-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Neuropathological diagnoses and clinical correlates in older adults in Brazil: A cross-sectional study
In a postmortem analysis, Lea Grinberg and colleagues examine neuropathological diagnoses in a sample of older adults from Brazil.
Published in the journal: . PLoS Med 14(3): e32767. doi:10.1371/journal.pmed.1002267
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002267Summary
In a postmortem analysis, Lea Grinberg and colleagues examine neuropathological diagnoses in a sample of older adults from Brazil.
Introduction
In 2010, 35.6 million people lived with dementia worldwide. This number is expected to double every 20 y and reach 115.4 million in 2050 [1]. Much of this increase will be due to a surge in dementia prevalence in low - and middle-income countries (LMICs). By 2050, 71% of people with dementia will live in LMICs, as oppose to 58% in 2010 [1]. Alzheimer disease (AD) is the leading cause of dementia worldwide, according to epidemiological studies, accounting for an average of 60% of cases in LMICs [2] and ranging from 50% to 84% of cases in Latin America [3].
The neuropathological changes underlying dementia are broad and comprise neurodegenerative and non-neurodegenerative conditions. Neurodegenerative conditions are classified by their molecular signature, represented by deposits of unfolded proteins, and the distribution of these proteins. For instance, AD features deposits of beta-amyloid extracellular plaques and intraneuronal fibrillary tangles enriched for phospho-tau species [4]. Lewy body dementia shows intraneuronal deposits of alpha-synuclein, known as Lewy bodies. Frontotemporal lobar degeneration (FTLD) encompasses a large number of distinct neuropathological entities that share superficial cortical vacuolation, gliosis, and neuronal loss [5]. FTLD may feature deposits of transactivation response DNA-binding protein of 43 kDa (TDP-43) in a variety of distribution called types A to D [6]. FTLD with TDP-43 inclusions (FTLD-TDP) may overlap with motor neuron disease, especially FTLD-TDP type B. Deposits of phospho-tau protein comprise the second main FTLD type. These tau deposits are in some cases enriched for 4-repeat tau or 3-repeat tau species. Among the FTLD-tau, the most common are corticobasal degeneration, progressive supranuclear palsy, and Pick disease. Among the non-neurodegenerative causes of dementia in senior adults, cognitive changes due to a variety of different cerebrovascular lesions are the most prevalent [7,8].
Clinicopathological studies, the gold-standard for determining the underlying cause of a dementing illness, point to the fact that vascular dementia (VaD), either alone or associated with AD, is at least as common as ‘‘pure” AD in high-income countries (HICs) [9,10]. VaD is greatly associated with cardiovascular risk factors (e.g., hypertension, diabetes mellitus, and dyslipidemia). Since such factors are less likely to be detected and treated in LMICs [11], it is reasonable to suppose that cerebrovascular disease causes more injuries in these countries than in HICs. In the first community-based clinicopathological study on dementia in a population from a LMIC (São Paulo, Brazil) [12], we found that VaD in isolation or overlapping with AD underlaid over one-third of the 113 dementia cases. Unfortunately, that sample size was too small for exploring the influence of other factors distinguishing HICs from LMICs that impact the clinical expression of dementia, such as low educational attainment [13] and different race composition [14–16]. For the same reason, the small sample size failed to offer enough power for examining the role of the coexistence of different neuropathological lesions contributing to cognitive impairment [9,10,17–21].
Here, we investigated the underlying causes of dementia, clinicopathological correspondence, and the association of specific neuropathological lesions in groups and in isolation with cognitive and neuropsychiatric symptoms in 1,092 participants of admixed races, of the Brain Bank of the Brazilian Aging Brain Study Group (BBBABSG) [22].
Methods
Participants
Autopsy verification is mandatory in Brazil to define the cause of death for most individuals who die of natural causes. The São Paulo Autopsy Service (SPAS) is the only morgue serving the metropolitan area of São Paulo (Brazil). Eligible participants to the BBBABSG have the brain donated by the deceased’s next-of-kin after death upon the signature of informed consent. All BBBABSG protocols, the informed consent form, and procedures follow international and Brazilian regulations for research involving humans and were approved by the local and federal research committees. A detailed description of the BBBABSG procedures can be found elsewhere [22].
This cross-sectional study includes participants recruited from 2004 to 2014. Enrollment eligibility includes age of death above 50 y and the presence of a knowledgeable informant to provide clinical and functional information. A knowledgeable informant was someone who had at least weekly contact with the deceased in the last 6 mo before death. Individuals were excluded if clinical data were inconsistent or if the brain tissue was incompatible for neuropathological analyses (e.g., cerebrospinal fluid pH < 6.5 or major acute brain lesions including hemorrhages) [22]. This study is reported as per Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (S1 Checklist).
Clinicofunctional assessments and definitions
Trained gerontologists supervised by a registered nurse with expertise in dementia performed the clinicofunctional assessments. After the informed consent had been signed, the most knowledgeable informant was interviewed to obtain the deceased’s past clinical history using a semistructured interview, which was previously validated for postmortem use and has shown good evidence of validity for the detection of cognitive impairment by informants in postmortem settings [23]. The clinicofunctional assessment lasts for about 40 min and consists of three parts:
Sociodemographics (age at death, sex, years of formal education, race, and the frequency of contact with the informant). Race was reported by the next-of-kin during the clinical interview, according to the following categories: white, black, brown, and other races (i.e., Asian and Brazilian Indian). Other demographic data were confirmed on government-issued documents;
Past medical history (hypertension, diabetes, coronary artery disease, heart failure, arrhythmia, and stroke), family history, and lifestyle data (smoking habits, alcohol use, and physical activity);
Clinicofunctional and neuropsychiatric assessment. Cognitive impairment was assessed using the Clinical Dementia Rating (CDR) [24] scale and the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) [25]. The CDR is a five-point scale used to stage dementia severity by assessing six domains: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. Because of the nature of this study, only the informant section of the CDR was applied. Participants were then classified into five categories: normal cognition (CDR 0); questionable dementia (CDR 0.5); mild dementia (CDR 1); moderate dementia (CDR 2); and severe dementia (CDR 3). Given that the CDR is a categorical scale, we also used the continuous CDR sum of boxes (CDR-SOB) scores ranging from 0 to 18 points in part of our analysis [26]. The retrospective form of the IQCODE was also applied as an alternative measure of cognitive function. The IQCODE’s 26 items assess whether the person had changes in cognition compared to 10 y ago and result in scores from 1 to 5. A score of 3 means no change, and a 5 means that an individual declined considerably in all items evaluated. The IQCODE has been widely validated for the Brazilian population [23,27]. Neuropsychiatric symptoms were assessed using the Neuropsychiatric Inventory (NPI) [28], which evaluates frequency and severity of a wide range of neuropsychiatric symptoms (delusions, hallucinations, dysphoria, anxiety, agitation, aggression, euphoria, disinhibition, irritability, lability, apathy, and aberrant motor activity). Scores vary from 0 to 144 points, and higher scores are associated with higher frequency and severity of symptoms. Upon autopsy, we also measured the deceased’s weight and height without clothes in the supine position, using an electronic scale and a stadiometer, respectively. Body mass index was calculated by dividing the weight in kilos by the square of the height in meters. In participants with CDR ≥ 1, the clinical diagnosis of the dementia subtype was defined by a panel consensus including a geriatric nurse with expertise in dementia (RELFR), the nurse supervisor (LS), and two neurologists (RN and SMDB). Dementia diagnosis followed the definitions from the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders [29]. The clinical diagnosis of the dementia subtype was based on international criteria for AD [30], VaD [31], dementia with Lewy body (DLB) [32], Parkinson disease dementia (PDD) [33], and other dementia [34,35]. In the CDR ≥ 1 cases, the final clinical diagnosis included six categories: possible AD, possible VaD, mixed dementia (AD + cerebrovascular disease), DLB or PDD, other dementia, and undefined (when it was not possible to fulfill any other of the previous criteria).
Neuropathological assessment and definitions
Brain tissue was obtained within 24 h of death. One hemisphere was fixed in 4% buffered paraformaldehyde, and selected brain areas from the other hemisphere were frozen at −80°C. The following samples from the fixed hemisphere were embedded in paraffin: middle frontal gyrus, middle and superior temporal gyri, angular gyrus, superior frontal and anterior cingulate gyrus, visual cortex, hippocampal formation at the level of the lateral geniculate body, amygdala, basal ganglia at the level of the anterior commissure, thalamus, midbrain, pons, medulla oblongata, and cerebellum. Blocks were sectioned into 5-μm-thick sections. All sections were stained with hematoxylin and eosin. Immunohistochemistry with antibodies against β-amyloid (4G8, 1 : 10.000; Signet Pathology Systems, Dedham, Massachusetts), phosphorylated tau (PHF-1, 1 : 2.000; gift from Peter Davies, New York), TDP-43 (1 : 500, Proteintech, Chicago, Illinois), and α-synuclein (EQV-1, 1 : 10.000; gift from Kenji Ueda, Tokyo, Japan) were performed in selected sections [22,36,37]. Internationally accepted neuropathological criteria were used to stage and diagnose the brain pathologies [38–42]. AD-related pathology was scored using the Braak and Braak staging system for neurofibrillary pathology and the Consortium to Establish a Registry for AD (CERAD) criteria for neuritic plaque [38,39]. The new National Institutes of Health-Alzheimer Association criteria [43] were adopted by the BBBABSG in 2013. Therefore, this scale was not used in this study. A neuropathological diagnosis of AD was ascertained for individuals with Braak stage III or above and with a CERAD neuritic plaque density of moderate or frequent.
The diagnosis of argyrophilic grain disease (AGD) was based on the presence of abundant phosphorylated tau-positive grains in the CA1 sector of the hippocampus; pretangles, especially in the hippocampal CA2 sector; and oligodendrocytes with coiled bodies in the hippocampal/temporal white matter [44]. Lewy-type pathology was classified according to the Braak et al. staging scheme for PD [40]. We used the neuropathological term Lewy body disease (LBD) for all diseases associated with Lewy bodies, thereby eliminating the distinction between PD, PDD, and DLB [45]. We considered the diagnosis of LBD when Braak PD stage ≥ 3 [46].
Assessment of cerebrovascular lesions was performed macroscopically by naked eye examination and microscopically using hematoxylin and eosin stained slides in all sampled areas. The presence of small vessel disease (SVD) was evaluated according to the degree of vessel changes, localization, and extension of disease. The SVD changes included small-vessel arteriolosclerosis/atherosclerosis and lipohyalinosis [47]. SVD diagnosis required at least moderate and/or severe microvascular changes in three or more cortical regions [12]. Additionally, lacunae and large infarcts were registered by topography, stage, size, and number [12,13]. Siderocalcinosis, a vascular mineralization with an encrustation of calcium and iron in the middle layer, was evaluated in the basal ganglia and classified as present/absent [47]. Hippocampal sclerosis, defined by pyramidal cell loss and gliosis in CA1 and subiculum of the hippocampal formation, was noted and scored as present/absent [48,49]. Moreover, cerebral amyloid angiopathy (CAA) was analyzed using β-amyloid immunostaining. The localization of CAA (meningeal, gray matter, and /or white matter) as well as the severity and presence of capillary amyloid deposition was noted [50]. CAA was considered as present when it was observed widespread in the parenchyma in at least three different cortical areas.
For the current study, the diagnosis of VaD was granted to participants with either one large chronic infarct (> 1 cm) or three lacunae (< 1 cm) in any of the following strategic areas: thalamus, frontocingular cortex, basal forebrain and caudate, medial temporal area, or angular gyrus [12]. SVD only was not enough for granting a VaD diagnosis. Neuropathological diagnoses were made blinded to clinical status.
Immunohistochemistry to detect TDP-43 (1 : 500, Proteintech, Chicago, Illinois), in at least hippocampal formation and amygdala, was introduced in our routine in 2012, and previous cases are being reassessed (to date, a total of 347 cases underwent TDP-43 assessment). Furthermore, all cases with an undetermined neuropathological diagnosis or a clinical diagnosis of frontotemporal dementia or primary progressive aphasia underwent extensive immunohistochemistry for TDP-43 [5].
Apolipoprotein E (APOE) genotyping
When DNA was available, APOE genotypes (single-nucleotide polymorphisms rs429358 and rs7412) were determined by allele-specific amplification real-time PCR assays, in duplicates, as previously described [51].
Statistical analysis
Study design and statistical analyses for this study were planned 2 y after obtaining the data. We compared participants in the three categories of dementia status (CDR = 0: no dementia; CDR = 0.5: questionable dementia; and CDR ≥ 1: dementia) regarding clinical, APOE allele ε4, and neuropathological variables using one-way ANOVA or Kruskal-Wallis when variables were quantitative and using chi-square or Fisher’s exact tests when variables were categorical. We had first planned to include in the multivariate model only the variables that were associated with dementia status in univariate analyses. However, following peer review request, we included all neuropathological variables measured in this study in a multivariate ordinal logistic regression model that had the three categories of cognitive status (normal, questionable dementia, and dementia) as the dependent variable, since these variables have been associated with higher risk of dementia in several studies [9,10,19,44,47]. The ordinal logistic model was adjusted for age, sex, and education. The variables associated with dementia status at the 0.05 alpha level were selected. We used the coefficients from the ordinal logistic regression model with the selected variables to develop a neuropathological comorbidity (NPC) score. Since each neuropathological lesion was associated with different odds of dementia, points were assigned to each neuropathological variable by dividing each coefficient by the lowest coefficient (i.e., siderocalcinosis) and rounding up or down to the nearest integer [52,53]. An NPC score was assigned for each participant by adding the points for each neuropathological variable present. We then investigated the association between NPC scores with three different outcomes (CDR-SOB, IQCODE, and NPI total score), using linear regression models adjusted for age, sex, and education. Additionally, we used linear regression models to investigate whether there was an interaction between the NPC score, excluding Braak neurofibrillary tangle (NFT) score, and cognitive scores to answer the question of whether these non-AD-related lesions have a synergic or additive effect to Braak NFT score on cognitive symptoms [20,54,55].
We described the absolute and relative frequencies of the neuropathological diagnoses in all participants and also stratified by dementia status. We then calculated the sensitivity (the number of true positives divided by the number of true positives plus false negatives), specificity (the number of true negatives divided by the number of true negatives plus positives), and accuracy (the number of true positives plus true negatives divided by the total number of participants) of the clinical diagnosis of dementia subtypes compared to the neuropathological diagnoses, which was considered the gold standard. We used Stata 13.0 (StataCorp, College Station, Texas) to perform the statistical analyses. The alpha level for all statistical tests was set at 0.05 level in two-tailed tests.
Results
Participants
Between January 2004 and December 2014, 650,837 people (50% male) died in the city of São Paulo with a mean age at death of 73±12 y [56]. During this period, 104,385 persons (16% of the total number of deaths) were autopsied in SPAS. The mean age of the 1,092 participants of this study was 74±12 y, and 49% were male, both similar to the death data from São Paulo city. Moreover, 69% were identified by next-of-kin as white, 11% black, 18% brown, and 2% were from other races. The mean years of education was 4.2±3.7 y. Cardiovascular risk factors and diseases were frequent, as 66% reportedly had hypertension, 27% diabetes, and 24% had coronary artery disease. Regarding cognitive outcomes, 61% had CDR = 0, the mean IQCODE score was 3.41±0.67, and the median NPI score was 5. APOE genotype was available for 524 participants. In this subsample, 155 individuals had at least one APOE allele ε4 (S1 and S2 Tables).
Regarding the dementia status, participants with dementia were older, predominantly female, and had a lower education level (Table 1). Interestingly, the dementia group had a lower prevalence of some cardiovascular risk factors and diseases, like hypertension, coronary artery disease, heart failure, smoking, and alcohol use (Table 1). As expected, IQCODE scores were higher and neuropsychiatric symptoms were more common in demented participants (CDR ≥ 1). APOE allele ε4 was more common in the dementia group (Table 1).
Tab. 1. Sociodemographics, clinical variables, and APOE genotype, according to dementia status (n = 1,092). 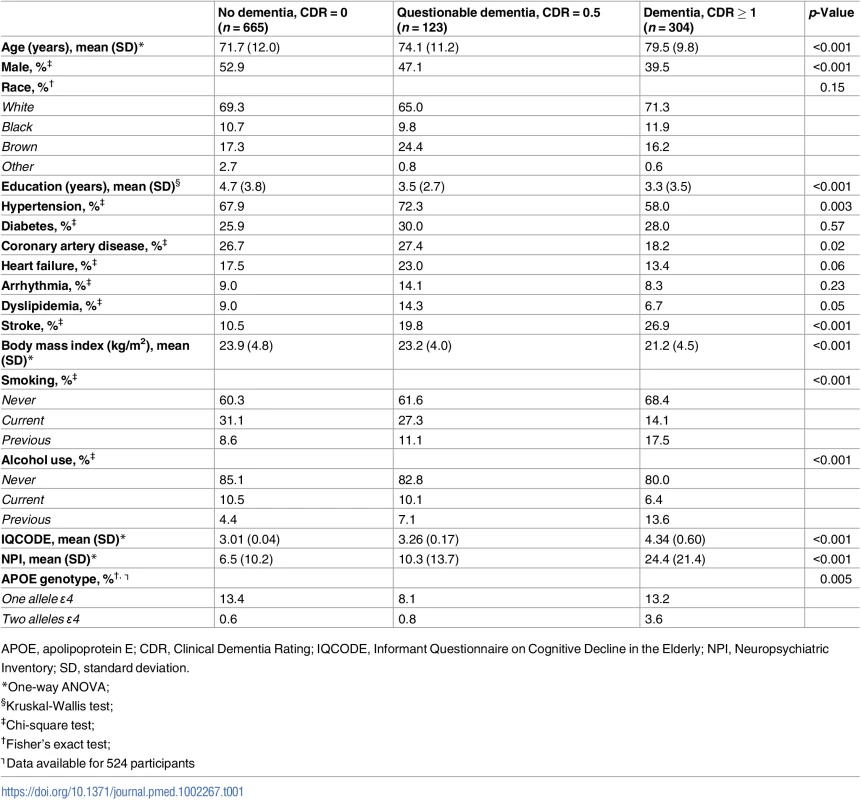
APOE, apolipoprotein E; CDR, Clinical Dementia Rating; IQCODE, Informant Questionnaire on Cognitive Decline in the Elderly; NPI, Neuropsychiatric Inventory; SD, standard deviation. Neuropathological diagnoses and cognitive status
Forty-four percent of the sample (n = 480), regardless of cognitive status, had enough lesions to meet criteria for a neuropathological diagnosis (S1 Fig). Among these 480 participants, mixed neuropathology was present in 20% of the sample. The most common neuropathological diagnosis was AD alone or in combination with other neuropathological diagnoses, which was present in 240 individuals (50% of the 480 participants with neuropathological diagnosis). The diagnosis of VaD was ascertained in 170 individuals (35%), LBD in 87 (18%), and other neuropathological diagnoses in 77 (16%). Within the category “Other,” we grouped neuropathological diagnoses with low frequencies, including four instances of FTLD-TDP, eight of FTLD with tau inclusions (two cases of progressive supranuclear palsy, one of corticobasal degeneration, one of Pick disease, and four unspecified tauopathies) [5], three pure CAA cases, five instances of tangle-only dementia [57], one case of multiple system atrophy, and one diagnosis of hippocampal sclerosis of aging [17].
Fig 1 shows the neuropathological diagnosis by dementia status. Among the 665 participants with normal cognition (CDR = 0), 145 (22%) met the criteria for a neuropathological diagnosis, with AD as the most common diagnosis (n = 82; 57% of the CDR = 0 individuals with a neuropathological diagnosis). Among the 123 participants with questionable dementia (CDR = 0.5), 74 (60%) met the criteria for a neuropathological diagnosis, with VaD as the most common one (n = 30; 41%). Finally, among the 304 participants with dementia (CDR ≥ 1), 261 (86%) met the criteria for at least one neuropathological diagnosis. AD alone or in combination with other neuropathological lesions was present in 139 CDR ≥ 1 participants (53%), VaD in 110 (42%), and LBD in 39 (15%). Forty-three CDR ≥ 1 participants (14%) did not have enough neuropathological lesions to fulfill criteria for any neuropathological diagnoses.
Fig. 1. Mosaic plot showing the relationship between neuropathological classification and dementia status according to the Clinical Dementia Rating (CDR) scale: CDR = 0: No dementia; CDR = 0.5: Questionable dementia; And CDR ≥ 1: Dementia. 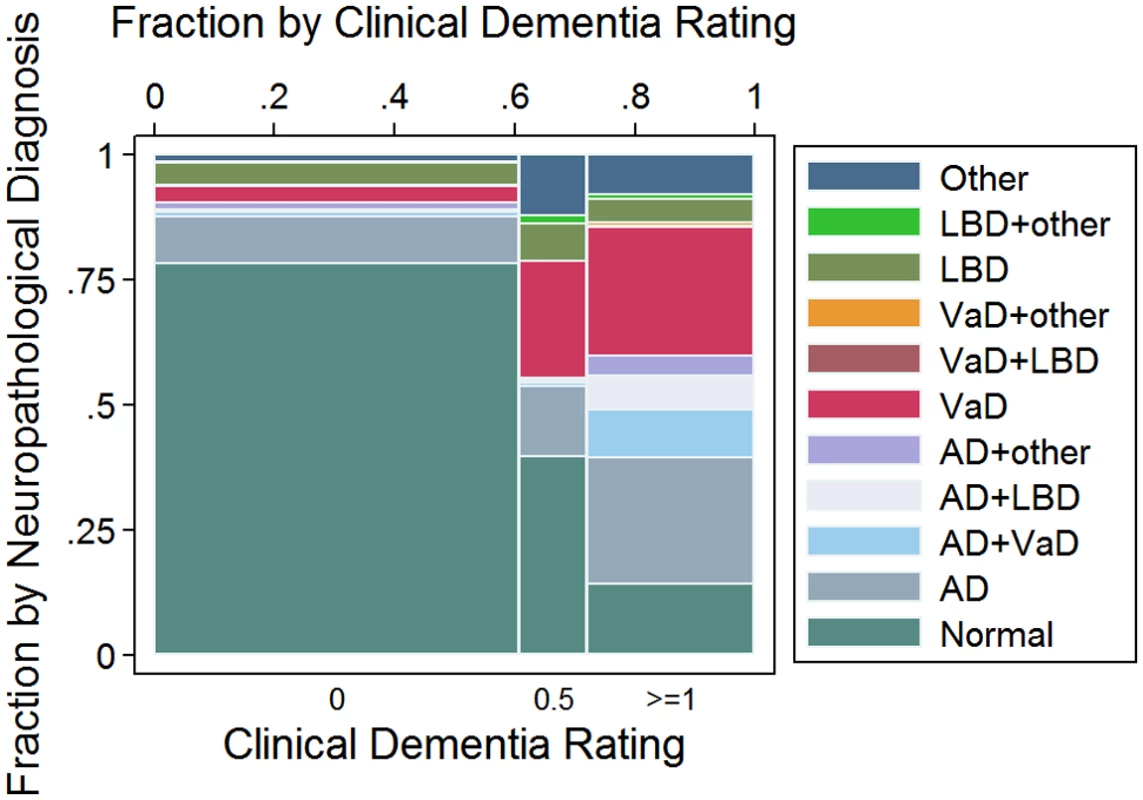
AD, Alzheimer disease; LBD, Lewy body disease; VaD, vascular disease. Association between neuropathological lesions and dementia status
In univariate analyses, all neuropathological lesions, except AGD, were associated with dementia (p < 0.001, except for CAA with p = 0.004) (Table 2). A multivariable ordinal logistic model including all neuropathological variables indicated that Braak NFT stage, hippocampal sclerosis, lacunar infarcts, hyaline arteriolosclerosis, siderocalcinosis, and Braak LBD stage were associated with dementia status at the 0.05 level (S3 Table). We used these variables to develop our NPC score (Table 3). Fig 2 shows the number of individuals at each strata of the NPC score, according to three categories of dementia status. Higher NPC scores were associated with higher CDR-SOB (β = 1.15; 95% CI 1.04–1.27; p < 0.001), IQCODE (β = 0.12; 95% CI 0.11–0.14; p < 0.001), and NPI (β = 1.50; 95% CI 1.14–1.85; p < 0.001) scores (S4 Table). Since the Braak NFT score showed a disproportionately higher weight than the other variables in the NPC score, we also tested the association between all the other elements of the NPC scores together and cognitive and neuropsychiatric outcomes. NPC scores devoid of the Braak stage element remained associated with worse CDR-SOB, IQCODE, and NPI scores (S4 Table). Next, we tested the interaction between the Braak NFT score in isolation and all the other elements of the NPC scores as a group on cognitive and neuropsychiatric outcomes in models adjusted for age, sex, and education. We failed to find a multiplicative interaction between Braak NFT scores and the other types of neuropathological lesions (S4 Table and Fig 3).
Tab. 2. Frequency of neuropathological lesions per dementia status (n = 1,092). 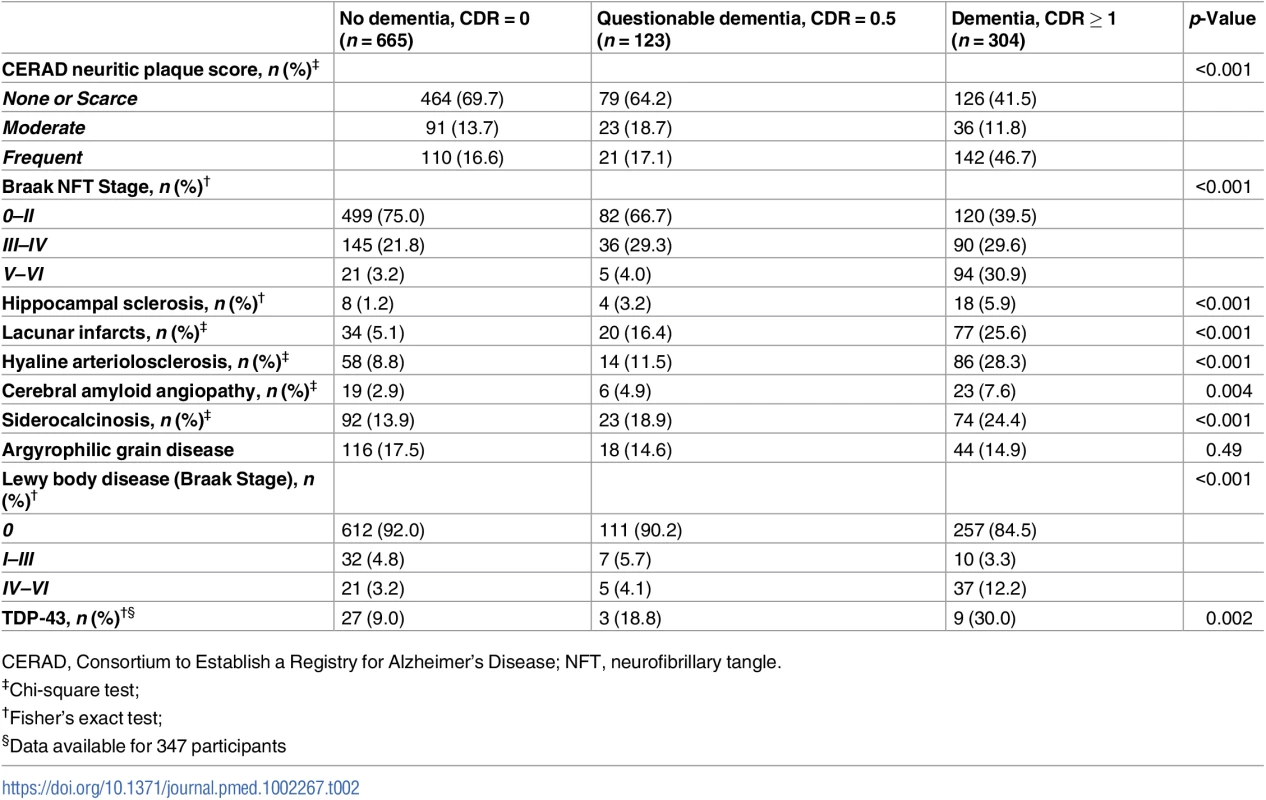
CERAD, Consortium to Establish a Registry for Alzheimer's Disease; NFT, neurofibrillary tangle. Tab. 3. Neuropathological lesions that were independently associated with dementia status in multivariate ordinal logistic regression (n = 1,092). 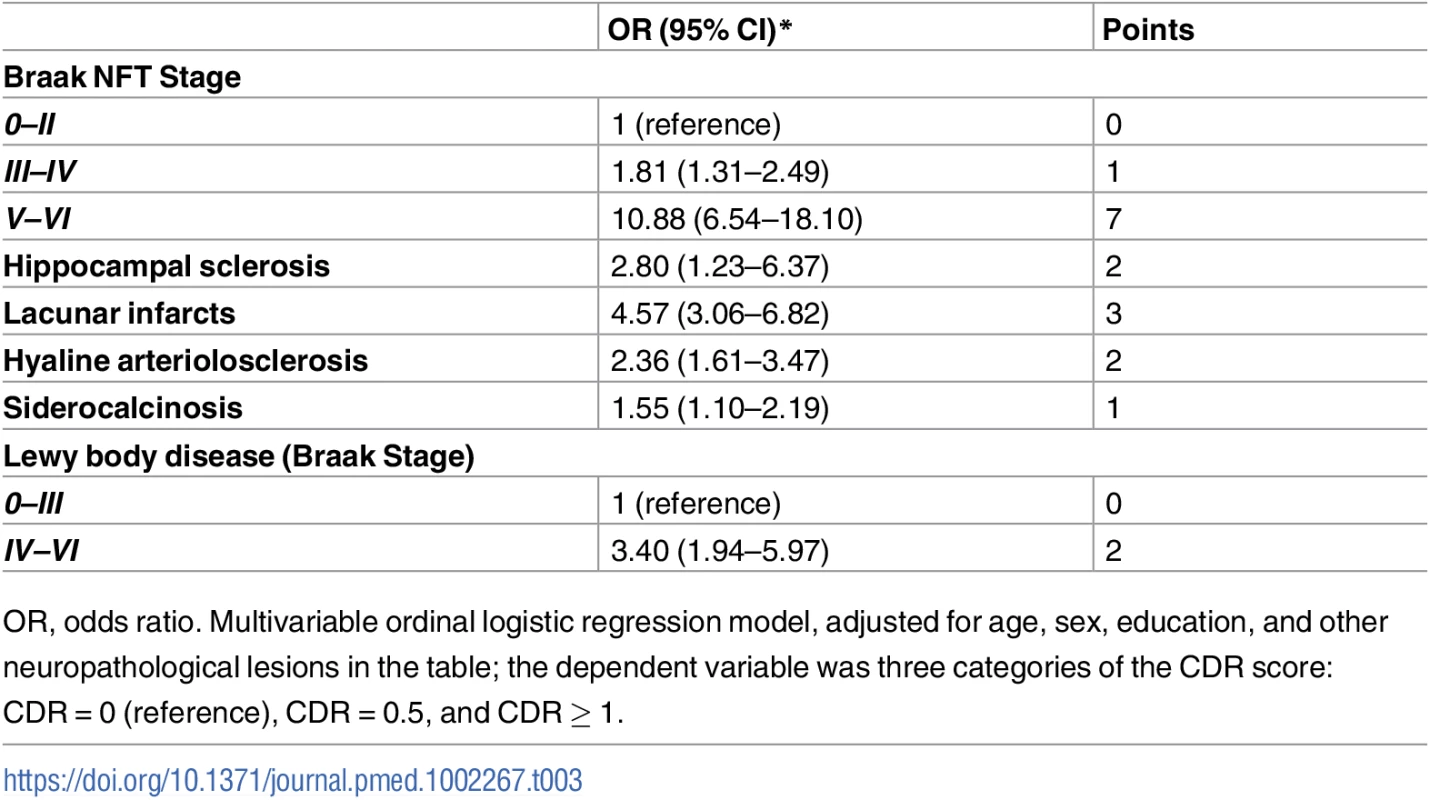
OR, odds ratio. Multivariable ordinal logistic regression model, adjusted for age, sex, education, and other neuropathological lesions in the table; the dependent variable was three categories of the CDR score: CDR = 0 (reference), CDR = 0.5, and CDR ≥ 1. Fig. 2. Number of participants in each stratum of the Neuropathological Comorbidity Score (NPCS) according to dementia status defined by the CDR scale (CDR = 0: No dementia; CDR = 0.5: Questionable dementia; CDR ≥ 1: Dementia). 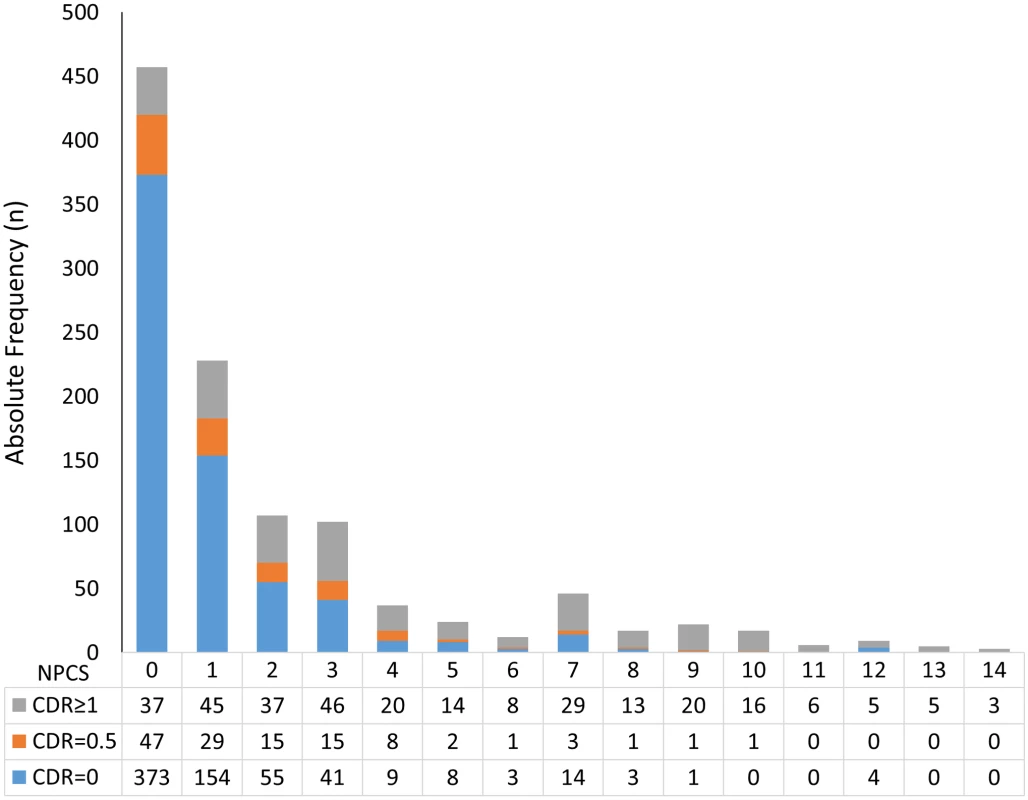
Fig. 3. Predicted values of (A) the CDR Sum of Boxes (CDR-SOB), (B) the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), and (C) the Neuropsychiatric Inventory (NPI) considering the Neuropathological Comorbidity Score (NPCS) without Alzheimer disease pathology for participants with Neurofibrillary Tangle (NFT) score = 0 (Braak NFT stage = 0–II: Blue line), NFT score = 1 (Braak NFT stage = III–IV: Red line), and NFT score = 7 (Braak NFT stage = V–VI: Green line). 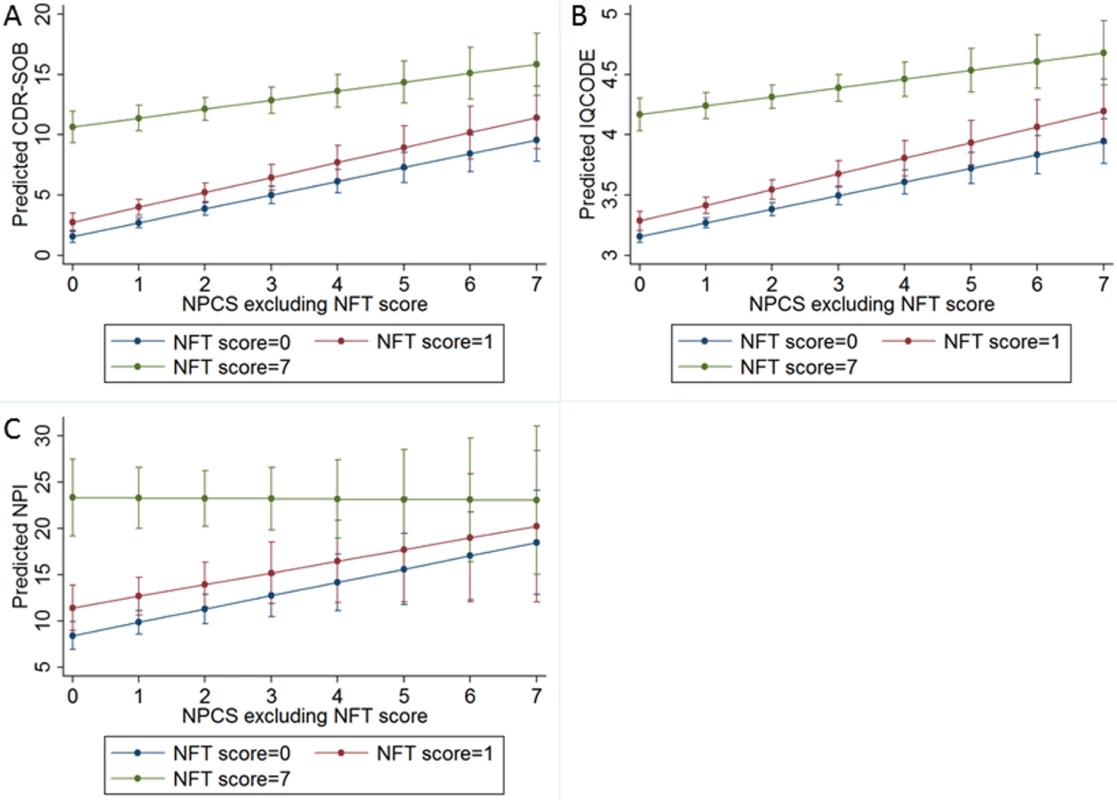
Predicted values of cognitive and neuropsychiatric outcomes were obtained by multivariate linear models adjusted for age, sex, and education. Clinicopathological correlations
Table 4 shows the correlation between clinical and neuropathological diagnoses in participants with CDR ≥ 1. According to the clinical consensus, 45% of the participants with dementia met clinical diagnosis for probable or possible AD, 20% met criteria for AD plus cerebrovascular disease, and 17% met criteria for VaD. Considering the neuropathological diagnosis as the gold standard, the sensitivity of the clinical diagnosis for AD (pure AD plus AD with cerebrovascular disease) was 83%, the specificity was 47%, and the accuracy was 61%. The clinical diagnosis of possible VaD (VaD alone or in combination with AD) had a sensitivity of 47%, specificity of 70%, and accuracy of 61%, while the clinical diagnosis of LBD or dementia related to PD had a sensitivity of 18%, specificity of 97%, and accuracy of 86% (Table 4).
Tab. 4. Comparison between clinical and neuropathological diagnoses among participants with dementia (CDR ≥ 1) (n = 287). 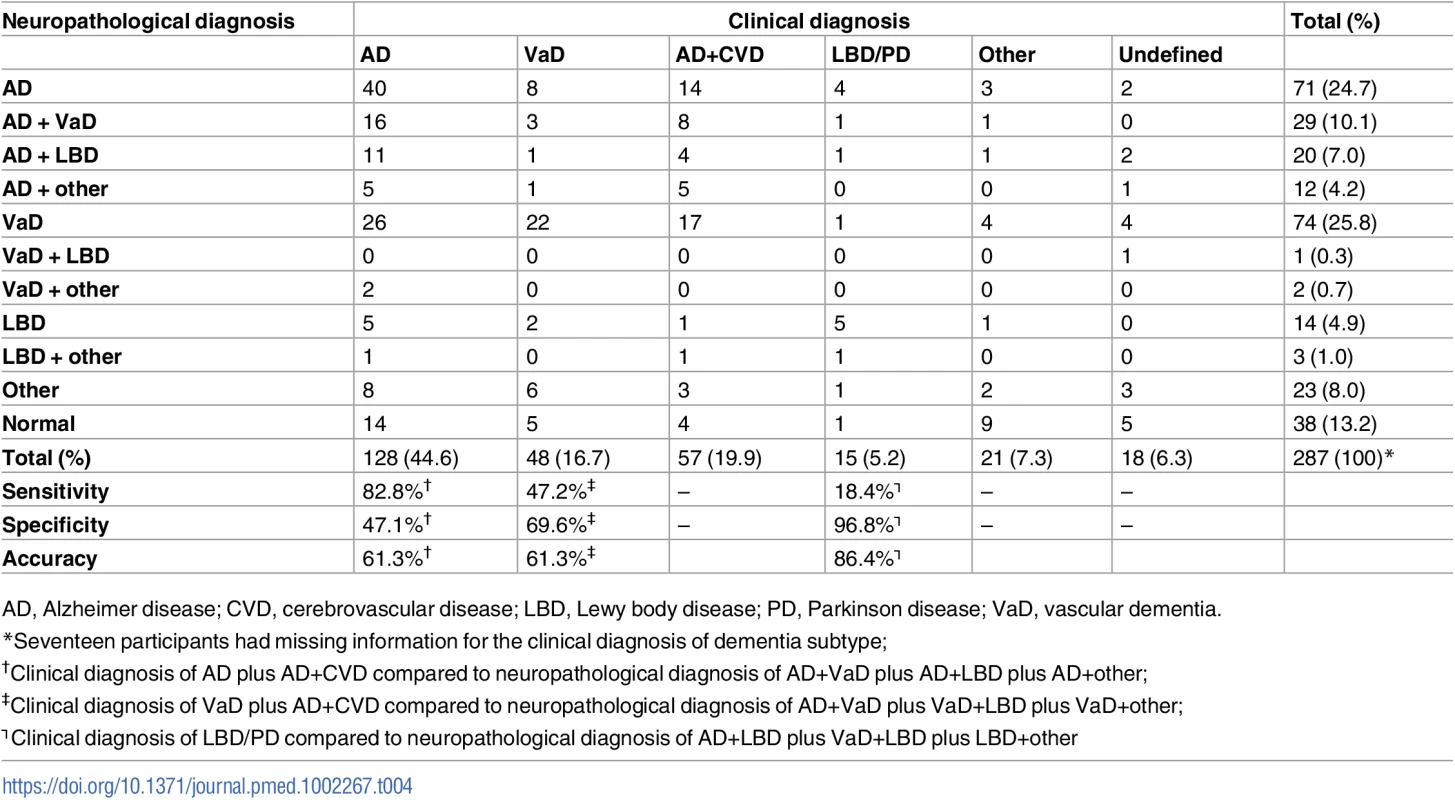
AD, Alzheimer disease; CVD, cerebrovascular disease; LBD, Lewy body disease; PD, Parkinson disease; VaD, vascular dementia. Discussion
Here we described neuropathological findings and clinical and neuropathological variables associated with cognitive impairment in a community-based sample of 1,092 older adults from a LMIC with broad educational attainment and admixed race background, a population profile much different compared to the samples enrolled in other neuropathologic studies conducted in developed countries. The main findings of this study were as follows:
Neurodegenerative and cerebrovascular lesions are common in older adults from a LMIC, similar to data from HICs. Furthermore, almost one-fourth of cognitively normal individuals met the criteria for a neuropathological diagnosis, and these numbers increase progressively in participants with questionable dementia and dementia, respectively. AD was the most common neuropathological diagnosis in the CDR = 0 and CDR ≥ 1 groups, followed by VaD. In fact, VaD was the most common diagnosis in the CDR = 0.5 group. Finally, a small fraction of individuals lack a neuropathological diagnosis to explain the cognitive impairment.
NFT burden, hippocampal sclerosis, lacunar infarcts, hyaline atherosclerosis, siderocalcinosis, and LBD were independently associated with cognitive impairment. NFT burden was the main driver of the association between higher NPC scores and worse cognitive and neuropsychiatric outcomes. Overlapping neuropathological lesions had an additive rather than a multiplicative impact on cognition.
The clinical criteria for LBD presented the highest specificity and accuracy, although the sensibility was very poor. Both clinical criteria for AD and VaD showed a moderate accuracy.
In our total sample, a large percentage of individuals met the criteria for a neuropathological diagnosis. Out of them, 50% met the criteria for AD, including AD associated with other diseases. These numbers are in accordance with other clinicopathological studies, in which the AD prevalence ranged from 19% to 65% [58–61]. In contrast, the prevalence of pathologically confirmed VaD alone or in combination with other pathology (35%) was on the higher end compared to other reported prevalence rates, which ranged from 8% to 45% [58–60,62]. Interestingly, these high frequencies of VaD corroborate our previous findings from a smaller sample of the same population [12]. The high prevalence of VaD alone or in combination with AD found in the present study might reflect the limited access of the study population to basic health care and, consequently, poor control of cerebrovascular risk factors. In addition, other factors might have contributed to the high proportion of VaD. The Brazilian population is highly mixed genetically, mainly due to historical waves of immigration from Africa, Europe, and Asia. We have shown before that African and Japanese ancestry may modify the risk for specific neurodegenerative lesions [36,63]. Quantitative studies are necessary to investigate if the same is true for cerebrovascular lesions. Nevertheless, other biases could explain the high prevalence of VaD in our sample. The use of different neuropathological criteria could also play a role [64,65], and although the BBBASG follows internationally accepted criteria and the neuropathologists (LTG and RDR) have broad experience in international centers, a universally accepted neuropathological criteria for VaD has yet to be established, and each center has adopted its own criteria [66,67]. Even the nature of the cerebrovascular lesions believed to cause cognitive decline varies considerably [47]. We used stringent criteria for VaD. Had isolated—but widespread—SVD been considered part of our criteria, the proportion of VaD diagnosed in our series would have been even greater (from 35% of the total number of cases fulfilling the criteria for a neuropathology diagnosis to 49%). The proportion of individuals with LBD (18%), including PD and DLB alone or in combination with other pathology, was similar to other studies [68,69] but lower than studies with older individuals [70].
When grouping the participants by cognitive status, we found that more than 20% of CDR = 0 individuals met the criteria for one or more neuropathological diagnoses. Among these individuals with CDR = 0 and neuropathological diagnoses, AD was the most common diagnosis (57%), which is in line with rates reported previously [71–73]. These numbers go in accordance with other studies in which the rates of neuropathological AD in the nondemented groups were 7% [74] and 22% [75], respectively, using the CERAD criteria plus Braak NFT stage, compared with 33% [74] and 55% [75], respectively, using the CERAD criteria alone. Had we considered only the CERAD scores, the prevalence of AD in the CDR = 0 group with neuropathological diagnoses would be 69%.
This asymptomatic sample with high neuropathological burden is one of the largest reported to date, and it offers a unique opportunity to investigate factors associated with cognitive reserve. For instance, participants without dementia had higher education attainment than participants with CDR ≥ 0.5 in this study. In fact, we have shown in one of our previous smaller studies that even a few years of education was associated with better cognitive abilities [13].
Sixty percent of participants with CDR = 0.5 met the criteria for at least one neuropathological diagnosis. VaD was the most common diagnosis in this subgroup, highlighting that cerebrovascular lesions may lead to milder cognitive impairment. Out of the 46 CDR = 0.5 individuals who did not meet criteria for a neuropathological diagnosis, two had major depression, one had schizophrenia, and one was a heavy alcohol user. Also, a large portion of these individuals had some degree of neurodegenerative lesions that could have impacted cognition. Future work quantifying these lesions for clinicopathological correlation may help to shed light on this question.
Among participants with CDR ≥ 1, AD alone or in combination accounted for 53% of the neuropathological diagnoses, followed closely by VaD alone or in combination (42%). The prevalence of AD is also in line with previous large autopsy studies, although the VaD prevalence is close to the higher rate of VaD reported [59]. Forty-three participants did not have enough cerebral lesions to ascertain a neuropathological diagnosis. Among them, 10 had clinical diseases that could explain the cognitive impairment: three had major depression, four were heavy alcohol users, one could have had cognitive symptoms secondary to cancer, and two had schizophrenia—although schizophrenia is an exclusion criterion for the diagnosis of dementia, these individuals presented cognitive deficiency, suggesting dementia; thus, they were included in the study. Dementia without a clear explanation has also been reported elsewhere, including in a series of 128 demented patients, in which 16% did not have enough brain lesions to explain the symptoms [76].
Our data corroborate that AD-related tau pathology (NFT burden) and cerebrovascular lesions (lacunar infarcts and SVD) were independently associated with cognitive impairment. Particularly, the presence of Braak NFT stages V/ VI was related to much higher odds of dementia in our sample and in other studies [9,18,74,77–81]. Moreover, hippocampal sclerosis, siderocalcinosis, and LBD were also independently related to moderately higher odds of dementia [9,19,47,82]. By using a NPC score and analyzing it against CDR-SOB, IQCODE, and NPI, we showed that neuropathological comorbidity is related to increased risk of dementia and the severity of neuropsychological symptoms. These results are in line with other cohorts from HICs [9,10,19,20,83]. One open question refers to whether neuropathological comorbidity results in a synergistic effect of worsening cognition in humans, as has been shown in animal models [55]. Our data failed to show a multiplicative effect of neuropathological comorbidity with cognition. Studies in humans assigning a weighted score to different neuropathological lesions are necessary to verify our findings.
Although beta-amyloid plaques are considered by many to be the main neuropathological hallmark of AD, beta-amyloid plaque burden did not affect cognitive or neuropsychiatric scores in this series or in other community-based studies [74,75,84]. Likewise, AGD, a common age-related tauopathy [44], failed to worsen cognitive scores. Other studies suggest that AGD can actually represent a protective factor against AD spread [85].
We detected relatively high sensitivity and low specificity for the clinical diagnosis of AD, while there was a high specificity with very low sensitivity for DLB and Parkinson disease dementia. The same findings were reported in a study that involved 31 United States academic medical centers as well as in another Swedish study [69,86]. We found low sensitivity and moderate specificity for the diagnosis of VaD. Low sensitivity of the clinical criteria for VaD has been found in other community-based studies [86,87], particularly those using the National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l'Enseignement en Neurosciences (NINDS-AIREN) criteria [88,89]. Additionally, we have adopted conservative neuropathological criteria for VaD, which could have contributed to the low sensitivity of the clinical criteria.
We found several associations between demographics and clinical variables and cognitive impairment. Older age, being a woman, lower educational attainment, and a previous diagnosis of stroke were associated with dementia status in our study and are in line with results from other studies [61,90,91]. Hypertension, coronary artery disease, higher body mass index, currently smoking, and alcohol use were more common among participants without dementia. Although paradoxal, the inverse associations between some risk factors and dementia in late life has been described before [92,93] and may reflect a secondary effect associated with dementia (e.g., low body mass index [BMI] due to appetite loss) or survival bias [94].
Strengths and limitations of the study
Our study has several advantages. We presented clinical and neuropathological data from a large sample of individuals who had a low educational level (mean education of 4 y) and were of admixed race. Previous large studies included mainly white or Asian participants with high education attainment (mean education of 16 y in most studies) [9,78]. We also collected comprehensive clinical and neuropathological data that allowed us to develop the NPC score and investigate its association with cognitive and neuropsychiatric scales. Moreover, we investigated the association between clinical symptoms and AGD, which has not been fully explored in other series [44]. In addition, the BBBABSG is a community-based autopsy study, which allows for the collection of a large number of brains from individuals with normal cognition and also an opportunity to study participants with dementia of unknown etiology.
However, our results should be examined considering the study limitations. We did not follow participants during life, and clinical variables were evaluated postmortem through an interview with an informant. To increase the reliability of these data, we included only participants who had at least weekly contact with the informant and excluded individuals when the informant provided conflicting information during the clinical interview. In addition, we have shown that the postmortem cognitive evaluation had a sensitivity of 87% and specificity of 84% for the clinical diagnosis of dementia [23]. Second, the neuropathological criteria for the diagnosis of VaD have not been standardized through different research centers [47]. We used a conservative neuropathological criterion for VaD. In addition, some recent neuropathological diagnoses, such as chronic traumatic encephalopathy or TDP-43 deposition [95], were not systematically investigated in our sample. Although we assessed all individuals with an undetermined neuropathological diagnosis or a clinical diagnosis of frontotemporal dementia or primary progressive aphasia to rule out the possibility of FTLD-TDP, we only completed TDP-43 assessment for 347 participants. Despite the fact that TDP-43 inclusions are found in a considerable percentage of brains of cognitively normal elderly, evidence shows that TDP-43 inclusions in limbic structures may worsen the cognition in the context of AD [95,96]. Finally, our sample is not representative of all deaths that occurred in São Paulo during the study period. SPAS receives people who die from natural causes, and cardiac arrests are common in this population; therefore, we might have over-represented individuals with cardiovascular disease and that might have increased the frequency of cerebrovascular lesions in our sample (participants with and without dementia), but it should be emphasized that cerebrovascular lesions were much more frequent in the group with dementia. On the other hand, individuals with macroscopically detectable acute brain infarctions, hemorrhages, or trauma were underrepresented in the BBBABSG, as an immediate examination was required for the completion of the death certificate, and this fact might have decreased the frequency of cerebrovascular lesions in our sample. Unfortunately, selection bias has been found in most population-based studies on dementia. Almost half of the first 209 individuals submitted to autopsy in the CFAS study had dementia, a much higher proportion than the one found in the general population [74], whereas the Honolulu-Asia Aging study only recruited Americans with Japanese ethnicity [97] and the Nun study only included white women [98]. The Religious Order study mainly included individuals who identified as white, and the mean age of death was higher than that of the general population [77,99]. Average older age at death was also present in other studies [20,100–102].
In conclusion, we described the association of neuropathological lesions and cognitive and neuropsychiatric outcomes in a unique sample of Brazilian individuals with low education attainment and admixed race. In concordance with previous studies, NFT deposition and high comorbidity neuropathological scores were highly associated with dementia status. We also found a higher frequency of cerebrovascular lesions than previously described. Future clinicopathological studies with populations in LMICs are important to confirm our findings.
Supporting Information
Zdroje
1. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9 : 63–75. doi: 10.1016/j.jalz.2012.11.007 23305823
2. Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall KT, et al. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurology. 2008;7 : 812–26. doi: 10.1016/S1474-4422(08)70169-8 18667359
3. Nitrini R, Bottino CMC, Albala C, Custodio Capunay NS, Ketzoian C, Llibre Rodriguez JJ, et al. Prevalence of dementia in Latin America: a collaborative study of population-based cohorts. International Psychogeriatrics. 2009;21 : 622–30. doi: 10.1017/S1041610209009430 19505354
4. Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118 : 5–36. doi: 10.1007/s00401-009-0532-1 19381658
5. Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114 : 5–22. doi: 10.1007/s00401-007-0237-2 17579875
6. Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122 : 111–3. doi: 10.1007/s00401-011-0845-8 21644037
7. Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta Neuropathol. 2010;119 : 277–90. doi: 10.1007/s00401-010-0652-7 20155424
8. McAleese KE, Alafuzoff I, Charidimou A, De Reuck J, Grinberg LT, Hainsworth AH, et al. Post-mortem assessment in vascular dementia: advances and aspirations. BMC Med. 2016;14 : 129. doi: 10.1186/s12916-016-0676-5 27600683
9. Matthews FE, Brayne C, Lowe J, McKeith I, Wharton SB, Ince P. Epidemiological pathology of dementia: attributable-risks at death in the Medical Research Council Cognitive Function and Ageing Study. PLoS Med. 2009;6(11):e1000180. doi: 10.1371/journal.pmed.1000180 19901977
10. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69 : 2197–204. doi: 10.1212/01.wnl.0000271090.28148.24 17568013
11. Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. 2007;370 : 1929–38. doi: 10.1016/S0140-6736(07)61696-1 18063029
12. Grinberg LT, Nitrini R, Suemoto CK, Lucena Ferretti-Rebustini RE, Leite RE, Farfel JM, et al. Prevalence of dementia subtypes in a developing country: a clinicopathological study. Clinics (Sao Paulo). 2013;68 : 1140–5.
13. Farfel JM, Nitrini R, Suemoto CK, Grinberg LT, Ferretti RE, Leite RE, et al. Very low levels of education and cognitive reserve: a clinicopathologic study. Neurology. 2013;81 : 650–7. doi: 10.1212/WNL.0b013e3182a08f1b 23873971
14. Schlesinger D, Grinberg LT, Alba JG, Naslavsky MS, Licinio L, Farfel JM, et al. African ancestry protects against Alzheimer's disease-related neuropathology. Mol Psychiatry. 2013;18 : 79–85. doi: 10.1038/mp.2011.136 22064377
15. Schmidt MI, Duncan BB, Azevedo e Silva G, Menezes AM, Monteiro CA, Barreto SM, et al. Chronic non-communicable diseases in Brazil: burden and current challenges. Lancet. 2011;377 : 1949–61. doi: 10.1016/S0140-6736(11)60135-9 21561658
16. Mehta KM, Yaffe K, Perez-Stable EJ, Stewart A, Barnes D, Kurland BF, et al. Race/ethnic differences in AD survival in US Alzheimer's Disease Centers. Neurology. 2008;70 : 1163–70. doi: 10.1212/01.wnl.0000285287.99923.3c 18003939
17. Nelson PT, Schmitt FA, Lin YS, Abner EL, Jicha GA, Patel E, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134 : 1506–18. doi: 10.1093/brain/awr053 21596774
18. Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH, et al. Neuropathological correlates of dementia in over-80-year-old brain donors from the population-based Cambridge city over-75s cohort (CC75C) study. J Alzheimers Dis. 2009;18 : 645–58. doi: 10.3233/JAD-2009-1182 19661624
19. White LR, Edland SD, Hemmy LS, Montine KS, Zarow C, Sonnen JA, et al. Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology. 2016;86 : 1000–8. doi: 10.1212/WNL.0000000000002480 26888993
20. Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology. 2015;85 : 535–42. doi: 10.1212/WNL.0000000000001831 26180144
21. Hou CE, Yaffe K, Perez-Stable EJ, Miller BL. Frequency of dementia etiologies in four ethnic groups. Dement Geriatr Cogn Disord. 2006;22 : 42–7. doi: 10.1159/000093217 16682792
22. Grinberg LT, Ferretti RE, Farfel JM, Leite R, Pasqualucci CA, Rosemberg S, et al. Brain bank of the Brazilian aging brain study group—a milestone reached and more than 1,600 collected brains. Cell Tissue Bank. 2007;8 : 151–62. doi: 10.1007/s10561-006-9022-z 17075689
23. Ferretti REL, Damim AE, Brucki SMD, Morillo LS, Perroco T, Campora F, et al. Post-moretem diagnosis of dementia by informant interview. Dementia and neuropshycologia. 2010;4 : 138–44.
24. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43 : 2412–4.
25. Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24 : 145–53. 8208879
26. O'Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, et al. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer's research consortium study. Arch Neurol. 2008;65 : 1091–5. doi: 10.1001/archneur.65.8.1091 18695059
27. Perroco TR, Bustamante SE, Moreno Mdel P, Hototian SR, Lopes MA, Azevedo D, et al. Performance of Brazilian long and short IQCODE on the screening of dementia in elderly people with low education. Int Psychogeriatr. 2009;21 : 531–8. doi: 10.1017/S1041610209008849 19323868
28. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44 : 2308–14. 7991117
29. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, DSM-IV. 4th edition. Washigton, D.C; 1994.
30. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers & Dementia. 2011;7 : 263–9.
31. Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43 : 250–60. 8094895
32. McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65 : 1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1 16237129
33. Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22 : 1689–707. doi: 10.1002/mds.21507 17542011
34. Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76 : 1006–14. doi: 10.1212/WNL.0b013e31821103e6 21325651
35. Rascovsky K, Hodges JR, Kipps CM, Johnson JK, Seeley WW, Mendez MF, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord. 2007;21:S14–8. 18090417
36. Nascimento C, Suemoto CK, Rodriguez RD, Alho AT, Leite RP, Farfel JM, et al. Higher Prevalence of TDP-43 Proteinopathy in Cognitively Normal Asians: A Clinicopathological Study on a Multiethnic Sample. Brain Pathol. 2016;26 : 177–85. doi: 10.1111/bpa.12296 26260327
37. Rodriguez JJL, Ferri CP, Acosta D, Guerra M, Huang YG, Jacob KS, et al. Prevalence of dementia in Latin America, India, and China: a population-based cross-sectional survey. Lancet. 2008;372 : 464–74. doi: 10.1016/S0140-6736(08)61002-8 18657855
38. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82 : 239–59. 1759558
39. Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41 : 479–86. 2011243
40. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24 : 197–211. 12498954
41. Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119 : 1–4. doi: 10.1007/s00401-009-0612-2 19924424
42. Braak H, Braak E. Cortical and subcortical argyrophilic grains characterize a disease associated with adult onset dementia. Neuropathol Appl Neurobiol. 1989;15 : 13–26. 2471109
43. Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123 : 1–11. doi: 10.1007/s00401-011-0910-3 22101365
44. Rodriguez RD, Suemoto CK, Molina M, Nascimento CF, Leite RE, de Lucena Ferretti-Rebustini RE, et al. Argyrophilic Grain Disease: Demographics, Clinical, and Neuropathological Features From a Large Autopsy Study. J Neuropathol Exp Neurol. 2016;75 : 628–35. doi: 10.1093/jnen/nlw034 27283329
45. Ince PG, Clark B, Holton J, Revez T, Wharton SB. Disorders with Lewy bodies, including Parkinson’s disease dementia. Love S, Louis DN, Ellison DW, editors. Greenfield’s neuropathology. 8th edn Edward Arnold; London: 2008. pp. 1073–1081.
46. Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 2008;116 : 1–16. doi: 10.1007/s00401-008-0406-y 18592254
47. Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta Neuropathologica. 2010;119 : 277–90. doi: 10.1007/s00401-010-0652-7 20155424
48. Rauramaa T, Pikkarainen M, Englund E, Ince PG, Jellinger K, Paetau A, et al. Consensus recommendations on pathologic changes in the hippocampus: a postmortem multicenter inter-rater study. J Neuropathol Exp Neurol. 2013;72 : 452–61. doi: 10.1097/NEN.0b013e318292492a 23656988
49. Nelson PT, Smith CD, Abner EL, Wilfred BJ, Wang WX, Neltner JH, et al. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta Neuropathol. 2013;126 : 161–77. doi: 10.1007/s00401-013-1154-1 23864344
50. Thal DR, Ghebremedhin E, Rub U, Yamaguchi H, Del Tredici K, Braak H. Two types of sporadic cerebral amyloid angiopathy. J Neuropathol Exp Neurol. 2002;61 : 282–93. 11895043
51. Calero O, Hortiguela R, Bullido MJ, Calero M. Apolipoprotein E genotyping method by real time PCR, a fast and cost-effective alternative to the TaqMan and FRET assays. J Neurosci Methods. 2009;183 : 238–40. doi: 10.1016/j.jneumeth.2009.06.033 19583979
52. Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. Jama. 2006;295 : 801–8. doi: 10.1001/jama.295.7.801 16478903
53. Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J Gen Intern Med. 2009;24 : 1115–22. doi: 10.1007/s11606-009-1073-y 19649678
54. Horvath J, Herrmann FR, Burkhard PR, Bouras C, Kovari E. Neuropathology of dementia in a large cohort of patients with Parkinson's disease. Parkinsonism Relat Disord. 2013;19 : 864–8; discussion. doi: 10.1016/j.parkreldis.2013.05.010 23746454
55. Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J Neurosci. 2010;30 : 7281–9. doi: 10.1523/JNEUROSCI.0490-10.2010 20505094
56. Tabnet DATASUS. Mortality data from Sao Paulo city: Prefeitura de Sao Paulo. 2016 http://tabnet.saude.prefeitura.sp.gov.br/cgi/deftohtm3.exe?secretarias/saude/TABNET/SIM/obito
57. Jellinger KA, Bancher C. Senile dementia with tangles (tangle predominant form of senile dementia). Brain Pathol. 1998;8 : 367–76. 9546293
58. Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16 : 203–12. 12468894
59. Brunnstrom H, Gustafson L, Passant U, Englund E. Prevalence of dementia subtypes: a 30-year retrospective survey of neuropathological reports. Arch Gerontol Geriatr. 2009;49 : 146–9. doi: 10.1016/j.archger.2008.06.005 18692255
60. Akatsu H, Takahashi M, Matsukawa N, Ishikawa Y, Kondo N, Sato T, et al. Subtype analysis of neuropathologically diagnosed patients in a Japanese geriatric hospital. J Neurol Sci. 2002;196 : 63–9. 11959158
61. Rahimi J, Kovacs GG. Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther. 2014;6 : 82. doi: 10.1186/s13195-014-0082-1 25419243
62. Jellinger KA, Attems J. Prevalence and Pathology of Vascular Dementia in the Oldest-Old. Journal of Alzheimers Disease. 2010;21 : 1283–93.
63. Schlesinger D, Grinberg LT, Alba JG, Naslavsky MS, Licinio L, Farfel JM, et al. African ancestry protects against Alzheimer's disease-related neuropathology. Mol Psychiatry. 2013; 18 : 79–85. doi: 10.1038/mp.2011.136 22064377
64. Alafuzoff I, Pikkarainen M, Al-Sarraj S, Arzberger T, Bell J, Bodi I, et al. Interlaboratory comparison of assessments of Alzheimer disease-related lesions: a study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol. 2006;65 : 740–57. doi: 10.1097/01.jnen.0000229986.17548.27 16896308
65. Alafuzoff I, Pikkarainen M, Arzberger T, Thal DR, Al-Sarraj S, Bell J, et al. Inter-laboratory comparison of neuropathological assessments of beta-amyloid protein: a study of the BrainNet Europe consortium. Acta Neuropathol. 2008;115 : 533–46. doi: 10.1007/s00401-008-0358-2 18343933
66. Grinberg LT, Heinsen H. Toward a pathological definition of vascular dementia. J Neurol Sci. 2010;299 : 136–8. doi: 10.1016/j.jns.2010.08.055 20920816
67. Thal DR, Grinberg LT, Attems J. Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol. 2012;47 : 816–24. doi: 10.1016/j.exger.2012.05.023 22705146
68. Jellinger KA. Prevalence and impact of cerebrovascular lesions in Alzheimer and lewy body diseases. Neurodegener Dis. 2010;7 : 112–5. doi: 10.1159/000285518 20173339
69. Nelson PT, Jicha GA, Kryscio RJ, Abner EL, Schmitt FA, Cooper G, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2010;257 : 359–66. doi: 10.1007/s00415-009-5324-y 19795154
70. Wakisaka Y, Furuta A, Tanizaki Y, Kiyohara Y, Iida M, Iwaki T. Age-associated prevalence and risk factors of Lewy body pathology in a general population: the Hisayama study. Acta Neuropathol. 2003;106 : 374–82. doi: 10.1007/s00401-003-0750-x 12904992
71. SantaCruz KS, Sonnen JA, Pezhouh MK, Desrosiers MF, Nelson PT, Tyas SL. Alzheimer disease pathology in subjects without dementia in 2 studies of aging: the Nun Study and the Adult Changes in Thought Study. J Neuropathol Exp Neurol. 2011;70 : 832–40. doi: 10.1097/NEN.0b013e31822e8ae9 21937909
72. Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. "Preclinical" AD revisited—Neuropathology of cognitively normal older adults. Neurology. 2000;55 : 370–6. 10932270
73. Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, et al. Neuropathology of cognitively normal elderly. Journal of Neuropathology and Experimental Neurology. 2003;62 : 1087–95. 14656067
74. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet. 2001;357 : 169–75. 11213093
75. Polvikoski T, Sulkava R, Myllykangas L, Notkola IL, Niinisto L, Verkkoniemi A, et al. Prevalence of Alzheimer's disease in very elderly people: a prospective neuropathological study. Neurology. 2001;56 : 1690–6. 11425935
76. Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of "dementia of unknown etiology" increases with age and is nearly 50% in nonagenarians. Arch Neurol. 2000;57 : 713–9. 10815138
77. Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66 : 1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6 16801647
78. Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 2016;15 : 934–43. doi: 10.1016/S1474-4422(16)30029-1 27312738
79. Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, et al. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles "do count" when staging disease severity. J Neuropathol Exp Neurol. 2007;66 : 1136–46. doi: 10.1097/nen.0b013e31815c5efb 18090922
80. Cholerton B, Larson EB, Baker LD, Craft S, Crane PK, Millard SP, et al. Neuropathologic correlates of cognition in a population-based sample. J Alzheimers Dis. 2013;36 : 699–709. doi: 10.3233/JAD-130281 23666176
81. Middleton LE, Grinberg LT, Miller B, Kawas C, Yaffe K. Neuropathologic features associated with Alzheimer disease diagnosis: age matters. Neurology. 2011;77(19):1737–44. doi: 10.1212/WNL.0b013e318236f0cf 22031532
82. Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol. 2013;126 : 365–84. doi: 10.1007/s00401-013-1157-y 23900711
83. Keage HA, Ince PG, Matthews FE, Wharton SB, McKeith IG, Brayne C, et al. Impact of less common and "disregarded" neurodegenerative pathologies on dementia burden in a population-based cohort. J Alzheimers Dis. 2012;28 : 485–93. doi: 10.3233/JAD-2011-111268 22045491
84. Giannakopoulos P, Gold G, Kovari E, von Gunten A, Imhof A, Bouras C, et al. Assessing the cognitive impact of Alzheimer disease pathology and vascular burden in the aging brain: the Geneva experience. Acta Neuropathol. 2007;113 : 1–12. doi: 10.1007/s00401-006-0144-y 17036244
85. Grinberg LT, Wang X, Wang C, Sohn PD, Theofilas P, Sidhu M, et al. Argyrophilic grain disease differs from other tauopathies by lacking tau acetylation. Acta Neuropathol. 2013;125 : 581–93. doi: 10.1007/s00401-013-1080-2 23371364
86. Brunnstrom H, Englund E. Clinicopathological concordance in dementia diagnostics. Am J Geriatr Psychiatry. 2009;17 : 664–70. 19634210
87. Wetterling T, Kanitz RD, Borgis KJ. Comparison of different diagnostic criteria for vascular dementia (ADDTC, DSM-IV, ICD-10, NINDS-AIREN). Stroke. 1996;27 : 30–6. 8553399
88. Pohjasvaara T, Mantyla R, Ylikoski R, Kaste M, Erkinjuntti T. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l'Enseignement en Neurosciences. Stroke. 2000;31 : 2952–7. 11108755
89. Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment—a critical update. Front Aging Neurosci. 2013;5 : 17. doi: 10.3389/fnagi.2013.00017 23596414
90. Bennett DA, Wilson RS, Schneider JA, Evans DA, de Leon CFM, Arnold SE, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60 : 1909–15. 12821732
91. Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, Dementia, and Alzheimer Disease in the Baltimore Longitudinal Study of Aging Cohort. Annals of Neurology. 2010;68 : 231–40. doi: 10.1002/ana.22055 20695015
92. Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12:e426–37. doi: 10.1111/j.1467-789X.2010.00825.x 21348917
93. Power MC, Weuve J, Gagne JJ, McQueen MB, Viswanathan A, Blacker D. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology. 2011;22 : 646–59. 21705906
94. Hernan MA, Alonso A, Logroscino G. Cigarette smoking and dementia: potential selection bias in the elderly. Epidemiology. 2008;19 : 448–50. 18414087
95. James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA. TDP-43 stage, mixed pathologies, and clinical Alzheimer's-type dementia. Brain. 2016 Sep 30. pii: aww224. [Epub ahead of print].
96. Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, et al. TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol. 2014;127 : 811–24. doi: 10.1007/s00401-014-1269-z 24659241
97. Gelber RP, Launer LJ, White LR. The Honolulu-Asia Aging Study: epidemiologic and neuropathologic research on cognitive impairment. Curr Alzheimer Res. 2012;9 : 664–72. 22471866
98. Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997;277 : 813–7. 9052711
99. Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18 : 691–701. doi: 10.3233/JAD-2009-1227 19749406
100. Polvikoski T, Sulkava R, Myllykangas L, Notkola IL, Niinisto L, Verkkoniemi A, et al. Prevalence of Alzheimer's disease in very elderly people—A prospective neuropathological study. Neurology. 2001;56 : 1690–6. 11425935
101. Dolan D, Troncoso J, Resnick SM, Crain BJ, Zonderman AB, O'Brien RJ. Age, Alzheimer's disease and dementia in the Baltimore Longitudinal Study of Ageing. Brain. 2010;133 : 2225–31. doi: 10.1093/brain/awq141 20647264
102. Green MS, Kaye JEA, Ball MJ. The Oregon Brain Aging Study—Neuropathology accompanying healthy aging in the oldest old. Neurology. 2000;54 : 105–13. 10636134
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 3- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- 2016 Reviewer and Editorial Board Thank You
- , , and mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases
- Differential associations of plasma lipids with incident dementia and dementia subtypes in the 3C Study: A longitudinal, population-based prospective cohort study
- Mixed pathologies and neural reserve: Implications of complexity for Alzheimer disease drug discovery
- -related risk of mild cognitive impairment and dementia for prevention trials: An analysis of four cohorts
- Neuropathological diagnoses and clinical correlates in older adults in Brazil: A cross-sectional study
- Early diagnosis of mild cognitive impairment and mild dementia through basic and instrumental activities of daily living: Development of a new evaluation tool
- Potentially modifiable lifestyle factors, cognitive reserve, and cognitive function in later life: A cross-sectional study
- Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study
- Fine-mapping of the human leukocyte antigen locus as a risk factor for Alzheimer disease: A case–control study
- What’s the “Take Home” from Research on Dementia Trends?
- Cultural representations of dementia
- Dementia and aging populations—A global priority for contextualized research and health policy
- Dementia in the oldest old: Beyond Alzheimer disease
- Rehabilitation for people living with dementia: A practical framework of positive support
- Dementia in low-income and middle-income countries: Different realities mandate tailored solutions
- Challenges and opportunities in understanding dementia and delirium in the acute hospital
- Dementia incidence trend over 1992-2014 in the Netherlands: Analysis of primary care data
- Association between delirium superimposed on dementia and mortality in hospitalized older adults: A prospective cohort study
- Development of an adaptive, personalized, and scalable dementia care program: Early findings from the Care Ecosystem
- Genetic assessment of age-associated Alzheimer disease risk: Development and validation of a polygenic hazard score
- Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study
- The impact of individual Cognitive Stimulation Therapy (iCST) on cognition, quality of life, caregiver health, and family relationships in dementia: A randomised controlled trial
- Effectiveness of an intervention to facilitate prompt referral to memory clinics in the United Kingdom: Cluster randomised controlled trial
- Subjective and objective cognitive function among older adults with a history of traumatic brain injury: A population-based cohort study
- Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: A community-based cohort study
- Multimorbidity and healthcare utilization among home care clients with dementia in Ontario, Canada: A retrospective analysis of a population-based cohort
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Effectiveness of an intervention to facilitate prompt referral to memory clinics in the United Kingdom: Cluster randomised controlled trial
- , , and mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases
- Challenges and opportunities in understanding dementia and delirium in the acute hospital
- Early diagnosis of mild cognitive impairment and mild dementia through basic and instrumental activities of daily living: Development of a new evaluation tool
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



