-
Články
- Vzdělávání
- Časopisy
Top články
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Providing Impetus, Tools, and Guidance to Strengthen National Capacity for Antimicrobial Stewardship in Viet Nam
article has not abstract
Published in the journal: . PLoS Med 10(5): e32767. doi:10.1371/journal.pmed.1001429
Category: Health in Action
doi: https://doi.org/10.1371/journal.pmed.1001429Summary
article has not abstract
Summary Points
-
Antimicrobial stewardship has been difficult to implement globally, and in emerging economies it is usually absent or inadequate.
-
Implementation of antibiotic stewardship programmes need not wait for “perfect local data” or funding. There is often sufficient local expertise to start a programme in the absence of additional resources. No immediate impact on resistance levels should be expected.
-
Antibiotic resistance is partly the result of a dysfunctional health system. Long-term commitment is necessary to improve healthcare infrastructure in order to establish a successful antibiotic stewardship programme and reduce resistance rates.
-
Healthcare professionals, preferably “respected” figures, should coordinate stewardship programmes. The programmes should be contextualised and compatible with local practices to encourage engagement and compliance of all healthcare workers.
Antimicrobial Resistance in Emerging Economies: An Urgency to Intervene
Antimicrobial resistance is a major global health threat. In the European Union an estimated 25,000 deaths occur annually secondary to multi-drug-resistant infections [1]. No reliable estimates for developing countries exist, but figures are likely to be higher. Strategies to contain antimicrobial resistance were comprehensively set forth by the World Health Organization (WHO) in 2001 [2]. However, implementation in low - and middle-income countries, where the need for effective antimicrobials is greatest, has thus far proved problematic [3],[4].
In Viet Nam, where resistance rates are among the highest in Asia [5], the challenge is urgent and great [6]. A large population, high infectious disease burden, and unrestricted access to antimicrobials make Viet Nam a hotspot for the emergence of drug resistance [7]. Adequate legislation to tackle antimicrobial resistance in Viet Nam already exists [7]–[9], but a lack of resources prevents effective policy enforcement [7].
Growing global consensus that antimicrobial resistance must be tackled provides opportunity to intervene. Interventions must account for limited resources available in many regions with the highest resistance burdens. In response to this we launched the Viet Nam Resistance (VINARES) project, a capacity-building initiative designed to strengthen antimicrobial stewardship in Viet Nam. The framework of VINARES may be transferable to other settings, and emerging economies in particular.
Conception of VINARES
In 2010 a situation analysis of antibiotic use and resistance in Viet Nam identified sub-optimal infection control, inadequate laboratory diagnostic capacity, and inappropriate antibiotic therapy as important drivers of bacterial resistance [7]. Parallels exist with the priorities in the WHO antimicrobial resistance policy package issued on World Health Day, 2011 [10]. However, a dysfunctional health system prevents translation of this research into action [4],[7].
VINARES aims to provide impetus and tools to support development of an effective antimicrobial stewardship programme for Viet Nam and thus bridge the “know–do” gap that often plagues implementation of health initiatives in emerging economies [11]. It is a partner-driven collaboration, conceived by Vietnamese healthcare professionals, the Oxford University Clinical Research Unit and Wellcome Trust Major Overseas Programme in Viet Nam, and Linköping University, Sweden. Together we invited 16 hospitals to participate (Figure 1). Practising Vietnamese healthcare professionals coordinate each arm of the project, which address the following areas: (1) infection control and healthcare-associated infections (HAI), (2) antibiotic consumption, and (3) microbiological analysis and reporting capacity.
Fig. 1. Location, speciality, and type of the 16 participating hospitals in the VINARES project. 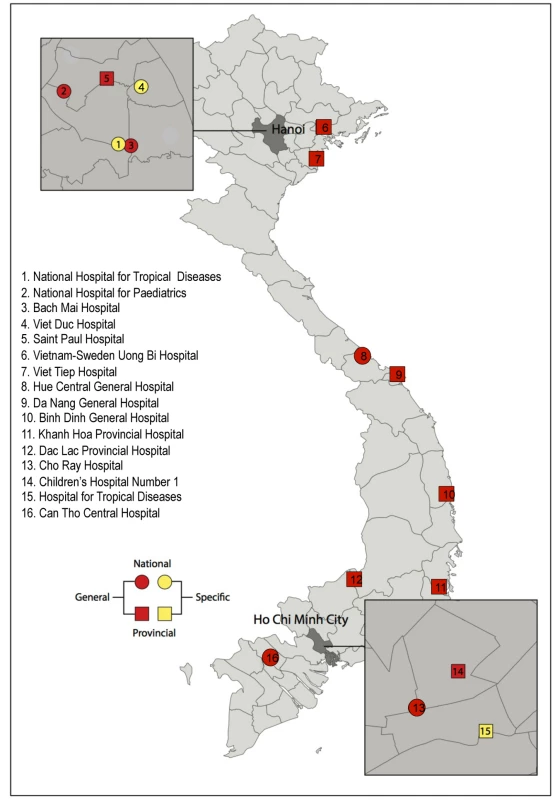
Infection Control and Healthcare-Associated Infections
With the new antibiotic development pipeline running dry, infection prevention is increasingly important [12]. Infection control is particularly problematic in Vietnamese intensive care units (ICUs) [6]. As measures of HAI control are a good surrogate for overall infection control [13], assessing the HAI burden in ICUs is an effective way to identify areas for improvement.
HAIs are assessed by monthly point prevalence surveys (PPSs) in the ICUs of participating hospitals. The surveillance design is similar to methodologies implemented by the European Centre for Disease Prevention and Control [14], contextualised to the Vietnamese setting. Data are entered into European Centre for Disease Prevention and Control software (HELICSwin), which has been translated into Vietnamese. Traditional HAI risks are recorded, as well as those specific to the Vietnamese healthcare system, for example, the hands-on involvement of family in patient care, the large numbers of staff and students accessing the ICU, and the high prevalence of tetanus patients requiring extended periods of invasive mechanical ventilation. Preliminary data show that 75% of ICU patients have family involved in caretaking. The aim is to emphasise the importance of surveillance, identify common problems in ICUs, and establish priorities accordingly. To date, data on over 1,300 patients in Vietnamese ICUs have been collected.
Antibiotic Consumption
Antibiotic use—appropriate and inappropriate—drives resistance [15]. We have designed a simple database to help pharmacists calculate monthly antibiotic consumption in defined daily dosages. In addition to reducing the number of prescriptions, improving prescribing rationale is essential [10]. For each patient receiving antimicrobials at the time of the PPS we collect information on route of administration, dose, and prescription indication. Inappropriate antimicrobial use, particularly for medical and surgical prophylaxis, is common in Viet Nam [16].
Microbiological Analysis and Reporting Capacity
Appropriate antibiotic use requires a hospital's healthcare professionals to act in unison. It cannot be achieved if physicians prescribe idiosyncratically. Standard treatment guidelines (STGs) are essential to coordinate prescribing, but their formulation requires reliable data on causative organisms and antibiotic susceptibility. To deliver this, enhancing laboratory capacity is crucial [17].
We have provided each laboratory with a computer, surveillance database software (WHONET), current antibiotic susceptibility testing guidelines from the Clinical and Laboratory Standards Institute translated into Vietnamese, and American Type Culture Collection reference strains for internal quality control. Many Vietnamese laboratories perform susceptibility testing to drugs that is not indicated, often giving discrepant, erroneous results and wasting resources [18]. The training and materials provided by VINARES aim to remedy this.
We have applied for all laboratories to participate in an external quality assurance programme (United Kingdom National External Quality Assessment Service). Laboratories can send important resistant strains (vancomycin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and carbapenem-resistant enterobacteria) to a reference laboratory for confirmation testing. If verified, dissemination of important information to the Vietnamese and international scientific community is possible. Furthermore, feedback and training are given if the resistance phenotype is not confirmed. In several laboratories we observed an improbably high rate of vancomycin-resistant S. aureus. We found this high rate to be due to erroneous testing methodology and gave recommendations to correct this.
Implementation of VINARES
In September 2012, 110 delegates from participating hospitals and the Viet Nam Ministry of Health attended inaugural workshops. Delegates included hospital directors, clinicians, infection control doctors, microbiologists, and pharmacists. Day one of the workshop provided a forum for each hospital to present important aspects of infection control, HAIs, antibiotic consumption, and antimicrobial stewardship at their respective hospitals. A key objective of VINARES is to motivate change by sharing information and learning from each other. The workshops were the first stage in this process.
Day two was primarily for training. Participants attended focus groups: use of antibiotic consumption software (pharmacists), resistance surveillance and training in microbiological techniques (microbiologists), and introduction to the PPS and HELICSwin (infection control and ICU doctors). Discussions stimulated modifications to the protocol. For example, a more detailed assessment of family involvement in patient care was included, and discrepancies between European brand names and the Vietnamese formulary were addressed. These modifications highlight the importance of consulting with local stakeholders when implementing an initiative such as VINARES.
After the workshops, implementation teams visited each hospital to deliver two computers containing databases and protocols, assist with the first PPS, and configure a functioning WHONET database for each laboratory. A dedicated help desk was established, which hospitals could contact by E-mail. Figure 2 provides a framework for designing and implementing a stewardship programme in a resource-limited country.
Fig. 2. Bridging the “know–do” gap: design and implementation of an antimicrobial stewardship programme in an emerging economy. 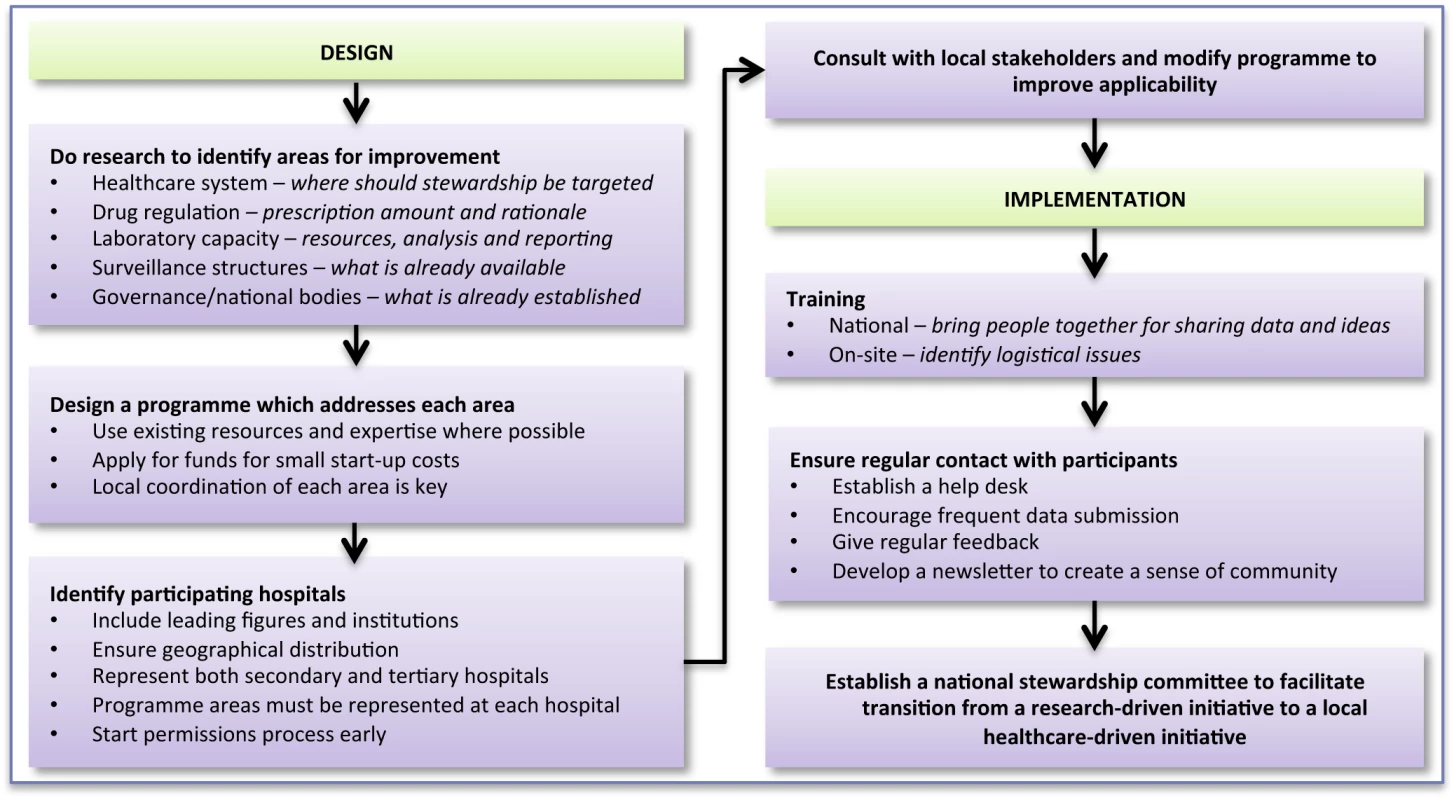
Challenges along the Way
Data Quality
Effective stewardship requires reliable data. However, in resource-limited settings poor data are initially acceptable as they identify where to improve. Emphasis is on encouraging stewardship and building capacity.
Modification of the PPS following suggestions from participants and on-site training in surveillance methodologies has reduced ambiguity and encouraged uniform cross-site data collection. Site visits have ensured a minimum operating standard of laboratories. VINARES coordinators perform monthly data quality checks.
Permissions
This project required permission from the Viet Nam Ministry of Health and approval from the Institutional Review Board of the National Hospital for Tropical Diseases. This process required close monitoring to ensure it progressed. Good relationships and patience were key. Another challenge was acquiring import permits for external quality assurance panels containing live bacteria. The government requested that we reveal the identity of the bacteria; however, the nature of proficiency panels is that their identity should remain unknown. This required further meetings to explain the purpose of external quality assurance.
Ownership of Data
Ownership of data is contentious, particularly for sensitive issues such as HAIs and infection control. Building trust is essential to achieve sharing of data, upon which interventions can be developed. VINARES aims to encourage sharing of data between institutions, but this is understandably approached with caution. However, most hospitals have been willing to share data.
Standard Treatment Guidelines
Early in 2013 local stakeholders from participating hospitals met to draft STGs for important ICU infections, including pneumonia (community - and hospital-acquired) and meningitis. Data submitted from VINARES were reviewed and used to begin formulation of the first evidence-based STGs for Viet Nam. Using national surveillance data as a primary source will permit current and contextualised STGs, which we aim to make readily available throughout Viet Nam with the expected launch of the national stewardship website in June 2013.
After VINARES
VINARES is a capacity-building initiative designed to equip hospitals with the tools to perform self-sufficient antimicrobial stewardship for the long term. Hospitals submit monthly data on antibiotic consumption, infection control surveillance, and susceptibility of bacterial pathogens. We provide hospitals with regular reports, comparing their results to the average performance of all hospitals. We hope personalised feedback, including newsletters, will motivate appropriately targeted service change. Figure 3 illustrates a timeline for the VINARES project.
Fig. 3. A timeline to illustrate the conception and implementation of the VINARES project. 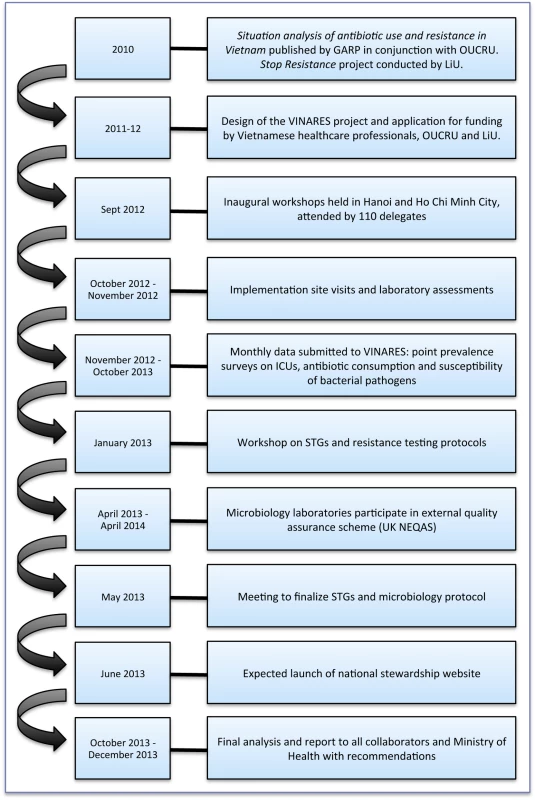
GARP, Global Antibiotic Resistance Partnership; LiU, Linköping University; OUCRU, Oxford University Clinical Research Unit; UK NEQAS, United Kingdom National External Quality Assessment Service. Hospitals will receive a full year of support to establish antimicrobial stewardship programmes. If local leaders and physicians are convinced that patient care has improved, they will be motivated to maintain the surveillance structures established during that year.
VINARES addresses almost all hospital-related priorities in the WHO policy package on antimicrobial resistance (Table 1) [10]. Its purpose is to bridge the gap between policy and action that has hampered antimicrobial stewardship in low - and middle-income countries [11]. Through VINARES, 16 hospitals have committed to improving antimicrobial stewardship in Viet Nam for the next 12 months. In “training the trainers”, we hope that other hospitals, in Viet Nam or abroad, will follow, with support from one of these 16 hospitals.
Tab. 1. Drivers of antibiotic resistance, hospital-related WHO policy package priorities, and how these are met by the VINARES project <em class="ref">[10]</em>. ![Drivers of antibiotic resistance, hospital-related WHO policy package priorities, and how these are met by the VINARES project <em class="ref">[10]</em>.](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/67b39d84ff4abd7681a81fc26a1fa384.png)
Zdroje
1. European Centre for Disease Prevention and Control (2009) The bacterial challenge: time to react. A call to narrow the gap between multidrug-resistant bacteria in the EU and the development of new antibacterial agents. Stockholm: European Centre for Disease Prevention and Control.
2. World Health Organization (2001) WHO global strategy for containment of antimicrobial resistance. Geneva: World Health Organization.
3. LopardoG, TitantiP, BerdinasV, BarcelonaL, CurcioD (2011) Antimicrobial stewardship program in a developing country: the epidemiological barrier. Rev Panam Salud Publica 30 : 667–668.
4. OkekeIN, AboderinOA, ByarugabaDK, OjoKK, OpintanJA (2007) Growing problem of multidrug-resistant enteric pathogens in Africa. Emerg Infect Dis 13 : 1640–1646.
5. KimSH, SongJH, ChungDR, ThamlikitkulV, YangY, et al. (2012) Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother 56 : 1418–1426.
6. JohanssonM, PhuongDM, WaltherSM, HanbergerH (2011) Need for improved antimicrobial and infection control stewardship in Vietnamese intensive care units. Trop Med Int Health 16 : 737–743.
7. GARP-Vietnam National Working Group (2010) Situation analysis: antibiotic use and resistance in Vietnam. Washington (District of Columbia): Center for Disease Dynamics, Economics and Policy.
8. Viet Nam Ministry of Health (2004) Chi thi cua bô ˙ truong Bô ˙ Y tê vê viê ˙c chân chinh công tác cung ú ¸ng, su dung thuôc trong bê ˙nh viê ˙n. No. 05/2004/CT-BYT. Hanoi: Viet Nam Ministry of Health.
9. Viet Nam Ministry of Health (2009) Regulation on infection control in healthcare institutions. No. 18/2008/TT-BYT. Hanoi: Viet Nam Ministry of Health.
10. LeungE, WeilDE, RaviglioneM, NakataniH (2011) The WHO policy package to combat antimicrobial resistance. Bull World Health Organ 89 : 390–392.
11. HainesA, KuruvillaS, BorchertM (2004) Bridging the implementation gap between knowledge and action for health. Bull World Health Organ 82 : 724–731; discussion 732.
12. DoronS, DavidsonLE (2011) Antimicrobial stewardship. Mayo Clin Proc 86 : 1113–1123.
13. EbnotherC, TannerB, SchmidF, La RoccaV, HeinzerI, et al. (2008) Impact of an infection control program on the prevalence of nosocomial infections at a tertiary care center in Switzerland. Infect Control Hosp Epidemiol 29 : 38–43.
14. European Centre for Disease Prevention and Control (2012) Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals—protocol version 4.3. Stockholm: European Centre for Disease Prevention and Control.
15. GoossensH, FerechM, Vander SticheleR, ElseviersM (2005) Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365 : 579–587.
16. ThuTA, RahmanM, CoffinS, Harun-Or-RashidM, SakamotoJ, et al. (2012) Antibiotic use in Vietnamese hospitals: a multicenter point-prevalence study. American J Infect Control 40 : 840–844.
17. WertheimHF, PuthavathanaP, NghiemNM, van DoornHR, NguyenTV, et al. (2010) Laboratory capacity building in Asia for infectious disease research: experiences from the South East Asia Infectious Disease Clinical Research Network (SEAICRN). PLoS Med 7: e1000231 doi:10.1371/journal.pmed.1000231.
18. Viet Nam Ministry of Health (2012) First report on antibiotic use and resistance in Vietnamese hospitals in 2009. Hanoi: Viet Nam Ministry of Health.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 5- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Ferinject: správně indikovat, správně podat, správně vykázat
- Hojení análních fisur urychlí čípky a gel
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
-
Všechny články tohoto čísla
- Measuring Coverage in MNCH: Challenges and Opportunities in the Selection of Coverage Indicators for Global Monitoring
- Measuring Coverage in MNCH: Tracking Progress in Health for Women and Children Using DHS and MICS Household Surveys
- Measuring Coverage in MNCH: Population HIV-Free Survival among Children under Two Years of Age in Four African Countries
- Tobacco Company Efforts to Influence the Food and Drug Administration-Commissioned Institute of Medicine Report An Analysis of Documents Released through Litigation
- Irreconcilable Conflict: The Tobacco Industry and the Public Health Challenge of Tobacco Use
- Providing Impetus, Tools, and Guidance to Strengthen National Capacity for Antimicrobial Stewardship in Viet Nam
- Measuring Coverage in MNCH: New Findings, New Strategies, and Recommendations for Action
- Grand Challenges: Integrating Maternal Mental Health into Maternal and Child Health Programmes
- Measuring Coverage in MNCH: Challenges in Monitoring the Proportion of Young Children with Pneumonia Who Receive Antibiotic Treatment
- Measuring Coverage in MNCH: Current Indicators for Measuring Coverage of Diarrhea Treatment Interventions and Opportunities for Improvement
- Measuring Coverage in MNCH: Evaluation of Community-Based Treatment of Childhood Illnesses through Household Surveys
- Measuring Coverage in MNCH: Accuracy of Measuring Diagnosis and Treatment of Childhood Malaria from Household Surveys in Zambia
- Grand Challenges: Integrating Mental Health Care into the Non-Communicable Disease Agenda
- Integrating Global and National Knowledge to Select Medicines for Children: The Ghana National Drugs Programme
- Grand Challenges: Integrating Mental Health Services into Priority Health Care Platforms
- Disability Transitions and Health Expectancies among Adults 45 Years and Older in Malawi: A Cohort-Based Model
- Comparative Efficacy of Seven Psychotherapeutic Interventions for Patients with Depression: A Network Meta-Analysis
- Measuring Coverage in MNCH: Design, Implementation, and Interpretation Challenges Associated with Tracking Vaccination Coverage Using Household Surveys
- Measuring Coverage in MNCH: A Prospective Validation Study in Pakistan and Bangladesh on Measuring Correct Treatment of Childhood Pneumonia
- Contribution of and Smoking Trends to US Incidence of Intestinal-Type Noncardia Gastric Adenocarcinoma: A Microsimulation Model
- Carriage of in the Upper Respiratory Tract of Symptomatic and Asymptomatic Children: An Observational Study
- Setting Research Priorities to Reduce Mortality and Morbidity of Childhood Diarrhoeal Disease in the Next 15 Years
- Grand Challenges: Improving HIV Treatment Outcomes by Integrating Interventions for Co-Morbid Mental Illness
- Gene Expression Classification of Colon Cancer into Molecular Subtypes: Characterization, Validation, and Prognostic Value
- Domestic Violence and Perinatal Mental Disorders: A Systematic Review and Meta-Analysis
- Intimate Partner Violence and Incident Depressive Symptoms and Suicide Attempts: A Systematic Review of Longitudinal Studies
- Effect of Facilitation of Local Maternal-and-Newborn Stakeholder Groups on Neonatal Mortality: Cluster-Randomized Controlled Trial
- Intimate Partner Violence and Population Mental Health: Why Poverty and Gender Inequities Matter
- Assessing Population Aging and Disability in Sub-Saharan Africa: Lessons from Malawi?
- The Paradox of Mental Health: Over-Treatment and Under-Recognition
- Measuring Coverage in MNCH: Determining and Interpreting Inequalities in Coverage of Maternal, Newborn, and Child Health Interventions
- Measuring Coverage in MNCH: Total Survey Error and the Interpretation of Intervention Coverage Estimates from Household Surveys
- Measuring Coverage in MNCH: Indicators for Global Tracking of Newborn Care
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gene Expression Classification of Colon Cancer into Molecular Subtypes: Characterization, Validation, and Prognostic Value
- Domestic Violence and Perinatal Mental Disorders: A Systematic Review and Meta-Analysis
- Intimate Partner Violence and Incident Depressive Symptoms and Suicide Attempts: A Systematic Review of Longitudinal Studies
- Measuring Coverage in MNCH: Challenges in Monitoring the Proportion of Young Children with Pneumonia Who Receive Antibiotic Treatment
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



