-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEarly Myeloid Dendritic Cell Dysregulation is Predictive of Disease Progression in Simian Immunodeficiency Virus Infection
Myeloid dendritic cells (mDC) are lost from blood in individuals with human immunodeficiency virus (HIV) infection but the mechanism for this loss and its relationship to disease progression are not known. We studied the mDC response in blood and lymph nodes of simian immunodeficiency virus (SIV)-infected rhesus macaques with different disease outcomes. Early changes in blood mDC number were inversely correlated with virus load and reflective of eventual disease outcome, as animals with stable infection that remained disease-free for more than one year had average increases in blood mDC of 200% over preinfection levels at virus set-point, whereas animals that progressed rapidly to AIDS had significant loss of mDC at this time. Short term antiretroviral therapy (ART) transiently reversed mDC loss in progressor animals, whereas discontinuation of ART resulted in a 3.5-fold increase in mDC over preinfection levels only in stable animals, approaching 10-fold in some cases. Progressive SIV infection was associated with increased CCR7 expression on blood mDC and an 8-fold increase in expression of CCL19 mRNA in lymph nodes, consistent with increased mDC recruitment. Paradoxically, lymph node mDC did not accumulate in progressive infection but rather died from caspase-8-dependent apoptosis that was reduced by ART, indicating that increased recruitment is offset by increased death. Lymph node mDC from both stable and progressor animals remained responsive to exogenous stimulation with a TLR7/8 agonist. These data suggest that mDC are mobilized in SIV infection but that an increase in the CCR7-CCL19 chemokine axis associated with high virus burden in progressive infection promotes exodus of activated mDC from blood into lymph nodes where they die from apoptosis. We suggest that inflamed lymph nodes serve as a sink for mDC through recruitment, activation and death that contributes to AIDS pathogenesis.
Published in the journal: . PLoS Pathog 6(12): e32767. doi:10.1371/journal.ppat.1001235
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001235Summary
Myeloid dendritic cells (mDC) are lost from blood in individuals with human immunodeficiency virus (HIV) infection but the mechanism for this loss and its relationship to disease progression are not known. We studied the mDC response in blood and lymph nodes of simian immunodeficiency virus (SIV)-infected rhesus macaques with different disease outcomes. Early changes in blood mDC number were inversely correlated with virus load and reflective of eventual disease outcome, as animals with stable infection that remained disease-free for more than one year had average increases in blood mDC of 200% over preinfection levels at virus set-point, whereas animals that progressed rapidly to AIDS had significant loss of mDC at this time. Short term antiretroviral therapy (ART) transiently reversed mDC loss in progressor animals, whereas discontinuation of ART resulted in a 3.5-fold increase in mDC over preinfection levels only in stable animals, approaching 10-fold in some cases. Progressive SIV infection was associated with increased CCR7 expression on blood mDC and an 8-fold increase in expression of CCL19 mRNA in lymph nodes, consistent with increased mDC recruitment. Paradoxically, lymph node mDC did not accumulate in progressive infection but rather died from caspase-8-dependent apoptosis that was reduced by ART, indicating that increased recruitment is offset by increased death. Lymph node mDC from both stable and progressor animals remained responsive to exogenous stimulation with a TLR7/8 agonist. These data suggest that mDC are mobilized in SIV infection but that an increase in the CCR7-CCL19 chemokine axis associated with high virus burden in progressive infection promotes exodus of activated mDC from blood into lymph nodes where they die from apoptosis. We suggest that inflamed lymph nodes serve as a sink for mDC through recruitment, activation and death that contributes to AIDS pathogenesis.
Introduction
Myeloid dendritic cells (mDC) are professional antigen-presenting cells that are critical for the induction of acquired immune responses to pathogens [1]. Depletion of mDC from blood in human immunodeficiency virus (HIV) infection has been well described and shown to be inversely correlated with virus load and absent from long-term non-progressors, suggesting a relationship between mDC and disease control [2]–[8]. A proposed mechanism to account for mDC loss from blood is their activation and subsequent recruitment to inflamed lymph nodes [9]. Increased expression of costimulatory molecules on blood mDC indicative of activation has been reported in HIV-infected individuals [3], [5], [10], as has accumulation of mDC in peripheral lymph nodes during acute infection [11]. However, findings relating to mDC in lymph nodes during chronic HIV infection are inconsistent, with both accumulation [12], [13] and substantial loss of mDC [14] being reported. mDC are depleted from both blood and lymph nodes of simian immunodeficiency virus (SIV)-infected rhesus macaques during AIDS [15] but data are lacking from earlier stages of infection. Few studies have evaluated mDC dynamics in both blood and lymph node in the same individuals [12], [15] and no longitudinal studies of mDC kinetics in both compartments have been reported. As such, the relationship between mDC loss and recruitment in infection remains ill-defined, and whether differences in mDC dynamics predict disease outcome is not known.
The impact of antiretroviral therapy (ART) on mDC loss and recovery in HIV infection is also unclear, as several studies indicate that ART is not effective at increasing blood mDC [6], [7], [16] while others suggest that ART significantly restores blood mDC numbers [3], [8], [17], [18]. ART rapidly resolves immune activation in lymphoid tissues [19] and may have beneficial effects on lymph node mDC activation and function [13], although this has not been well characterized.
In the present study we followed the mDC response in blood and lymph nodes over time in two cohorts of SIV-infected animals that received ART and adenovirus (Ad)-based immunotherapy with different disease outcomes. We find that loss of blood mDC at virus set-point is predictive of disease progression, whereas an increase in blood mDC is predictive of long-term absence of disease, and that even relatively short periods of ART are beneficial to mDC homeostasis. In animals that progress to AIDS the early loss of mDC from blood is associated with evidence of increased CCR7-CCL19-mediated recruitment to lymph nodes and increased apoptosis within these tissues.
Results
Disease progression is independent of Ad-based immunotherapy
Animals in this study were enrolled in an immunotherapy protocol using Ad-based vectors the majority of which has been previously described [20]. Animals were infected with the pathogenic isolate SIVmac251 by intravenous inoculation and received ART consisting of a combination of two reverse transcriptase inhibitors, 9-[2-(phosphonyl-methoxy)propyl]adenine (PMPA) and 2′-deoxy-5-fluoro-3′-thia-cytidine (FTC), from weeks 12 to 24 and weeks 32 to 44, depending on survival. Immunotherapy consisted of priming with Ad serotype 5 (Ad5)-based vectors expressing SIV Gag, Env and Nef with or without IL-15 at weeks 16 and 22 followed by boosting with Ad35-based vectors expressing the same transgenes at weeks 36 and 42. Control-treated animals were given the same regimen of Ad5-ψ5 and Ad35-ψ5 vectors that lacked transgenes [20]. Ad-based immunotherapy boosted T cell responses to SIV but had no effect on virus load, progression to disease or survival [20](and data not shown). However, when analyzed independent of immunotherapy, animals in the cohort could be readily separated into two groups based on disease progression, with one group remaining healthy until elective sacrifice at a mean of 60 weeks post infection (n = 11, ‘stable’ group), and the other succumbing to AIDS with a mean survival time of 32 weeks (n = 10, ‘progressor’ group, Table 1). AIDS was defined clinically by lymphadenopathy, persistent weight loss and anorexia, with or without opportunistic infections [15]. Equal numbers of animals in the stable and progressor groups received Ad-based immunotherapy with the remainder receiving control vectors or no treatment, confirming the lack of association between immunotherapy and disease outcome (Table 1). The MHC class I molecule Mamu-A*01 was expressed by 3/11 and 0/10 animals in the stable and progressor groups, respectively, consistent with an association of this molecule with control of SIV infection (Table 1) [21].
Tab. 1. Characteristics of animal cohort. 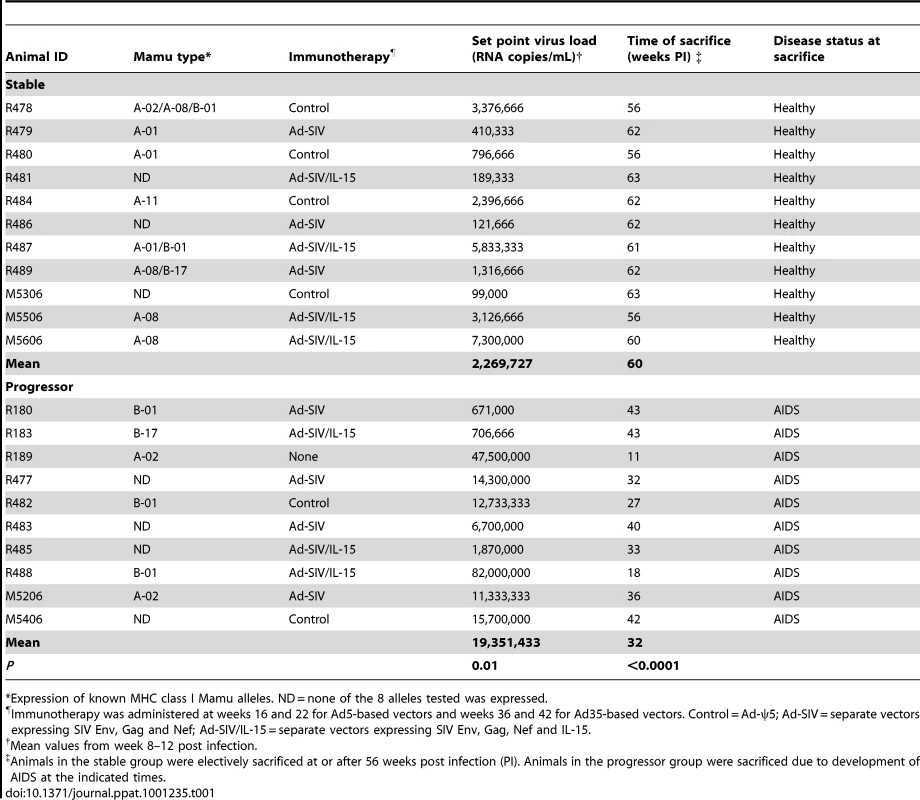
*Expression of known MHC class I Mamu alleles. ND = none of the 8 alleles tested was expressed. Peak plasma virus loads in stable and progressor animals at 2 weeks post infection were similar at around 2×107 RNA copies/ml plasma; however virus loads began to diverge by week 4 and at virus set point virus loads differed by ∼1 log between groups (Table 1 and Figure 1). Survival time was inversely correlated with virus load at set-point (Table 1 and Figure 1B), consistent with previous reports [22], [23]. ART beginning at week 12 had parallel although modest effects on virus load in both groups, with an immediate decrease of ∼1.5 logs that fluctuated over the course of therapy (Figure 1). Virus load persisted at ∼0.5 logs below set-point levels after discontinuation of ART and then decreased by ∼1.5 logs with initiation of the second cycle of ART at week 32, again with fluctuations over the course of therapy (Figure 1). Only animals in the stable group survived beyond the second cycle of ART and in these animals virus load remained at ∼1.5 logs below set-point until sacrifice (Table 1 and Figure 1). These data show that disease progression and survival in this cohort of animals correlated with virus load at set-point prior to initiation of ART and not with Ad-based immunotherapy, and that ART was effective at inducing modest but similar decreases in virus load in both groups.
Fig. 1. Relationship between virus load and disease progression in SIV infected macaques. 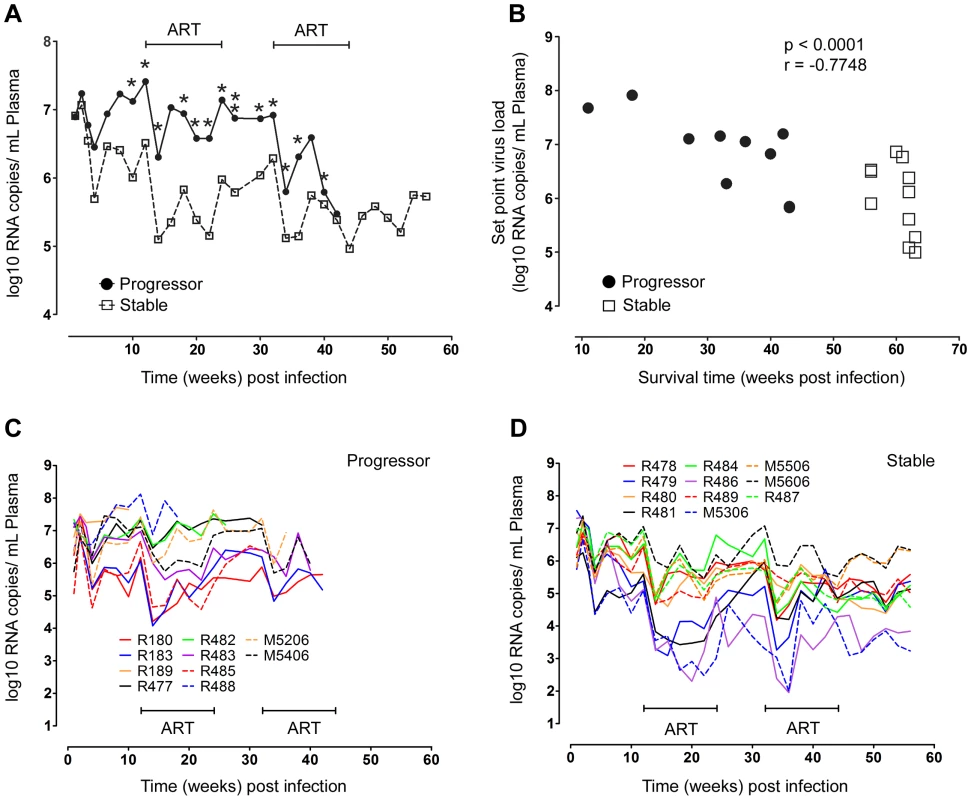
(A) Mean virus load in plasma for SIV-infected animals that progress rapidly to disease (progressor, n = 10) or remain disease free for more than one year (stable, n = 11). *P<0.05, **P<0.01 (week 26). (B) Correlation between survival time and virus load at set-point, taken as the mean virus load from weeks 8 to 12 post infection, for all animals. Data points for animals R180 and R183, which died at week 43 with very similar virus loads, appear as a single data point. (C, D) Individual virus load measurements for progressor (C) and stable animals (D). ART = intervals of antiretroviral therapy. Disease progression correlates with a divergent mDC response in SIV-infected macaques
The characteristics of this cohort allowed us to ask whether differences in eventual disease outcome were reflected in earlier changes in the mDC response and whether short-term exposure to ART was beneficial to this response. Blood mDC were identified in peripheral blood mononuclear cells (PBMC) as CD45+ lineage− HLA-DR+ CD11c+ cells (Figure 2A) and were enumerated based on the ratio of mDC to CD4+ T cells [20], [24]. Staining of blood cells with antibody to CD11c was inconsistent in animals R487 (stable group) and M5406 (progressor group) making it difficult to delineate mDC at all time points (data not shown), and as a result these animals were not studied further. The median number of blood mDC in the remaining 19 animals prior to infection was 51 cells/ul with a relatively large range from 16 to 202 cells/ul, consistent with our previous findings (Figure 2C, D) [24]. Blood mDC were reduced at 2 weeks post infection relative to baseline levels when all animals were analyzed together (P = .03), although when each group was analyzed separately this decrease was not significant. However, in the post-acute period the mDC response diverged, as mDC in progressor animals continued to decline to week 12 when they were significantly reduced in number relative to preinfection time points. In contrast, mDC in stable animals significantly increased from weeks 2 to 12 (Figure 2B–D). The relative change in the number of blood mDC in individual animals over the first 12 weeks of infection was significant, as mDC dropped to around 30% of preinfection levels in some progressor animals (mean for the group 60%) but increased to nearly 500% in some stable animals (mean for the group 206%) (Figure 2E). This change was inversely correlated with virus load at week 12 post infection, revealing a relationship between viral burden and mDC homeostasis (Figure 2E). Exposure to the first round of ART in progressor animals resulted in an increase in mDC number from weeks 12 to 20, when virus load was near its lowest point, and appeared to stabilize the number of blood mDC in the stable group (Figure 2B–D). However, after ART was discontinued at week 24 the number of mDC in stable but not progressor animals rose markedly reaching a mean increase of 3.5 fold over baseline at week 32, with individual increases approaching 10-fold in some animals (Figure 2B–D). The magnitude of the mDC response after ART was not influenced by the vaccination regimen received during ART, as a comparison of mean mDC counts for all animals from weeks 28 to 32 (the period of greatest response) based on the type of immunotherapy received revealed no statistically significant differences (data not shown, Kruskal-Wallis test, P = 0.3). Initiation of the second round of ART again reduced mDC number in the majority of stable animals, concurrent with the reduction in virus load, after which the number of mDC remained relatively constant (Figure 2B, D). The divergent mDC response contrasted with changes in CD4+ T cells, which did not statistically differ between groups at any time before or after infection (Figure 2F). These data indicate that differences in eventual disease outcome in SIV infection are reflected by differences in the blood mDC response that are apparent relatively early in infection. They also indicate that short-term ART may be effective at transiently restoring blood mDC in animals with the most severe disease.
Fig. 2. Divergent mDC response in blood correlates with virus load and disease progression. 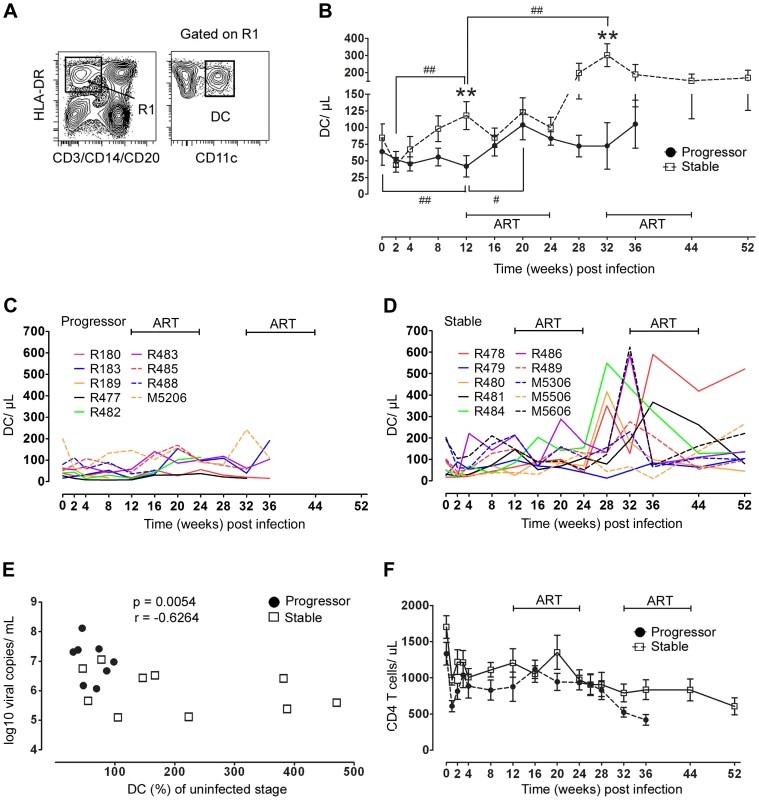
(A) Representative flow cytometry plots showing the gating strategy used to define CD11c+ mDC within the Lineage− HLA-DR+ fraction of PBMC. (B) Changes in the absolute number of mDC in blood over the course of SIV infection in animals with stable (n = 10) and progressive (n = 9) infection. Shown are mean ± SEM. **P<0.01 between groups; #P<0.05; ##P<0.01 within groups relative to week 12 post infection. (C, D) Individual mDC counts in blood for progressor animals (C, n = 9) and stable animals (D, n = 10). (E) Correlation between percent change of mDC in blood from week 0 to 12 and virus load at week 12 (n = 18, R189 died at week 11 and is not included). (F) Change in absolute number of CD4 T cells in blood over the course of SIV infection in animals with stable (n = 10) and progressive (n = 9) infection. Shown are mean ± SEM. ART = intervals of antiretroviral therapy. Blood mDC are rapidly and differentially activated in SIV infection
We next asked whether differences in disease progression were reflected in earlier differences in activation of circulating mDC in SIV-infected macaques. For these and subsequent analyses we focused on the first 32 weeks of infection incorporating one 12-week cycle of ART and one 8-week period of treatment interruption, as after this time the number of animals surviving in the progressor group rapidly diminished (Table 1). Expression of the costimulatory molecules CD80 and CD86 was markedly increased in all animals at 2 weeks post infection indicative of rapid mDC activation (Figure 3A–D). However, by 12 weeks post infection differences in mDC activation were evident between groups particularly with respect to the chemokine receptor CCR7, which was expressed by a significantly greater proportion of mDC in animals that progressed to AIDS relative to animals with stable infection (Figure 3A). A majority of CCR7+ mDC in progressor animals also expressed CD86 with a smaller proportion expressing CD80, consistent with activation (Figure 3E). The 12-week course ART was effective at reducing blood mDC activation, particularly with respect to CD80, and in animals in the stable group expression of all markers of activation returned to preinfection levels during ART (Figure 3A–C). The increase in CD80 at week 32 suggested mDC were again activated during the period of ART discontinuation, although no increase in CCR7 or CD86 expression was noted at this time (Figure 3A–C). These findings indicate that during chronic infection animals that progress to AIDS have increased blood mDC activation relative to animals with stable infection. They also confirm that ART has the beneficial effect of reducing mDC activation, consistent with findings in HIV-infected humans [3], [12].
Fig. 3. Differential activation of blood mDC in stable and progressive SIV infection. 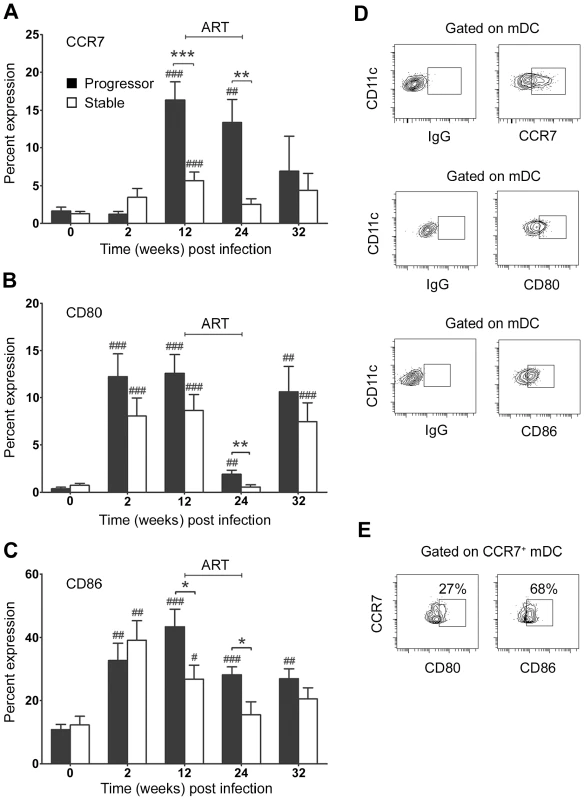
The proportion of blood mDC expressing CCR7 (A), CD80 (B) and CD86 (C) relative to staining with a control antibody before and at various times after SIV infection. Shown are mean ± SEM for naïve (n = 19), progressor (n = 8) and stable animals (n = 10). (D) Representative flow cytometry plots of the gating strategy to identify positive populations. (E) Flow cytometry plots of CCR7+ mDC from progressor animal R180 at 12 weeks post infection labeled with antibodies to CD80 and CD86. Numbers represent the percentage of cells that co-express both markers relative to staining with a control antibody. Data are representative of three experiments on separate animals. *P<0.05, **P<0.01, ***P<0.005 between groups; #P<0.05, ##P<0.01, ###P<0.005 within groups relative to week 0. ART = interval of antiretroviral therapy. mDC do not accumulate in lymph nodes despite increased tissue CCL19 expression
The finding that loss of blood mDC in progressor animals occurs as the proportion of mDC expressing CCR7 increases is consistent with excessive mDC recruitment to lymph nodes via the CCR7/CCL19/CCL21 pathway, as has been suggested by in vitro studies [9]. To examine this potential in vivo, we used flow cytometry to identify mDC in lymph node cell suspensions taken prior to infection and at intervals after infection in our two groups of animals. mDC were defined as lineage− HLA-DR+ CD11c+ cells (Figure 4A) and enumerated as a proportion of all cells in the lineage− HLA-DR+ gate, which we have previously shown to be an accurate indicator of the absolute number of mDC per unit of weight [15]. Surprisingly, we found no significant difference in the number of lymph node mDC as a result of SIV infection regardless of disease progression, indicating a lack of mDC accumulation (Figure 4B). However, the phenotype of mDC within lymph nodes was significantly different as a function of disease, as animals with stable but not progressive infection had a lower percentage of mDC expressing CCR7, CD40 and CD86 and reduced mDC expression of MHC class II at 12 weeks relative to preinfection time points, reflecting reduced mDC activation (Figure 4C, D). To address the issue of mDC recruitment further, we next used real time PCR to determine the relative expression of CCR7 ligands in lymph node tissues. We found that CCL19 but not CCL21 mRNA was increased 8-fold in lymph nodes at 12 weeks post infection, but only in animals that progressed to AIDS (Figure 4E). Together with our findings in blood, these data suggest that mDC are recruited to lymph nodes in progressive disease via an enhanced CCR7/CCL19 pathway, but that expanded mDC recruitment fails to result in mDC accumulation.
Fig. 4. Differential mDC activation and tissue chemokine expression in lymph nodes correlates with disease progression. 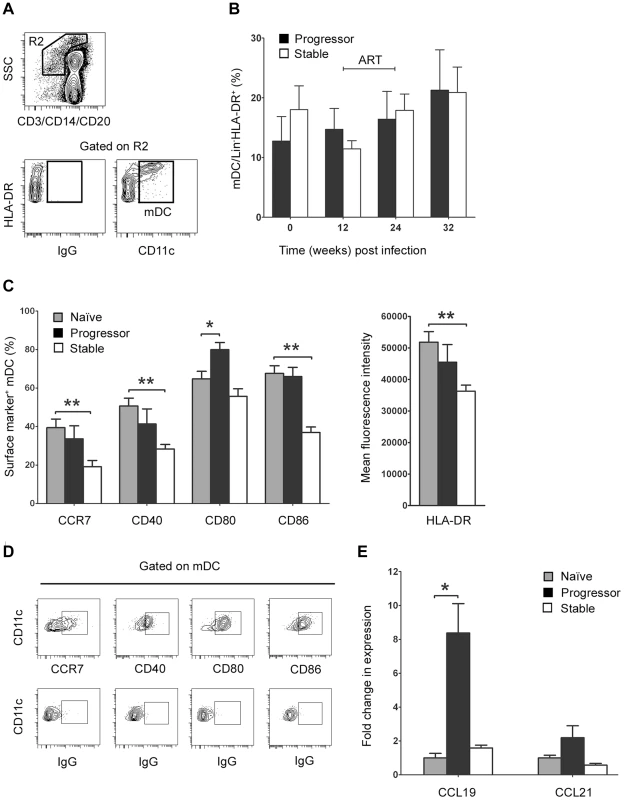
(A) Representative flow cytometry plots showing the gating strategy used to define CD11c+ mDC within the Lineage− HLA-DR+ fraction of peripheral lymph node cell suspensions. (B) The number of mDC in lymph nodes as a proportion of Lineage− HLA-DR+ cells at intervals before and after SIV infection. Shown are mean ± SEM for naïve (T = 0, n = 18), progressor (n = 7) and stable animals (n = 10). (C) The proportion of lymph node mDC expressing CCR7, CD40, CD80 and CD86 relative to staining with a control antibody before and 12 weeks after SIV infection (left) and the mean fluorescence intensity of HLA-DR expression on lymph node mDC at week 12 post infection (right). Shown are mean ± SEM for naïve (n = 8), progressor (n = 6) and stable animals (n = 6). (D) Representative flow cytometry plots showing gating strategy to identify positive cell populations. (E) Expression of CCL19 and CCL21 mRNA in lymph node cell suspensions prior to infection (naïve) and at 12 weeks post infection for animals with stable and progressive infection. mRNA expression levels were calculated using the 2−ΔCT method using [beta]-GUS as the endogenous control. The fold change in expression was calculated by normalizing to the mean 2−ΔCT of the naïve group. Shown are mean ± SEM of 4 animals in each group. *P<0.05, **P<0.01. ART = interval of antiretroviral therapy. Increased mDC apoptosis in lymph nodes during progressive SIV infection
The lack of mDC accumulation in lymph nodes despite evidence for enhanced CCR7/CCL19-mediated recruitment in progressive infection led us to suspect that lymph node mDC were dying at an increased rate in these tissues. To examine this possibility we identified live mDC in lymph node cell suspensions as being lineage− HLA-DR+ CD11c+ cells that lacked staining with a fixable dead-cell dye, and then identified cells undergoing early apoptosis within this gate using an antibody to active caspase-3 (Figure 5A). At week 12 post infection, 15% of lymph node mDC in animals that eventually progressed to AIDS were entering apoptosis, representing a 3-fold increase from preinfection levels, whereas lymph node mDC from animals with stable infection had no significant change in apoptosis (Figure 5B). ART given from week 12 to 24 post infection decreased the frequency of apoptotic mDC in progressor animals, although this did not reach statistical significance (Figure 5B). To determine whether apoptosis was mediated by extrinsic or intrinsic pathways we exposed lymph node cells from progressor animals taken at week 12 post infection to small molecule inhibitors of caspase-8 or caspase-9, respectively. The presence of caspase-8 inhibitor Z-IETD-FMK reduced apoptosis by more than 50% relative to a control peptide whereas the caspase-9 inhibitor had minimal effect (Figure 5C), suggesting that cell-extrinsic mediators of apoptosis were predominant. Consistent with this finding, lymph node mDC taken from progressor but not stable animals at week 12 post infection showed a significant increase in the proportion of mDC expressing CD95 relative to preinfection samples (Figure 5D). Together, these data suggest that increased mDC apoptosis in lymph nodes during chronic infection in animals that progress to AIDS offsets the increase in mDC recruitment from blood, resulting in no net accumulation of mDC.
Fig. 5. Apoptosis of lymph node mDC increases in progressive but not stable SIV infection. 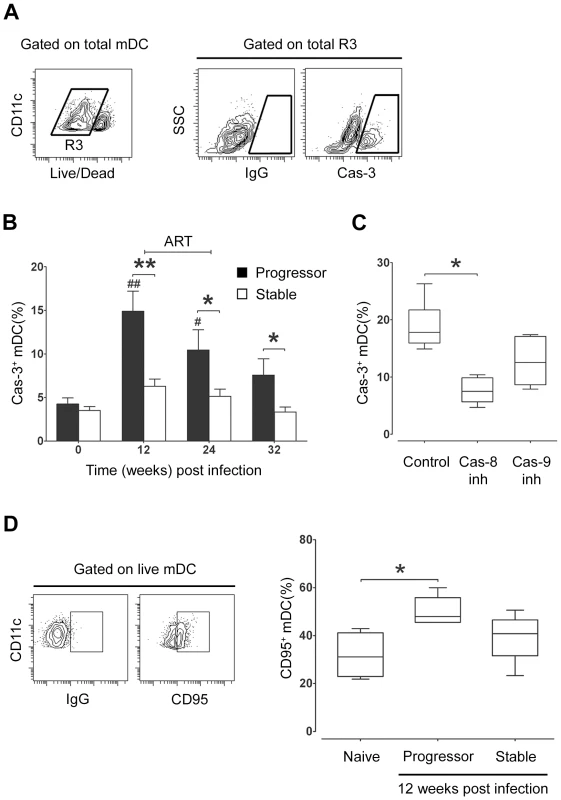
(A) Representative flow cytometry plots showing the gating strategy used to identify mDC expressing active caspase-3 (Cas-3). Live mDC were defined as Lineage− HLA-DR+ CD11c+ cells that lacked staining with a fixable dead-cell dye. (B) The proportion of lymph node mDC expressing active caspase-3 relative to staining with a control antibody at intervals before and after SIV infection. Cells were cultured for 3 hours prior to analysis. Shown are mean ± SEM for naïve (T = 0, n = 14), progressor (n = 8) and stable n = 10). (C) The proportion of lymph node mDC, taken at 12 weeks post infection from animals with progressive infection, expressing active caspase-3 after 3 hours in the presence of caspase-8 inhibitor (Cas-8 inh), caspase-9 inhibitor (Cas-9 inh) or control inhibitor (Control). Boxes represent 25th to 75th percentile and median values, and whiskers represent the minimum and maximum values, using data from 6 animals. (D) Left: Representative dot plots showing expression of CD95 relative to isotype control antibody to define positively staining cells. Right: The proportion of lymph node mDC at 12 weeks post infection that express CD95 in naïve animals (n = 4) and animals with progressive (n = 5) and stable infection (n = 5). Boxes represent 25th to 75th percentile and median values, and whiskers represent minimum and maximum values. *P<0.05, **P<0.01 between groups; #P<0.05, ##P<0.01, within groups relative to week 0. ART = intervals of antiretroviral therapy. Evidence of increased responsiveness of lymph node mDC to stimulation with TLR7/8 agonist during infection
Changes in mDC activation and apoptosis within lymph nodes during SIV infection could impact the capacity of these cells to respond to microbial stimuli and subsequently induce adaptive T cell immune responses. To investigate the functional capacity of mDC following SIV infection in our two groups of animals we stimulated lymph node cell suspensions taken at intervals before and after infection with 3M-007, a small molecule synthetic agonist of TLR7/8, which, like HIV and SIV RNA, activates mDC through their engagement of TLR8 [25]–[27]. We analyzed mDC for expression of two key immunoregulatory cytokines, TNF-α and IL-12 (p40/p70). Interestingly, lymph node mDC taken prior to infection responded relatively poorly to short-term stimulation with a small proportion of cells producing TNF-α and IL-12 (Figure 6). In contrast, stimulation of mDC taken at 12 weeks post infection resulted in 20 to 30% of cells producing TNF-α and a smaller but significant percentage producing IL-12, representing a 4 - to 5-fold increase above preinfection levels regardless of disease outcome (Figure 6). ART reduced mDC responsiveness to TLR8 stimulation although this did not reach statistical significance (Figure 6B). These data indicate that mDC resident in lymph nodes of SIV-infected rhesus macaques are functional capable of responding to stimulation, and may even be hyperresponsive as a consequence of SIV infection.
Fig. 6. Lymph node mDC from SIV infected animals remain responsive to exogenous TLR8 stimulation. 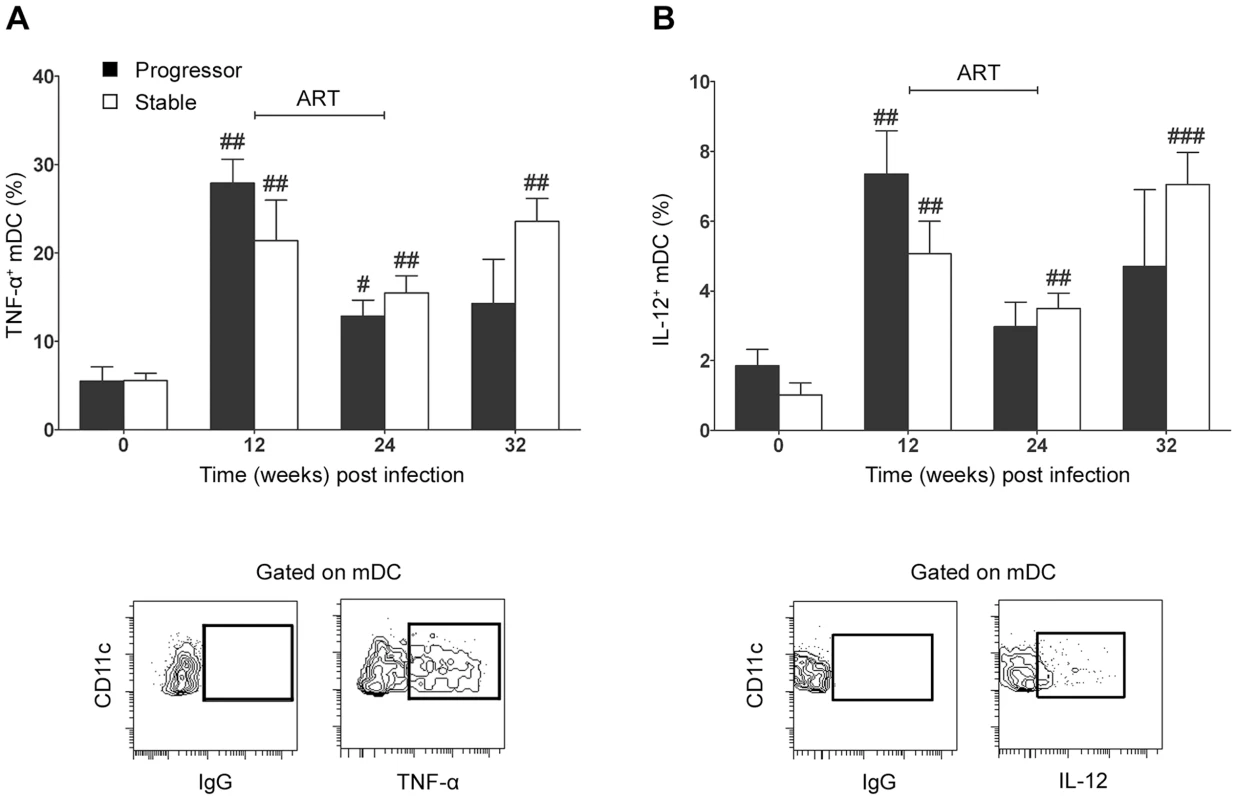
The proportion of lymph node mDC before and after SIV infection that express (A) TNF-α (A) or IL-12 (B) following stimulation with the TLR7/8 agonist 3M-007 for 5 hours. Shown are mean ± SEM for naïve (T = 0, n = 13), progressor (n = 7) and stable animals (n = 10). Also shown are representative flow cytometry plots of the gating strategy to identify positive populations. #P<0.05, ##P<0.01 within groups relative to week 0. ART = interval of antiretroviral therapy. Discussion
In this study we examined the relationship between mDC dynamics and disease progression over time in pathogenic SIV infection of rhesus macaques. We show for the first time that mDC are preferentially lost from blood in animals that progress to AIDS but are increased in blood of animals with long-term stable infection. This divergent mDC response was apparent at virus set-point, indicating that changes in blood mDC number over the first 3 months of infection are predictive of eventual disease progression.
mDC are recruited from blood to lymphoid tissues through upregulation of CCR7, the ligand for chemokines CCL19 and CCL21 that are expressed in the lymph node paracortex [28]. In animals with progressive infection, mDC loss from blood was associated with an increase in the frequency of blood mDC expressing CCR7 and an increase in expression of CCL19 in lymph nodes, consistent with increased extravasation to lymph nodes that exceeded the rate of mDC production from bone marrow. Expression of CCL19 has been shown previously to be markedly increased in lymph nodes during the acute phase of SIV infection [29], and our data suggest that expression in lymph nodes remains high into chronic infection as a function of virus load. Indeed, recent studies have shown that increased levels of CCL19 and CCL21 in blood correlate with higher virus loads and disease progression in HIV infected humans [30]. In vitro exposure to CCL19 and CCL21 also promotes an inflammatory response in PBMC from HIV-infected individuals with high virus loads [31]. We now provide evidence of a functional link between CCL19 upregulation in lymph nodes and increased expression of CCR7 on circulating mDC that promotes mDC recruitment to lymph nodes in progressive SIV infection. While not examined in this study, the potential exists for proinflammatory factors to promote differential emigration of mDC to lymph nodes in progressive relative to stable SIV infection. In particular, lipopolysaccharide is increased in the circulation during chronic HIV and pathogenic SIV infection as a consequence of microbial translocation through increased gut permeability [32]. Lipopolysaccharide activates mDC via engagement of TLR4 [33] and is a potent activator of DC migration in vivo [34], [35].
In contrast to progressive infection, we found that mDC in animals that controlled SIV infection had significant increases in blood mDC over time, with increases of up to 5-fold by virus set-point and nearly 10-fold in some cases at 32 weeks of infection. Studies in HIV infected individuals have indicated that mDC loss is inversely proportional to virus load, as we have shown, and is not observed in long-term non-progressors [2], [5], [8], but such cross sectional studies have by design not revealed changes over time. Increased blood mDC may arise from increased hematopoiesis in bone marrow in response to inflammatory cytokines such as TNF-α and IL-1 that are elevated during HIV infection and promote DC generation [36], [37]. The lack of an upregulated CCR7-CCL19 axis in this group would exacerbate the impact of enhanced DC production and mobilization into blood by limiting mDC exodus into tissues.
Paradoxically, there was no net increase in mDC within lymph nodes in monkeys with progressive SIV infection, associated with an increase in mDC apoptosis, suggesting that increased recruitment to lymph nodes is offset by increased cell death in severe infection. mDC apoptosis was caspase-8-dependent and associated with increased CD95 expression, similar to the findings for plasmacytoid DC in HIV and SIV infection [38], [39], consistent with a cell-extrinsic mechanism of apoptosis involving CD95 ligation. Apoptosis through virus infection of mDC is unlikely to be a significant factor, as previous studies indicate that only a minor fraction of lymph node mDC contain incorporated viral DNA during peak viremia [38]. HIV and SIV clearly affect mDC in the absence of productive infection, in particular through interactions of viral RNA with endosomal TLR8; however this interaction tends to promote cell survival rather than apoptosis [9], [27]. While the increase in mDC recruitment appears to keep pace with apoptosis in tissues during the chronic stages of SIV infection studied here it is clear that mDC are ultimately lost from lymph nodes as AIDS is established, as previously reported [15]. This eventual decline may be associated with a similar decline in lymph node expression of CCL19 in the final stages of disease [29].
Several reports have described the presence of semimature mDC with reduced expression of costimulatory molecules and/or CD83 in lymph node and spleen of HIV-infected humans [11], [13], [40] and SIV-infected macaques [41], [42]. Our data now suggest that these cells may have a beneficial function in vivo, as lymph node mDC with significantly lower expression of CCR7 and costimulatory molecules consistent with a semimature state were present only in animals with long-term stable infection. In vitro, semimature DC with tolerogenic function are derived from exposure to immunoregulatory cytokines including IL-10 and transforming growth factor-β [43], however whether these factors modulate DC maturation and function in progressive versus stable SIV and HIV infection is not known. Semimature mDC from HIV-infected lymph nodes have been shown to promote regulatory T cell function [13]. While we were not able to examine the effect of these cells on regulatory T cells in this study, the prevalence of semimature mDC in stable but not progressive infection might suggest a role for enhanced regulatory T cell responses in disease control. The role of regulatory T cells in pathogenic and nonpathogenic SIV infection is currently controversial [44]–[46], and the interplay between mDC and regulatory T cells in control and progression to disease deserves attention. In contrast to stable infection, mDC in lymph nodes of animals with progressive infection showed essentially no difference in expression of CCR7 and activation markers relative to naïve animals, although the proportion of cells expressing these markers was substantially greater than in blood. It is possible that activated mDC undergo apoptosis immediately upon entering lymph nodes, or alternatively that other newly identified costimulatory molecules from the CD28 and TNFR families not examined here may be differentially expressed in progressively infected lymph nodes [47].
In our study the two short courses of ART had only modest although similar effects on virus load in both groups of animals, reducing virus levels in plasma by ∼1.5 logs. This may be due to the fact that ART was initiated in chronic as opposed to acute infection and that therapy was limited to PMPA and FTC which both target the same viral protein, reverse transcriptase. Similarly limited effects of ART on virus load in SIV infection have been reported by others [48], [49]. Despite this, ART had noticeably beneficial effects on mDC homeostasis. In blood, ART reduced mDC activation and transiently restored mDC numbers in monkeys with progressive infection, consistent with reports in HIV-infected individuals [8], [17], [18]. Most strikingly, discontinuation of ART in stable animals led to a marked increase in the number of mDC in blood. In lymph nodes, ART resulted in a decrease in mDC apoptosis in animals with progressive infection and a reduction in mDC responsiveness overall. Consistent with this finding, expression of proinflammatory factors and CD95L that likely induce functional activation and apoptosis of mDC are substantially reduced in SIV - and HIV-infected lymph nodes in response to ART [19], [50], [51]. Animals in the progressive infection group died at a median time of 34 weeks post infection and did not receive the full second course of ART initiated at week 32. We do not believe this difference in treatment interval was a determining factor in survival, as disease status and time to sacrifice were correlated with virus load at set-point, before initiation of any therapy, and thus were independent of ART. HIV-infected individuals with higher baseline virus loads and immune activation have poorer reconstitution of innate immune cells in response to ART [16], [52]. In our study, differences in baseline virus load influenced the response to ART, as animals with stable and progressive infection had transient increases and decreases, respectively, in the number of blood mDC, although this could clearly be influenced by the differences in virus load in the two groups while on ART. It will be important to determine the impact of improved antiretroviral drug regimens on mDC dynamics in SIV infection, including the orally available integrase inhibitors that are highly active in monkeys [53].
Our data indicate that mDC present in lymph nodes in SIV infected monkeys remain functionally responsive to exogenous stimulation regardless of disease outcome. The finding that ex vivo stimulation through TLR8 induced a five-fold increase in expression of TNF-α relative to naïve animals suggests that these cells may in fact be hyperresponsive, although testing with a more extensive panel of agonists targeting different TLR ligands is needed to confirm this. CCL19 induces terminal activation of DC and promotes DC production of proinflammatory cytokines within lymph nodes [54], although this effect would not explain the finding of increased responsiveness of mDC in animals with stable infection that had normal levels of CCL19 in our study. It is possible that other proinflammatory factors such as IFN-γ that induce DC activation [55] and are markedly increased in lymph nodes during pathogenic SIV infection [56] are responsible for mDC increased responsiveness in SIV infection.
An increasing emphasis in HIV and SIV pathogenesis is now placed on the role of gut mucosa in disease, as this is a major site of virus replication and CD4+ T cell depletion [57]–[59]. mDC are recruited to inflamed respiratory mucosal surfaces in children with respiratory viral infections [60], and it is likely mDC and other DC subsets are similarly recruited to gut and vaginal mucosa in SIV infection [61]. It will be important to evaluate the mDC response in gut mucosa and its relationship to disease progression in SIV infection. However, such quantitative studies are technically difficult to perform as the DC is a relatively rare cell that can only be isolated in sufficient numbers through gut resection surgeries as opposed to the more commonly performed endoscopic biopsies.
Collectively, these data suggest that the inflammatory response associated with increased virus load during progressive SIV infection leads to an increase in the CCR7-CCL19 chemokine axis that serves to accelerate mDC recruitment to lymph nodes. Apoptosis of mDC within tissues during this chronic phase, which was found only in animals with progressive infection, would compromise the innate and adaptive immune response to opportunistic pathogens promoting disease progression. It is currently not clear whether recently recruited and activated mDC produce increased levels of proinflammatory cytokines in vivo that may mediate immune activation characteristic of HIV and pathogenic SIV infection [62]. Interestingly, increased turnover of blood monocytes associated with apoptosis of tissue macrophages has been shown to correlate with progression to disease in SIV-infected macaques and is a better predictive marker than viral load or lymphocyte activation [63], [64]. This response is not likely limited to lymph nodes, as evidenced by the fact that increased monocyte turnover and recruitment to brain correlates with the severity of SIV encephalitis [65]. These findings point to a broad-based dysregulation of mDC and monocytes in blood and tissues as a significant factor in the pathogenesis of AIDS.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh (Assurance Number A3187-01). Surgeries were performed under anesthesia induced and maintained with ketamine hydrochloride and medetomidine hydrochloride, and all efforts were made to minimize suffering.
Animal manipulations
Twenty one Indian-origin rhesus macaques (Macaca mulatta) used in this study were housed at the University of Pittsburgh Primate Facility for Infectious Disease Research. All animals were infected by intravenous inoculation with 1,000 TCID50 of uncloned, pathogenic SIVmac251 (provided by Christopher J. Miller, California National Primate Research Center). Virus load in plasma was determined as described previously [66]. ART consisted of PMPA (20 mg/kg/d, subcutaneous injection) and FTC (30 mg/kg/d, subcutaneous injection; both provided by Michael Miller, Gilead Sciences) and was given from weeks 12–24 and from weeks 32–44 as described [20]. All animals except R189 (sacrificed at week 11 post infection) received one or more administrations of Ad-based vectors during the study depending on survival. Priming injections of separate Ad5-based vectors expressing codon-optimized SIVmac239 gag, env and nef with and without rhesus IL-15.FLAG or empty Ad-ψ5 were given by intramuscular injection at week 16 (5×1010 total viral particles) and week 22 (1×1011 total viral particles), and boosting injections of the same quantity of Ad35-based vectors expressing the same transgenes were given at week 36 and 42. All Ad vectors were E1/E3-deleted with the exception of Ad35 containing the env gene which was E3 deleted. Lymph nodes were taken from the axillary or inguinal regions prior to infection and at weeks 12, 24 and 32 post infection and single cell suspensions were generated by disruption and digestion with collagenase D, as described [67].
Cell identification and enumeration
Identification of mDC was performed as previously described with some modifications [15], [24]. Briefly, PBMC or lymph node cell suspensions were stained with fluorescently-labeled antibodies to Lineage markers [CD3 (clone SP34-2; all antibodies from BD Bioscience unless otherwise noted), CD14 (M5E2), and CD20 (2H7)], HLA-DR (G46-6) and CD11c (S-HCL-3), with and without antibodies to CD45 (D058-1283), CD80 (L307.4), CD86 (FUN-1), CCR7 (150503, R&D Systems), CD40 (5C3) and CD95 (DX2). An amine-reactive fixable dead-cell dye (Invitrogen) was used to discriminate live from dead cells. mDC were defined as Lineage− HLA-DR+ cells expressing CD11c. In lymph nodes a broad Lineage− HLA-DR+/++ gate was used to include all mDC as described previously The number of blood CD4+ T cells was quantified using a precise volume of blood stained with antibodies in the absence of any wash step in TruCOUNT tubes (BD Biosciences) that contained a known number of fluorescent beads to provide internal calibration, as previously reported [20]. The number of blood mDC was then calculated based on the ratio of mDC to CD4+ T cells in PBMCs at the same time point [24]. All analyses were done on an LSR II flow cytometer with FACSDiva software (BD Bioscience).
Cytokine expression and apoptosis
Intracellular cytokine production by lymph node mDC was measured as described previously for plasmacytoid DC with minor modifications [38]. Briefly, cell suspensions were cultured for 5 hours with 10 µM of the TLR7/8 agonist 3M-007 (3 M Pharmaceuticals) with and without the addition of 10 µg/mL brefeldin A (Sigma) after 1 hour. Cells were stained with surface-labeling antibodies as above and fixed and permeabilized prior to incubation with antibody to TNF-α (MAb11) and IL-12 (8.6, Mitenyi Biotec) and analysis by flow cytometry. To detect apoptosis in mDC, lymph node cell suspensions were cultured in media for 3 hours with and without caspase-8 inhibitor Z-IETD-FMK, caspase-9 inhibitor Z-LEHD-FMK or irrelevant peptide Z-Fa-FMK (BD Biosciences). Cells were stained with surface-labeling antibodies as above and fixed and permeabilized prior to incubation with antibody to active caspase-3 (C92-605) and analysis by flow cytometry.
Detection of chemokine mRNA expression
Total lymph node RNA was extracted and purified from cell suspensions generated from biopsies taken prior to or 12 weeks after infection using the RNAeasy kit (Qiagen) after treatment with DNAse I (Invitrogen). cDNA was synthesized using random primers and Superscript II reverse transcriptase (Invitrogen). Primers and probes from Taqman human gene expression arrays (Applied Biosystems, Foster City, CA) were utilized for real time PCR analysis of CCL19, CCL21 and β-glucuronidase expression as previously described [68]. mRNA expression levels for each gene were calculated with the 2−ΔCT method using β-glucuronidase as the endogenous control [69].
Statistical analysis
Comparisons between two groups were carried out using the Mann-Whitney U test. Comparison of DC numbers across different time points was carried out using the Wilcoxon signed-rank test. Correlations were determined using the non-parametric Spearman rank test. Graphpad Prism 5 (Graphpad Software) was used for statistical analysis. All P values are two-sided with significance considered to be P<0.05.
Gene identification
The identification of genes analyzed in this paper as defined by Entrez-Gene are 574386 (CCL19), 574183 (CCL21) and 677692 (β-glucuronidase).
Zdroje
1. ShortmanK
LiuYJ
2002
Mouse and human dendritic cell subtypes.
Nat Rev Immunol
2
151
161
2. AlmeidaM
CorderoM
AlmeidaJ
OrfaoA
2005
Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection.
AIDS
19
261
271
3. BarronMA
BlyveisN
PalmerBE
MaWhinneyS
WilsonCC
2003
Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals.
J Infect Dis
187
26
37
4. DonaghyH
PozniakA
GazzardB
QaziN
GilmourJ
2001
Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load.
Blood
98
2574
2576
5. GrassiF
HosmalinA
McIlroyD
CalvezV
DebreP
1999
Depletion in blood CD11c-positive dendritic cells from HIV-infected patients.
AIDS
13
759
766
6. PacanowskiJ
KahiS
BailletM
LebonP
DeveauC
2001
Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection.
Blood
98
3016
3021
7. FontaineJ
CoutleeF
TremblayC
RoutyJP
PoudrierJ
2009
HIV infection affects blood myeloid dendritic cells after successful therapy and despite nonprogressing clinical disease.
J Infect Dis
199
1007
1018
8. ChehimiJ
CampbellDE
AzzoniL
BachellerD
PapasavvasE
2002
Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals.
J Immunol
168
4796
4801
9. FonteneauJF
LarssonM
BeignonAS
McKennaK
DasilvaI
2004
Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells.
J Virol
78
5223
5232
10. JonesGJ
WateraC
PattersonS
RutebemberwaA
KaleebuP
2001
Comparative loss and maturation of peripheral blood dendritic cell subpopulations in African and non-African HIV-1-infected patients.
Aids
15
1657
1663
11. LoreK
SonnerborgA
BrostromC
GohLE
PerrinL
2002
Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection.
Aids
16
683
692
12. DillonSM
RobertsonKB
PanSC
MawhinneyS
MeditzAL
2008
Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection.
J Acquir Immune Defic Syndr
48
1
12
13. KrathwohlMD
SchackerTW
AndersonJL
2006
Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects.
J Infect Dis
193
494
504
14. BiancottoA
GrivelJC
IglehartSJ
VanpouilleC
LiscoA
2007
Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1.
Blood
109
4272
4279
15. BrownKN
TrichelA
Barratt-BoyesSM
2007
Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS.
J Immunol
178
6958
6967
16. AzzoniL
ChehimiJ
ZhouL
FoulkesAS
JuneR
2007
Early and delayed benefits of HIV-1 suppression: timeline of recovery of innate immunity effector cells.
Aids
21
293
305
17. FinkeJS
ShodellM
ShahK
SiegalFP
SteinmanRM
2004
Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy.
J Clin Immunol
24
647
652
18. AlmeidaM
CorderoM
AlmeidaJ
OrfaoA
2006
Persistent abnormalities in peripheral blood dendritic cells and monocytes from HIV-1-positive patients after 1 year of antiretroviral therapy.
J Acquir Immune Defic Syndr
41
405
415
19. LiQ
SchackerT
CarlisJ
BeilmanG
NguyenP
2004
Functional genomic analysis of the response of HIV-1-infected lymphatic tissue to antiretroviral therapy.
J Infect Dis
189
572
582
20. SoloffAC
LiuX
GaoW
DayRD
GambottoA
2009
Adenovirus 5 - and 35-based immunotherapy enhances the strength but not breadth or quality of immunity during chronic SIV infection.
Eur J Immunol
39
2437
2449
21. MotheBR
WeinfurterJ
WangC
RehrauerW
WilsonN
2003
Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication.
J Virol
77
2736
2740
22. WatsonA
RanchalisJ
TravisB
McClureJ
SuttonW
1997
Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival.
J Virol
71
284
290
23. StapransSI
DaileyPJ
RosenthalA
HortonC
GrantRM
1999
Simian immunodeficiency virus disease course is predicted by the extent of virus replication during primary infection.
J Virol
73
4829
4839
24. BrownKN
Barratt-BoyesSM
2009
Surface phenotype and rapid quantification of blood dendritic cell subsets in the rhesus macaque.
J Med Primatol
38
272
278
25. BeignonAS
McKennaK
SkoberneM
ManchesO
DaSilvaI
2005
Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions.
J Clin Invest
115
3265
3275
26. MandlJN
BarryAP
VanderfordTH
KozyrN
ChavanR
2008
Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections.
Nat Med
14
1077
1087
27. MeierA
AlterG
FrahmN
SidhuH
LiB
2007
MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands.
J Virol
81
8180
8191
28. ForsterR
Davalos-MisslitzAC
RotA
2008
CCR7 and its ligands: balancing immunity and tolerance.
Nat Rev Immunol
8
362
371
29. ChoiYK
FallertBA
Murphey-CorbMA
ReinhartTA
2003
Simian immunodeficiency virus dramatically alters expression of homeostatic chemokines and dendritic cell markers during infection in vivo.
Blood
101
1684
1691
30. DamasJK
LandroL
FevangB
HeggelundL
FrolandSS
2009
Enhanced levels of the CCR7 ligands CCL19 and CCL21 in HIV infection: correlation with viral load, disease progression and response to highly active antiretroviral therapy.
Aids
23
135
138
31. DamasJK
LandroL
FevangB
HeggelundL
TjonnfjordGE
2009
Homeostatic chemokines CCL19 and CCL21 promote inflammation in human immunodeficiency virus-infected patients with ongoing viral replication.
Clin Exp Immunol
157
400
407
32. BrenchleyJM
PriceDA
SchackerTW
AsherTE
SilvestriG
2006
Microbial translocation is a cause of systemic immune activation in chronic HIV infection.
Nat Med
12
1365
1371
33. KadowakiN
HoS
AntonenkoS
MalefytRW
KasteleinRA
2001
Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens.
J Exp Med
194
863
869
34. De SmedtT
PajakB
MurailleE
LespagnardL
HeinenE
1996
Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo.
J Exp Med
184
1413
1424
35. RoakeJA
RaoAS
MorrisPJ
LarsenCP
HankinsDF
1995
Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1.
J Exp Med
181
2237
2247
36. GeissmannF
ManzMG
JungS
SiewekeMH
MeradM
2010
Development of monocytes, macrophages, and dendritic cells.
Science
327
656
661
37. RimaniolAC
ZylberbergH
ZavalaF
ViardJP
1996
Inflammatory cytokines and inhibitors in HIV infection: correlation between interleukin-1 receptor antagonist and weight loss.
Aids
10
1349
1356
38. BrownKN
WijewardanaV
LiuX
Barratt-BoyesSM
2009
Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection.
PLoS Pathog
5
e1000413
39. MeyersJH
JustementJS
HallahanCW
BlairET
SunYA
2007
Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells.
PLoS One
2
e458
40. McIlroyD
AutranB
ClauvelJP
OksenhendlerE
DebreP
1998
Low CD83, but normal MHC class II and costimulatory molecule expression, on spleen dendritic cells from HIV+ patients.
AIDS Res Hum Retroviruses
14
505
513
41. SoderlundJ
NilssonC
LoreK
Castanos-VelezE
EkmanM
2004
Dichotomy between CD1a+ and CD83+ dendritic cells in lymph nodes during SIV infection of macaques.
J Med Primatol
33
16
24
42. ZimmerMI
LarreginaAT
CastilloCM
CapuanoS3rd
FaloLDJr
2002
Disrupted homeostasis of Langerhans cells and interdigitating dendritic cells in monkeys with AIDS.
Blood
99
2859
2868
43. RutellaS
DaneseS
LeoneG
2006
Tolerogenic dendritic cells: cytokine modulation comes of age.
Blood
108
1435
1440
44. QinS
SuiY
SoloffAC
JuneckoBA
KirschnerDE
2008
Chemokine and cytokine mediated loss of regulatory T cells in lymph nodes during pathogenic simian immunodeficiency virus infection.
J Immunol
180
5530
5536
45. EstesJD
LiQ
ReynoldsMR
WietgrefeS
DuanL
2006
Premature induction of an immunosuppressive regulatory T cell response during acute simian immunodeficiency virus infection.
J Infect Dis
193
703
712
46. PereiraLE
VillingerF
OnlamoonN
BryanP
CardonaA
2007
Simian immunodeficiency virus (SIV) infection influences the level and function of regulatory T cells in SIV-infected rhesus macaques but not SIV-infected sooty mangabeys.
J Virol
81
4445
4456
47. DuttaguptaPA
BoesteanuAC
KatsikisPD
2009
Costimulation signals for memory CD8+ T cells during viral infections.
Crit Rev Immunol
29
469
486
48. BeqS
NugeyreMT
Ho Tsong FangR
GautierD
LegrandR
2006
IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy.
J Immunol
176
914
922
49. KarlssonI
MalleretB
BrochardP
DelacheB
CalvoJ
2007
Dynamics of T-cell responses and memory T cells during primary simian immunodeficiency virus infection in cynomolgus macaques.
J Virol
81
13456
13468
50. HerbeuvalJP
NilssonJ
BoassoA
HardyAW
VaccariM
2009
HAART reduces death ligand but not death receptors in lymphoid tissue of HIV-infected patients and simian immunodeficiency virus-infected macaques.
Aids
23
35
40
51. BehbahaniH
LandayA
PattersonBK
JonesP
PottageJ
2000
Normalization of immune activation in lymphoid tissue following highly active antiretroviral therapy.
J Acquir Immune Defic Syndr
25
150
156
52. ChehimiJ
AzzoniL
FarabaughM
CreerSA
TomescuC
2007
Baseline viral load and immune activation determine the extent of reconstitution of innate immune effectors in HIV-1-infected subjects undergoing antiretroviral treatment.
J Immunol
179
2642
2650
53. HazudaDJ
YoungSD
GuareJP
AnthonyNJ
GomezRP
2004
Integrase inhibitors and cellular immunity suppress retroviral replication in rhesus macaques.
Science
305
528
532
54. MarslandBJ
BattigP
BauerM
RuedlC
LassingU
2005
CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells.
Immunity
22
493
505
55. HilkensCM
KalinskiP
de BoerM
KapsenbergML
1997
Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype.
Blood
90
1920
1926
56. AbelK
La Franco-ScheuchL
RourkeT
MaZM
De SilvaV
2004
Gamma interferon-mediated inflammation is associated with lack of protection from intravaginal simian immunodeficiency virus SIVmac239 challenge in simian-human immunodeficiency virus 89.6-immunized rhesus macaques.
J Virol
78
841
854
57. LiQ
DuanL
EstesJD
MaZM
RourkeT
2005
Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells.
Nature
434
1148
1152
58. MattapallilJJ
DouekDC
HillB
NishimuraY
MartinM
2005
Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection.
Nature
434
1093
1097
59. VeazeyRS
DeMariaM
ChalifouxLV
ShvetzDE
PauleyDR
1998
Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection.
Science
280
427
431
60. GillMA
PaluckaAK
BartonT
GhaffarF
JafriH
2005
Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections.
J Infect Dis
191
1105
1115
61. LiQ
EstesJD
SchlievertPM
DuanL
BrosnahanAJ
2009
Glycerol monolaurate prevents mucosal SIV transmission.
Nature
458
1034
1038
62. SodoraDL
SilvestriG
2008
Immune activation and AIDS pathogenesis.
Aids
22
439
446
63. HasegawaA
LiuH
LingB
BordaJT
AlvarezX
2009
The level of monocyte turnover predicts disease progression in the macaque model of AIDS.
Blood
114
2917
2925
64. KurodaMJ
2010
Macrophages: do they impact AIDS progression more than CD4 T cells?
J Leukoc Biol
87
569
573
65. BurdoTH
SoulasC
OrzechowskiK
ButtonJ
KrishnanA
2010
Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma.
PLoS Pathog
6
e1000842
66. Barratt-BoyesSM
SoloffAC
GaoW
NwanegboE
LiuX
2006
Broad cellular immunity with robust memory responses to simian immunodeficiency virus following serial vaccination with adenovirus 5 - and 35-based vectors.
J Gen Virol
87
139
149
67. Barratt-BoyesSM
ZimmerMI
HarshyneLA
MeyerEM
WatkinsSC
2000
Maturation and trafficking of monocyte-derived dendritic cells in monkeys: implications for dendritic cell-based vaccines.
J Immunol
164
2487
2495
68. FallertBA
PovedaS
SchaeferTM
PfeiferME
SanghaviSK
2008
Virologic and immunologic events in hilar lymph nodes during simian immunodeficiency virus infection: development of polarized inflammation.
J Acquir Immune Defic Syndr
47
16
26
69. SchmittgenTD
LivakKJ
2008
Analyzing real-time PCR data by the comparative C(T) method.
Nat Protoc
3
1101
1108
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 12- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Is Adherence to Erythrocytes a Factor in Extrapulmonary Dissemination?
- Identifying the Age Cohort Responsible for Transmission in a Natural Outbreak of
- Blockade of Immunosuppressive Cytokines Restores NK Cell Antiviral Function in Chronic Hepatitis B Virus Infection
- Metaeffector Exploits Host Proteasome to Temporally Regulate Cognate Effector
- The p53-Target Gene Drives Neutrophil-Mediated Protection against Lethal Bacterial Sepsis
- Development of an RNAi Protocol to Investigate Gene Function in the Filarial Nematode,
- Lysine Acetyltransferase GCN5-A Functions in the Cellular Response to Alkaline Stress and Expression of Cyst Genes
- Structural Basis for Apoptosis Inhibition by Epstein-Barr Virus BHRF1
- Molecular Architectures of Trimeric SIV and HIV-1 Envelope Glycoproteins on Intact Viruses: Strain-Dependent Variation in Quaternary Structure
- Interaction of c-Cbl with Myosin IIA Regulates Bleb Associated Macropinocytosis of Kaposi's Sarcoma-Associated Herpesvirus
- Glacial Refugia in Pathogens: European Genetic Structure of Anther Smut Pathogens on and
- Role for Sumoylation in Systemic Inflammation and Immune Homeostasis in Larvae
- Inflammasome Sensor Nlrp1b-Dependent Resistance to Anthrax Is Mediated by Caspase-1, IL-1 Signaling and Neutrophil Recruitment
- Noise Cancellation: Viral Fine Tuning of the Cellular Environment for Its Own Genome Replication
- Infectious Speciation Revisited: Impact of Symbiont-Depletion on Female Fitness and Mating Behavior of
- Eis Regulates Autophagy, Inflammation, and Cell Death through Redox-dependent Signaling
- NleC, a Type III Secretion Protease, Compromises NF-κB Activation by Targeting p65/RelA
- Early Myeloid Dendritic Cell Dysregulation is Predictive of Disease Progression in Simian Immunodeficiency Virus Infection
- HIV Capsid is a Tractable Target for Small Molecule Therapeutic Intervention
- Structural and Functional Studies of Nonstructural Protein 2 of the Hepatitis C Virus Reveal Its Key Role as Organizer of Virion Assembly
- Coming of Age—Sexual Reproduction in Species
- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Compartmentation of Redox Metabolism in Malaria Parasites
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Rapid End-Point Quantitation of Prion Seeding Activity with Sensitivity Comparable to Bioassays
- Dimeric 2G12 as a Potent Protection against HIV-1
- Hypoxia Induces an Immunodominant Target of Tuberculosis Specific T Cells Absent from Common BCG Vaccines
- CD4 Natural Regulatory T Cells Prevent Experimental Cerebral Malaria via CTLA-4 When Expanded In Vivo
- The Killing of African Trypanosomes by Ethidium Bromide
- H2A.Z Demarcates Intergenic Regions of the Epigenome That Are Dynamically Marked by H3K9ac and H3K4me3
- Large-Scale Field Application of RNAi Technology Reducing Israeli Acute Paralysis Virus Disease in Honey Bees (, Hymenoptera: Apidae)
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Coming of Age—Sexual Reproduction in Species
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Compartmentation of Redox Metabolism in Malaria Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



