-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Pregnancy Weight Gain and Childhood Body Weight: A Within-Family Comparison
Background:
Excessive pregnancy weight gain is associated with obesity in the offspring, but this relationship may be confounded by genetic and other shared influences. We aimed to examine the association of pregnancy weight gain with body mass index (BMI) in the offspring, using a within-family design to minimize confounding.Methods and Findings:
In this population-based cohort study, we matched records of all live births in Arkansas with state-mandated data on childhood BMI collected in public schools (from August 18, 2003 to June 2, 2011). The cohort included 42,133 women who had more than one singleton pregnancy and their 91,045 offspring. We examined how differences in weight gain that occurred during two or more pregnancies for each woman predicted her children's BMI and odds ratio (OR) of being overweight or obese (BMI≥85th percentile) at a mean age of 11.9 years, using a within-family design. For every additional kg of pregnancy weight gain, childhood BMI increased by 0.0220 (95% CI 0.0134–0.0306, p<0.0001) and the OR of overweight/obesity increased by 1.007 (CI 1.003–1.012, p = 0.0008). Variations in pregnancy weight gain accounted for a 0.43 kg/m2 difference in childhood BMI. After adjustment for birth weight, the association of pregnancy weight gain with childhood BMI was attenuated but remained statistically significant (0.0143 kg/m2 per kg of pregnancy weight gain, CI 0.0057–0.0229, p = 0.0007).Conclusions:
High pregnancy weight gain is associated with increased body weight of the offspring in childhood, and this effect is only partially mediated through higher birth weight. Translation of these findings to public health obesity prevention requires additional study.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 10(10): e32767. doi:10.1371/journal.pmed.1001521
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001521Summary
Background:
Excessive pregnancy weight gain is associated with obesity in the offspring, but this relationship may be confounded by genetic and other shared influences. We aimed to examine the association of pregnancy weight gain with body mass index (BMI) in the offspring, using a within-family design to minimize confounding.Methods and Findings:
In this population-based cohort study, we matched records of all live births in Arkansas with state-mandated data on childhood BMI collected in public schools (from August 18, 2003 to June 2, 2011). The cohort included 42,133 women who had more than one singleton pregnancy and their 91,045 offspring. We examined how differences in weight gain that occurred during two or more pregnancies for each woman predicted her children's BMI and odds ratio (OR) of being overweight or obese (BMI≥85th percentile) at a mean age of 11.9 years, using a within-family design. For every additional kg of pregnancy weight gain, childhood BMI increased by 0.0220 (95% CI 0.0134–0.0306, p<0.0001) and the OR of overweight/obesity increased by 1.007 (CI 1.003–1.012, p = 0.0008). Variations in pregnancy weight gain accounted for a 0.43 kg/m2 difference in childhood BMI. After adjustment for birth weight, the association of pregnancy weight gain with childhood BMI was attenuated but remained statistically significant (0.0143 kg/m2 per kg of pregnancy weight gain, CI 0.0057–0.0229, p = 0.0007).Conclusions:
High pregnancy weight gain is associated with increased body weight of the offspring in childhood, and this effect is only partially mediated through higher birth weight. Translation of these findings to public health obesity prevention requires additional study.
Please see later in the article for the Editors' SummaryIntroduction
The adverse effects of undernutrition during pregnancy on the long-term health of the offspring have been extensively studied [1],[2]. With onset of the epidemic of obesity in children, much attention has been focused on the consequences of maternal overnutrition, including high prepregnancy body mass index (BMI) and pregnancy weight gain [3]–[8]. Overnutrition during pregnancy has been hypothesized to alter fetal growth, body composition, metabolism, hormonal responses, and brain pathways regulating body weight or epigenetic patterns in ways that would increase long-term risk for obesity and related diseases.
Translational research provides evidence for this hypothesis [9]–[12]. In one study, offspring of female rats that were overfed before and during pregnancy gained more weight than offspring whose mothers were not overfed, even though both groups of progeny had the same genetic background [10]. Other animal studies reported that offspring of overfed mothers have increased expression of appetite stimulating neuropeptides, lower physical activity level, and changes in skeletal muscle structure and function [9],[11],[12].
Human studies have found associations between high prepregnancy BMI or pregnancy weight gain and body weight in the offspring [13]–[27]. However, the possibility of confounding must be carefully considered in these observational analyses of unrelated mother-child pairs. The child of an obese mother (or one who has gained excessive weight during her pregnancy) may also be obese, not because of the intrauterine effects of overnutrition, but rather because of shared obesity-promoting genetic or environmental factors. Indeed, among 2,758 families in the Collaborative Perinatal Project, the associations of prepregnancy BMI and pregnancy weight gain with child BMI z-score at age 4 years observed in conventional generalized equations became statistically nonsignificant in fixed effects models that account for shared familial influences [28]. Similarly, a study of 4,091 families found that the association between prepregnancy BMI and offspring adiposity at age 9 to 11 years became nonsignificant using the FTO (fat mass and obesity associated) genotype as an instrumental variable for maternal adiposity [29]. In addition, a sibling study of Swedish males born between 1973 and 1988 found no association between pregnancy weight gain and offspring BMI at age 18 years for the full cohort, though a positive association was observed among the subgroup of 21,146 offspring whose mothers had high prepregnancy BMI [30]. Moreover, most [31]–[36] but not all [37] analyses involving both parents demonstrate associations in BMI between father and offspring equal to, or greater than, between mother and offspring—findings that further discount a special influence of the intrauterine environment in this regard.
A recent observational study reported improved measures of adiposity among children born to 20 women after bariatric surgery, compared to their siblings born before surgery [38]. Here too, confounding by biological and behavioral factors cannot be excluded. Bariatric surgery may have produced changes in the mother (e.g., micro - or macronutrient malabsorption) or the household (e.g., greater awareness of the importance of weight control) that affected the offspring independent of maternal weight. Moreover, the children born after maternal bariatric surgery were substantially younger at the time of measurement (and their mothers were substantially older at the time of these children's birth), producing another source of potential confounding. Thus, apart from the special case of diabetes during pregnancy [39] in which severe metabolic aberrations may occur, the role of maternal overnutrition in childhood obesity remains unproven. Therefore, we aimed to examine the independent effects of pregnancy weight gain in a large, contemporary, population-based cohort with follow-up to a mean age of 11.9 years in the offspring, using a within-family design to minimize confounding.
Methods
Ethics Statement
These analyses were conducted under an approved Institutional Review Board (IRB) protocol through the University of Arkansas for Medical Sciences. The IRB at Princeton University also provided approval for this study. HLR had full access to, and takes responsibility for the integrity of, all primary data in the study. All authors vouch for the accuracy and completeness of the data analyses.
Study Design
The purpose of this study was to determine whether the relationship between pregnancy weight gain and offspring weight previously observed at birth [40] persists into mid-childhood, using a similar, within-family analytic approach. We utilized Vital Statistics Natality records covering all live births in Arkansas from January 1, 1989 to December 31, 2005 and data on children's BMI mandated to be collected in all public schools from August 18, 2003 to June 2, 2011. Data for the current study were provided through a data use agreement with the Arkansas Center for Health Improvement (ACHI). ACHI is a legislatively governed entity authorized to collect and integrate statewide administrative datasets from the Departments of Health and Education, among others, through the use of strict data use agreements that protect the confidentiality and privacy of individual information contained in these records. ACHI conducted all data matching and de-identification of datasets prior to statistical analysis. Biological siblings (with the same mother) were matched using information contained in birth records based on maternal identifiers including name, date of birth, social security numbers, and sometimes addresses. Birth data were then linked to school BMI records using child-level identifiers including names, dates of birth, social security numbers, and sometimes addresses.
Data Collection
Birth outcomes and maternal characteristics were obtained from Vital Statistics Natality records, which are based on the Certificate of Live Birth, a legal and medical record required for all births. (A summary of the data files is available from the US National Center for Health Statistics: http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm.) The variables of interest included pregnancy weight gain, birth weight, diabetes during pregnancy, week of gestation at delivery, maternal age, maternal education, maternal marital status, maternal smoking, child gender, child parity, and year of birth. Data for birth weight obtained by this method are highly reliable [41]. Prepregnancy weight is obtained from mothers (who complete a worksheet at the hospital) and weight at delivery is obtained from a worksheet completed by hospital personnel using labor and delivery records. Examples of the worksheets are available on the Centers for Disease Control and Prevention (CDC) Vital Statistics web page: (www.cdc.gov/nchs/data/dvs/momskf_improv.pdf and www.cdc.gov/nchs/data/dvs/facwksBF04.pdf).
Weight gain was calculated as weight at delivery minus maternally reported pre-pregnancy weight. Until very recently, prepregnancy weight and height were not included on the Certificate of Live Birth. Because prepregnancy weight is based on maternal self-report, it may have lower reliability than weight at delivery. However, an exact concordance between certificate of live birth data and medical record data for pregnancy weight gain was found in 82.8% of a random sample in North Carolina [42]. Another study found concordances between data on prepregnancy weight from the Certificate of Live Birth records and from medical records of 82.0% in New York City and 99.0% in Vermont [43].
A legislative act in Arkansas (Arkansas Annotated Code 20-7-133-135, 2003) mandated collection of height and weight for all public school children beginning in kindergarten. From 2004 through 2007, the state collected data for all children every year; beginning in 2008, data were collected every other year (i.e., kindergarten, 2nd, 4th, 6th, 8th, and 10th grades). During the fall semester of each school year, a data file of all public school students' demographic information was used to pre-populate data entry screens for schools to enter height and weight. Trained school personnel or student-health professionals obtained one weight and two height measurements at each time, as described [44]. BMI was calculated as (weight in pounds/[height in inches]2)×703. Gender - and age-specific BMI percentiles were calculated according to CDC definitions [45]. Students were classified as overweight or obese if BMI was ≥85th percentile.
Our study involved merging two administrative datasets collected by government agencies in Arkansas: the school student BMI records and the Vital Statistics Natality Data. Table 1 summarizes the sequential merger and exclusion process to obtain the study cohort. Beginning with 2,688,625 child BMI records from the Arkansas Dept. of Education, we excluded records lacking valid date of birth, height, or weight. Of these 2,222,521 school records, we were able to merge 1,044,086 (47.0%) with Vital Statistics Natality records. We then excluded multiple births, gestational age <37 weeks or >42 weeks, maternal diabetes, birth weight <500 g or ≥7,000 g, missing data for pregnancy weight, and weight gain outliers. Next, we deleted multiple records for the same child, including only the last record available, with the aim of assessing BMI of the offspring as late as possible in childhood. (Restricting measurement of BMI to a narrow age window would have substantially reduced the number of sibling pairs available, thereby reducing sample size and statistical power.) To control flexibly for differences in age, we added dummy variables for each month of age. Moreover, the median age difference between siblings was zero at the time of measurement (see below). Finally, per study design, we excluded children without siblings in the database, yielding a cohort of 91,045 offspring and their 42,133 mothers.
Tab. 1. Cohort selection and exclusion criteria. 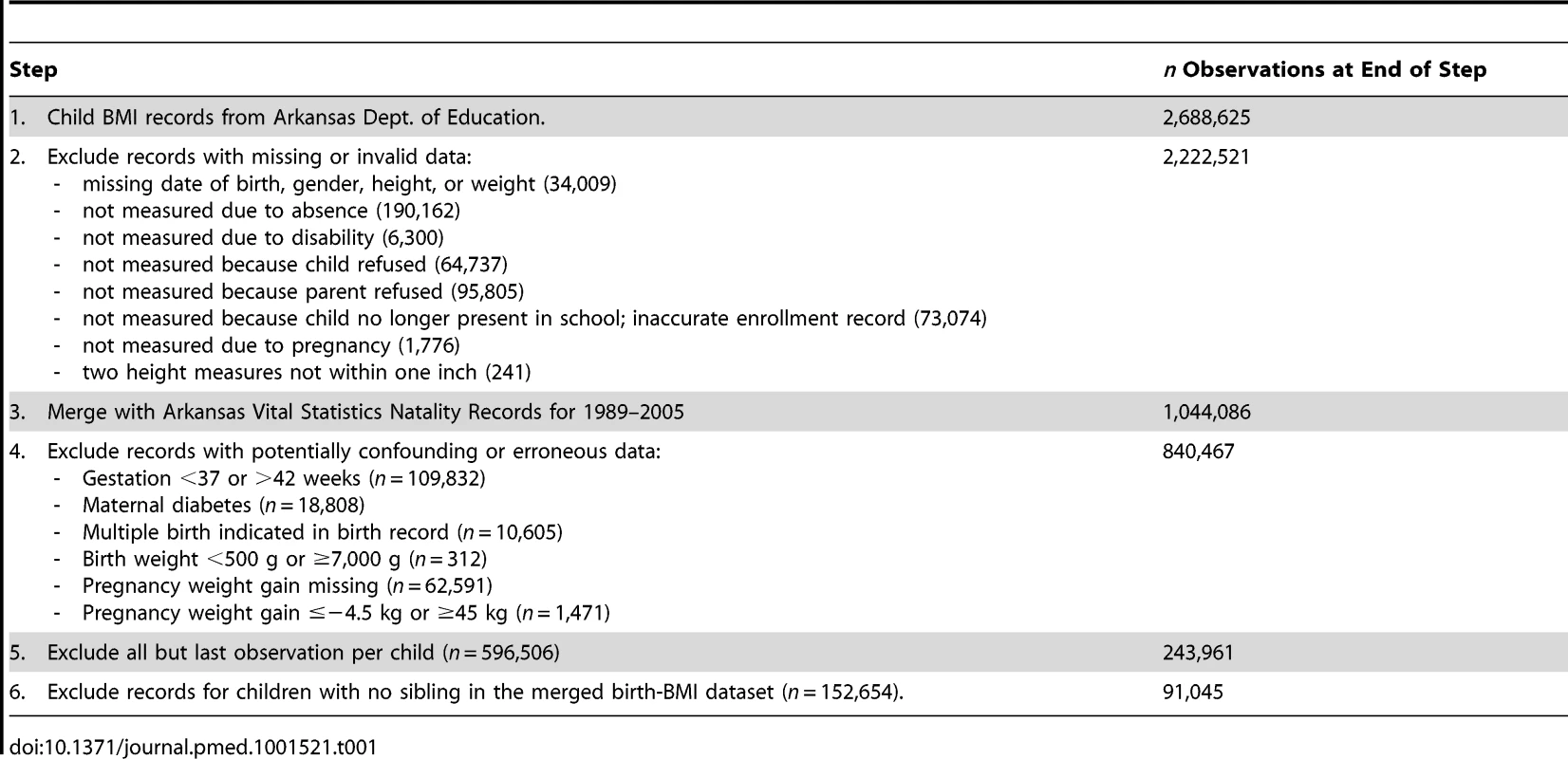
Table 2 presents descriptive statistics for the children in our sample compared to all children born in Arkansas between 1989 and 2005 who satisfied similar sample exclusion restrictions (e.g., singleton births with gestation 37–42 weeks, excluding those with extreme values for birth weight). The characteristics of both groups were similar in most ways, including weight gain during pregnancy, although the study cohort had a slightly larger proportion of African Americans and married women, and a slightly lower proportion of smokers.
Tab. 2. Comparison of the study cohort with all Arkansas births 1989–2005. 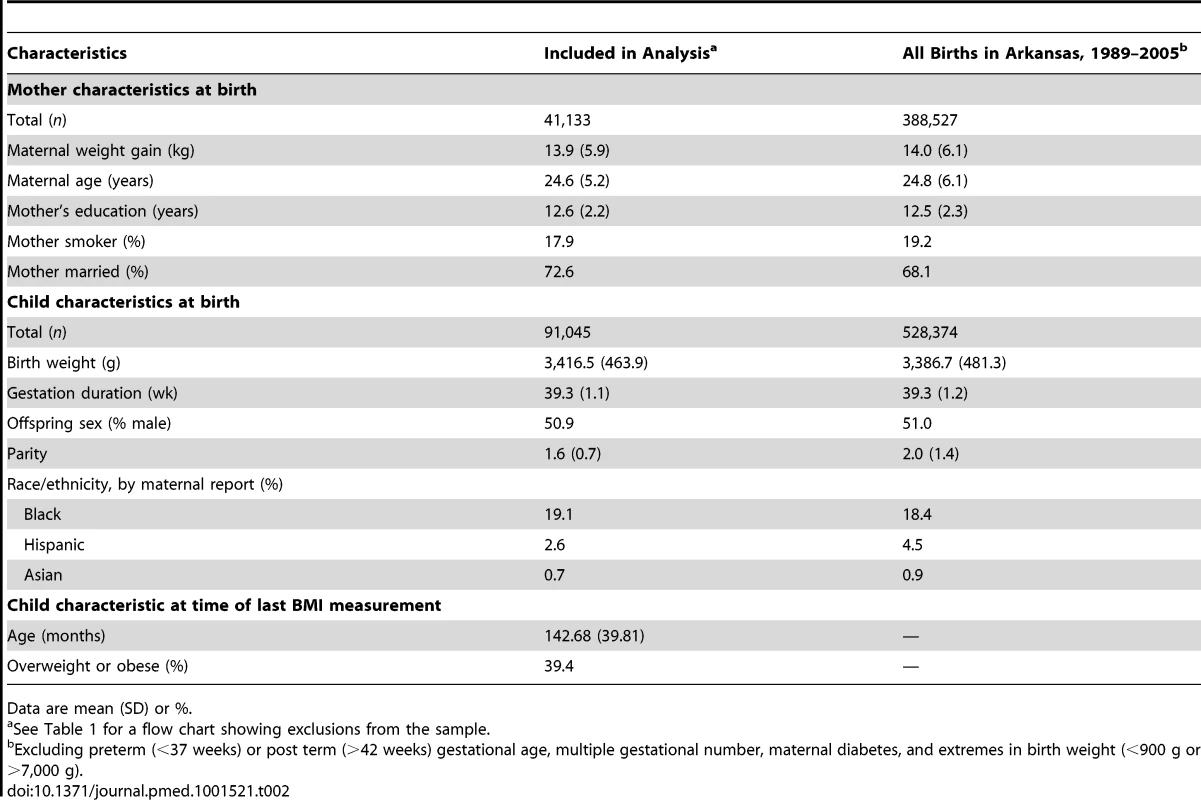
Data are mean (SD) or %. Statistical Analysis
The primary hypothesis was that pregnancy weight gain predicts child BMI, independent of confounding factors. We aimed to minimize confounding in three ways, following the approached used in our prior study of pregnancy weight gain and birth weight [40]. First, we eliminated some sources of potential confounding through sample exclusion criteria, including preterm (<37 weeks) or post term (>42 weeks) gestational age, multiple gestational number, maternal diabetes, and extremes in birth weight that may represent data entry error (<500 g or ≥7,000 g). Second, we incorporated measured confounders in our statistical models. Third, we controlled for residual confounding by measured and unmeasured (e.g., shared genetic and environmental) covariates by comparing offspring born to the same mother. Although we lacked data on prepregnancy BMI and paternal BMI because these variables are not included in the birth records in Arkansas, our inability to consider these covariates would not have produced spurious associations between pregnancy weight gain and childhood BMI (see Discussion).
The dependent variables were child BMI (expressed in a linear fashion) and the odds ratio (OR) for being overweight or obese, both determined at the last available measurement. Our main model regresses the dependent variable on continuous maternal weight gain. We also estimated models that regressed the dependent variable on indicators for the following categories of pregnancy weight gain: <6 kg, ≥12–18 kg, and >18 kg (using ≥6–12 kg as the reference category), an analytic approach that does not constrain the effects of weight gain to be linear. Covariates included in our models were sex of child, maternal marital status, an indicator for maternal smoking during pregnancy, child parity (indicators for parity of 1, 2, 3, 4 or more), the mother's age at the birth (<20, 20–24, 25–29, 30–34, ≥35), indicators for each month of child's age, indicators for each week of gestational age, and indicators for each year of birth (taking into account potential secular trends in childhood BMI unrelated to pregnancy weight gain). Where data on smoking and marital status were missing, we created a category for “missing” in addition to categories for other values, rather than deleting these observations from the dataset (n = 173 for smoking and n = 464 for marital status).
The models of child BMI as a continuous variable include maternal fixed effects and were estimated using the XTREG command in the STATA statistical program (release 11). In this way, we control for any maternal covariate that does not change with time (e.g., maternal height and weight before the first observed pregnancy, although neither was recorded in our database) [46]. XTREG “absorbs” the fixed effects by calculating the mean of the variable for each family and then subtracting the mean for the family from each individual observation. This procedure is mathematically identical, and produces identical estimates, to adding a separate indicator variable for each family, or (in the case of two-child families) to taking the difference between the two siblings. When there are more than two children per family (as is sometimes the case here), our procedure is more efficient than taking differences between adjacent siblings. We estimated standard errors that are robust to heteroskedasticity (i.e., subpopulations with different error variances) using the vce(robust) command in STATA [47]. For the binary outcome of overweight/obese or not, Fixed Effects Logit models were estimated using CLOGIT in STATA [48]. Further information about this statistical procedure is provided in Text S1.
To examine for effect mediation, we added birth weight of the child to the model involving BMI in two ways: as a continuous variable (in grams) and as categories, allowing for non-linear effects. Both models produced qualitatively similar results, and therefore only the linear model is presented. All data are presented as means and standard deviations (SDs) for maternal cohort characteristics or 95% confidence intervals (CI) for outcome data.
Results
As illustrated in Figure 1, the modal weight gain was 12 to 15 kg, accounting for 22.4% of pregnancies. The modal childhood BMI was between 17 and 19 kg/m2, with this category accounting for 17.7% of the sample. The distribution was right-skewed with significant numbers of children in the BMI categories 29–31 (3.2%), 31–33 (2.2%), 33–35 (1.5%), and >35 (3.0%).
Fig. 1. Distribution of maternal weight gain and child BMI in the study cohort. 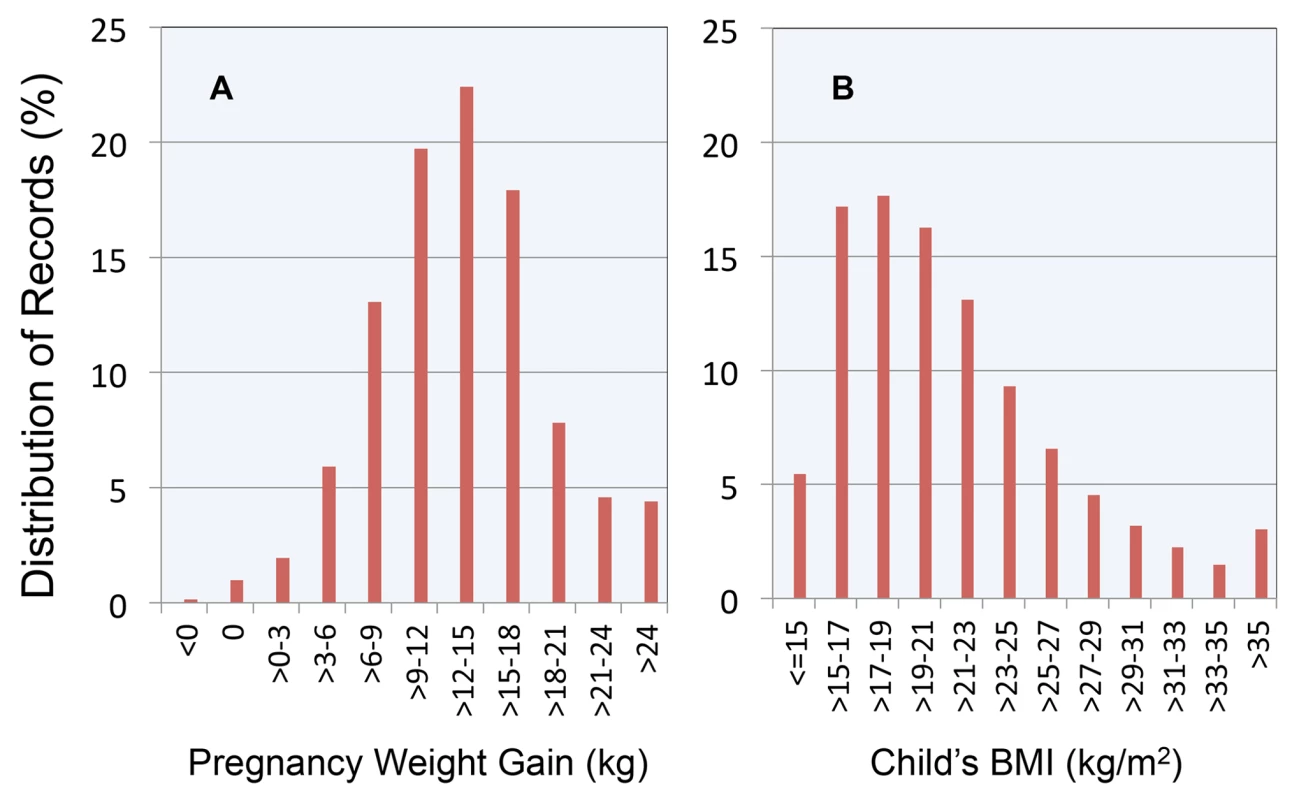
(A) Maternal weight gain; (B) child BMI. Between successive pregnancies, the 10th percentile, 25th percentile, median, 75th percentile, and 90th percentile of changes in key covariates were: maternal pregnancy weight gain (−8.2, −4.1, 0.0, 3.6, 8.2 kg), birth weight (−595, −312, 0.0, 312, 595 g), child's last BMI (−7.5, −3.4, −0.1, 3.3, 7.4 kg/m2), and age of BMI measurement (−4.5, −2,4, 0.0, 2.5, 4.5 y). Thus, we found no evidence of systematic effects of birth order on any of these variables.
Figure 2 replicates findings from our previous study [40], depicting the relationship between pregnancy weight gain and OR for birth weight >4,000 g. The OR of having a child with high birth weight, relative to the reference category, was 1.57 (CI 1.40–1.75, p<0.0001) for women who gained >18 kg.
Fig. 2. Relationship between pregnancy weight gain and odds ratio for high birth weight infant. 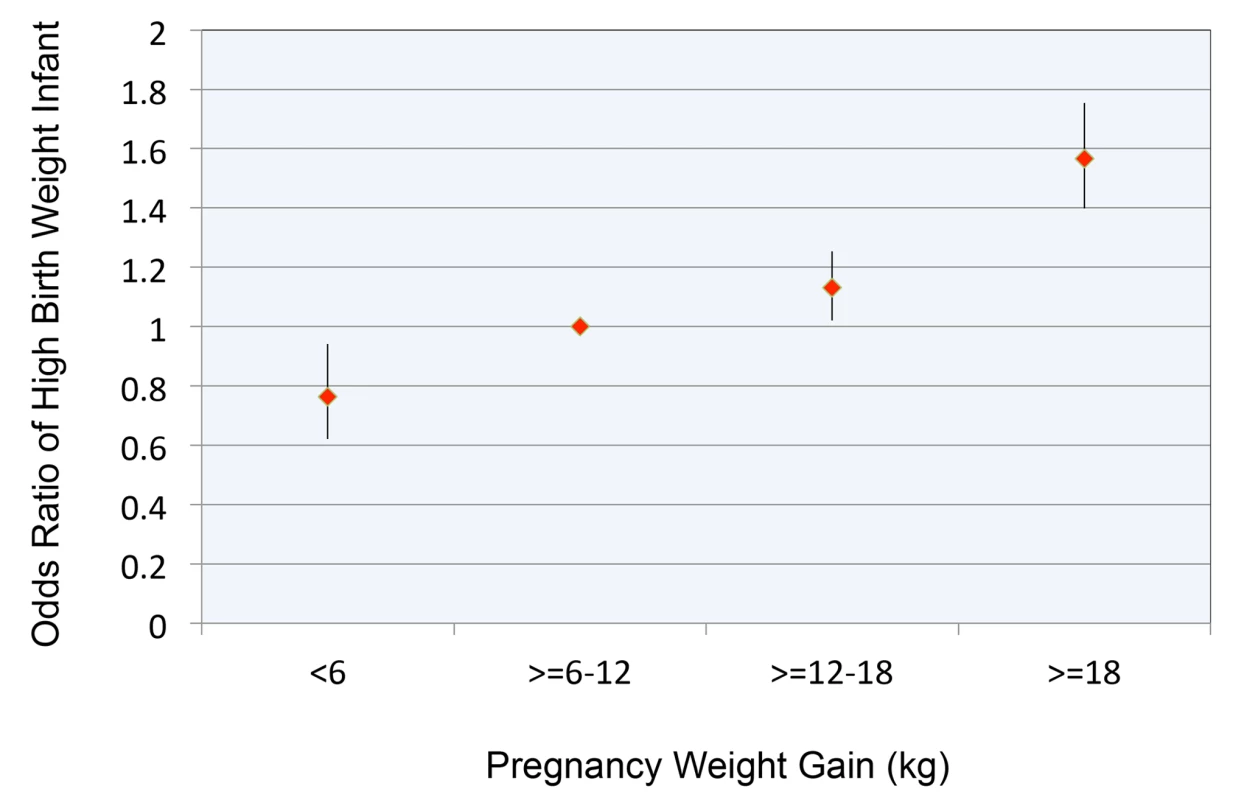
Pregnancy weight gain expressed in kg. Reference range for pregnancy weight gain ≥6 to 12 kg. High birth weight defined as >4,000 g. Error bars are 95% confidence intervals. In our primary analyses, each additional 1 kg of pregnancy weight gain as a continuous variable was associated with a 0.0220 (CI 0.0134–0.0306, p<0.0001) increase in childhood BMI and an OR of 1.007 (CI 1.003–1.012, p = 0.0008) for childhood overweight or obesity. (Tables S1 and S2 provide coefficient estimates for the models of BMI and OR for overweight/obese, respectively.) Adjustment for birth weight modestly attenuated (by approximately 35%) the association between pregnancy weight gain and child BMI. With this adjustment, childhood BMI increased by 0.0143 (CI 0.0057–0.0229, p = 0.0007) for every 1 kg of pregnancy weight gain.
The results of models involving pregnancy weight gain as a categorical variable demonstrated a nearly linear relationship with childhood BMI (Figure 3A) and the OR for childhood overweight or obesity (Figure 3B). The difference in childhood BMI associated with the lowest versus highest category of pregnancy weight gain was 0.43 kg/m2. A child whose mother gained >18 kg versus the reference range had a 1.08 OR (CI 1.01–1.15, p = 0.02) of overweight/obesity.
Fig. 3. Relationship between pregnancy weight gain and body weight in childhood. 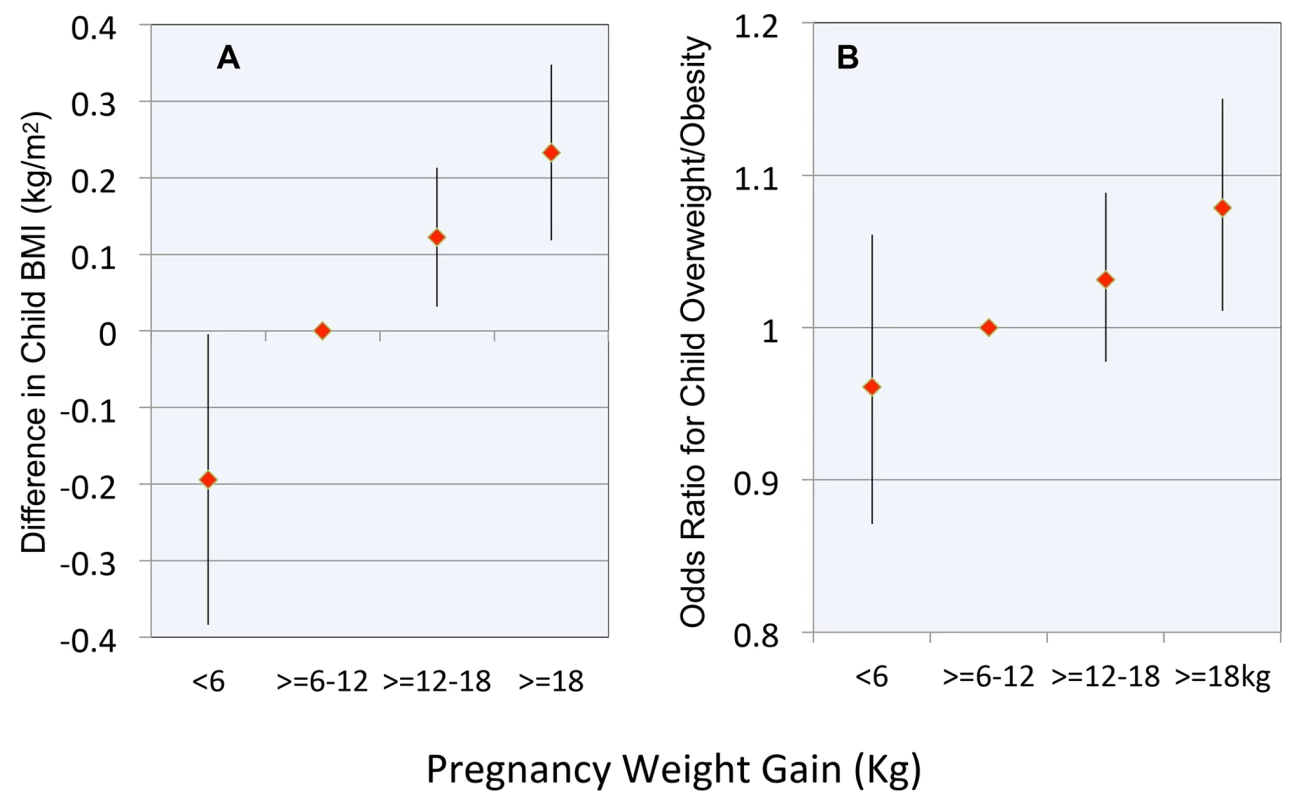
(A) Difference in child BMI; (B) OR for child overweight or obesity. Reference range for pregnancy weight gain is ≥6 to 12 kg. Error bars are 95% confidence intervals. Discussion
Using a population-based cohort contemporaneous with the obesity epidemic and a within-family design to minimize confounding, we found evidence for an independent association between pregnancy weight gain and body weight in childhood. We also observed an association between pregnancy weight gain and birth weight, replicating the finding from a prior study of births in New Jersey and Michigan [40]. However, birth weight mediated less than half of the association between pregnancy weight gain and child BMI. Because childhood body weight predicts adult body weight [49], our study suggests that overnutrition in pregnancy may program the fetus for an increased lifetime risk for obesity, though the magnitude of this effect may be small.
Excessive body weight in childhood is recognized to be complex in origin, and prevention of this highly prevalent medical problem will likely require attention to many modifiable factors. From this perspective, the magnitude of effects in our study, though small on an individual basis, could have important public health implications. Compared to the reference range, the 8% increase in risk among offspring of mothers with high pregnancy weight gain would account for several hundred thousand annual cases of pediatric overweight or obesity worldwide. In addition, for children whose mothers had high versus low pregnancy weight gain, the 0.43 kg/m2 increase in BMI could represent a significant component of the estimated 2 kg/m2 increase in mean childhood BMI in the US since the 1970s [50].
The main limitation of this study is our inability to consider the effect of prepregnancy BMI, because data on height and weight prior to conception were unavailable. Prepregnancy BMI could confound our findings if it were related to both pregnancy weight gain and childhood body weight. Indeed, prepregnancy BMI is positively associated with weight of the offspring later in life [16],[17],[19],[20],[23]–[26]. However, prepregnancy BMI is inversely associated with pregnancy weight gain [19]–[21],[24],[51]–[53]. Because heavier women gain less weight during pregnancy, on average, than normal weight women, our models would therefore tend to underestimate the magnitude of the associations involving pregnancy weight gain. Thus, our findings could not falsely arise from failure to consider prepregnancy BMI and the effect of including prepregnancy BMI into the statistical models, if any, would be to increase the magnitude of the associations. In any event, variations in prepregnancy BMI within individuals in our study would be substantially smaller than variations in conventional analyses involving comparisons between individuals, and the significance of weight change (typically weight gain) over time among women in our study would be further reduced by controlling for parity. Although adequately powered studies are needed to examine the joint effects of pregnancy weight gain and prepregnancy BMI, the former would probably have greater public health significance than the latter for several reasons. Weight management is easier over the short term (i.e., during pregnancy) than the long term (a woman's reproductive years), pregnant women tend to be especially motivated to pursue a healthful lifestyle out of concern for the well-being of their offspring, and many women do not know when they will become pregnant.
Several other study methodological issues warrant comment. The independent variable, pregnancy weight gain, is subject to reporting error that might vary by prepregnancy BMI, education, or levels of prenatal care, but any selective bias would be attenuated by our within-individual design. Random measurement error in any variable would tend to diminish the strengths of the associations, producing bias towards acceptance of the null hypothesis. In contrast, measurement of birth weight has high reliability [41], providing confidence in our estimates of effect mediation. We have no information about the Tanner stage in the BMI records, which are from an administrative school database. Conceivably, pregnancy weight gain could affect pubertal timing, and pubertal timing in turn could affect BMI. In addition, we have no information about paternal BMI. However, the increased genetic variation among siblings with different fathers would tend to decrease the precision, but not the accuracy, of our estimates. Furthermore, although the study cohort was derived from a state-wide registry, the generalizability of these findings to other populations with different baseline characteristics is not known.
Various biological or behavioral factors may mediate or modify our findings, warranting further mechanistically-oriented research. Pregnancy weight gain may influence childhood BMI, in part, through effects on infant body composition independent of birth weight or through programmed changes in body weight regulatory systems, as suggested by animal studies [9],[11],[12]. The effects of pregnancy weight gain on the offspring may also vary by weight status (prepregnancy BMI), diet quality, physical activity levels, genetic background, or other characteristics in the mother, and evidence of such effect modification could help identify populations at special risk of transgenerational obesity propagation.
In conclusion, this study suggests that high pregnancy weight gain increases body weight in childhood and that measures to limit pregnancy weight gain may help prevent obesity in the subsequent generation. However, in view of the observational nature of this study, additional research will be required to assess the relevance of these findings to obesity prevention on a public health basis.
Supporting Information
Zdroje
1. BarkerDJ, GluckmanPD, GodfreyKM, HardingJE, OwensJA, et al. (1993) Fetal nutrition and cardiovascular disease in adult life. Lancet 341 : 938–941.
2. HalesCN, BarkerDJ (1992) Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35 : 595–601.
3. AdamoKB, FerraroZM, BrettKE (2012) Can we modify the intrauterine environment to halt the intergenerational cycle of obesity? Int J Environ Res Public Health 9 : 1263–1307.
4. BarkerDJ (2007) Obesity and early life. Obes Rev 8 Suppl 1 : 45–49.
5. EbbelingCB, PawlakDB, LudwigDS (2002) Childhood obesity: public-health crisis, common sense cure. Lancet 360 : 473–482.
6. HerringSJ, RoseMZ, SkouterisH, OkenE (2012) Optimizing weight gain in pregnancy to prevent obesity in women and children. Diabetes Obes Metab 14 : 195–203.
7. RheeKE, PhelanS, McCafferyJ (2012) Early determinants of obesity: genetic, epigenetic, and in utero influences. Int J Pediatr 2012 : 463850.
8. WhitakerRC, DietzWH (1998) Role of the prenatal environment in the development of obesity. J Pediatr 132 : 768–776.
9. BayolSA, SimbiBH, SticklandNC (2005) A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol 567 : 951–961.
10. LevinBE, GovekE (1998) Gestational obesity accentuates obesity in obesity-prone progeny. Am J Physiol 275: R1374–1379.
11. MuhlhauslerBS, AdamCL, FindlayPA, DuffieldJA, McMillenIC (2006) Increased maternal nutrition alters development of the appetite-regulating network in the brain. FASEB J 20 : 1257–1259.
12. SamuelssonAM, MatthewsPA, ArgentonM, ChristieMR, McConnellJM, et al. (2008) Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: a novel murine model of developmental programming. Hypertension 51 : 383–392.
13. CrozierSR, InskipHM, GodfreyKM, CooperC, HarveyNC, et al. (2010) Weight gain in pregnancy and childhood body composition: findings from the Southampton Women's Survey. Am J Clin Nutr 91 : 1745–1751.
14. FraserA, TillingK, Macdonald-WallisC, SattarN, BrionMJ, et al. (2010) Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation 121 : 2557–2564.
15. HinkleSN, SharmaAJ, SwanDW, SchieveLA, RamakrishnanU, et al. (2012) Excess gestational weight gain is associated with child adiposity among mothers with normal and overweight prepregnancy weight status. J Nutr 142 : 1851–1858.
16. HochnerH, FriedlanderY, Calderon-MargalitR, MeinerV, SagyY, et al. (2012) Associations of maternal prepregnancy body mass index and gestational weight gain with adult offspring cardiometabolic risk factors: the Jerusalem Perinatal Family Follow-up Study. Circulation 125 : 1381–1389.
17. LaitinenJ, JaaskelainenA, HartikainenAL, SovioU, VaarasmakiM, et al. (2012) Maternal weight gain during the first half of pregnancy and offspring obesity at 16 years: a prospective cohort study. BJOG 119 : 716–723.
18. MamunAA, O'CallaghanM, CallawayL, WilliamsG, NajmanJ, et al. (2009) Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation 119 : 1720–1727.
19. Margerison ZilkoCE, RehkopfD, AbramsB (2010) Association of maternal gestational weight gain with short - and long-term maternal and child health outcomes. Am J Obstet Gynecol 202: e571–578, 574, e571-578.
20. Margerison-ZilkoCE, ShrimaliBP, EskenaziB, LahiffM, LindquistAR, et al. (2012) Trimester of maternal gestational weight gain and offspring body weight at birth and age five. Matern Child Health J 16 : 1215–1223.
21. OkenE, Rifas-ShimanSL, FieldAE, FrazierAL, GillmanMW (2008) Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol 112 : 999–1006.
22. OkenE, TaverasEM, KleinmanKP, Rich-EdwardsJW, GillmanMW (2007) Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol 196: e321–328, 322, e321-328.
23. OlsonCM, StrawdermanMS, DennisonBA (2009) Maternal weight gain during pregnancy and child weight at age 3 years. Matern Child Health J 13 : 839–846.
24. Schack-NielsenL, MichaelsenKF, GamborgM, MortensenEL, SorensenTI (2010) Gestational weight gain in relation to offspring body mass index and obesity from infancy through adulthood. Int J Obes (Lond) 34 : 67–74.
25. StuebeAM, FormanMR, MichelsKB (2009) Maternal-recalled gestational weight gain, pre-pregnancy body mass index, and obesity in the daughter. Int J Obes (Lond) 33 : 743–752.
26. WhitakerRC (2004) Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 114: e29–36.
27. WrotniakBH, ShultsJ, ButtsS, StettlerN (2008) Gestational weight gain and risk of overweight in the offspring at age 7 y in a multicenter, multiethnic cohort study. Am J Clin Nutr 87 : 1818–1824.
28. BranumAM, ParkerJD, KeimSA, SchempfAH (2011) Prepregnancy body mass index and gestational weight gain in relation to child body mass index among siblings. Am J Epidemiol 174 : 1159–1165.
29. LawlorDA, TimpsonNJ, HarbordRM, LearyS, NessA, et al. (2008) Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med 5: e33 doi:10.1371/journal.pmed.0050033
30. LawlorDA, LichtensteinP, FraserA, LangstromN (2011) Does maternal weight gain in pregnancy have long-term effects on offspring adiposity? A sibling study in a prospective cohort of 146,894 men from 136,050 families. Am J Clin Nutr 94 : 142–148.
31. Davey SmithG, SteerC, LearyS, NessA (2007) Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC). Arch Dis Child 92 : 876–880.
32. FletenC, NystadW, StigumH, SkjaervenR, LawlorDA, et al. (2012) Parent-offspring body mass index associations in the Norwegian Mother and Child Cohort Study: a family-based approach to studying the role of the intrauterine environment in childhood adiposity. Am J Epidemiol 176 : 83–92.
33. KivimakiM, LawlorDA, SmithGD, ElovainioM, JokelaM, et al. (2007) Substantial intergenerational increases in body mass index are not explained by the fetal overnutrition hypothesis: the Cardiovascular Risk in Young Finns Study. Am J Clin Nutr 86 : 1509–1514.
34. KnightB, ShieldsBM, HillA, PowellRJ, WrightD, et al. (2007) The impact of maternal glycemia and obesity on early postnatal growth in a nondiabetic Caucasian population. Diabetes Care 30 : 777–783.
35. LearyS, Davey SmithG, NessA (2010) No evidence of large differences in mother-daughter and father-son body mass index concordance in a large UK birth cohort. Int J Obes (Lond) 34 : 1191–1192.
36. PatelR, MartinRM, KramerMS, OkenE, BogdanovichN, et al. (2011) Familial associations of adiposity: findings from a cross-sectional study of 12,181 parental-offspring trios from Belarus. PLoS ONE 6: e14607 doi:10.1371/journal.pone.0014607
37. LawlorDA, SmithGD, O'CallaghanM, AlatiR, MamunAA, et al. (2007) Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the mater-university study of pregnancy and its outcomes. Am J Epidemiol 165 : 418–424.
38. GuenardF, DeshaiesY, CianfloneK, KralJG, MarceauP, et al. (2013) Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proc Natl Acad Sci U S A 110 : 11439–1144.
39. LawlorDA, LichtensteinP, LangstromN (2011) Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation 123 : 258–265.
40. LudwigDS, CurrieJ (2010) The association between pregnancy weight gain and birthweight: a within-family comparison. Lancet 376 : 984–990.
41. NorthamS, KnappTR (2006) The reliability and validity of birth certificates. J Obstet Gynecol Neonatal Nurs 35 : 3–12.
42. BuescherPA, TaylorKP, DavisMH, BowlingJM (1993) The quality of the new birth certificate data: a validation study in North Carolina. Am J Public Health 83 : 1163–1165.
43. Dietz PM (2012) Evaluating the 2003 Birth Certificate Data: Preliminary Findings from the PRAMS Data Quality Improvement Project. Available: http://www.cdc.gov/nchs/ppt/nchs2012/SS-19_DIETZ.pdf. Accessed 28 August 2013.
44. JustusMB, RyanKW, RockenbachJ, KatterapalliC, Card-HigginsonP (2007) Lessons learned while implementing a legislated school policy: body mass index assessments among Arkansas's public school students. J Sch Health 77 : 706–713.
45. CDC (2011) About BMI for Children and Teens. Available: http://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html. Accessed 28 August 2013.
46. Allison PD (2009) Fixed effects regression models. Los Angeles: Sage Publications.
47. Wooldridge JM (2002) Econometric analysis of cross sectional and panel data. Cambridge (Massachusetts): MIT Press.
48. ChamberlainG (1980) Analysis of Covariance with Qualitative Data. Rev Economic Studies 47 : 225–238.
49. WhitakerRC, WrightJA, PepeMS, SeidelKD, DietzWH (1997) Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 337 : 869–873.
50. WangYC, OrleansCT, GortmakerSL (2012) Reaching the healthy people goals for reducing childhood obesity: closing the energy gap. Am J Prev Med 42 : 437–444.
51. DietzPM, CallaghanWM, SharmaAJ (2009) High pregnancy weight gain and risk of excessive fetal growth. Am J Obstet Gynecol 201: e51–56, 51, e51-56.
52. NohrEA, VaethM, BakerJL, SorensenTI, OlsenJ, et al. (2009) Pregnancy outcomes related to gestational weight gain in women defined by their body mass index, parity, height, and smoking status. Am J Clin Nutr 90 : 1288–1294.
53. WiseLA, PalmerJR, HeffnerLJ, RosenbergL (2010) Prepregnancy body size, gestational weight gain, and risk of preterm birth in African-American women. Epidemiology 21 : 243–252.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2013 Číslo 10- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Modelling the Strategic Use of Antiretroviral Therapy for the Treatment and Prevention of HIV
- Psychosocial Interventions for Perinatal Common Mental Disorders Delivered by Providers Who Are Not Mental Health Specialists in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis
- Predicting Patterns of Long-Term CD4 Reconstitution in HIV-Infected Children Starting Antiretroviral Therapy in Sub-Saharan Africa: A Cohort-Based Modelling Study
- Use of Expert Panels to Define the Reference Standard in Diagnostic Research: A Systematic Review of Published Methods and Reporting
- Elimination of HIV in South Africa through Expanded Access to Antiretroviral Therapy: A Model Comparison Study
- A Transcriptional Signature for Active TB: Have We Found the Needle in the Haystack?
- Poor Health in Rich Countries: A Role for Open Access Journals
- The 2003 Iraq War and Avoidable Death Toll
- Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data
- The Final Push for Polio Eradication: Addressing the Challenge of Violence in Afghanistan, Pakistan, and Nigeria
- Saving Lives in Health: Global Estimates and Country Measurement
- Complexity in Mathematical Models of Public Health Policies: A Guide for Consumers of Models
- Mortality in Iraq Associated with the 2003–2011 War and Occupation: Findings from a National Cluster Sample Survey by the University Collaborative Iraq Mortality Study
- Why We Must Provide Better Support for Pakistan's Female Frontline Health Workers
- Effect on Postpartum Hemorrhage of Prophylactic Oxytocin (10 IU) by Injection by Community Health Officers in Ghana: A Community-Based, Cluster-Randomized Trial
- The Prevention of Postpartum Hemorrhage in the Community
- Pregnancy Weight Gain and Childhood Body Weight: A Within-Family Comparison
- Detection of Tuberculosis in HIV-Infected and -Uninfected African Adults Using Whole Blood RNA Expression Signatures: A Case-Control Study
- A New Approach to Psychiatric Drug Approval in Europe
- Utility of the Xpert MTB/RIF Assay for Diagnosis of Tuberculous Meningitis
- Methodological and Policy Limitations of Quantifying the Saving of Lives: A Case Study of the Global Fund's Approach
- Diagnostic Accuracy of Quantitative PCR (Xpert MTB/RIF) for Tuberculous Meningitis in a High Burden Setting: A Prospective Study
- Assessing Optimal Target Populations for Influenza Vaccination Programmes: An Evidence Synthesis and Modelling Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Effect on Postpartum Hemorrhage of Prophylactic Oxytocin (10 IU) by Injection by Community Health Officers in Ghana: A Community-Based, Cluster-Randomized Trial
- Utility of the Xpert MTB/RIF Assay for Diagnosis of Tuberculous Meningitis
- Modelling the Strategic Use of Antiretroviral Therapy for the Treatment and Prevention of HIV
- Diagnostic Accuracy of Quantitative PCR (Xpert MTB/RIF) for Tuberculous Meningitis in a High Burden Setting: A Prospective Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



