-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Melanoma Differentiation-Associated Gene 5 (MDA5) Is Involved in the Innate Immune Response to Infection In Vivo
The early host response to pathogens is mediated by several distinct pattern recognition receptors. Cytoplasmic RNA helicases including RIG-I and MDA5 have been shown to respond to viral RNA by inducing interferon (IFN) production. Previous in vitro studies have demonstrated a direct role for MDA5 in the response to members of the Picornaviridae, Flaviviridae and Caliciviridae virus families ((+) ssRNA viruses) but not to Paramyxoviridae or Orthomyxoviridae ((−) ssRNA viruses). Contrary to these findings, we now show that MDA5 responds critically to infections caused by Paramyxoviridae in vivo. Using an established model of natural Sendai virus (SeV) infection, we demonstrate that MDA5−/− mice exhibit increased morbidity and mortality as well as severe histopathological changes in the lower airways in response to SeV. Moreover, analysis of viral propagation in the lungs of MDA5−/− mice reveals enhanced replication and a distinct distribution involving the interstitium. Though the levels of antiviral cytokines were comparable early during SeV infection, type I, II, and III IFN mRNA expression profiles were significantly decreased in MDA5−/− mice by day 5 post infection. Taken together, these findings indicate that MDA5 is indispensable for sustained expression of IFN in response to paramyxovirus infection and provide the first evidence of MDA5-dependent containment of in vivo infections caused by (−) sense RNA viruses.
Published in the journal: . PLoS Pathog 6(1): e32767. doi:10.1371/journal.ppat.1000734
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000734Summary
The early host response to pathogens is mediated by several distinct pattern recognition receptors. Cytoplasmic RNA helicases including RIG-I and MDA5 have been shown to respond to viral RNA by inducing interferon (IFN) production. Previous in vitro studies have demonstrated a direct role for MDA5 in the response to members of the Picornaviridae, Flaviviridae and Caliciviridae virus families ((+) ssRNA viruses) but not to Paramyxoviridae or Orthomyxoviridae ((−) ssRNA viruses). Contrary to these findings, we now show that MDA5 responds critically to infections caused by Paramyxoviridae in vivo. Using an established model of natural Sendai virus (SeV) infection, we demonstrate that MDA5−/− mice exhibit increased morbidity and mortality as well as severe histopathological changes in the lower airways in response to SeV. Moreover, analysis of viral propagation in the lungs of MDA5−/− mice reveals enhanced replication and a distinct distribution involving the interstitium. Though the levels of antiviral cytokines were comparable early during SeV infection, type I, II, and III IFN mRNA expression profiles were significantly decreased in MDA5−/− mice by day 5 post infection. Taken together, these findings indicate that MDA5 is indispensable for sustained expression of IFN in response to paramyxovirus infection and provide the first evidence of MDA5-dependent containment of in vivo infections caused by (−) sense RNA viruses.
Introduction
Innate pathogen sensors detect viral products and respond by initiating a signaling cascade that leads to rapid anti-viral response involving secretion of type I IFNs (i.e. IFN-α and IFN-β) and inflammatory cytokines (i.e. IL-6 and TNF-α) [1]. In particular, type I IFNs restrict infection by inhibiting viral replication within cells and by stimulating the innate and adaptive immune responses. Once induced, secreted IFN-α and IFN-β bind to the IFNα receptor on the cell surface in an autocrine or paracrine manner. Activation of this receptor initiates the JAK/STAT signal transduction pathways [2],[3] and the expression of IFN-inducible genes [4]. These gene products increase the cellular resistance to viral infection and sensitize virally-infected cells to apoptosis [5]. In addition, type I IFNs directly activate DC and NK cells, and promote effector functions of T and B cells, thus providing a link between the innate response to infection and the adaptive immune response [6],[7].
Several viral sensors have been identified that belong to the Toll-like receptor (TLR) and RIG-I like receptor (RLR) families [8]. TLRs are expressed on the cell surface and/or in endosomal compartments [9]. TLR3 recognizes double stranded RNA (dsRNA), a molecular pattern associated with replication of single stranded RNA (ssRNA) viruses as well as the genomic RNA of dsRNA viruses [10]. TLR7 and TLR8 recognize ssRNA [9],[11],[12], whereas TLR9 recognizes unmethylated CpG-containing DNA [13]. RLRs are cytoplasmic proteins that recognize viral nucleic acids that have gained access to the cytosol [14]–[19]. The RLR family consists of three known members: retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated gene 5 (MDA5), and LGP2. RIG-I and MDA5 both contain a DExD/H box helicase domain that binds dsRNA, a C-terminal domain and two N-terminal caspase recruitment domains (CARDs) that are involved in signaling [8],[17],[20],[21]. LGP2 contains a helicase domain but lacks the CARDs, and its precise contribution to antiviral signaling remains ambiguous [17],[22].
Though RIG-I and MDA5 share common downstream signaling via activation of IPS-1 (also called MAVS, VISA or Cardif) and IRF3 [23]–[26], these helicases exhibit distinct substrate specificity. In this regard, RIG-I has been shown to preferentially recognize ssRNA that is phosphorylated at the 5′ end [27],[28] and dsRNA molecules which are relatively short [29]–[31]. In contrast, MDA5 recognizes long dsRNAs but does not discern 5′ phosphorylation[30],[32],[33]. This distinct ligand preference has been shown to confer specific recognition of individual viruses: RIG-I has been shown to detect Influenza A and B viruses, paramyxovirus, vesicular stomatitis virus (all (−) ssRNA virues) and some Flaviviruses ((+) ssRNA viruses including Japanese encephalitis virus, Hepatitis C virus and West Nile virus)[16],[33],[34]. In comparison, MDA5 has been shown to selectively detect (+) ssRNA viruses including picornaviruses (encephalomyocarditis virus, Mengo virus and Theilers virus) [32],[33], Caliciviridae (murine norovirus-1) [35], and Flaviridae (West Nile Virus and Dengue Virus) [34],[36]. Accordingly, it is believed that the presence of different classes of sensors may reflect the need for multiple mechanisms to effectively control the wide variety of viral pathogens.
Paramyxoviruses are (−) ssRNA viruses that are responsible for a number of human diseases including those caused by measles, mumps, parainfluenza virus and respiratory syncytial virus (RSV). Importantly, infections caused by paramyxoviruses are the most frequent cause of serious respiratory illness in childhood and are associated with an increased risk of asthma [37],[38]. Sendai virus (SeV) is a murine parainfluenza virus which causes an acute respiratory disease in mice that resembles severe paramyxoviral bronchiolitis found in humans following RSV infection [39]. To date, RIG-I is the only dsRNA sensor that has been implicated in the veritable detection of paramyxoviruses [33],[40]. The importance of RIG-I in the containment of SeV infection is underscored by capacity of SeV C proteins to directly antagonize RIG-I signaling [41] in addition to their ability to inhibit IFN signal transduction [42],[43]. However, paramyxovirus-encoded V proteins are known to directly interfere with MDA5 function by blocking binding of dsRNA [14],[44], thus implicating MDA5 in the containment of paramyxovirus infection as well. In addition, SeV defective interfering (DI) particles have been shown to engage MDA5 in vitro [45], though the in vivo relevancy of this detection mode is unknown. Thus, to determine whether MDA5 functions during natural infection with paramyxovirus in vivo, we assessed mice deficient in MDA5 (MDA5−/− mice) following respiratory tract infection with SeV.
Results
Infection with SeV causes increased morbidity and mortality in MDA5−/− mice
In order to assess an in vivo role for MDA5 in containment of paramyxovirus infection, we infected MDA5−/− mice with Sendai virus (SeV). Mice on a C57BL/6 (B6) background were selected for these experiments as the 129 strain is lethally susceptible to SeV at extremely low inocula [46], thus prohibiting assessment of loss of MDA5 function on this background. A dose of 200,000 pfu was administered to mice by intranasal delivery, an infection method that typically results in acute, non-lethal bronchiolitis in B6 mice. As a gross determinant of virus-induced morbidity, % body weight for infected WT and MDA5−/− mice was monitored for 2 weeks post infection (PI). Though essentially identical % weight loss values were observed up until day 8 PI; onwards, weight loss in MDA5−/− mice was significantly more severe (p<0.05) (Figure 1A). Correspondingly, histological analysis of lung sections obtained from day 12 PI MDA5−/− mice revealed consolidation of the lung parenchyma as well as notable PAS-positive airway cells, an indication of mucus hyper-secretion (Figure 1B). Severe histopathology was not observed in the lung sections obtained from control mice at this time point. In addition, we compared survival following increasing inocula of SeV (Figure 1C). Though MDA5−/− mice were not susceptible to the 200K pfu SeV dose, MDA5−/− mice fully succumbed to 400K and 600K pfu SeV, between 9–14 days PI. In contrast, control mice were fully resistant to the 400K pfu dose, though 40% mortality was observed for controls infected with the 600K dose. Thus MDA5−/− mice exhibit enhanced morbidity and susceptibility to SeV infection relative to control mice.
Fig. 1. Infection with SeV causes increased morbidity and mortality in MDA5−/− mice. 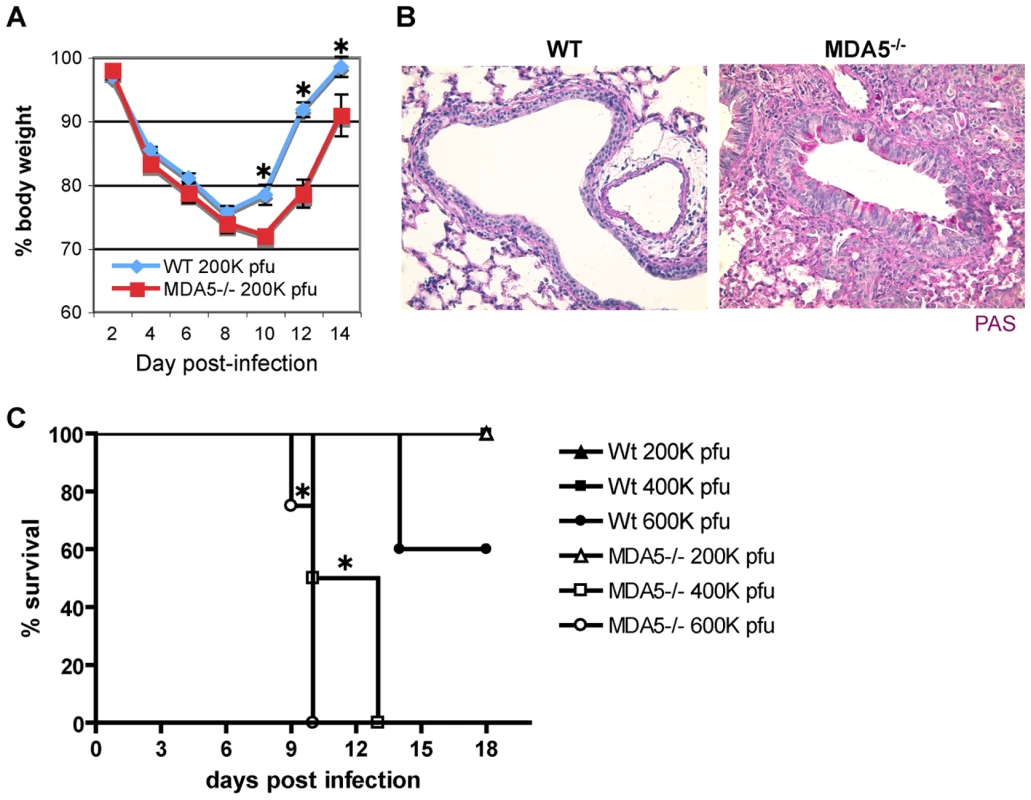
WT and MDA5−/− mice were infected with 200K pfu SeV and assessed for A) loss of body weight over the PI period and B) mucus production (PAS reactivity). C) WT and MDA5−/− mice were infected with 200K, 400K and 600K pfu SeV and assessed for viability. N = 4–16 mice, error bars refer to SEM, * P≤0.05. To more fully assess SeV susceptibility, we extended our analysis of the histological changes seen in the lungs of SeV-infected MDA5−/− mice. H&E stained sections obtained from day 2 PI (not shown) and day 5 PI (Figure 2A) lungs demonstrated similar patterns of bronchiolitis, though peribronchiolar lymphoid cuffing that formed in the lungs of control mice appeared moderately thicker and more densely populated than those of MDA5−/− mice (Figure 2A). FACS analysis of lung-derived leukocytes at d2, d5, and d8 PI revealed no significant differences in lymphoid and myeloid subpopulations (neutrophils, cDC, macrophage and alveolar macrophage; data not shown). Significantly, at d5 and d8 PI, FACS analysis revealed equal relative numbers of lymphoid subpopulations (CD3+, CD19+ and NK1.1+); CD69 expression profiles on these subsets were comparable between strains (data not shown). By d8-9 PI, significant pathology was observed in the lungs of SeV-infected MDA5−/− mice (Figure 2A), despite the fact that comparable numbers of SeV-specific CTL were generated in both strains at this time point (Figure 2B). Grossly, lungs dissected from SeV-infected MDA5−/− mice exhibited enhanced areas of hemorrhage relative to control lungs (data not shown). Microscopic analysis revealed epithelial cells that were notably hyperplastic with abundant micropapillary projections. Additionally, severe bronchointerstitial pneumonia was observed, with alveolar walls adjacent to affected airways thickened and congested with chronic inflammatory cell infiltrates and hyperplastic type II pneumocytes, a lung injury pattern consistent with SeV susceptibility [46],[47]. In comparison, sections obtained from control mice at these later time points exhibited moderate changes to the airway epithelium and mild interstitial infiltration (Figure 2A).
Fig. 2. Increased histopathology in MDA5−/− mice. 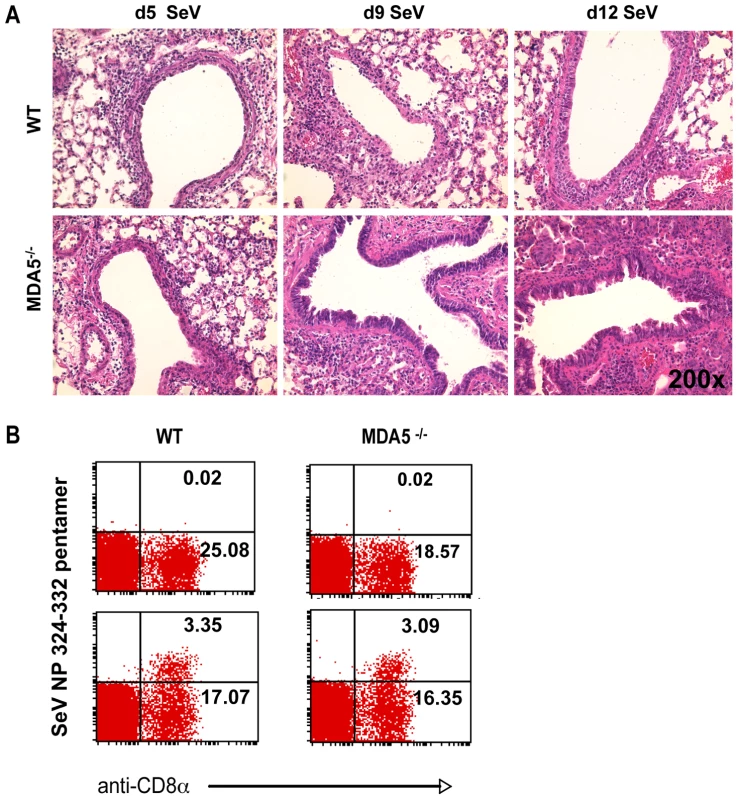
A) H&E micrographs of lung sections obtained from WT and MDA5−/− mice infected with 400K pfu SeV on d5, d9, d12 PI. B) FACS analysis of lymphocytes derived from the lungs of WT and MDA5−/− mice, uninfected (top panels) and d5 post infected (bottom panels) stained with anti-CD8 and H-2Kb: FAPGNYPAL pentamer. MDA5−/− mice demonstrate increased susceptibility to SeV propagation
As susceptibility to SeV infection correlates with increased viral burden [48], we next assessed viral replication in wild type and MDA5−/− mice using a combined approach of real-time PCR analysis and specific staining for SeV antigens. Initially, at d2 PI, IF staining of viral antigens in lung sections appeared comparable between the two strains. By d5 PI, SeV antigens exhibited restrained expression in the airways of control mice (Figure 3A top of panel). In contrast, the bronchioles of MDA5−/− mice remained notably positive for SeV antigens at this time point (Figure 3A bottom of panel). More striking however, was the observation that parenchyma tissues proximal to infected airways stained conspicuously for SeV antigens in MDA5−/− mice at d5 PI. In SeV resistant strains of mice, SeV infection is typically restricted to the mucociliary epithelium of the conducting airways, including the trachea, bronchi and bronchioles [49],[50]. Viral replication that extends to the alveolar spaces is a feature commonly seen in susceptible strains of mice [51]. Accordingly, this pattern of infection supports a role for MDA5 in controlling the replication of SeV during in vivo infection.
Fig. 3. SeV replication is enhanced in MDA5−/− mice. 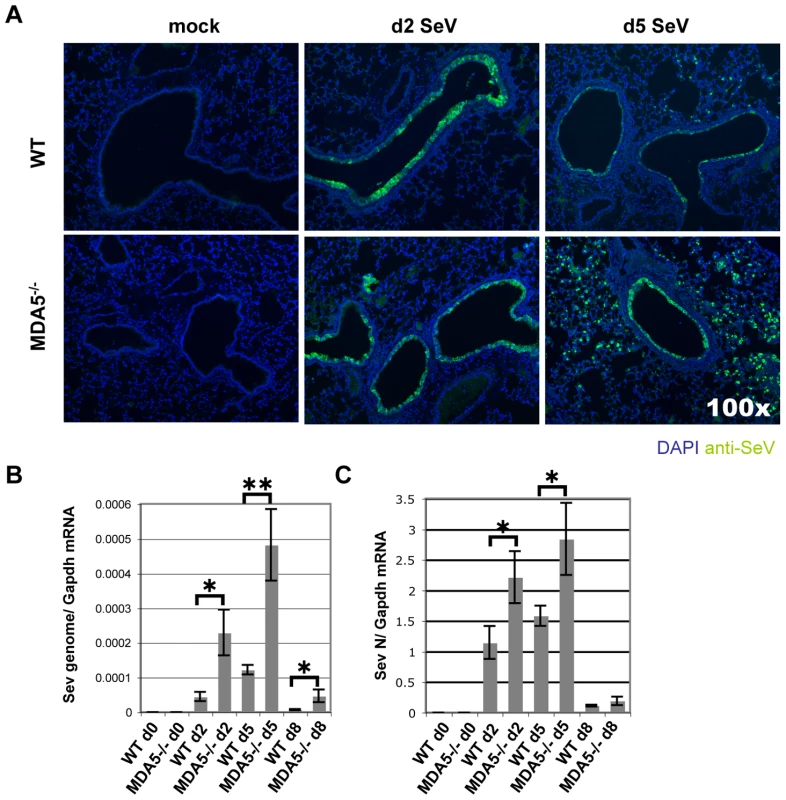
WT and MDA5−/− mice infected with 200K pfu SeV were assessed for A) SeV replication by IF detection of SeV antigens and by real time PCR analysis of B) SeV genome and C) SeV N gene expression. N = 4, error bars refer to SEM, * P<0.05; ** P<0.005. To confirm this finding, we measured viral RNA levels from WT and MDA5−/− mice infected with 200K pfu SeV using real-time PCR analysis. Assessment was made using primer/probe sets designed to amplify SeV genome (3′ untranslated region) and SeV N gene (genomic and transcript) (Figure 3B and C). Using this strategy, an approximate 5 fold increase in SeV genome copy number/Gapdh mRNA was detected on d5 PI, though significant differences were also observed on d2 and d8 PI. Analysis of N gene revealed ∼2 fold increase in expression on days 2 and 5, though there were no significant differences by d8 PI. Thus it appears that MDA5 contributes in part to the containment of SeV replication in vivo.
Cytokine response to SeV infection is altered in MDA5−/− mice
Though SeV is a potent inducer of type I IFN in the mouse, functioning via several distinct pathways, it possesses several mechanisms by which it can counteract the IFN response. Despite this property, induction of IFN expression [14],[52], particularly type I and II, is critical in the containment of SeV infection in vivo as underscored by the profound SeV susceptibility seen for mice deficient in STAT1−/− mice [53]. As MDA5 is known to induce expression of type I IFN in vitro in response to polyI:C stimulation and viral infection [17], we sought to directly assess the ability of MDA5−/− mice to express IFN in response to SeV infection. In this regard, WT and MDA5−/− mice were infected with 200K pfu SeV and subsequently assessed for cytokine expression by real-time PCR analysis over the acute period. While both strains demonstrated comparable mRNA levels at d2 PI, type I IFN expression was dramatically dampened in MDA5−/− mice at d5 PI (Figure 4A and B). Unexpectedly, significant decreases in expression of Ifn-γ, Il-28b (Ifn-λ3) and Tnf-α mRNA were also observed in the lungs of MDA5−/− mice compared to the WT cohort, with the most dramatic difference observed for Il-28b mRNA expression (Figure 4C, D and E). In contrast, Il-1β, and Il-10 mRNA levels were not significantly different across strains, though the levels of Il-6 mRNA was markedly increased in MDA5−/− mice following infection (Figure 4F, G and H). Accordingly, MDA5 appears to control the expression of SeV-induced anti-viral cytokines, particularly type I, II and III IFNs, during the late acute period (d5 PI), but does not appear to be involved during the immediate early response. Importantly, the decrease in IFN expression coincides with expanded viral propagation in the MDA5−/− mice, suggesting that reduced IFN expression during this time point accounts for the corresponding increased viral burden.
Fig. 4. MDA5 is required for sustained expression of cytokines in response to SeV infection. 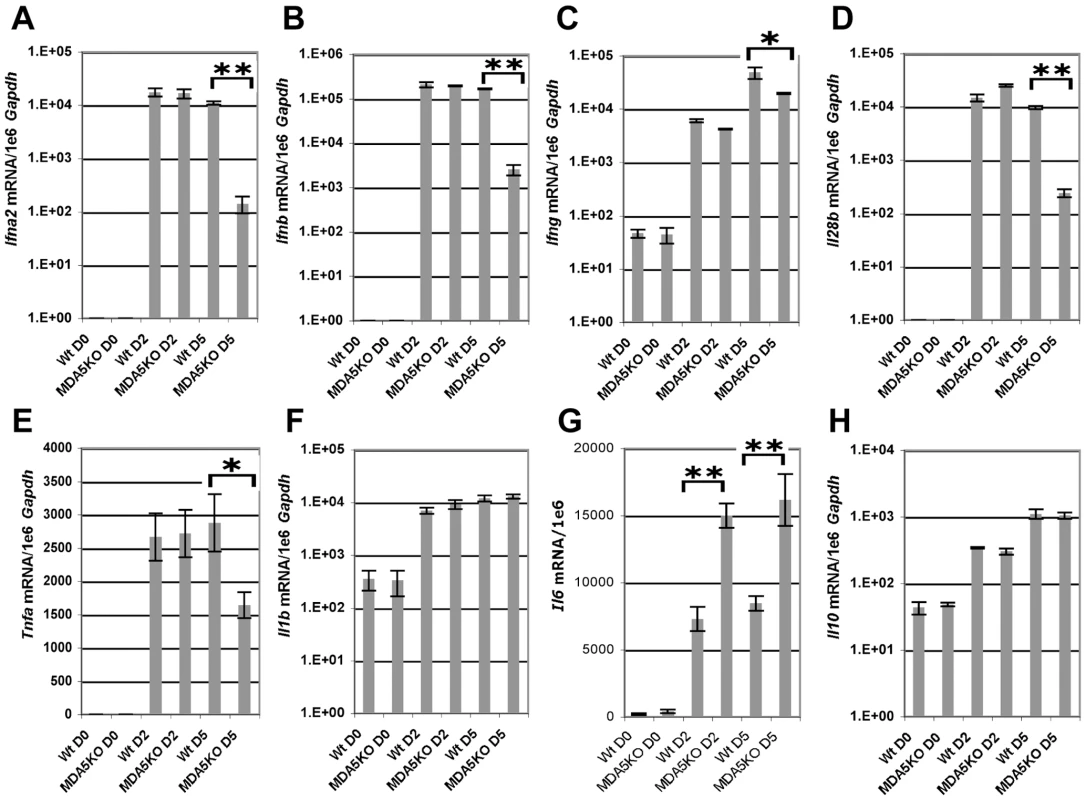
Real time PCR analysis of whole lung homogenates obtained from WT and MDA5−/− mice infected with 200K pfu SeV for expression levels of A) Ifn-α2, B) Ifn-β, C) Ifn-γ, D) Il-28b, E) Tnf-α, F) Il-1β, G) Il-6 and H) Il-10 mRNA. N = 4, error bars refer to SEM, * P<0.05, ** P<0.00001. Induction of IFN expression transactivates expression of a number of IFN response genes through a signal transduction cascade involving JAK/STAT activation. MDA5 and RIG-I are among the genes induced by IFN signaling in vitro [20]. To determine the expression profile of MDA5 and RIG-I in the airways of mice infected with SeV, mRNA was measured by real-time PCR analysis from whole lung homogenates obtained from WT mice infected with 200K pfu SeV. Expression of Mda5 and Rig-i mRNA was significantly increased at d2 and d5 PI, though the levels began to decline by d8 PI (Figure 5A). Lastly, to determine the tissue distribution of MDA5 expression, lung sections from d5 PI mice were stained with anti-MDA5 polyclonal antibodies. Visualization of MDA5-specific staining was performed using tyramide-based amplification. IF microscopic analysis of affected airways revealed a pattern of MDA5 expression that was primarily restricted to the airway epithelium, though expression was also detected in cells of the proximal interstitum, in particular, in cells that appeared to resemble type II pneumocytes and alveolar macrophage (Figure 5B). Sections from MDA5−/− mice did not stain for MDA5, confirming the specificity of anti-MDA5 staining. Accordingly these findings indicate that MDA5 is induced following SeV infection and that the lack of expression in MDA5−/− mice accounts for the phenotype described at the later time point.
Fig. 5. Infection with SeV results in induction of antiviral sensor expression. 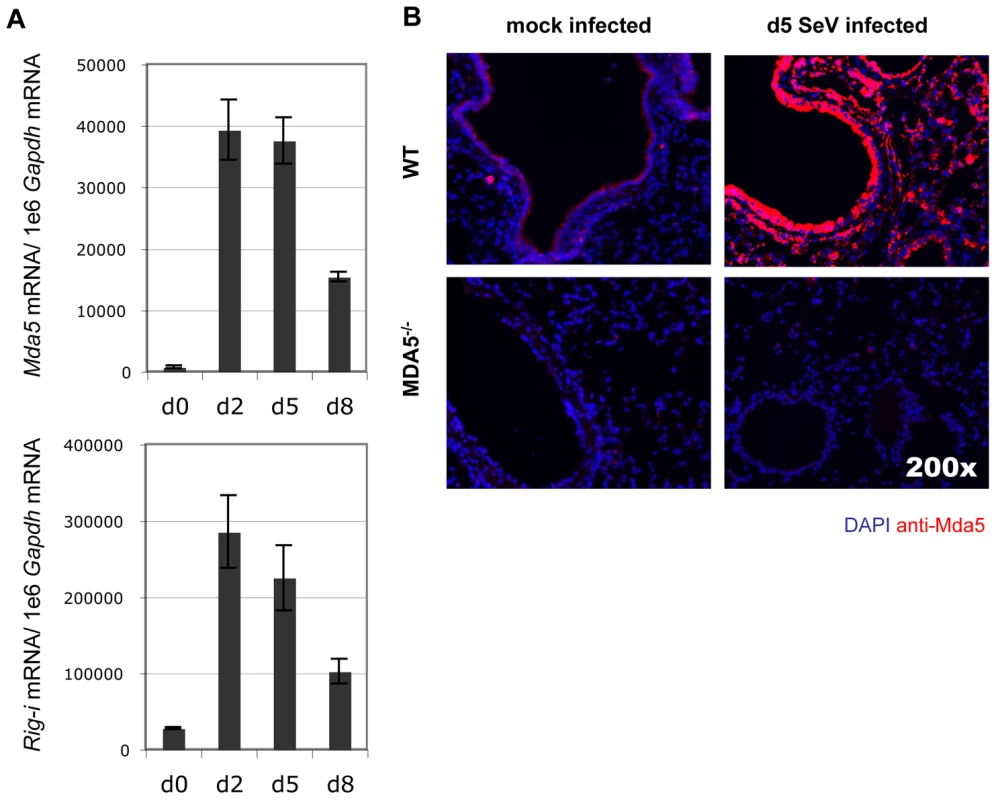
A) Analysis of Mda5 and Rig-I mRNA expression in WT mice during the acute SeV infection period as determined by real-time PCR analysis. B) Micrographs taken of lung sections obtained from WT and MDA5−/− mice infected with 200K pfu SeV and stained for MDA5 expression. N = 4, error bars refer to SEM, * P<0.05. Discussion
Our understanding of innate immune factors that recognize and respond to pathogens has greatly expanded over the last decade. A major component of the RNA virus detection system in mammals involves members of the RLR family, including RIG-I, MDA5, and LGP2 [1]. Elucidating a role for the RLRs in virus-induced IFN production has been facilitated by the availability of RIG-I−/− and MDA5−/− mice [32],[33]. Initial observations using embryonic fibroblasts and bone marrow derived DCs generated from these mice revealed striking phenotypes including a failure to produce IFN in response to a wide cross-section of viruses and nucleic acids, and an inability to contain viral replication. Specifically, MDA5 was found to be the sole receptor for picornaviruses and caliciviruses ((+) ssRNA viruses) [32],[33],[35], whereas RIG-I was described as the receptor for (−) ssRNA viruses such as paramyxoviruses and orthomyxoviruses, as well as for (+) ssRNA viruses belonging to the Flaviridae family [16],[33]. However, our understanding of these virus recognition systems in vivo is limited, in part because RIG-I−/− mice die perinatally.
The precise molecular patterns of virus replication recognized by RIG-I and MDA5 are still not fully clear. Initially, a mimic of viral dsRNA, polyI:C, was found to bind and activate RIG-I. However, ensuing research identified 5′-triphosphate-linked ssRNA as the major RIG-I inducer [27],[28]. Furthermore, in vitro data obtained using knockout mice suggested in fact that MDA5, and not RIG-I, recognizes polyI:C, thereby formulating a recognition model whereby RIG-I recognizes short 5′-triphosphorylated RNAs, while MDA5 recognizes dsRNA structures irrespective of the 5′ cap [8], [30]–[32]. However, more thorough dissection of the helicase binding function and activation process has recently determined that the picture is indeed more complex than previously thought [29],[40]. In this regard, both helicases have been shown to recognize dsRNA, in a manner that is likely dependent on its length, while RIG-I demonstrates the added ability to respond to 5′-triphosphate ssRNA products. To complicate these paradigms, there is increasing evidence that viruses have evolved various properties aimed at antagonizing or degrading viral sensors. Thus, our understanding of viral recognition by the RLR helicases is evolving.
With respect to molecular sensing of paramyxovirus infection, both 5′-triphosphorylated ssRNA and long dsRNA species are likely present in SeV-infected cells, thereby implicating both MDA5 and RIG-I in the antiviral sensing process. However, in vitro studies concur that cultured embryonic fibroblasts and bone marrow-derived DC cells detect SeV RNA chiefly through RIG-I, whereas MDA5 and TLR3 are dispensable [33],[34],[41],[54]. TLR7 and TLR8 in myeloid cells have also been shown to recognize SeV RNA in vitro as well [55]. Regardless, it cannot be excluded that in vivo, other RNA sensors, including MDA5, may contribute, at least in part, to anti-SeV responses. Indeed, a recent study by Yount et al. has demonstrated that MDA5 can detect SeV DI particles in vitro [45]. The relevancy of this recognition system in vivo is uncertain; certainly in our hands, using SeV/52, which has a limited ability to form DI particles, as per PCR-based analysis (data not shown), MDA5 appears to exert a significant effect on viral containment. Most importantly however, SeV encodes a V protein that specifically binds to and blocks MDA5 signaling in vitro [14],[44]. Thus, it is possible that MDA5 does, indeed, detect SeV in vitro, but that it is functionally curtailed by the V protein in this circumstance. Interestingly, in our hands, in vivo infections using SeV with V protein deletion resulted in no real effect on mortality or type I IFN induction across strains (data not shown), likely explained by the fact that deletion of V protein in SeV markedly attenuates virulence and pathogenicty in vivo [56].
While initial characterization of MDA5-deficient cells has not supported a role for MDA5 in containment of SeV, these studies have been limited to observations made in cultured embryonic fibroblasts and in vitro-derived dendritic cells; populations which are not primary targets for SeV replication in the course of the natural infection. Rather, SeV replication mostly occurs in the airway epithelium of the conducting airways [49],[50]. For these reasons, we hypothesized that SeV propagation would be sensitive to the MDA5 status of the host in the context of an in vivo infection. Indeed, the epithelial cells constitutively express MDA5 at low levels and subsequently up-regulate expression in response to SeV (Figure 5B), a finding that supports the relevance of this RNA helicase to SeV and other airborne infections. Interestingly, MDA5 deficiency did not influence the composition of the inflammatory infiltrate (data not shown), implying that the immune defect is largely restricted to the airway epithelium, the site of viral replication. This is compatible with our earlier findings using STAT-1−/− chimeras, wherein we observed that loss of IFN response in the stromal compartment alone accounted for the immune deficiency to SeV [53]. We therefore sought to further assess the significance of MDA5 in the control of SeV infection in vivo. In this regard we have demonstrated that MDA5 controls SeV replication and spread through induction of type I IFNs, but that this effect appears late (d5 PI), as IFN gene transcription is not impaired on d2 PI (Figure 4). It is likely that the initial IFN response is sufficient to initiate a range of immune responses, such that the late reduction in IFN transcripts results only in a 2–3 fold change in LD50 (Figure 1). Whether this specific IFN pattern remains true for other viruses as well remains to be tested. This surprising collapse of the host type I IFN response at d5 PI is accompanied by parallel decreases in the level of Il-28b and Tnf-α expression (Figure 4), and, curiously, decreased Ifn-γ transcript levels. This later observation may reflect a selective role for MDA5 in the induction of IFN-γ expression by NK cells. Lastly, the MDA5 status does not appear to influence the levels of IL-1β, or IL-10 or the ability of the host to mount a virus-specific CTL response. However, the levels of Il-6 mRNA in whole lung homogenates derived from d2 and d5 PI MDA5−/− was markedly increased, suggesting the induction of compensatory mechanisms in the context of MDA5 deficiency that could potentially account for the enhanced morbidity and mortality seen in the MDA5−/− mice.
An additional concern raised by these data is the relative contribution of MDA5 and RIG-I in the response to virus. In light of the existing literature [33],[40], it seems likely that RIG-I is responsible for the normal IFN response to SeV early in the infection. Indeed, as depicted in Figure 5A, RIG-I is strongly induced early on during infection. Why the later IFN response depends on MDA5 is not known. MDA5 is encoded by an IFN-upregulated transcript, and it remains possible that it is the accumulation of MDA5 that allows for the subsequent MDA5-dependent IFN response on d5 PI. Yet other IFN-induced genes, notably RIG-I, are also upregulated by IFN, which should provide additional antiviral protection in vivo. Interestingly, SeV encodes a nested set of C proteins that have been shown to impede IFN signaling through direct inhibition of STAT signaling [41],[42] and which are also known to strongly antagonize RIG-I function [41]. Furthermore, SeV-V proteins have been shown to have direct inhibitory effects on both MDA5 and RIG-I signaling [41],[44]. Thus it remains possible that the effects of SeV V and C proteins have an accumulative effect on RIG-I function that essentially overwhelms this sensor at d5 PI, and that in this context, MDA5 plays an essential role in containment of SeV. Since assessment of the relative contribution of RIG-I and MDA5 in containment of SeV infection in vivo is not possible, a possible next step in assessing the importance of MDA5 function would involve assessment in MDA5−/− and IPS-1−/− strains.
We envision several possibilities that could potentially explain this dramatic effect of MDA5. The first is that, in the absence of MDA5, the balance between virus replication and the IFN response is disrupted sufficiently, such that by d5 PI, virus replication has overwhelmed the response in a qualitative fashion –presumably through direct cytotoxic effects or via the overproduction of immunosuppressive C proteins. This possibility is supported by the fact that SeV is replicating to higher levels in the MDA5−/− lung already by d2 PI (Figure 3B). Indeed, in support of this hypothesis, we observe a striking increase in SeV replication that spreads extensively into the interstitium of MDA5−/− lungs compared to controls. Another possible explanation, which we have not assessed, is an apoptotic response potentially mediated by MDA5. In this scenario, MDA5 would instruct or sensitize infected cells to commit suicide so as to shut down viral replication in infected cells. Indeed, ectopic expression of MDA5 in a melanoma cell line has been shown to inhibit colony formation, presumably through induction of apoptosis [20], and IPS-1 overexpression induces cell death, as well [57]. In fact, SeV-dependent apoptotic signaling requires IRF3 [58]. In the case of MDA5 deficiency, loss of pro-apoptotic activity could lead to a robust increase in viral replication and enhanced IFN blockade through overexpression of SeV C proteins. This possibility is favored by the fact that, despite a normal IFN response on d2 PI (Figure 4), the virus is found to be replicating at higher titers (Figure 3B).
It seems likely that the inability of the MDA5−/− animals to sustain an IFN response leads to increased viral replication and dissemination on d5 PI, thus causing significantly higher morbidity and mortality in the knockout cohort (Figure 1). It is important to note, however, that the effects of MDA5 deficiency on SeV replication are much milder than expected if MDA5 were the sole target of the V protein. Indeed, SeV V mutants (SeV-ΔV) are severely attenuated; replication is demonstrably abrogated in the lungs by d2 PI [56]. IRF3 deficiency of the host restores SeV-ΔV pathogenicity, suggesting that the mutant virus acts by blocking IRF3 signaling [59]. Yet disease caused by SeV in MDA5−/− mice is milder than the disease seen in the IRF3−/− animals. Consequently, we believe that the V protein must have additional targets besides MDA5. In this regard, it has recently been demonstrated that Lgp2 encodes a helicase epitope that is akin to the MDA5 helicase, the portion of MDA5 that binds paramyxovirus V proteins [60], thereby suggesting that LGP2 may be an additional V protein target. In this case, a MDA5-LGP2 double knockout mouse may potentially phenocopy the IRF3 mutation in its response to SeV infection.
Taken together, our findings demonstrate that MDA5 significantly contributes to the response to paramyxovirus and constitute the first in vivo demonstration of MDA5 activity against a negative-strand virus. As such, it appears likely that MDA5 has a wider specificity as a viral nucleic acid receptor than initially believed, and that the initial clear-cut cases of either MDA5 or RIG-I being the sole receptor for a given virus will prove to be exceptions rather than rules when studied in the context of in vivo infections.
Materials and Methods
Mouse generation, maintenance and infection
Control C57BL/6J (B6) mice used in these experiments were purchased from JAX. MDA5−/− mice [32] were backcrossed onto the B6 background to 99.9% congenicity. For in vivo SeV infection, Sendai/52 Fushimi strain was instilled intranasally into deeply anesthetized mice and at the indicated time points, mice were humanly sacrificed for harvest of lung tissue. Virus was purchased from the ATCC and subject to two rounds of in vitro plaque purification in Vero cells to eliminate the presence of DI particles. A clone thus identified was then subject to a single round of amplification in embryonated chicken eggs following inoculation of ∼1000 PFU. 24–36 hr post inoculation, SeV was isolated from the allantoic fluids and diluted in phosphate-buffered solution to generate a viral stock that was subsequently characterized on the basis of in vivo infectious properties. Calculation of PFU was performed by standard plaque assay using either Vero E6 cells or LLC-MK2 cells. Importantly, propagation under these conditions does not favor the formation of DI particles, a process that occurs most frequently when virus is repeatedly passaged at high MOI. Indeed, PCR analysis of stock virus indicated the absence of DI genomes. The methods for mice use and care were approved by the Washington University Animal Studies Committee and are in accordance with NIH guidelines.
FACS
Single cell lung suspensions were made from minced lung tissue subjected to collagenase/hyaluronidase/DNAse I digestion. Staining of surface markers was performed using FcR block and fluorochrome-conjugated mAbs. To immunophenotype the immune infiltrate, specific combinations of mAbs were chosen which discern granulocytes (Ly6G+), macrophages (F4/80+), cDC (CD11c+F4/80−Siglec-H−), pDC (Siglec-H+ CD11cmid), NK cells (NK1.1+NKp46+), T cells (CD3+CD4+/−CD8+/−) and B cells (CD19+). SeV-specific PE-labeled pentamer Kb:FAPGNYPAL (NP 324-332) was purchased from Proimmune; cells were stained with CD8 and counterstained with propidium iodide, F4/80 and CD19 to eliminate background. Activation status was determined using specific mAbs for MHC-II, NKG2D and CD69. Samples were acquired on a FACScalibur (BD Biosciences) and analyzed using Cellquest software.
Analysis of mRNA and virus-specific RNA
RNA was purified from lung homogenate using Trizol Reagent (Invitrogen). RNA was treated with RNAse-free DNAse I (Ambion) to eliminate genomic DNA. RNA was converted to cDNA using the High-Capacity cDNA Archive kit (Applied Biosystems). Target mRNA and viral RNAs were quantified by real-time PCR using specific fluorogenic probes and primers and the Fast Universal PCR Master Mix system (Applied Biosystems). Primer sets and probes for mouse Ifn-α2 (Mm00833961_s1), Ifn-β (Mm00439552_s1), Ifn-γ (Mm00801778-m1), Il-28b (Mm00663660_g1), Tnf-α (Mm00443259_g1), Mda5 (Mm00459183_m1), Il-1β (Mm00434227_g1), Il-6 (Mm00446190_m1), Il-10 (Mm00439616_m1) mRNA and SeV genome and Gapdh mRNA were purchased from Applied Biosystems. Samples were assayed on the 7500 Fast Real-Time PCR System and analyzed using the 7500 Fast System Software (Applied Biosystems). Levels of specific gene expression were standardized to Gapdh mRNA expression levels.
Histology
Lungs were perfused and fixed with 4% paraformaldehyde. Tissue was embedded in paraffin, cut into 5 um sections and adhered to charged slides. Sections were deparaffinized in Citrosolv (Fisherbrand), hydrated, and in the case of IF-microscopy, treated to heat-activated antigen unmasking solution (Vector Laboratories, Inc). H&E and PAS sections were visualized by brightfield microscopy. Expression analysis was performed by IF using chicken polyclonal anti-SeV (Jackson ImmunoResearch Laboratories, Inc) and rabbit polyclonal anti-mouse MDA5 (Axxora Life Sciences, Inc). Biotinylated secondary antibodies were purchased from Vector Laboratories, Inc). SeV and MDA5 expression was visualized using tyramide-based signal amplification with Alexa Fluor 488 or 594 fluorochromes (Invitrogen). Slides were counterstained with DAPI mounting media (Vector Laboratories, Inc). Microscopy was performed using an Olympus BX51 microscope.
Statistical analyses
Real-time PCR data was analyzed using an unpaired Student's t-test. If variances were unequal, Welch's correction was applied. Charted values represent mean ± SEM. Survival statistics were determined using by Kaplan-Meier analysis of paired cohorts. P values below 0.05 were regarded as being significant for all analyses. Experiments were repeated a minimum of three times.
Zdroje
1. KawaiT
AkiraS
2008 Toll-like Receptor and RIG-1-like Receptor Signaling. Annals of the New York Academy of Sciences 1143 1 20
2. KotenkoSV
PestkaS
2000 Jak-Stat signal transduction pathway through the eyes of cytokine class II receptor complexes. Oncogene 19 2557 2565
3. SchindlerC
DarnellJE
1995 Transcriptional Responses to Polypeptide Ligands: The JAK-STAT Pathway. Annual Review of Biochemistry 64 621 652
4. de VeerMJ
HolkoM
FrevelM
WalkerE
DerS
2001 Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol 69 912 920
5. StetsonDB
MedzhitovR
2006 Type I Interferons in Host Defense. Immunity 25 373 381
6. BraunD
CaramalhoI
DemengeotJ
2002 IFN-{alpha}/{beta} enhances BCR-dependent B cell responses. Int Immunol 14 411 419
7. ToughDF
2004 Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leukemia & Lymphoma 45 257 264
8. SaitoT
HiraiR
LooY-M
OwenD
JohnsonCL
2007 Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proceedings of the National Academy of Sciences 104 582 587
9. IwasakiA
MedzhitovR
2004 Toll-like receptor control of the adaptive immune responses. Nat Immunol 5 987 995
10. AlexopoulouL
HoltAC
MedzhitovR
FlavellRA
2001 Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413 732 738
11. DieboldSS
KaishoT
HemmiH
AkiraS
Reis e SousaC
2004 Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA.[see comment]. Science 303 1529 1531
12. HeilF
HemmiH
HochreinH
AmpenbergerF
KirschningC
2004 Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8.[see comment]. Science 303 1526 1529
13. BauerS
KirschningCJ
HäckerH
RedeckeV
HausmannS
2001 Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proceedings of the National Academy of Sciences of the United States of America 98 9237 9242
14. AndrejevaJ
ChildsKS
YoungDF
CarlosTS
StockN
2004 The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proceedings of the National Academy of Sciences of the United States of America 101 17264 17269
15. RothenfusserS
GoutagnyN
DiPernaG
GongM
MonksBG
2005 The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. Journal of Immunology 175 5260 5268
16. SumpterRJr
LooYM
FoyE
LiK
YoneyamaM
2005 Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. Journal of Virology 79 2689 2699
17. YoneyamaM
KikuchiM
MatsumotoK
ImaizumiT
MiyagishiM
2005 Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. Journal of Immunology 175 2851 2858
18. YoneyamaM
KikuchiM
NatsukawaT
ShinobuN
ImaizumiT
2004 The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses.[see comment]. Nature Immunology 5 730 737
19. KomuroA
HorvathCM
2006 RNA and Virus-Independent Inhibition of Antiviral Signaling by RNA Helicase LGP2. Journal of Virology
20. KangDC
GopalkrishnanRV
WuQ
JankowskyE
PyleAM
2002 mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proceedings of the National Academy of Sciences of the United States of America 99 637 642
21. KovacsovicsM
MartinonF
MicheauO
BodmerJL
HofmannK
2002 Overexpression of Helicard, a CARD-containing helicase cleaved during apoptosis, accelerates DNA degradation.[erratum appears in Curr Biol. 2002 Sep 17;12(18):1633.]. Current Biology 12 838 843
22. VenkataramanT
ValdesM
ElsbyR
KakutaS
CaceresG
2007 Loss of DExD/H Box RNA Helicase LGP2 Manifests Disparate Antiviral Responses. J Immunol 178 6444 6455
23. KawaiT
TakahashiK
SatoS
CobanC
KumarH
2005 IPS-1, an adaptor triggering RIG-I - and Mda5-mediated type I interferon induction.[see comment]. Nature Immunology 6 981 988
24. MeylanE
CurranJ
HofmannK
MoradpourD
BinderM
2005 Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437 1167 1172
25. SethRB
SunL
EaCK
ChenZJ
2005 Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3.[see comment]. Cell 122 669 682
26. XuLG
WangYY
HanKJ
LiLY
ZhaiZ
2005 VISA is an adapter protein required for virus-triggered IFN-beta signaling. Molecular Cell 19 727 740
27. HornungV
EllegastJ
KimS
BrzozkaK
JungA
2006 5′-Triphosphate RNA Is the Ligand for RIG-I. Science 314 994 997
28. PichlmairA
SchulzO
TanCP
NaslundTI
LiljestromP
2006 RIG-I-Mediated Antiviral Responses to Single-Stranded RNA Bearing 5′-Phosphates. Science 314 997 1001
29. KatoH
TakeuchiO
Mikamo-SatohE
HiraiR
KawaiT
2008 Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med 205 1601 1610
30. SaitoT
GaleMJr
2008 Differential recognition of double-stranded RNA by RIG-I-like receptors in antiviral immunity. J Exp Med 205 1523 1527
31. SaitoT
OwenDM
JiangF
MarcotrigianoJ
GaleMJr
2008 Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature 454 523 527
32. GitlinL
BarchetW
GilfillanS
CellaM
BeutlerB
2006 Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proceedings of the National Academy of Sciences of the United States of America 103 8459 8464
33. KatoH
TakeuchiO
SatoS
YoneyamaM
YamamotoM
2006 Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441 101 105
34. LooY-M
FornekJ
CrochetN
BajwaG
PerwitasariO
2008 Distinct RIG-I and MDA5 Signaling by RNA Viruses in Innate Immunity. J Virol 82 335 345
35. McCartneySA
ThackrayLB
GitlinL
GilfillanS
Virgin IvHW
2008 MDA-5 Recognition of a Murine Norovirus. PLoS Pathog 4 e1000108 doi:10.1371/journal.ppat.1000108
36. FredericksenBL
KellerBC
FornekJ
KatzeMG
GaleMJr
2008 Establishment and Maintenance of the Innate Antiviral Response to West Nile Virus Involves both RIG-I and MDA5 Signaling through IPS-1. J Virol 82 609 616
37. CollinsPL
ChanockRM
McIntoshK
1996 Parainfluenza Viruses.
Fields
Fields Virology Philadelphia Lippincott-Raven
38. CollinsPL
ChanockRM
McIntoshK
1996 Respiratory Syncytial Virus. Fields Virology Philadelphia Lippincott-Raven
39. WalterMJ
KajiwaraN
KaranjaP
CastroM
HoltzmanMJ
2001 Interleukin 12 p40 Production by Barrier Epithelial Cells during Airway Inflammation. J Exp Med 193 339 352
40. HausmannS
MarqJ-B
TapparelC
KolakofskyD
GarcinD
2008 RIG-I and dsRNA-Induced IFNÎ2 Activation. PLoS ONE 3 e3965 doi:10.1371/journal.pone.0003965
41. StrahleL
MarqJ-B
BriniA
HausmannS
KolakofskyD
2007 Activation of the Beta Interferon Promoter by Unnatural Sendai Virus Infection Requires RIG-I and Is Inhibited by Viral C Proteins. J Virol 81 12227 12237
42. GarcinD
LatorreP
KolakofskyD
1999 Sendai Virus C Proteins Counteract the Interferon-Mediated Induction of an Antiviral State. J Virol 73 6559 6565
43. GotohB
TakeuchiK
KomatsuT
YokooJ
KimuraY
1999 Knockout of the Sendai virus C gene eliminates the viral ability to prevent the interferon-[alpha]/[beta]-mediated responses. FEBS Letters 459 205 210
44. ChildsK
StockN
RossC
AndrejevaJ
HiltonL
2007 mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology 359 190 200
45. YountJS
GitlinL
MoranTM
LopezCB
2008 MDA5 Participates in the Detection of Paramyxovirus Infection and Is Essential for the Early Activation of Dendritic Cells in Response to Sendai Virus Defective Interfering Particles. J Immunol 180 4910 4918
46. ParkerJC
WhitemanMD
RichterCB
1978 Susceptibility of inbred and outbred mouse strains to Sendai virus and prevalence of infection in laboratory rodents. Infect Immun 19 123 130
47. ItohT
IwaiH
UedaK
1991 Comparative lung pathology of inbred strain of mice resistant and susceptible to Sendai virus infection. Journal of Veterinary Medical Science 53 275 279
48. MoXY
SarawarSR
DohertyPC
1995 Induction of cytokines in mice with parainfluenza pneumonia. J Virol 69 1288 1291
49. BlandfordG
HeathRB
1972 Studies on the immune response and pathogenesis of Sendai virus infection of mice. I. The fate of viral antigens. Immunology 22 637 649
50. DegréM
MidtvedtT
1971 Respiratory infection with parainfluenza 1, Sendai virus in gnotobiotic and conventional mice. Acta pathologica et microbiologica Scandinavica Section B: Microbiology and immunology 79 123 124
51. BrownsteinDGSA
JohnsonEA
1981 Sendai virus infection in genetically resistant and susceptible mice. The American journal of pathology 105 156 163
52. TakeuchiK
KomatsuT
YokooJ
KatoA
ShiodaT
2001 Sendai virus C protein physically associates with Stat1. Genes to Cells 6 545 557
53. ShornickLP
WellsAG
ZhangY
PatelAC
HuangG
2008 Airway Epithelial versus Immune Cell Stat1 Function for Innate Defense against Respiratory Viral Infection. J Immunol 180 3319 3328
54. LopezCB
MoltedoB
AlexopoulouL
BonifazL
FlavellRA
2004 TLR-Independent Induction of Dendritic Cell Maturation and Adaptive Immunity by Negative-Strand RNA Viruses. J Immunol 173 6882 6889
55. MelchjorsenJ
JensenSB
MalmgaardL
RasmussenSB
WeberF
2005 Activation of innate defense against a paramyxovirus is mediated by RIG-I and TLR7 and TLR8 in a cell-type-specific manner. Journal of Virology 79 12944 12951
56. KatoA
KiyotaniK
SakaiY
YoshidaT
NagaiY
1997 The paramyxovirus, Sendai virus, V protein encodes a luxury function required for viral pathogenesis. EMBO (European Molecular Biology Organization) Journal 16 578 587
57. LeiY
MooreCB
LiesmanRM
O'ConnorBP
BergstralhDT
2009 MAVS-Mediated Apoptosis and Its Inhibition by Viral Proteins. PLoS ONE 4 e5466 doi:10.1371/journal.pone.0005466
58. PetersK
ChattopadhyayS
SenGC
2008 IRF-3 Activation by Sendai Virus Infection Is Required for Cellular Apoptosis and Avoidance of Persistence. J Virol 82 3500 3508
59. KiyotaniK
SakaguchiT
KatoA
NagaiY
YoshidaT
2007 Paramyxovirus Sendai virus V protein counteracts innate virus clearance through IRF-3 activation, but not via interferon, in mice. Virology 359 82 91
60. ParisienJ-P
BammingD
KomuroA
RamachandranA
RodriguezJJ
2009 A Shared Interface Mediates Paramyxovirus Interference with Antiviral RNA Helicases MDA5 and LGP2. J Virol JVI.00153 00109
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of MetastasisČlánek Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF AirwayČlánek Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- CD8+ T Cell Control of HIV—A Known Unknown
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
- Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- Within-Host Evolution of in Four Cases of Acute Melioidosis
- The Type III Secretion Effector NleE Inhibits NF-κB Activation
- Protease-Sensitive Synthetic Prions
- Histone Deacetylases Play a Major Role in the Transcriptional Regulation of the Life Cycle
- Parasite-Derived Plasma Microparticles Contribute Significantly to Malaria Infection-Induced Inflammation through Potent Macrophage Stimulation
- β-Neurexin Is a Ligand for the MSCRAMM SdrC
- Structure of the HCMV UL16-MICB Complex Elucidates Select Binding of a Viral Immunoevasin to Diverse NKG2D Ligands
- Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF Airway
- Like Will to Like: Abundances of Closely Related Species Can Predict Susceptibility to Intestinal Colonization by Pathogenic and Commensal Bacteria
- Importance of the Collagen Adhesin Ace in Pathogenesis and Protection against Experimental Endocarditis
- N-glycan Core β-galactoside Confers Sensitivity towards Nematotoxic Fungal Galectin CGL2
- Two Plant Viral Suppressors of Silencing Require the Ethylene-Inducible Host Transcription Factor RAV2 to Block RNA Silencing
- A Small-Molecule Inhibitor of Motility Induces the Posttranslational Modification of Myosin Light Chain-1 and Inhibits Myosin Motor Activity
- Temporal Proteome and Lipidome Profiles Reveal Hepatitis C Virus-Associated Reprogramming of Hepatocellular Metabolism and Bioenergetics
- Marburg Virus Evades Interferon Responses by a Mechanism Distinct from Ebola Virus
- B Cell Activation by Outer Membrane Vesicles—A Novel Virulence Mechanism
- Killing a Killer: What Next for Smallpox?
- PPARγ Controls Dectin-1 Expression Required for Host Antifungal Defense against
- TRIM5α Modulates Immunodeficiency Virus Control in Rhesus Monkeys
- Immature Dengue Virus: A Veiled Pathogen?
- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- In Vivo CD8+ T-Cell Suppression of SIV Viremia Is Not Mediated by CTL Clearance of Productively Infected Cells
- Placental Syncytiotrophoblast Constitutes a Major Barrier to Vertical Transmission of
- Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
- The M/GP Glycoprotein Complex of Porcine Reproductive and Respiratory Syndrome Virus Binds the Sialoadhesin Receptor in a Sialic Acid-Dependent Manner
- Social Motility in African Trypanosomes
- Melanoma Differentiation-Associated Gene 5 (MDA5) Is Involved in the Innate Immune Response to Infection In Vivo
- Protection of Mice against Lethal Challenge with 2009 H1N1 Influenza A Virus by 1918-Like and Classical Swine H1N1 Based Vaccines
- Upregulation of xCT by KSHV-Encoded microRNAs Facilitates KSHV Dissemination and Persistence in an Environment of Oxidative Stress
- Persistent ER Stress Induces the Spliced Leader RNA Silencing Pathway (SLS), Leading to Programmed Cell Death in
- Evolutionary Trajectories of Beta-Lactamase CTX-M-1 Cluster Enzymes: Predicting Antibiotic Resistance
- Nucleoporin 153 Arrests the Nuclear Import of Hepatitis B Virus Capsids in the Nuclear Basket
- CD8+ Lymphocytes Control Viral Replication in SIVmac239-Infected Rhesus Macaques without Decreasing the Lifespan of Productively Infected Cells
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- CD8+ T Cell Control of HIV—A Known Unknown
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



