-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRoss River Virus: Many Vectors and Unusual Hosts Make for an Unpredictable Pathogen
article has not abstract
Published in the journal: . PLoS Pathog 11(9): e32767. doi:10.1371/journal.ppat.1005070
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1005070Summary
article has not abstract
Introduction
While most mosquito-borne viruses are associated with a narrow range of vector and reservoir host species, some pathogens have much larger vector and host assemblages. One such group is the Alphaviruses (including chikungunya virus [CHIKV]), with Ross River virus (RRV), endemic to Australia, providing a fascinating example of the complicated relationship between vector and reservoir host species across different environments (Fig 1). RRV is responsible for the most commonly reported mosquito-borne disease in Australia, and as both a reservoir host and vector generalist, the virus has complex spatial and temporal activity that makes outbreak prediction, vector and pathogen surveillance, and public health risk mitigation strategies difficult. Here, we review the unique ecology of RRV and the challenges it presents for local health authorities.
Fig. 1. There are complex relationships between the vectors and the zoonotic reservoirs of Ross River virus across coastal, inland, and metropolitan regions of Australia. 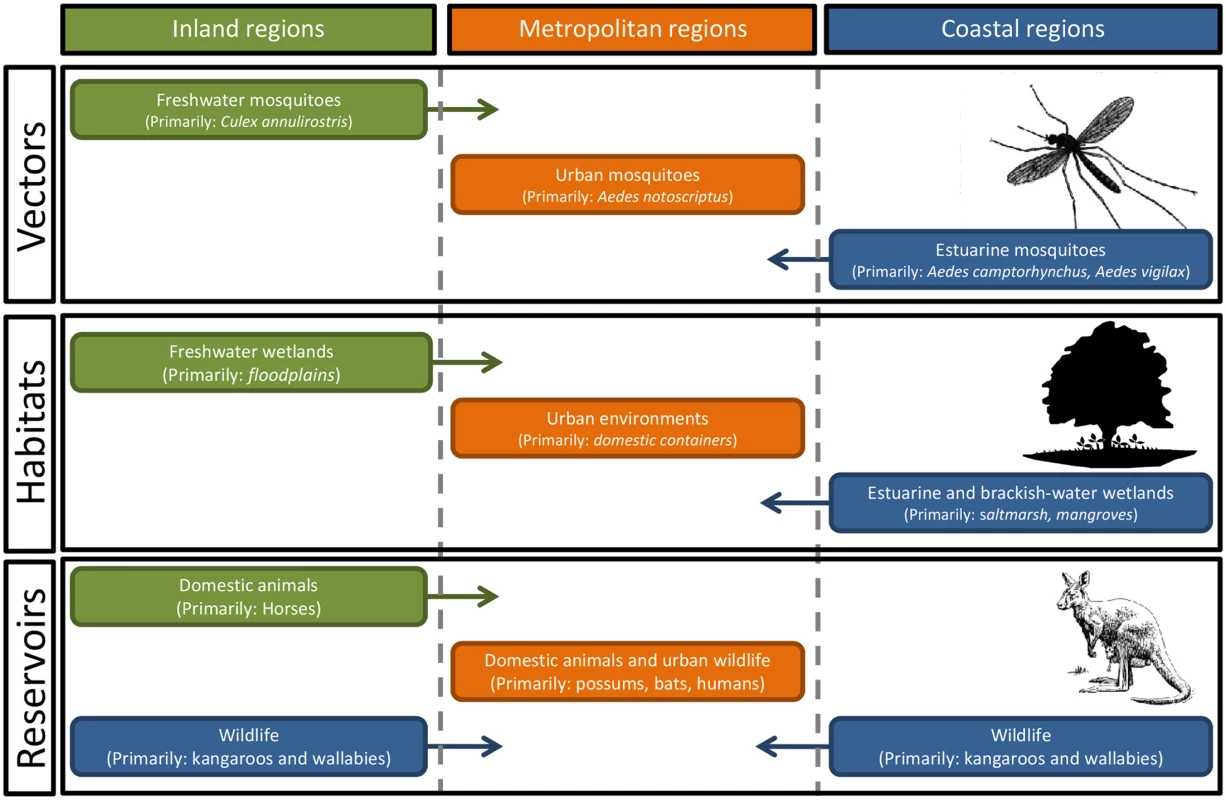
With around 40 different mosquito species implicated as potential vectors, the environmental drivers of mosquito abundance will vary, not only with the abundance and distribution of habitats within each region but also temporally, with differences in temperature, rainfall, and tidal inundations of estuarine wetlands. Ross River Virus Disease
Ross River virus disease (also commonly known as Ross River Fever) is not fatal. However, the associated arthralgia can be seriously debilitating. While there are a wide range of disease symptoms, they typically include arthritic joint pain, usually of the peripheral joints, which affects 83%–98% of patients; fatigue and rash, both of which affect over 50% of patients; and fever, which affects 20%–60% of patients [1]. The severity of symptoms varies, as does their duration, which can range from a few weeks to several months [2]; several studies indicate that chronic joint pain affects over 50% of RRV disease patients, which can persist for years after diagnosis [1]. The public health impacts of RRV disease are significant: it is estimated to cost Australia at least US$4.1–US$4.7 million per year [3].
The incidence of RRV disease varies regionally [2]. Roughly 5,000 cases of RRV disease are officially notified each year [4]. There are generally more cases recorded in northern Australia, but the virus still poses a substantial threat in the temperate southern regions of the country. RRV disease case numbers are generally thought to be an underestimate [5]. Accurately quantifying the scope of RRV disease is difficult, as the variability in symptom severity and the requirement of a blood test to confirm infection may cause many milder cases to go undiagnosed. As a consequence, official statistics may only represent the most severe cases.
There are no specific treatments available for the disease; patients are usually given supportive care and prescribed general analgesics and anti-inflammatory agents to treat symptoms [1]. While a vaccine is in development [6], current prevention strategies rely primarily on mosquito avoidance and control.
Reservoir Hosts
It is rare that a national icon is also a key reservoir host for a mosquito-borne pathogen. Yet, native Australian macropods, such as the beloved kangaroo and wallaby, are currently thought to be the most significant of RRV’s large suite of reservoir hosts, for both the maintenance of the virus in nature and its transmission to humans [7]. Serological studies and laboratory investigations have indicated that several other domestic and wild animals serve as RRV reservoirs, including dogs, cats, possums, and horses [5]. Humans have been implicated as critical reservoir hosts in significant RRV outbreaks across the Pacific Islands [1] and within metropolitan areas in Australia [8], regions where no macropods are present. High viremia in human patients and low seroprevalence in nonhuman vertebrates during outbreaks provide strong evidence for human–mosquito–human transmission in these cases [1]. Under these conditions, RRV is capable of epidemic local spread, as in the South Pacific outbreak of 1979–1989, allowing it to expand its range and re-emerge outside of Australia—regardless of the presence of preferred enzootic hosts—so long as competent mosquito vectors are present.
Vectors
Ross River virus is a vector generalist. It was first isolated from mosquitoes (Aedes vigilax) collected in Queensland in 1959 [9], and since that time, over 40 species of mosquitoes across Australia have been identified that may play a role in transmission. While most mosquito species have been incriminated by the isolation of RRV from field-collected specimens, laboratory vector competence experiments have confirmed effective transmission of the virus by more than ten species [5].
Field surveillance and laboratory testing have identified a diverse range of particularly influential species, including Aedes camptorhynchus, Aedes notoscriptus, Aedes vigilax, and Culex annulirostris. A. camptorhynchus and A. vigilax are closely associated with estuarine wetlands, with local population abundance determined by local tidal flooding and rainfall inundating habitats. The abundance of C. annulirostris, associated with freshwater ephemeral and permanent habitats, is determined by rainfall and riverine flooding. Finally, the metropolitan species A. notoscriptus is associated with water-holding containers [10]. There is also a suite of mosquito species found in freshwater and brackish environments that may play an important role in local enzootic and epidemic transmission. The diverse range of vector habitats, together with concomitant environmental drivers of mosquito abundance across coastal, freshwater, and urban environments, clearly illustrates the ecological complexity of RRV transmission cycles and the challenges faced by those attempting to manage the associated public health risks.
Management and Surveillance of Public Health Risks
Traditionally, RRV was considered a greater risk in rural regions, where there are both large mosquito populations and an abundance of suitable reservoir hosts. However, recently there have been RRV disease outbreaks on the outskirts of several major metropolitan regions, including Brisbane, Sydney, and Perth [5]. As urbanization of coastal regions increases, human populations will continue to encroach on mosquito and wildlife habitats, increasing the risk of mosquito-borne disease. Mosquito control can be an effective strategy for disease mitigation [11], but few local authorities have the capacity to maintain broadscale treatment programs. In most regions, disease prevention continues to rely heavily on personal protection measures, such as insect repellents, promoted by local health authorities [12].
The diversity of RRV vector species and the associated environmental drivers of vector abundance create spatiotemporal complexity, which further impedes outbreak prediction efforts [13,14]. The activity of RRV within local reservoir host populations, a potentially significant factor in predicting outbreaks [7], is also incredibly difficult to quantify. In the absence of reliable predictive models for RRV activity, health authorities rely on surveillance programs to provide an early warning of mosquito and pathogen activity [15]. However, these programs can be logistically and financially demanding, and incorporating surveillance data into public health prevention strategies may become even more fraught in the future, as urban development and climate change alter RRV disease dynamics.
Broader Applications and Future Directions
While the Australian experience with RRV provides valuable insight for the management of other mosquito-borne enzootic pathogens, particularly those associated with urban wildlife, it may be of only limited usefulness for other arthritic alphaviruses, such as CHIKV, in which humans are the predominate reservoir host. The dramatic rise in activity of CHIKV internationally is due primarily to the mosquito–human–mosquito transmission cycle, with anthropophilic mosquitoes, such as Aedes aegypti and Aedes albopictus, playing critical roles in outbreaks [16]. However, in the case of RRV, it is primarily the enzootic vectors that drive outbreaks of disease, given the primarily wildlife–mosquito–human transmission cycles. Consequently, the RRV system illustrates the importance of pathogen - and region-specific prediction and control measures, with the ecological and environmental variability of RRV resulting in multiple epidemiologies in Australia and limiting the utility of generic prediction and control approaches at the local level [13]. In response to any mosquito-borne disease outbreak risk, local authorities must consider the local aspects of the transmission cycle and the RRV system illustrates the importance of region-specific prediction and control measures.
Future work is required to assess the impact of climate change, increasing human populations, and urban development on RRV disease prevalence. Climate change is expected to increase RRV prevalence by changing weather patterns—including rainfall—expanding mosquito ranges, and lengthening warm periods, which may increase active mosquito periods through an extension of the “mosquito season” [14,17]. The growing Australian population and related urbanization may increase the contact rate between humans and reservoir hosts and vectors, increasing disease risk [18]. This is particularly true where constructed and rehabilitated wetlands, which provide suitable habitats for both mosquitoes and wildlife, are located close to growing residential populations. Significant gaps exist in the understanding of wildlife dynamics; further elucidation of the interactions among the virus, mosquitoes, and wildlife is essential for the development of more effective outbreak prediction models. These trends present a daunting picture for future disease mitigation efforts, but analytical innovations, such as those in geospatial information systems (GIS) [19] and emerging vector and pathogen surveillance systems [15], may assist outbreak prevention and prediction efforts in adjusting to these ecological shifts.
Conclusion
The unique ecology of RRV as a vector and reservoir host generalist makes it a moving target for health authorities. The variability in disease presentation and disease prevalence between vector and host species, within vector and host species, and in space and time makes the virus extremely ecologically complex and limits the scope of predictive models, which in turn impedes control efforts. The challenges presented by RRV offer a unique example of the importance of understanding the ecological underpinnings of disease systems for effective management.
Zdroje
1. Harley D, Sleigh A, Ritchie S (2001) Ross River virus transmission, infection, and disease: a cross-disciplinary review. Clin Microbiol Rev 14 : 909–932. 11585790
2. Condon RJ, Rouse IL (1995) Acute symptoms and sequalae of Ross River virus infection in South-Western Australia: A follow-up study. Clin Diag Vir 3 : 273–284.
3. Woodruff R, Bambrick H (2008) Climate change impacts on the burden of Ross River virus disease. Garnaut Clim Change Rev 115.
4. Russell RC, Kay BH (2004) Medical entomology: changes in the spectrum of mosquito-borne disease in Australia and other vector threats and risks, 19722004. Aus J Ent 43 : 271–282.
5. Russell RC (2002) Ross River virus: Ecology and distribution. Annu Rev Entomol 47 : 131.
6. Aaskov J, Williams L, Yu S (1997) A candidate Ross River virus vaccine: preclinical evaluation. Vaccine 15 : 1396–1404. 9302751
7. Potter A, Johansen CA, Fenwick S, Reid SA, Lindsay MDA (2014) The seroprevalence and factors associated with Ross River virus infection in western grey kangaroos (Macropus fuliginosus) in Western Australia. Vector-borne Zoo Dis 14 : 740–745.
8. Ritchie SA, Fanning ID, Phillips DA, Standfast HA, McGinn D, Kay BH (1997) Ross River Virus in Mosquitoes (Diptera: Culicidae) During the 1994 Epidemic Around Brisbane, Australia. J Med Ent 34 : 156–159.
9. Doherty RL, Whitehead RH, Gorman BM, O’Gower AK (1963) The isolation of a third group A arbovirus in Australia, with preliminary observation on its relationship to epidemic polyarthritis. Aust J Sci 26 : 183–184.
10. Watson and Kay BH (1998) Vector Competence of Aedes notoscriptus (Diptera: Culicidae) for Ross River Virus in Queensland, Australia. J Med Ent 35 : 104–106.
11. Tomerini DM, Dale PE, Sipe N (2011) Does mosquito control have an effect on mosquito-borne disease? The case of Ross River virus disease and mosquito management in Queensland, Australia. J Am Mos Con Assoc 27 : 39–44.
12. Webb CE (2015) Are we doing enough to promote the effective use of mosquito repellents? Med J Aus 202 : 128–129.
13. Kelly-Hope LA, Purdie DM, Kay BH (2004) Ross River virus disease in Australia, 1886–1998, with analysis of risk factors associated with outbreaks. J Med Entomol 41 : 133–150. 15061271
14. Jacups SP, Whelan PI, Currie BJ (2008) Ross River virus and Barmah Forest virus infections: A review of history, ecology, and predictive models, with implications for tropical northern Australia. Vector-Borne Zoo Dis 8 : 283–297.
15. van den Hurk AF, Hall-Mendelin S, Townsend M, Kurucz N, Edwards J (2011) Applications of a sugar-based surveillance system to track arboviruses in wild mosquito populations. Vector-Borne Zoo Dis 14 : 66–72.
16. Pialoux G, Gaüzère B-A, Jauréguiberry S, Strobel M (2007) Chikungunya, an epidemic arbovirus. Lancet Inf Dis 7 : 319–327.
17. Faddy H, Dunford M, Seed C, Olds A, Harley D, et al. (2014) Seroprevalence of antibodies to Ross River and Barmah Forest viruses: Possible implications for blood transfusion safety after extreme weather events. EcoHealth. E-pub ahead of print. doi: 10.1007/s10393-014-1005-0
18. Weinstein P, Judge D, Carver S (2011) Biological and cultural coevolution and emerging infectious disease: Ross River virus in Australia. Med Hypotheses 76 : 893–896. doi: 10.1016/j.mehy.2011.03.001 21435794
19. Vally H, Peel M, Dowse GK, Cameron S, Codde JP (2012) Geographic Information Systems used to describe the link between the risk of Ross River virus infection and proximity to the Leschenautl estuary, WA. Aus New Zea J Pub Hea 36 : 229–235.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Ross River Virus: Many Vectors and Unusual Hosts Make for an Unpredictable Pathogen
- Distinct but Spatially Overlapping Intestinal Niches for Vancomycin-Resistant and Carbapenem-Resistant
- Intracellular Survival of Depends on Uptake and Degradation of Extracellular Matrix Glycosaminoglycans by Macrophages
- Type IX Secretion Substrates Are Cleaved and Modified by a Sortase-Like Mechanism
- Structural and Functional Characterization of Anti-A33 Antibodies Reveal a Potent Cross-Species Orthopoxviruses Neutralizer
- Suppression of a Natural Killer Cell Response by Simian Immunodeficiency Virus Peptides
- Inhibition of Translation Initiation by Protein 169: A Vaccinia Virus Strategy to Suppress Innate and Adaptive Immunity and Alter Virus Virulence
- Enteropathogenic Uses NleA to Inhibit NLRP3 Inflammasome Activation
- Flavodoxin-Like Proteins Protect from Oxidative Stress and Promote Virulence
- Cullin4 Is Pro-Viral during West Nile Virus Infection of Mosquitoes
- The NLRP3 Inflammasome and IL-1β Accelerate Immunologically Mediated Pathology in Experimental Viral Fulminant Hepatitis
- DYRK2 Negatively Regulates Type I Interferon Induction by Promoting TBK1 Degradation via Ser527 Phosphorylation
- A KSHV microRNA Directly Targets G Protein-Coupled Receptor Kinase 2 to Promote the Migration and Invasion of Endothelial Cells by Inducing CXCR2 and Activating AKT Signaling
- The Operon Essential for Biofilm and Rugose Colony Development in
- ADAP2 Is an Interferon Stimulated Gene That Restricts RNA Virus Entry
- The Role of the Antiviral APOBEC3 Gene Family in Protecting Chimpanzees against Lentiviruses from Monkeys
- The Deacetylase Sirtuin 1 Regulates Human Papillomavirus Replication by Modulating Histone Acetylation and Recruitment of DNA Damage Factors NBS1 and Rad51 to Viral Genomes
- Experimental Malaria in Pregnancy Induces Neurocognitive Injury in Uninfected Offspring via a C5a-C5a Receptor Dependent Pathway
- Intrahepatic Transcriptional Signature Associated with Response to Interferon-α Treatment in the Woodchuck Model of Chronic Hepatitis B
- Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection
- Infection Is Associated with Impaired Hepatic Dimethylarginine Dimethylaminohydrolase Activity and Disruption of Nitric Oxide Synthase Inhibitor/Substrate Homeostasis
- Conserved Motifs within Hepatitis C Virus Envelope (E2) RNA and Protein Independently Inhibit T Cell Activation
- The RelA/SpoT Homolog and Stringent Response Regulate Survival in the Tick Vector and Global Gene Expression during Starvation
- Hybridization in Parasites: Consequences for Adaptive Evolution, Pathogenesis, and Public Health in a Changing World
- KSHV Latency Locus Cooperates with Myc to Drive Lymphoma in Mice
- Immunostimulatory Defective Viral Genomes from Respiratory Syncytial Virus Promote a Strong Innate Antiviral Response during Infection in Mice and Humans
- Retraction: Extreme Resistance as a Host Counter-counter Defense against Viral Suppression of RNA Silencing
- Appetite for a Foodborne Infection
- Here I Am, Despite Myself
- Microbial Regulation of p53 Tumor Suppressor
- Fiat Luc: Bioluminescence Imaging Reveals In Vivo Viral Replication Dynamics
- Knocking on Closed Doors: Host Interferons Dynamically Regulate Blood-Brain Barrier Function during Viral Infections of the Central Nervous System
- Rapid Lymphatic Dissemination of Encapsulated Group A Streptococci Lymphatic Vessel Endothelial Receptor-1 Interaction
- Simian Immunodeficiency Virus Infection of Chimpanzees () Shares Features of Both Pathogenic and Non-pathogenic Lentiviral Infections
- Epicellular Apicomplexans: Parasites “On the Way In”
- The Depsipeptide Romidepsin Reverses HIV-1 Latency
- Skin-Derived C-Terminal Filaggrin-2 Fragments Are -Directed Antimicrobials Targeting Bacterial Replication
- Type IV Pili Composed of Sequence Invariable Pilins Are Masked by Multisite Glycosylation
- Heterosexual Transmission of Subtype C HIV-1 Selects Consensus-Like Variants without Increased Replicative Capacity or Interferon-α Resistance
- Prevention of Influenza Virus-Induced Immunopathology by TGF-β Produced during Allergic Asthma
- Global Analysis of Mouse Polyomavirus Infection Reveals Dynamic Regulation of Viral and Host Gene Expression and Promiscuous Viral RNA Editing
- Modulation of the Host Lipid Landscape to Promote RNA Virus Replication: The Picornavirus Encephalomyocarditis Virus Converges on the Pathway Used by Hepatitis C Virus
- Intrinsic MyD88-Akt1-mTOR Signaling Coordinates Disparate Tc17 and Tc1 Responses during Vaccine Immunity against Fungal Pneumonia
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Epicellular Apicomplexans: Parasites “On the Way In”
- Fiat Luc: Bioluminescence Imaging Reveals In Vivo Viral Replication Dynamics
- Knocking on Closed Doors: Host Interferons Dynamically Regulate Blood-Brain Barrier Function during Viral Infections of the Central Nervous System
- A KSHV microRNA Directly Targets G Protein-Coupled Receptor Kinase 2 to Promote the Migration and Invasion of Endothelial Cells by Inducing CXCR2 and Activating AKT Signaling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



