-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRegulation of Lifespan, Metabolism, and Stress Responses by the SH2B Protein, Lnk
Drosophila Lnk is the single ancestral orthologue of a highly conserved family of structurally-related intracellular adaptor proteins, the SH2B proteins. As adaptors, they lack catalytic activity but contain several protein–protein interaction domains, thus playing a critical role in signal transduction from receptor tyrosine kinases to form protein networks. Physiological studies of SH2B function in mammals have produced conflicting data. However, a recent study in Drosophila has shown that Lnk is an important regulator of the insulin/insulin-like growth factor (IGF)-1 signaling (IIS) pathway during growth, functioning in parallel to the insulin receptor substrate, Chico. As this pathway also has an evolutionary conserved role in the determination of organism lifespan, we investigated whether Lnk is required for normal lifespan in Drosophila. Phenotypic analysis of mutants for Lnk revealed that loss of Lnk function results in increased lifespan and improved survival under conditions of oxidative stress and starvation. Starvation resistance was found to be associated with increased metabolic stores of carbohydrates and lipids indicative of impaired metabolism. Biochemical and genetic data suggest that Lnk functions in both the IIS and Ras/Mitogen activated protein Kinase (MapK) signaling pathways. Microarray studies support this model, showing transcriptional feedback onto genes in both pathways as well as indicating global changes in both lipid and carbohydrate metabolism. Finally, our data also suggest that Lnk itself may be a direct target of the IIS responsive transcription factor, dFoxo, and that dFoxo may repress Lnk expression. We therefore describe novel functions for a member of the SH2B protein family and provide the first evidence for potential mechanisms of SH2B regulation. Our findings suggest that IIS signaling in Drosophila may require the activity of a second intracellular adaptor, thereby yielding fundamental new insights into the functioning and role of the IIS pathway in ageing and metabolism.
Published in the journal: . PLoS Genet 6(3): e32767. doi:10.1371/journal.pgen.1000881
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000881Summary
Drosophila Lnk is the single ancestral orthologue of a highly conserved family of structurally-related intracellular adaptor proteins, the SH2B proteins. As adaptors, they lack catalytic activity but contain several protein–protein interaction domains, thus playing a critical role in signal transduction from receptor tyrosine kinases to form protein networks. Physiological studies of SH2B function in mammals have produced conflicting data. However, a recent study in Drosophila has shown that Lnk is an important regulator of the insulin/insulin-like growth factor (IGF)-1 signaling (IIS) pathway during growth, functioning in parallel to the insulin receptor substrate, Chico. As this pathway also has an evolutionary conserved role in the determination of organism lifespan, we investigated whether Lnk is required for normal lifespan in Drosophila. Phenotypic analysis of mutants for Lnk revealed that loss of Lnk function results in increased lifespan and improved survival under conditions of oxidative stress and starvation. Starvation resistance was found to be associated with increased metabolic stores of carbohydrates and lipids indicative of impaired metabolism. Biochemical and genetic data suggest that Lnk functions in both the IIS and Ras/Mitogen activated protein Kinase (MapK) signaling pathways. Microarray studies support this model, showing transcriptional feedback onto genes in both pathways as well as indicating global changes in both lipid and carbohydrate metabolism. Finally, our data also suggest that Lnk itself may be a direct target of the IIS responsive transcription factor, dFoxo, and that dFoxo may repress Lnk expression. We therefore describe novel functions for a member of the SH2B protein family and provide the first evidence for potential mechanisms of SH2B regulation. Our findings suggest that IIS signaling in Drosophila may require the activity of a second intracellular adaptor, thereby yielding fundamental new insights into the functioning and role of the IIS pathway in ageing and metabolism.
Introduction
SH2B proteins are a recently identified family of intracellular adaptor proteins that transduce signals downstream of a number of receptor tyrosine kinases (RTKs). These include the receptors for insulin, insulin-like growth factor-1, Janus kinase 2 (Jak2), platelet derived growth factor, fibroblast growth factor and nerve growth factor [1]–[5]. Consequently, SH2B proteins have been shown to function during multiple physiological processes including glucose homeostasis, energy metabolism, hematopoesis and reproduction [6]–[9]. Moreover, mutations in SH2B orthologues in humans are associated with metabolic disregulation and obesity. Several SH2B family members have been identified in mammals so far including SH2B1 (of which there are four splice variants: SH2B1α, SH2B1β, SH2B1γ and SH2B1δ), SH2B2 (APS) and SH2B3 (Lnk). They are characterised by a number of conserved domains including a central pleckstrin homology (PH-) domain, a C-terminal Src Homology 2 (SH2-) domain, an N-terminal proline rich region, multiple consensus sites for tyrosine and serine/threonine phosphorylation and a highly conserved C-terminal c-Cbl recognition motif [6], [10]–[12]. These domains function as protein-protein interaction motifs and so allow SH2B proteins to integrate and transduce intracellular signals from multiple signaling networks in the absence of intrinsic catalytic activity [6], [10]–[12].
Biochemical studies have demonstrated that SH2B proteins bind via their SH2 domains to phosphotyrosine residues within the intracellular tails of several activated RTKs thereby contributing to receptor activation [10],[13],[14]. Once bound, SH2B proteins have been shown to undergo RTK-stimulated tyrosine phosphorylation although they might also be serine/threonine phosphorylated in their basal state as they show anomalous migration on SDS/PAGE indicative of protein structural modifications [13],[15],[16]. In vitro binding assays have identified interactions between SH2B proteins and a number of other intracellular adaptor proteins including the insulin receptor substrates IRS1 and IRS2, Grb2, Shc and c-Cbl [2],[17],[18]. These interactions may or may not require tyrosine phosphorylation of SH2B depending on the isoform studied [2],[18]. Interactions with IRS proteins promote activation of the phosphoinositol-3 kinase (PI3K) pathway and overexpression in cell culture has been show to enhance activation of both the PI3K and the Ras/MapK pathways [17],[19]. Binding to the proto-oncogene product, c-Cbl, a RING-type E2-dependent ubiquitin protein ligase, may facilitate either endocytosis or degradation of the receptor through receptor ubiquitination [16],[20]. Thus, SH2B proteins may have dual functionality in both positively and negatively regulating RTK signaling.
Mammalian SH2B family members are widely expressed in a number of tissues suggesting that they may share some overlapping, redundant functions [13],[21],[22]. For example, mice carrying a genetic deletion for SH2B3 show a selective defect in the regulation of B cell lymphopoeisis. This is consistent with the high levels of SH2B3 expression observed in hematopoetic organs such as the bone marrow and lymph nodes [22] and suggests that SH2B3 plays a specific, non-redundant role in the development of a subset of immune cells. However, SH2B3 mRNA is also abundant in non-hematopoetic tissues such as testis, brain and muscle and so presumably the absence of phenotype in these tissues indicates redundancy with other SH2B family members [22]–[24]. Studies into the physiological functions of SH2B1 and SH2B2 have produced contradictory results. Genetic deletion of SH2B1 in mice produces neonatal growth retardation and infertility probably due to impaired responses to GH or IGF-1 [7]. It was reported that SH2B1 null mice rapidly increase their body mass and develop obesity as a result of significantly impaired hypothalamic leptin signaling resulting in hyperleptinemia and hyperphagia [8],[25]. These mice were also shown to have attenuated insulin signaling in muscle, liver and fat resulting in insulin resistance and diabetes. More recently, a second model showed that SH2B1 null mice actually have decreased fat mass possibly caused by a reduction in adipogenesis as SH2B1 deficiency was associated with reduced expression of adipogenic genes such as peroxisome proliferator-activated receptor γ (PPARγ) and impaired adipocyte differentiation in cell culture [26]. In the case of SH2B2, it was reported that SH2B2 null mice develop hypoinsulinemia and show increased insulin sensitivity at young ages [27]. However, more recent reports saw no effect of SH2B2 deletion on fasted blood glucose, insulin levels, glucose or insulin tolerance [9]. The reasons for this apparent discrepancy between studies is unclear but may be confounded by differences in genetic backgrounds, diet or housing conditions.
Understanding the physiological functions of SH2B proteins in mammals has therefore been complicated by the presence of multiple SH2B isoforms and conflicting data from genetic analyses. The genome of Drosophila melanogaster encodes a single SH2B homologue (Lnk) that shares a similar domain structure to its mammalian counterparts, with 36% sequence identity to human SH2B proteins in its PH-domain and 74% sequence identity in its PTB domain as well as containing a highly conserved c-Cbl binding motif. Furthermore, most of the basic metabolic and signaling pathways that maintain homeostasis are conserved in the fly providing an ideal context for in vivo studies of SH2B biological function.
Recent evidence has shown that Drosophila Lnk is a key regulator of cell growth and proliferation during development [28]. Loss-of-function mutations in Lnk produce phenotypes reminiscent of reduced IIS signalling such as growth reduction, developmental delay and female sterility. Genetic epistasis experiments indicated that Lnk functions downstream of the Drosophila Insulin Receptor (dInR) and upstream of PI3K in IIS-mediated growth control. Genetic epistasis suggested that Lnk may play a similar role as the insulin receptor substrate, Chico, in the activation of PI3K upon dInR stimulation during growth [28]. Mutations that reduce IIS activity in C. elegans, Drosophila and mouse can increase lifespan in all three organisms, demonstrating that the IIS pathway has evolutionary conserved roles in the determination of adult lifespan. In Drosophila, the effects of insulin receptor activity on lifespan determination are mediated via the Chico/PI3K/forkhead transcription factor [29]–[32]. Therefore, we investigated whether Lnk also plays a role in the determination of adult lifespan. Here, we show that Lnk mutant flies exhibit increased lifespan as well as improved survival under conditions of oxidative stress and starvation. We also show that Lnk loss-of-function results in increased stored energy reserves associated with transcriptional changes in genes involved in both lipid and carbohydrate metabolism. Biochemical and genetic data indicate that Lnk functions within both the IIS and Ras/MapK signaling cascades and is itself a direct target for transcriptional regulation by the dFoxo transcription factor.
Results
Lnk mutants have increased lifespan
Novel alleles of Lnk were recently isolated in a genetic screen looking for new regulators of growth in flies as Lnk loss-of-function clones were found to cause cell-autonomous growth inhibition in the developing eye [28]. We have characterised two additional mutant alleles of Lnk: Lnkd07478 containing a P-element insertion within the first intron of the Lnk locus and LnkDel29, a small deletion generated by FLP-FRT recombination between two pBAC elements that removes the first two exons of Lnk including the predicted translational start site (Figure 1A). Homozygous mutants had significantly reduced levels of Lnk transcripts as measured by quantitative RT-PCR (Figure 1B). Homozygous and transheterozygous mutants under normal culture conditions were adult viable but developmentally delayed with an overall reduction in body size as a result of reduced cell size and cell number (Figure 1C and 1D). No further reductions in growth were observed in hemizygous combinations over a deficiency that removes the entire Lnk locus suggesting that they represent strong loss-of-function alleles (Figure 1C). In addition, we were able to fully rescue the growth defects of LnkDel29 homozygotes by introducing a genomic rescue construct containing the entire Lnk locus indicating that these growth defects are specific to Lnk (Figure 1C).
Fig. 1. Lnk loss-of-function mutations reduce body size. 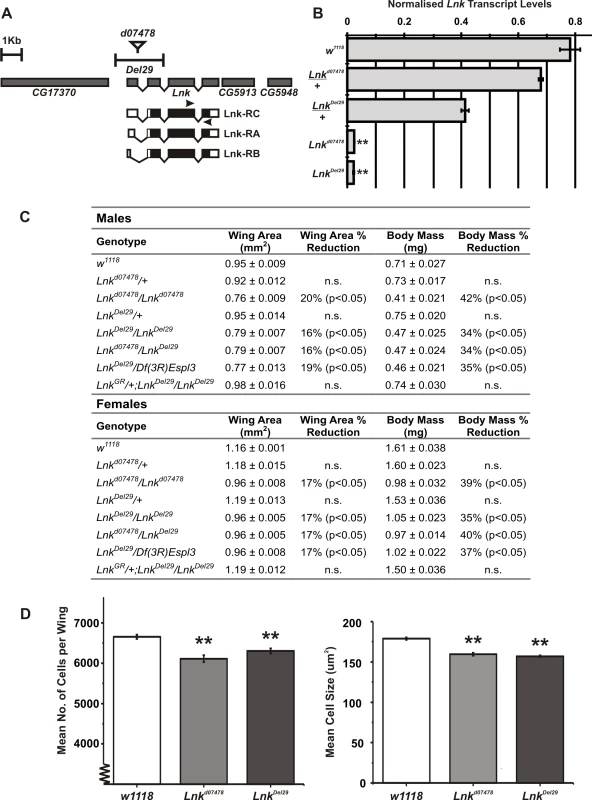
(A) Schematic representation of the Lnk locus along with the flanking genes CG17370, CG5913 and CG5948. The three Lnk transcripts (RA, RB, and RC) are shown along with the positions of the P-element insertion d07478 and the deletion Del29. Arrows show the position of primers used for the quantitative RT–PCR in (B). (B) Quantitative RT–PCR analysis of Lnk transcript expression in 7-day old female flies. Lnk transcripts were amplified using the primers indicated in (A) and normalised to actin5C. (C) Mean wing area and body mass for male and female flies of the indicated genotypes. Data are represented as means ± SEM (n = 10 for each measurement). Percentage differences compared to w1118 controls are indicated (n.s. = not significantly different). (D) The wings of homozygous Lnk mutant females contain fewer and smaller cells compared to the w1118 controls. Data are shown as means ±SEM (n = 10). ** denotes statistically significant difference (p<0.05). Both alleles were backcrossed for more than eight generations into two distinct genetic backgrounds: the inbred w1118 strain and the outbred wDahomey (wDah) strain. We then assayed heterozygotes and homozygotes of both alleles for longevity. After backcrossing into the w1118 genetic background, heterozygosity for either Lnkd07478 or LnkDel29 did not result in any significant differences in lifespan in either males or females (Figure 2A, 2B, 2E, and 2F). In contrast, we observed significant increases in both median and maximum lifespan in both males and females homozygous mutant for either allele when compared to wild-type controls (Figure 2A, 2B, 2E, and 2F). Furthermore, the longevity effects of LnkDel29 males and females were fully reproducible after backcrossing into wDah (Figure 2C and 2G) and the lifespan extension observed in females homozygous mutant for LnkDel29 was fully rescued by the introduction of a Lnk genomic rescue construct (Figure 2I), thereby confirming a role for Lnk in lifespan determination.
Fig. 2. Mutation of Lnk extends lifespan in both sexes and reduces female fecundity. 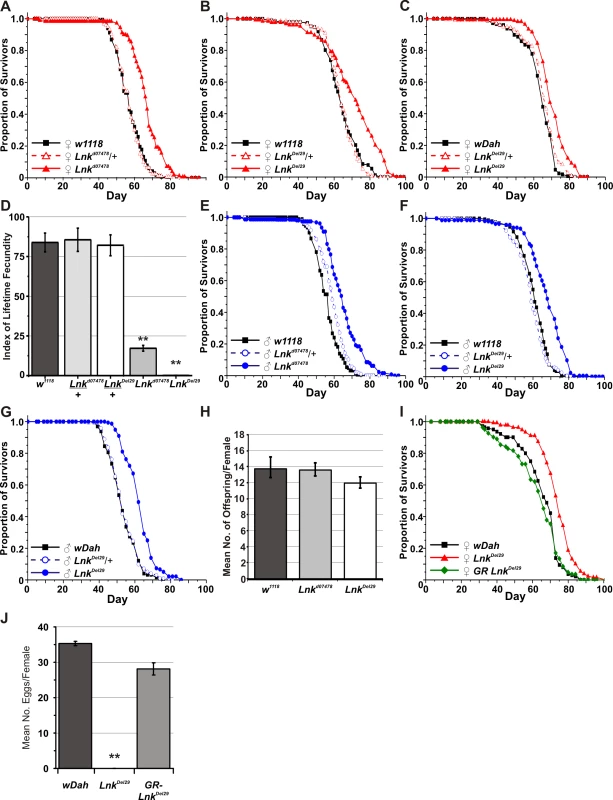
(A) w1118-backcrossed females. Median lifespans are: 57 days for w1118 (n = 196), 58 days for Lnkd07478/+ (n = 203) and 65 days for Lnkd07478/Lnkd07478 (n = 163). Log rank test χ2 and p-values: w1118 versus Lnkd07478/+ (χ2 = 1.7, p = 0.192) and w1118 versus Lnkd07478/Lnkd07478 (χ2 = 90.2 , p<0.0001). (B) w1118-backcrossed females. Median lifespans are 61 days for w1118 (n = 173), 59 days for LnkDel29/+ (n = 200) and 68 days for LnkDel29/LnkDel29 (n = 182). Log rank test χ2 and p-values: w1118 versus LnkDel29/+ (χ2 = 2.2, p = 0.139) and w1118 versus LnkDel29/LnkDel29 (χ2 = 65.2, p<0.0001). (C) wDah-backcrossed females. Median lifespans are 63 days for wDah (n = 179), 66 days for LnkDel29/+ (n = 177) and 68 days for LnkDel29/LnkDel29 (n = 178). Log rank test χ2 and p-values: wDah versus LnkDel29/+ (χ2 = 4.67, p = 0.03) and wDah versus LnkDel29/LnkDel29 (χ2 = 53.09, p<0.0001). (D) Reduced fecundity in Lnk homozygous mutant females. Index of lifetime fecundity represents the mean number of eggs laid per female per day at 10 time points during the first 40 days of life. Index of lifetime fecundity in Lnk homozygous mutant females (white bars) is significantly reduced compared to control flies (w1118; dark grey bars) and heterozygotes (light grey bars). Data are shown as means ±SEM. ** denotes statistically significant difference (p<0.05). (E) w1118-backcrossed males. Median lifespans are 57 days for w1118 (n = 198), 57 days for Lnkd07478/+ (n = 196) and 67 days for Lnkd07478/Lnkd07478 (n = 190). Log rank test χ2 and p-values: w1118 versus Lnkd07478/+ (χ2 = 0.1 , p = 0.778) and w1118 versus Lnkd07478/Lnkd07478 (χ2 = 107.0, p<0.0001). (F) w1118-backcrossed males. Median lifespans are 63 days for w1118 (n = 192), 63 days for LnkDel29/+ (n = 181) and 71 days for LnkDel29/LnkDel29 (n = 144). Log rank test χ2 and p-values: w1118 versus LnkDel29/+ (χ2 = 0.5, p = 0.463) and w1118 versus LnkDel29/LnkDel29 (χ2 = 37.0, p<0.0001). (G) wDah-backcrossed males. Median lifespans are 52 days for wDah (n = 169), 52 days for LnkDel29/+ (n = 168) and 61 days for LnkDel29/LnkDel29 (n = 171). Log rank test χ2 and p-values: wDah versus LnkDel29/+ (χ2 = 0.24, p = 0.627) and wDah versus LnkDel29/LnkDel29 (χ2 = 44.89, p<0.0001). (H) Mean number of eggs laid per w1118 female crossed to males of the indicated genotype. Data are shown as mean number of eggs laid per female fly over a four day period ± SEM. (I) Genomic rescue (GR) of Lnk in wDah-backcrossed females. Median lifespans are 67 days for wDah (n = 151), 74 days for LnkDel29/LnkDel29 (n = 150) and 66 days for LnkGR;LnkDel29/LnkDel29 (n = 92). Log rank test χ2 and p-values: wDah versus LnkDel29/LnkDel29 (χ2 = 33.87, p<0.0001) and wDah versus. LnkGR;LnkDel29/LnkDel29 (χ2 = 0.71, p = 0.40). (J) Genomic rescue of female fertility defects. Eggs were counted on day 7 of the lifespan experiment shown in (I). Data are shown as mean number of eggs/female over a 24 hour period ± SEM. ** denotes statistically significant difference (p<0.05). In addition to increased lifespan, homozygous Lnk females produced significantly fewer eggs compared to their wild-type counterparts, especially LnkDel29 homozygous females, which were practically sterile (Figure 2D). Furthermore, Lnk mutant ovaries were dramatically reduced in size and contained immature oocytes that were arrested in previtellogenic stages of oogenesis (data not shown) and the egg laying defects observed in LnkDel29 homozygous females were fully rescued in the presence of a Lnk genomic rescue construct (Figure 2J). We observed no obvious defects in the fertility of Lnk homozygous males and females mated to Lnk mutant males produced comparable numbers of eggs as females mated to w1118 males (Figure 2H).
Lnk mutants are stress resistant and show metabolic disregulation
Interventions that extend lifespan are often associated with enhanced resistance to various stresses [29],[33]. We therefore tested the ability of Lnk mutant flies to survive under conditions of oxidative stress and starvation. To induce oxidative stress, flies were starved for 5 hours and then fed 5% hydrogen peroxide in a sucrose/agar media. Both males and females, homozygous mutant for Lnk, showed significantly increased median survival times when fed 5% hydrogen peroxide compared to control flies under an identical regime (Figure 3A and 3B). Furthermore, this increased resistance to hydrogen peroxide was fully rescued in both sexes upon introduction of the Lnk genomic rescue construct (Figure 3A and 3B). We also observed a significant increase in survival times when Lnk mutant males and females were maintained on an agar-only diet to induce starvation (Figure 3C and 3D). Again, the starvation resistance observed in Lnk mutants was fully rescued in both sexes in the presence of the Lnk genomic rescue construct (Figure 3C and 3D). Moreover, resistance to hydrogen peroxide and starvation were observed with both Lnk mutant alleles and in both genetic backgrounds (Figure S1).
Fig. 3. Lnk mediates responses to oxidative stress and starvation and is required for metabolic regulation. 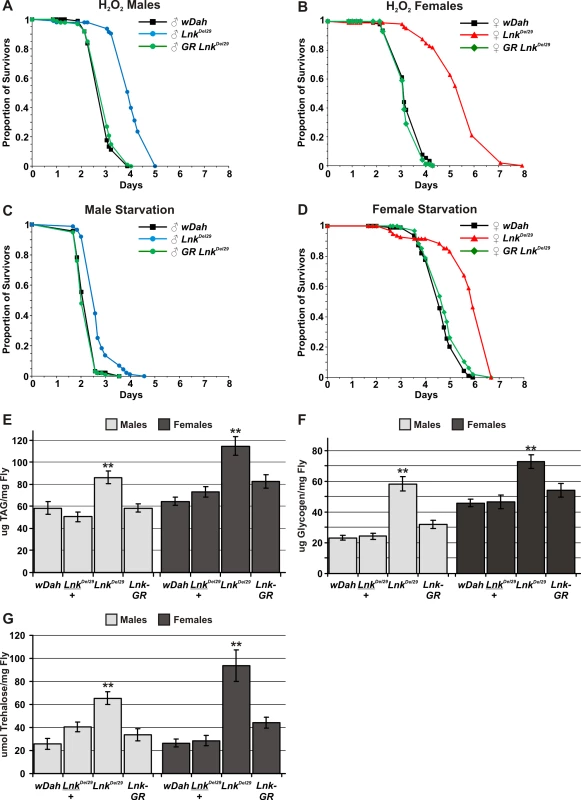
(A) Survival of male flies fed 5% hydrogen peroxide. Lnk mutant males live approximately 50% longer than controls and mutants carrying a Lnk genomic rescue (GR) construct (p<0.001). Median survival times are 2.6 days for wDah (black line; n = 97), 3.9 days for LnkDel29 (blue line; n = 93) and 2.6 days for LnkGR; LnkDel29/LnkDel29 (green line; n = 100). (B) Survival of female flies fed 5% hydrogen peroxide. Lnk mutant females live approximately 50% longer than controls and mutants carrying a Lnk genomic rescue (GR) construct (p<0.001). Median survival times are 3.1 days for wDah (black line; n = 93), 5.6 days for LnkDel29 (red line; n = 105) and 3.1 days for LnkGR; LnkDel29/LnkDel29 (green line; n = 100). (C) Survival of male flies under starvation conditions. Lnk mutant males live 30% longer than controls and mutants carrying a Lnk genomic rescue (GR) construct (p<0.001). Median survival times are as follows: 2.0 days for wDah (black line; n = 92), 2.6 days for LnkDel29 (blue line; n = 86) and 1.9 days for LnkGR; LnkDel29/LnkDel29(green line; n = 97). (D) Survival of female flies under starvation conditions. Lnk mutant females live 35% longer than controls and mutants carrying a Lnk genomic rescue (GR) construct (p<0.001). Median survival times are as follows: 4.3 days for wDah (black line; n = 94), 5.8 days for LnkDel29 (red line; n = 95) and 4.7 days for LnkGR; LnkDel29/LnkDel29 (green line; n = 95). (E) Whole-fly content of triglycerides (TAG) per mg of fly (fresh weight). Data are presented as means (n = 10) ± SEM. ** denotes statistically significant difference (p<0.05). (F) Whole-fly glycogen content per mg of fly (fresh weight). Data are presented as means (n = 10) ± SEM. ** denotes statistically significant difference (p<0.05). (G) Whole-fly trehalose content per mg of fly (fresh weight). Data are presented as means (n = 10) ± SEM. ** denotes statistically significant difference (p<0.05). Enhanced survival under conditions of starvation is often associated with increased levels of stored energy resources indicative of a disruption to metabolic homeostasis. In flies, metabolised nutrients are primarily stored as triglycerides (TAG) and glycogen in the fat body, the insect equivalent of the mammalian liver and white adipose tissue. We observed significantly elevated levels of both TAG and glycogen in whole-fly extracts of both males and females when we compared Lnk mutants to wild-type controls (Figure 3E and 3F). These elevated levels of TAG and glycogen were restored back down to those observed in wild-type flies in the presence of a Lnk genomic rescue construct (Figure 3E and 3F) Despite the observed differences in metabolic stores, we did not detect any obvious differences in the feeding behaviour of Lnk mutant flies compared to age-matched controls (Figure S2) suggesting that this increase in metabolic stores is unlikely to be mediated by increased feeding but by changes in cellular metabolism.
In addition to glycogen, adult insects possess a second metabolic pool of carbohydrate in the form of the disaccharide trehalose which is a major sugar in the fat body, thorax muscles and hemolymph and is rapidly consumed during certain energy-requiring activities such as flight. We found that whole-body levels of trehalose were also significantly increased in Lnk mutant males and females when compared to controls (Figure 3G) and again, these elevated levels of trehalose were restored to those observed in wild-type flies by the introduction of the Lnk genomic rescue construct (Figure 3G). However, when we measured trehalose levels in hemolymph extracted from either third instar or adult flies we found no significant differences between Lnk mutants and controls (data not shown). The total volume of hemolymph in an adult fly is extremely small (approximately 0.1 µl) and so the contribution of hemolymph trehalose to the total trehalose content can be regarded as negligible. Thus, the increase in trehalose content in whole fly extracts is almost certainly caused by increased tissue trehalose stores. Insect hemolymph also contains circulating glucose which is obtained from the diet and again, we found no significant differences in circulating glucose levels in Lnk mutants compared to controls (data not shown).
Transcriptional changes in Lnk mutants
In order to investigate further the molecular mechanisms of Lnk function, we performed microarray studies comparing the transcriptome of homozygous Lnk mutants to controls. FlyAtlas, a microarray-based atlas of adult gene expression in multiple Drosophila tissues (http://www.flyatlas.org; [34]), shows that Lnk mRNA is widely expressed in the adult fly but that transcripts are particularly enriched in the central nervous system. We therefore performed our transcriptome analysis on RNAs extracted from the heads of control and Lnk homozygous mutant females. After extraction, RNAs were labelled and hybridised to Affymetrix Drosophila 2.0 microarrays. All experiments were conducted in quadruplicate to facilitate statistical analysis. The raw data files were background corrected and normalised using the R programming language (see Material and Methods).
Using biological annotation available through the Gene Ontology (GO), we analysed our dataset using Catmap analysis. Catmap assigns significance to functional categories based on their representation within a ranked list of differentially expressed genes. This generated a list of GO terms associated with genes that show altered expression in Lnk mutants compared to controls (Table S1). The majority of the downregulated GO terms are involved in the metabolism of carbohydrates, amino acids, lipids and fatty acids suggesting that cellular metabolic processes are downregulated in Lnk mutant animals. Among the most significant upregulated GO terms are many linked to signal transduction and transcription indicating that these processes are upregulated in Lnk mutants compared to controls.
We then determined gene expression changes in our data set using a linear model. This study revealed that 2483 transcripts show significant differential expression (p<0.05; >0.1-fold) between Lnk mutants and controls with 1768 genes showing increased expression and 715 genes with decreased expression (Table S2). We compared this data set to a previously reported list of 484 transcripts that function in Drosophila metabolic pathways [35] and found that a number of genes in our differentially expressed gene list overlap with genes that regulate carbohydrate and lipid catabolism (Table S3). Downregulated genes included genes encoding several enzymes of the glycolytic pathway and the mitochondrial β-oxidation pathway while genes involved in glycogen synthesis and lipid storage showed upregulated expression. These changes in gene expression are consistent with an overall metabolic switch from catabolism to synthesis/storage and are congruent with our findings that Lnk mutants show increased levels of metabolic stores.
Interestingly, a number of transcripts that function in the IIS pathway were found to be upregulated in Lnk mutants compared to controls. The mammalian SH2B proteins have been shown biochemically to function as intracellular adaptors for the mammalian insulin receptor and recent genetic data from Drosophila has shown that Lnk may play a similar role to chico during IIS-mediated growth control. We therefore compared our data set to a comprehensive list of transcripts that function in the IIS pathway in Drosophila (Figure 4A and Table S4). We found upregulation of transcripts encoding positive regulators of IIS including the insulin-like ligands dilp2, dilp3, dilp5 and dilp6 as well as chico, Dp110, PDK-1 and dAkt. In contrast, we found downregulation of transcripts that encode negative regulators of the IIS pathway such as the IGFBP-like, ImpL2, and the PI3kinase inhibitor, Susi. Several of these IIS gene expression changes were further confirmed by quantitative RT-PCR (Figure 4B).
Fig. 4. Regulation of signal transduction pathway gene expression in Lnk mutants. 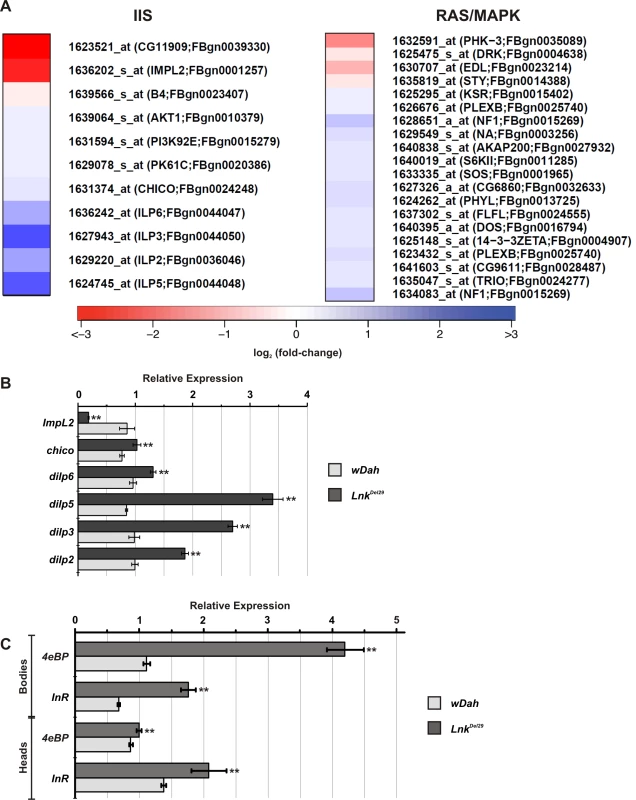
(A) Heat map showing expression of genes within the IIS and Ras/MapK signal transduction pathways that were significantly altered in Lnk mutants compared to controls. Red indicates lower expression; blue indicates higher expression (scale = log2 fold change). (B) Transcript levels of six IIS pathway genes measured by qRT-PCR and normalised to actin5C. RNA was extracted from adult female heads. Data are represented as mean normalised transcript level ± SEM (n = 4; ** p<0.05). (C) 4eBP and dInR mRNA levels measured by qRT-PCR and normalised to actin5C. RNA was extracted from either heads or bodies of adult females. Data are represented as mean normalised transcript level ± SEM (n = 4; ** p<0.05). Transcriptional outputs of the IIS pathway are mediated via several downstream effectors including the forkhead transcription factor, dFoxo, the Ras/MapK signaling pathway and the protein kinase complex TORC1, all of which have been shown to regulate gene expression either directly or indirectly. Further examination of our microarray data set identified four known dFoxo target genes with upregulated expression: split-ends (CG18497), ches-1-like (CG12690), eIF-4E (CG4035) and CG9009 (Table S4). In addition, we observed increased expression of two additional well-characterised dFoxo target genes, 4eBP and dInR by qRT-PCR (Figure 4C). These data therefore suggest that dFoxo activity is increased in Lnk mutant flies.
Using EASE analysis followed by Fisher's exact test for statistical significance and Bonferroni correction for multiple comparisons, we found that IIS pathway genes and genes classified by Flybase in the functional category of Ras signal transduction were over-represented in our data set (p = 0.002 and p = 0.004, respectively) (Table S4). In contrast, genes of the canonical TOR signaling pathway were not significantly over-represented in our data set (p = 0.784) (Table S4). Taken together, these data suggest significant transcriptional feedback onto the IIS via dFoxo and the Ras/MapK pathway but not via TOR signaling in Lnk mutant animals.
The potential for transcriptional feedback by dFoxo onto upstream components of the IIS cascade suggested that expression of Lnk itself may be regulated by dFoxo activity. We therefore looked for perfect matches to the mouse Foxo1/Foxo4 consensus binding site (RWWAACA) within 3Kb upstream of the Lnk translational start site and identified eight putative dFoxo binding sites in the Lnk promoter. To determine if dFoxo is indeed bound at the Lnk promoter, we performed chromatin immunoprecipitation (ChIP) using a specific dFoxo antibody [36]. Quantitative PCR (qPCR) was used to compare the relative DNA binding of dFoxo at a 5′ region of the Lnk promoter to two negative control genomic regions: a region within the U6 snRNA promoter and a region 3′ to the Lnk locus just downstream of the last exon (Figure 5A). We observed a significant increase in the relative DNA binding at the Lnk promoter region compared to the negative controls (Figure 5B). The magnitude of this increase in relative DNA binding (approximately 2-fold) was comparable to that observed for Lk6, a known dFoxo target gene (Figure 5B). In addition, we observed further increases in dFoxo DNA-binding at both loci in flies that had been starved or treated with paraquat prior to chromatin extraction, conditions in which dFoxo is activated (Figure 5B). Furthermore, quantitative RT-PCR analysis of Lnk expression in RNA extracts from foxo mutant flies revealed that Lnk transcript levels are significantly elevated the absence of dFoxo (Figure 5C) suggesting that dFoxo may normally function to repress Lnk expression.
Fig. 5. dFoxo binds to the promoter of Lnk and regulates Lnk expression. 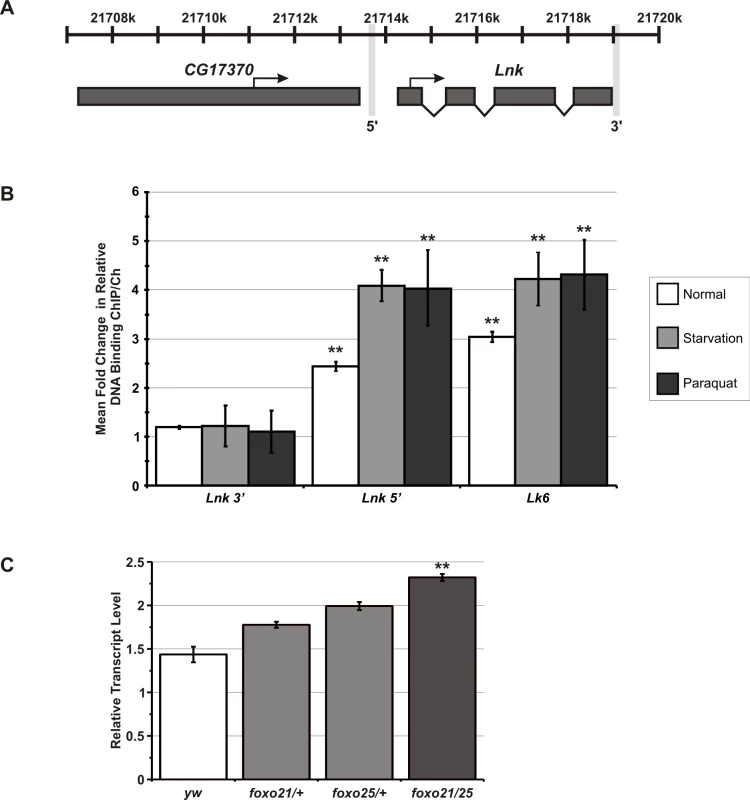
(A) Schematic diagram showing in vivo binding region for dFoxo at the Lnk locus as detected by chromatin immunoprecipitation (ChIP). Transcriptional start sites and the direction of transcription are indicated by arrows. The genomic region 3′ to the Lnk locus is also marked. (B) Quantitative PCR (qPCR) on the Lnk promoter, a 3′ region of the Lnk gene [as highlighted in (A)], the known dFoxo target gene, Lk6 and the U6 snRNA promoter (-87 to +61 from transcriptional start) to determine the proportion of DNA recovered after ChIP using anti-dFoxo antibody under normal, starvation and paraquat-treated conditions. Relative DNA binding was calculated as the proportion of chromatin recovered in the ChIP divided by that in the total chromatin preparation. Data are presented as mean fold-changes in relative DNA–binding compared to U6 ± SEM of three biological repeats (** p<0.05). (C) Lnk mRNA levels in dFoxo mutant flies measured by qRT–PCR and normalised to actin5C. Data are represented as mean normalised transcript level ± SEM (n = 3; **p<0.05). Lnk as a component of the Drosophila IIS and Ras/MapK pathways
To assess the biological significance of a regulatory role for Lnk in the IIS and Ras/MapK pathways, we examined the effects of RNAi-mediated knockdown of Lnk expression on insulin-stimulated signaling in insect cells. Activation of the dInR by insulin triggers activation of both the PI3K and the Ras/MapK branches of the insulin signaling pathways resulting in the phosphorylation of various intracellular effectors, including Akt and the MapK, Erk-A [37]. RNAi-mediated knockdown of Lnk resulted in reduced levels of phosphorylated Akt and Erk-A upon insulin stimulation with no significant change in the levels of total protein (Figure 6). This reduction in phosphorylated Akt and Erk-A was comparable to that caused by RNAi-mediated knockdown of either the dInR or its intracellular substrate, chico, suggesting that Lnk expression is required for full insulin signaling transduction via both the PI3K and MAPK branches of the IIS pathway in cultured cells.
Fig. 6. Lnk expression is required for insulin signaling and Ras/MapK signal transduction in flies. 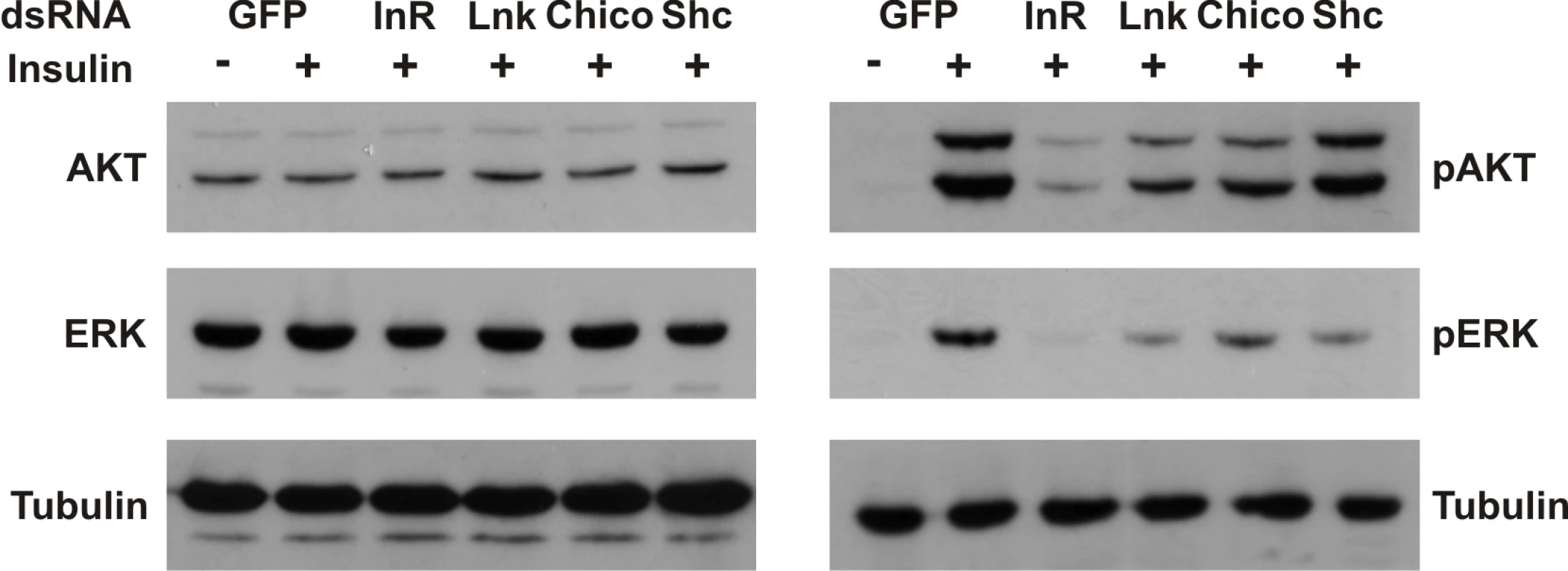
Western blot analysis of Akt and Erk-A phosphorylation in protein extracts of Drosophila S2 cells after treatment with the indicated dsRNAs before (-) and after (+) stimulation with human insulin. Knockdown of dInR, chico, or Lnk expression inhibits both Akt and Erk-A phosphorylation after insulin stimulation whereas knockdown of Shc inhibits phosphorylation of Erk-A only consistent with its role as an adapter for Ras/MAPK signaling. dsRNA against GFP was used as a negative control. Blots were probed with anti-tubulin as a loading control. Knockdowns of Lnk and chico transcripts were confirmed by Northern blot; knockdowns of dInR and Shc expression were confirmed by western blot (data not shown). Discussion
Our understanding of the physiological roles of the SH2B family of intracellular adaptors has been complicated by the presence of multiple family members in mammals. Furthermore, phenotypic analysis of genetic knockouts in mice has produced contradictory results. Recent genetic evidence has described a role for the single ancestral SH2B protein in Drosophila (Lnk) during IIS-mediated growth control. Here, we have characterised a critical role for Lnk in the regulation of lifespan, stress responses and cellular metabolism. Our results support a model in which Lnk functions as an intracellular adaptor for transduction of the IIS and Ras/MapK signaling cascades to mediate these physiological processes.
A recent genetic study has shown that mutations in Drosophila Lnk produce phenotypes reminiscent of reduced IIS during development including impaired growth, developmental delay and female sterility. Genetic epistasis experiments placed Lnk downstream of dInR and upstream of PI3K at the same level as Chico, the single fly insulin receptor substrate. Mutations in both chico and Lnk produce similar phenotypes and display similar reductions in IIS activity. Furthermore, flies homozygous mutant for both genes are lethal suggesting that they may be functionally redundant. The precise mechanisms whereby mammalian SH2B proteins transduce intracellular signaling from the insulin receptor remain unclear although like the IRS proteins, they have been shown to bind to multiple downstream mediators such as PI3K and Grb2 [6]. However, Drosophila Lnk lacks a consensus binding site for PI3K which is present in Chico so it is unlikely that they regulate similar downstream mechanisms.
The IIS pathway has an evolutionary conserved role in the determination of adult lifespan mediated by the Chico/PI3K/dFoxo branch of the IIS cascade. Previous studies have shown that flies either homozygous or heterozygous for chico1, a strong loss-of-function allele of chico, show increased lifespan [38]. We have shown that Lnk homozygotes also show increased lifespan although no obvious effects on lifespan were observed in heterozygous animals. Interestingly, the effects of Lnk mutation on lifespan extension were similar in both males and females, which is uncommon in Drosophila, even for IIS mutants. This data therefore suggests that as during growth regulation, signaling via the activated dInR during lifespan determination may require a second intracellular adaptor in addition to the insulin receptor substrate, Chico, and provides the first evidence of a role for SH2B proteins in lifespan determination.
Lifespan extension in females was associated with reduced fecundity as a result of an arrest in oogenesis. However, there were no visible effects of Lnk mutation on male fertility as measured by offspring production. As male homozygous mutants were also long-lived, this suggests that the extended lifespan of Lnk mutant females is not simply due to reduced fecundity. Genetic knockouts of SH2B1 in mice also show infertility due to impaired signal transduction from the IGF-1 receptor resulting in poor gonad development [7]. The sex-specific differences on fertility observed in Lnk mutants are probably due to sex-specific differences in Lnk transcript expression as microarray analyses of Drosophila gene expression has shown that Lnk transcripts are enriched within the female ovary but not in the male testis or accessory glands (http://www.flyatlas.org; [34]).
A comparison of the transcriptomes of Lnk mutant flies to controls revealed a number of gene expression changes associated with genes that encode components of the Drosophila IIS pathway. Hence, we observed upregulation of a number of factors that potentiate IIS such as the insulin-like ligands dilp2, dilp3, dilp5 and dilp6, as well as the insulin receptor substrate chico, the Drosophila class I PI3K, Dp110, phosphoinositide-dependent protein kinase PDK-1 and dAkt. In contrast, the expression of negative regulators of IIS such as the IGFBP-like ImpL2 and the PI3kinase inhibitor susi were downregulated. Several of these changes in expression were confirmed by qRT-PCR analysis and these data suggest that IIS transduction is affected by Lnk mutation, further strengthening the genetic evidence that Lnk is a component of the IIS pathway in flies. Transcriptional regulation downstream of IIS is in part mediated by the dFoxo transcription factor which is activated in response to low IIS by dAkt-mediated phosphorylation. While we did not observe any differences in dFoxo mRNA or protein levels in Lnk mutants compared to controls (data not shown), a number of dFoxo target genes did show changes in expression. Thus, split-ends (CG18497), ches-1-like (CG12690), eIF-4E (CG4035) and CG9009 all showed upregulated expression in our microarray data set. We also observed increased expression of two well-characterised dFoxo target genes, 4eBP and dInR, by quantitative RT-PCR. Taken together, these data suggest that dFoxo activity may be increased in Lnk mutant animals.
Interestingly, we observed a marked difference in the magnitude of increased expression of both 4eBP and dInR between different body parts. Thus, for 4eBP we observed a 1.1-fold increase in expression in head RNA extracts compared to a 3.8-fold increase in RNA extracts from bodies. Similarly, for dInR we observed a 1.5-fold increase in expression in head RNA extracts compared to a 2.6-fold increase in body RNA extracts. These data suggest that different tissues may exhibit differences in the magnitude of the transcriptional response to Lnk loss of function. As our microarray experiments were performed on RNA isolated from adult heads only, this may explain why 4eBP and dInR were not identified in the microarray data set as microarray analysis of gene expression is generally regarded as less sensitive than qRT-PCR especially when changes in expression are small.
The observations that upstream components of the IIS pathway show transcriptional upregulation in response to Lnk loss of function suggest that transcriptional feedback back onto multiple components of the pathway may play an important regulatory role in IIS signal transduction. Previous studies have shown that dInR is itself a direct target of dFoxo so that when IIS levels are low, activated dFoxo increases dInR expression. In this study, we have shown that dFoxo also binds to the Lnk promoter in vivo suggesting that Lnk itself may be a direct target of dFoxo. dFoxo activity may also regulate transcription of IIS genes under basal conditions. Previous studies have shown that dFoxo is required for the basal expression of the dilp3 ligand [39]. In our study, we found that in the absence of dFoxo, Lnk transcript expression increases suggesting that dFoxo activity is normally required for Lnk repression. Thus, regulation by dFoxo may involve both positive and negative effects on gene expression.
Our microarray data set also contained a number of differentially expressed genes that function within the Ras/MapK signal transduction pathway. Previous studies have shown that the Ras binding domain of Drosophila PI3K is required for maximal PI3K activity during growth and female egg laying linking Ras/MapK and IIS during growth and development in Drosophila [40]. Furthermore, we have shown that RNAi-mediated knockdown of Lnk inhibits insulin-stimulated Erk phosphorylation in insect cells. We cannot exclude the possibility that Lnk may play an adaptor function for Ras signaling downstream of other RTKs in addition to the insulin receptor. However, it should be noted that Lnk RNAi knockdown has no effect on Spitz-stimulated Erk phosphorylation via activation of the Drosophila EGF receptor [41].
Despite their small body size, Lnk mutants contain elevated levels of both lipid and carbohydrate stores. Consistent with their increased metabolic stores, Lnk mutants also showed increased survival under starvation conditions. Transcriptome analysis revealed gene expression changes in a number of components of metabolic regulation in Lnk mutants compared to controls. Thus, we observed reduced expression of several enzymes that function in the glycolytic pathway and upregulation of genes that function in glycogen synthesis. In addition, several genes in the mitochondrial β-oxidation pathway were downregulated whereas genes involved in the regulation of lipid storage showed increased expression. Taken together, these changes in gene expression are consistent with an overall inhibition of catabolic processes and upregulation of pathways that regulate the synthesis and storage of carbohydrates and lipids.
Studies on the metabolic defects of SH2B knockouts in mice have proved inconsistent. One group has shown that genetic deletion of SH2B1 impairs adipogenesis by downregulating adipogenic gene expression including PPARγ resulting in mice with decreased fat mass [26]. A Drosophila PPAR homolog has yet to identified but the closest Drosophila relative is the orphan receptor, E75 [42]. This gene was not among the differentially expressed gene list from our microarray data. Other studies have shown that SH2B1 null mice actually increase their body mass and develop obesity as a result of hyperphagia [8],[9]. In mammals, feeding is regulated by hypothalmic leptin signaling. Binding of leptin to its receptor results in receptor activation which in turn interacts with the non-receptor Janus kinase (Jak) stimulating downstream signaling events. Leptin stimulation of Jak is strongly potentiated by SH2B1 binding and so SH2B1 deletion impairs leptin signaling via Jak [4],[43],[44]. We did not observe any obvious differences in the feeding behaviour of Lnk mutant flies and there is no evidence to date that a leptin-like hormone exists in Drosophila. A functional Jak has been identified encoded by the hopscotch (hop) gene that has a well characterised role in hematopoesis in flies. We did not observe any obvious hematopoetic defects in Lnk mutants and Lnk was not found to genetically interact with any of the core JAK/STAT pathway components (data not shown). Our data therefore suggests that the increased adiposity in Lnk mutant flies is unlikely to be mediated by increased feeding or by defects in Jak signaling. In fact, our data suggest that the ancestral function of Lnk in Drosophila is to regulate carbohydrate and fat storage by regulating gene expression of several key metabolic regulatory pathways.
In mammalian cells, SH2B proteins have been shown to have dual functions during insulin signaling transduction by both activating and inhibiting downstream intracellular signaling events. Phosphorylation of SH2B2 by the activated insulin receptor creates a binding site for the proto-oncogene product c-Cbl. This promotes the ubiquitination of tyrosine kinase receptors by functioning as a RING-type E2-dependent ubiquitin protein ligase facilitating either endocytosis or proteasomal degradation of the receptor [16],[20]. The c-Cbl binding motif is conserved in Drosophila Lnk and so it will be of interest to determine whether the interaction with c-Cbl is important for Lnk function especially during lifespan regulation.
Materials and Methods
Fly stocks and husbandry
w1118 and Lnkd07478 were obtained from the Bloomington Drosophila Stock Centre. yw, dfoxo21a/TM3 and dfoxo25c/TM3 were a gift from the Hafen lab [45]. The dfoxo21a and dfoxo25c alleles were backcrossed for at least 6 generations into the yw background before use. The LnkDel29 deletion was generated using the pBAC insertions Lnke01414 and Lnkf02642 (obtained from the Exelixis Collection at Harvard Medical School) according to published protocols [46]. A 6 kb fragment spanning from the 3′ end of CG17370 to the beginning of the first exon of CG5913 was used for the genomic rescue construct. This was inserted by means of ΦC31 mediated integration into a landing site on the second chromosome at 51D [28]. The wild-type stock Dahomey was collected in 1970 in Dahomey (now Benin) and has since been maintained in large population cages with overlapping generations on a 12L∶12D cycle at 25°C. The white Dahomey (wDah) stock was derived by incorporation of the w1118 deletion into the outbred Dahomey background by successive backcrossing. Both w1118 and wDah stocks were negative for the endosymbiont Wolbachia as determined by PCR using primers specific to Wolbachia genomic DNA. Lnk mutants were backcrossed for at least 8 generations into both w1118 and wDah genetic backgrounds before phenotypic analyses. Stocks were maintained and all experiments were conducted at 25°C on a 12h∶12h light:dark cycle at constant humidity using standard sugar/yeast/agar (SYA) medium [47]. For all experiments including lifespan experiments flies were reared at standard larval density and eclosing adults were collected over a 12 hour period. Flies were mated for 48 hours before sorting into single sexes.
Body size measurements
Body weights of individual male and female 7-day old flies (n = 10 for each genotype) were measured using a precision balance. Wing areas, cell numbers and cell sizes were measured as previously described [48].
Fertility tests
For female fecundity tests, female flies were housed with males for 48 hours post-eclosion and then separated into vials at a density of 5 or 10 females per vial. Eggs were collected over two 24-hour periods per week for 4 weeks. The number of eggs laid per vial at each time point was counted. For male fertility tests, individual 3 day old males were mated to 30 virgin females, 3 to 5 days of age. Matings were observed and then females were separated from the males and housed in vials at a density of 3 females per vial. Eggs were collected and counted over four consecutive 24 hour periods.
Lifespan experiments
For lifespan experiments, flies were maintained in vials at a density of 10 flies per vial on standard SYA medium. Flies were transferred to new vials three times per week.
Stress experiments
For all stress assays, flies were reared and housed as for lifespan experiments. For oxidative stress assays, 4-day old flies were first starved for 5 hours on 1% agar and then transferred onto 5% sucrose/agar containing 5% hydrogen peroxide. For starvation experiments, 7-day old flies were transferred to 1% agar.
Feeding experiments
Feeding rates of flies were measured using a proboscis-extension assay in undisturbed conditions as previously described [49] using 7-day-old mated flies. Flies were housed at a density of 5 flies of the same sex per vial and transferred to new food on the evening before the assay. Feeding data is expressed as a proportion by experimental group (sum of scored feeding events divided by total number of feeding opportunities, where total number of feeding opportunities = number of flies in vial×number of vials in the group×number of observations). For statistical analyses, comparisons between experimental groups were made on the totals of feeding events by all flies within a vial, to avoid pseudoreplication.
S2 cell culture and western blots
Drosophila S2 cell culture, dsRNA treatment and insulin treatment were as described in [37]. For western blots, 40 µg of total protein were resolved on 10% Tris-Glycine-SDS. Proteins were transferred to PVDF membranes and probed for total Akt (1∶1000; Cell Signaling), phospho-Akt (1∶1000; Cell Signaling), Erk (1∶1000; Cell Signaling), phospho-Erk (1∶1000; Cell Signaling) and tubulin (1∶5000; Sigma). Secondary antibodies conjugated to HRP were purchased from Biorad.
Glucose and trehalose measurements
Hemolymph was collected and pooled from either 5 third instar larvae or 12 3-day old adult female flies. Glucose and trehalose levels were measured using the Glucose Infinity Reagent (ThermoScientific) as described in [33]. Whole fly trehalose in 7-day old adult males was measured as described in [33] and normalised to body weight.
Glycogen and triglyceride measurements
Glycogen content of 7-day old adult males was measured as described in [33] and normalised to body weight. Levels of TAG in 7-day old adult males were measured using the Tryglyceride Infinity Reagent (ThermoScientific) and also normalised to body weight.
Transcript expression analysis
Total RNA was extracted from 10 whole adult flies, 10 adult bodies or 25 adult heads per genotype using standard Trizol (Invitrogen) protocols. cDNA was prepared using oligod(T) primer and Superscript II reverse transcriptase according to the manufacturer's protocol (Invitrogen). Quantitative RT-PCR was performed using the PRISM 7000 sequence-detection system and Power SYBR® Green PCR Master Mix (ABI). Relative quantities of transcripts were determined using the relative standard curve method and normalized to actin5C. Three or four independent RNA extractions were used for each genotype. Primer sequences are available upon request.
Microarray data analyses
Whole organism microarray experiments are generally only useful for detecting concerted changes of expression of widely expressed genes and most tissues will be under-represented in the array signal from a whole fly. Further complications arise from whole organism arrays when there are significant structural differences between treatments. Lnk transcripts are widely expressed but are particularly enriched within the central nervous system and as Lnk mutant ovaries show significant structural differences compared to controls, we restricted our microarray expression analysis to isolated heads.
Raw data (cel files) were processed to correct for probe-sequence biases, and R's implementation of the Affymetrix's MicroArray Suite 5.0 software was used to determine present target transcripts [50]. A transcript was considered present if the p-value was <0.111, and absent otherwise. The data was normalized using loess normalization and a linear model was fitted to identify a set of differentially expressed genes using the R limma package [51]. All individual probes have been mapped against all known and predicted transcripts of the Drosophila melanogaster genome release version 5.4. Promiscuous (some or all probes within a probe set map to more than one gene in the genome) and orphan (no probes in the probe set map to any known or predicted gene in the genome) probe sets were excluded from further analysis. FlyBase gene ids were mapped to Gene Ontology (GO) ids (version 1.107).
For functional analysis using all expressed genes, we used the Wilcoxon rank sum test implemented in Catmap [52]. Ranks of genes were based on the Bayes t-statistic for differential expression and, for a given functional category, the significance of the rank sum for all genes in the category was calculated analytically based on a random gene-rank distribution.
Identification of dFoxo binding sites in the Lnk promoter
Sequence analysis was performed using Regulatory Sequence Analysis Tools [53] looking for perfect matches to the mouse Foxo1/Foxo4 binding sites [RWWAACA] [54].
Chromatin Immunoprecipitation (ChIP)
Chromatin immunoprecipitations were carried out essentially as described by [55]. For starvation and paraquat treatments, flies were either starved for 24 hours or fed 20 mM paraquat for 16 hours. 1000 adult female flies were crushed to a fine powder under liquid nitrogen and suspended in 6 ml of PBS supplemented with Protease Inhibitor Cocktail (Sigma). Cross-linking was performed with 0.5% formaldehyde for 10 minutes and quenched by the addition of 1.5 ml of 2.5M glycine. The cross-linked chromatin was recovered by centrifugation and washed twice with FA/SDS buffer (50 mM Hepes-KOH, 150 mM NaCl, 1 mM EDTA, 0.1% Na Deoxycholate, 0.1% SDS, 1% Triton-X100 and 1 mM PMSF). Samples were resuspended in FA/SDS buffer and incubated for 1 hour at 4C. Chromatin was recovered by centrifugation and sheared to an average size of 400 bp by sonication, giving on average 6 ml of chromatin in FA/SDS. For immunoprecipitations (IPs), 1 µl of affinity purified rabbit anti-Foxo antibody [36] was bound to Protein-G Dynabeads (Invitrogen) and incubated with 450 µl of chromatin for 2 hours at room temperature. Beads were washed three times with FA/SDS, once with TE, and once with 10 mM Tris-HCl pH 8, 250 mM LiCl, 1 mM EDTA, 1% NP40, 0.5% Na Deoxycholate. DNA was recovered, treated with proteases, de-cross-linked, treated with RNase and purified using the Qiagen PCR purification kit (Qiagen). For quantitative PCR, a suitable dilution of total chromatin and IP was used for quantification using the PRISM 7000 sequence-detection system and Power SYBR® Green PCR Master Mix (ABI). For ChIP analysis, relative amounts of the target DNA recovered after ChIP compared to total chromatin were determined using three independent biological replicates. The relative proportion of DNA binding was calculated by dividing the proportion of DNA binding in the ChIP for a single region by the average recovered for all regions for that chromatin to normalise for plate-plate differences.
Other statistical analyses
Statistical analyses were performed using JMP software (version 4.0.5; SAS Institute). Log rank tests were performed on lifespan and stress survival curves. Other data were tested for normality using the Shapiro-Wilk W test on studentised residuals and where appropriate log-transformed. One-way analyses of variance (ANOVA) and planned comparisons of means were made using Tukey-Kramer HSD test.
Supporting Information
Zdroje
1. RuiL
Carter-SuC
1998 Platelet-derived growth factor (PDGF) stimulates the association of SH2-Bbeta with PDGF receptor and phosphorylation of SH2-Bbeta. J Biol Chem 273 21239 21245
2. QianX
RiccioA
ZhangY
GintyDD
1998 Identification and characterization of novel substrates of Trk receptors in developing neurons. Neuron 21 1017 1029
3. RuiL
HerringtonJ
Carter-SuC
1999 SH2-B is required for nerve growth factor-induced neuronal differentiation. J Biol Chem 274 10590 10594
4. RuiL
Carter-SuC
1999 Identification of SH2-bbeta as a potent cytoplasmic activator of the tyrosine kinase Janus kinase 2. Proc Natl Acad Sci U S A 96 7172 7177
5. KongM
WangCS
DonoghueDJ
2002 Interaction of fibroblast growth factor receptor 3 and the adapter protein SH2-B. A role in STAT5 activation. J Biol Chem 277 15962 15970
6. HuangX
LiY
TanakaK
MooreKG
HayashiJI
1995 Cloning and characterization of Lnk, a signal transduction protein that links T-cell receptor activation signal to phospholipase C gamma 1, Grb2, and phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A 92 11618 11622
7. OhtsukaS
TakakiS
IsekiM
MiyoshiK
NakagataN
2002 SH2-B is required for both male and female reproduction. Mol Cell Biol 22 3066 3077
8. DuanC
YangH
WhiteMF
RuiL
2004 Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol Cell Biol 24 7435 7443
9. LiM
RenD
IsekiM
TakakiS
RuiL
2006 Differential role of SH2-B and APS in regulating energy and glucose homeostasis. Endocrinology 147 2163 2170
10. RiedelH
WangJ
HansenH
YousafN
1997 PSM, an insulin-dependent, pro-rich, PH, SH2 domain containing partner of the insulin receptor. J Biochem 122 1105 1113
11. YokouchiM
SuzukiR
MasuharaM
KomiyaS
InoueA
1997 Cloning and characterization of APS, an adaptor molecule containing PH and SH2 domains that is tyrosine phosphorylated upon B-cell receptor stimulation. Oncogene 15 7 15
12. AhmedZ
PillayTS
2001 Functional effects of APS and SH2-B on insulin receptor signalling. Biochem Soc Trans 29 529 534
13. KotaniK
WildenP
PillayTS
1998 SH2-Balpha is an insulin-receptor adapter protein and substrate that interacts with the activation loop of the insulin-receptor kinase. Biochem J 335 (Pt 1) 103 109
14. NelmsK
O'NeillTJ
LiS
HubbardSR
GustafsonTA
1999 Alternative splicing, gene localization, and binding of SH2-B to the insulin receptor kinase domain. Mamm Genome 10 1160 1167
15. AhmedZ
SmithBJ
KotaniK
WildenP
PillayTS
1999 APS, an adapter protein with a PH and SH2 domain, is a substrate for the insulin receptor kinase. Biochem J 341 (Pt 3) 665 668
16. AhmedZ
SmithBJ
PillayTS
2000 The APS adapter protein couples the insulin receptor to the phosphorylation of c-Cbl and facilitates ligand-stimulated ubiquitination of the insulin receptor. FEBS Lett 475 31 34
17. DuanC
LiM
RuiL
2004 SH2-B promotes insulin receptor substrate 1 (IRS1) - and IRS2-mediated activation of the phosphatidylinositol 3-kinase pathway in response to leptin. J Biol Chem 279 43684 43691
18. YokouchiM
WakiokaT
SakamotoH
YasukawaH
OhtsukaS
1999 APS, an adaptor protein containing PH and SH2 domains, is associated with the PDGF receptor and c-Cbl and inhibits PDGF-induced mitogenesis. Oncogene 18 759 767
19. AhmedZ
PillayTS
2003 Adapter protein with a pleckstrin homology (PH) and an Src homology 2 (SH2) domain (APS) and SH2-B enhance insulin-receptor autophosphorylation, extracellular-signal-regulated kinase and phosphoinositide 3-kinase-dependent signalling. Biochem J 371 405 412
20. AhnMY
KatsanakisKD
BhedaF
PillayTS
2004 Primary and essential role of the adaptor protein APS for recruitment of both c-Cbl and its associated protein CAP in insulin signaling. J Biol Chem 279 21526 21532
21. MoodieSA
Alleman-SposetoJ
GustafsonTA
1999 Identification of the APS protein as a novel insulin receptor substrate. J Biol Chem 274 11186 11193
22. VelazquezL
ChengAM
FlemingHE
FurlongerC
VeselyS
2002 Cytokine signaling and hematopoietic homeostasis are disrupted in Lnk-deficient mice. J Exp Med 195 1599 1611
23. TakakiS
SauerK
IritaniBM
ChienS
EbiharaY
2000 Control of B cell production by the adaptor protein lnk. Definition Of a conserved family of signal-modulating proteins. Immunity 13 599 609
24. RuddCE
2001 Lnk adaptor: novel negative regulator of B cell lymphopoiesis. Sci STKE 2001 PE1
25. RenD
LiM
DuanC
RuiL
2005 Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab 2 95 104
26. YoshigaD
SatoN
TorisuT
MoriH
YoshidaR
2007 Adaptor protein SH2-B linking receptor-tyrosine kinase and Akt promotes adipocyte differentiation by regulating peroxisome proliferator-activated receptor gamma messenger ribonucleic acid levels. Mol Endocrinol 21 1120 1131
27. MinamiA
IsekiM
KishiK
WangM
OguraM
2003 Increased insulin sensitivity and hypoinsulinemia in APS knockout mice. Diabetes 52 2657 2665
28. WerzCKK
HafenE
StockerH
The Drosophila SH2B Family Adaptor Lnk Acts in Parallel to Chico in the Insulin Signaling Pathway. PLoS Genet 5(8) e1000596 doi:10.1371/journal.pgen.1000596
29. ClancyDJ
GemsD
HafenE
LeeversSJ
PartridgeL
2002 Dietary restriction in long-lived dwarf flies. Science 296 319
30. TatarM
KopelmanA
EpsteinD
TuMP
YinCM
2001 A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 292 107 110
31. HwangboDS
GershmanB
TuMP
PalmerM
TatarM
2004 Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature 429 562 566
32. GiannakouME
GossM
JungerMA
HafenE
LeeversSJ
2004 Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science 305 361
33. BroughtonSJ
PiperMD
IkeyaT
BassTM
JacobsonJ
2005 Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A 102 3105 3110
34. ChintapalliVR
WangJ
DowJA
2007 Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39 715 720
35. BakerKD
ThummelCS
2007 Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab 6 257 266
36. GiannakouME
GossM
JacobsonJ
VintiG
LeeversSJ
2007 Dynamics of the action of dFOXO on adult mortality in Drosophila. Aging Cell 6 429 438
37. ClemensJC
WorbyCA
Simonson-LeffN
MudaM
MaehamaT
2000 Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc Natl Acad Sci U S A 97 6499 6503
38. ClancyDJ
GemsD
HarshmanLG
OldhamS
StockerH
2001 Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science 292 104 106
39. BroughtonS
AlicN
SlackC
BassT
IkeyaT
2008 Reduction of DILP2 in Drosophila triages a metabolic phenotype from lifespan revealing redundancy and compensation among DILPs. PLoS ONE 3 e3721 doi:10.1371/journal.pone.0003721
40. OrmeMH
AlrubaieS
BradleyGL
WalkerCD
LeeversSJ
2006 Input from Ras is required for maximal PI(3)K signalling in Drosophila. Nat Cell Biol 8 1298 1302
41. FriedmanA
PerrimonN
2006 A functional RNAi screen for regulators of receptor tyrosine kinase and ERK signalling. Nature 444 230 234
42. SchoonjansK
StaelsB
AuwerxJ
1996 Role of the peroxisome proliferator-activated receptor (PPAR) in mediating the effects of fibrates and fatty acids on gene expression. J Lipid Res 37 907 925
43. RuiL
GunterDR
HerringtonJ
Carter-SuC
2000 Differential binding to and regulation of JAK2 by the SH2 domain and N-terminal region of SH2-bbeta. Mol Cell Biol 20 3168 3177
44. O'BrienKB
O'SheaJJ
Carter-SuC
2002 SH2-B family members differentially regulate JAK family tyrosine kinases. J Biol Chem 277 8673 8681
45. JungerMA
RintelenF
StockerH
WassermanJD
VeghM
2003 The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol 2 20
46. ThibaultST
SingerMA
MiyazakiWY
MilashB
DompeNA
2004 A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet 36 283 287
47. BassTM
GrandisonRC
WongR
MartinezP
PartridgeL
2007 Optimization of dietary restriction protocols in Drosophila. J Gerontol A Biol Sci Med Sci 62 1071 1081
48. BohniR
Riesgo-EscovarJ
OldhamS
BrogioloW
StockerH
1999 Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell 97 865 875
49. WongR
PiperMD
WertheimB
PartridgeL
2009 Quantification of food intake in Drosophila. PLoS ONE 4 e6063 doi:10.1371/journal.pone.0006063
50. SchusterEF
BlancE
PartridgeL
ThorntonJM
2007 Correcting for sequence biases in present/absent calls. Genome Biol 8 R125
51. SmythGK
2004 Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3 Article3
52. BreslinT
EdenP
KroghM
2004 Comparing functional annotation analyses with Catmap. BMC Bioinformatics 5 193
53. van HeldenJ
2003 Regulatory sequence analysis tools. Nucleic Acids Res 31 3593 3596
54. BiggsWH3rd
CaveneeWK
ArdenKC
2001 Identification and characterization of members of the FKHR (FOX O) subclass of winged-helix transcription factors in the mouse. Mamm Genome 12 416 425
55. KurasL
StruhlK
1999 Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature 399 609 613
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 3- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Příjem alkoholu a menstruační cyklus
- Doporučení pro diagnostiku a léčbu akutních jaterních porfyrií
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Parental Genome Dosage Imbalance Deregulates Imprinting in
- Identification and Functional Analysis of the Vision-Specific BBS3 (ARL6) Long Isoform
- HAP2(GCS1)-Dependent Gamete Fusion Requires a Positively Charged Carboxy-Terminal Domain
- Initial Genomics of the Human Nucleolus
- Role of RecA and the SOS Response in Thymineless Death in
- PPS, a Large Multidomain Protein, Functions with Sex-Lethal to Regulate Alternative Splicing in
- Mislocalization of XPF-ERCC1 Nuclease Contributes to Reduced DNA Repair in XP-F Patients
- Transgenic Rat Model of Neurodegeneration Caused by Mutation in the Gene
- Human Population Differentiation Is Strongly Correlated with Local Recombination Rate
- Local-Scale Patterns of Genetic Variability, Outcrossing, and Spatial Structure in Natural Stands of
- Arginylation-Dependent Neural Crest Cell Migration Is Essential for Mouse Development
- HP1 Recruitment in the Absence of Argonaute Proteins in
- MiR-218 Inhibits Invasion and Metastasis of Gastric Cancer by Targeting the Robo1 Receptor
- Bias and Evolution of the Mutationally Accessible Phenotypic Space in a Developmental System
- Papillorenal Syndrome-Causing Missense Mutations in / Result in Hypomorphic Alleles in Mouse and Human
- Rapid Assessment of Genetic Ancestry in Populations of Unknown Origin by Genome-Wide Genotyping of Pooled Samples
- Regulation of Lifespan, Metabolism, and Stress Responses by the SH2B Protein, Lnk
- KRAB–Zinc Finger Proteins and KAP1 Can Mediate Long-Range Transcriptional Repression through Heterochromatin Spreading
- Identification of the Regulatory Logic Controlling Pathoadaptation by the SsrA-SsrB Two-Component System
- Drosophila Xpd Regulates Cdk7 Localization, Mitotic Kinase Activity, Spindle Dynamics, and Chromosome Segregation
- Multiple Signals Converge on a Differentiation MAPK Pathway
- In the Tradition of Science: An Interview with Victor Ambros
- Association of the Polymorphism His615Arg with Melanin Content in East Asian Populations: Further Evidence of Convergent Evolution of Skin Pigmentation
- Fatal Cardiac Arrhythmia and Long-QT Syndrome in a New Form of Congenital Generalized Lipodystrophy with Muscle Rippling (CGL4) Due to Mutations
- Deciphering Normal Blood Gene Expression Variation—The NOWAC Postgenome Study
- Derepression of the Plant Chromovirus Induces Germline Transposition in Regenerated Plants
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Deciphering Normal Blood Gene Expression Variation—The NOWAC Postgenome Study
- Transgenic Rat Model of Neurodegeneration Caused by Mutation in the Gene
- Papillorenal Syndrome-Causing Missense Mutations in / Result in Hypomorphic Alleles in Mouse and Human
- Fatal Cardiac Arrhythmia and Long-QT Syndrome in a New Form of Congenital Generalized Lipodystrophy with Muscle Rippling (CGL4) Due to Mutations
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



