-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
N-glycan Core β-galactoside Confers Sensitivity towards Nematotoxic Fungal Galectin CGL2
The physiological role of fungal galectins has remained elusive. Here, we show that feeding of a mushroom galectin, Coprinopsis cinerea CGL2, to Caenorhabditis elegans inhibited development and reproduction and ultimately resulted in killing of this nematode. The lack of toxicity of a carbohydrate-binding defective CGL2 variant and the resistance of a C. elegans mutant defective in GDP-fucose biosynthesis suggested that CGL2-mediated nematotoxicity depends on the interaction between the galectin and a fucose-containing glycoconjugate. A screen for CGL2-resistant worm mutants identified this glycoconjugate as a Galβ1,4Fucα1,6 modification of C. elegans N-glycan cores. Analysis of N-glycan structures in wild type and CGL2-resistant nematodes confirmed this finding and allowed the identification of a novel putative glycosyltransferase required for the biosynthesis of this glycoepitope. The X-ray crystal structure of a complex between CGL2 and the Galβ1,4Fucα1,6GlcNAc trisaccharide at 1.5 Å resolution revealed the biophysical basis for this interaction. Our results suggest that fungal galectins play a role in the defense of fungi against predators by binding to specific glycoconjugates of these organisms.
Published in the journal: . PLoS Pathog 6(1): e32767. doi:10.1371/journal.ppat.1000717
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000717Summary
The physiological role of fungal galectins has remained elusive. Here, we show that feeding of a mushroom galectin, Coprinopsis cinerea CGL2, to Caenorhabditis elegans inhibited development and reproduction and ultimately resulted in killing of this nematode. The lack of toxicity of a carbohydrate-binding defective CGL2 variant and the resistance of a C. elegans mutant defective in GDP-fucose biosynthesis suggested that CGL2-mediated nematotoxicity depends on the interaction between the galectin and a fucose-containing glycoconjugate. A screen for CGL2-resistant worm mutants identified this glycoconjugate as a Galβ1,4Fucα1,6 modification of C. elegans N-glycan cores. Analysis of N-glycan structures in wild type and CGL2-resistant nematodes confirmed this finding and allowed the identification of a novel putative glycosyltransferase required for the biosynthesis of this glycoepitope. The X-ray crystal structure of a complex between CGL2 and the Galβ1,4Fucα1,6GlcNAc trisaccharide at 1.5 Å resolution revealed the biophysical basis for this interaction. Our results suggest that fungal galectins play a role in the defense of fungi against predators by binding to specific glycoconjugates of these organisms.
Introduction
Lectins are defined as non-immunoglobulin, carbohydrate-binding proteins without catalytic activity towards the recognized carbohydrate [1]. They occur in all domains of life and are very diverse with respect to structure, carbohydrate-binding specificity and function [2]. Specific lectins play an important role in the defense against predators, parasites and pathogens due to their ability to recognize specific carbohydrate signatures displayed on the cell surfaces of these organisms. They act either as direct effectors or as opsonins, i.e., by recruiting other effectors of the immune system. Examples of the former type are bacterial toxins directed against bacterivores (Bacillus thuringiensis crystal toxins), plant lectins directed against herbivores (Ricinus communis (Ricin) toxin), and mammalian lectins directed against bacterial and fungal mucosal pathogens (RegIIIγ [3] and galectin-3 [4], respectively). The effector role of all these lectins is based on their toxicity towards the predator or pathogen. In only a few cases is the molecular basis of this toxicity known in terms of identification of the target glycoconjugate in the predator/pathogen recognized by the lectin. For example, the nematicidal crystal toxin Cry5B from B. thuringiensis was shown to bind a specific glycosphingolipid on the intestinal epithelium of the bacterivorous nematode Caenorhabditis elegans [5] and antifungal mammalian galectin-3 was shown to bind β1,2-linked mannans on the surface of Candida albicans cells [4]. It should be noted here that Ricin and many bacterial toxins contain additional domains with pore-forming or catalytic activity which contribute to the toxicity of these lectins [6].
Fungal fruiting-bodies are a rich source for lectins of various structures and specificities [7],[8],[9],[10]. A specific subgroup of fungal lectins, the so-called fruiting-body lectins, are characterized by their specific expression and abundance in the fruiting body and their cytoplasmic subcellular localization. The latter property is concluded from the absence of a signal peptide for classical secretion and the concomitant lack of disulphide bridges or protein glycosylation. The physiological function of these fruiting body lectins, however, remains unknown. A suggested role in fruiting body formation, based on the developmental regulation of their expression, has become unlikely based on recent studies in the ascomycete (sac fungus) Sordaria macrospora and the homobasidiomycete (mushroom) Coprinopsis cinerea [11],[12]. Given the frequent role of lectins in defense of other organisms and the reported toxicity of fruiting-body lectins towards insects and mammalian cells [13],[14],[15],[16],[17],[18],[19], we and others hypothesized that this group of lectins may be effectors of a fungal defense system against fungivores. Examples for predators that have specialized on fungi as food source are larvae of some flies and fungal-feeding nematodes [20],[21],[22]. In comparison to insects, fungal-feeding nematodes represent, due to their high number in soil and any kind of decaying organic matter, a major threat to fungi in their ecological environment [23],[24].
The fungus C. cinerea expresses several fruiting body lectins including two isogalectins, CGL1 and CGL2, and a galectin-related lectin, CGL3 [25],[26],[27]. In agreement with above hypothesis, we demonstrate that both C. cinerea isogalectins displayed toxicity towards the model soil nematode C. elegans. Based on a number of approaches, including the use of E. coli expressing these fungal lectins to mimic the in vivo situation, we conclude that this nematotoxicity is dependent on the specific interaction between these proteins and a β-galactoside on the core of N-glycans in the C. elegans intestine.
Results
CGL2 inhibits C. elegans development and reproduction dependent on its carbohydrate-binding ability
To test the C. cinerea galectins for nematotoxic activity in a genetically tractable system, the bacterivorous model nematode C. elegans was fed with E. coli cells expressing either the authentic CGL1 and CGL2 proteins or the carbohydrate-binding defective variant CGL2(W72G) in the cytoplasm in comparison to ‘empty vector’-containing E. coli transformants. The essential role of W72 in the coordination of β-galactosides by CGL2 was demonstrated previously [28]. For CGL2 and CGL2(W72G), the effect of this diet on C. elegans development, reproduction and survival was examined by determining the fraction of L1 larvae developing to the L4 stage within 72 h, the brood size per hermaphrodite and the survival of L4 larvae, respectively. The results of all three assays showed a strong effect of the fungal galectin, i.e., the animals that fed on CGL2-expressing bacteria were severely inhibited in larval development and showed a reduced brood size and survival (Fig. 1, panels A to C). The nematotoxicity was dependent on the ability of CGL2 to bind carbohydrates since the CGL2(W72G) variant, which does not possess β-galactoside binding activity [28], did not show any effect in either assay. For CGL1, only larval development was scored, yielding similar results as in the case of CGL2 (Fig. 1A, left panel).
Fig. 1. Dose and carbohydrate-binding dependent toxicity of C. cinerea galectin CGL2 towards C. elegans. 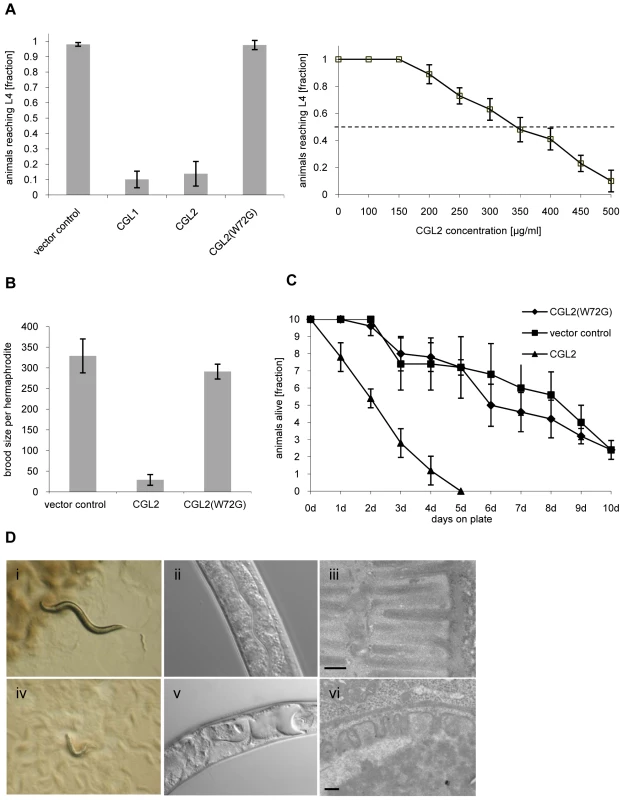
In all of the experiments, E. coli BL21(DE3) cells expressing either the authentic CGL1 or CGL2 proteins or the carbohydrate-binding defective variant CGL2(W72G), or control transformants were fed to C. elegans wild type N2. (A) CGL2 inhibits C. elegans development. C. elegans L1 larvae were seeded onto lawns of above bacteria (left panel) or fed with increasing concentrations of purified CGL2 together with equal amounts of empty vector-containing BL21(DE3) in liquid culture (right panel) and scored for the fraction developing to the L4 stage within 72 h and 96 h, respectively. Columns represent the average of 6 and 12 replicates, respectively. Error bars indicate standard deviations. The fraction of animals reaching L4 was significantly lower on CGL1- or CGL2-expressing bacteria (p<0.01) than on bacteria expressing CGL2(W72G) or containing empty vector. No significant difference was observed between latter two conditions (p>0.5). In the liquid assay, worm development decreases significantly at CGL2 concentrations higher than 150 µg/ml. (B) CGL2 inhibits C. elegans reproduction. C. elegans hermaphrodites were placed as L4 animals on plates seeded with above bacteria and scored for total progeny counts per hermaphrodite. The broods of eight to ten hermaphrodites were averaged per data point. The standard deviations are indicated. The differences between the L4 fractions on CGL2 and vector control and CGL2(W72G) were statistically significant (p>0.01). None of the progeny on wild type CGL2 developed to adulthood within 96 h post hatch. (C) CGL2 ultimately kills C. elegans. 10 L4 staged wild type C. elegans (N2) were seeded onto lawns of CGL2-, CGL2(W72G)-expressing and empty vector control-containing E. coli BL21(DE3) cells. Each day, the plates were checked for surviving animals which were then transferred to novel bacterial lawns of the same type. The data points represent the average of five replicates. Error bars indicate standard deviations. The survival rate of the worms was significantly impaired on CGL2-expressing bacteria compared to CGL2(W72G) and vector control (p<0.01), whereas there was no significant difference between latter two conditions (p>0.5). (D) CGL2 damages C. elegans intestine. C. elegans L4 larvae were fed with CGL2-expressing (panels i-vi) and control E. coli BL21(DE3) cells (panels i-iii) and examined after 24 h under the stereomicroscope (panels i,iv), by differential interference contrast (DIC) microscopy (panels ii, v) and by transmission electron microscopy (TEM) (panels iii,vi). The size bars in panels iii and vi are 200 nm. The effect of using bacteria expressing CGL2 was verified using the purified recombinant lectin. Incubation of L1 larvae with different concentrations of purified CGL2 with empty vector-containing bacteria demonstrated that the lectin-mediated arrest in larval development was dose-dependent with a median lethal dose (LD50) of approximately 350 µg/ml (Fig. 1, panel A right). Comparative light - and electron-microscopy of C. elegans L4 larvae fed with CGL2-expressing or empty vector-containing E. coli cells revealed that the lumen of the anterior intestine was expanded in the former animals and that the microvillar ultrastructure of the epithelium was damaged (Fig. 1, panel D). These results demonstrated that the C. cinerea galectin CGL2 had a pronounced nematotoxic activity that was dependent on the ability of the protein to bind carbohydrates.
The CGL2-ligand in C. elegans is different from the Cry5B-ligand but its biosynthesis also relies on fucose biosynthesis
The phenotype of CGL2-mediated nematotoxicity was reminiscent of the intoxication of C. elegans induced by feeding with E. coli cells expressing the nematicidal pore-forming crystal toxin Cry5B from Bacillus thuringiensis [29]. Indicative of the role of carbohydrate binding in their function, crystal toxins contain lectin domains that are necessary for toxicity [30] and indeed a genetic screen for Cry5B-resistant C. elegans mutants yielded mutations in five different genes, bre-1(ye4), bre-2(ye31), bre-3(ye26), bre-4(ye27) and bre-5(ye17) [29]. Four of these C. elegans genes code for glycosyltransferases involved in the biosynthesis of a specific, β-galactoside-containing glycosphingolipid (bre-2 to bre-5) and one gene encodes an enzyme involved in the conversion of GDP-mannose into GDP-fucose (bre-1) [31]. Given the specificity of C. cinerea galectins for β-galactoside-containing oligosaccharides [27],[28], we tested each bre mutant for resistance to CGL2. Unexpectedly, we found that the bre-1 mutation conferred almost complete resistance whereas the other bre mutants, with the exception of the bre-4 mutant, that was as sensitive as wild type C. elegans, appeared even more sensitive to CGL2 than wild type (Fig. 2). The bre-1(ye4) mutant also showed resistance towards CGL1-mediated nematotoxicity suggesting that CGL1 acts via the same mechanism (data not shown). These results demonstrated that the ligand of C. cinerea galectins in C. elegans was different from the Cry5B-ligand but that its biosynthesis relied on fucose biosynthesis as in the case of the bacterial toxin.
Fig. 2. Resistance of bre-1 mutant towards CGL2-mediated toxicity. 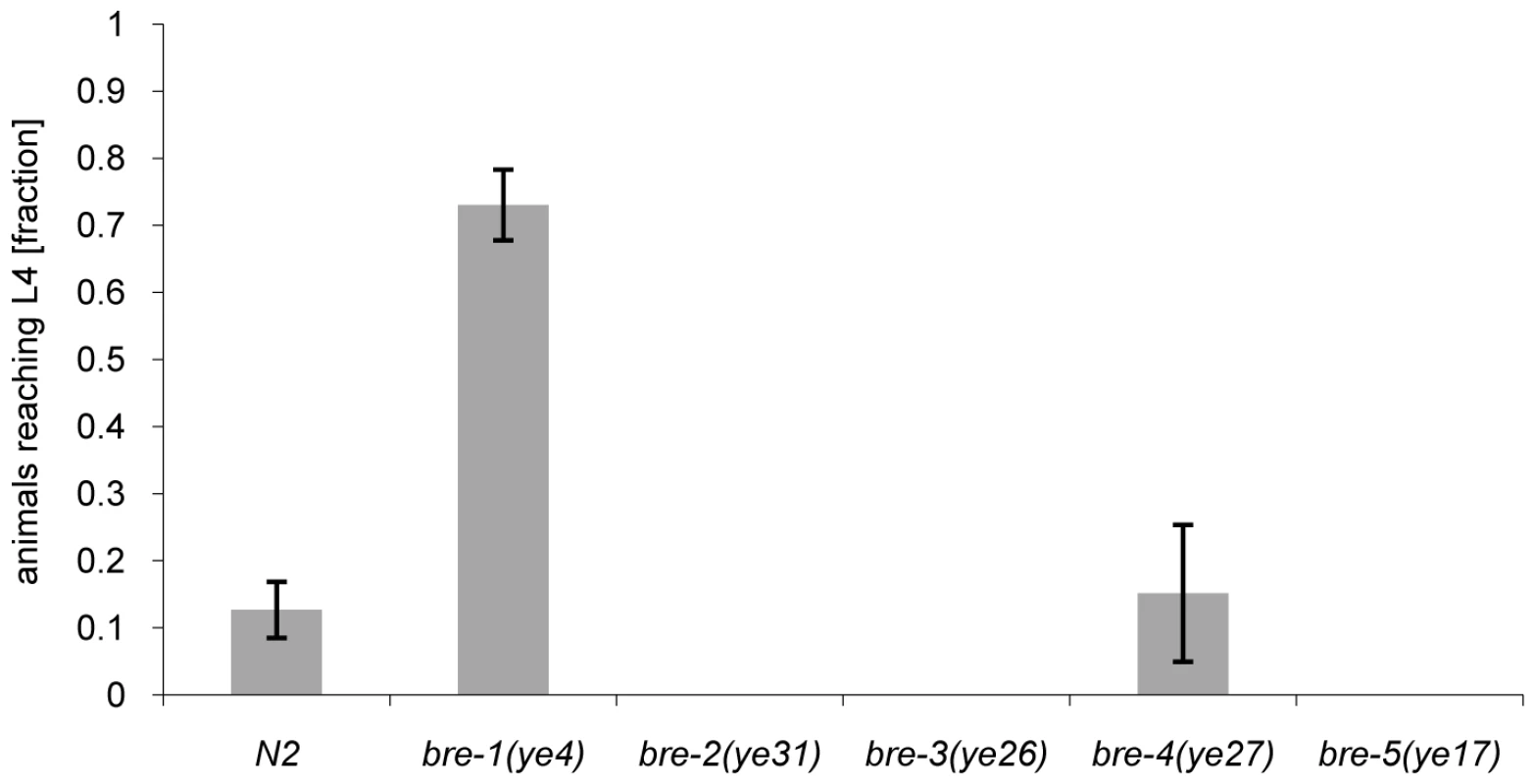
L1 larvae of C. elegans bre-1 to bre-5 mutant strains as well as wild-type N2 were seeded onto a lawn of CGL2-expressing E. coli BL21(DE3) cells and scored for the fraction developing to the L4 stage within 72 h. Columns represent the average of 6 replicates. Error bars indicate standard deviations. The fraction of animals reaching L4 was significantly higher for the bre-1 mutant (p<0.01) than for the wild type (N2) or the bre-4 mutant. No significant difference was observed between latter two strains (p>0.5). For the rest of the mutants, not a single larva developed, so results were not compared statistically. Mutations in the C. elegans p38 MAPK pathway cause hypersensitivity to CGL2
Based on the resistance of the C. elegans bre-1(ye4) mutant towards feeding of CGL2-expressing bacteria we initiated a forward genetic screen for mutations conferring CGL2-resistance in order to identify the target glycoconjugate of CGL2 in C. elegans. For an efficient selection of CGL2-resistant mutants, it was essential to lower the background of 10% surviving worms in case of C. elegans wild type strain (N2) (Fig. 4, panel A). Since mutants in the p38 mitogen-activated protein kinase (MAPK) pathway of C. elegans were previously shown to be hypersensitive towards pathogenic bacteria and Cry5B-expressing E. coli cells [32],[33], we tested whether these strains would also be more susceptible towards feeding with the CGL2-expressing E. coli cells. For this purpose, L4 larvae derived from N2 wild type and isogenic pmk-1(km25) (p38 MAPK), sek-1(ag1) (MAPK kinase) and nsy-1(ag3) (MAPK kinase kinase) mutant worms were tested for survival on a lawn of CGL2-expressing bacteria over time. We found that all of the mutations increased the sensitivity of C. elegans towards the fungal galectin but that the effect of the pmk-1 mutation was most pronounced (Fig. 3, panel A). As a proof of principle for the subsequent screen, we constructed a worm carrying the bre-1(ye4) mutation in a pmk-1(km25) mutant genetic background and tested this strain for its sensitivity to CGL2 in comparison to the pmk-1(km25) single mutant strain. The double mutant strain exhibited significant resistance towards CGL2 confirming the previous results in the wild type background and suggesting that a forward genetic screen for mutations conferring CGL2-resistance in a pmk-1(km25) mutant genetic background was feasible (Fig. 4, panel A). The screen, as outlined in Fig. 3 (panel B), was based on an insertional mutagenesis using the Mos1 transposon to facilitate the identification of the obtained mutations.
Fig. 3. Workflow of the forward genetic screen for CGL2-resistant C. elegans mutants. 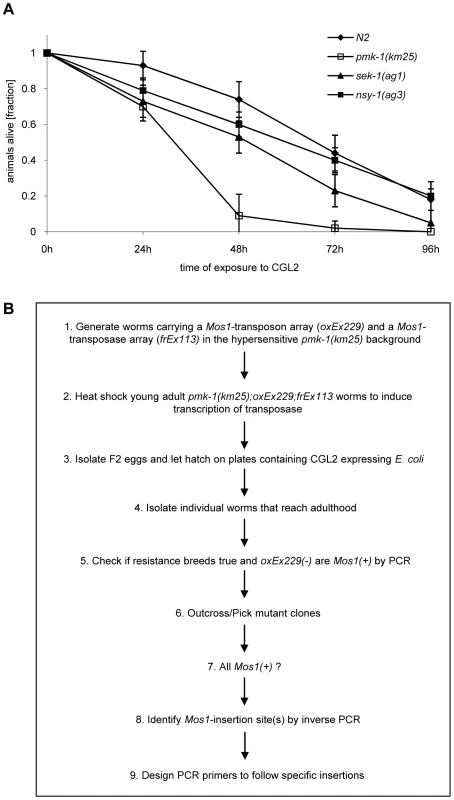
(A) CGL2-sensitivity test of pmk-1, sek-1 and nsy-1 mutant worms defective in the p38 MAPK pathway. 10 L4 staged worms of the indicated genotypes were seeded onto a lawn of CGL2-expressing E. coli BL21(DE3) cells. The plates were checked for surviving animals at the indicated time points. The data points represent the average of ten replicates. Error bars indicate standard deviations. The pmk-1 and sek-1 mutants were significantly hypersensitive compared to the N2 wild type strain (p<0.01), whereas the slightly higher sensitivity of the nsy-1 mutant is less significant (p<0.2). (B) Mos1 insertional mutagenesis workflow. Worms that carry the Mos1 transposon array oxEx229 and the Mos1 transposase array frEx113 in the CGL2-hypersensitive pmk-1(km25) background were generated. Fig. 4. Results of the forward genetic screen for CGL2-resistant C. elegans mutants. 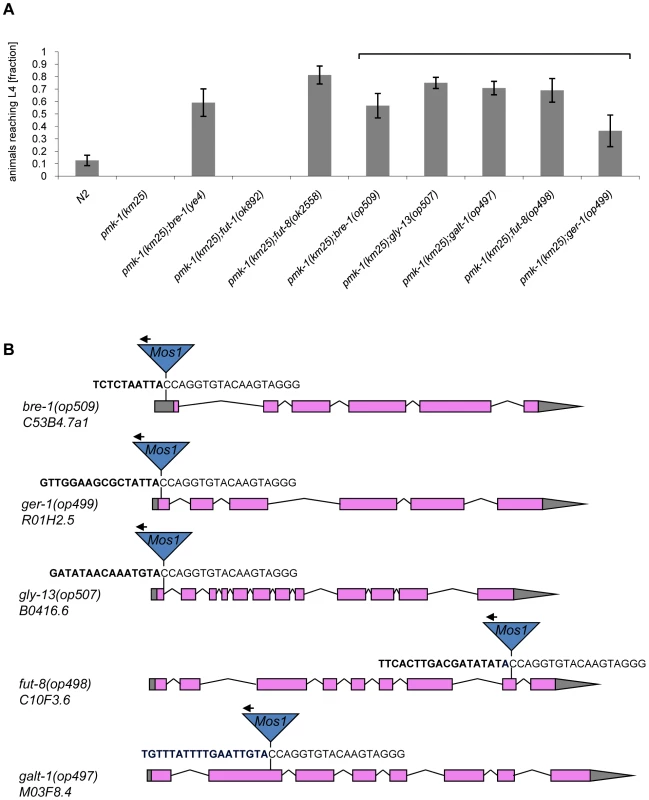
(A) Resistance of isolated and constructed C. elegans mutants towards CGL2-mediated toxicity. C. elegans mutants of the indicated genotypes were analysed for development from L1 to L4 as outlined above. The gene appendix (op) and the bracket above the histogram indicate mutants isolated in the Mos1-screen. The other mutants were constructed by crossing. The increase in the fractions of animals reaching L4 of the various mutants compared to N2 worms was statistically significant (p<0.05). In case of the pmk-1 and the pmk-1;fut-1 mutant, not a single larva developed, so results were not compared statistically. (B) Insertion sites of Mos1 elements in CGL2-resistant mutants. Arrows above Mos1 elements indicate the orientation of the Mos1 primer oJL115 used for sequencing iPCR products of mutant lysates. Bold letters indicate C. elegans genomic sequences and are followed by Mos1 sequence. Gene models are taken from WormBase Release WS207. bre-1(op509) mutants have a Mos1 insertion in the 5′-UTR of C53B4.7a1, located 184 bp upstream of the translational start codon. ger-1(op499) mutants have a Mos1 insertion in the first exon of R01H2.5, located 50 bp downstream of the translational start codon. gly-13(op507) mutants have a Mos1 insertion in a conserved splicing donor site flanking the first exon of B0416.6. fut-8(op498) mutants have a Mos1 insertion in the eighth exon of C10F3.6. galt-1(op497) mutants have a Mos1 insertion in the second exon of M03F8.4. Mutations affecting α1,6-core fucosylation of C. elegans N-glycans confer resistance to CGL2 and prevent binding of CGL2 to the intestine
The screen yielded Mos1-insertions in five different genes: bre-1, ger-1, gly-13, fut-8 and M03F8.4. The degree of resistance towards CGL2-intoxication was determined for each of the outcrossed mutants and is shown in Fig. 4 (panel A). The identified Mos1-insertions are located in 5′-untranslated regions (bre-1(op509)), at an exon-intron junction (gly-13(op507)) and in exons (ger-1(op499), fut-8(op498) and M03F8.4(op497)), respectively (Fig. 3, panel D). bre-1 and ger-1 code for the GDP-mannose-4,6-dehydratase and GDP-4-keto-6-deoxymannose-3,5-epimerase-4-reductase, respectively, catalyzing the two enzymatic steps in the conversion from GDP-mannose to GDP-fucose [34]. The link between the biosynthesis of GDP-fucose and the biosynthesis of a specific glycoconjugate was offered by the data indicating a role for the fut-8 and gly-13 genes in toxicity, suggesting that N-glycans are the targets of CGL2. The fut-8 gene encodes the only fucosyltransferase capable of transferring fucose in α1,6-configuration from GDP-fucose to the asparagine-linked GlcNAc in C. elegans N-glycan cores [35], whereas the gly-13 gene codes for the major GlcNAc-transferase I (GnTI) in C. elegans [36], which transfers GlcNAc in β1,2-configuration to the α1,3-linked mannose of pentamannosidic N-glycan. The modification of N-glycans by GnTI is the prerequisite for many of the subsequent modifications of N-glycans including the action of core α1,6-fucosyltransferase [37]; on the other hand, GnTI is not required in C. elegans for the core modification by another fucosyltransferase, encoded by the fut-1 gene, which forms the so-called HRP epitope consisting of fucose α1,3-linked to the core asparagine-linked GlcNAc [38].
In order to confirm and corroborate these data, two available mutations affecting both types of core fucosylation, fut-8(ok2558) and fut-1(ok892), were each crossed into the pmk-1 mutant background and the resulting double mutants were tested for CGL2-sensitivity. In agreement with the results of the forward genetic screen, the fut-8(ok2558) mutation conferred clear resistance towards CGL2, recapitulating the phenotype shown by the fut-8(op498) allele, whereas the fut-1 pmk-1 double mutant was as susceptible as the pmk-1 single mutant (Fig. 4, panel A). Analogous tests for CGL1-sensitivity yielded similar results providing additional evidence that CGL1 and CGL2 confer nematotoxicity via the same mechanism (data not shown).
In addition, available mutants of all other characterized C. elegans fucosyltransferase (fut) - and N-acetylglucosaminyltransferase (gly)-encoding genes were tested for CGL2-sensitivity in comparison to wild type N2 worms. With exception of fut-8(ok2558) and gly-13(ok712) and consistent with the putative biosynthetic roles of the relevant genes, none of the tested mutations conferred resistance in this background suggesting that the effects of the mutations identified in the screen were highly specific (Fig. 5). In the case of gly-13, the tested strain background carried a mutation in an additional gene, dpy-6, that is required for normal body morphology but is not involved in glycosylation [39]. Thus, all of the identified genes conferring CGL2-resistance, with the exception of the previously uncharacterized gene M03F8.4, suggested a specific role of α1,6-linked fucosylation at the asparagine-linked GlcNAc of C. elegans N-glycans in CGL2-mediated nematotoxicity.
Fig. 5. CGL2-sensitivity test and biosynthetic context of available C. elegans fucosyltransferase and GlcNAc-transferase mutants. 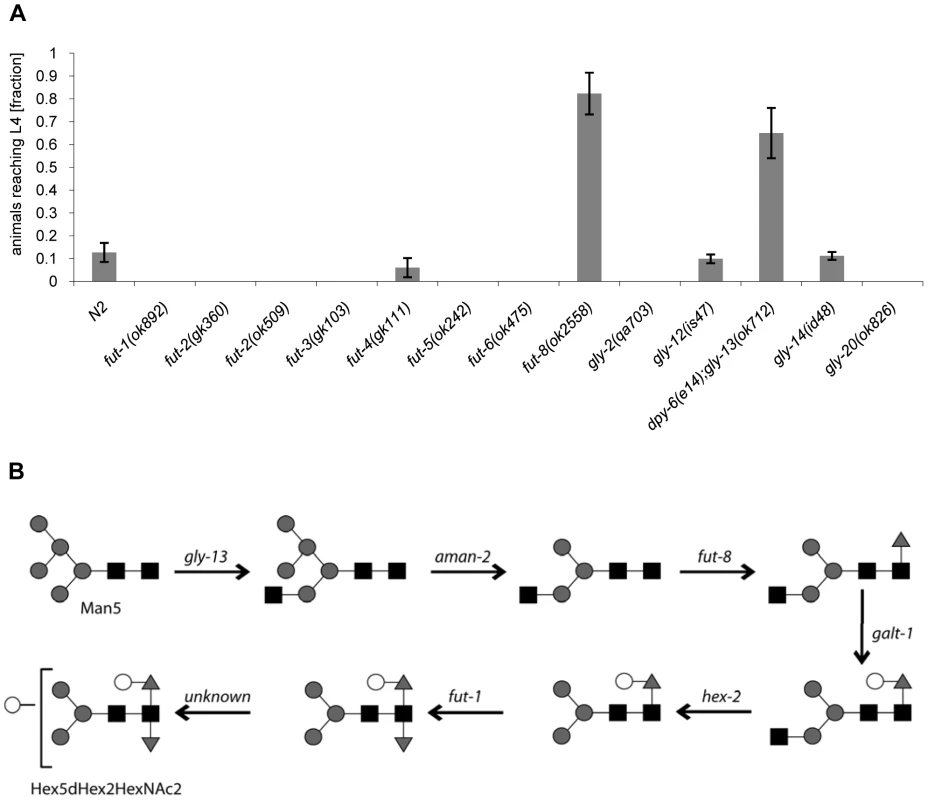
(A) CGL2-sensitivity test. C. elegans mutants of the indicated genotypes were analysed for development from L1 to L4 as outlined above. The differences between the L4 fractions of the fut-4(gk111) and dpy-6(e14);gly-13(ok712) mutants to N2 are statistically significant (p<0.01) in contrast to the differences between the L4 fractions of the fut8(ok2558), gly-12(is47) and gly-14(id48) mutants to N2 (p<0.9). The differences of the residual mutants to N2 could not be evaluated due to the lack of variance. (B) Biosynthetic context. The putative pathway for biosynthesis of the CGL2 epitope, based on previous in vivo and in vitro data, indicates the key roles of the enzymes encoded by the gly-13, fut-8 and galt-1(M03F8.4) genes; on the other hand, FUT-1 acts after the processing by hexosaminidases such as HEX-2. Other fucosyltransferases such as FUT-2 through to FUT-6 are not involved in modification of the reducing-terminal GlcNAc residue of N-glycans, whereas the gly-2 and gly-20 encoded enzymes are not prerequisites for α1,6-fucosylation by FUT-8. Further modifications by other enzymes, encoded by unknown genes, are possible and result in structures such as the depicted Hex5dHex2HexNAc2 glycan. To confirm these data and localize the target glycoconjugate of CGL2 in situ, L1 larvae of pmk-1 and pmk-1;fut-8(op498) animals were incubated for 24 h in a solution containing tetramethylrhodamine(TAMRA)-labeled CGL2, transferred to plates seeded with standard OP50 bacteria and examined by fluorescence microscopy thereafter. The pictures revealed a distinct red fluorescent signal on the intestinal epithelium of pmk-1 but not of pmk-1;fut-8(op498) animals suggesting that core α1,6-fucosylated N-glycans are the in vivo ligands of CGL2 and that this glycoepitope recognized by CGL2 localizes mainly to the intestinal epithelium of C. elegans (Supplementary Table S1; Fig. 6).
Fig. 6. In situ localization of the glycoepitope recognized by CGL2. 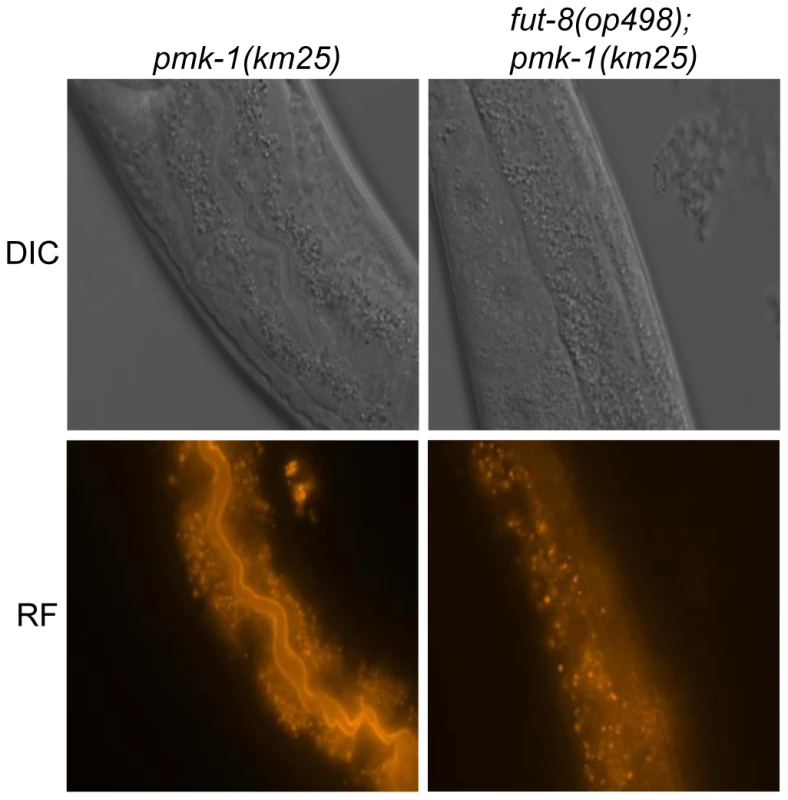
C. elegans CGL2-sensitive pmk-1(km25) and CGL2-resistant pmk-1(km25);fut-8(op498) worms were fed with TAMRA-labeled CGL2 and examined by differential interference contrast (DIC) and red fluorescence (RF) microscopy. 32 and 25 animals, respectively, were scored for staining of the intestinal epithelium (see Supplementary Table S1). The numbers of worms with stained intestinal epithelium was significantly different between the two genetic backgrounds (p<0.01). Identified M03F8.4 mutants are defective in galactose capping of α1,6-core fucose in C. elegans N-glycans
Based on these results, the specificity of CGL2 for β-galactosides and the reported capping of core α1,6-fucose on C. elegans N-glycans with galactose residues in β1,4-configuration [40], we hypothesized that binding of CGL2 to this Galβ1,4Fucα1,6 epitope was required for its nematotoxicity. We surmised that the product of the M03F8.4 gene, which encodes a putative type II membrane protein with a predicted glycosyltransferase GT-A domain [41] may be, directly or indirectly, involved in the biosynthesis of this N-glycan core modification. In order to validate this hypothesis, the N-glycomes of the pmk-1 single mutant and the identified CGL2-resistant pmk-1(km25);fut-8(op498) and pmk-1(km25);M03F8.4(op497) double mutants were analysed. N-glycans were released sequentially by PNGase F and PNGase A to separate core α1,3-fucosylated structures from those devoid of this epitope, labeled with 2-aminopyridine (PA) and analysed by comparative two-dimensional HPLC combined with MALDI-TOF mass spectrometric and enzymatic analysis of the individual glycans. N-glycans of the analysed strains showed significant differences in the normal phase 1st-dimension chromatograms (Fig. 7, panel A). Among the PNGase F resistant N-glycans, i.e. those bearing an α1,3 fucose at the reducing end GlcNAc, peaks eluting at high retention times (tR = 40−50 min) were strongly reduced in the identified M03F8.4 mutant and were completely absent in the identified fut-8 mutant; such a shift in NP-HPLC chromatograms is consistent with a loss of higher molecular weight glycans.
Fig. 7. Comparative analysis of the N-glycome in CGL2-resistant C. elegans double mutants pmk-1;fut-8(op498) (red trace) and pmk-1(km25);M03F8.4(op497) (green trace) and the isogenic CGL2-hypersensitive single mutant strain pmk-1(km25) (black trace). 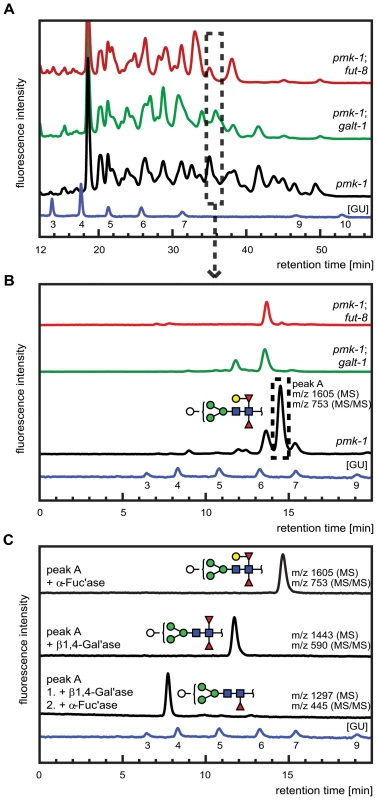
(A) and (B) HPLC of released and fluorescently labeled N-glycans. Upon enzymatic release (here the PNGase F resistant, but PNGase A sensitive glycome fraction is shown) and fluorescent labeling, N-glycans were separated by normal phase HPLC and analysed by mass spectrometry (A). Fractions at similar retention times (e.g., dashed rectangle) were further separated by reversed phase HPLC and the resulting pure glycans were analysed by mass spectrometry (MS) and for selected fractions by MS/MS (B). (C) Structural characterization of a selected isolated peak. Peak A found in the pmk-1 strain but not in the two double mutant strains was treated with β1,4-galactosidase (β1,4-Gal'ase) and α-fucosidase (α-Fuc'ase). The reaction products were analysed by reversed phase HPLC and MS and MS/MS (see Supplementary Figure S2). The blue HPLC trace represents the glycan standard in glucose units (GU). Monosaccharides are represented as symbols: Man (green circle), Gal (yellow circle), GlcNAc (blue square), Fuc (red triangle), Hex (white circle). The individually obtained fractions were subsequently resolved in a second dimension by reversed phase HPLC into pure glycans suitable for structural characterization. The comparison of the 2nd dimension chromatograms of 1st dimension fractions of equal retention times revealed pronounced differences between the N-glycomes of the pmk-1 strain and the two identified CGL2-resistant mutant strains also for the region with less obvious differences in the 1st dimension chromatograms (i.e., tR = 20−40 min). For example, fractions at tR = 34.0−35.5 min in the 1st dimension yielded comparable 2nd dimension patterns (Fig. 7, panel B) with a highly abundant sub-fraction (tR = 14.5 min) being only present in the pmk-1 pseudo wild type strain. MS analysis identified this peak as a pyridylaminated glycan with the net composition Hex5dHex2HexNAc2-2-amino-pyridine(PA) (m/z 1605). By MS/MS a daughter ion at m/z 753 was observed, compatible with this glycan containing a chitobiose core carrying α1,3 - and α1,6-difucosylation at the reducing end GlcNAc and an additional hexose linked to this reducing end pyridylaminated trisaccharide.
The purified Hex5dHex2HexNAc2 PA-glycan was subsequently treated with exoglycosidases and the reaction products were further analysed by HPLC and MALDI-TOF MS and MS/MS (Fig. 7, panel C). First, this saccharide was exposed to α-fucosidase which prefers terminal α1,6 linked over α1,3 linked fucoses but no shift in its HPLC elution characteristics was observed (tR = 14.5 min) and the mass was unaltered as determined by mass spectrometry. When this glycan was treated with a fungal (Aspergillus) β1,4-specific galactosidase instead, a shift in retention time was observed (tR = 12.5 min) and a loss of one hexose was revealed by MS (m/z 1443) and assigned to the hexose linked to the difucosylated reducing-terminal GlcNAc due to the absence of the m/z 753 daughter ion in the reaction product. The octasaccharide obtained (tR = 12.5 min) then proved sensitive towards α-fucosidase and loss of the ‘decapped’ terminal α1,6-linked fucose was observed (tR = 7.5 min, m/z 1297). These results demonstrated the presence of a β1,4-linked galactose epitope on the α1,6-linked fucose, a modification described previously by Reinhold and co-workers [40]. Analysis of the complete set of 2D-HPLC-MS-MS/MS data (Supplementary Figure S2) confirmed the absence of the galactosylated fucose epitope in the identified M03F8.4 mutant, suggesting that this gene encodes a glycosyltransferase that is required for the biosynthesis of the β1,4-galactoside linked to the core α1,6 - fucose residue. The biochemical characterization of this enzyme, termed GALT-1, is published elsewhere [42]. Based on the available evidence, a pathway for the biosynthesis of Hex5dHex2HexNAc2 can be proposed (Fig. 5, panel B).
Structural basis for the recognition of Galβ1,4Fuc by CGL2
In order to provide biochemical and structural evidence for the interaction between CGL2 and this nematode-specific β-galactoside, the trisaccharide Galβ1,4Fucα1,6GlcNAc was chemically synthesized with a linker at the reducing end and used as ligand in in vitro binding experiments with affinity-purified CGL2. Isothermal titration microcalorimetry measurements confirmed binding of this trisaccharide to CGL2 and suggested a dissociation constant of approximately 100 µM (Supplementary Figure S1). The molecular basis of this interaction was investigated by determining the X-ray structure of cocrystals between CGL2 and the trisaccharide at 1.5 Å resolution (Fig. 8, panel A; Supplementary Table S2). Comparisons with the previously determined structures of complexes between CGL2 and the Thomsen-Friedenreich antigen (Galβ1,3GalNAc) and lactose (Galβ1,4Glc) [28] revealed that the structure of CGL2 as well as the direct hydrogen bonds between CGL2 and the β-galactoside of the different carbohydrate ligands were fully superimposable (Fig. 8, panel B). A major difference between these structures lies in the orientation of the preceding monosaccharide subunits to the protein, e.g. the plane of Glc in lactose is perpendicular to the plane of Fuc in the trisaccharide. Due to the low number of direct contacts, there seems to exist a high degree of flexibility on the side of CGL2 with regard to the identity of this monosaccharide subunit. The only constraint is the orientation of the 3′ (in glucose and fucose) or 4′ (in GalNAc) OH of the linked monosaccharide to allow interaction with the conserved carbohydrate-coordinating residues, Arg55 and Glu75. In addition, these hydroxyl groups make additional contact to Arg77 in case of Glc (in lactose) and Fuc (in Galβ1,4Fucα1,6GlcNAc) but not in case of GalNAc (in Thomsen-Friedenreich antigen).
Fig. 8. Detailed view of the interaction between CGL2 and Galβ1,4Fucα1,6GlcNAc (A) and comparison with two other CGL2/carbohydrate complexes (B). 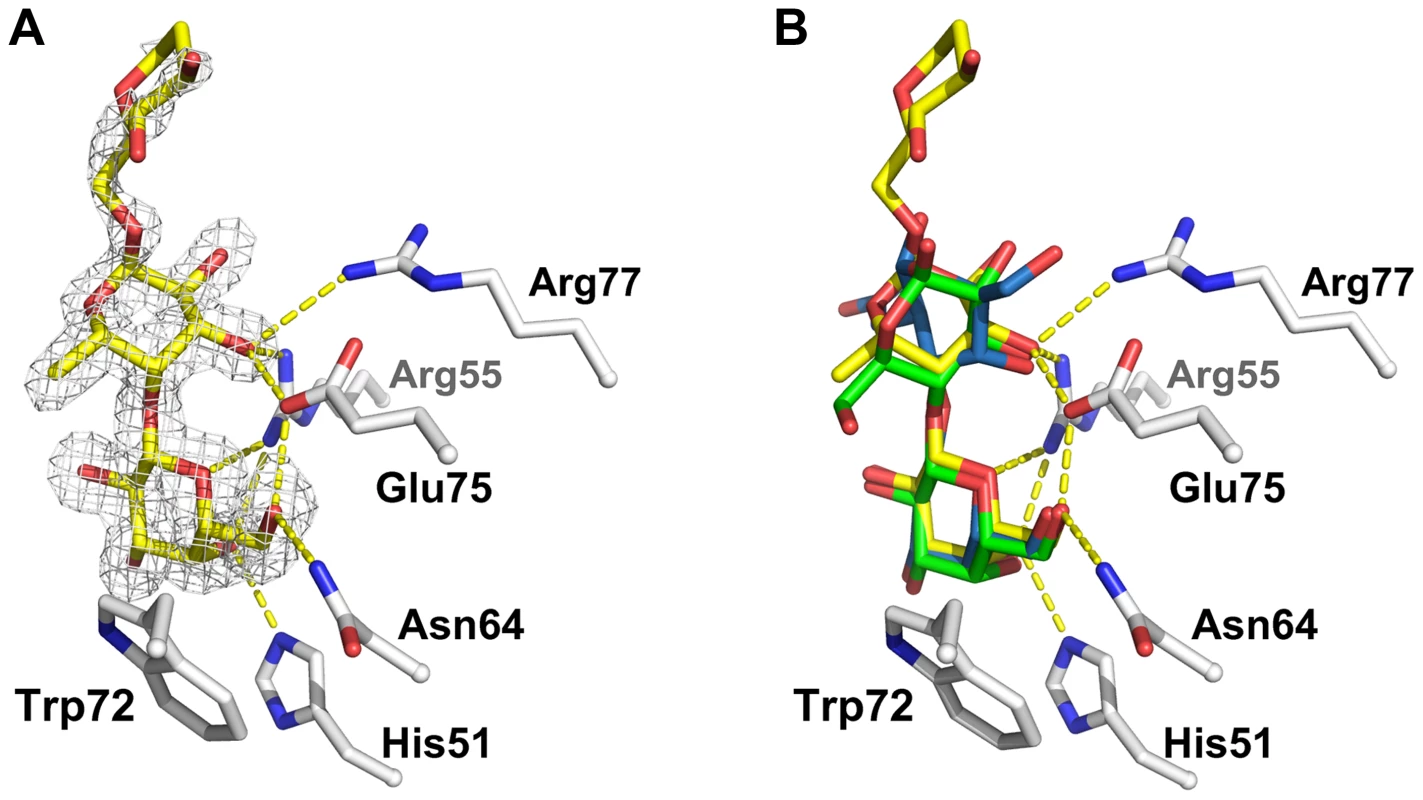
(A) Fourier difference map (with Fo - Fc coefficients) around the visible part of ligand contoured at 3 σ. Residues belonging to the binding pocket are displayed as sticks and H-bonds as dashed yellow lines. (B) Superimposition of the lactose (Galβ1,4Glc) (green, PDB ID 1ULC) and of the Thomsen-Friedenreich antigen (Galβ1,3GalNAc) (blue, PDB ID 1ULG) onto the CGL2/Galβ1,4Fucα1,6GlcNAc structure (yellow, PDB ID 2WKK). The binding pocket is almost identical in all three structures. Discussion
Galectins are β-galactoside-binding lectins known primarily from animals and fungi. In animals, galectins appear to have important roles in development and immunity [43],[44],[45],[46]. The function of the fungal representatives of this lectin family, however, has remained unclear. A role of fungal galectins in fruiting body development, as suggested by their specific expression in these reproductive structures [26] and the identification of a putative endogenous ligand [47] could not be confirmed: Neither constitutive expression nor silencing of the two C. cinerea isogalectins, CGL1 and CGL2, affected fruiting body formation [11]. These results together with the toxicity of another mushroom galectin, Agrocybe aegerita AAL, towards HeLa cells and mice [14],[48] prompted us to follow the hypothesis of a role of fungal galectins in the defense of higher fungi against predators such as fungal-feeding nematodes. In agreement with this hypothesis, we demonstrate that the C. cinerea galectin CGL2 exhibited a pronounced toxicity towards the model nematode C. elegans. The nematotoxic activity of CGL2 with a LD50 of 350 µg/ml was comparable to the entomotoxic activity of XCL, a fruiting body lectin from another homobasidiomycete and of plant lectins [13].
A role of fungal galectins in defense against predatory nematodes is in line with their predicted cytoplasmic localization, since fungal-feeding as well as plant-feeding nematodes feed by adsorbing the cellular content using a mouth organ called stylet that acts like a syringe [24]. In this light, the cytoplasm, which is essentially free of complex glycoconjugates, is an ideal storage compartment for lectins that are destined to find their ligands in the intestine of predators [Comment: in this study, the anatomical limitation of C. elegans, as regards a stylet, is circumvented by expression of the recombinant lectin in the cytoplasm of bacteria]. Indeed, a growing number of cytoplasmic lectins has been identified in plants, possibly complementing the classical vacuolar lectins in the defense of plants against herbivores [49]. Since the cytoplasmic localization is a hallmark of fruiting body lectins, we are currently testing a whole series of such lectins for toxicity towards various organisms including C. elegans to provide additional experimental support for a role of these fungal lectins in defense. A recent survey of insecticidal activities in fruiting bodies suggests that most of these activities are protein-based and, thus, that the protein-mediated defense may be at least as significant for fungal physiology and ecology as the more established chemical defense of fungi by secondary metabolites [50],[51],[52].
The identified ligand of CGL2 in C. elegans, Galβ1,4Fucα1,6 modification of the proximal GlcNAc of N-glycan cores, was originally discovered as a feature of octopus and squid rhodopsin [53],[54] and keyhole limpet hemocyanin [55]. The physiological significance of this N-glycan modification is unknown and the identification of a novel, putative glycosyltransferase required for this modification may shed light both on its distribution and function. The biochemical characterization of the relevant C. elegans enzyme is published elsewhere [42]. None of the mutations affected in the biosynthesis of this specific glycoepitope showed obvious defects in development under standard laboratory conditions. In the light of the fact that these mutations were identified, because they conferred resistance towards CGL2-expressing E. coli, it is noteworthy that mutations in gly-12, gly-13 and gly-14, coding for the three GnTI isoenzymes of C. elegans, were previously reported to affect the response of C. elegans to pathogenic bacteria [39]. This could suggest that the Galβ1,4Fucα1,6GlcNAc epitope, or some other GnTI-dependent modification, plays a specific role in the defense of C. elegans against bacterial pathogens. The fact that, of these three mutations, only gly-13 led to resistance against CGL2-expressing E. coli may reflect the ubiquitous expression of this gene and the function of its product as the major GnTI in C. elegans [36]. On the other hand, even though the gly-14 gene was reported to be specifically expressed in the intestine (i.e., the same tissue which we observe expresses CGL2 epitopes), the lack of resistance shown by the gly-14 mutant can be explained by the ‘overruling’, ubiquitous expression of gly-13 [56]. As noted above, core α1,6-fucosylation by the FUT-8 enzyme, required as the basis for subsequent galactosylation by GALT-1, the M03F8.4 gene product, is itself dependent on the prior action of GnTI. On the other hand, GlcNAc transferases II and V (encoded by the gly-20 and gly-2 genes, respectively) are not prerequisites for the action of FUT-8 and, thus, the lack of resistance of gly-2 and gly-20 mutants to CGL2 is not surprising. The other tested fucosyltransferase mutants (fut-1 through to fut-6) affect other forms of fucosylation and are not involved in the formation of the Galβ1,4Fucα1,6 epitope.
Analysis of the C. elegans glycome suggests that there are, besides the Galβ1,4Fucα1,6GlcNAc epitope, additional β-galactosides on N-glycans [37], on O-glycans [57] and on glycosphingolipids [5] representing potential CGL2-ligands based on the carbohydrate-binding specificity of this galectin [27]. However, the exclusive and almost complete resistance of C. elegans mutants affected in the biogenesis of the β1,4-galactoside on the α1,6-bound core fucose of N-glycans and the almost full sensitivity of all other tested mutants suggest that these other potential ligands are either not accessible for CGL2, not bound by CGL2 or their binding by CGL2 does not lead to toxicity. From the experiments with B. thuringiensis Cry5B toxin [58] and our own experiments with Thomsen-Friedenreich antigen (Galβ1,3GalNAc)-binding fungal lectins (S. Bleuler, unpublished), it can be concluded that at least some of these additional β-galactoside-containing glycoconjugates are present in the C. elegans intestine, making the first possibility unlikely. Since mutations in the GDP-fucose biosynthetic pathway still allowed formation of residual β-galactoside-containing glycosphingolipids [59] but led to complete loss of CGL2-binding to the intestinal epithelium (data not shown), we conclude that CGL2 does not bind these β-galactosides in vivo, at least not in sufficient degree to be visualized. This explanation may also apply for Thomsen-Friedenreich antigen, an epitope which is associated with C. elegans O-glycans and was shown to bind CGL2 in vitro, albeit with low affinity [28],[47],[57].
On the other hand, the sensitivity of the bre-mutants, except for bre-1, towards CGL2, suggests that the resistance of these mutants towards the B. thuringiensis crystal toxin Cry5B is highly specific and that such resistances depend on the carbohydrate-binding specificity of the respective lectin/toxin. This conclusion is further validated by the sensitivity of some of the reported CGL2-resistant mutants towards other nematoxic fruiting body lectins (S. Bleuler, unpublished).
Analogous to our own results, the Galβ1,4Fucα1,6GlcNAc epitope was recently identified as a ligand for the endogenous C. elegans galectin LEC-6 in vitro [60]. It is not known, however, whether this interaction occurs also in vivo and what the consequences of such an interaction would be. Similar to mutations interfering with the formation of the glycoepitope (see above), knockdown of lec-6 mRNA did not result in any obvious phenotype in C. elegans high-throughput functional studies [61]. In the light of the proposed role of the Galβ1,4Fucα1,6GlcNAc epitope in the response of C. elegans to pathogenic bacteria, it is noteworthy that expression of the lec-6 gene was induced upon exposure of C. elegans to Photorhabdus luminescens [62].
The molecular details of the interaction between CGL2 and Galβ1,4Fucα1,6GlcNAc and other characterized ligands suggest that CGL2, and possibly many other galectins, have a relaxed specificity in vitro with regard to the type of linkage and the type of sugar attached to the galactose as long as the 3′ (in glucose and fucose) or 4′ (in GalNAc) OH of the linked monosaccharide is in the right orientation to interact with the conserved carbohydrate-coordinating residues, Arg55 and Glu75; of the other residues involved in binding, Arg77 only makes contact with Glc in lactose and fucose in Galβ1,4Fucα1,6GlcNAc but not with GalNAc in Thomsen-Friedenreich antigen. Our isothermal titration microcalorimetry (ITC) data does not allow conclusive statements about the relative affinity of CGL2 to Galβ1,4Fucα1,6GlcNAc vs. other non-substituted, terminal β-galactosides but taking the structural data into account, it can be estimated that the affinity to Galβ1,4Fuc is in the same range or even slightly higher than to other ligands. In an in vivo context, the CGL2 epitope is part of a larger N-glycan structure, which may indeed have a higher affinity towards the lectin than the trisaccharide tested here. Analogously, the role of an entire structure in optimal binding to N-glycans was shown by studies on anti-HRP which recognises core α1,3-fucosylated determinants [63]. Such a binding mode would help to explain why there is apparently no biological significance to the in vitro binding of CGL2 to the Thomsen-Friedenreich antigen (see discussion above).
Apart from the identity of the target glycoconjugate, we can only speculate about the mechanism of CGL2-mediated nematotoxicity: Based on the in situ localization of the glycoconjugate using fluorescently labeled CGL2, we assume that the recognized N-glycan is bound to one or several proteins on the epithelial membrane or the peritrophic membrane of the intestine. The fact that we isolated in our screen exclusively mutations in carbohydrate-active enzymes, suggests that toxicity is not based on the interaction between CGL2 and a particular “receptor” N-glycoprotein but is rather caused by binding of CGL2 to different N-glycoproteins characterized by the Galβ1,4Fucα1,6 epitope. A recent proteomic approach identified a number of such glycoproteins based on recognition by LEC-6 [64]. The caveats of such a conclusion are that our results are still compatible with a specific “receptor” N-glycoprotein for CGL2 and that the screen is probably not saturated and, by design, not able to identify mutations in genes that are essential for worm viability.
The intestinal localization of the CGL2-ligand in C. elegans coincides with the morphological changes of the intestinal epithelium upon feeding with CGL2-expressing E. coli. Similar morphological changes were reported in C. elegans in response to feeding with E. coli expressing the B. thuringiensis crystal toxin Cry5B [29] and other toxin-producing, gram-positive and gram-negative bacteria [65],[66]. Enlargement of the intestinal lumen and damage of microvillar structure of the epithelium was also observed in insect larvae upon feeding with the entomotoxic mammalian galectin-1 and snow drop lectin (GNL), respectively [67],[68]. The morphological changes are usually more pronounced in the anterior part of the intestine possibly because this part receives the highest concentration of the lectins/toxins upon feeding.
Preliminary experiments with purified CGL2 and HeLa cells suggest that the lectin is cytotoxic, rapidly endocytosed and ends up in a perinuclear compartment (M. Garbani, unpublished). This is in contrast to the A. aegerita galectin (AAL) which is also toxic for HeLa cells but enters the cytoplasm and nucleoplasm of the cells [48]. This difference in behavior between two different fungal galectins might be due to differences in oligomerization: AAL is a dimer whereas CGL2 forms a tetramer in solution, which might restrict its access to the cyto - and nucleoplasm [15],[28]. The cytotoxicity of AAL was recently shown to be dependent on a conserved hydrophobic patch on the protein surface in addition to domains involved in oligomerization and carbohydrate-binding [15]. At this point, we do not know whether this patch is also required for CGL2-mediated toxicity. Cytotoxicity towards mammalian cells is shared between fungal and mammalian galectins. However, ligands and toxicity mechanisms appear to vary between the different mammalian galectins and cell types [69],[70],[71],[72],[73],[74]. It remains to be tested, whether CGL2 uses one of the ligands and toxicity mechanisms identified for the mammalian galectins, whether endocytosis is necessary for cytotoxicity and how these processes relate to the nematotoxicity of CGL2.
Materials and Methods
Strains and cultivation conditions
Escherichia coli strains DH5α and BL21(DE3) were used for cloning and amplification of plasmids and bacterial expression of proteins, respectively. E. coli was cultivated on standard media as described [75]. Caenorhabditis elegans strains were maintained on nematode growth media (NGM) and fed with E. coli strain OP50 as described [76]. The Bristol isolate N2 was used as the wild type strain. Strains pmk-1(km25), sek-1(ag1), nsy-1(ag3), bre-1(ye4), bre-2(ye31), bre-3(ye26), bre-4(ye27), bre-5(ye17), fut-1(ok892), fut-2(gk360), fut-2(ok509), fut-3(gk103), fut-4(gk111), fut-5(ok242), fut-6(ok475), fut-8(ok2558), gly-2(qa703), gly-12(is47), dpy-6(e14);gly-13(ok712), gly-14(id48), gly-20(ok826), unc-119(ed3) were obtained from the Caenorhabditis Genetics Center (CGC) at the University of Minnesota (USA). The strain carrying the two extrachromosomal constructs frEx113[hsp::MosTransposase;Pcol12::DsRed] and oxEx229[Mos1;Pmyo-2::gfp] was kindly provided by Jonathan Ewbank. For Mos1-mediated mutagenesis, we generated the strains pmk-1(km25);frEx113 and pmk-1(km25);oxEx229. Strains resulting from Mos1-mediated mutagenesis and subsequent outcrossing were pmk-1(km25); M03F8.4(op497), pmk-1(km25); fut-8(op498), pmk-1(km25); ger-1(op499), pmk-1(km25);gly-13(op507) and pmk-1(km25);bre-1(op509). Primers used for genotyping are listed in Supplementary Table S3.
Cloning and expression
Plasmid pET24-CGL2 for expression of authentic CGL2 in BL21(DE3) was described previously [27]. The plasmid for bacterial expression of C. cinerea CGL2(W72G) was constructed by amplifying the respective open reading frame from pYADE4-CGL2(W72G) [28] using the primers NdeI-CGL2N and BamHI-CGL2C [27] and ligating the resulting fragment into pET24a (Invitrogen) using the introduced restriction sites. Plasmid pET24-CGL1 for expression of authentic CGL1 in BL21(DE3) was constructed analogously using cgl1-specific primers and plasmid pBCG1 as template [26]. Expression of CGL2 and CGL2(W72G) in liquid culture was performed as described for CGL3 [27]. For the C. elegans bioassays, 300 µl of a overnight culture of the respective BL21(DE3) transformants were spread on NGM-plates containing 1 mM isopropyl-β-D-thiogalactoside (IPTG) and 50 µg/ml Kanamycin and incubated overnight at 23°C before addition of the nematodes. Lectin expression was verified by separating whole cell extracts of induced BL21(DE3)-transformants on Coomassie blue-stained SDS-polyacrylamide gels and immunoblotting using anti-CGL2 antiserum [26] (data not shown).
Protein purification and labeling
Bacterial cell pellets were resuspended in ice-cold phosphate-buffered saline (PBS) [30 mM Na-phosphate pH 7.3, 150 mM NaCl] containing 1 mM phenylmethylsulfonyl fluoride and ruptured using a French press. Cell debris was removed in two consecutive steps of centrifugation at 12000 g for 15 min and 27000 g for 30 min. The supernatant was incubated with lactosyl-sepharose at 4°C for 1 h and CGL2 was finally eluted at room temperature in PBS containing 200 mM lactose. After size exclusion chromatography on Superose 6 10/300 GL (GE Healthcare) equilibrated in PBS, fractions containing the protein were pooled and concentrated using an Amicon Ultra-4 centrifugal filter device (Millipore) with a molecular weight cutoff of 10 kDa. Protein concentration was calculated by measuring the absorbance at 280 nm, assuming a relation of 1.25 mg/ml to 1 unit absorbance at 280 nm for a path length of 1 cm.
Conjugation of purified CGL2 to tetramethylrhodamine (TAMRA) (Molecular Probes) was performed as described [47].
C. elegans toxicity assays
A plate assay was devised to examine the toxicity of authentic CGL1 and CGL2 and mutant CGL2 towards C. elegans. NGM plates were seeded with E. coli BL21(DE3) expressing either authentic CGL1 or CGL2 or mutant CGL2(W72G) as described above. As a control, plates were seeded with E. coli BL21(DE3) containing the vector pET24a. The plates were incubated overnight at 23°C and seeded with synchronized populations of C. elegans [77] for the different toxicity assays:
Microscopic analysis
For microscopic analysis of the toxic effect of CGL2 towards C. elegans, L4 animals were seeded onto the plates and examined after 24 h at 23°C.
Developmental assay
Quantitative data on the effect of CGL1 and CGL2 on C. elegans development was acquired by placing 50 to 100 newly hatched L1 larvae of the indicated genotypes on the plates. After 72 h, the fraction of animals that reached L4 stage was determined.
Brood size assay
The effect of CGL2 on C. elegans reproduction was assayed by picking individual L4 wild type hermaphrodites onto plates. Thereafter, the mothers were transferred to new plates daily until the mother either stopped producing offspring or died. The progeny of the previous plate were counted the next day. The number of progeny from the various plates were added up to give the final brood size.
Determination of the CGL2 median lethal dose (LD50)
We defined the LD50 as the concentration of toxin at which >50% of the animals fail to reach larval stage 4 (L4) within 96 h in liquid culture. 20 L1 staged C. elegans wild type worms were placed in wells containing S-medium [78], E. coli BL21 containing empty vector pET24a with a KanR gene as a food source, kanamycin and chloramphenicol (30 µg/ml each) and purified CGL2 protein in the concentrations indicated. After 96 h, the worms were transferred to NGM plates and the number of worms that reached L4 stage was determined.
Survival curves
For the comparison between CGL2-, CGL2(W72G)-expressing and empty vector control-containing bacteria (Fig. 1, panel C), 10 L4 stage wild type C. elegans (N2) were seeded onto plates with the respective bacterial lawns. Surviving animals were transferred each day onto a novel plate with the same type of bacterial lawn. For the hypersensitivity assay (Fig. 3, panel A), 10 L4 stage C. elegans of the indicated genotypes were transferred to plates with CGL2-expressing bacteria. The plates were checked for surviving animals every 24 h. A worm was considered dead when it did not react to touching with a worm pick.
Statistical analysis
The statistical significance of the results was evaluated using appropriate tests. The results of the developmental toxicity assay were analyzed by the non-parametric Kolmogorov-Smirnov test, comparing pairwise each lectin to the vector control and each C. elegans mutant to the wildtype strain. The differences in the brood-size were compared, also pairwise, using a t test. For the statistical evaluation of the survival curves a Kaplan-Meyer analysis in combination with log rank tests was used. The difference in the CGL2-TAMRA staining between two different C. elegans strains was assessed using a chi square test of the respective numbers of animals showing specific staining of the intestinal epithelium (see Supplemental Table S1).
Screen for CGL2-resistant C. elegans mutants using Mos1 insertional mutagenesis
Mos1 insertional mutagenesis was in principle performed as published [79]. We used the extrachromosomal arrays frEx113, which carries the Mos1 transposase under the control of a heat-shock promoter, and oxEx229, which carries multiple copies of the substrate Mos1 transposon. The two extrachromosomal constructs were crossed into CGL2-hypersensitive pmk-1(km25) worms to generate the two starting strains pmk-1(km25);frEx113 and pmk-1(km25);oEx229 for the screen. To generate double-array carrying animals, pmk-1(km25);oxEx229 males were crossed to L4 pmk-1(km25);frEx113 hermaphrodites. Progeny containing both arrays, recognized by the concurrent expression of GFP in the pharynx (Pmyo-2::gfp, contained in oxEx229) and DsRed in the epidermis (Pcol12::DsRed, contained in frEx113), were propagated for approximately six generations before subjecting them to heat-shock. Several hundred double-transgenic animals per mutagenesis round were subjected to heat-shock for 1 h at 33°C, 1 h at 20°C, and 1 h at 33°C and then allowed to recover overnight at 20°C. P0 were distributed to 90 mm NGM plates and eggs were collected 12–40 h after heat shock. P0 were then removed. After 3 days, gravid F1 were washed off the plates in M9 buffer and F2 eggs were isolated as described [76]. The synchronized population of F2 L1 animals was distributed on 30 plates containing CGL2-expressing E. coli BL21(DE3). After 3 to 7 days, these plates were screened for CGL2-resistant animals that had reached adulthood. Each plate with resistant animals was treated as an individual hit to avoid redundant Mos1 insertions. Candidate worms were propagated on CGL2-expressing E. coli to confirm the resistance phenotype. Once resistance was confirmed, mutants that had lost the extrachromosomal Mos1-bearing array were outcrossed 2 to 6 times before assaying for the presence of Mos1 elements and trying to locate the site of insertion. For those mutants that still contained a Mos1 element, we determined the insertion site through inverse PCR on worm lysates as published [79].
In total, approximately 500′000 haploid genomes were screened. The transposition efficiency was meassured as 50%. In total, 14 CGL2-resistant worms were isolated. Only 5 of them, pmk-1(km25);M03F8.4(op497), pmk-1(km25);fut-8(op498), ger-1(op499); pmk-1(km25), pmk-1(km25);gly-13(op507) and pmk-1(km25);bre-1(op509), contained the Mos1 transposon insertion.
Light microscopy of C. elegans
For general worm handling, a Leica MZ 12.5 stereomicroscope was used. To select double-array carrying worms (pmk-1(km25);oxEx229;frEx113), we used a Leica MZ 16 FA stereomicroscope equipped with appropriate filtersets (DsRed and GFP filter). Pictures were taken with a Nikon Coolpix 990 digital camera.
For DIC and fluorescence microscopy, worms were placed on 2% agarose pads in M9 [75], anaesthesized with levamisole (3–5 mM) (Sigma) and mounted under a coverslip for observation using a Leica DM-RA or Zeiss Axiovert 200 microscope equipped with DIC (Nomarski) optics and standard epifluorescence with a DsRed filterset for detection of TAMRA. Pictures were taken with a Hamamatsu ORCA-ER camera. Images were false-coloured using OpenLab software.
For the in situ localization of the CGL2-ligand, L4 staged C. elegans were placed in wells containing S-medium [78], BL21(DE3) E. coli harbouring empty KanR-vector, kanamycin and chloramphenicol at 30 µg/ml each and TAMRA-labeled CGL2 at 100 µg/ml. After 24 hrs, worms were transferred to standard OP50 plates and screened for TAMRA fluorescence 2 h thereafter.
Electron microscopy of C. elegans
For electron microscopic examination, worms were prepared by a described two-step chemical fixation [80]. Fixation and slicing of the samples was kindly carried out by Garry Barmettler at the Center for Microscopy and Image Analysis (University of Zurich, Switzerland). The samples were examined using a Philips CM100 transmission electron microscope equipped with a side mounted digital camera (Gatan).
Isolation of C. elegans N-glycans
C. elegans strains pmk-1(km25), pmk-1(km25);M03F8.4(op497) and pmk-1(km25);fut-8(op498) were grown for 5 days at room temperature in liquid culture with E. coli OP50 and afterwards separated from bacteria and debris by 30% (w/v) sucrose gradient centrifugation [76]. N-glycan preparation was performed as previously published [81] by enzymatic release of glycans from partially purified glycopeptides using peptide-N-glycanase (PNGase) F and subsequently PNGase A in order to separate core α1,3-fucosylated glycans from other core fucosylated glycans. Briefly, approximately 3 g of worms (wet weight) were boiled prior to grinding. The extract was adjusted to contain 5% (v/v) formic acid and incubated with 3 mg pepsin (Sigma) at 37°C overnight. After centrifugation at 15′000 g for 15 min, the supernatant was applied to 15 ml Dowex AG WX2 equilibrated with 2% acetic acid. The glycopeptides were eluted with ammonium acetate (0.6 M, pH 6). Orcinol-positive fractions were pooled and lyophilized overnight. The samples were then desalted by application to a Sephadex G25 column and eluted with 1% acetic acid. The orcinol-positive fractions were again pooled and lyophilized. The samples were dissolved in 250 µl water. After heat treatment at 95°C for 5 min to inactivate residual pepsin, the samples were cooled prior to addition of 250 µl ammonium carbonate buffer pH 8 and 3 U PNGase F (Roche) and incubated overnight at 37°C. The samples were then acidified with 400 µl 10% acetic acid and applied to 5 ml Dowex AG WX2. The unretained free glycans were lyophilized and dried for subsequent fluorescent labeling whereas the retained Orcinol positive fractions eluting with ammonium acetate (0.6 M, pH 6) were desalted as above, dissolved in ammonium acetate buffer (50 mM, pH 5) and treated with PNGase A (0.6 mU) overnight at 37°C. Again the samples were acidified with 400 µl 10% acetic acid and applied to 5 ml Dowex AG WX2. The unretained free glycans were lyophilized and also dried for subsequent fluorescent labeling.
Labeling and structural analysis of C. elegans N-glycans
Fluorescent labeling of the N-glycans was performed as previously described using 2-amino-pyridine (PA). Complete N-glycomes of either PNGase A or F released and pyridylaminated glycans were fractionated by 2D-HPLC using a Shimadzu HPLC system (consisting of a SCL-10A controller, two LC10AP pumps and a RF-10AXL fluorescence detector controlled by a personal computer using Class-VP software (V6.13SP2)) at room temperature and fluorescence detection (excitation at 310 or 320 nm, emission detected at 380 or 400 nm). The N-glycans were first fractionated on a normal phase HPLC (Tosoh TSK gel Amide-80, 4.6×250 mm, 5 µm; flow 1 ml/min, elution: 5 min isocratic 71.3% MeCN, 10 min gradient from 71.3% to 61.8% MeCN, 25 min isocratic 61.8% MeCN, 15 min 61.8% to 54.2% MeCN using ammonium formate (10 mM, pH 7) as buffer). The fractions were lyophilized and further fractionated on reversed phase HPLC (Hypersil ODS C-18; 4×250 mm, 5 µm; flow 1.5 ml/min, gradient of 0–9% MeOH over 30 min using ammonium formate (0.1 M, pH 4) as buffer). HPLC chromatograms were visualized using the opensource program PLOT (Version 0.997 by Wesemann and Thijsse). Each fraction was subjected to monoisotopic MALDI-TOF MS using a Bruker Ultraflex TOF/TOF with 2,5-dihydroxybenzoic acid as matrix. In general, all fractions with fucose containing N-glycans were subjected to MS/MS to elucidate their composition. A peptide standard mixture (Bruker) was used for external calibration. MS data were analysed using Bruker software and the mMass V2.4 software package [82].
Selected isolated N-glycans were examined for the presence of either terminal β-galactosides by treatment with Aspergillus oryzae β1,4-galactosidase (27 mU, 50 mM sodium citrate, pH 4.5) [83] for 2 days at 37°C or terminal α-fucosides by use of bovine kidney α-fucosidase (Sigma, 15 mU, 50 mM ammonium acetate, pH 5). Digestion products were subsequently analysed for altered structural characteristics by RP-HPLC (see above) and MALDI-TOF MS.
Chemical synthesis of Galβ1,4Fucα1,6GlcNAcβOC5H10NH2
See Supplementary Figure S3 and Supplementary Text S1.
Crystallization of CGL2 in complex with carbohydrate ligand
Crystallization conditions were screened using the PEG/Ion Screen from Hampton Research with the hanging-drop vapor-diffusion method at 18°C. The best crystals were obtained by mixing 2.5 µl of protein solution (10 mg/ml) containing 1 mM Galβ1,4Fucα1,6GlcNAcβOC5H10NH2 with 2.5 µl mother liquor consisting of 0.2 M magnesium acetate, 20% (w/v) PEG 3350. Drops were equilibrated against 500 µl reservoir solution. After 3 weeks, crystals were cryostabilized in mother liquor supplemented with 25% glycerol and flash frozen in liquid nitrogen.
Data collection and structure determination
Diffraction data were collected at the Swiss Light Source, beamline X06DA (Villigen, Switzerland) at 100 K and processed with XDS, XSCALE and XDSCONV [84]. The structure was readily solved by molecular replacement with MOLREP [85], using the known CGL2 structure as a search model PDB ID 1UL9 [28]. The structure was refined anisotropically with Phenix [86] to 1.5 Å resolution and iterative model rebuilding was performed using Coot [87]. Model statistics were obtained with Procheck/Sfcheck as part of the CCP4 suite [85]. Molecular visualizations and structures illustrations were performed using PyMOL [88]. Data processing and refinement statistics are summarized in Supplementary Table S2. Coordinate and structure factors have been deposited with the PDB under code 2WKK.
Supporting Information
Zdroje
1. PeumansWJ
Van DammeEJ
1995 Lectins as plant defense proteins. Plant Physiol 109 347 352
2. LorisR
2002 Principles of structures of animal and plant lectins. Biochim Biophys Acta 1572 198 208
3. CashHL
WhithamCV
BehrendtCL
HooperLV
2006 Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313 1126 1130
4. KohatsuL
HsuDK
JegalianAG
LiuFT
BaumLG
2006 Galectin-3 induces death of Candida species expressing specific β1,2-linked mannans. J Immunol 177 4718 4726
5. GriffittsJS
HaslamSM
YangT
GarczynskiSF
MulloyB
2005 Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science 307 922 925
6. RappuoliR
MontecuccoC
1997 Guidebook to Protein Toxins and Their Use in Cell Biology. Oxford Oxford University Press
7. GoldsteinIJ
WinterHC
2007 Mushroom Lectins.
KamerlingJP
Comprehensive Glycoscience: From Chemistry to Systems biology Amsterdam Elsevier Ltd
8. GuillotJ
KonskaG
1997 Lectins in Higher Fungi. Biochem Syst Ecol 25 203 230
9. FumioY
SakaiT
ShiraishiN
YotsumotoM
MukoyoshiR
2000 Hemagglutinins (lectins) in fruit bodies of Japanese higher fungi. Mycoscience 41 323 330
10. WangH
NgTB
OoiVE
1998 Lectins from mushrooms. Mycol Res 102 897 906
11. WaltiMA
VillalbaC
BuserRM
GrunlerA
AebiM
2006 Targeted gene silencing in the model mushroom Coprinopsis cinerea (Coprinus cinereus) by expression of homologous hairpin RNAs. Eukaryot Cell 5 732 744
12. NowrousianM
CebulaP
2005 The gene for a lectin-like protein is transcriptionally activated during sexual development, but is not essential for fruiting body formation in the filamentous fungus Sordaria macrospora. BMC Microbiol 5 64 74
13. TriguerosV
LougarreA
Ali-AhmedD
RahbeY
GuillotJ
2003 Xerocomus chrysenteron lectin: identification of a new pesticidal protein. Biochim Biophys Acta 1621 292 298
14. SunH
ZhaoCG
TongX
QiYP
2003 A Lectin with Mycelia Differentiation and Antiphytovirus Activities from the Edible Mushroom Agrocybe Aegerita. J Biochem Mol Biol 36 214 222
15. YangN
LiDF
FengL
XiangY
LiuW
2009 Structural basis for the tumor cell apoptosis-inducing activity of an antitumor lectin from the edible mushroom Agrocybe aegerita. J Mol Biol 387 694 705
16. WarnerRL
WinterHC
SpeyerCL
VaraniJ
OldsteinIJ
2004 Marasmius oreades lectin induces renal thrombotic microangiopathic lesions. Exp Mol Pathol 77 77 84
17. HarperSM
CrenshawRW
MullinsMA
PrivalleLS
1995 Lectin binding to insect brush border membranes. J Econ Entomol 88 1197 1202
18. PohlevenJ
ObermajerN
SaboticJ
AnzlovarS
SepcicK
2008 Purification, characterization and cloning of a ricin B-like lectin from mushroom Clitocybe nebularis with antiproliferative activity against human leukemic T cells. Biochim Biophys Acta 1790 173 181
19. FunkPE
ThompsonCB
1998 Identification of a lectin that induces cell death in developing chicken B cells. Cell Immunol 186 75 81
20. OkadaH
KadotaI
2003 Host status of 10 fungal isolates for two nematode species, Filenchus misellus and Aphelenchus avenae. Soil Biology & Biochemistry 35 1601 1607
21. SmithJE
ChallenMP
WhitePF
EdmondsonRN
ChandlerD
2006 Differential effect of Agaricus host species on the population development of Megaselia halterata (Diptera: Phoridae). Bull Entomol Res 96 565 571
22. O'ConnorL
KeilCB
2005 Mushroom host influence on Lycoriella mali (Diptera: Sciaridae) life cycle. J Econ Entomol 98 342 349
23. WalkerGE
1984 Ecology of the mycophagous nematode Aphelenchus avenae in wheat-field and pine-forest soils. Plant and Soil 78 417 428
24. YeatesGW
BongersT
De GoedeRG
FreckmanDW
GeorgievaSS
1993 Feeding habits in soil nematode families and genera-an outline for soil ecologists. J Nematol 25 315 331
25. CooperDN
BoulianneRP
CharltonS
FarrellEM
SucherA
1997 Fungal galectins, sequence and specificity of two isolectins from Coprinus cinereus. J Biol Chem 272 1514 1521
26. BoulianneRP
LiuY
AebiM
LuBC
KuesU
2000 Fruiting body development in Coprinus cinereus: regulated expression of two galectins secreted by a non-classical pathway. Microbiology 146 (Pt8) 1841 1853
27. WaltiMA
WalserPJ
ThoreS
GrunlerA
BednarM
2008 Structural basis for chitotetraose coordination by CGL3, a novel galectin-related protein from Coprinopsis cinerea. J Mol Biol 379 146 159
28. WalserPJ
HaebelPW
KunzlerM
SargentD
KuesU
2004 Structure and functional analysis of the fungal galectin CGL2. Structure 12 689 702
29. MarroquinLD
ElyassniaD
GriffittsJS
FeitelsonJS
AroianRV
2000 Bacillus thuringiensis (Bt) toxin susceptibility and isolation of resistance mutants in the nematode Caenorhabditis elegans. Genetics 155 1693 1699
30. de MaagdRA
BravoA
CrickmoreN
2001 How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet 17 193 199
31. BarrowsBD
GriffittsJS
AroianRV
2007 Resistance is non-futile: resistance to Cry5B in the nematode Caenorhabditis elegans. J Invertebr Pathol 95 198 200
32. KimDH
FeinbaumR
AlloingG
EmersonFE
GarsinDA
2002 A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297 623 626
33. HuffmanDL
AbramiL
SasikR
CorbeilJ
van der GootFG
2004 Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proc Natl Acad Sci U S A 101 10995 11000
34. RhombergS
FuchslugerC
RendicD
PaschingerK
JantschV
2006 Reconstitution in vitro of the GDP-fucose biosynthetic pathways of Caenorhabditis elegans and Drosophila melanogaster. FEBS J 273 2244 2256
35. PaschingerK
StaudacherE
StemmerU
FabiniG
WilsonIB
2005 Fucosyltransferase substrate specificity and the order of fucosylation in invertebrates. Glycobiology 15 463 474
36. ChenS
SpenceAM
SchachterH
2003 Isolation of null alleles of the Caenorhabditis elegans gly-12, gly-13 and gly-14 genes, all of which encode UDP-GlcNAc: α-3-D-mannoside β1,2-N-acetylglucosaminyltransferase I activity. Biochimie 85 391 401
37. PaschingerK
GutterniggM
RendicD
WilsonIB
2008 The N-glycosylation pattern of Caenorhabditis elegans. Carbohydr Res 343 2041 2049
38. PaschingerK
RendicD
LochnitG
JantschV
WilsonIB
2004 Molecular basis of anti-horseradish peroxidase staining in Caenorhabditis elegans. J Biol Chem 279 49588 49598
39. ShiH
TanJ
SchachterH
2006 N-glycans are involved in the response of Caenorhabditis elegans to bacterial pathogens. Methods Enzymol 417 359 389
40. HannemanAJ
RosaJC
AshlineD
ReinholdVN
2006 Isomer and glycomer complexities of core GlcNAcs in Caenorhabditis elegans. Glycobiology 16 874 890
41. Marchler-BauerA
AndersonJB
ChitsazF
DerbyshireMK
DeWeese-ScottC
2009 CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res 37 D205 210
42. TitzA
ButschiA
HenrissatB
FanYY
HennetT
2009 Molecular basis for galactosylation of core fucose residues in invertebrates: Identification of Caenorhabditis elegans N-glycan core α1,6-fucoside-β1,4-galactosyltransferase GALT-1 as a member of a novel glycosyltransferase family. J Biol Chem: in press
43. VastaGR
2009 Roles of galectins in infection. Nat Rev Microbiol 7 424 438
44. RabinovichGA
ToscanoMA
2009 Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol 9 338 352
45. AhmedH
DuSJ
VastaGR
2009 Knockdown of a galectin-1-like protein in zebrafish (Danio rerio) causes defects in skeletal muscle development. Glycoconj J 26 277 283
46. GeorgiadisV
StewartHJ
PollardHJ
TavsanogluY
PrasadR
2007 Lack of galectin-1 results in defects in myoblast fusion and muscle regeneration. Dev Dyn 236 1014 1024
47. WalserPJ
KuesU
AebiM
KunzlerM
2005 Ligand interactions of the Coprinopsis cinerea galectins. Fungal Genet Biol 42 293 305
48. LiangY
FengL
TongX
WangK
LiDF
2009 Importance of nuclear localization for the apoptosis-induced activity of a fungal galectin AAL (Agrocybe aegerita lectin). Biochem Biophys Res Commun 386 437 442
49. Van DammeEJ
BarreA
RougeP
PeumansWJ
2004 Cytoplasmic/nuclear plant lectins: a new story. Trends Plant Sci 9 484 489
50. WangM
TriguerosV
PaquereauL
ChavantL
FournierD
2002 Proteins as active compounds involved in insecticidal activity of mushroom fruitbodies. J Econ Entomol 95 603 607
51. FoxEM
HowlettBJ
2008 Secondary metabolism: regulation and role in fungal biology. Curr Opin Microbiol 11 481 487
52. SpitellerP
2008 Chemical defence strategies of higher fungi. Chemistry 14 9100 9110
53. ZhangY
IwasaT
TsudaM
KobataA
TakasakiS
1997 A novel monoantennary complex-type sugar chain found in octopus rhodopsin: occurrence of the Galβ1,4Fuc group linked to the proximal N-acetylglucosamine residue of the trimannosyl core. Glycobiology 7 1153 1158
54. TakahashiN
MasudaK
HirakiK
YoshiharaK
HuangHH
2003 N-Glycan structures of squid rhodopsin. Eur J Biochem 270 2627 2632
55. WuhrerM
RobijnML
KoelemanCA
BalogCI
GeyerR
2004 A novel Gal(β1-4)Gal(β1-4)Fuc(α1-6)-core modification attached to the proximal N-acetylglucosamine of keyhole limpet haemocyanin (KLH) N-glycans. Biochem J 378 625 632
56. ChenS
ZhouS
SarkarM
SpenceAM
SchachterH
1999 Expression of three Caenorhabditis elegans N-acetylglucosaminyltransferase I genes during development. J Biol Chem 274 288 297
57. JuT
ZhengQ
CummingsRD
2006 Identification of core 1 O-glycan T-synthase from Caenorhabditis elegans. Glycobiology 16 947 958
58. GriffittsJS
WhitacreJL
StevensDE
AroianRV
2001 Bt toxin resistance from loss of a putative carbohydrate-modifying enzyme. Science 293 860 864
59. BarrowsBD
HaslamSM
BischofLJ
MorrisHR
DellA
2007 Resistance to Bacillus thuringiensis toxin in Caenorhabditis elegans from loss of fucose. J Biol Chem 282 3302 3311
60. TakeuchiT
HayamaK
HirabayashiJ
KasaiK
2008 Caenorhabditis elegans N-glycans containing a Gal-Fuc disaccharide unit linked to the innermost GlcNAc residue are recognized by C. elegans galectin LEC-6. Glycobiology 18 882 890
61. SonnichsenB
KoskiLB
WalshA
MarschallP
NeumannB
2005 Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature 434 462 469
62. WongD
BazopoulouD
PujolN
TavernarakisN
EwbankJJ
2007 Genome-wide investigation reveals pathogen-specific and shared signatures in the response of Caenorhabditis elegans to infection. Genome Biol 8 R194
63. WilsonIB
HarthillJE
MullinNP
AshfordDA
AltmannF
1998 Core α1,3-fucose is a key part of the epitope recognized by antibodies reacting against plant N-linked oligosaccharides and is present in a wide variety of plant extracts. Glycobiology 8 651 661
64. KajiH
KamiieJ
KawakamiH
KidoK
YamauchiY
2007 Proteomics reveals N-linked glycoprotein diversity in Caenorhabditis elegans and suggests an atypical translocation mechanism for integral membrane proteins. Mol Cell Proteomics 6 2100 2109
65. GarsinDA
SifriCD
MylonakisE
QinX
SinghKV
2001 A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci U S A 98 10892 10897
66. KotheM
AntlM
HuberB
StoeckerK
EbrechtD
2003 Killing of Caenorhabditis elegans by Burkholderia cepacia is controlled by the cep quorum-sensing system. Cell Microbiol 5 343 351
67. GatehouseAM
GatehouseJA
BharathiM
SpenceJ
PowellKS
1998 Immunohistochemical and developmental studies to elucidate the mechanism of action of the snowdrop lectin on the rice brown planthopper, Nilaparvata lugens (Stal). J Insect Physiol 44 529 539
68. ChenSJ
ChenNT
WangSH
HsuJC
DingWH
2009 Insecticidal action of mammalian galectin-1 against diamondback moth (Plutella xylostella). Pest Manag Sci 65 923 930
69. HernandezJD
NguyenJT
HeJ
WangW
ArdmanB
2006 Galectin-1 binds different CD43 glycoforms to cluster CD43 and regulate T cell death. J Immunol 177 5328 5336
70. BiS
EarlLA
JacobsL
BaumLG
2008 Structural features of galectin-9 and galectin-1 that determine distinct T cell death pathways. J Biol Chem 283 12248 12258
71. StowellSR
KarmakarS
ArthurCM
JuT
RodriguesLC
2009 Galectin-1 induces reversible phosphatidylserine exposure at the plasma membrane. Mol Biol Cell 20 1408 1418
72. PatnaikSK
PotvinB
CarlssonS
SturmD
LefflerH
2006 Complex N-glycans are the major ligands for galectin-1, -3, and -8 on Chinese hamster ovary cells. Glycobiology 16 305 317
73. StillmanBN
HsuDK
PangM
BrewerCF
JohnsonP
2006 Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol 176 778 789
74. StowellSR
ArthurCM
MehtaP
SlaninaKA
BlixtO
2008 Galectin-1, -2, and -3 exhibit differential recognition of sialylated glycans and blood group antigens. J Biol Chem 283 10109 10123
75. SambrookJ
RussellDW
2001 Molecular Cloning: A Laboratory Manual. New York Cold Spring Harbor Laboratory Press 999 p.
76. Stiernagle T (February 11, 2006) Maintenance of C. elegans. In: The C. elegans Research Community, WormBook, doi/10.1895/wormbook.1.101.1, http://www.wormbook.org
77. BarrowsBD
GriffittsJS
AroianRV
2006 Caenorhabditis elegans carbohydrates in bacterial toxin resistance. Methods Enzymol 417 340 358
78. SulstonJ
HodgkinJ
1988 Methods.
WoodWB
The nematode Caenorhabditis elegans New York Cold Spring Harbor Laboratory Press 587 606
79. BoulinT
BessereauJL
2007 Mos1-mediated insertional mutagenesis in Caenorhabditis elegans. Nat Protoc 2 1276 1287
80. HallDH
1995 Electron microscopy and three-dimensional image reconstruction. Methods Cell Biol 48 395 436
81. PoltlG
KernerD
PaschingerK
WilsonIB
2007 N-glycans of the porcine nematode parasite Ascaris suum are modified with phosphorylcholine and core fucose residues. Febs J 274 714 726
82. StrohalmM
HassmanM
KosataB
KodicekM
2008 mMass data miner: an open source alternative for mass spectrometric data analysis. Rapid Commun Mass Spectrom 22 905 908
83. GutterniggM
Kretschmer-LubichD
PaschingerK
RendicD
HaderJ
2007 Biosynthesis of truncated N-linked oligosaccharides results from non-orthologous hexosaminidase-mediated mechanisms in nematodes, plants, and insects. J Biol Chem 282 27825 27840
84. KabschW
1993 Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Crystallogr 26 795 800
85. CollaborativeComputationalProject, No.4 1994 The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr D50 760 763
86. AdamsPD
Grosse-KunstleveRW
HungLW
IoergerTR
McCoyAJ
2002 PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58 1948 1954
87. EmsleyP
CowtanK
2004 Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60 2126 2132
88. DeLanoWL
2008 The PyMOL Molecular Graphics System. Palo Alto, CA, USA DeLano Scientific LLC
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of MetastasisČlánek Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF AirwayČlánek Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- CD8+ T Cell Control of HIV—A Known Unknown
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
- Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- Within-Host Evolution of in Four Cases of Acute Melioidosis
- The Type III Secretion Effector NleE Inhibits NF-κB Activation
- Protease-Sensitive Synthetic Prions
- Histone Deacetylases Play a Major Role in the Transcriptional Regulation of the Life Cycle
- Parasite-Derived Plasma Microparticles Contribute Significantly to Malaria Infection-Induced Inflammation through Potent Macrophage Stimulation
- β-Neurexin Is a Ligand for the MSCRAMM SdrC
- Structure of the HCMV UL16-MICB Complex Elucidates Select Binding of a Viral Immunoevasin to Diverse NKG2D Ligands
- Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF Airway
- Like Will to Like: Abundances of Closely Related Species Can Predict Susceptibility to Intestinal Colonization by Pathogenic and Commensal Bacteria
- Importance of the Collagen Adhesin Ace in Pathogenesis and Protection against Experimental Endocarditis
- N-glycan Core β-galactoside Confers Sensitivity towards Nematotoxic Fungal Galectin CGL2
- Two Plant Viral Suppressors of Silencing Require the Ethylene-Inducible Host Transcription Factor RAV2 to Block RNA Silencing
- A Small-Molecule Inhibitor of Motility Induces the Posttranslational Modification of Myosin Light Chain-1 and Inhibits Myosin Motor Activity
- Temporal Proteome and Lipidome Profiles Reveal Hepatitis C Virus-Associated Reprogramming of Hepatocellular Metabolism and Bioenergetics
- Marburg Virus Evades Interferon Responses by a Mechanism Distinct from Ebola Virus
- B Cell Activation by Outer Membrane Vesicles—A Novel Virulence Mechanism
- Killing a Killer: What Next for Smallpox?
- PPARγ Controls Dectin-1 Expression Required for Host Antifungal Defense against
- TRIM5α Modulates Immunodeficiency Virus Control in Rhesus Monkeys
- Immature Dengue Virus: A Veiled Pathogen?
- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- In Vivo CD8+ T-Cell Suppression of SIV Viremia Is Not Mediated by CTL Clearance of Productively Infected Cells
- Placental Syncytiotrophoblast Constitutes a Major Barrier to Vertical Transmission of
- Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
- The M/GP Glycoprotein Complex of Porcine Reproductive and Respiratory Syndrome Virus Binds the Sialoadhesin Receptor in a Sialic Acid-Dependent Manner
- Social Motility in African Trypanosomes
- Melanoma Differentiation-Associated Gene 5 (MDA5) Is Involved in the Innate Immune Response to Infection In Vivo
- Protection of Mice against Lethal Challenge with 2009 H1N1 Influenza A Virus by 1918-Like and Classical Swine H1N1 Based Vaccines
- Upregulation of xCT by KSHV-Encoded microRNAs Facilitates KSHV Dissemination and Persistence in an Environment of Oxidative Stress
- Persistent ER Stress Induces the Spliced Leader RNA Silencing Pathway (SLS), Leading to Programmed Cell Death in
- Evolutionary Trajectories of Beta-Lactamase CTX-M-1 Cluster Enzymes: Predicting Antibiotic Resistance
- Nucleoporin 153 Arrests the Nuclear Import of Hepatitis B Virus Capsids in the Nuclear Basket
- CD8+ Lymphocytes Control Viral Replication in SIVmac239-Infected Rhesus Macaques without Decreasing the Lifespan of Productively Infected Cells
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- CD8+ T Cell Control of HIV—A Known Unknown
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



