-
Články
- Vzdělávání
- Časopisy
Top články
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Development of an RNAi Protocol to Investigate Gene Function in the Filarial Nematode,
Our ability to control diseases caused by parasitic nematodes is constrained by a limited portfolio of effective drugs and a paucity of robust tools to investigate parasitic nematode biology. RNA interference (RNAi) is a reverse-genetics tool with great potential to identify novel drug targets and interrogate parasite gene function, but present RNAi protocols for parasitic nematodes, which remove the parasite from the host and execute RNAi in vitro, are unreliable and inconsistent. We have established an alternative in vivo RNAi protocol targeting the filarial nematode Brugia malayi as it develops in an intermediate host, the mosquito Aedes aegypti. Injection of worm-derived short interfering RNA (siRNA) and double stranded RNA (dsRNA) into parasitized mosquitoes elicits suppression of B. malayi target gene transcript abundance in a concentration-dependent fashion. The suppression of this gene, a cathepsin L-like cysteine protease (Bm-cpl-1) is specific and profound, both injection of siRNA and dsRNA reduce transcript abundance by 83%. In vivo Bm-cpl-1 suppression results in multiple aberrant phenotypes; worm motility is inhibited by up to 69% and parasites exhibit slow-moving, kinked and partial-paralysis postures. Bm-cpl-1 suppression also retards worm growth by 48%. Bm-cpl-1 suppression ultimately prevents parasite development within the mosquito and effectively abolishes transmission potential because parasites do not migrate to the head and proboscis. Finally, Bm-cpl-1 suppression decreases parasite burden and increases mosquito survival. This is the first demonstration of in vivo RNAi in animal parasitic nematodes and results indicate this protocol is more effective than existing in vitro RNAi methods. The potential of this new protocol to investigate parasitic nematode biology and to identify and validate novel anthelmintic drug targets is discussed.
Published in the journal: . PLoS Pathog 6(12): e32767. doi:10.1371/journal.ppat.1001239
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001239Summary
Our ability to control diseases caused by parasitic nematodes is constrained by a limited portfolio of effective drugs and a paucity of robust tools to investigate parasitic nematode biology. RNA interference (RNAi) is a reverse-genetics tool with great potential to identify novel drug targets and interrogate parasite gene function, but present RNAi protocols for parasitic nematodes, which remove the parasite from the host and execute RNAi in vitro, are unreliable and inconsistent. We have established an alternative in vivo RNAi protocol targeting the filarial nematode Brugia malayi as it develops in an intermediate host, the mosquito Aedes aegypti. Injection of worm-derived short interfering RNA (siRNA) and double stranded RNA (dsRNA) into parasitized mosquitoes elicits suppression of B. malayi target gene transcript abundance in a concentration-dependent fashion. The suppression of this gene, a cathepsin L-like cysteine protease (Bm-cpl-1) is specific and profound, both injection of siRNA and dsRNA reduce transcript abundance by 83%. In vivo Bm-cpl-1 suppression results in multiple aberrant phenotypes; worm motility is inhibited by up to 69% and parasites exhibit slow-moving, kinked and partial-paralysis postures. Bm-cpl-1 suppression also retards worm growth by 48%. Bm-cpl-1 suppression ultimately prevents parasite development within the mosquito and effectively abolishes transmission potential because parasites do not migrate to the head and proboscis. Finally, Bm-cpl-1 suppression decreases parasite burden and increases mosquito survival. This is the first demonstration of in vivo RNAi in animal parasitic nematodes and results indicate this protocol is more effective than existing in vitro RNAi methods. The potential of this new protocol to investigate parasitic nematode biology and to identify and validate novel anthelmintic drug targets is discussed.
Introduction
Lymphatic filariasis is a disease caused by filarial nematodes including Wuchereria bancrofti and Brugia malayi, transmitted through the bite of infected mosquitoes. These parasites perpetuate socioeconomic instability in developing countries by inflicting crippling morbidity and debilitating stigmatization. The impact of this disease is vast - over 120 million people are infected in 81 endemic countries [1]. In an effort to alleviate morbidity and eliminate transmission of this disease, the Global Program for the Elimination of Lymphatic Filariasis (GPELF) has orchestrated a systematic mass drug administration (MDA) program centered on the repeated dosing of either diethylcarbamazine citrate (DEC) and albendazole or albendazole and ivermectin in areas where the other filarial parasites, Onchocerca volvulus and Loa loa are co-endemic. This strategy has reduced prevalence in many areas [2] but lymphatic filariasis remains a significant global health concern. Many factors contribute to continued transmission, but central is the inadequate portfolio of effective drugs; none of the MDA drugs are effective against all life stages of the parasite with notable inefficacy against adult worms [3]–[5]. This means MDA must be provided annually for the duration of the lifespan of adult parasites. This situation is compounded by gaps in our understanding of mechanisms of drug action and pharmacology – the site of action of DEC is unknown despite being the drug of choice for lymphatic filariasis control for decades, and the filaricidal mechanism of ivermectin at therapeutic concentrations is also equivocal. There is a very real and significant need for additional and more effective antifilarial drugs, and a better understanding of the mode of action of existing drugs [6].
A major obstacle to the rational development of such drugs is the experimental intractability of parasitic nematodes. An example of this complication is RNA interference (RNAi), a reverse genetic tool that allows researchers to rapidly and specifically ‘turn off’ genes of interest. RNAi has fast become a standard tool in rational drug discovery for the identification and validation of potential new drug targets [7], [8]. By suppressing specific genes and examining the resulting phenotype, it is possible to delineate gene function and appraise the potential value of encoded proteins as drug targets. Successful applications of present RNAi protocols to parasitic nematodes have been sporadically reported, limited in their effectiveness and seldom repeated [9]. Some success has been achieved with Nippostrongylus brasiliensis [10], Ascaris suum [11], Trichostrongylus colubriformis [12], Ostertagia ostertagi [13] and Haemonchus contortus [14], [15]. Germane to the study of filarial worms, RNAi has been described in B. malayi [16], [17], Onchocerca volvulus [18], [19] and Litomosoides sigmodontis [20]. The conclusion has been reached, however, that successful RNAi “only works on a limited number of genes, and in some cases the effect is small and difficult to reproduce” [14]. The inability to depend on present RNAi protocols with parasitic nematodes has proved a major stumbling block to the identification and validation of new drug targets, to a better understanding of anthelmintic mode of action, and to advancing our comprehension of parasite biology.
The recalcitrance of animal parasitic nematodes to RNAi is perplexing, given that Caenorhabditis elegans, a free-living nematode, and plant parasitic nematodes are readily susceptible to the technique [21]–[28]. One hypothesis advanced to explain this recalcitrance is that because present RNAi protocols employ in vitro approaches including soaking nematodes in an RNAi trigger, feeding nematodes bacteria producing the trigger, or electroporating of the trigger into the parasite, the RNAi trigger is not provided in a manner conducive to systemic gene suppression [29]. Implicit in the use of these protocols is the removal of a parasite from the host and its maintenance in a liquid culture. Therefore these protocols have distinct drawbacks such as difficulty maintaining healthy, viable worms that behave normally in vitro, limitation of use of parasites or life stages for which in vitro culture is defined, and poor efficacy in RNAi trigger delivery methods that can prove lethal to the parasite [30].
The aim of this study was to develop an innovative in vivo approach to RNAi in parasitic nematodes that overcomes the drawbacks associated with present in vitro experimental paradigms. Our approach is based on the filarial nematode B. malayi. We establish a B. malayi infection in an intermediate host, the mosquito Aedes aegypti, and then initiate suppression of parasite genes by injecting an RNAi trigger directly into the mosquito. The mosquito acts as an ideal culture and delivery system, ensuring the RNAi trigger is exposed to healthy, developing parasites. Using this approach we have effectively and quantifiably suppressed expression of Bm-cpl-1, a B. malayi gene encoding a cathepsin L-like cysteine protease. Dramatic aberrant phenotypes accompany this suppression, including a marked retardation of motility, an inhibition of normal parasite migration behavior within the mosquito and impaired parasite growth and development. Suppression is specific; non-target RNAi has no effect on nematode viability or behavior, and the level of gene suppression and extent of the resultant phenotypes suggest this new protocol is more effective than previous methods. The development of an in vivo RNAi protocol to reliably suppress gene expression in filarial worms has great potential for the identification and validation of novel drug targets, and more broadly, to explore parasitic nematode biology and host-parasite interactions.
Results
A Brugia RNAi trigger rapidly disseminates throughout the mosquito host
Our hypothesis is that mosquitoes provide an optimal culture and delivery system for an RNAi trigger targeted to developing Brugia malayi parasites. Healthy, viable, developing parasites are subjected to the RNAi trigger because the parasites undergo growth and development in the mosquito intermediate host. In order to test the extent of dissemination of the RNAi trigger from the site of intrathoracic injection, 150 ng of an equimolar mix of four 3′ Cy 3-labelled Bm-cpl-1 siRNAs was injected into adult Aedes aegypti mosquitoes as described. The dissemination of this RNAi trigger through the mosquito was tracked over 15 d post-injection by periodic microdissection of the mosquito and evaluation of internal fluorescence compared to saline injected controls. The labeled siRNA mix spread rapidly from the site of injection and maximal fluorescence was observed 24 h post-injection (Fig. 1). The intensity of fluorescence slowly decreased until reaching basal levels at five d post-injection after which fluorescence intensity was not appreciably different from control mosquitoes. Our observations closely parallel those of a previous report that describes the spread of 140 ng AlexaFluor 555-labeled siRNA in the mosquito Anopheles gambiae from an injection site to the midgut and pericardial cells 36 h post-injection [31]. Systemic dispersion and persistence of RNAi signal from the site of injection suggests B. malayi larvae are likely to be exposed to the RNAi trigger in our experimental model.
Fig. 1. Dissemination and persistence of intrathoracically injected Cy 3-labelled Brugia malayi Cathepsin-L1 siRNAs in Aedes aegypti. 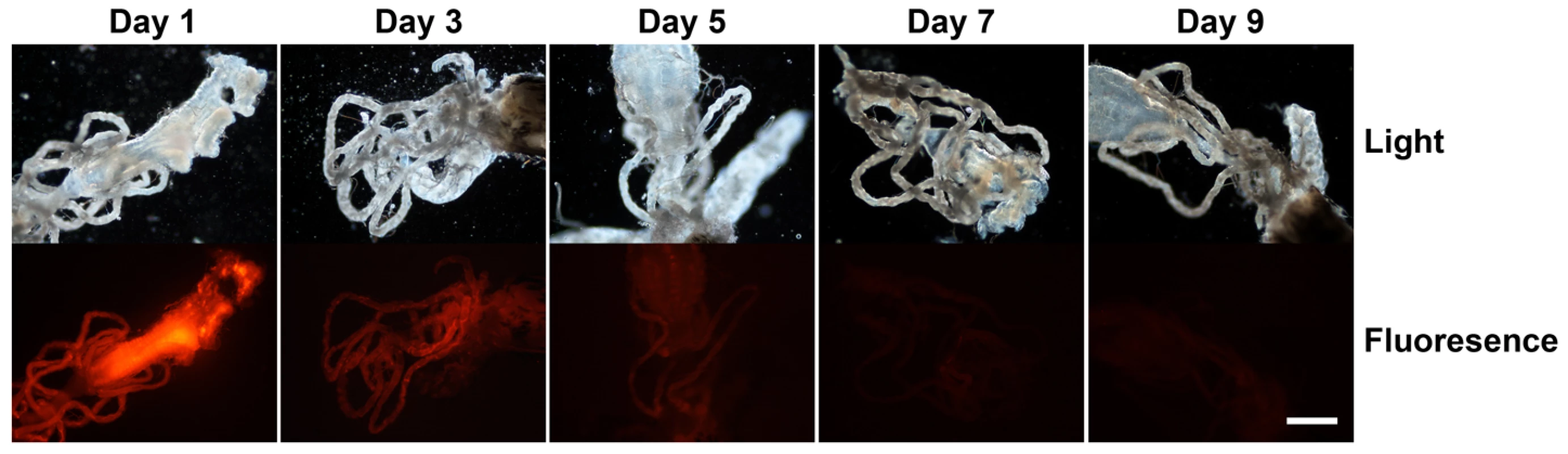
Midgut and Malpighian tubule tissues are shown in light (upper panel) and fluorescence (lower panel) micrographs from 1 to 9 days post-injection (scale bar 100 µm). Brugia gene suppression in vivo is potent and specific
Recently it has been shown that B. malayi genes encoding cathepsin L-like enzymes can be suppressed in vitro by soaking adult parasites in culture media containing siRNA [17]. We tested the capacity of our novel methodology to suppress larval stage B. malayi gene expression in vivo by injecting mixed siRNAs specific to the cathepsin L-like Bm-cpl-1 gene directly into Ae. aegypti mosquitoes harboring L3 stage B. malayi parasites. Gene suppression was assayed 48 h post-injection using a semi-quantitative RT-PCR in which the intensity of Bm-cpl-1 amplification in the linear phase of the reaction was compared to an internal B. malayi reference gene (Bm-flp-14) that is expressed stably and at comparable levels to Bm-cpl-1. Control mosquitoes were injected with equal volumes of Aedes physiologic saline. This methodology was optimized to amplify Bm-cpl-1 from a heterogeneous mosquito/parasite total RNA preparation from a single mosquito. Suppression was concentration-dependent because injection of 0.15 ng siRNA did not appear to reduce Bm-cpl-1 transcript levels. However, injection of 15 ng or 1.5 ng of siRNA decreased transcript levels, and injection of 150 ng mixed siRNA into mosquitoes profoundly suppressed Bm-cpl-1 expression; the target parasite gene could not be amplified (Fig. 2). This suppression was also specific; expression of the Bm-flp-14 reference gene was unaffected by siRNA injection and target gene expression was normal in saline-injected controls.
Fig. 2. Concentration-dependent, in vivo suppression of Brugia malayi Cathepsin-L1 (Bm-cpl-1) using siRNA (Top) or dsRNA (Bottom) RNAi triggers. 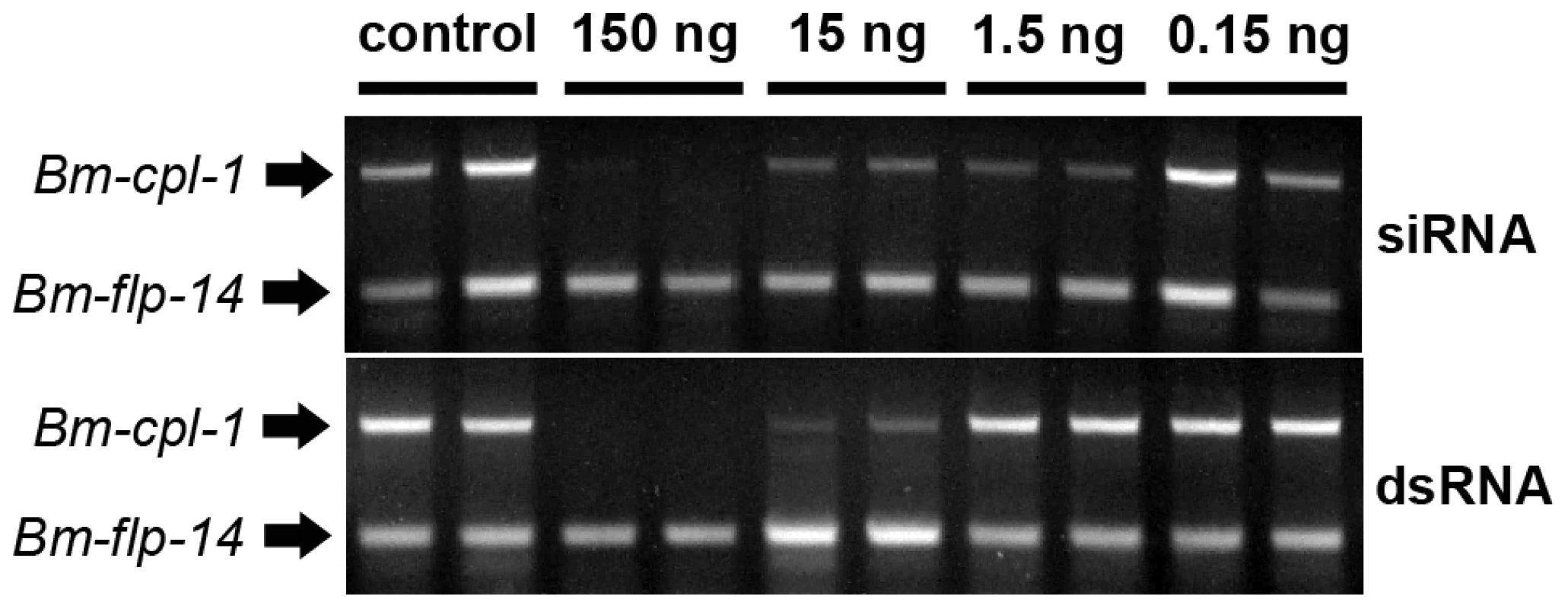
Micrograph shows ethidium bromide stained agarose gel electrophoresis of relative RT-PCR analysis of individual, B. malayi-infected mosquitoes 48 h post-injection of RNAi trigger at 10 d post-infection. Amplified product for the target gene, Bm-cpl-1, is shown above a neuropeptide reference gene (Bm-flp-14). Application of dsRNA is the commonly used method for triggering RNAi in parasitic nematodes and has advantages over siRNA; dsRNA can be generated in-house more quickly than commercially produced siRNAs at lower cost. B. malayi-infected mosquitoes were also subjected to treatment with dsRNA as an RNAi trigger. The effect of dsRNA was concentration-dependent such that injection of 15 ng dsRNA results in Bm-cpl-1 suppression but 1.5 ng dsRNA had no appreciable effect. Injection of 150 ng of dsRNA potently suppressed Bm-cpl-1 transcript abundance and suppression appeared specific, with Bm-flp-14 expression unaffected by dsRNA (Fig. 2).
RT-qPCR was used to quantify the level of Bm-cpl-1 suppression relative to two reference genes (Bm-flp-14 and Bm-tph-1) using the efficiency-corrected (EΔΔCq) relative quantification method [32]. PREXCEL-Q software was used to optimize the performance of the RT-qPCR assay; and important data pertinent to PCR efficiency, linear dynamic range and normalization of the assay are documented in Table 1. Bm-tph-1 showed stable Cq values across the experiment and therefore was the most appropriate reference gene for these studies, as shown previously [33]. The suppressive effects of both RNAi treatments were almost identical; injection of 150 ng siRNA reduced Bm-cpl-1 transcript by 83% compared to saline-injected controls (P<0.0001) and 150 ng dsRNA also reduced Bm-cpl-1 transcript by 83% (P<0.0001) (Fig. 3). Bm-flp-14 reference gene transcript was slightly reduced by both RNAi treatments but these reductions were not significant (siRNA, 9%, P = 0.38; dsRNA, 12%, P = 0.17). These data support the gel-based semi-quantitative RT-PCR experimental findings and demonstrate the efficacy of this novel method of RNAi delivery.
Fig. 3. Quantitative PCR demonstrates significant reduction in Bm-cpl-1 transcript levels as a result of siRNA and dsRNA RNAi trigger injection into B. malayi-infected Ae. aegypti. 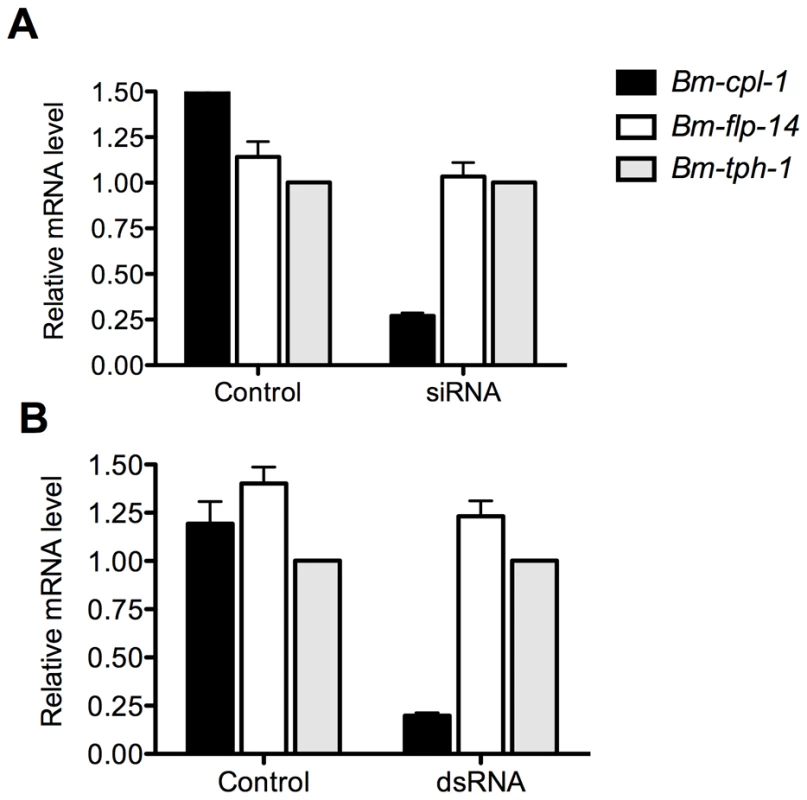
Both siRNA (A) and dsRNA (B) injection reduces Bm-cpl-1 transcript by 83% compared to controls (saline injected Ae. aegypti infected with B. malayi). Bm-cpl-1 and control gene, Bm-flp-14, are normalized to a reference gene Bm-thp-1. qPCR was performed 48 h post-injection of RNAi trigger at 10 d post-infection. Each bar represents 13 mosquitoes from three biological replicates. Tab. 1. Reportable information on RT-qPCR experiment. 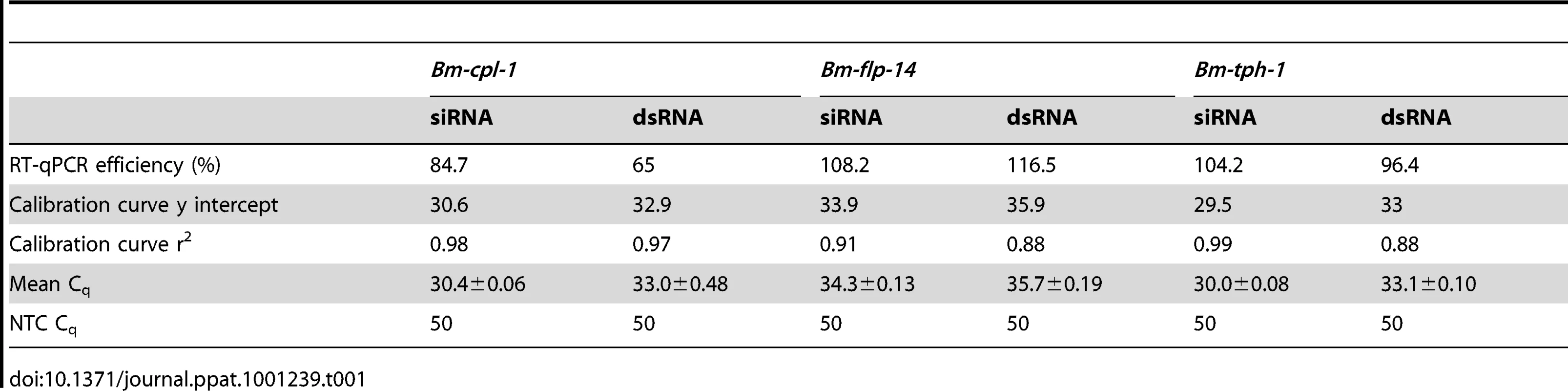
Bm-cpl-1 suppression elicits marked motility and developmental phenotypes
Previous studies have described aberrant filarial worm phenotypes associated with cathepsin L-like gene suppression in vitro including decreased microfilariae (mf) release from adult B. malayi [17] and an inhibition of the L3 to L4 molt in Onchocerca volvulus [18]. Based on these data, we predicted that Bm-cpl-1 suppression would produce a phenotype in vivo in the mosquito host. Mosquitoes were injected with 150 ng Bm-cpl-1 dsRNA 10 d post-infection (dpi) then microdissected four d post-injection to harvest L3-stage parasites. Worm motility was digitally recorded and scored according to a five-point schema of one (immobile), to five (all parts of the worm in constant motion). 100% of control worms from mosquitoes injected with Aedes physiologic saline were categorized as four or five on this scale. Bm-cpl-1 suppression significantly inhibited this normal worm motility (P<0.001), with only 67% of worms ranked as four or five on the scale (Fig. 4). To confirm that this effect was Bm-cpl-1 specific and not due to exogenous dsRNA impairing worm viability, this experiment was repeated with dsRNA for enhanced GFP (eGFP) as a random exogenous RNA. These worms were phenotypically identical to saline-injected controls (100% category four or five), confirming the specificity of the aberrant phenotype in Bm-cpl-1 suppressed worms.
Fig. 4. Aberrant motility of dsRNA Bm-cpl-1-exposed B. malayi. 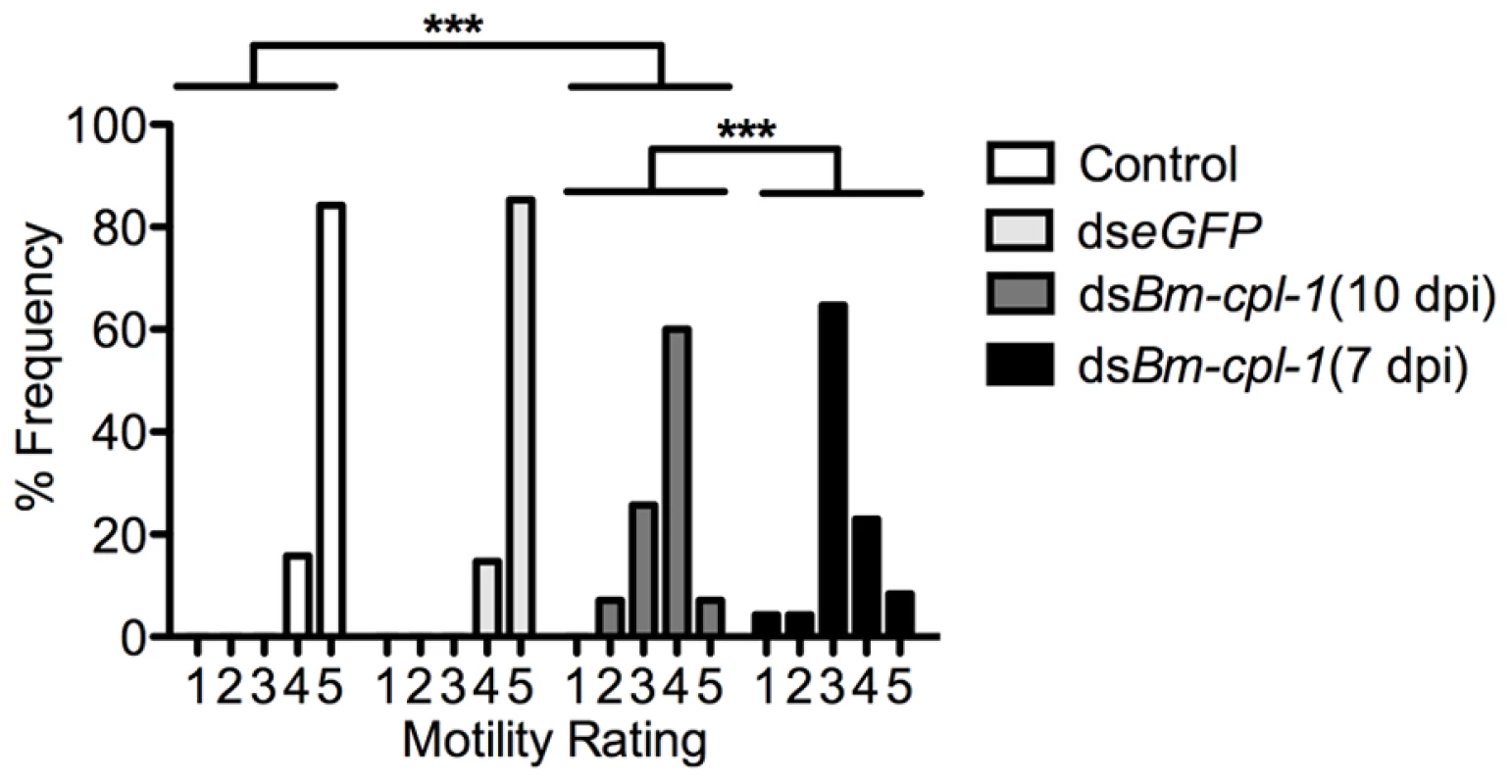
Frequency distribution for motility of L3 stage B. malayi recovered from Ae. aegypti showing significantly reduced motility of Bm-cpl-1 suppressed worms. Parasitized mosquitoes were injected with saline (control), 150 ng eGFP dsRNA, or 150 ng Bm-cpl-1 dsRNA at 7 or 10 d post-infection (dpi), then dissected to obtain parasites at 14 dpi. Parasite motility was scored on a 1–5 scale, with 1 = immobile and 5 = all parts of worm in constant motion (control n = 101, dseGFP n = 68, dsBm-cpl-1 10 dpi n = 70, dsBm-cpl-1 7 dpi n = 48, P<0.001). The effect of changing the timing of Bm-cpl-1 suppression on worm motility was also examined. Bm-cpl-1 transcript levels are elevated in L3 stage parasites, such that this gene has a purported role in the L3 to L4 molt [18]. The temporal expression of Bm-cpl-1 was reported to be up-regulated during the L2 to L3 transition, at six to seven dpi [34]. Based on the timing of Brugia development in Ae. aegypti [35], infected mosquitoes were injected with Bm-cpl-1 dsRNA at 10 dpi in order to target L3-stage worms (described above) and at seven dpi to target the L2 to L3 transition. Parasites exposed to Bm-cpl-1 dsRNA at seven dpi showed significantly inhibited motility compared to saline controls (P<0.001) with only 31% of worms displaying normal motility. The difference between parasites exposed to dsRNA at seven and 10 dpi was significant (P<0.001), and may reflect an important biological role for Bm-cpl-1 during the transition from L2 to L3 stages. More explicitly, earlier exposure to the RNAi trigger could impose more significant detrimental impact on the parasite by disrupting the L2 to L3 molt, or it may simply be a consequence of the longer period of time from gene suppression to phenotype assay, allowing Bm-CPL-1 rundown and maturation of the phenotype.
In addition to depressed activity, other morphological and motility phenotypes were apparent in Bm-cpl-1 suppressed worms. A highly active, convoluted body form characterizes motility of healthy B. malayi L3s, both the heads and tails of the parasites in particular are conspicuously tortuous – curvature we described as ‘knotted’. Control worms from saline-injected mosquitoes frequently (86% of worms) displayed knotting at both ends. Suppression of Bm-cpl-1 10 dpi significantly inhibited this motility, because only 14% of worms presented with both ends knotted (P<0.001) (Fig. 5). This phenotype was enhanced by an early suppression of Bm-cpl-1 at seven dpi such that no Bm-cpl-1 suppressed parasites exhibited this knotting morphology. The difference between L2 and L3 Bm-cpl-1 suppression was significant (P = 0.005). Worms exposed to the exogenous eGFP dsRNA control confirmed that this phenotype was gene-specific because parasite motility was not significantly different from saline controls (85% knotted at both ends, P = 0.2). Another aberrant motility observed was the presence of a perturbed section of body wall slightly caudal to the midpoint of the worm. This abnormal kinked morphology was absent from control worms (4% of worms from saline-injected and 0% from eGFP-injected mosquitoes displayed this morphology), but evident with significantly greater frequency in 10 dpi Bm-cpl-1 suppressed worms (47%, P<0.001) (Fig. 6). This kink rate increased with Bm-cpl-1 suppression at seven dpi (63%), but compared to 10 dpi this was not significant (P = 0.08). Finally, partial paralysis of Bm-cpl-1 suppressed worms was evident, presenting as immobility in the caudal third of the worm. This paralysis was observed in 61% of 10 dpi Bm-cpl-1 suppressed worms, and 83% of seven dpi suppressed worms (this increase was significant, P = 0.005) but was generally absent from control worms (5% of worms from saline-injected mosquitoes and 3% of worms from eGFP-injected mosquitoes) (Fig. 6).
Fig. 5. Disrupted motile phenotypes of dsRNA Bm-cpl-1-exposed B. malayi. 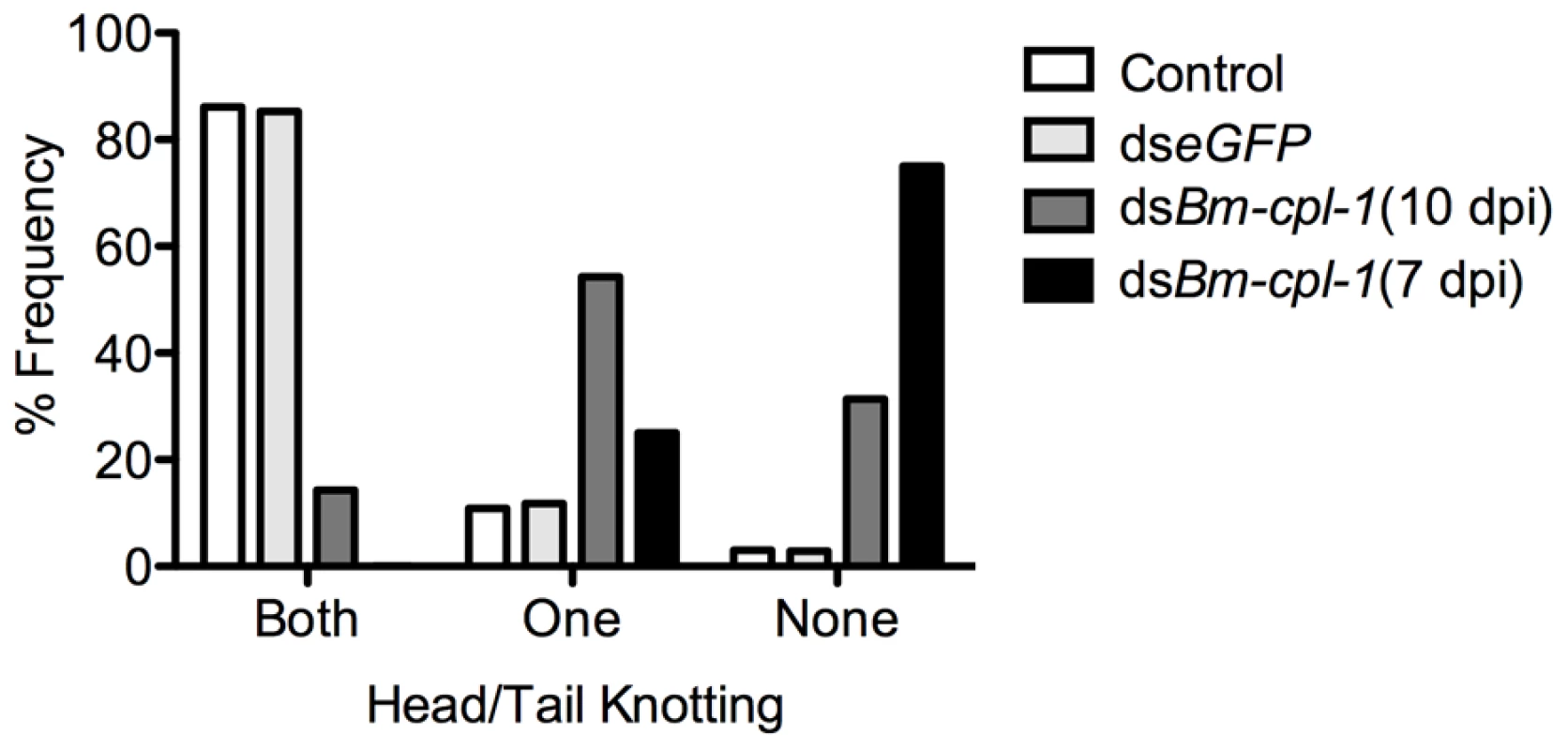
Frequency distribution for parasites exhibiting rigorous knotting behavior at both ends of worm, one end, or not at all showing normal terminal curvature is inhibited by Bm-cpl-1 suppression. Parasitized mosquitoes were injected with saline (control), 150 ng eGFP dsRNA, or 150 ng Bm-cpl-1 dsRNA at 7 or 10 d post-infection (dpi), then dissected to obtain parasites at 14 dpi (control n = 101, dseGFP n = 68, dsBm-cpl-1 10 dpi n = 70, dsBm-cpl-1 7 dpi n = 48). Fig. 6. The frequency of caudal paralysis and kinked posture of dsRNA Bm-cpl-1-exposed B. malayi. 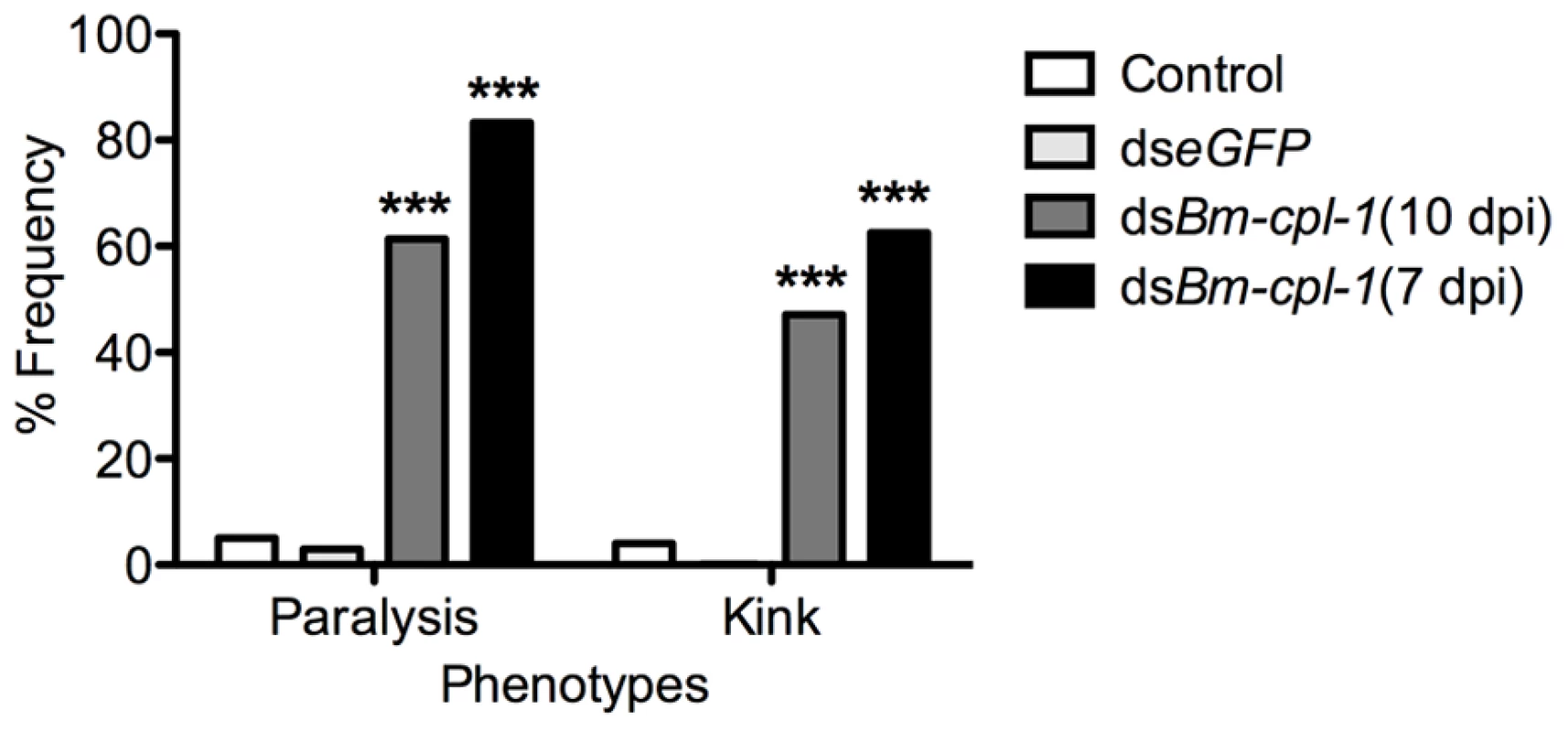
The frequency of both caudal paralysis and kinked posture is significantly increased with Bm-cpl-1 suppression. Parasitized mosquitoes were injected with saline (control), 150 ng eGFP dsRNA, or 150 ng Bm-cpl-1 dsRNA at 7 or 10 d post-infection (dpi), then dissected to obtain parasites at 14 dpi (control n = 101, dseGFP n = 68, dsBm-cpl-1 10 dpi n = 70, dsBm-cpl-1 7 dpi n = 48, P<0.001). To examine the consequence of this aberrant motility on B. malayi development, mosquitoes were injected with 150 ng Bm-cpl-1 dsRNA 10 dpi then microdissected four d post-injection, partitioning the mosquitoes into head, thorax and abdomen preparations. Control worms from mosquitoes injected with either saline or eGFP dsRNA were found exclusively (100%) in head preparations as expected. Bm-cpl-1 suppressed worms were most frequently observed escaping from the thorax and abdomen (Fig. 7). Parasites in Bm-cpl-1 dsRNA-injected mosquitoes, however, did not leave the thorax (94% of worms were found here) or abdomen (6%). Bm-cpl-1 suppression, therefore, prevents worm migration to the head of the mosquito, effectively preventing normal progression of the parasite life cycle and thus abolishing the potential for parasite transmission.
Fig. 7. dsRNA Bm-cpl-1-exposed B. malayi fail to migrate to the head of the mosquito. 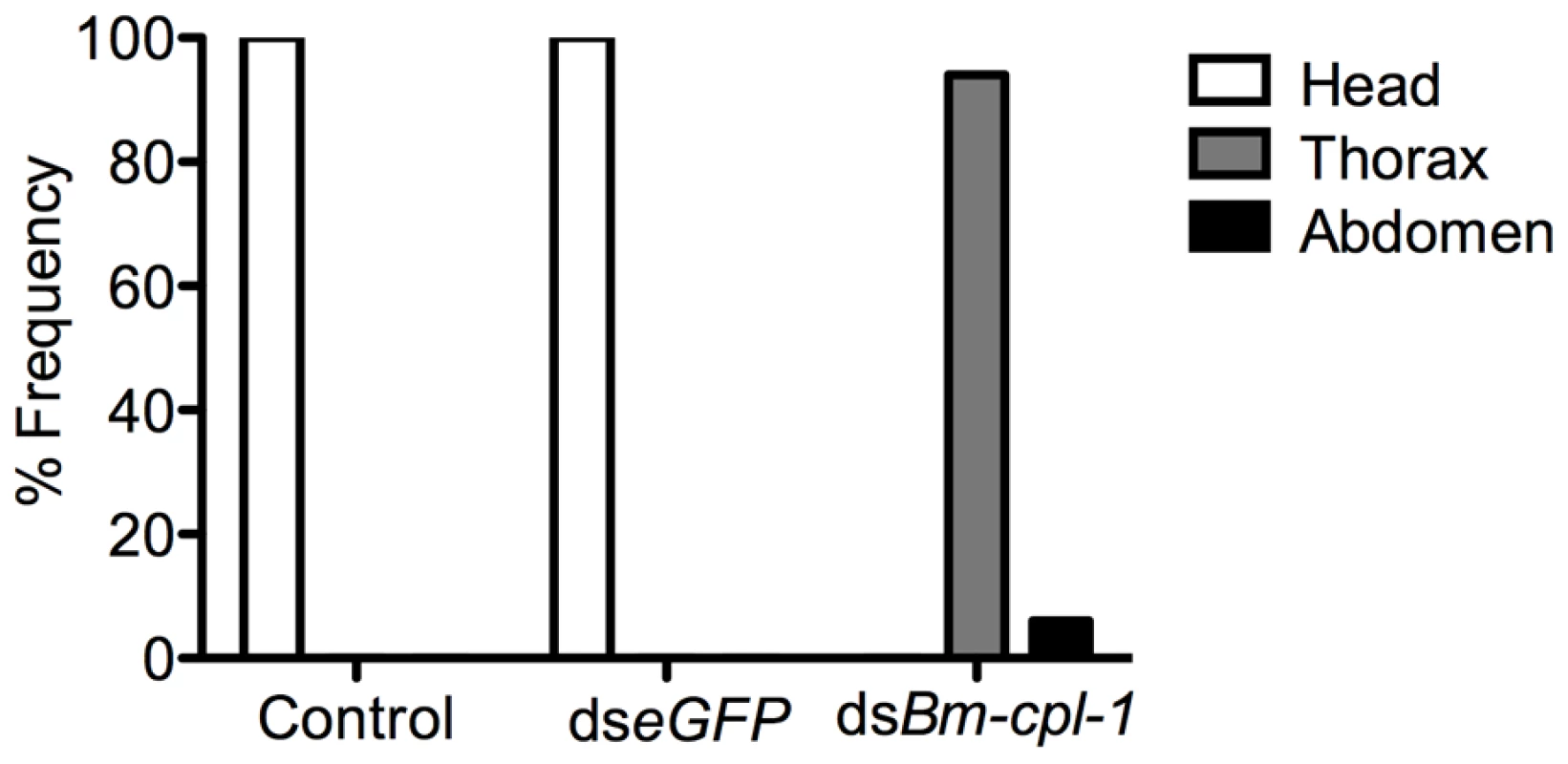
Frequency distribution of infectious (L3) stage B. malayi recovered from Ae. aegypti in the head, thorax or abdomen of the mosquito host. Parasites were dissected from Ae. aegypti mosquitoes 14 d post-infection and 4 d post-injection of saline (control), 150 ng eGFP dsRNA or 150 ng Bm-cpl-1 dsRNA. One worm was recovered from each mosquito. Numbers of mosquitoes dissected from three biological replicates: n = 18 (control), n = 20 (dseGFP), and n = 31 (dsBm-cpl-1). A significant negative effect also was seen on growth and development of parasites subjected to Bm-cpl-1 suppression. Parasitized mosquitoes were injected with 150 ng Bm-cpl-1 dsRNA seven dpi then microdissected 14 dpi and the length, width and appearance of the worms recorded. Mosquitoes were injected seven dpi because the previous motility experiments dictated that this experimental timing generated the most pronounced motility phenotypes. Bm-cpl-1 suppression significantly reduced the length of L3 worms by 48% (P<0.0001)(Fig. 8). The mean length of control L3, removed from mosquitoes seven d after saline injection and 14 dpi, was 1347±18 µm. This was reduced to 700±49 µm after RNAi treatment. Unlike parasite length, width was not significantly affected by Bm-cpl-1 suppression, although a slight decrease of 5% was observed (P = 0.39) from 31±1 µm in control worms to 30±2 µm in RNAi worms. In addition to worm length, the majority of Bm-cpl-1 dsRNA parasites also presented with additional aberrant developmental phenotypes. Most evident was a disruption of the cuticle (Fig. 8B), which extended significantly beyond the body of the worm. Some degree of this cuticular sloughing was noticed in most worms but the severity of this phenotype was variable. Finally, the integrity of the gut appeared compromised in Bm-cpl-1 worms. In such instances, the gastrointestinal tract of the parasites appeared incomplete and porous when examined at the light microscope level.
Fig. 8. Bm-cpl-1-suppressed B. malayi are significantly shorter than control worms. 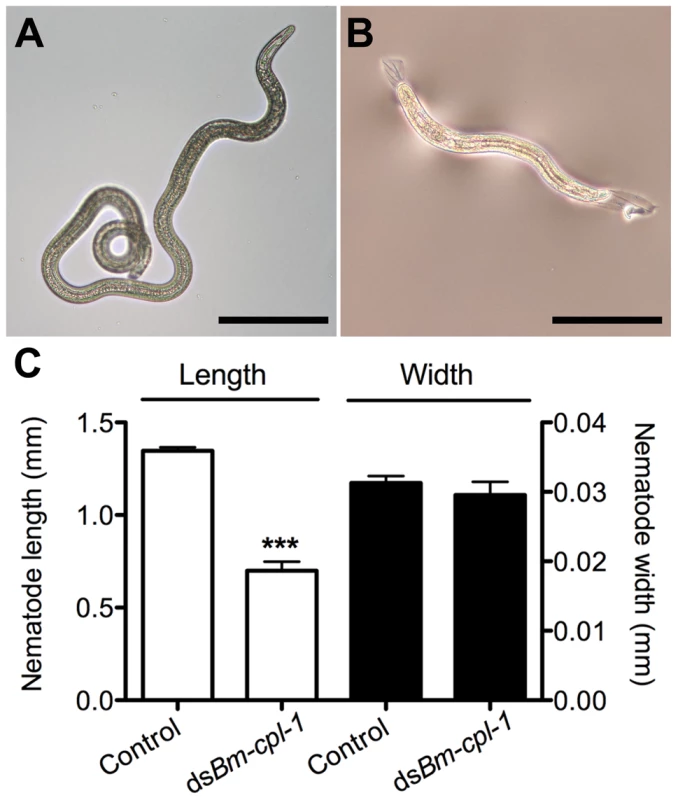
Parasites were dissected from Ae. aegypti mosquitoes 14 days post-infection and 4 d post-injection of saline (control, A) or 150 ng Bm-cpl-1 dsRNA (B). Scale bar 250 µm. (C) RNAi-exposed parasites are significantly shorter in length, but not width, than control worms. Numbers of parasites from three biological replicates: n = 19 (control) and n = 13 (dsBm-cpl-1) (P<0.001). Bm-cpl-1 suppression enhances mosquito survival and decreases parasite prevalence
Phenotype data resoundingly demonstrate that Bm-cpl-1 suppression decreases B. malayi viability. It is logical to predict that this decreased viability would also have an impact on mosquito survival and prevalence of parasite infection. To examine this, mosquitoes were injected with 150 ng Bm-cpl-1 dsRNA 10 dpi and the number of mosquitoes that survived through the development of parasites to the infectious stage, 14 dpi, was counted. Surviving mosquitoes then were microdissected to determine the proportion that harbored parasite infections. Bm-cpl-1 suppression increases host mosquito survival. The survival rate of control mosquitoes injected with saline or eGFP dsRNA was 62% and 65% respectively (P = 0.6) as compared to 80% in Bm-cpl-1 RNAi-exposed mosquitoes (P<0.001) (Fig. 9A). Early suppression of Bm-cpl-1 at seven dpi enhanced the phenotype even more significantly such that 93% of mosquitoes were alive at the termination of the experiment (P = 0.007). This increased mosquito survival rate after Bm-cpl-1 suppression may be as a result of the parasite's compromised ability to feed on, and migrate through, the host or may result from a more successful or effective host response against parasites with decreased viability. This hypothesis is supported by our observation that Bm-cpl-1 suppression also decreased prevalence of infection – fewer surviving mosquitoes harbored parasites after Bm-cpl-1 RNAi (Fig. 9B). Every surviving mosquito injected with saline or eGFP was found to contain parasites 14 dpi, but 14 dpi Bm-cpl-1 suppressed parasites (exposed to dsRNA at 10 dpi) were found in just 76% of mosquitoes, a significant reduction in prevalence (P<0.001). Prevalence was further reduced to 62% in parasites exposed to Bm-cpl-1 dsRNA at seven dpi, a statistically significant decrease compared to worms exposed to the dsRNA trigger at 10 dpi (P = 0.03).
Fig. 9. Ae. aegypti survival significantly increases as infection prevalence decreases in Bm-cpl-1-dsRNA exposed mosquitoes. 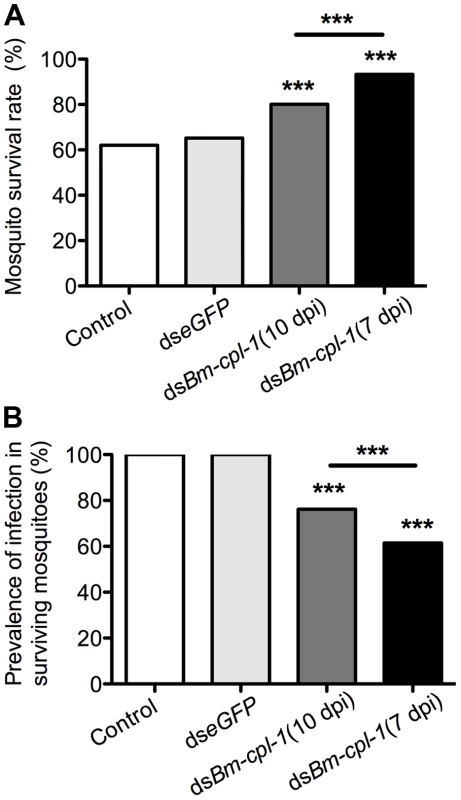
The effects on survival (A) and infection prevalence (B) are most profound in parasites exposed to Bm-cpl-1 dsRNA at the L2/L3 transition (7 d post-infection, dpi). Parasites were exposed to saline (control), 150 ng eGFP dsRNA or 150 ng Bm-cpl-1 dsRNA at 7 or 10 dpi, then were dissected from Ae. aegypti mosquitoes at 14 dpi. Discussion
Here we report the development of a novel in vivo approach to RNAi in the filarial nematode Brugia malayi, and describe its application first to suppress the expression of Bm-cpl-1, a B. malayi gene encoding a cathepsin L-like cysteine protease, then to validate this gene as a potentially potent anthelmintic drug target. To the best of our knowledge, this is the first description of in vivo RNAi in parasitic nematodes and represents an advance in the study of filarial nematode biology that may aid in the development of drugs to combat parasitic nematode infection. The rationale for developing an in vivo RNAi protocol stems from the hypothesis that RNAi is ineffective in animal parasitic nematodes because the supply of an RNAi trigger to the worms is inappropriate [29]. Our overarching hypothesis was that RNAi would work effectively and robustly if a trigger is supplied to healthy, viable worms in a host environment. Supporting this hypothesis, we were able to specifically reduce target gene transcript abundance in B. malayi larvae by 83% by supplying an RNAi trigger to parasites developing within the mosquito host. This level of transcript knockdown has not previously been reported using current in vitro RNAi soaking methods. The ‘in squito’ approach to RNAi we describe is effective for the specific suppression of cathepsin genes in Brugia larval stages as they develop within their cognate mosquito host; it is therefore possible that this in vivo approach may represent a more effective means of eliciting gene suppression in filarial nematodes.
The mechanism by which the RNAi trigger is delivered to the parasites ‘in squito’ is unclear, but could be a result of bathing the parasite in the trigger within a cell, or as a result of uptake by tissue ingestion. In support of the former, Cy3-labeled siRNA injected into the haemocoel rapidly disseminates throughout the mosquito supporting a hypothesis that the developing parasites are effectively incubating in a host milieu containing an RNAi trigger, essentially a scenario analogous to in vitro RNAi by soaking. If this is the case, the ‘in squito’ approach represents an efficient way to generate gene suppression by soaking. Most successful animal parasitic nematode in vitro soaking protocols use large amounts of ds - or siRNA with concentrations of 1 mg/ml typical, meaning anywhere between 25 µg and 2 mg of RNAi trigger are required per suppression experiment [10]–[19], with the exception of one report showing that lower trigger concentrations could still be effective at producing gene suppression by soaking [20]. Here we showed that gene suppression can be achieved using just 150 ng of ds - or siRNA per RNAi event, and indeed, a reduction in transcript abundance was observed after injecting as little as 15 ng dsRNA. In addition to the obvious cost saving advantages to performing RNAi experiments in this manner, such low RNA concentrations may also improve the specificity of gene suppression. Soaking plant parasitic nematodes in serial dilutions of ds - and siRNAs has been shown to reduce off-target effects in RNAi experiments [28], [36]. A second delivery hypothesis is that the developing parasites are ingesting the RNAi trigger. Microfilariae taken in during the blood meal rapidly penetrate the mosquito midgut [37], and migrate to the thoracic musculature where they grow and develop to the L3 stage [38], [39], a process completed in under two weeks [35]. From the L2 stage, the developing larvae are active feeders and consume host tissue [35], [40], [41], a behavior that would lead to the ingestion of an injected RNAi trigger in our experimental model. RNAi by feeding is a well-established method in free-living nematodes [22], [23], [42], [43]; by feeding these worms bacteria expressing dsRNA, systemic gene suppression can be effected in a relatively simple and efficient manner. This approach has not been successful with parasitic nematodes, however, as most parasitic species are not bacteriotrophic, and even for those species with bacteriotrophic life stages, this method is unreliable [30]. Resolution of the RNAi trigger delivery mechanism afforded by our in vivo protocol may come through targeting B. malayi L1 worms in the mosquito. If target gene expression can be reduced in this non-feeding stage, this would support soaking as the prime mechanism.
The in squito suppression of Bm-cpl-1 reveals new phenotypes associated with molting, growth and development, and motility that shed light on the important biological functions of this gene family in larval stages of B. malayi. Nematode molting is a three-stage process characterized by a shedding or separation of the old cuticle from the epidermis (apolysis), generation of a new cuticle, then the shedding of the old cuticle (ecdysis). The use of specific cysteine protease inhibitors markedly inhibits the L3 to L4 molt in filarial worms implicating cysteine proteases in general in this process [34], [44], [45]. More explicitly, both apolysis and ecdysis are disrupted giving rise to L4 parasites constrained within an L3 cuticle, termed an ‘accordion’ phenotype [34]. Multiple members of the cathepsin L-like family appear to be involved in molting as the specific suppression of cpl-1 alone in Onchocerca vovlvulus reduced but did not abrogate the L3 to L4 molt [18]. We show that Bm-cpl-1 is also involved in similar processes in B. malayi as its suppression manifested an aberrant cuticular phenotype in L3 worms. Examination of worms suppressed at seven dpi revealed an apparent sloughing of the cuticle without the accordion phenotype. As the L3 to L4 molt occurs in the vertebrate host this phenotype is not a disruption of the L3 to L4 molt, but rather a dysfunction in L3 cuticle formation, maintenance or development. Alternatively, we could be observing a disruption of the L2 to L3 molt. Bm-cpl-1 expression is up-regulated in the L3 stage but the exact timing of this up-regulation as it relates to the transition from L2 to L3 stages is unclear. Guiliano et al. [34] report Bm-cpl-1 up-regulation at six dpi, a window consistent with the L2 to L3 transition. If Bm-cpl-1 performs the same function for the L2 to L3 molt as for the L3 to L4 molt, then the sloughed cuticle we observe upon dsRNA injection at seven dpi could be that of the L2, with Bm-cpl-1 suppression preventing ecdysis. Further examination of cuticle ultrastructure in these suppressed worms at the electron microscope level could provide evidence to this effect.
We observed a stunting of Bm-cpl-1 suppressed L3 growth compared to control worms, a phenotype previously unreported either after chemical inhibition of cysteine proteases or gene suppression in other parasite stages. Normally at the end of the L2 stage parasites are 750–795 µm long and increase in length to approximately 1350 µm at the L3 stage within four d [38]. Our control L3, taken from mosquitoes injected with saline, had a mean length of 1347 µm corresponding closely with the published data. The mean length of Bm-cpl-1 suppressed L3, however, was significantly shorter (700 µm). Suppression of this gene at the L2/L3 interface (seven dpi) arrests parasite growth and the L3 worms remain L2-sized within the mosquito. One explanation for this observation is that Bm-cpl-1 suppression at the L2/L3 interface is inhibiting the L2 to L3 molt, L2 cuticle ecdysis is not successful and therefore the worms are constrained within it, unable to increase their length. Alternatively, the stunting may not be due to aberrant molting but rather an inhibition of normal CPL-regulated development or cellular remodeling post-molt as is seen in other nematodes [46]. RNAi suppression of cpl-1 in C. elegans L3 by soaking produced significantly shorter and thinner adults [46] and the localization of cpl-1 to the hypodermis in C. elegans, O. volvulus and B. malayi is consistent with a developmental role in nematodes [18], [34], [46]. Further, germline suppression of cpl-1 in C. elegans by dsRNA injection generated an embryonic lethal phenotype but some embryos did progress to the L1 stage and those had incomplete gut development [46]. A repeatedly observed phenotype in our Bm-cpl-1 suppressed L3 was a compromised gut that appeared fenestrated and poorly developed.
Finally, Bm-cpl-1 suppression reduced normal L3 motility by up to 69%, increased atypical postural phenotypes including caudal paralysis, kinked appearance and reduced normal convolution at the head and tail of B. malayi L3 as compared to control worms. These behaviors made it impossible for the treated L3 to progress through to the culmination of development in the mosquito host, i.e., transfer to the definitive host. The dystaxic behaviors produced by the suppression of Bm-cpl-1 suggest this gene has some role, directly or indirectly, in the neuromuscular activity of B. malayi L3 in the mosquito. It is certainly true that cathepsins are required for normal neuromuscular behavior in other helminths; suppression of a cathepsin L-like gene in the flatworm Fasciola hepatica generated several aberrant motile phenotypes including paralysis [47].
This study is the first to use the host as a delivery mechanism for animal parasitic nematode RNAi. The model of using the host as a delivery mechanism for RNAi has been established but has been restricted to plant pathology where the concept has an applied use with transgenic plants helping to control nematode infestation by RNAi mechanisms in planta [48]–[50]. An alluring corollary is that by generating transgenic mosquitoes capable of suppressing key nematode genes in vivo we may be able to abolish parasite transmission. We have already demonstrated here that Bm-cpl-1 suppression in vivo prevents parasites migrating to the mosquito head and proboscis thus eliminating transmission potential. Transformation of a mosquito with an inverted-repeat (IR) transgene derived from Bm-cpl-1 may result in endogenous transcription of a hairpin dsRNA, a trigger that conceptually would induce RNAi in vivo as described here and produce a mosquito incapable of transmitting lymphatic filariasis-causing worms. Methods to introduce transgenes into mosquito germlines are well established [51]–[55] and proof of this principle has already been demonstrated for a mosquito-borne virus; transgenic lines of Dengue virus-resistant mosquitoes were generated using a Dengue virus IR transgene driven by the carboxypeptidase A promoter, reducing virus transmission by an RNAi mechanism [56]. The viability of this approach is enhanced not only by the ability to transform important vector species but also by the identification of tissue-specific promoters to drive transgene expression in favorable tissues, for example, act88F [57] is a fly-specific promoter that drives gene expression in the flight musculature – the precise site of parasite development. Another positive impact this protocol may have on lymphatic filariasis control is as a means of better understanding the biology of current putative drug targets and generating new data that may validate proposed novel drug targets. This protocol introduces the ability to investigate mosquito-borne parasite life stages, allowing the critical examination of gene function in worms growing and developing in an optimum environment. This makes it possible to assay genes that encode known or proposed drug targets in a parasite within its native intermediate host, contextualizing the null phenotypes in vivo and accurately determining the consequences of target gene suppression producing a more valuable target validation. As an illustration, nematode cathepsins have been proposed as attractive novel drug targets [58] and we have further validated these drug targets in vivo, revealing new phenotypes, defining new biological roles and showing that B. malayi sans Bm-cpl-1 are incapable of completing their life cycle. These data enhance the appeal of cathepsins as novel anthelmintic drug targets. Beyond cathepsins, this technique will have most utility in the investigation of known and potential antifilarial drug target genes expressed in both the mosquito-borne life stages and those life stages that are vulnerable to chemotherapeutic intervention.
In summary, we have developed an innovative RNAi protocol using B. malayi that differs conceptually from present RNAi protocols in that parasite gene expression is suppressed within the mosquito intermediate host. Using this protocol we suppressed a B. malayi gene in vivo, eliciting aberrant developmental and motility phenotypes in the parasite – phenotypes that eliminate transmission potential. In contrast to present RNAi methods, we have found the protocol to be reliable and effective, providing a major advancement in our capability to better understand filarial nematode gene function to the benefit of human health.
Materials and Methods
Mosquito maintenance and injection protocol
Aedes aegypti (Liverpool strain), previously selected for susceptibility to filarial parasites [59], were maintained in a contained environment at a constant temperature of 25°C, 80% relative humidity and a 14 h light to 10 h dark photoperiod. The mosquitoes were fed a diet of 0.3 M sucrose. Throughout the study mosquitoes to be injected were anaesthetized on ice and immobilized on a vacuum saddle before being microinjected intrathoracically at the base of the head using a pulled borosilicate glass pipette attached to a manual syringe for injection by volume displacement. A maximum volume of 0.5 µL can be injected using this approach with a high mosquito survival rate (>95%).
Establishing Brugia infection
B. malayi microfilaria (mf) infected cat blood was obtained from the University of Georgia NIH/NIAID Filariasis Research Reagent Resource Center. To establish a consistent and repeatable parasitemia, mf were first purified using a filtration protocol [60]. Blood containing the parasites was diluted with phosphate buffered saline (1∶5 ratio, blood:PBS) then syringe filtered through a 0.45 µm Millipore filter. Captured mf were washed three to five times with PBS then a further three to five times with Aedes physiologic saline [61] before centrifugation at 6,800× g for five min. Supernatant was removed and the pelleted mf resuspended in fresh Aedes saline to a concentration of 40 worms per µL. To inoculate mosquitoes, 20 mf were injected as described. Microdissection of the mosquitoes throughout a 14 dpi period confirmed this method established a controlled infection that progressed in a predictable and consistent manner. We also tried a blood feeding approach to establish infection but this produced an inconsistent worm burden that is too variable to reliably assess subsequent gene suppression experiments.
siRNA and dsRNA generation and injection
Short interfering RNAs (siRNA) targeting a B. malayi cathepsin L-like gene (Bm-cpl-1 AF331035 [34]) were generated commercially (Qiagen, CA) and modified with a 3′-Cy3 fluorophore on the sense strand. The location of each siRNA was optimized using a proprietary algorithm and the sequence of each siRNA is as follows: BmCL1-1, AAGGCTTAGTTTCTTATACAA; BmCL1-2, CCGAATGGAAAGATTATGTAA; BmCL1-3, CAGAAGTGCATTGAAGGAATA; and BmCL1-4, CCGGTATTTACTCCAGTAATA. Equimolar amounts of each siRNA were combined and this mix was used for injection and gene suppression experiments. dsRNA duplexes were generated in-house using a T7 transcription-based approach. A 410 base pair transcription template was polymerase chain reaction (PCR) amplified from a B. malayi L3 stage cDNA library (kindly provided by Dr. S. Williams, Smith College, MA) using gene specific oligonucleotides designed to incorporate a T7 promoter sequence (TAATACGACTCACTATAGGGTACT) at both the 5′ and 3′ ends of the amplicon. For the Bm-cpl-1 template, oligonucleotide sequence was: L1T7dsRNAF 5′ TAATACGACTCACTATAGGGTACTACGGTTACCAAATTC 3′ and L1T7dsRNAR 5′ TAATACGACTCACTATAGGGTACTCGACAACAACAGGTC 3′. The location of this transcription template was carefully chosen so as to exclude the pro region of Bm-cpl-1, a domain with high sequence homology to other cathepsin L family genes, and consequently increase the specificity of this dsRNA duplex. Transcription templates were gel purified and dsRNA duplexes synthesized using the MEGAscript RNAi Kit (Ambion, TX) according to manufacturer's protocols. dsRNA species were quantified with a NanoVue spectrophotometer (GE Healthcare, NJ) prior to use. The timing of siRNA or dsRNA injection into B. malayi-infected mosquitoes coincided with the presence of the parasite stage of interest: to target second larval stage (L2) parasites siRNA or dsRNA were injected five to eight dpi; to target third larval stage (L3) parasites siRNA or dsRNA were injected nine to 12 dpi (and for the lifespan of mosquito) [35]. The mosquitoes were processed to confirm suppression of the target gene, as described below, 48 h post-injection of siRNA or dsRNA.
Relative quantitative RT-PCR
Brugia infected, RNA-treated and control mosquitoes were cold-anesthetized on ice. Total RNA was extracted from individual mosquitoes using RNAqueous Kit (Ambion, TX) before DNase treatment using the TURBO DNA-free Kit (Ambion, TX) in thin-walled PCR tubes. The RNA was stabilized with RNase Out Inhibitor (Invitrogen, CA) and stored in RNase-free microcentrifuge tubes at 4°C. This RNA was used as a template for a relative semi-quantitative multiplex RT-PCR using the SuperScript III One-Step RT-PCR System with Platinum Taq DNA Polymerase (Invitrogen, CA). The principle of this reaction is to amplify a target gene of interest and compare its intensity with a multiplexed and normalized internal standard during the linear phase of product amplification. A putative neuropeptide encoding gene, Bm-flp-14 (Accession number AI508026) served this role. This gene was chosen as we had previously determined its stable transcript production during the B. malayi L3 stage by PCR (C. Song, unpublished). The oligonucleotide primers used to amplify Bm-cpl-1 were: CPL-1 F 5′ ACAGGGCAATATGACGAGAC 3′ and CPL-1 R 5′ ATCGAAGCAACGTGGCACAT 3′. These primer locations flank the region of Bm-cpl-1 homologous to the dsRNA construct. The oligonucleotide primers used to amplify the Bm-flp-14 internal standard were: FLP-14 F 5′ CTCGTCCACTCTTATCACTG 3′ and FLP-14 R 5′ ACCGCAATGATATACAACATATA 3′. The profile for this PCR was: cDNA synthesis at 50°C for 30 minutes; an initial denaturation phase of 94°C for 2 min; 38 cycles of 94°C for 30 s, 60°C for 30 s, 68°C for 1 min and a final extension phase of 68°C for 5 min. Reactions were visualized on a 1.2% agarose gel containing ethidium bromide.
Quantitative RT-PCR
Total RNA was extracted from individual mosquitoes and DNase-treated as described above for three replicated RNAi experiments and before addition of RNase Out Inhibitor and storage, each RNA sample was quantified spectrophometrically per a previous report [62]. This RNA served as a template in our RT-qPCR assays using the qScript One-Step Fast RT-PCR Kit with ROX (Quanta BioSciences, MD).
Establishing PREXCEL-Q parameters
PREXCEL-Q, a qPCR assay development and project management software, was used to establish our RT-qPCR parameters and to determine valid working ranges for all of our samples per target and reference genes. A mixture of the RNA samples was used to determine the optimum template to use while avoiding RT-qPCR inhibition for each of the three targets at concentration ranges of between 0.01 and 0.08 ng/µL for the siRNA experiments and between 0.02 to 0.14 ng/µL for the dsRNA experiments. For subsequent quantitative assessment of transcript abundance, each RNA sample was diluted to 0.06 ng/µL for the siRNA RT-qPCR study and 0.11 ng/µL for the dsRNA RT-qPCR study, with 6 µL of sample used per 25 µL reaction.
Primers and probes
The target sequence under evaluation in the RT-qPCR study was the B. malayi cathepsin L-like transcript previously described. Two reference genes were used, a neuropeptide-encoding gene Bm-flp-14 also previously described, and Bm-tph-1 (Accession number U80971), a tumor protein homolog-encoding gene that is a proven reference gene for qPCR of Brugia development in mosquitoes [33]. TaqMan minor groove binding (MGB) probes were used in this study to facilitate the use of shorter gene specific primer-probe sets. All probes and primers were designed using Primer Express v. 2.0 software (Applied Biosystems, CA) and synthesized by Applied Biosystems. The primer and probe sequences used are shown in Table 2.
Tab. 2. Primer and TaqMan probe sequences for RT-qPCR experiment. 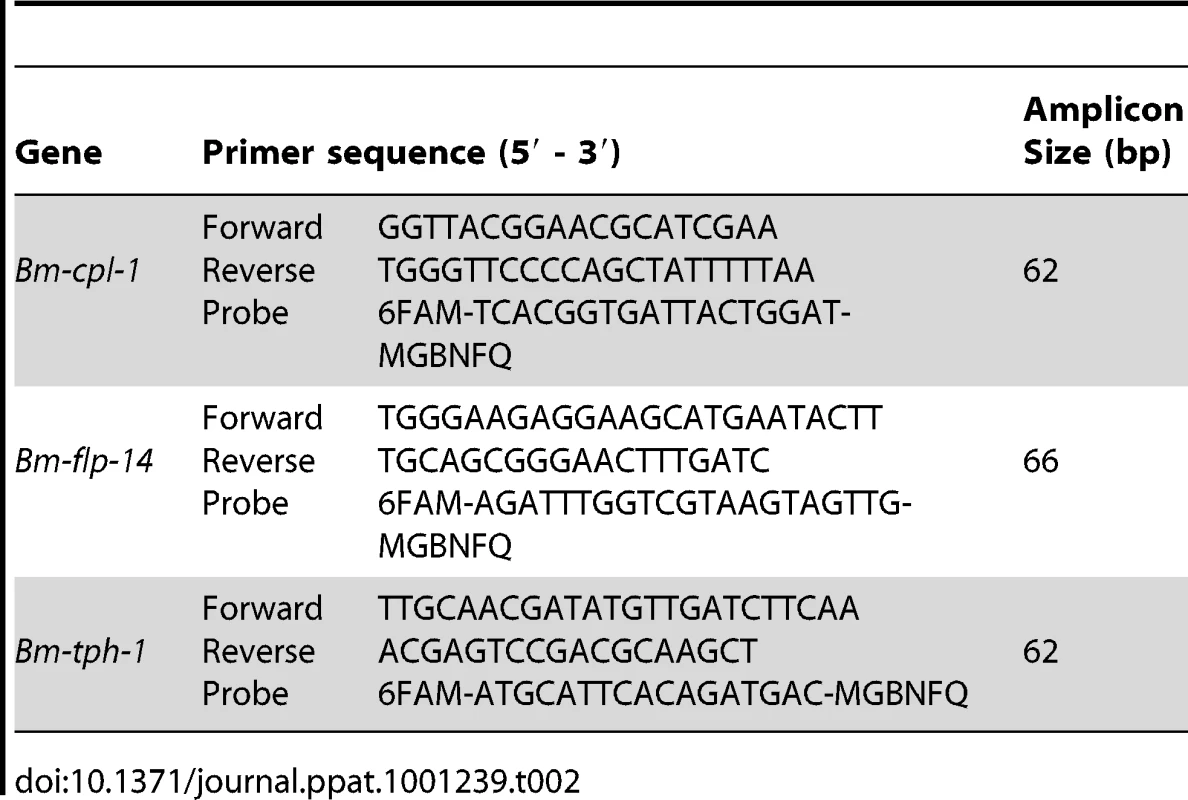
TaqMan RT-qPCR
25 µL volume reactions were prepared in duplicate for each RNA sample, and 20 µL of this reaction mixture is applied per well on a 96-well plate (using white-well reaction plates, Eppendorf, NY). Individual components of each RT-qPCR reaction were as follows: 6 µL prediluted RNA (as determined by PREXCEL-Q), 6.25 µL 4X One-step Fast Master Mix with ROX, 1.25 µL qScript One-Step Fast RT, 775 nM each primer, 150 nM probe, nuclease-free water to 25 µL. Cycling conditions included an initial cDNA synthesis step of 50°C for 5 min followed by an RT denaturation/Taq activation phase of 95°C for 30 s then 45 cycles of 95°C for 3 s and 58°C for 30 s. Four point standard curves were created for each target (within the ng/µL ranges already specified above) by diluting the RNA sample mixture in each case according to precise, PREXCEL-Q-determined parameters (eight-fold dilution from highest to lowest concentration). No-template control reactions substituted nuclease-free water for RNA, and thermocycling was performed on an ABI GeneAmp 5700 SDS (Applied Biosystems). Quantification cycle (Cq) values were obtained at an appropriate threshold per each target (∼0.1 DRn in all cases), and data were processed using custom Excel files by the efficiency-corrected (EΔΔCq) relative quantification method [32].
Phenotype analysis
After confirmation of Bm-cpl-1 suppression, multiple assays were performed to describe worm phenotypes. Each phenotypic assay was performed 14 dpi and at either four or seven d post-injection. Mosquitoes were cold-anesthetized then the wings and legs removed and discarded using a dissecting microscope. The head, thorax and abdomen were partitioned and further dissected to release the parasites. The following characteristics of dissected parasites were observed: (1) Parasite location. In order to be successfully transmitted, these parasites have to actively migrate to the head of the mosquito and vigorously writhe free of the proboscis. Parasite migration through the mosquito was recorded and measured according to escape point from the mosquito body (abdomen, thorax or head). (2) Worm motility. A scoring schema of: one (immobile), two (compromised motility, immobile for stretches of time), three (sluggish, partial movement), four (in motion, some straight segments), or five (all parts of the worm in constant motion) was used to quantify parasite movement in a blind fashion by an independent evaluator. Additional observations of aberrant motility included knotting at one or both ends, paralysis of caudal region and presence of a distinct angular kink were also recorded. (3) Parasite growth and development. Digital images of RNAi and control worms were captured so that length and diameter could be calculated using NIS Elements D 2.30 software (Nikon, NY). (4) Parasite viability. The number of parasites that survived to the infectious stage was recorded so that infection prevalence and mean intensity could be calculated. (5) Mosquito viability. We documented the number of mosquitoes that survived through the development of parasites to the infectious stage because these parasites inflict significant pathology and decrease mosquito survival.
Microscopy
Nikon Eclipse 50i fluorescence microscope under UV light (EXFO, ON) equipped with a Hy-Q FITC filter set (Chroma, VT). Images were captured using a Digital Sight DS-2Mv camera and NIS Elements D 2.3 software (Nikon, NY).
Statistical analysis
t-tests were used to analyze the effect of RNAi treatment on gene expression in the RT-qPCR experiments and parasite size, and ANOVA to analyze the effect of RNAi treatment on worm motility based on our one through five blind-scoring schema. Chi square tests were used to analyze the effect of RNAi treatment on all other worm and mosquito behaviors assayed. In all tests, P values ≤0.05 were considered statistically significant.
Zdroje
1. World Health Organization 2009 Global programme to eliminate lymphatic filariasis. Weekly epidemiological record 42 84 437 444
2. OttesenEA
HooperPJ
BradleyM
BiswasG
2008 The global programme to eliminate lymphatic filariasis: health impact after 8 years. PLoS Negl Trop Dis 2 10 e317
3. BennettJL
WilliamsJF
DaveV
1988 Pharmacology of ivermectin. Parasitol Today 4 8 226 228
4. PaxRA
WilliamsJF
GuderianRH
1988 In vitro motility of isolated adults and segments of Onchocerca volvulus, Brugia pahangi and Acanthocheilonema viteae. Trop Med Parasitol 39 Suppl 4 450 455
5. NoroesJ
DreyerG
SantosA
MendesVG
MedeirosZ
1997 Assessment of the efficacy of diethylcarbamazine on adult Wuchereria bancrofti in vivo. Trans R Soc Trop Med Hyg 91 1 78 81
6. BartzS
JacksonAL
2005 How will RNAi facilitate drug development? Sci STKE 2005 295 pe39
7. GearyTG
WooK
McCarthyJS
MackenzieCD
HortonJ
2010 Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int J Parasitol 40 1 1 13
8. BlakeR
2007 Target validation in drug discovery.
TaylorD
HaskinsJ
GuilianoK
Methods in Molecular Biology: High Content Screening: Humana Press 367 377
9. GeldhofP
VisserA
ClarkD
SaundersG
BrittonC
2007 RNA interference in parasitic helminths: current situation, potential pitfalls and future prospects. Parasitology 134 Pt 5 609 619
10. HusseinAS
KicheninK
SelkirkME
2002 Suppression of secreted acetylcholinesterase expression in Nippostrongylus brasiliensis by RNA interference. Mol Biochem Parasitol 122 1 91 94
11. IslamMK
MiyoshiT
YamadaM
TsujiN
2005 Pyrophosphatase of the roundworm Ascaris suum plays an essential role in the worm's molting and development. Infect Immun 73 4 1995 2004
12. IssaZ
GrantWN
StasiukS
ShoemakerCB
2005 Development of methods for RNA interference in the sheep gastrointestinal parasite, Trichostrongylus colubriformis. Int J Parasitol 35 9 935 940
13. VisserA
GeldhofP
de MaereV
KnoxDP
VercruysseJ
2006 Efficacy and specificity of RNA interference in larval life-stages of Ostertagia ostertagi. Parasitology 133 Pt 6 777 783
14. GeldhofP
MurrayL
CouthierA
GilleardJS
McLauchlanG
2006 Testing the efficacy of RNA interference in Haemonchus contortus. Int J Parasitol 36 7 801 810
15. KotzeAC
BagnallNH
2006 RNA interference in Haemonchus contortus: suppression of beta-tubulin gene expression in L3, L4 and adult worms in vitro. Mol Biochem Parasitol 145 1 101 110
16. AboobakerAA
BlaxterML
2003 Use of RNA interference to investigate gene function in the human filarial nematode parasite Brugia malayi. Mol Biochem Parasitol 129 1 41 51
17. FordL
ZhangJ
LiuJ
HashmiS
FuhrmanJA
2009 Functional analysis of the cathepsin-like cysteine protease genes in adult Brugia malayi using RNA interference. PLoS Negl Trop Dis 3 2 e377
18. LustigmanS
ZhangJ
LiuJ
OksovY
HashmiS
2004 RNA interference targeting cathepsin L and Z-like cysteine proteases of Onchocerca volvulus confirmed their essential function during L3 molting. Mol Biochem Parasitol 138 2 165 170
19. FordL
GuilianoDB
OksovY
DebnathAK
LiuJ
2005 Characterization of a novel filarial serine protease inhibitor, Ov-SPI-1, from Onchocerca volvulus, with potential multifunctional roles during development of the parasite. J Biol Chem 280 49 40845 40856
20. PfarrK
HeiderU
HoeraufA
2006 RNAi mediated silencing of actin expression in adult Litomosoides sigmodontis is specific, persistent and results in a phenotype. Int J Parasitol 36 6 661 669
21. FireA
XuS
MontgomeryMK
KostasSA
DriverSE
1998 Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391 6669 806 811
22. FraserAG
KamathRS
ZipperlenP
Martinez-CamposM
SohrmannM
2000 Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408 6810 325 330
23. KamathRS
Martinez-CamposM
ZipperlenP
FraserAG
AhringerJ
2001 Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol 2 1 RESEARCH0002
24. UrwinPE
LilleyCJ
AtkinsonHJ
2002 Ingestion of double-stranded RNA by preparasitic juvenile cyst nematodes leads to RNA interference. Mol Plant Microbe Interact 15 8 747 752
25. BakhetiaM
CharltonW
AtkinsonHJ
McPhersonMJ
2005 RNA interference of dual oxidase in the plant nematode Meloidogyne incognita. Mol Plant Microbe Interact 18 10 1099 1106
26. KimberMJ
McKinneyS
McMasterS
DayTA
FlemingCC
2007 flp gene disruption in a parasitic nematode reveals motor dysfunction and unusual neuronal sensitivity to RNA interference. Faseb J 21 4 1233 1243
27. ShinglesJ
LilleyCJ
AtkinsonHJ
UrwinPE
2007 Meloidogyne incognita: molecular and biochemical characterisation of a cathepsin L cysteine proteinase and the effect on parasitism following RNAi. Exp Parasitol 115 2 114 120
28. DalzellJJ
McMasterS
FlemingCC
MauleAG
2010 Short interfering RNA-mediated gene silencing in Globodera pallida and Meloidogyne incognita infective stage juveniles. Int J Parasitol 40 1 91 100
29. VineyME
ThompsonFJ
2008 Two hypotheses to explain why RNA interference does not work in animal parasitic nematodes. Int J Parasitol 38 1 43 47
30. LendnerM
DoligalskaM
LuciusR
HartmannS
2008 Attempts to establish RNA interference in the parasitic nematode Heligmosomoides polygyrus. Mol Biochem Parasitol 161 1 21 31
31. BoissonB
CJaquesJ
ChoumetV
MartinE
ZXuJ
2006 Gene silencing in mosquito salivary glands by RNAi. FEBS Letters 580 1988 1992
32. PfafflMW
2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29 9 e45
33. LaneySJ
ButtaroCJ
ViscontiS
PilotteN
RamzyRM
2008 A reverse transcriptase-PCR assay for detecting filarial infective larvae in mosquitoes. PLoS Negl Trop Dis 2 6 e251
34. GuilianoDB
HongX
McKerrowJH
BlaxterML
OksovY
2004 A gene family of cathepsin L-like proteases of filarial nematodes are associated with larval molting and cuticle and eggshell remodeling. Mol Biochem Parasitol 136 2 227 242
35. EricksonSM
XiZ
MayhewGF
RamirezJL
AliotaMT
2009 Mosquito infection responses to developing filarial worms. PLoS Negl Trop Dis 3 10 e529
36. DalzellJJ
McMasterS
JohnstonMJ
KerrR
FlemingCC
2009 Non-nematode-derived double-stranded RNAs induce profound phenotypic changes in Meloidogyne incognita and Globodera pallida infective juveniles. Int J Parasitol 39 13 1503 1516
37. ChristensenBM
SutherlandDR
1984 Brugia pahangi: Exsheathment and midgut penetration in Aedes aegypti. Transactions of the American Microscopical Society 103 4 423 433
38. MurthyPK
SenAB
1981 Sequential development changes in microfilariae of subperiodic Brugia malayi to infective larvae in susceptible strain of Aedes aegypti (Macdonald). J Commun Dis 13 2 102 109
39. BartholomayLC
ChristensenBM
2002 Vector-parasite interactions in mosquito-borne filariasis.
KleiT
RajanT
The Filaria. 1 ed Boston Kluwer Academic Publishers 9 19
40. BeckettEB
BoothroydB
1970 Mode of nutrition of the larvae of the filarial nematode Brugia pahangi. Parasitol 60 21 26
41. AliotaMT
FuchsJF
RocheleauTA
ClarkAK
HillyerJF
2010 Mosquito transcriptome profiles and filarial worm susceptibility in Armigeres subalbatus. PLoS Negl Trop Dis 4 4 e666
42. TimmonsL
CourtDL
FireA
2001 Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263 1–2 103 112
43. ShannonAJ
TysonT
DixI
BoydJ
BurnellAM
2008 Systemic RNAi mediated gene silencing in the anhydrobiotic nematode Panagrolaimus superbus. BMC Mol Biol 9 58
44. RicherJK
HuntWG
SakanariJA
GrieveRB
1993 Dirofilaria immitis: effect of fluoromethyl ketone cysteine protease inhibitors on the third - to fourth-stage molt. Exp Parasitol 76 3 221 231
45. LustigmanS
McKerrowJH
ShahK
LuiJ
HuimaT
1996 Cloning of a cysteine protease required for the molting of Onchocerca volvulus third stage larvae. J Biol Chem 271 47 30181 30189
46. HashmiS
BrittonC
LiuJ
GuilianoDB
OksovY
2002 Cathepsin L is essential for embryogenesis and development of Caenorhabditis elegans. J Biol Chem 277 5 3477 3486
47. McGonigleL
MousleyA
MarksNJ
BrennanGP
DaltonJP
2008 The silencing of cysteine proteases in Fasciola hepatica newly excysted juveniles using RNA interference reduces gut penetration. Int J Parasitol 38 2 149 155
48. HuangG
AllenR
DavisEL
BaumTJ
HusseyRS
2006 Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc Natl Acad Sci U S A 103 39 14302 14306
49. SteevesRM
ToddTC
EssigJS
TrickHN
2006 Transgenic soybeans expressing siRNAs specific to a major sperm protein gene suppress Heterodera glycines reproduction. Funct Plant Biol 33 991 999
50. SindhuAS
MaierTR
MitchumMG
HusseyRS
DavisEL
2009 Effective and specific in planta RNAi in cyst nematodes: expression interference of four parasitism genes reduces parasitic success. J Exp Bot 60 1 315 324
51. CoatesCJ
JasinskieneN
MiyashiroL
JamesAA
1998 Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci U S A 95 7 3748 3751
52. JasinskieneN
CoatesCJ
BenedictMQ
CornelAJ
RaffertyCS
1998 Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. PNAS 95 7 3743 3747
53. CatterucciaF
NolanT
LoukerisTG
BlassC
SavakisC
2000 Stable germline transformation of the malaria mosquito Anopheles stephensi. Nature 405 959 962
54. KokozaV
AhmedA
ChoWL
JasinskieneN
JamesAA
2000 Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci U S A 97 16 9144 9149
55. MoreiraLA
EdwardsMJ
AdhamiF
JasinskieneN
JamesAA
2000 Robust gut-specific gene expression in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A 97 20 10895 10898
56. FranzAW
Sanchez-VargasI
AdelmanZN
BlairCD
BeatyBJ
2006 Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc Natl Acad Sci U S A 103 11 4198 4203
57. AllenML
ChristensenBM
2004 Flight muscle-specific expression of act88F: GFP in transgenic Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Int 53 307 314
58. McKerrowJH
1999 Development of cysteine protease inhibitors as chemotherapy for parasitic diseases: insights on safety, target validation, and mechanism of action. Int J Parasitol 29 6 833 837
59. MacDonaldWW
1962 The selection of a strain of Aedes aegypti susceptible to infection with semi-periodic Brugia malayi. Ann Trop Med Parasit 56 368 372
60. BeerntsenBT
BartholomayLC
LoweryRJ
2007 Penetration of the mosquito midgut is not required for Brugia pahangi microfilariae to avoid the melanotic encapsulation response of Armigeres subalbatus. Vet Parasitol 144 3-4 371 374
61. HayesRO
1953 Determination of a physiological saline solution for Aedes aegypti (L.). Journal of Economic Entomology 46 4 624 627
62. GallupJM
SowFB
Van GeelenA
AckermannMR
2009 SPUD qPCR Assay Confirms PREXCEL-Q Softwares Ability to Avoid qPCR Inhibition. Curr Issues Mol Biol 12 3 129 134
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
-
Všechny články tohoto čísla
- Is Adherence to Erythrocytes a Factor in Extrapulmonary Dissemination?
- Identifying the Age Cohort Responsible for Transmission in a Natural Outbreak of
- Blockade of Immunosuppressive Cytokines Restores NK Cell Antiviral Function in Chronic Hepatitis B Virus Infection
- Metaeffector Exploits Host Proteasome to Temporally Regulate Cognate Effector
- The p53-Target Gene Drives Neutrophil-Mediated Protection against Lethal Bacterial Sepsis
- Development of an RNAi Protocol to Investigate Gene Function in the Filarial Nematode,
- Lysine Acetyltransferase GCN5-A Functions in the Cellular Response to Alkaline Stress and Expression of Cyst Genes
- Structural Basis for Apoptosis Inhibition by Epstein-Barr Virus BHRF1
- Molecular Architectures of Trimeric SIV and HIV-1 Envelope Glycoproteins on Intact Viruses: Strain-Dependent Variation in Quaternary Structure
- Interaction of c-Cbl with Myosin IIA Regulates Bleb Associated Macropinocytosis of Kaposi's Sarcoma-Associated Herpesvirus
- Glacial Refugia in Pathogens: European Genetic Structure of Anther Smut Pathogens on and
- Role for Sumoylation in Systemic Inflammation and Immune Homeostasis in Larvae
- Inflammasome Sensor Nlrp1b-Dependent Resistance to Anthrax Is Mediated by Caspase-1, IL-1 Signaling and Neutrophil Recruitment
- Noise Cancellation: Viral Fine Tuning of the Cellular Environment for Its Own Genome Replication
- Infectious Speciation Revisited: Impact of Symbiont-Depletion on Female Fitness and Mating Behavior of
- Eis Regulates Autophagy, Inflammation, and Cell Death through Redox-dependent Signaling
- NleC, a Type III Secretion Protease, Compromises NF-κB Activation by Targeting p65/RelA
- Early Myeloid Dendritic Cell Dysregulation is Predictive of Disease Progression in Simian Immunodeficiency Virus Infection
- HIV Capsid is a Tractable Target for Small Molecule Therapeutic Intervention
- Structural and Functional Studies of Nonstructural Protein 2 of the Hepatitis C Virus Reveal Its Key Role as Organizer of Virion Assembly
- Coming of Age—Sexual Reproduction in Species
- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Compartmentation of Redox Metabolism in Malaria Parasites
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Rapid End-Point Quantitation of Prion Seeding Activity with Sensitivity Comparable to Bioassays
- Dimeric 2G12 as a Potent Protection against HIV-1
- Hypoxia Induces an Immunodominant Target of Tuberculosis Specific T Cells Absent from Common BCG Vaccines
- CD4 Natural Regulatory T Cells Prevent Experimental Cerebral Malaria via CTLA-4 When Expanded In Vivo
- The Killing of African Trypanosomes by Ethidium Bromide
- H2A.Z Demarcates Intergenic Regions of the Epigenome That Are Dynamically Marked by H3K9ac and H3K4me3
- Large-Scale Field Application of RNAi Technology Reducing Israeli Acute Paralysis Virus Disease in Honey Bees (, Hymenoptera: Apidae)
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Coming of Age—Sexual Reproduction in Species
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Compartmentation of Redox Metabolism in Malaria Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



