-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Human Population Differentiation Is Strongly Correlated with Local Recombination Rate
Allele frequency differences across populations can provide valuable information both for studying population structure and for identifying loci that have been targets of natural selection. Here, we examine the relationship between recombination rate and population differentiation in humans by analyzing two uniformly-ascertained, whole-genome data sets. We find that population differentiation as assessed by inter-continental FST shows negative correlation with recombination rate, with FST reduced by 10% in the tenth of the genome with the highest recombination rate compared with the tenth of the genome with the lowest recombination rate (P≪10−12). This pattern cannot be explained by the mutagenic properties of recombination and instead must reflect the impact of selection in the last 100,000 years since human continental populations split. The correlation between recombination rate and FST has a qualitatively different relationship for FST between African and non-African populations and for FST between European and East Asian populations, suggesting varying levels or types of selection in different epochs of human history.
Published in the journal: . PLoS Genet 6(3): e32767. doi:10.1371/journal.pgen.1000886
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000886Summary
Allele frequency differences across populations can provide valuable information both for studying population structure and for identifying loci that have been targets of natural selection. Here, we examine the relationship between recombination rate and population differentiation in humans by analyzing two uniformly-ascertained, whole-genome data sets. We find that population differentiation as assessed by inter-continental FST shows negative correlation with recombination rate, with FST reduced by 10% in the tenth of the genome with the highest recombination rate compared with the tenth of the genome with the lowest recombination rate (P≪10−12). This pattern cannot be explained by the mutagenic properties of recombination and instead must reflect the impact of selection in the last 100,000 years since human continental populations split. The correlation between recombination rate and FST has a qualitatively different relationship for FST between African and non-African populations and for FST between European and East Asian populations, suggesting varying levels or types of selection in different epochs of human history.
Introduction
Single Nucleotide Polymorphism (SNP) allele frequency differentiation between human populations, usually measured by Wright's FST [1],[2], has been extensively studied both for characterizing population structure and demographic history [3]–[10], and for detecting loci that have experienced the effects of natural selection [8], [11]–[15]. In a recent study, Barreiro et al. found that global, inter-continental human population differentiation has been shaped in an important way by natural selection, with negative selection reducing population differentiation and mostly affecting coding regions, and positive selection increasing population differentiation and affecting primarily SNPs that are nonsynonymous or in the 5′ untranslated regions of genes [16]. In the current study, we used the variability of recombination rate across the genome as a tool to learn about how substantially the forces of natural selection have shaped patterns of inter-continental allele frequency differentiation across populations. Nucleotides that are in regions of the genome with low recombination rates are expected to be more affected by natural selection (due to hitchhiking and background selection from sites in the vicinity) than nucleotides in regions of the genome with high recombination rates. By measuring the extent to which regions of high and low recombination rate differ in their FST, it may be possible to learn about how much of the overall differentiation in allele frequencies across populations can be accounted for by natural selection.
Many studies have documented a positive correlation between nucleotide diversity and recombination rate in many species, notably in Drosophila [17]–[19], humans [10], [20]–[25], and Arabidopsis lyrata [26]. In Drosophila, humans, and maize, a positive correlation was also observed between interspecific sequence divergence and recombination rate [17], [22], [27]–[29]. The observed correlation between nucleotide diversity or divergence and recombination rate has often been ascribed to natural selection [18],[20],[21],[23],[24],[29],[30] since directional selection reduces diversity and its effect on linked neutral sites extends further in regions of lower recombination rate. Thus, both hitchhiking (associated with positive selection) [31] and background selection (the “hitchhiking” associated with negative selection) [32], are expected to give rise to a positive correlation between diversity and recombination rate [32]–[35]. A recent analysis in humans modeled the slightly different effects of hitchhiking and background selection on the shape of the correlation between nucleotide diversity and recombination rate and concluded that positive selection better explains the data [20], while another recent study concluded that either hitchhiking or background selection explain their results [22].
A substantial body of research has explored an alternative, mechanistic explanation for the observed positive correlation between nucleotide diversity and recombination, which is the mutagenic effect of recombination [10],[17],[21],[27],[36],[37],[38]. One way to partially disentangle the mutagenic effect of recombination from the effect of selection is to normalize nucleotide diversity by interspecific divergence such as human-chimpanzee divergence, while making the assumption that large-scale recombination rates and mutation rates have been unchanged since species divergence [10],[20],[22]. The two aforementioned recent genome-wide studies reported that a significant correlation of human nucleotide diversity with recombination rate is preserved following normalization by human-chimpanzee divergence [20],[22]. Another recent study that focused on 40 regions with medium to high recombination rate reported that a significant correlation of human diversity with recombination rate vanished following such normalization [10]. A secondary concern with the approach of normalizing by interspecific divergence is that it does not take into account the effect that hitchhiking and background selection may have had in the ancestral population of humans and chimpanzees [39]. This effect, which can contribute to the observed correlation between interspecific divergence and recombination rate, is expected to weaken the observed normalized correlation of human nucleotide diversity with recombination rate.
In this study, we examine the relationship between recombination rate and population differentiation in allele frequencies of ascertained polymorphisms. For this type of analysis, the mutagenic effect of recombination is not a confounder, and any observed correlation is expected to be the result of selection in human history since the split of the analyzed populations. Different types of natural selection are expected to affect patterns of allele frequency differentiation: Positive selection is predicted to produce a negative correlation between FST and recombination rate if adaptation is local and selective sweeps drive alleles to high frequency in some but not all of the populations between which FST is measured. Selective sweeps are expected to extend less far in regions of higher recombination rate, and thus allele frequency differentiation is expected to be higher on average in regions of low recombination rate [16],[30],[40],[41],[42],[43]. Negative (purifying) selection can also produce a negative correlation between FST and recombination rate. While negative selection is expected to decrease population differentiation at the site under selection itself [16],[40], background selection decreases the effective population size at sites linked to negatively selected alleles, thereby increasing their allele frequency differentiation between populations as captured by relative measures such as FST [41], [43]–[45]. As the effect of background selection extends further in regions of lower recombination rate, higher population differentiation on average is predicted in such regions. There is also another important difference between our approach of examining the relationship between population differentiation and recombination rate, to previous approaches of comparing nucleotide diversity and recombination rate. Population differentiation only reflects events that occurred since two populations split, and hence allows us to study the effects of natural selection over a circumscribed time period (as opposed to averaging over the hundreds of thousands of years of a population's history).
To empirically examine the correlation of recombination rate and allele frequency differentiation in human populations, we needed to examine data sets that were ascertained in a uniform way across the genome, in a manner that is independent of local recombination rate. We focused on two data sets: 1,110,338 Perlegen “class A” SNPs [46], as well as 248,886 uniformly-ascertained SNPs that we previously reported [4] and that are mostly a subset of the International Haplotype Map (HapMap) Phase II [9]. These two data sets allowed us to interrogate population differentiation between North Europeans, East Asians, African Americans, and West Africans. The aims of this paper are to probe the correlation between global population differentiation and recombination rate, and to test whether it is attributable to natural selection. Once we establish that a strong correlation exists and that it likely reflects a history of natural selection, we explore how the impact of selection has varied in different epochs of human history by characterizing the differentiation between different pairs of populations that separated at different times.
Results
Population differentiation is inversely correlated with recombination rate
Our primary set of analyses were carried out on 1,110,338 Perlegen “class A” autosomal SNPs [46]. We used this set of SNPs since it is the largest data set that we are aware of for which the ascertainment of SNPs is uniform across the genome. Perlegen “class A” SNPs were discovered by array-based resequencing of 24 human samples of diverse ancestry [46],[47]. While this ascertainment scheme is expected to introduce a complicated ascertainment bias in terms of allele frequency correlations across populations—since different SNPs were ascertained in different numbers of samples from different ancestries—this bias is expected to be the same at every point on the autosomes [46]. Due to the uniform nature of SNP ascertainment independent of recombination rate, any correlations that are observed between allele frequency patterns at these SNPs and recombination rate are not expected to be due to locus specific differences in SNP ascertainment. For each SNP, we estimated recombination rate in a 3 Mb stretch centered on the SNP based on the deCODE genetic map [48] (Methods), which results in a median across all SNPs of 1.23 cM/Mb (mean of 1.39 cM/Mb).
To examine whether and how population differentiation depends on recombination rate, we assigned SNPs to equally-sized bins according to the recombination rate around them. For each bin, we estimated global population differentiation for the SNPs in that bin as the FST between the three population samples, which consisted of 24 individuals of European ancestry, 24 individuals of Han Chinese ancestry, and 23 African Americans [46]. Population differentiation shows a strong dependence on recombination rate, ranging from 0.1248±0.0009 for the SNPs in the bin of lowest recombination rate to 0.1125±0.0006 for the SNPs in the bin of highest recombination rate, and 0.1213 for all SNPs combined (Figure 1A). More generally, we find a striking correlation between population differentiation of SNPs in a bin and the recombination rate in that bin, with a correlation coefficient between the two of r = −0.96 (P = 8.9×10−6). A linear regression of population differentiation as a function of recombination rate provides a reasonably good fit to the data, and predicts an average decrease of 0.0048 (4%) in FST for every 1 cM/Mb increase in recombination rate (Figure 1A). As binning the data by recombination rate averages out some of the variability across individual SNPs, we repeated the analysis without binning and observed a very significant correlation also between FST of single SNPs and recombination rate around them (r = −0.015; P≪10−12).
Fig. 1. Population differentiation in allele frequencies is inversely correlated with recombination rate. 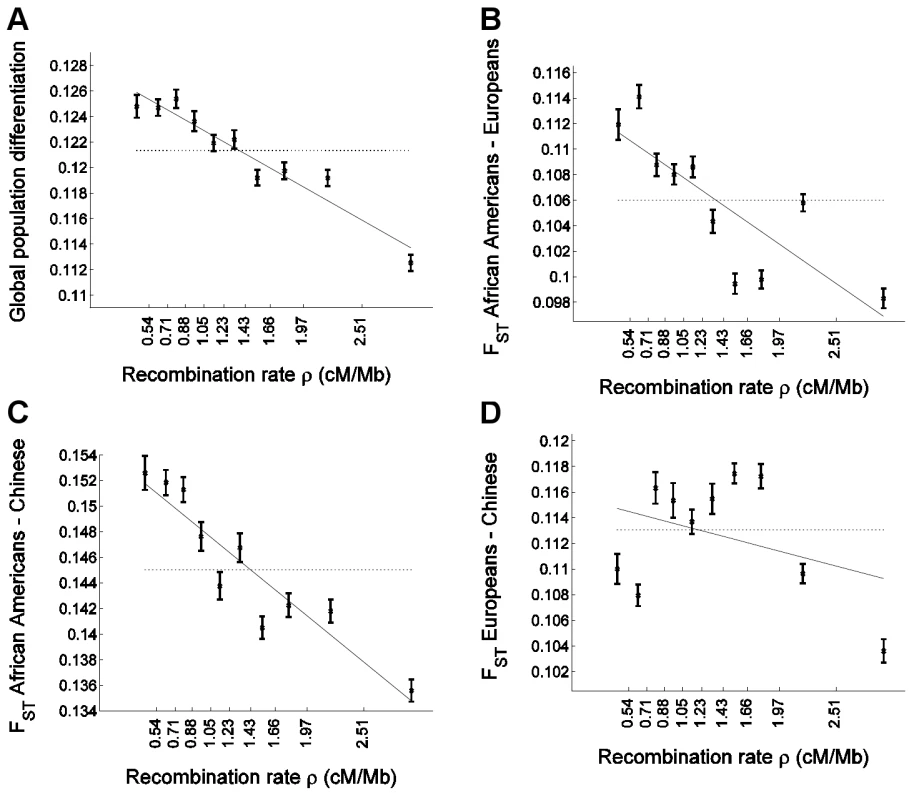
We placed 1,110,338 SNPs into 10 bins according to the recombination rate in a 3 Mb window centered on each SNP. The x-axis of all panels indicates the recombination rate, with the values indicated on the ticks corresponding to the edges between 10 bins. For each bin, at an x-axis position corresponding to the median recombination rate across the SNPs at that bin, the figure presents (A) global population differentiation between African Americans, Europeans, and Chinese; (B) FST between African Americans and Europeans; (C) FST between African Americans and Chinese; and (D) FST between Europeans and Chinese. Error bars indicate ±1 standard error, which is estimated based on 1,000 moving block bootstraps over the SNPs in the bin. Linear regression of FST estimates as a function of median recombination rate in each bin is also presented (solid line) and corresponds to (A) 0.1280–0.0048ρ (B) 0.1138–0.0057ρ (C) 0.1546–0.0067ρ and (D) 0.1156–0.0022ρ. The corresponding correlation coefficient estimates between FST and median recombination rate are (A) r = −0.962 (P = 8.9×10−6), (B) −0.815 (P = 0.0041), (C) −0.931 (P = 0.0001), and (D) −0.361 (P = 0.306). For comparison, population differentiation based on all SNPs in all bins combined is also presented (horizontal dotted line). The y-axis range is different between the four panels but spans 0.02 units in all. Figure S1 repeats Figure 1A for sets of SNPs of different minor allele frequency categories. Statistical framework for relating population differentiation and recombination rate
To characterize in more detail the relationship between population differentiation and recombination rate, we designed a more elaborate statistical framework that overcame three limitations of the correlation and regression analysis described above: (i) The previous analysis did not incorporate uncertainty in FST estimation that is due to a limited number of SNPs in each bin. (We did estimate standard errors in each bin via bootstrapping as presented in Figure 1, but did not incorporate these errors into the inference of the relationship between FST and recombination rate.) (ii) Because the regression did not incorporate this uncertainty, we could not apply the analysis to many recombination rate bins since as the number of SNPs per bin decreased the noise obscuring the signal increased. (iii) The analysis did not account for correlation between SNPs due to linkage disequilibrium (LD), which is important especially since LD is itself correlated to recombination rate.
To overcome these limitations, we developed a bootstrapping framework for estimating several statistics that capture the relationship between recombination rate and population differentiation. The framework generates many data sets of the same size as the original using a Moving Block Bootstrap (MBB) [49],[50],[51]. The MBB works by resampling contiguous runs of SNPs from the data (with replacement) to appropriately take into account the effect of correlation between SNPs due to LD [4]. Within each bootstrap, our statistical framework places the randomly selected set of SNPs into their associated recombination rate bins, estimates population differentiation for the SNPs, and measures correlation and regression statistics between differentiation and recombination rate along the same lines as the analysis described above. We estimated statistics by averaging across many bootstraps and estimated standard errors as the standard deviation of the estimates across bootstraps. Our estimates thus account for uncertainty in population differentiation estimation and also account for dependence among neighboring SNPs. They thus provide standard errors that we can use for hypothesis testing.
After applying the bootstrapping framework to the data set, the correlation between FST and recombination rate remains extremely significant: r = −0.89±0.06 (P≪10−12). Linear regression of FST as a function of recombination rate results in a best fitting relationship of 0.1280–0.0049ρ, which is very similar to the naïve analysis; but, importantly, the bootstrapping framework allows us to perform hypothesis testing based on the standard error estimates across bootstraps (Table 1). In particular, the recombination rate regression coefficient of −0.0049±0.0007 is significantly different from zero (P = 2.6×10−12) as is a t-statistic for the significance of the linear regression coefficient: −6.09±2.18 (P = 0.0051; Table 1). This further supports the conclusion that recombination rate is a significant explanatory variable of population differentiation. Increasing the number of recombination rate bins from 10 to 40 yielded similar results (Table 1), except that the greater number of bins allows for a more consistent application of the regression analysis, resulting in a more significant departure of the t-statistic from zero: −5.45±0.99 (P = 3.5×10−8). We use 10 recombination rate bins in all following analyses.
Tab. 1. Bootstrapped correlation and regression coefficient estimates of FST as a function of recombination rate in Perlegen data. 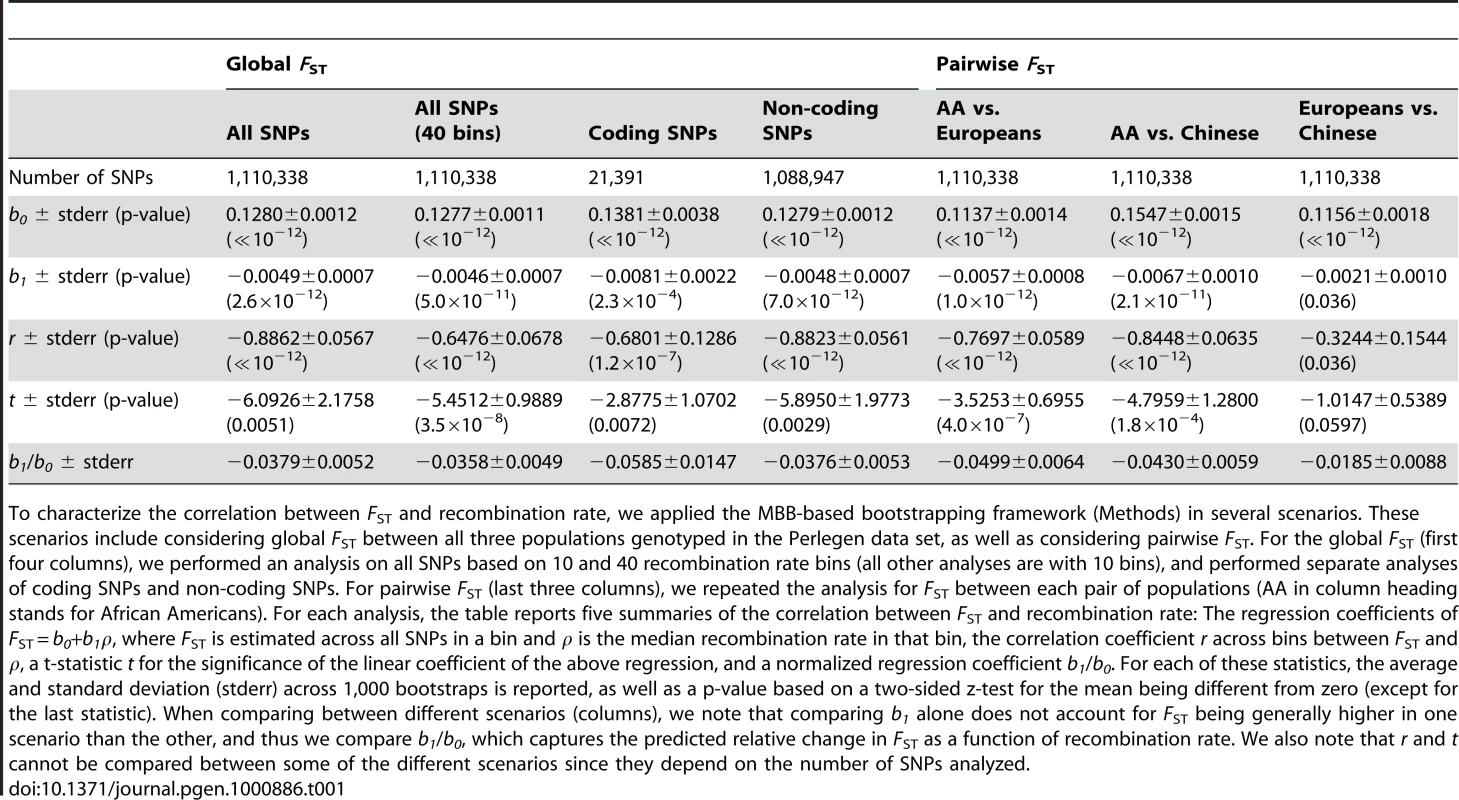
To characterize the correlation between FST and recombination rate, we applied the MBB-based bootstrapping framework (Methods) in several scenarios. These scenarios include considering global FST between all three populations genotyped in the Perlegen data set, as well as considering pairwise FST. For the global FST (first four columns), we performed an analysis on all SNPs based on 10 and 40 recombination rate bins (all other analyses are with 10 bins), and performed separate analyses of coding SNPs and non-coding SNPs. For pairwise FST (last three columns), we repeated the analysis for FST between each pair of populations (AA in column heading stands for African Americans). For each analysis, the table reports five summaries of the correlation between FST and recombination rate: The regression coefficients of FST = b0+b1ρ, where FST is estimated across all SNPs in a bin and ρ is the median recombination rate in that bin, the correlation coefficient r across bins between FST and ρ, a t-statistic t for the significance of the linear coefficient of the above regression, and a normalized regression coefficient b1/b0. For each of these statistics, the average and standard deviation (stderr) across 1,000 bootstraps is reported, as well as a p-value based on a two-sided z-test for the mean being different from zero (except for the last statistic). When comparing between different scenarios (columns), we note that comparing b1 alone does not account for FST being generally higher in one scenario than the other, and thus we compare b1/b0, which captures the predicted relative change in FST as a function of recombination rate. We also note that r and t cannot be compared between some of the different scenarios since they depend on the number of SNPs analyzed. Stronger impact of recombination rate on population differentiation in genes
We cannot envision any demographic or mechanistic explanation that would produce a correlation between recombination rate and allele frequency differentiation as observed and we hypothesize that our observations reflect a history of natural selection. Natural selection is usually expected to increase population differentiation at linked neutral sites [16],[30],[40],[41],[42],[43],[44],[45], an effect that is expected to extend over longer physical distances in regions of lower recombination rate. A prediction of an explanation based on natural selection is that the effect would be more marked in regions that are more likely to be influenced by selection, such as genes. To test this prediction, we identified a subset of 21,391 SNPs in the data that are in coding exons (cSNPs) and first considered the differentiation of this subset of SNPs without regard to recombination rate. Consistent with the results of Barreiro et al. [16], we found that the set of cSNPs exhibits a significantly higher (P = 9.0×10−4) population differentiation: 0.1265±0.0016 compared with 0.1212±0.0006 for non-coding SNPs (ncSNPs).
The novel signal of a negative correlation to recombination rate that we observed is more pronounced in genes: The slope of the regression of FST as a function of recombination rate is steeper for cSNPs than for ncSNPs (Figure 2). Interestingly, the regression predicts that for very high recombination rate, where the hitchhiking/background selection effect is expected to be weak, population differentiation is similar between cSNPs and ncSNPs (Figure 2). This suggests that selection may be the driving force behind both the higher population differentiation generally observed in genes and the correlation of population differentiation and recombination rate.
Fig. 2. Population differentiation is more strongly correlated with recombination rate in genes than outside of genes. 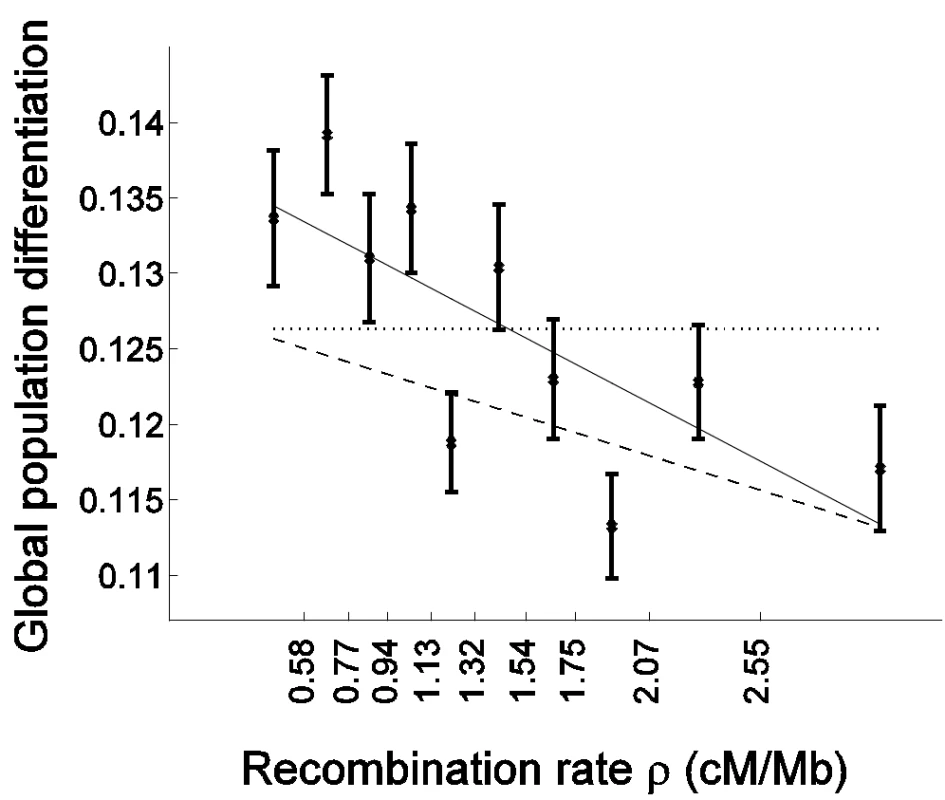
Global population differentiation between African Americans, Europeans, and Chinese is presented for coding SNPs (cSNPs). Except for focusing on the 21,391 SNPs in coding exons, the figure is identical to Figure 1A. In addition to the linear regression of FST estimates as a function of the median recombination rate in each bin (solid line; 0.1381–0.0081ρ), the linear regression for the rest of the data set (non-coding SNPs) is provided (dashed line; 0.1278–0.0048ρ), which is very similar to the regression based on the entire data set (Figure 1A). The correlation coefficient between FST of cSNPs and median recombination rate is −0.752 (P = 0.012). To test formally whether selection has an impact on the correlation with recombination rate above and beyond the general effect of increased population differentiation in genes, while controlling for the characteristically different recombination rate in genes, we repeated the bootstrapping framework analysis on cSNPs and compared it with analysis of ncSNPs (Table 1). As expected from the higher differentiation in genes, the constant coefficient of the linear regression is larger for cSNPs (Table 1; P = 0.005). Furthermore, as predicted if selection explains the correlation with recombination rate, the regression's slope is also steeper for cSNPs, −0.0081±0.0022 vs. −0.0048±0.0007, though due to the limited number of cSNPs this result is only borderline significant (P = 0.076; Table 1). To account for the possibility that the regression slope is steeper merely due to the differentiation in genes being generally higher, we compared the slope normalized by the regression constant coefficient, which captures the percentage change in population differentiation for each 1 cM/Mb (Table 1). This normalized slope is also steeper in genes (−0.0585±0.0147 vs. −0.0376±0.0053), though the result is only suggestive (P = 0.09).
Characteristically different correlation for different population pairs
We next considered the effect of recombination rate on population differentiation between each pair of populations separately. A negative correlation is observed between all pairs of populations, but the pattern is qualitatively different across population pairs (Figure 1B–1D and Table 1). The regression predicts a larger effect size, as assessed by the percentage change in FST per cM/Mb (to account for the varying levels of population differentiation between different populations), for FST between African Americans and Europeans and for FST between African Americans and Chinese, than for FST between Europeans and Chinese (P = 0.004 and P = 0.021; Table 1). We observed no significant difference in effect size for FST between African Americans and Europeans and for FST between African Americans and Chinese (P = 0.43; Table 1). When we considered single SNPs, without binning the data by recombination rate, we observed a very significant correlation with recombination rate for African American–European FST (r = −0.0183; P≪10−12), as well as for African American–Chinese FST (r = −0.0147; P≪10−12), but no correlation with recombination rate for European–Chinese FST (r = 0.0002; P = 0.81).
The weaker correlation for the FST between European and Chinese populations is driven by a dip in differentiation at very low recombination rate loci (Figure 1D), which is not at all what is seen in the comparison of African and non-African populations (Figure 1B and 1C). This curve shows a qualitatively non-monotonic pattern, which motivated us to perform a quadratic regression fitted within the bootstrapping framework. The regression is concave and includes very significant linear (P = 3.0×10−4) as well as quadratic (P = 1.8×10−5) terms. Conversely, quadratic regression gives a non-significant quadratic term for FST between African Americans and each of the other two populations and if anything is slightly convex. As expected, for single SNP analysis (without binning by recombination rate), linear regression is very significant for FST between African Americans and either non-African population (P≪10−12). For FST between Chinese and Europeans, however, linear regression is not significant (P = 0.81), while a quadratic regression is very significant (P≪10−12).
These results suggest a qualitatively different effect of recombination rate on allele frequency differentiation for different pairs of human populations and in different epochs of human history. In particular, most of the signal we observed of a correlation between recombination rate and FST of all three populations (Figure 1A) is attributable to selection that occurred either on the non-African lineage before the split of Europeans and Chinese or on the African lineage. While African American allele frequencies are a mixture of African and European allele frequencies, which are hence averaged in this analysis, a non-monotonic pattern such as observed between Europeans and Chinese is not observed in comparisons of African Americans and non-Africans.
Replication with uniformly ascertained subsets of HapMap
Considering the complexity of the ascertainment scheme [46], it is plausible that some features of the ascertainment of Perlegen “class A” SNPs would be correlated with recombination rate. In particular, though an effort has been made to mask repeat intervals [46], it is still possible that repeat content and GC content affect sequencing depth in the ascertainment panel [52]. If the same genomic features are also correlated with recombination rate, this could affect the correlation between FST and recombination rate. However, GC and repeat content are expected if anything to produce a correlation in the opposite direction to the one we observed, as these features are associated with lower resequencing depth, which would be expected to bias SNPs toward having a higher minor allele frequency. High minor allele frequency SNPs are empirically observed to be more differentiated than lower frequency SNPs (Figure S1), which would be expected to generate a positive correlation between recombination rate and FST since these features are associated with higher recombination rate.
To replicate our results in an independent data set with less complex ascertainment, we applied similar analyses on a data set of uniformly-ascertained SNPs that we previously reported, where ascertainment was carried out in two chromosomes of known ancestry in a way that is independent of the effect of genomic features on coverage and where the discovery in two chromosomes cannot result in a frequency bias associated with recombination rate [4],[5]. This data set is mostly composed of subsets of SNPs from HapMap II and for which genotype information is available in 60 unrelated West Africans (YRI), 60 unrelated European Americans (CEU), and 90 unrelated East Asians (ASN, denoting the combined CHB and JPT samples). Another advantage of this data set, in addition to the simplified ascertainment process, is that it includes a West African population, as opposed to the African American population of the Perlegen data set, for which analysis has been complicated by the admixture of European ancestry. We estimated recombination rate around 248,886 such uniformly-ascertained autosomal HapMap SNPs, which we then used to repeat all analyses that we had applied in the previous sections to the Perlegen SNPs (Figure 3; Table S1).
Fig. 3. Confirmation of the correlation of allele frequency differentiation and recombination rate in uniformly-ascertained subsets of HapMap. 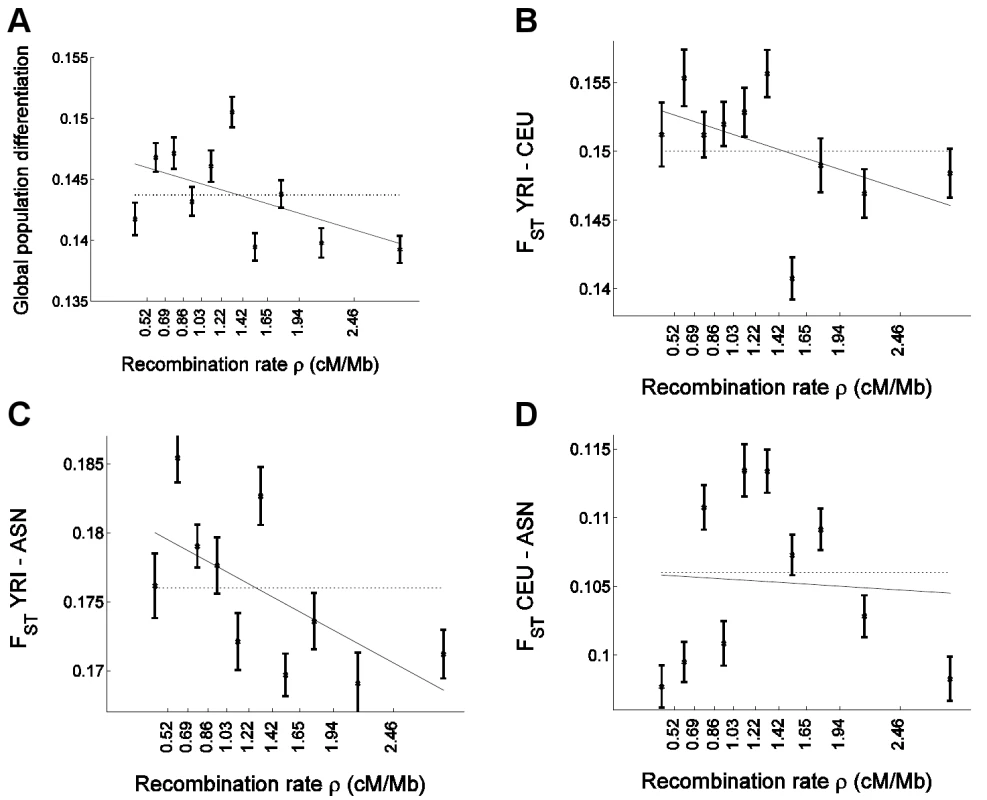
Similar to Figure 1, we placed 248,886 uniformly-ascertained HapMap SNPs into 10 bins according to the recombination rate and estimated for each bin (A) global population differentiation between YRI, CEU, and ASN (ASN denotes the combined CHB and JPT samples); (B) FST between YRI and CEU; (C) FST between YRI and ASN; and (D) FST between CEU and ASN. Linear regression as a function of the median recombination rate (solid line) is (A) 0.1473–0.0026ρ (B) 0.1541–0.0028ρ (C) 0.1819–0.0046ρ, and (D) 0.1060–0.0005ρ. The corresponding correlation coefficient estimate between FST and median recombination rate is (A) r = −0.526 (P = 0.118), (B) −0.482 (P = 0.158), (C) −0.634 (P = 0.049), and (D) −0.066 (P = 0.857). Results from the analysis with uniformly-ascertained subsets of HapMap replicated the previous results based on the Perlegen data. In particular, we replicated the strong negative correlation between global population differentiation and recombination rate (Figure 3A), and obtained a significant negative correlation coefficient (P = 0.001) and recombination rate regression coefficient (P = 0.004) based on the bootstrapping framework (Table S1). Furthermore, similar to the pattern in the Perlegen data set, we observed significant linear correlations with recombination rate for pairwise FST between YRI and CEU (P = 0.008 for correlation and P = 0.024 for regression coefficient) and for FST between YRI and ASN (P = 1.0×10−5 and P = 3.0×10−4), but not for FST between CEU and ASN (P = 0.59 and P = 0.59; Table S1), with the latter exhibiting the same non-monotonic relationship (Figure 3D) that we observed in the Perlegen data (Figure 1D). However, the differences between the populations are not significant, which we hypothesize is due to the smaller number of SNPs that we analyzed compared with the Perlegen data.
Discussion
In this study, we have explored whether local recombination rate is associated with allele frequency differentiation across human populations when examined on a genome-wide scale. The negative correlation we find in the Perlegen data set (the larger of the two data sets we analyzed) corresponds to an average decrease of 4% in FST for every 1 cM/Mb increase in recombination rate. This correlation is mostly driven by the differentiation between African and non-African populations, where the decrease in FST is 5% for every cM/Mb. The differentiation of European and East Asian populations shows a qualitatively different, inverse U-shaped relationship with recombination rate. These results are present in both the data sets we analyzed, and unlike similar results for nucleotide diversity are not sensitive to the mutagenic effect of recombination. By considering only data sets that have been uniformly-ascertained, we also ruled out the possibility that the correlation is due to ascertainment biases correlating with recombination rate.
We considered various explanations for these observations, all involving natural selection. We first considered evolution favoring higher recombination rate in functionally important elements, which could potentially contribute to the higher recombination rate observed in genes, and which could also generate a correlation between recombination rate and allele frequency differentiation. However, we realized that this would generate a correlation in the opposite direction to what we observe: This is expected to result in a higher recombination rate in functionally important regions, which exhibit higher differentiation on average (Figure 2), leading to a positive correlation between the two, while we observe the opposite pattern.
The only force we could identify that can explain the observation of a negative correlation between recombination rate and FST is directional selection; that is, hitchhiking linked to positively selected alleles (sweeps) or background selection linked to negatively selected alleles. Evidence for this explanation comes from the stronger negative correlation between population differentiation and recombination rate in coding regions, though we did not have enough data to establish the difference between coding SNPs and the rest of the genome with high statistical significance. Hitchhiking of recent, geographically localized selective sweeps, after the split of African and non-African populations is a potential explanation of these results, especially considering the magnitude of the effect we observed, since it is expected to increase population differentiation and to have a more marked effect in regions of lower recombination. Alternatively, background selection also has the potential to increase population differentiation, because it is expected to decrease within-population diversity at regions linked to loci under negative selection, which will have a more marked effect in regions of lower recombination.
The different nature of the effect these two selective forces have on population differentiation should make it possible to distinguish them in finer-scale studies [41]. Specifically, selective sweeps are expected to act relatively quickly and to affect large distance scales, whereas background selection is expected to act slowly on slightly deleterious alleles, suggesting that these two forces will operate on different size scales. Thus, it may be possible to distinguish their impact by repeating our analyses at different distance scales once a genetic map with higher resolution becomes available.
A striking observation in our study is the qualitatively different relationships between recombination rate and allele frequency differentiation for different pairs of populations, suggesting that selection has acted in different ways over different epochs of history. A possible explanation for the stronger correlation observed between African and non-African populations, compared to that between Europeans and East Asians, is the smaller effective population size in non-African population history compared with African history since they diverged. Although in general a reduced effective population size makes selection less efficient, it can increase the impact of background selection on patterns of genetic variation since weakly deleterious mutations are less efficiently purged from the population, thereby reaching higher frequencies which results in more extensive background selection when they are purged [32],[53]. More background selection and less efficient positive selection in populations with smaller size may in principle explain the qualitatively different relationship that we observed between recombination rate and allele frequency differentiation of European and East Asian populations vs. African and non-African populations. While this explanation remains speculative, a recent study supports this possibility by showing that there are proportionally more deleterious mutations segregating in Europeans than in African Americans [54].
Another scenario that could potentially produce different FST patterns between different pairs of populations is if selective sweeps were shared to different extents across populations. When an allele that arose in one population and is under selection enters a second population via migration, FST at linked neutral sites can actually be reduced between the two populations since hitchhiking effectively takes variation from the between-population component and injects it into the within-population component [43],[55],[56]. Such global selective sweeps predict a positive correlation between FST and recombination rate. Europeans and East Asians exchanged genes more recently than both did with Africans, and hence a larger fraction of selective sweeps are expected to be shared between these two populations, introducing a component of positive correlation between FST and recombination rate. The signature of global selective sweeps is expected to decay differently with genetic distance from the selected site than the decay due to local sweeps [56], and thus in principle could result in an inverse U-shaped relationship between FST and recombination rate. A limitation of this explanation for our observations, however, is that the phenomenon of reduced FST due to a global selective sweep has only been demonstrated for populations that are much more diverged than human populations [56].
To further study the pattern observed between different pairs of populations, we explored the relationship between FST and recombination rate in additional populations by studying data from HapMap 3, which genotyped 1,184 individuals from 11 populations [57]. A concern with the HapMap 3 data for our analysis is that the genotyping was carried out using SNP arrays (Affymetrix Human SNP array 6.0 and Illumina Human1M) that are affected by SNP ascertainment biases in a way that correlates with recombination rate [9]. Nevertheless, we observe the same qualitative results in the HapMap 3 data: We replicated our finding of a very strong negative correlation between FST and recombination rate, with an average decrease of 3% in global FST between all 11 populations for every 1 cM/Mb increase in recombination rate (Figure 4A). For pairwise FST, the same observations we made with the Perlegen data are also observed, with a quadratic regression being concave only for inter-continental FST between European and Asian populations (Figure 4B and 4C).
Fig. 4. The relationship between population differentiation and recombination rate in the larger set of HapMap 3 populations. 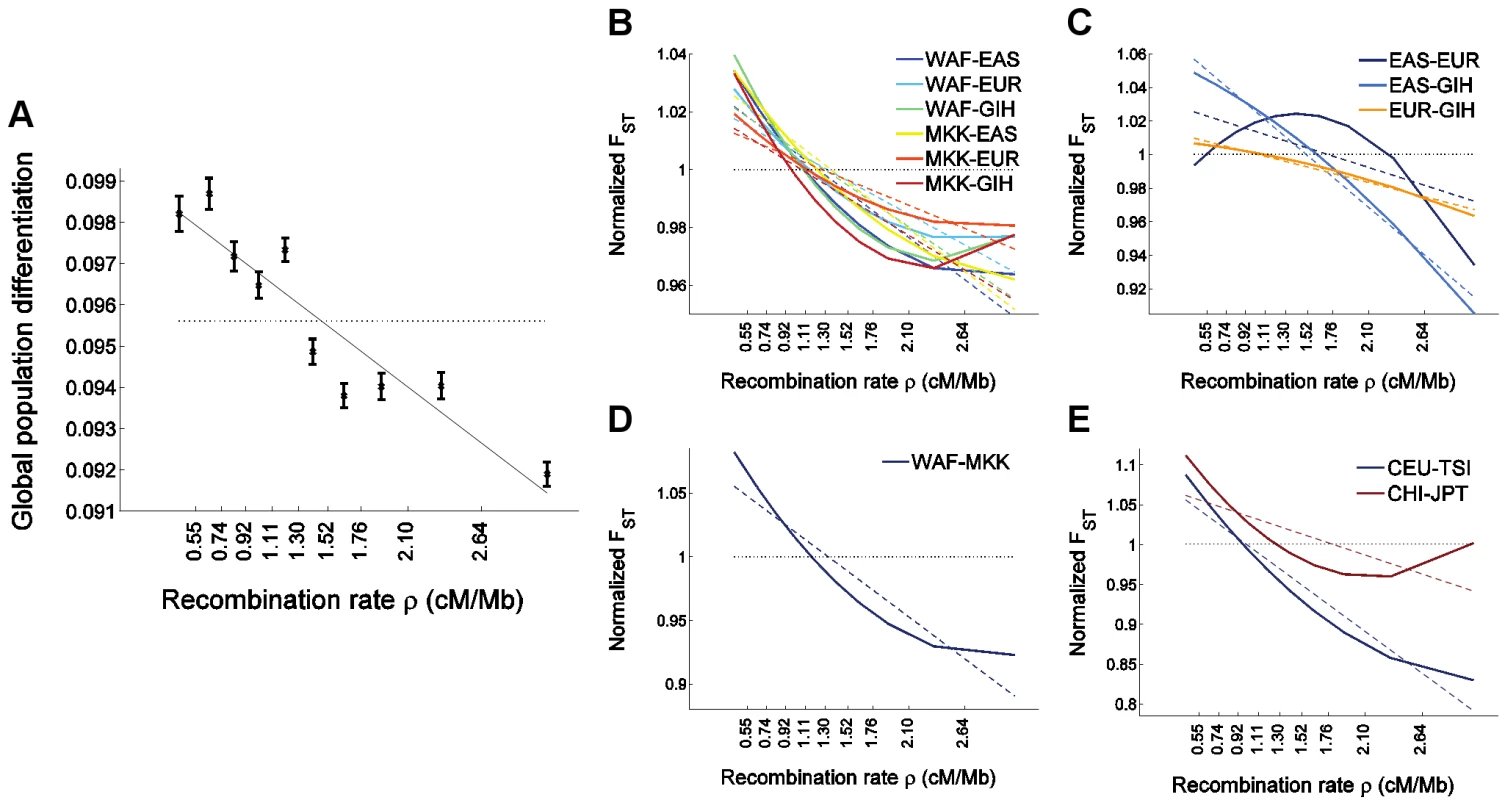
We placed 1,326,404 autosomal HapMap 3 SNPs (release 2) [57] into 10 bins according to recombination rate and estimated for each bin (A) global population differentiation between all 11 populations, and (B–E) population differentiation between pairs of populations. To avoid clutter, (B–E) depict only the linear (dashed lines) and quadratic (solid lines) regression of FST estimates as a function of the median recombination rate in each bin and partition the populations-pairs as follows: (B) FST between an African and a non-African population, where a negative correlation is observed with recombination rate, and where the quadratic regression is convex, (C) FST between a population of European, East Asian, or South Asian ancestry and a second population of a different one of these three ancestries, which shows a concave quadratic regression for all pairs of populations, and which recapitulates the result observed between North Europeans and East Asians in the uniformly-ascertained datasets (Figure 1D and Figure 3D). (A weaker phenomenon is observed for the South Asian GIH sample, which may be due to this population being somewhat related to both Europeans and East Asians [70], thereby confounding the North European–East Asian signal), (D) FST between two African populations, which shows a much steeper linear regression compared to intercontinental FST, as well as a convex quadratic regression, and (E) FST between closely-related non-African populations (within either Europe or East Asia; genome-wide FST<0.008), showing a very steep linear regression and a convex quadratic regression. FST based on all SNPs in all bins combined is presented as a horizontal dotted line and is equal to 1 in panels B–E since these present normalized FST values obtained by dividing each value by the genome-wide FST for the same pair of populations. Population codes are as follows: WAF (“West African”) is a combined sample of YRI (Yoruba in Ibadan, Nigeria) and LWK (Luhya in Webuye, Kenya); EAS (“East Asia”) is a combined sample of CHB (Han Chinese in Beijing, China), CHD (Chinese in Metropolitan Denver, CO, USA), and JPT (Japanese in Tokyo, Japan); EUR (“Europe”) is a combined sample of CEU (ancestry from Northern and Western Europe) and TSI (Toscani in Italia); GIH is a sample of Gujarati Indians in Houston, TX, USA; MKK is a sample of Maasai in Kinyawa, Kenya; and CHI (Chinese) is a combined sample of CHB and CHD. In addition to qualitatively replicating our findings, analysis of HapMap 3 data allows us to generalize them to additional populations. A striking result is that the relationship between FST and recombination rate is stronger for FST between pairs of closely-related populations, whether within or outside Africa: FST between a West African sample and Maasai (of mixed West African and East African ancestry [57]) decreases by an average of 6% for every 1 cM/Mb (Figure 4D), FST between Italians and individuals of North-Western European ancestry decreases by 10% for every cM/Mb (Figure 4E), and FST between Japanese and individuals of Chinese ancestry decreases by 4% (Figure 4E). In view of the large effective population size in recent human history since each of these pairs of populations have split, these observations support the possibility that the different patterns observed between different pairs of populations are due to natural selection operating more efficiently in the context of larger population sizes. We observed a weak convex relationship with recombination rate for FST between closely-related populations in a quadratic regression analysis (Figure 4D–4E), which is intriguingly opposite to what was observed between Europeans and Asians (Figure 1D and Figure 4C). On the other hand, these observations do not seem to support the possibility that the different patterns are due to selective sweeps being shared to different extent across different pairs of populations since the level of gene flow between HapMap 3 closely-related populations likely have had been higher than that between continents. These results, while interesting, should be viewed with caution due to the confounder of ascertainment bias. It will be possible to test these observations further by analyzing data from the 1000 Genomes Project, where whole-genome sequencing will generate data that is largely free of ascertainment bias for many of the HapMap 3 populations as well as additional populations [58].
The approach presented in this study allows not only a comparison of the effect selection has on allele frequency differentiation at different historical times, but also a comparison across different compartments of the genome. Repeating the analysis on Perlegen “class A” X-linked SNPs (to contrast with the autosomal analyses we report above), we observed a very significant correlation between global population differentiation of X-linked SNPs and recombination rate, with a correlation coefficient of −0.86 (P = 0.001) when partitioning the data into 10 bins (Figure S2). The small number of X-linked SNPs available for analysis (26,074) does not allow any conclusive results in comparison with the autosomes. However, the relationship between X-linked population differentiation and recombination rate is suggestively more marked than the relationship we observed for autosomal population differentiation with linear regression predicting a decrease of 24% in FST for every 1 cM/Mb increase in recombination rate (compared with 4% predicted for the autosomes). If this suggestive result is verified, it will point to natural selection playing more of a role in allele frequency differentiation on chromosome X than on the autosomes. This observation is especially interesting in light of our recent finding that chromosome X exhibits higher allele frequency differentiation between Africans and non-Africans than would be expected from ¾ the effective population size of the autosomes [5]. If natural selection operated more powerfully in some sense on chromosome X than on the autosomes during the human dispersal out of Africa, it could be a plausible explanation for the increased frequency differentiation on chromosome X. From a theoretical point of view, we are not aware of a model that can explain how the effects of selection could be amplified on chromosome X relative to the autosomes during the out of Africa dispersal. However, it should be possible to test this hypothesis empirically by analyzing data from whole-genome sequencing, which will allow a much more accurate analysis for chromosome X.
More generally, these results show that comparing the relationship of differentiation and recombination rate between different genomic regions and in different populations is a promising direction to be explored in future studies with larger data sizes. In addition to using this approach to study natural selection, by extrapolating the prediction of population differentiation to “infinite” recombination rate it might be possible to predict the level population differentiation that is due to genetic drift alone, separate from the effect of selection, since every nucleotide becomes independent of selection at nearby sites. (Population differentiation is still affected by natural selection at the sites directly under selection.) Models of demographic history would be expected to be more accurate if one used the prediction of FST for high recombination rate, rather than the genome-wide average. This is in the same spirit of studies targeting regions of high recombination rate and far from functional elements to infer human demographic history [10], [59]–[61]. To illustrate this type of analysis, we note that for global FST in the Perlegen data set, the genome-wide estimate is 0.121, while it is 0.113 for the SNPs in the bin with 10% highest recombination rate (Figure 1). For studies that attempt to infer history—for example, the time of split between human populations—this adjustment in FST would have a profound effect. The effect would be even larger if extrapolating to higher recombination rates.
A limitation of this study is the genetic map available. We chose to use a pedigree-derived human genetic map [48] since it is based on the direct observation of recombination events. Population-based genetic maps that are based on patterns of LD in a population [62]–[65] are sensitive to the increase in LD due to natural selection [66],[67], the very force which effect we sought to analyze. In future studies of these phenomena, it will be valuable to use genetic maps that are based on the direct observation of recombination events and that have a higher spatial resolution. As recombination rates vary on a fine-scale [68] and background selection may occur at a somewhat different genetic distance scale than hitchhiking, a finer-scale map should allow a better characterization of the relationship between population differentiation and recombination rate and improved statistical power in capturing its causes. Supporting this view, a recent study examining the correlation between nucleotide diversity and recombination rate in Drosophila found it to be non-significant when recombination rate was estimated in 2 Mb windows and to be significant when finer-scale heterogeneity in recombination rate was considered in the analysis [17].
In conclusion, we have shown that genome-wide human population differentiation in allele frequencies is significantly correlated with recombination rate on a megabase scale, demonstrating that natural selection has had a profound effect on allele frequency distributions averaged over the last hundred thousand years. While these results likely reflect the effects of hitchhiking and background selection, disentangling the strengths of these two forces will require extending the analyses presented in this paper. One important direction is to use genetic maps that have fine spatial resolution, which may shed light on the detailed distribution of selective coefficients that have shaped allele frequency differentiation. A second direction in which these results can be extended is to compare more populations of continentally diverse ancestry. This should facilitate an exploration of the relationship between recombination rate and population differentiation during different epochs of human evolution, and should allow a better understanding of how demographic history has shaped the impact of natural selection on patterns of human genetic variation.
Methods
Perlegen data
To examine the correlation between SNP allele frequency differentiation and recombination rate in a way that is not sensitive to the confounder of recombination rate-dependent SNP ascertainment, we limited our main analysis to autosomal Perlegen “class A” SNPs [46]. These SNPs, encompassing ∼69% of SNPs in the Perlegen data set [46], were uniformly discovered by array-based resequencing of 24 human samples of diverse ancestry from the NIH Polymorphism Discovery Resource [47]. All SNPs discovered in this way were genotyped in 24 European Americans, 23 African Americans, and 24 Han Chinese from the Los Angeles area, which were all unrelated to each other and also unrelated to the individuals in the discovery set [46]. We used liftOver (http://genome.ucsc.edu/cgi-bin/hgLiftOver) to convert genomic positions from build 34 (hg16) used in the Perlegen data set to build 35 (hg17) and discarded SNPs for which this conversion failed. We then estimated recombination rate around each SNP and discarded SNPs for which it could not be accurately estimated (see below). Following these filters, we were left with 1,110,338 autosomal SNPs with recombination rate estimates. Out of these, we also identified 21,391 coding SNPs according to the UCSC Known Genes track [69] for separate analysis.
Uniformly ascertained HapMap data
We replicated our results in a data set of SNPs that were uniformly ascertained as polymorphic in exactly two chromosomes of the same ancestry and genotyped in all HapMap samples [5], including 60 West Africans from Ibadan, Nigeria (YRI), 60 European Americans from Utah, USA (of North European ancestry; CEU), and 90 East Asians (ASN; 45 Han Chinese from Beijing, China (CHB) and 45 Japanese from Tokyo, Japan (JPT), which we pooled for analysis). We considered only samples that were unrelated to each other and also unrelated to the few individuals in the discovery sets. In a previous study using these uniformly ascertained SNP sets, we were interested in learning about features of human genetic variation that are not due to known effects of natural selection, and hence in that study we filtered genes, conserved non-coding loci, and regions that were putatively affected by selective sweeps [5]. As we were interested in exploring the effect of selection on population differentiation in the current study, we relaxed these filters. Furthermore, we pooled together SNPs discovered using different pairs of chromosomes since we were only concerned with the ascertainment being uniform genome-wide and independent of LD, which resulted in 248,886 SNPs for which we could accurately estimate recombination rate.
Recombination rate estimation
We determined recombination rate around each SNP based on the deCODE genetic map [48]. We considered a three megabase (Mb) window centered on each SNP and determined the genetic length in centimorgan (cM) for each window by subtracting the genetic position of the start from that of the end position of the window. The genetic position of the start and end positions were each determined by linearly interpolating, according to distance, the genetic position of the nearest marker in the genetic map on each side. We discarded the SNP from further analysis in cases where (i) the window's start position is before the chromosome start position, (ii) the window overlaps a centromere, or (iii) the window overlaps a telomere. To place SNPs into bins according to recombination rate, we chose bin boundaries such that an equal number of SNPs fell into each bin. We also repeated the main analyses while modifying the window length around each SNP from 3 Mb to either 1 Mb or 5 Mb and obtained similar results (Figure S3 and Figure S4). Similar results were also obtained when we masked out an additional 5 Mb of each telomeric region and on each side of each centromere, showing that our results are not explained by unusual patterns at these regions (Figure S5).
Allele frequency differentiation estimates
To estimate pairwise allele frequency differentiation between populations, we used the FST statistic as formulated in ref. [4]. In the context of the current study, these FST estimates are almost identical to the estimates obtained based on the estimator of Weir and Cockerham [1]. Global allele frequency differentiation was calculated as the average FST over all population-pairs. We estimated pairwise and global FST across all SNPs in each recombination rate bin. We also estimated FST standard errors (for presentation in Figure 1, Figure 2, Figure 3, Figure 4, and Figures S1, S2, S3, S4, S5) based on bootstrapping 1,000 random sets of SNPs from each bin using the moving block bootstrap (MBB) [49]–[51], randomly resampling contiguous runs of SNPs to take into account the effect of correlation between SNPs in LD [4]. We applied the same bootstrapping procedure to estimate FST for all SNPs irrespective of bins, and used the standard errors for z-tests to determine whether the genome-wide FST values are different, e.g. between cSNPs and ncSNPs.
Bootstrapping framework
We randomly resampled 1,000 data sets of SNPs from the set of all SNPs using MBB. For each of these sets, we started by stratifying resampled SNPs into bins according to their recombination rate, and then repeated the procedure that was used to analyze the original data set. Specifically, we estimated FST, and then estimated the correlation and regression between FST in a bin and the bin's median recombination rate ρ. Averaging across all 1,000 resamplings, we obtained accurate estimates for several correlation and regression statistics: (i) The coefficients of the linear regression FST = b0+b1ρ, (ii) the correlation coefficient across bins, (iii) a t-statistic for the significance of the linear coefficient of the above regression, and (iv) a normalized regression coefficient b1/b0. We estimated standard errors of these statistics as the standard deviation across resamplings. Hence, these standard errors account for the problem that nearby SNPs do not provide independent observations due to LD.
Based on averaged estimates across resamplings and their standard errors, we performed two-sided z-tests for the significance of each statistic (Table 1 and Table S1). We also compared estimates from analyses of different data: Testing for a significantly higher ratio of the regression coefficients (b1/b0) for cSNPs than for ncSNPs (one-sided z-test) and for a significantly different ratio between different pairs of populations (two-sided z-test). We also reported hypothesis testing for whether b0 and b1 by themselves are higher in cSNPs than ncSNPs (one-sided z-test).
Supporting Information
Zdroje
1. WeirBS
CockerhamCC
1984 Estimating F-statistics for the analysis of population structure. Evolution 38 1358 1370
2. WrightS
1943 Isolation by Distance. Genetics 28 114 138
3. JakobssonM
ScholzSW
ScheetP
GibbsJR
VanLiereJM
2008 Genotype, haplotype and copy-number variation in worldwide human populations. Nature 451 998 1003
4. KeinanA
MullikinJC
PattersonN
ReichD
2007 Measurement of the human allele frequency spectrum demonstrates greater genetic drift in East Asians than in Europeans. Nat Genet 39 1251 1255
5. KeinanA
MullikinJC
PattersonN
ReichD
2009 Accelerated genetic drift on chromosome X during the human dispersal out of Africa. Nat Genet 41 66 70
6. LewontinRC
1972 The apportionment of human diversity.
DobzhanskyT
HechtMK
SteereWC
Evolutionary Biology 6 New York Appleton-Century-Crofts 381 398
7. LiJZ
AbsherDM
TangH
SouthwickAM
CastoAM
2008 Worldwide human relationships inferred from genome-wide patterns of variation. Science 319 1100 1104
8. The International HapMap Consortium 2005 A haplotype map of the human genome. Nature 437 1299 1320
9. The International HapMap Consortium 2007 A second generation human haplotype map of over 3.1 million SNPs. Nature 449 851 861
10. WallJD
CoxMP
MendezFL
WoernerA
SeversonT
2008 A novel DNA sequence database for analyzing human demographic history. Genome Res 18 1354 1361
11. AkeyJM
ZhangG
ZhangK
JinL
ShriverMD
2002 Interrogating a high-density SNP map for signatures of natural selection. Genome Res 12 1805 1814
12. NielsenR
HellmannI
HubiszM
BustamanteC
ClarkAG
2007 Recent and ongoing selection in the human genome. Nat Rev Genet 8 857 868
13. SabetiPC
VarillyP
FryB
LohmuellerJ
HostetterE
2007 Genome-wide detection and characterization of positive selection in human populations. Nature 449 913 918
14. WeirBS
CardonLR
AndersonAD
NielsenDM
HillWG
2005 Measures of human population structure show heterogeneity among genomic regions. Genome Res 15 1468 1476
15. ChenH
PattersonN
ReichDE
2010 Population differentiation as a test for selective sweeps. Genome Res
16. BarreiroLB
LavalG
QuachH
PatinE
Quintana-MurciL
2008 Natural selection has driven population differentiation in modern humans. Nat Genet 40 340 345
17. KulathinalRJ
BennettSM
FitzpatrickCL
NoorMA
2008 Fine-scale mapping of recombination rate in Drosophila refines its correlation to diversity and divergence. Proc Natl Acad Sci U S A 105 10051 10056
18. BegunDJ
AquadroCF
1992 Levels of naturally occurring DNA polymorphism correlate with recombination rates in D. melanogaster. Nature 356 519 520
19. BegunDJ
HollowayAK
StevensK
HillierLW
PohYP
2007 Population genomics: whole-genome analysis of polymorphism and divergence in Drosophila simulans. PLoS Biol 5 e310 doi:10.1371/journal.pbio.0050310
20. HellmannI
MangY
GuZ
LiP
de la VegaFM
2008 Population genetic analysis of shotgun assemblies of genomic sequences from multiple individuals. Genome Res 18 1020 1029
21. NachmanMW
2001 Single nucleotide polymorphisms and recombination rate in humans. Trends Genet 17 481 485
22. CaiJJ
MacphersonJM
SellaG
PetrovDA
2009 Pervasive hitchhiking at coding and regulatory sites in humans. PLoS Genet 5 e1000336 doi:10.1371/journal.pgen.1000336
23. NachmanMW
BauerVL
CrowellSL
AquadroCF
1998 DNA variability and recombination rates at X-linked loci in humans. Genetics 150 1133 1141
24. HammerMF
GarriganD
WoodE
WilderJA
MobasherZ
2004 Heterogeneous patterns of variation among multiple human x-linked Loci: the possible role of diversity-reducing selection in non-Africans. Genetics 167 1841 1853
25. SpencerCC
DeloukasP
HuntS
MullikinJ
MyersS
2006 The influence of recombination on human genetic diversity. PLoS Genet 2 e148 doi:10.1371/journal.pgen.0020148
26. WrightSI
FoxeJP
DeRose-WilsonL
KawabeA
LooseleyM
2006 Testing for effects of recombination rate on nucleotide diversity in natural populations of Arabidopsis lyrata. Genetics 174 1421 1430
27. HellmannI
EbersbergerI
PtakSE
PaaboS
PrzeworskiM
2003 A neutral explanation for the correlation of diversity with recombination rates in humans. Am J Hum Genet 72 1527 1535
28. HellmannI
PruferK
JiH
ZodyMC
PaaboS
2005 Why do human diversity levels vary at a megabase scale? Genome Res 15 1222 1231
29. TenaillonMI
U'RenJ
TenaillonO
GautBS
2004 Selection versus demography: a multilocus investigation of the domestication process in maize. Mol Biol Evol 21 1214 1225
30. StephanW
MitchellSJ
1992 Reduced levels of DNA polymorphism and fixed between-population differences in the centromeric region of Drosophila ananassae. Genetics 132 1039 1045
31. SmithJM
HaighJ
1974 The hitch-hiking effect of a favourable gene. Genet Res 23 23 35
32. CharlesworthB
MorganMT
CharlesworthD
1993 The effect of deleterious mutations on neutral molecular variation. Genetics 134 1289 1303
33. KauerM
ZangerlB
DieringerD
SchlottererC
2002 Chromosomal patterns of microsatellite variability contrast sharply in African and non-African populations of Drosophila melanogaster. Genetics 160 247 256
34. WallJD
AndolfattoP
PrzeworskiM
2002 Testing models of selection and demography in Drosophila simulans. Genetics 162 203 216
35. FayJC
WuCI
2000 Hitchhiking under positive Darwinian selection. Genetics 155 1405 1413
36. LercherMJ
HurstLD
2002 Human SNP variability and mutation rate are higher in regions of high recombination. Trends Genet 18 337 340
37. MagniGE
Von BorstelRC
1962 Different Rates of Spontaneous Mutation during Mitosis and Meiosis in Yeast. Genetics 47 1097 1108
38. StrathernJN
ShaferBK
McGillCB
1995 DNA synthesis errors associated with double-strand-break repair. Genetics 140 965 972
39. McVickerG
GordonD
DavisC
GreenP
2009 Widespread genomic signatures of natural selection in hominid evolution. PLoS Genet 5 e1000471 doi:10.1371/journal.pgen.1000471
40. NielsenR
2005 Molecular signatures of natural selection. Annu Rev Genet 39 197 218
41. CharlesworthB
NordborgM
CharlesworthD
1997 The effects of local selection, balanced polymorphism and background selection on equilibrium patterns of genetic diversity in subdivided populations. Genet Res 70 155 174
42. BegunDJ
AquadroCF
1993 African and North American populations of Drosophila melanogaster are very different at the DNA level. Nature 365 548 550
43. StephanW
XingL
KirbyDA
BravermanJM
1998 A test of the background selection hypothesis based on nucleotide data from Drosophila ananassae. Proc Natl Acad Sci U S A 95 5649 5654
44. CharlesworthB
CoyneJA
BartonNH
1987 The relative rates of evolution of sex chromosomes and autosomes. The American Naturalist 130 113 146
45. CharlesworthB
1998 Measures of divergence between populations and the effect of forces that reduce variability. Mol Biol Evol 15 538 543
46. HindsDA
StuveLL
NilsenGB
HalperinE
EskinE
2005 Whole-genome patterns of common DNA variation in three human populations. Science 307 1072 1079
47. PatilN
BernoAJ
HindsDA
BarrettWA
DoshiJM
2001 Blocks of limited haplotype diversity revealed by high-resolution scanning of human chromosome 21. Science 294 1719 1723
48. KongA
GudbjartssonDF
SainzJ
JonsdottirGM
GudjonssonSA
2002 A high-resolution recombination map of the human genome. Nat Genet 31 241 247
49. LahiriSN
2003 Resampling methods for dependent data. New York Springer
50. KunschHR
1989 The jackknife and the bootstrap for general stationary observations. The Annals of Statistics 17 1217 1241
51. LiuRY
SinghK
1992 Moving blocks jackknife and bootstrap capture weak dependence.
LePageR
BillardL
Exploring the limits of bootstrap New York John Wiley 225 248
52. GajerP
SchatzM
SalzbergSL
2004 Automated correction of genome sequence errors. Nucleic Acids Res 32 562 569
53. CharlesworthB
1996 Background selection and patterns of genetic diversity in Drosophila melanogaster. Genet Res 68 131 149
54. LohmuellerKE
IndapAR
SchmidtS
BoykoAR
HernandezRD
2008 Proportionally more deleterious genetic variation in European than in African populations. Nature 451 994 997
55. ChenY
MarshBJ
StephanW
2000 Joint effects of natural selection and recombination on gene flow between Drosophila ananassae populations. Genetics 155 1185 1194
56. SantiagoE
CaballeroA
2005 Variation after a selective sweep in a subdivided population. Genetics 169 475 483
57. The International HapMap 3 Consortium 2010 An integrated haplotype map of rare and common genetic variation in diverse human populations. In revision
58. KaiserJ
2008 DNA sequencing. A plan to capture human diversity in 1000 genomes. Science 319 395
59. FrisseL
HudsonRR
BartoszewiczA
WallJD
DonfackJ
2001 Gene conversion and different population histories may explain the contrast between polymorphism and linkage disequilibrium levels. Am J Hum Genet 69 831 843
60. PluzhnikovA
Di RienzoA
HudsonRR
2002 Inferences about human demography based on multilocus analyses of noncoding sequences. Genetics 161 1209 1218
61. VoightBF
AdamsAM
FrisseLA
QianY
HudsonRR
2005 Interrogating multiple aspects of variation in a full resequencing data set to infer human population size changes. Proc Natl Acad Sci U S A 102 18508 18513
62. StumpfMP
McVeanGA
2003 Estimating recombination rates from population-genetic data. Nat Rev Genet 4 959 968
63. LiN
StephensM
2003 Modeling linkage disequilibrium and identifying recombination hotspots using single-nucleotide polymorphism data. Genetics 165 2213 2233
64. FearnheadP
HardingRM
SchneiderJA
MyersS
DonnellyP
2004 Application of coalescent methods to reveal fine-scale rate variation and recombination hotspots. Genetics 167 2067 2081
65. McVeanGA
MyersSR
HuntS
DeloukasP
BentleyDR
2004 The fine-scale structure of recombination rate variation in the human genome. Science 304 581 584
66. O'ReillyPF
BirneyE
BaldingDJ
2008 Confounding between recombination and selection, and the Ped/Pop method for detecting selection. Genome Res 18 1304 1313
67. ReedFA
TishkoffSA
2006 Positive selection can create false hotspots of recombination. Genetics 172 2011 2014
68. MyersS
BottoloL
FreemanC
McVeanG
DonnellyP
2005 A fine-scale map of recombination rates and hotspots across the human genome. Science 310 321 324
69. HsuF
KentWJ
ClawsonH
KuhnRM
DiekhansM
2006 The UCSC Known Genes. Bioinformatics 22 1036 1046
70. ReichD
ThangarajK
PattersonN
PriceAL
SinghL
2009 Reconstructing Indian population history. Nature 461 489 494
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 3- Akutní intermitentní porfyrie
- Souvislost haplotypu M2 genu pro annexin A5 s opakovanými reprodukčními ztrátami
- Vztah užívání alkoholu a mužské fertility
- Doc. Miloš Kubánek: Nemocní se srdeční amyloidózou jsou často skryti a sledováni pod jinými diagnózami
- Hysteroskopická resekce děložního septa zlepšuje šanci na graviditu žen s jinak nevysvětlenou infertilitou
-
Všechny články tohoto čísla
- Parental Genome Dosage Imbalance Deregulates Imprinting in
- Identification and Functional Analysis of the Vision-Specific BBS3 (ARL6) Long Isoform
- HAP2(GCS1)-Dependent Gamete Fusion Requires a Positively Charged Carboxy-Terminal Domain
- Initial Genomics of the Human Nucleolus
- Role of RecA and the SOS Response in Thymineless Death in
- PPS, a Large Multidomain Protein, Functions with Sex-Lethal to Regulate Alternative Splicing in
- Mislocalization of XPF-ERCC1 Nuclease Contributes to Reduced DNA Repair in XP-F Patients
- Transgenic Rat Model of Neurodegeneration Caused by Mutation in the Gene
- Human Population Differentiation Is Strongly Correlated with Local Recombination Rate
- Local-Scale Patterns of Genetic Variability, Outcrossing, and Spatial Structure in Natural Stands of
- Arginylation-Dependent Neural Crest Cell Migration Is Essential for Mouse Development
- HP1 Recruitment in the Absence of Argonaute Proteins in
- MiR-218 Inhibits Invasion and Metastasis of Gastric Cancer by Targeting the Robo1 Receptor
- Bias and Evolution of the Mutationally Accessible Phenotypic Space in a Developmental System
- Papillorenal Syndrome-Causing Missense Mutations in / Result in Hypomorphic Alleles in Mouse and Human
- Rapid Assessment of Genetic Ancestry in Populations of Unknown Origin by Genome-Wide Genotyping of Pooled Samples
- Regulation of Lifespan, Metabolism, and Stress Responses by the SH2B Protein, Lnk
- KRAB–Zinc Finger Proteins and KAP1 Can Mediate Long-Range Transcriptional Repression through Heterochromatin Spreading
- Identification of the Regulatory Logic Controlling Pathoadaptation by the SsrA-SsrB Two-Component System
- Drosophila Xpd Regulates Cdk7 Localization, Mitotic Kinase Activity, Spindle Dynamics, and Chromosome Segregation
- Multiple Signals Converge on a Differentiation MAPK Pathway
- In the Tradition of Science: An Interview with Victor Ambros
- Association of the Polymorphism His615Arg with Melanin Content in East Asian Populations: Further Evidence of Convergent Evolution of Skin Pigmentation
- Fatal Cardiac Arrhythmia and Long-QT Syndrome in a New Form of Congenital Generalized Lipodystrophy with Muscle Rippling (CGL4) Due to Mutations
- Deciphering Normal Blood Gene Expression Variation—The NOWAC Postgenome Study
- Derepression of the Plant Chromovirus Induces Germline Transposition in Regenerated Plants
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Deciphering Normal Blood Gene Expression Variation—The NOWAC Postgenome Study
- Transgenic Rat Model of Neurodegeneration Caused by Mutation in the Gene
- Papillorenal Syndrome-Causing Missense Mutations in / Result in Hypomorphic Alleles in Mouse and Human
- Fatal Cardiac Arrhythmia and Long-QT Syndrome in a New Form of Congenital Generalized Lipodystrophy with Muscle Rippling (CGL4) Due to Mutations
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



