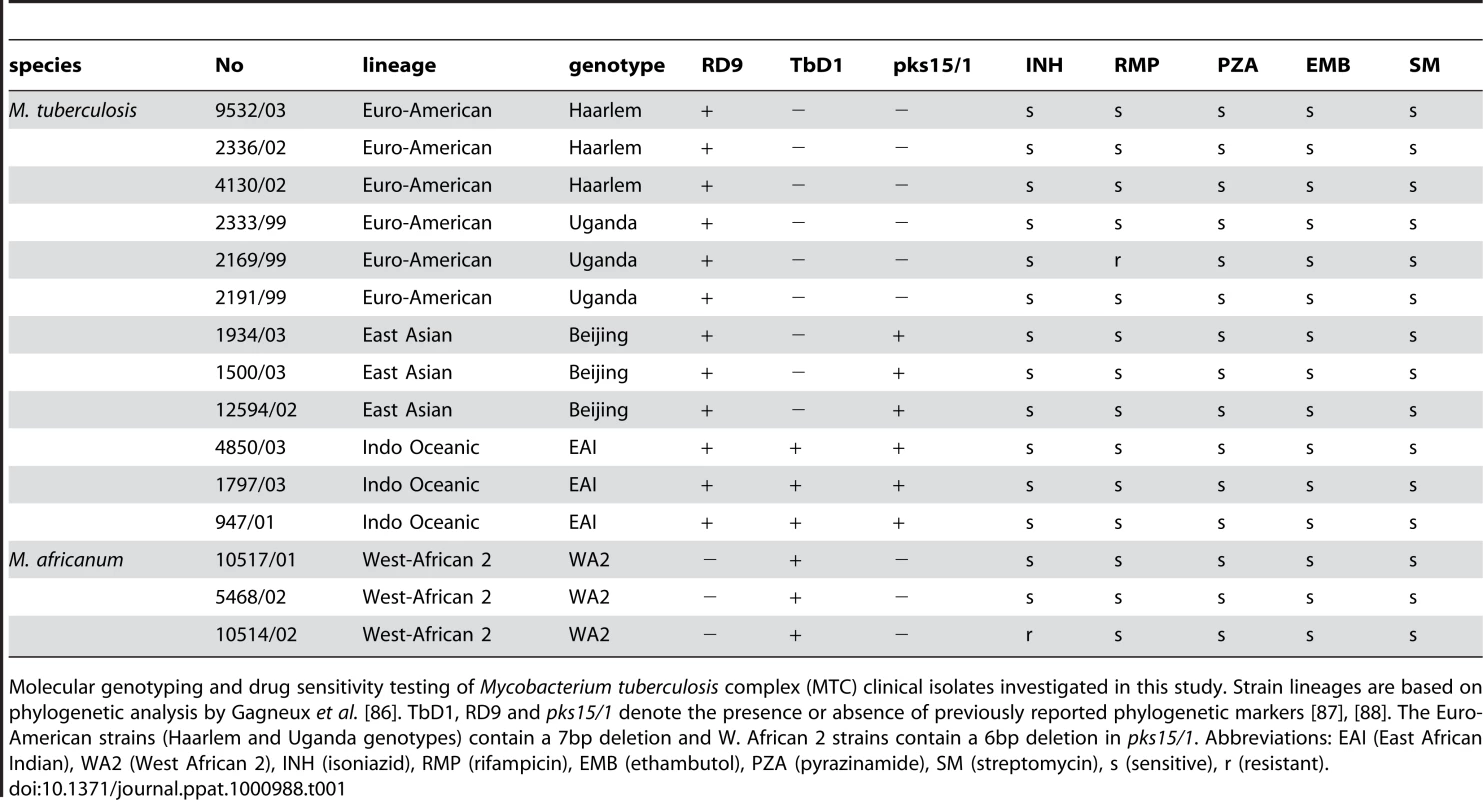
Molecular genotyping and drug sensitivity testing of Mycobacterium tuberculosis complex (MTC) clinical isolates investigated in this study. Strain lineages are based on phylogenetic analysis by Gagneux et al. [86]. TbD1, RD9 and pks15/1 denote the presence or absence of previously reported phylogenetic markers [87], [88]. The Euro-American strains (Haarlem and Uganda genotypes) contain a 7bp deletion and W. African 2 strains contain a 6bp deletion in pks15/1. Abbreviations: EAI (East African Indian), WA2 (West African 2), INH (isoniazid), RMP (rifampicin), EMB (ethambutol), PZA (pyrazinamide), SM (streptomycin), s (sensitive), r (resistant). We identified 364 genes (∼10% of all genes) with significant expression differences (p<0.01) between strains of different genotypes in at least one pair-wise comparison (Figure 2A). Our analyses delineated genotype-specific signatures (Figure 2B) like the virS-mym operon (Rv3082c-3089) dysegulated in all EAI strains and the dosRS two-component regulator (Rv3132c-3133c) overexpressed in all Beijing strains (Figure 2B). Semi-quantitative qRT-PCR verified the ∼10-fold higher basal expression level of dosR in Beijing isolates (Supplementary Figure S8 and S9B), the elevated transcription of virS (3-fold) and concomitant repression (∼50-fold) of Rv3083 in EAI strains (Supplementary Figure S8 and S9C). Additionally, we identified profiles shared between genotypes such as the moa operon (moaE3, moaC3, gphA, MT3427, MT3427.1) hyper-expressed in Beijing and Haarlem strains. Similarly, transcripts of the Rv3612c-3616c operon, involved in secretion of the virulence factor ESAT-6, were more abundant in the Haarlem and Uganda genotypes while exhibiting diminished expression in EAI strain 1797/03 (Figure 2C). Subsequent 1-way ANOVA analysis (p<0.01) identified a total of 195 genes with strain-specific gene expression profiles (Supplementary Figure S4). The elevated expression of the fabG1-inhA-hemZ operon (Rv1483-1485) in a single West African 2 strain, for example, highlights the potential for genetic variation to alter a drug target (InhA) involved in cell wall biosynthesis (Figure 2C). In fact, subsequent analysis of strain 10514/01 revealed a previously described C→T substitution at position −15 upstream of this operon [25] accompanied by isoniazid resistance (data not shown). The negligible detection of eleven IS6110 related genes in strain 1797/03, defined as a subgroup of the EAI genotype by unique IS6110 RFLP fingerprinting, illustrates the potential to identify possible deletions among clinical isolates based on gene expression data (Figure 2C, Supplementary Figure S1).
Fig. 2. Identification of genotype- and strain- specific in vitro transcription profiles. 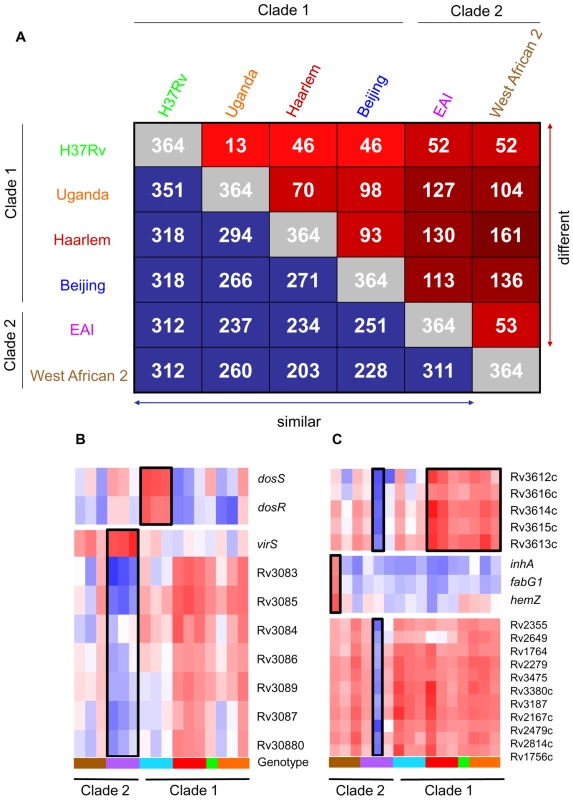
(A) Pair-wise comparison of genes with significant genotype-dependent expression. Includes genes identified by one-way ANOVA of quality-filtered geneset (present in 42 of 48 samples) using Benjamini and Hochberg False Discovery Rate (p<0.01). The matrix of intragenotype comparisons was generated by Tukey post hoc test. The numbers within red squares indicate genes with unique expression patterns between the two intersecting genotypes. (B) Gene tree of select virulence-associate transcriptional regulators with genotype-specific expression signatures. The dosRS two-component regulator, overexpressed in Beijing strains, controls the expression of ∼50 genes in response to multiple signals including nitric oxide, hypoxia, and carbon monoxide and is required for infection in animal models [20], [36], [70], [80], [81], [82], [83]. VirS is overexpressed in EAI strains with concomitant decreased transcript levels of the mymA operon (Rv3083-Rv3089). virS and mymA genes play a role in cell wall ultrastructure and are required for growth in activated macrophages and in mouse spleen [52], [53]. (C) Gene tree of select loci exhibiting strain-specific expression and profiles shared across multiple genotypes. Expression ratios indicated by color gradient are in vitro log phase transcript levels relative to CDC1551 reference strain (see Fig. 1B for color key). This remarkable degree of diversity in the basal transcriptome of MTC clinical isolates in vitro provides compelling evidence that genomic variation among strains results in a greater functional impact than appreciated previously.
Variable survival of MTC clinical isolates in murine macrophages
To test the hypothesis that the disparate patterns of gene expression described above would influence the ability of MTC strains to survive in their intracellular niche, we analyzed the growth of our panel of 15 clinical isolates and reference strains CDC1551 and H37Rv in resting and activated murine macrophages for 11 days. We found that intracellular growth revealed clade-, genotype - and strain-specific fitness profiles for survival within macrophages even more clearly than in vitro growth (Figure 3, Supplementary Figure S7B–C).
Fig. 3. Long-term survival and growth of MTC clinical isolates in macrophages. 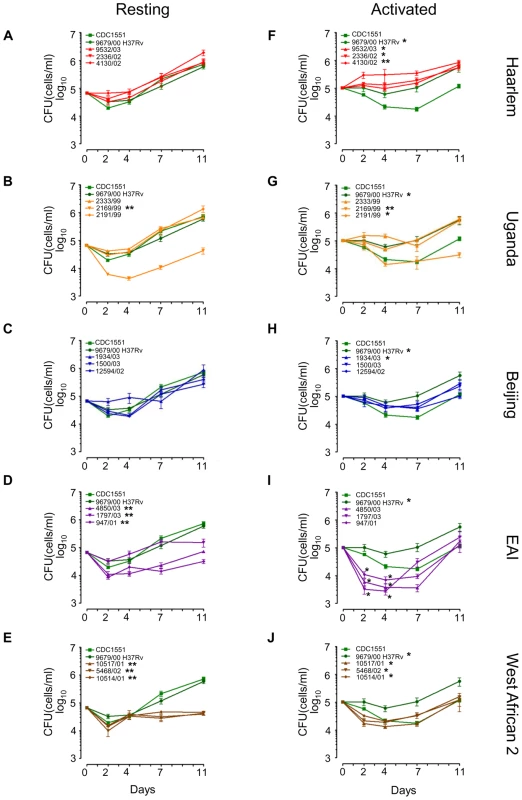
Resting (A–E) and IFN-γ + LPS activated (F–J) murine bone-marrow derived macrophages were infected at low MOI (∼1∶1) with MTC strains. Quantification of viable CFU at day 0,2,4,7, and 11 post-infection were conducted by lysis of monolayers, serial dilution, and plating on 7H10 medium. Error bars indicate standard error of the mean from two independent biological replicates each consisting of three technical replicates per strain (total of 6 wells/strain). Growth profiles of reference strains, H37Rv and CDC1551, are shown in green in all panels. Asterisks in the legend indicate strains determined to exhibit growth profiles significantly different compared to CDC1551 by ANOVA (* = p<0.05, ** = p<0.001). Although EAI strains did not reach p<0.05 when time was modeled as a continuous variable, modeling time as a nominal variable yielded differences of statistical significance at specific time points, indicated by asterisks at day 2 and day 4 in (I). In resting macrophages, intracellular survival of most clade 1 clinical isolates was similar to CDC1551 and H37Rv (Figure 3A–C, Supplementary Figure S7B). This was not surprising given that molecular genotyping indicates CDC1551 and H37Rv are related to the Haarlem and Uganda genotypes, respectively (Figure 1A). Consistent with Theus et al. [26], we did not observe the previously reported hypervirulence of Beijing strains in our infection model [27], [28] (Figure 3C). It is notable that most clade 2 strains exhibited either an early survival defect (EAI 4850/03, 947/01) and/or significantly impaired replication in resting macrophages compared to clade 1 or reference strains (Figure 3D–E, Supplementary Figure S7B). A single clade 1 isolate within the Uganda genotype (2169/99) was severely attenuated for intramacrophage survival during early stages of infection perhaps linked to its poor in vitro replication (Figure 3B, Supplementary Figure S2 and S7B). However, overall there was no direct correlation between in vitro growth rates and intracellular fitness. For example, EAI and West African 2 isolates were clearly impaired for survival in resting macrophages compared to Beijing strains, despite similar replication kinetics in vitro.
Activation of the host macrophages with interferon-g and LPS accentuated the differences in intracellular growth between clinical isolates (Figure 3F–J). Note the divergence of CDC1551 and H37Rv intracellular growth profiles compared to resting macrophages (Figure 3A and 3F). Although activated macrophages were more effective at suppressing mycobacterial growth until day 7, we did note a late rebound by most MTC isolates after day 7 perhaps indicating delayed bacterial adaptation or a waning of antimicrobial effectors in the activated macrophages. Pair-wise comparisons between all strains using the Tukey-Kramer HSD test delineated significant genotype - and strain-dependent intracellular growth profiles (Supplementary Figure S7C). For example, the Haarlem genotype proved to be better adapted for phagosome survival than Beijing, EAI, or West African 2 isolates (Figure 3F, Supplementary Figure S7C). Contrary to previous reports of enhanced intracellular replication [9], [27], [28], Beijing isolates grew only slightly better than CDC1551 (Figure 3H). Uganda strain 2169/99 survived but failed to grow in activated macrophages, consistent with its subpar growth in vitro and in resting macrophages (Figure 3G). In activated macrophages, the growth profiles of clade 2 isolates were distinct from Haarlem, Uganda (except 2169/99), and H37Rv (Supplementary Figure S7C). Strains of the EAI genotype presented a unique profile, experiencing an acute early decline in CFU followed by a rebound to CDC1551 levels by day 11. Intriguingly, West African 2 strains (M. africanum) appear better adapted for growth in activated versus resting macrophages (compare Figure 3E and 3J).
MTC strains exhibit a highly conserved core intracellular transcriptome
To determine the effect of natural genomic variation on the response of MTC isolates to the intracellular niche, we conducted microarray analysis of intracellular MTC bacilli 24h after infection of resting and activated murine macrophages. This facilitated identification of a highly-conserved, core intramacrophage transcriptome which included 280 genes either universally induced (168 genes) or repressed (112 genes) in all strains, regardless of activation state of the host cell (Figure 4A–B, Supplementary Figure S5, Supplementary Tables S3, S4, S5, S6, S7 and S8). The induction of genes with roles in hypoxia, oxidative and nitrosative stress, cell wall remodeling, regulation, and fatty acid metabolism across the panel of clinical isolates confirms previous studies using single strains [22], [23] and affirms their association with the adoption of an intracellular lifestyle (Supplementary Figure S5A, Supplementary Tables S3, S4 and S5). The 112 universally repressed genes, in addition to reflecting suppression of growth and energy metabolism, indicate modulation of cell wall composition (acpM, kasA, fabD, lipoproteins), lipid and cholesterol transport (mmpL9, mmpL12, mce1 genes lprK, Rv0167, Rv0170, Rv0172, Rv0176, and mce4 genes Rv3492c, Rv3493c, Rv3497c) [29], [30], and transcriptional regulation (sigD, cspA, Rv3676) (Supplementary Figure S5B, Supplementary Tables S6, S7 and S8). Approximately 40% (114/280) of the “universal intracellular transcriptome” that exhibited conserved regulation among clinical isolates were genes of unknown function. The core intracellular transcriptome highlights fundamental stress responses, pathways, and genes involved in intracellular survival that are conserved across the MTC.
Fig. 4. The MTC universal intracellular transcriptome: identification of genes with conserved expression and regulation inside macrophage phagosome across global panel of MTC clinical isolates. 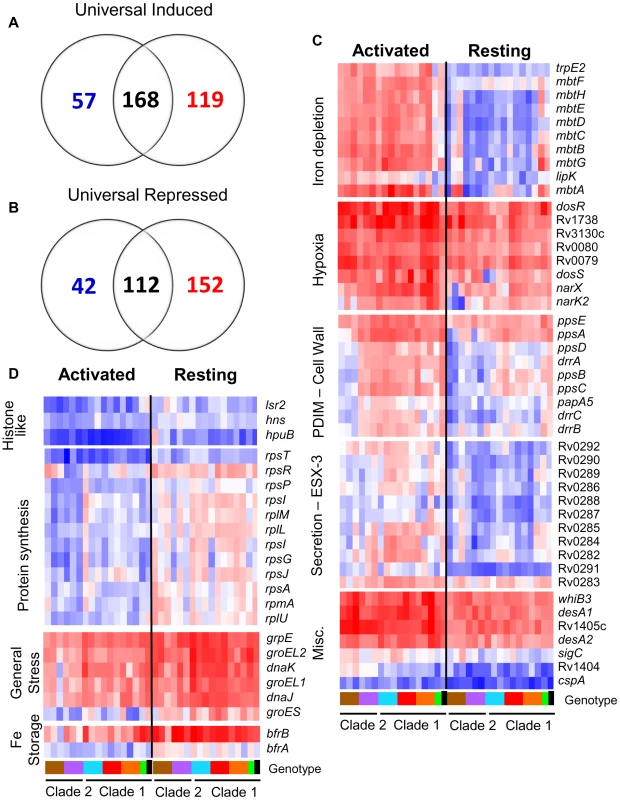
Global gene expression profiles of 17 MTC strains 24h post-infection of resting and activated macrophages were determined by microarray. Normalized expression ratios for each strain were determined by comparison of RNA from intracellular bacteria versus control bacteria of the same strain treated identically except for phagocytosis by macrophages. This serves to identify relative responses to phagosomal cues rather than inherent strain-dependent differences in gene expression. (A–B) Venn diagrams showing activation-dependent (red = activated, blue = resting) and independent (black) genes with conserved expression patterns across clinical isolates. “Universal genes” were selected based on trending up or down in all strains (up or down >1.2-fold in 15 of 17 strains) and significant induction or repression in >50% of strains (up or down >1.5× in 8 of 17 strains) in each macrophage type. The starting gene list for this analysis included only genes flagged as present in the majority of samples from both macrophage types. (C) Select genes with higher expression levels in activated versus resting macrophages conserved across all or most clinical isolates. Represents subset of activation-dependent genes identified by one-way ANOVA analysis of all intracellular transcription profiles (Benjamini and Hochberg False Discovery Rate p<0.01). (D) Select genes with higher expression in resting versus activated macrophages. See (C) above for analysis description. Refer to Fig 1B for genotype color bar definition (black box denotes reference strain CDC1551). Following the initial encounter of pathogenic mycobacteria with resting alveolar macrophages, the onset of the adaptive immune response presents an enhanced “stress test” for MTC. Classical activation of macrophages by IFN-γ constrains bacterial growth by overriding the block in phagolysosome fusion and upregulating antimicrobial effectors, such as ROI and RNI [31]. However, despite controlling the infection, the failure to eradicate MTC indicates that the pathogen is capable of adapting to or modulating this environment to survive. To identify transcriptional responses that correlated with activation status of the macrophage, we used 1-way ANOVA analysis of intracellular array data to identify 719 genes whose expression was significantly influenced by macrophage activation (p<0.01) across the panel of strains.
Responses to known phagosome parameters enhanced by activation, such as nitric oxide production (dosR regulon) and iron limitation (mycobactin operon), were evident among the 317 genes with elevated induction in activated macrophages (Figure 4C, Supplementary Table S10). The conserved induction of certain transcriptional regulators (i.e. whiB3, cpsA), transporters (i.e.ESX-3), lipid metabolism enzymes (i.e. desA1, desA2, ppsABCDE), and 109 genes of unknown function in all 15 clinical MTC isolates within activated macrophages indicates a role for these loci in survival under immune pressure (Figure 4C, Supplementary Table S9).
Our analyses also delineated 402 genes with higher induction ratios in resting versus activated macrophages (Figure 4D, Supplementary Table S10). Many genes, such as rps and rpl genes encoding ribosomal proteins involved in protein synthesis and histone-like proteins (lsr2, hns, hupB) also fell into this category because they were highly repressed in activated macrophages (Figure 4D). The enhanced expression of iron storage genes bfrA and bfrB are indicative of the iron-replete environment of the transferrin-accessible resting phagosome [32]. Interestingly, markers of the general stress response (grpE, groEL, groEL2, groES) were induced more highly by MTC strains in resting macrophages. This would imply that these chaperones are required to sustain growth because many of the genes that exhibited higher expression in resting macrophages are clearly linked to bacterial growth and nutrient acquisition consistent with replicating organisms.
Lineage specific transcriptional responses to intracellular cues
The disparate abilities of MTC clinical isolates to survive and replicate in macrophages could be attributable to both the distinct baseline patterns of in vitro gene expression (described above) and/or to strain-dependent transcriptional adaptation to phagosome-derived cues. Intracellular transcriptome data, which were normalized to strain-matched extracellular controls to minimize the contribution of in vitro gene expression differences, revealed that MTC clinical isolates also exhibited distinct responses to environmental cues within the phagosome. Although the responses were more divergent than observed from in vitro microarrays, the changes in gene expression induced by macrophage invasion still reflected the phylogenetic relationship of strains with greater correlation between intragenotypic (Figure 5A) versus intergenotypic (Figure 5B) profiles. One-way ANOVA analyses (p<0.01) identified 625 genes with genotype-specific and 293 genes with strain-specific expression across both resting and activated macrophage datasets. Similar analysis of each macrophage state separately revealed 114 and 266 genes with genotype-specific expression patterns in resting and activated macrophages, respectively. The failure of M. africanum (West African 2) strains to induce the phthiocerol dimycocerosate (PDIM) locus, a complex cell wall lipid unique to mycobacteria, exemplifies the genotype-specific regulation of virulence factors revealed by this study (Figure 5C, Supplementary Figure S10B). The unique responses of individual strains (Figure 5D) provided further evidence of the impact of natural genetic variation on environmental sensing and phagosome adaptation across the panel of MTC clinical isolates.
Fig. 5. Lineage-specific transcriptional responses of MTC clinical isolates to macrophage invasion. 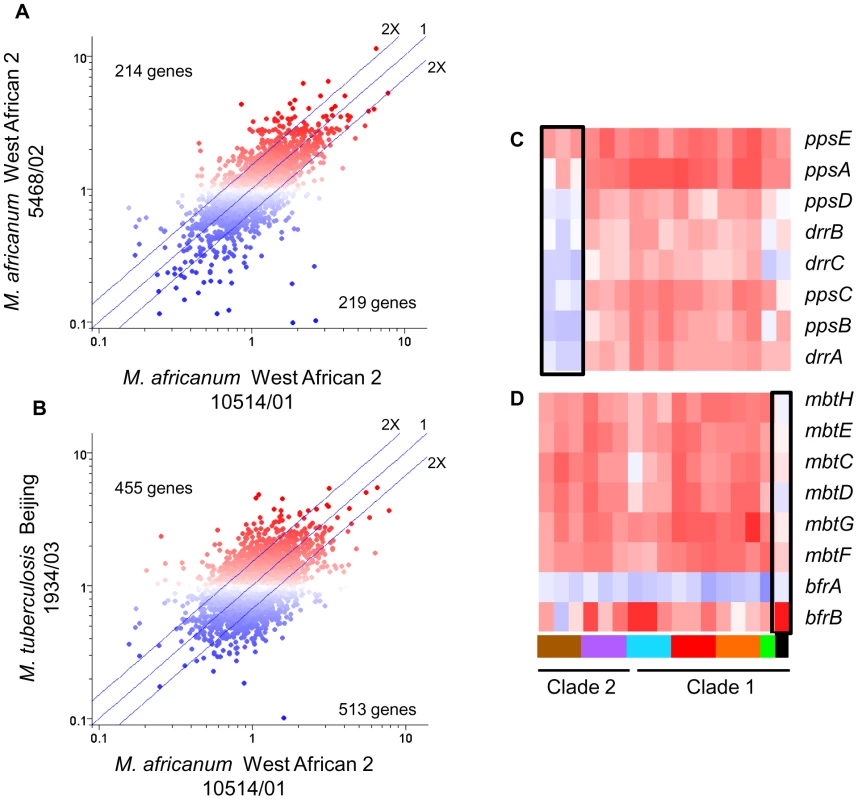
(A) Intragenotype comparison of gene expression profiles 24h post-infection of resting macrophages. Scatter plot showing similarity of transcriptome modulation of two M. africanum (genotype West African 2) strains 10514/01 (x-axis) versus 5468/02 (y-axis). (B) Intergenotype comparison of gene expression profiles 24hr post-infection of resting macrophages. Scatter plot showing M. africanum, genotype West African 2 strain 10514/01 (x-axis) versus M. tuberculosis Beijing strain 1934/03 (y-axis). Each spot represents a single gene with expression ratios >1 indicating intracellular induction (red spots) and <1 indicating intracellular repression (blue spots). The degree of similarity in the regulation of a gene between two strains is shown by proximity to the middle diagonal line (ratio = 1). Axes show normalized expression ratio relative to strain-matched extracellular control in a log10 scale. The number of genes with >2-fold differences in intracellular regulation are indicated on the graph. The increased scatter and number of differentially regulated genes seen in strains of different genotypes (B) reflects the diversity of responses to the vacuole environment observed in strains from genetically diverse lineages. Gene trees showing examples genotype- (C), and strain- (D) specific gene regulation after 24h infection of activated macrophages. Refer to Fig 1B for genotype color bar definition (black box denotes reference strain CDC1551). The success of mycobacteria/host cell interactions is likely impacted by both absolute transcript levels as well as the appropriate, temporal regulation of gene expression. In the previous section, we normalized intracellular array data to strain-matched extracellular controls to focus on relative responses to the phagosomal environment rather than inherent strain-dependent differences. To assess the additive effect of baseline in vitro differences and phagosomal changes in transcription, we directly compared transcript levels from intracellular MTC isolates. Although there are potential caveats this in silico analysis, such as skewing of values due to normalization of data from separate hybridizations, the constitutive expression of housekeeping genes (i.e. sigA) and qRT-PCR analyses discussed below (Supplementary Figure S10) served to validate this approach. This analysis yielded a robust differentiation of phylogenetic relationships which discriminated between strains, genotypes, and clades (Supplementary Figure S6). For example, the clade 2-specific underexpression of the nitrate reductase (narGHJI) and overexpression of the mce4 locus (Rv3492c-3501c) (Figure 6A) present candidate mechanisms for the reduced fitness of these strains for intramacrophage survival (Figure 3). Closer examination of narG expression by qRT-PCR revealed that diminished in vitro expression coupled with lack of induction inside macrophages both contribute to >10-fold lower transcript levels in West African 2 strains within the phagosome (Supplementary Figure S10A). This analysis identified 499 and 463 genes from resting and activated macrophages, respectively, with significant (p<0.01) genotype-dependent differences in phagosomal transcript levels. Differences in the expression level of other genes involved in toxin-antitoxin pairs (Rv0549c-0550c), metabolism (glgC), DNA repair (nrdF,nrdEIH), and transcriptional regulation (furB, whiB4, Rv0452) point to additional mechanisms by which natural variation in gene expression may impact mycobacterial pathogenesis.
Fig. 6. The additive effect of strain-specific differences in basal in vitro gene expression and transcriptional responses to intracellular cues. 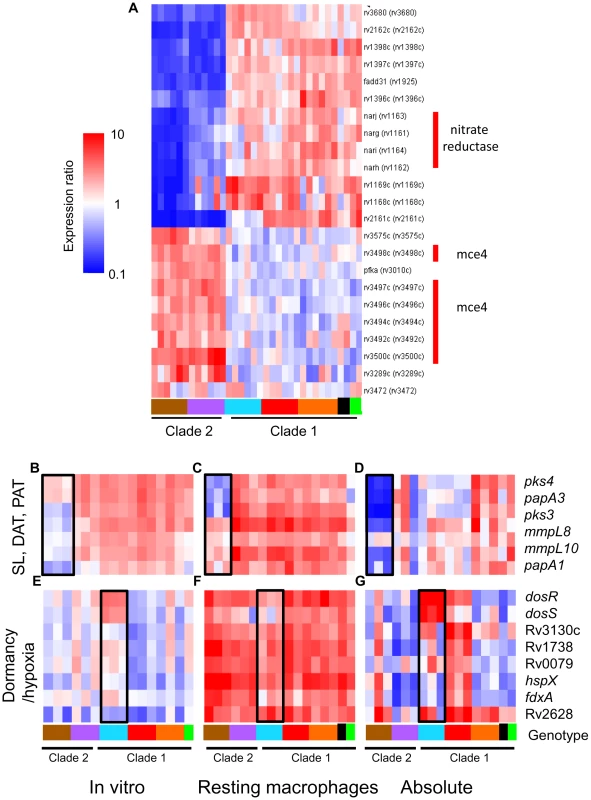
Microarray data from RNA derived from intracellular MTC 24h post-infection were compared directly following median normalization. This allows quantitative estimation of cumulative “absolute” intracellular transcript levels resulting from in vitro differences plus phagosome regulation. (A) Gene tree showing clade-specific intracellular expression levels of putative virulence factors. The narGHJI nitrate reductase operon, implicated in anaerobic nitrate respiration [84] and required for tissue-specific persistence in animal models [65], [85], is expressed at lower levels in all clade 2 versus clade 1 strains. The mce4 operon, encoding a cholesterol import system required for growth in activated macrophages and persistence in mouse lungs [30], is overexpressed in clade 2 strains. (B–D) Reduced expression of sulfolipid (SL), diacyltrehalose (DAT), and polyacyltrehalose (PAT) synthesis genes in intracellular M. africanum West African 2 strains (B) due to lower in vitro expression (B) coupled with lower induction (mmpL8, mmpL10, papA1) or repression (pks4, papA3, pks3) in phagosomes of resting macrophage (C). (E–G) Expression and regulation of DosRS dormancy regulon in MTC clinical isolates. Despite in vitro overexpression of the dosRS two-component regulator in Beijing strains, transcripts of downstream effectors were not notably elevated (E). dosRS and effectors were induced in all strains by phagosomal cues in resting (F) and activated macrophages (data not shown). Although “absolute” levels of dosRS are highest in Beijing strains, DosR-dependent genes are expressed more highly in Haarlem strains (G). Black box in genotype legend denotes reference strain CDC1551. A wider scale for gene expression (0.1–10) was used for (A,D,G). To further illustrate the integration of our multilevel array analysis of MTC clinical isolates, Figure 6B–G depicts a comprehensive examination of the in vitro, intracellular, and cumulative transcriptional profiles of two sets of genes implicated in mycobacterial pathogenesis. The polyketide synthesis loci mmpL8-papA1-pks2 and pks3-pks4-papA3-mmpL10 are required for the synthesis of sulfolipid (SL) and di - and poly-acyltrehaloses (DAT, PAT) respectively [33], [34], [35]. The in vitro underexpression of SL synthesis genes and the repression of DAT/PAT gene expression in macrophages in West African 2 strains results in dramatic cumulative differences in the transcript levels of these genes when the bacilli are inside phagosomes (Figure 6B–D, Supplementary Figure S10C). The DosR regulon, induced by the DosRS two-component regulator in response to hypoxia, carbon monoxide, and nitrosative stress (nitric oxide), is important for survival in animal disease models [36]. Despite the constitutive overexpression of dosRS by Beijing strains in vitro (Figure 6E), the dosR regulon was induced by phagosome cues in all strains (Figure 6F), with relative transcript levels of dosR-dependent effectors actually higher in several non-Beijing vs Beijing isolates (Figure 6G, Supplementary Figure S10D, E). Our findings argue that further characterization of MTC clinical isolates is warranted to understand the interplay between genotype, transcriptional regulation of gene expression, and virulence associated phenotypes.
Discussion
The longstanding view of pathogenic mycobacteria as a homogeneous clonal population with negligible functional genetic diversity has been challenged by recent molecular genotyping studies [2] and whole genome sequencing of members of the MTC [37]. This new appreciation of the significant genetic diversity among MTC isolates has prompted studies documenting strain-dependent differences in resistance to in vitro stress [6], [7], [38], interactions with host cells [8], [26], [27], [39], [40], [41], and virulence in animal infection models [9], [10], [11], [12], [13], [42], [43], [44]. Phylogenetic analyses of the MTC have delineated multiple distinct lineages broadly divided into “modern” (clade 1) and “ancient” (clade 2) strains, with the latter including animal pathogens with distinct host tropisms [1], [2], [45], [46]. Although molecular genotyping provides a robust framework for understanding evolution and epidemiology of MTC, the functional impact of genetic diversity remains poorly characterized. Our microarray data revealed phylogenetically-constrained patterns of in vitro gene expression including clade-, genotype-, and strain-specific signatures. The dramatic effect of natural genetic diversity on transcriptome profiles, brought about by gene deletion, promoter SNPs, or missense mutations in regulators or other proteins, has yielded a functionally heterogeneous population of pathogenic mycobacteria.
The constitutive dysregulation of loci known to impact mycobacterial pathogenesis highlights putative mechanisms underlying the spectrum of host interactions and disease outcomes caused by MTC clinical isolates. For example, clade 2 isolates of the West African 2 and EAI genotypes showed elevated transcription of the mce4 locus (Figure 6, Supplementary Figure S9A). The 11-gene mce4 operon encodes a cholesterol import system that is dispensable for growth in resting macrophages during early infection but is essential for growth in interferon-γ activated macrophages in order to maintain persistent infections in the mouse model [30], [47], [48], [49], [50]. Given the repression of the cholesterol uptake genes by all strains upon macrophage infection, perhaps to counter the toxicity associated with utilization of cholesterol [24], the elevated basal expression of the mce4 locus could conceivably contribute to the reduced fitness of clade 2 strains in macrophages. Altered expression of virulence regulators could also profoundly influence mycobacteria-host interactions. Clinical isolates of the EAI genotype constitutively overexpressed virS (Figure 2B, Supplementary Figure S8 and S9C), an AraC-family transcriptional regulator found only in the MTC [51]. VirS is reportedly required for the expression of the adjacent, divergently transcribed mymA operon (Rv3083-Rv3089) which acts to modify mycolic acid components of the cell wall during acid stress [52], [53]. Disruption of either virS or mymA results in altered cell wall composition and structure, increased sensitivity to antibiotics and surface-acting stresses, and attenuation of virulence in activated macrophages and guinea pigs [52], [53]. In contrast to Singh et al. [53], our data suggest that VirS represses rather than activates transcription of the mymA locus. The dramatic repression of the mymA operon in EAI strains overexpressing virS is likely to have deleterious effects on virulence-associated phenotypes.
Following establishment of the baseline transcriptomes of the clinical strains in rich medium, we characterized the transcriptional responses of the bacteria following infection of resting and activated macrophages in the context of their intracellular growth profiles. This analysis revealed both common and divergent responses that paralleled the capacity of MTC isolates to survive intracellularly, albeit with varying degrees of success. The common responses constitute a rigorous delineation of the core transcriptome associated with intracellular survival of Mycobacteria. Many of the “themes” revealed in the core transcriptome, consisting of 280 genes with 168 genes induced and 112 genes repressed in the intracellular environment, validate the findings reported in earlier studies [22], [23]. The ubiquitous expression and conserved regulation of these genes within macrophages across a global panel of strains should inform the prioritization of targets for the development of novel biomarkers, drugs, or vaccines. This data also suggest that the 45% (75/168) of the core up-regulated genes with no known function, many of which are unique to Mycobacteria, warrant further investigation.
In contrast to the common responses, the distinct intramacrophage fitness levels and intra-strain diversity of transcription profiles, especially between clade 1 and clade 2 strains, were clear indicators of unique lineage-specific adaptation to the phagosome environment. It is notable that gene expression differences were evident as early as 24hr post-infection before growth/survival of strains diverged significantly. The identification of lineage-specific regulons (both known virulence factors and genes of unknown function) that correlate with phenotypes relevant to pathogenesis (i.e. survival in macrophages) provides a powerful approach for discovery of novel strategies of host adaptation and virulence. Differential regulation of virulence factors by M. africanum, the most phylogenetically distant genotype in this study, may provide insight into the evolution of Mtb-host interactions. Notably, the West African 2 strains were distinguished by their failure to upregulate genes required to synthesize sulfolipids (SL-1, pks2-papA1-mmpL8) and phthiocerol dimycocerosates (PDIM, ppsABCDE,drrABC) within macrophage phagosomes (Supplementary Figure S10B, S10C). The importance of SL-1 and PDIM during infection [54], [55], [56] stem from multiple roles including cell well integrity, modulation of the immune response, resistance of interferon-γ independent defenses, and detoxification of proprionate and maintaining redox balance during lipid metabolism [55], [56], [57], [58], [59]. Similarly, high levels of hypoxia-inducible nitrate reductase activity, which allows nitrate respiration in the absence of oxygen, are associated with the globally dominant lineages of the MTC [60], [61], [62]. West African 2 isolates, however, expressed the narGHJI nitrate reductase at depressed levels in vitro and failed to induce both narGHJI and the nitrate/nitrite transporter narK2X inside phagosomes (Supplementary Figure S10A). This is consistent with clade 2-specific SNPs in the promoters of both narGHJI and narK2X [61], [63], [64]. Although animal studies suggest nitrate reductase is required for virulence [65], the exact role of this virulence factor is unclear given that nitrate reductase does not appear to support actual anaerobic growth [66]. Thus, evolving the ability to respond to host-derived and endogenous metabolic cues and adapt to conditions within the host represent key steps in the continuing evolution of the MTC.
On the other hand, the Beijing genotype represents a modern lineage whose growing predominance in the clinical setting suggests a selective advantage over other M. tuberculosis strains. Speculated mechanisms underlying the success of Beijing isolates have included escape from BCG vaccination, enhanced multidrug resistance, and expression of unique virulence lipids [67]. A recent study by Reed et al. found that a large subset of Beijing strains constitutively overexpress the dosRS two-component regulator and 48-gene regulon [20]. The resulting induction of a triacylglycerol (TAG) synthase (Rv3130c) and accumulation of fatty acids in the form of TAG was hypothesized to augment virulence by affording a selective advantage during intracellular survival or latency. As shown in Figure 3, the survival and intracellular replication of Beijing isolates within murine macrophages was unremarkable relative to other lineages, regardless of macrophage activation state. Although our data confirmed the elevated basal transcription levels of dosRS in Beijing isolates (Figure 2B and 6F, Supplementary Figures S8 and S9B), transcript levels of dosRS-dependent effectors such as hspX and Rv3130c were comparable to reference strains (CDC1551 and H37Rv). The use of microaerophilic static growth conditions in this study, leading to moderate activation of the DosR regulon and accumulation of TAG (data not shown) compared to aerated growth in roller-bottles would explain the discrepancy with the findings of Reed et al. [20], [68]. During intracellular adaptation, the induction and overall expression of dosRS and downstream genes was lower in Beijing strains likely as a consequence of genotype-specific frameshift mutations in dosT [68], encoding a histidine kinase that acts on DosR [69], [70]. Our findings do not support the hypothesis that overexpression of dosRS by Beijing strains provides a selective advantage for survival within macrophages. The molecular mechanisms behind the success of this lineage remain to be elucidated.
The global predominance of strains belonging to “modern” clade 1 compared to “ancestral” clade 2 lineages would support the hypothesis that transcriptome features associated with these strains represent strategies evolved for enhanced human infection and transmission. Recent evidence suggests that a strong and stable association exists between MTC strains and distinct geographic regions and human populations [71], [72], [73]. Thus, selective pressures unique to their “native” host environment may be driving the genetic divergence of MTC strains.
Finally, the global resurgence of tuberculosis necessitates a concerted effort to improve the outdated and inadequate strategies of prevention, detection, and treatment that are currently available. Characterization of the molecular and phenotypic differences and similarities between clinical isolates targeted by such interventions is a crucial component in the design of new control measures. In fact, mathematical modeling analyses by Cohen et al. [74] predicted that a failure to consider mycobacterial strain diversity could have a significant negative impact on vaccine efficacy, due to strain replacement by MTC variants not targeted by the vaccine. The functional genetic diversity and distinct macrophage interactions evident in our panel of strains reinforces the need to include challenges with diverse MTC strains as a routine step in vaccine testing [74] and drug screening.
Materials and Methods
Bacterial and cell culture
Fifteen well-characterized clinical isolates of the M. tuberculosis complex (MTC) representing five different phylogenetic genotypes, were selected based on previous studies on the genetic diversity of the MTC. All strains were characterized by various genotyping methods and susceptibility testing as described elsewhere (Supplementary Figure S1, Supplemental Table S1) [2]. Two fully sequenced reference M. tuberculosis strains, H37Rv and CDC1551, and all clinical isolates were routinely cultured in 7H9-OADC medium in 25-cm2 vented flasks without shaking. The MTC clinical isolates used in this study were handled to minimize in vitro passaging. Briefly, strains were initially cultured from clinical samples on Lowenstein-Jensen (LJ) medium for routine diagnostic testing at the National Reference Center in Borstel, Germany. Clinical isolate cultures used in this study were derived from frozen stocks prepared after a single in vitro passage of original archived samples. The clinical isolate reference strain CDC1551 was also maintained to minimize serial passaging. The virulent lab strain H37Rv, however, has undergone countless rounds of in vitro passaging. For in vitro growth assays, the OD600 (BioRad Smart Spec 3000) of 15ml cultures inoculated at OD600 ∼0.05 from frozen stocks grown to mid-log phase was monitored. Bone marrow-derived macrophages (MØ) were isolated from C57BL/6 mice and grown in DMEM supplemented with 10% FCS, 1% sodium pyruvate, 1% L - glutamine, 20% L cell-conditioned medium and 1% penicillin and streptomycin. Media lacking antibiotics was added at least 24h before initiation of mycobacterial infections and maintained during infection.
Macrophage survival assays
To monitor the survival/growth of MTC strains in macrophages, confluent macrophage monolayers (∼5×105 cells) in 24-well dishes were infected at an MOI ∼1∶1. At 0, 2, 4, 7, and 11 days post-infection, intracellular MTC were released from monolayers by lysis with ddH2O+0.05% Tween-80, serially diluted in PBS+0.05% Tween-80, and plated on 7H10 + cycloheximide (10mg/ml) agar. Colony forming units were enumerated after ∼3–4 weeks incubation at 37°C. Activated macrophages were primed with 100 units/ml of recombinant mouse interferon-γ (Peprotech) for 24h followed by pre-activation with 10ng/ml of LPS (Sigma) for 16hr. The integrity of the monolayer was confirmed at each time point by visual microscopic inspection. A total of 6 wells/strain were analyzed (two independent biological replicates with three wells/strain in each assay).
Macrophage infections and RNA isolation
The protocol for RNA isolation from intracellular MTC has been previously described [22]. Briefly, confluent monolayers of ∼2×107 MØ in 75-cm2 vented tissue culture flasks were infected (MOI ∼4∶1) with MTC strains grown to mid-log phase (OD = 0.6–0.8) in standing 75-cm2 vented flasks. Control samples consisted of an aliquot of the same bacterial preparation used to infect macrophages that was instead placed in a 75 - cm2 flask with no macrophages. Care was taken to treat control bacteria identically with the exception of the condition being tested to minimize detection of spurious changes in gene expression due to irrelevant stimuli. Prior to infections, bacteria were declumped by 10 passages through a 21 gauge needle in PBS + 0.01% Tween-80 and diluted in 1ml of uptake buffer (PBS with 4.5mg/ml glucose, 5mg/ml defatted BSA, and 1mg/ml gelatin) and 5ml binding medium (DMEM with 5% fetal calf serum, 10mM HEPES, pH 7.4). After 4h infection, extracellular bacteria were removed and replaced with fresh macrophage medium without antibiotics. At 24h post-infection, addition of GTC lysis buffer (4M guanidine thiocyanate, 0.5% Na N-lauryl sarcosine, 25mM sodium citrate, and 0.1M β-mercaptoethanol) selectively lysed MØ, halted RNA transcription/degradation while leaving mycobacteria intact as previously described [75], [76]. Extracellular controls remained in macrophage medium for the same 24h time course before being stopped by the addition of GTC lysis buffer. RNA from intracellular MTC strains and extracellular controls was isolated and linearly amplified (using 250ng total RNA template per reaction) as described previously [22]. Intracellular transcriptome profiling was conducted on two independent biological replicates for each strain in each macrophage type.
For in vitro array analyses, log-phase mycobacteria in starter cultures grown to mid-log from frozen stocks were inoculated into 7H9-OADC medium in 25-cm2 vented flasks at an OD ∼0.05 and grown without shaking for ∼1 week to an OD∼0.5–0.6. As described above, GTC lysis buffer added at a 5∶1 ratio to culture aliquots served to preserve RNA transcripts for RNA isolation and amplification. The results presented represent the average of three independent biological replicates.
Target preparation and microarray hybridization
Amino-allyl modified amplified RNA (aRNA) were labeled with Alexa Fluor 555 (extracellular controls) and Alexa Fluor 647 (intracellular) (Invitrogen) and purified using a MegaClear kit (Ambion). 10µg of Alexa-labeled aRNA from paired samples was dried using a Speedvac and resuspended in 75µl of hybridization buffer (5× SSC, 25% formamide, 0.1% SDS, and 25µg salmon sperm DNA). Samples were denatured at 95°C for 5min and briefly cooled to 60°C before being applied to arrays under a glass LifterSlip (Erie Scientific). Slides were prehybridized for 1h in 25% formamide, 5× SSC, 0.1% SDS, 1% BSA and washed with H2O and isopropanol. Labeled targets were hybridized to microarrays in humidified slide chambers (Corning) at 45°C for 16–18 h. Arrays were washed sequentially with buffer 1 (2× SSC, 0.1% SDS) pre-warmed to 45°C, buffer 2 (0.2× SSC, 0.1% SDS), buffer 3 (0.2× SSC), and buffer 4 (0.05× SSC). Slides were briefly dipped in de-ionized ultrafiltered water before being dried by centrifugation (700×g, 10min).
For in vitro arrays, aRNA from clinical isolates was labeled with Alexa 647 while aRNA from log-phase strain CDC1551 labeled with Alexa 555 served as a common denominator on all slides. For macrophage infection arrays, the intracellular transcriptome of each strain was compared to a matched sample of the same strain from a macrophage-free control to measure relative changes in gene expression in response to the phagosome environment.
Microarray fabrication
The microarray platform used can be accessed via NCBI's Gene Expression Omnibu (GEO) database [77] under platform accession number GPL5754.
Microarray data analysis
Microarrays were scanned with a GenePix 4000B instrument (Axon Instruments, Inc.) with preliminary image analysis, spot intensity determination, background measurements, spot quality assessment and flagging conducted using Imagene software (version 6.0, Biodiscovery). Poor quality spots with signal intensities less than three standard deviations above background were excluded from further analysis. Subsequent normalization, statistical analysis, and visualization of array data were performed with Genespring 7.3 (Agilent). Genes with significant changes in expression levels relative to controls were identified based on fold change (1.5-fold) and reproducibility between replicates (p-value <0.05). One-way ANOVA and Tukey post-hoc tests were used to identify genes with significant differences in expression between conditions. For most analyses, multiple testing corrections (Benjamini and Hochberg false discovery rate <0.01) were applied. For the direct comparison of intracellular transcript levels (see Figure 6), instead of comparing with cohybridized extracellular control samples, preprocessed images from the channel representing intracellular samples were loaded into Genespring as a single-color experiment. Per chip (normalized to 50th percentile) and per gene (normalized to median) normalizations were conducted on the assembled datasets. The same approach was employed to directly compare transcriptomes of strains from extracellular macrophage-free controls. A high correlation between the relative transcript levels of constitutive housekeeping genes (i.e. sigA) across the panel of clinical isolates analyzed in this manner served to validate this approach. The relative abundance of select transcripts expressed by intracellular MTC was further confirmed by qRT-PCR (Supplementary Figure S10).
All microarray data has been deposited in the GEO database under the accession number GSE21114 and is also available through the TBDB database at www.tbdb.org [78].
Quantitative real-time RT-PCR (qRT-PCR)
RNA amplification and microarray methodology used herein were previously validated by qRT-PCR of select genes differentially regulated by the reference strain CDC1551 during infection of bone marrow derived macrophages [22]. Additional qRT-PCR validation of target gene expression of clinical isolates in this study was conducted by two-step real-time RT-PCR using iScript and iTaq SYBR Green reagents (Biorad). Each sample was analyzed in triplicate on an ABI 7500 starting with 100ng of total RNA (amplified and unamplified). CT values were normalized to values obtained for sigA, a constitutively expressed Mtb gene, and relative changes in gene expression were calculated using the 2−ΔΔCT method [79].
The faithful representation of transcript levels following linear amplification was further validated in this study by qRT-PCR of select genes by comparing expression ratios derived from unamplified versus amplified RNA templates prepared from clinical isolates (Supplementary Figure S8). For qRT-PCR analysis of in vitro and intracellular gene expression, aRNA from a single representative strain from each genotype served as template – Haarlem (4130/02), Beijing (12594/02), Uganda (2333/99), EAI (4850/03), and West African 2 (10514/01).
Statistical analysis
Preliminary analysis of in vitro growth data and intramacrophage survival/growth data was performed using GraphPad Prism software (version 4). Statistical analysis of growth data shown in Figure 3 and Supplemental Figure S2 were conducted using JMP 8.0 software (SAS) with assistance provided by the Cornell Statistical Consulting Unit (CSCU). Significant differences (p<0.05) were determined by one-way ANOVA with pair-wise comparisons between strains made using the Tukey-Kramer HSD post-hoc test.
Supporting Information
Zdroje
1. HershbergR
LipatovM
SmallPM
ShefferH
NiemannS
2008 High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol 6 e311
2. WirthT
HildebrandF
Allix-BeguecC
WolbelingF
KubicaT
2008 Origin, spread and demography of the Mycobacterium tuberculosis complex. PLoS Pathog 4 e1000160
3. SousaAO
SalemJI
LeeFK
VerçosaMC
CruaudP
1997 An epidemic of tuberculosis with a high rate of tuberculin anergy among a population previously unexposed to tuberculosis, the Yanomami Indians of the Brazilian Amazon. Proceedings of the National Academy of Sciences of the United States of America 94 13227 13232
4. AchtmanM
2008 Evolution, population structure, and phylogeography of genetically monomorphic bacterial pathogens. Annu Rev Microbiol 62 53 70
5. AllandD
WhittamTS
MurrayMB
CaveMD
HazbonMH
2003 Modeling bacterial evolution with comparative-genome-based marker systems: application to Mycobacterium tuberculosis evolution and pathogenesis. J Bacteriol 185 3392 3399
6. FirmaniMA
RileyLW
2002 Mycobacterium tuberculosis CDC1551 is resistant to reactive nitrogen and oxygen intermediates in vitro. Infect Immun 70 3965 3968
7. O'BrienL
CarmichaelJ
LowrieDB
AndrewPW
1994 Strains of Mycobacterium tuberculosis differ in susceptibility to reactive nitrogen intermediates in vitro. Infect Immun 62 5187 5190
8. RhoadesER
OrmeIM
1997 Susceptibility of a panel of virulent strains of Mycobacterium tuberculosis to reactive nitrogen intermediates. Infect Immun 65 1189 1195
9. BarczakAK
DomenechP
BoshoffHI
ReedMB
MancaC
2005 In vivo phenotypic dominance in mouse mixed infections with Mycobacterium tuberculosis clinical isolates. J Infect Dis 192 600 606
10. ManabeYC
DannenbergAMJr
TyagiSK
HatemCL
YoderM
2003 Different strains of Mycobacterium tuberculosis cause various spectrums of disease in the rabbit model of tuberculosis. Infect Immun 71 6004 6011
11. Marquina-CastilloB
Garcia-GarciaL
Ponce-de-LeonA
Jimenez-CoronaME
Bobadilla-Del ValleM
2008 Virulence, immunopathology and transmissibility of selected strains of Mycobacterium tuberculosis in a murine model. Immunology
12. PalanisamyGS
DuteauN
EisenachKD
CaveDM
TheusSA
2009 Clinical strains of Mycobacterium tuberculosis display a wide range of virulence in guinea pigs. Tuberculosis (Edinb)
13. TsenovaL
EllisonE
HarbacheuskiR
MoreiraAL
KurepinaN
2005 Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J Infect Dis 192 98 106
14. van der SpuyGD
KremerK
NdabambiSL
BeyersN
DunbarR
2009 Changing Mycobacterium tuberculosis population highlights clade-specific pathogenic characteristics. Tuberculosis (Edinb) 89 120 125
15. MalikAN
Godfrey-FaussettP
2005 Effects of genetic variability of Mycobacterium tuberculosis strains on the presentation of disease. Lancet Infect Dis 5 174 183
16. NicolMP
WilkinsonRJ
2008 The clinical consequences of strain diversity in Mycobacterium tuberculosis. Trans R Soc Trop Med Hyg 102 955 965
17. ReedMB
DomenechP
MancaC
SuH
BarczakAK
2004 A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431 84 87
18. SinsimerD
HuetG
MancaC
TsenovaL
KooMS
2008 The phenolic glycolipid of Mycobacterium tuberculosis differentially modulates the early host cytokine response but does not in itself confer hypervirulence. Infect Immun 76 3027 3036
19. KeatingLA
WheelerPR
MansoorH
InwaldJK
DaleJ
2005 The pyruvate requirement of some members of the Mycobacterium tuberculosis complex is due to an inactive pyruvate kinase: implications for in vivo growth. Mol Microbiol 56 163 174
20. ReedMB
GagneuxS
DeriemerK
SmallPM
BarryCE3rd
2007 The W-Beijing lineage of Mycobacterium tuberculosis overproduces triglycerides and has the DosR dormancy regulon constitutively upregulated. J Bacteriol 189 2583 2589
21. GaoQ
KripkeKE
SaldanhaAJ
YanW
HolmesS
2005 Gene expression diversity among Mycobacterium tuberculosis clinical isolates. Microbiology 151 5 14
22. RohdeKH
AbramovitchRB
RussellDG
2007 Mycobacterium tuberculosis invasion of macrophages: linking bacterial gene expression to environmental cues. Cell Host Microbe 2 352 364
23. SchnappingerD
EhrtS
VoskuilMI
LiuY
ManganJA
2003 Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J Exp Med 198 693 704
24. ChangJC
MinerMD
PandeyAK
GillWP
HarikNS
2009 igr Genes and Mycobacterium tuberculosis cholesterol metabolism. J Bacteriol 191 5232 5239
25. MusserJM
KapurV
WilliamsDL
KreiswirthBN
van SoolingenD
1996 Characterization of the catalase-peroxidase gene (katG) and inhA locus in isoniazid-resistant and -susceptible strains of Mycobacterium tuberculosis by automated DNA sequencing: restricted array of mutations associated with drug resistance. J Infect Dis 173 196 202
26. TheusS
EisenachK
FomukongN
SilverRF
CaveMD
2007 Beijing family Mycobacterium tuberculosis strains differ in their intracellular growth in THP-1 macrophages. Int J Tuberc Lung Dis 11 1087 1093
27. LiQ
WhalenCC
AlbertJM
LarkinR
ZukowskiL
2002 Differences in rate and variability of intracellular growth of a panel of Mycobacterium tuberculosis clinical isolates within a human monocyte model. Infect Immun 70 6489 6493
28. ZhangM
GongJ
YangZ
SamtenB
CaveMD
1999 Enhanced capacity of a widespread strain of Mycobacterium tuberculosis to grow in human macrophages. J Infect Dis 179 1213 1217
29. MohnWW
van der GeizeR
StewartGR
OkamotoS
LiuJ
2008 The actinobacterial mce4 locus encodes a steroid transporter. J Biol Chem 283 35368 35374
30. PandeyAK
SassettiCM
2008 Mycobacterial persistence requires the utilization of host cholesterol. Proc Natl Acad Sci U S A 105 4376 4380
31. SchaibleUE
Sturgill-KoszyckiS
SchlesingerPH
RussellDG
1998 Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol 160 1290 1296
32. Sturgill-KoszyckiS
SchaibleUE
RussellDG
1996 Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. Embo J 15 6960 6968
33. BhattK
GurchaSS
BhattA
BesraGS
JacobsWRJr
2007 Two polyketide-synthase-associated acyltransferases are required for sulfolipid biosynthesis in Mycobacterium tuberculosis. Microbiology 153 513 520
34. HatziosSK
SchelleMW
HolsclawCM
BehrensCR
BotyanszkiZ
2009 PapA3 is an acyltransferase required for polyacyltrehalose biosynthesis in Mycobacterium tuberculosis. J Biol Chem 284 12745 12751
35. SirakovaTD
ThirumalaAK
DubeyVS
SprecherH
KolattukudyPE
2001 The Mycobacterium tuberculosis pks2 gene encodes the synthase for the hepta - and octamethyl-branched fatty acids required for sulfolipid synthesis. J Biol Chem 276 16833 16839
36. ConversePJ
KarakousisPC
KlinkenbergLG
KesavanAK
LyLH
2009 Role of the dosR-dosS two-component regulatory system in Mycobacterium tuberculosis virulence in three animal models. Infect Immun 77 1230 1237
37. NiemannS
KoserCU
GagneuxS
PlinkeC
HomolkaS
2009 Genomic diversity among drug sensitive and multidrug resistant isolates of Mycobacterium tuberculosis with identical DNA fingerprints. PLoS One 4 e7407
38. FirmaniMA
RileyLW
2002 Reactive nitrogen intermediates have a bacteriostatic effect on Mycobacterium tuberculosis in vitro. J Clin Microbiol 40 3162 3166
39. Chacon-SalinasR
Serafin-LopezJ
Ramos-PayanR
Mendez-AragonP
Hernandez-PandoR
2005 Differential pattern of cytokine expression by macrophages infected in vitro with different Mycobacterium tuberculosis genotypes. Clin Exp Immunol 140 443 449
40. ParkJS
TamayoMH
Gonzalez-JuarreroM
OrmeIM
OrdwayDJ
2006 Virulent clinical isolates of Mycobacterium tuberculosis grow rapidly and induce cellular necrosis but minimal apoptosis in murine macrophages. J Leukoc Biol 79 80 86
41. TorrellesJB
KnaupR
KolarethA
SlepushkinaT
KaufmanTM
2008 Identification of Mycobacterium tuberculosis clinical isolates with altered phagocytosis by human macrophages due to a truncated lipoarabinomannan. J Biol Chem 283 31417 31428
42. Boschi-MullerS
AzzaS
BranlantG
2001 E. coli methionine sulfoxide reductase with a truncated N terminus or C terminus, or both, retains the ability to reduce methionine sulfoxide. Protein Sci 10 2272 2279
43. LopezB
AguilarD
OrozcoH
BurgerM
EspitiaC
2003 A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol 133 30 37
44. MancaC
TsenovaL
BergtoldA
FreemanS
ToveyM
2001 Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proc Natl Acad Sci U S A 98 5752 5757
45. GagneuxS
SmallPM
2007 Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis 7 328 337
46. GutierrezMC
BrisseS
BroschR
FabreM
OmaisB
2005 Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis. PLoS Pathog 1 e5
47. JoshiSM
PandeyAK
CapiteN
FortuneSM
RubinEJ
2006 Characterization of mycobacterial virulence genes through genetic interaction mapping. Proc Natl Acad Sci U S A 103 11760 11765
48. KendallSL
WithersM
SoffairCN
MorelandNJ
GurchaS
2007 A highly conserved transcriptional repressor controls a large regulon involved in lipid degradation in Mycobacterium smegmatis and Mycobacterium tuberculosis. Mol Microbiol 65 684 699
49. SenaratneRH
SiddersB
SequeiraP
SaundersG
DunphyK
2008 Mycobacterium tuberculosis strains disrupted in mce3 and mce4 operons are attenuated in mice. J Med Microbiol 57 164 170
50. Van der GeizeR
YamK
HeuserT
WilbrinkMH
HaraH
2007 A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci U S A 104 1947 1952
51. GuptaS
JainS
TyagiAK
1999 Analysis, expression and prevalence of the Mycobacterium tuberculosis homolog of bacterial virulence regulating proteins. FEMS Microbiol Lett 172 137 143
52. SinghA
GuptaR
VishwakarmaRA
NarayananPR
ParamasivanCN
2005 Requirement of the mymA operon for appropriate cell wall ultrastructure and persistence of Mycobacterium tuberculosis in the spleens of guinea pigs. J Bacteriol 187 4173 4186
53. SinghA
JainS
GuptaS
DasT
TyagiAK
2003 mymA operon of Mycobacterium tuberculosis: its regulation and importance in the cell envelope. FEMS Microbiol Lett 227 53 63
54. CoxJS
ChenB
McNeilM
JacobsWRJr
1999 Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402 79 83
55. DomenechP
ReedMB
DowdCS
MancaC
KaplanG
2004 The role of MmpL8 in sulfatide biogenesis and virulence of Mycobacterium tuberculosis. J Biol Chem 279 21257 21265
56. MurryJP
PandeyAK
SassettiCM
RubinEJ
2009 Phthiocerol dimycocerosate transport is required for resisting interferon-gamma-independent immunity. J Infect Dis 200 774 782
57. CamachoLR
ConstantP
RaynaudC
LaneelleMA
TriccasJA
2001 Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. J Biol Chem 276 19845 19854
58. JainM
PetzoldCJ
SchelleMW
LeavellMD
MougousJD
2007 Lipidomics reveals control of Mycobacterium tuberculosis virulence lipids via metabolic coupling. Proc Natl Acad Sci U S A 104 5133 5138
59. SinghA
CrossmanDK
MaiD
GuidryL
VoskuilMI
2009 Mycobacterium tuberculosis WhiB3 maintains redox homeostasis by regulating virulence lipid anabolism to modulate macrophage response. PLoS Pathog 5 e1000545
60. GohKS
RastogiN
BerchelM
HuardRC
SolaC
2005 Molecular evolutionary history of tubercle bacilli assessed by study of the polymorphic nucleotide within the nitrate reductase (narGHJI) operon promoter. J Clin Microbiol 43 4010 4014
61. StermannM
SedlacekL
MaassS
BangeFC
2004 A promoter mutation causes differential nitrate reductase activity of Mycobacterium tuberculosis and Mycobacterium bovis. J Bacteriol 186 2856 2861
62. WayneLG
HayesLG
1998 Nitrate reduction as a marker for hypoxic shiftdown of Mycobacterium tuberculosis. Tuber Lung Dis 79 127 132
63. ChauhanS
SinghA
TyagiJS
2010 A single-nucleotide mutation in the -10 promoter region inactivates the narK2X promoter in Mycobacterium bovis and Mycobacterium bovis BCG and has an application in diagnosis. FEMS Microbiol Lett 303 190 196
64. StermannM
BohrssenA
DiephausC
MaassS
BangeFC
2003 Polymorphic nucleotide within the promoter of nitrate reductase (NarGHJI) is specific for Mycobacterium tuberculosis. J Clin Microbiol 41 3252 3259
65. FritzC
MaassS
KreftA
BangeFC
2002 Dependence of Mycobacterium bovis BCG on anaerobic nitrate reductase for persistence is tissue specific. Infect Immun 70 286 291
66. SohaskeyCD
WayneLG
2003 Role of narK2X and narGHJI in hypoxic upregulation of nitrate reduction by Mycobacterium tuberculosis. J Bacteriol 185 7247 7256
67. ParwatiI
van CrevelR
van SoolingenD
2010 Possible underlying mechanisms for successful emergence of the Mycobacterium tuberculosis Beijing genotype strains. Lancet Infect Dis 10 103 111
68. FallowA
DomenechP
ReedMB
2010 Strains of the East Asian (W/Beijing) lineage of Mycobacterium tuberculosis are DosS/DosT-DosR two-component regulatory system natural mutants. J Bacteriol 192 2228 2238
69. HonakerRW
LeistikowRL
BartekIL
VoskuilMI
2009 Unique roles of DosT and DosS in DosR regulon induction and Mycobacterium tuberculosis dormancy. Infect Immun 77 3258 3263
70. KumarA
ToledoJC
PatelRP
LancasterJRJr
SteynAJ
2007 Mycobacterium tuberculosis DosS is a redox sensor and DosT is a hypoxia sensor. Proc Natl Acad Sci U S A 104 11568 11573
71. CheepsattayakornA
CheepsattayakornR
2009 Human genetic influence on susceptibility of tuberculosis: from infection to disease. J Med Assoc Thai 92 136 141
72. HirshAE
TsolakiAG
DeRiemerK
FeldmanMW
SmallPM
2004 Stable association between strains of Mycobacterium tuberculosis and their human host populations. Proc Natl Acad Sci U S A 101 4871 4876
73. ReedMB
PichlerVK
McIntoshF
MattiaA
FallowA
2009 Major Mycobacterium tuberculosis lineages associate with patient country of origin. J Clin Microbiol 47 1119 1128
74. CohenT
ColijnC
MurrayM
2008 Modeling the effects of strain diversity and mechanisms of strain competition on the potential performance of new tuberculosis vaccines. Proc Natl Acad Sci U S A 105 16302 16307
75. ButcherPD
ManganJA
MonahanIM
1998 Intracellular gene expression. Analysis of RNA from mycobacteria in macrophages using RT-PCR. Methods Mol Biol 101 285 306
76. ManganJA
MonahanIM
ButcherPD
2002 Gene Expression during host-pathogen interactions: approaches to bacterial mRNA extraction and labeling for microarray analysis.
WrenBW
DorrellN
Methods in Microbiology London Academic Press 137 151
77. EdgarR
DomrachevM
LashAE
2002 Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30 207 210
78. ReddyTB
RileyR
WymoreF
MontgomeryP
DeCaprioD
2009 TB database: an integrated platform for tuberculosis research. Nucleic Acids Res 37 D499 508
79. LivakKJ
SchmittgenTD
2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 402 408
80. KendallSL
MovahedzadehF
RisonSC
WernischL
ParishT
2004 The Mycobacterium tuberculosis dosRS two-component system is induced by multiple stresses. Tuberculosis (Edinb) 84 247 255
81. KumarA
DeshaneJS
CrossmanDK
BolisettyS
YanBS
2008 Heme oxygenase-1-derived carbon monoxide induces the Mycobacterium tuberculosis dormancy regulon. J Biol Chem 283 18032 18039
82. ParkHD
GuinnKM
HarrellMI
LiaoR
VoskuilMI
2003 Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol 48 833 843
83. VoskuilMI
SchnappingerD
ViscontiKC
HarrellMI
DolganovGM
2003 Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med 198 705 713
84. KhanA
SarkarD
2006 Identification of a respiratory-type nitrate reductase and its role for survival of Mycobacterium smegmatis in Wayne model. Microb Pathog 41 90 95
85. WeberI
FritzC
RuttkowskiS
KreftA
BangeFC
2000 Anaerobic nitrate reductase (narGHJI) activity of Mycobacterium bovis BCG in vitro and its contribution to virulence in immunodeficient mice. Mol Microbiol 35 1017 1025
86. GagneuxS
DeRiemerK
VanT
Kato-MaedaM
de JongBC
2006 Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 103 2869 2873
87. BroschR
GordonSV
MarmiesseM
BrodinP
BuchrieserC
2002 A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A 99 3684 3689
88. MarmiesseM
BrodinP
BuchrieserC
GutierrezC
SimoesN
2004 Macro-array and bioinformatic analyses reveal mycobacterial ‘core’ genes, variation in the ESAT-6 gene family and new phylogenetic markers for the Mycobacterium tuberculosis complex. Microbiology 150 483 496
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



