-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Papillary Carcinoma of Thyroid Gland in a Two-year-old Child
Papilárny karcinóm štítnej žľazy u dvojročného dieťaťa
Cieľ:
Ochorenia štítnej žľazy u detí sú zriedkavé. Na rozdiel od prevalencie, malígne nádory sú častejšie u detí (26 % tyreopatií) v porovnaní s dospelými (5 – 10 % tyreopatií). K rizikovým faktorom ich vzniku patrí ženské pohlavie, postpubertálny vek, už existujúce ochorenie štítnej žľazy, predchádzajúce ožarovanie oblasti krku a pozitívna rodinná anamnéza tyreopatie. U detí mladších ako 10 rokov, bez prítomnosti rizikových faktorov, sa malígne nádory štítnej žľazy vyskytujú raritne.Kazuistika:
Dvojročné zdravé dievčatko bolo prijaté na naše pracovisko pre uzol laloka štítnej žľazy vpravo. Hladina tyreoidálnych hormónov bola v norme. Ultrasonografické vyšetrenie opísalo nehomogénny uzol veľkosti 4,8 × 3,2 × 2,5 cm. Dieťa podstúpilo pravostrannú hemityreoidektómiu. Na základe výsledku histopatologického vyšetrenia, ktoré odhalilo papilárny karcinóm štítnej žľazy, bola následne vykonaná reoperácia v zmysle dokončenia tyreoidektómie a selektívna disekcia lymfatických uzlín krku.Záver:
Prezentujeme veľmi vzácny prípad papilárneho karcinómu štítnej žľazy u dvojročného dieťaťa bez prítomnosti rizikových faktorov. Výskyt uzla štítnej žľazy u takto malých detí môže byť podmienený malignitou, preto vyžaduje podrobnú diagnostiku a vhodnú liečbu.Kľúčové slová:
papilárny karcinóm – štítna žľaza – detský vek – epidemiológia – diagnostika – liečba
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.Obdržané:
3. 11. 2015Prijaté:
7. 12. 2015
Authors: B. Uhliarová 1; A. Hajtman 2
Authors place of work: Department of Otorhinolaryngology, FD Roosevelt Faculty Hospital, Banska Bystrica, Slovak Republic 1; Department Of Otorhinolaryngology, Head and Neck Surgery, Comenius University, Jessenius Faculty of Medicine, University Hospital, Martin, Slovak Republic 2
Published in the journal: Klin Onkol 2016; 29(2): 139-144
Category: Kazuistika
doi: https://doi.org/10.14735/amko2016139Summary
Background:
Thyroid nodules are less common among children than among adults. By contrast, thyroid nodules are more often malignant in childhood than in adulthood. In children, 26% of thyroid nodules are malignant, while in adults the corresponding value is 5–10%. Risk factors for developing thyroid nodules in children are female sex, post-pubertal age, previous or co-existing thyroid disease, previous irradiation of the neck, and a family history of thyroid disease. In children younger than 10 years, when no risk factors are present, the incidence rates are practically negligible.Case report:
A two-year-old girl presented with a right thyroid mass. Laboratory evaluation revealed normal levels of triiodothyronine and thyroid-stimulating hormone. Thyroid ultrasonography revealed a 4.8 × 3.2 × 2.5 cm nonhomogenous nodule. The patient underwent right hemithyroidectomy. The pathology was consistent with papillary thyroid carcinoma; therefore, total thyroidectomy and selective neck dissection were performed.Conclusions:
We report a very rare case of papillary thyroid carcinoma in a two-year-old child with no risk factors. The detection of a thyroid nodule in such a young child with no pre-disposing risk factors does not exclude the possibility of thyroid carcinoma and warrants careful evaluation and appropriate therapy.Key words:
papillary carcinoma – thyroid gland – children – epidemiology – diagnosis – treatmentIntroduction
Thyroid nodules are less common in children and adolescents and have a prevalence between 0.2% and 1.8%, whereas in adults it is around 4 – 7%. In contrast to prevalence, thyroid nodules are more often malignant in children than in adults. In children, 26% of thyroid nodules are malignant, while in adults the corresponding value is 5–10% [1]. Risk factors for developing thyroid nodules in children are female sex, post-pubertal age, previous or co-existing thyroid disease, previous irradiation of the neck, and a family history of thyroid disease [2,3].
We present a very rare case of papillary carcinoma of thyroid gland in a two-year-old child with no risk factors.
Case report
A two-year-old girl was referred to a pediatric endocrinologist by a pediatrician where she was evaluated for growing painless right thyroid mass of six weeks duration. No change in her appetite or weight had been noted. Her mother denied hoarseness, heat intolerance, changes in bowel habits, hair loss, difficulty swallowing, or prior radiation exposure of her daughter. Her medical history included a normal birth and was unremarkable. Her family history was not significant for thyroid diseases.
She had a visible swelling of the right lobe of her thyroid with a palpable nontender 5 × 3 cm nodule. The thyroid moved with swallowing. No lymph nodes were palpated. She had no lid lag or tongue fasciculations. Heart, lung, and abdominal examinations were normal. On neurological examination, she had normal deep tendon reflexes and no weakness.
The thyroid ultrasonography showed a large nonhomogenous 4.8 × 3.2 × 2.5 cm nodule in the right lobe, left lobe had normal echostructure with normal size of 1.3 × 0.9 × 2.3 cm.
Thyroid function tests revealed that her thyroid-stimulating hormone (TSH) was 2.234 mIU/ L (reference range, 0.5 – 4.70 mIU/ L; International System of Units (SI)), free thyroxine was 14.7 pmol/ L (reference range, 10 – 23 pmol/ L; SI), and her calcitonine was 3.6 pg/ mL (reference range, < 10 pg/ mL; SI). Her TSH receptor antibody was undetectable (< 6%). Her thyroid peroxidase antibody and thyroglobulin antibody were negative. Her thyroglobulin concentration was 37 µg/ L (reference range, ≤ 60 µg/ L; SI).
Correlation of clinical features, imaging results, and laboratory data led to the conclusion of an euthyroid solitary nodule and child was referred to Department of Otorhinolaryngology, Head and Neck Surgery, Comenius University, Jessenius Faculty of Medicine, University Hospital in Martin, Slovak Republic, for surgery. Subsequently, she underwent a surgical revision with peroperative excision of the thyroid nodule. No specific patterns of papillary thyroid carcinoma (PTC) were observed on peroperative frozen section examination. Thus, based on the clinical and peroperative histological findings, right hemithyroidectomy was performed (Fig. 1 – 3). The final pathologic examination of thyroid nodule revealed follicular variant of papillary carcinoma with incomplete infiltration of thyroid capsule, without extrathyroidal or intravascular propagation of the tumour. Upon diagnosisof PTC, additional evaluation was conducted. Chest X-ray showed no evidence of metastatic disease. Approxi-mately two weeks after her initial surgery, a complete thyroidectomy with selective lymph node dissection was performed (Fig. 4). The final pathology from the left thyroid completion thyroidectomy showed normofollicular tissue of thyroid gland, and PTC was not detected. No metastasis in lymph nodes was identified. The disease was staged as stage I (T4a N0 M0). Subsequently, she underwent 131I radioactive ablation.
Fig. 1. Right hemithyroidectomy. 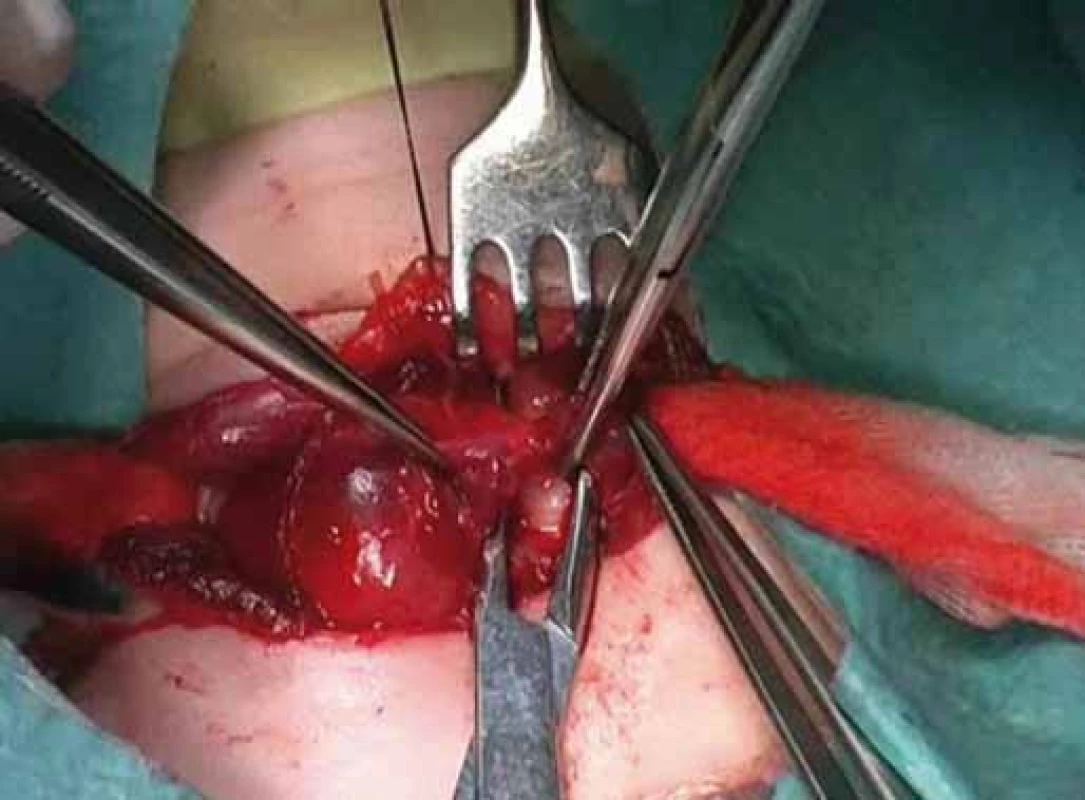
Fig. 2. Thyroid nodule of right lobe. 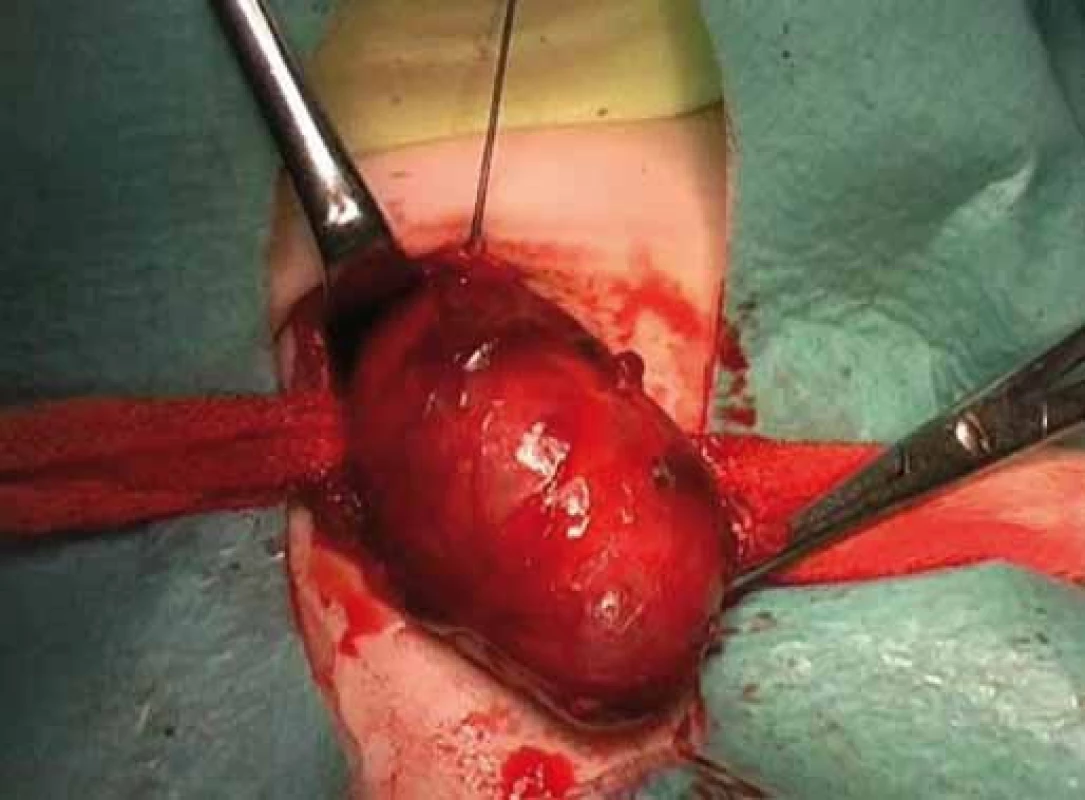
Fig. 3. Excision of thyroid nodule. 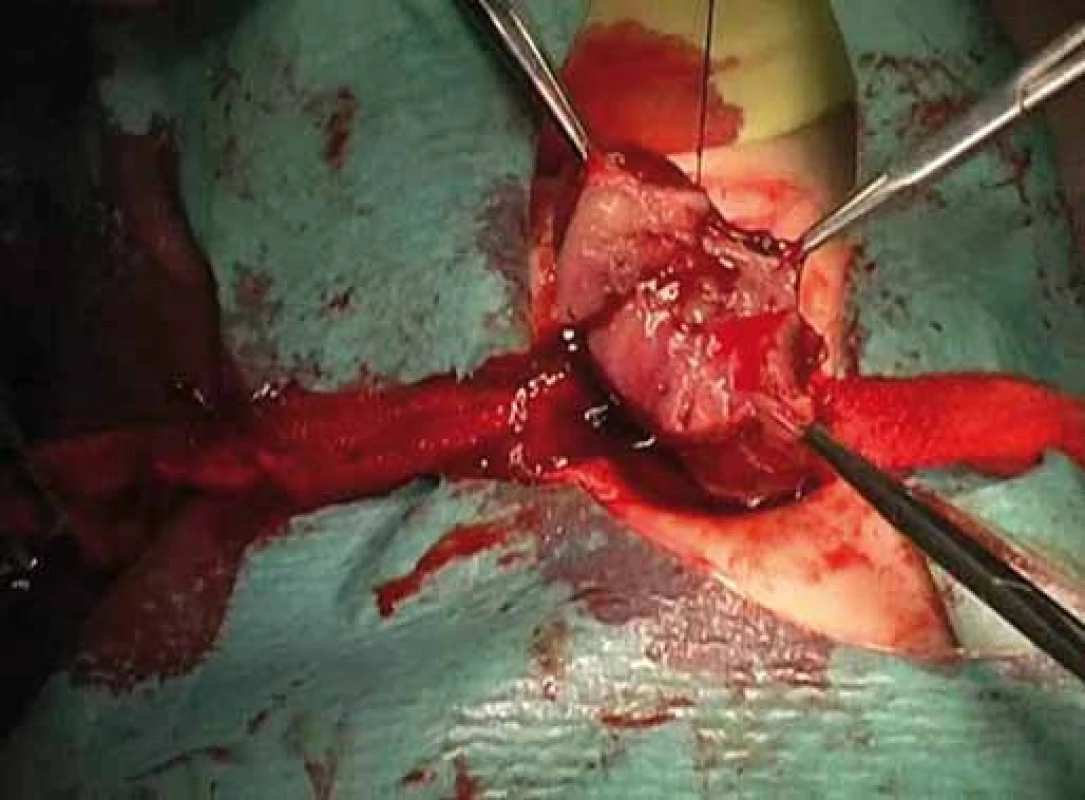
Fig. 4. Completion of thyroidectomy. 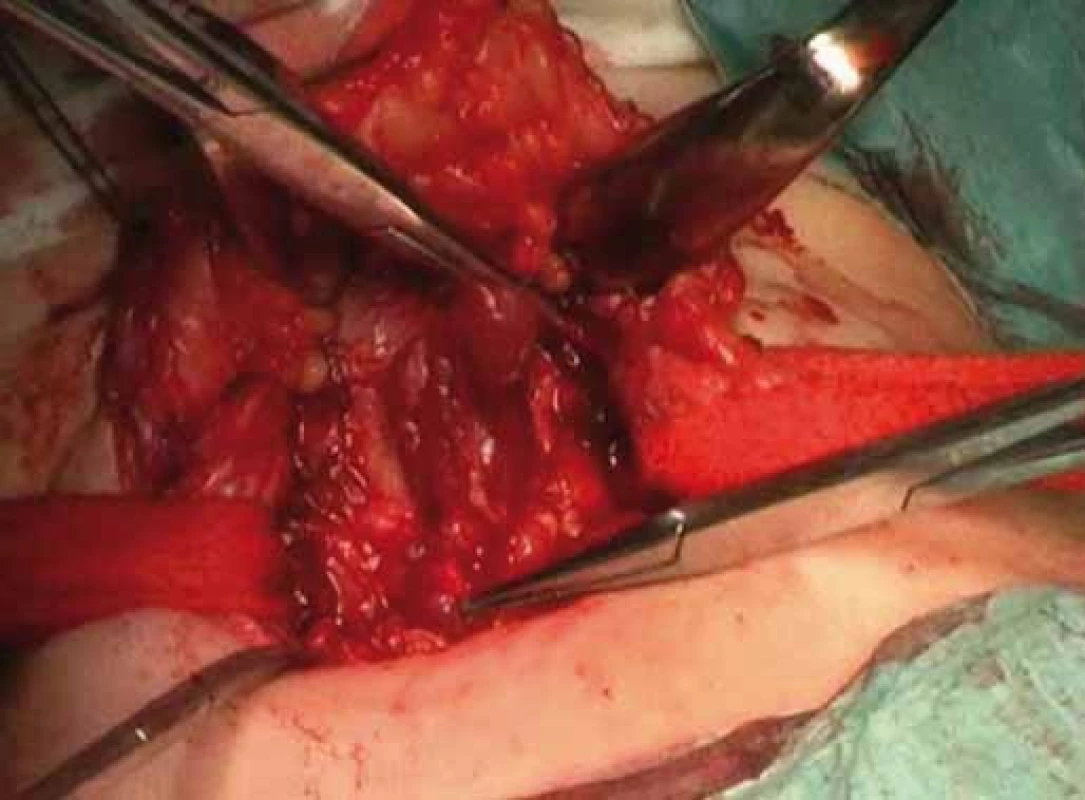
Two years after thyroidectomy, thyroid scan showed no significant uptake in the thyroid bed or elsewhere in her body, and thyroglobulin concentration was < 1 µg/ L (reference range, ≤ 60 µg/ L; SI) consistent with remission. She is maintained on thyroid hormone replacement therapy for thyroidectomy and postablative hypothyroidism.
Discussion
Epidemiology
Endocrine cancers are very rare in children, with thyroid cancer being the most common one constituting 0.5% to 3% of all childhood malignancies. The incidence in females is higher, with a 3 : 1 ratio before 15 years and 6 : 1 in the 15 – 19 year age group [4–6]. In very young children, incidence rates are practically negligible.
Thyroid carcinomas in childhood are almost always well differentiated. Recently, a multicentric study conducted on 120 pediatric patients with a thyroid nodule not associated with risk factors, such as autoimmune thyroid diseases or radiotherapy revealed a 16% occurrence of thyroid carcinoma, with 12% papillary, 2.5% follicular, and 1.7% medullary histotypes [7].
Familial occurrence for thyroid neoplasms is striking and well-documented for medullary thyroid cancer, and it is also frequently present in other histotypes [4]. Preexisting thyroid diseases represent a further risk factor. Cases of cancer have also been described in congenital hypothyroidism due to dyshormonogenesis, in iodide organification defects, in ectopy, as well as in thyroid hemiagenesis and thyroglossal duct cysts, usually of follicular histotypes [3].
Exposure to ionizing radiations is another well-known risk factor for thyroid cancer, especially for papillary carcinoma. Children’s thyroid gland is much more sensitive to carcinogenic effects of ionizing radiation than that of adults. One explanation of this interesting biologic phenomenon is that thyrocytes have a very low division rate at adult age compared to younger age groups. Radiation-induced mutations are thus less likely to be transmitted to later generations of cells in higher age groups in view of the early expiration of the potency of thyrocytes to divide [8,9].
Lower doses than those observed for most other radiation induced cancers may induce thyroid carcinogenesis (to the order of 0.10 Gy). High doses (> 30 Gy) result in a lowered risk of thyroid cancer, probably due to cell killing [10]. From a historical viewpoint, the high incidence of thyroid cancers between 1940 and 1960 following the extensive use of low dose radiotherapy for benign pathologies of the head, neck, and chest like tinea capitis, scrofula, and an enlarged thymus is worth mentioning. In one of the earliest reports on this subject, Duffy et al. [11] showed how 35% of the pediatric patients with thyroid cancer had previously been irradiated for thymus enlargement.
A great deal of our knowledge about radiation effects on thyroid carcinogenesis arises from evidence from exposure to radioactive isotopes in the fallout from Chernobyl in April 1996. The relative incidence of thyroid cancer had increased from 0.1 – 0.3/ 100 000 before the accident to 3.3 – 13.5/ 100,000 in 1990 – 1996 [12]. Almost exclusively, a papillary histotype developed [13] that exhibited aggressive growth and early metastasis [14].
Nowadays, history of previous neckirradiation is obtained mostly in childhood cancer survivors. The risk of thyroid cancer increases in parallel to radiation dosages of up to 20 – 29 Gy, but then falls at higher doses [10]. Furthermore, there is increasing evidence that chemotherapy might also be a causative factor [15].
There has been ongoing debate regarding the existence of distinct genetic alterations. Efforts have been made to identify acquired genetic abnormalities that will determine the tumour biological behavior and ultimately allow molecular prognostication. Most of the studies have been performed in radiation-exposed pediatric thyroid carcinoma, while most routine cases of thyroid cancer are indeed sporadic cases. New driver genes that are altered in radiation-exposed pediatric PTC cases have been identified. Nearly 84% had fusion of genes, most oncoproteins activate MAPK pathways, suggesting that pediatric PTC are also MAPK-driver cancer. Conversely, the prevalence of drive fusion oncogenes in sporadic pediatric PTC was much lower. Nearly 30% of cases are negative for fusion events and/ or point mutations found in radiation-induced pediatric cohort [16,17]. As the risk factor for the development of sporadic pediatric thyroid carcinoma is not known and the landscape of sporadic pediatric cancer likely differs significantly from the landscape of the radiation-exposed pediatric cases, it is expected that sporadic cases might have higher prevalence of point mutations than radiation-induced pediatric thyroid carcinomas.
In our case report, the child was a very young two-year-old girl, with no history of previous irradiation of the neck nor family history of thyroid disease. Papillary carcinoma developed in eufunctioning thyroid gland. Genetic investigation of thyroid tissue was not performed.
Considering the fact that this malignancy occurred in a two-year-old child, it can be hypothesized that so far unrecognized germinal mutations/ tumor predisposition could initiate the disease. Therefore, the child is in secondary prevention program (not only to detect recurrence but event. also second primary tumor) and is followed by pediatric oncologist.
Differences in thyroid carcinoma between children and adults
Several studies have shown that thyroid carcinoma in pediatric patients differs from thyroid carcinoma in adults with respect to its presentation and its outcome. Compared with adults, thyroid cancers occurring in children are more aggressive, are discovered in advanced stages, and are associated with higher rates of recurrences [18 – 20].
Lymph node involvement at diagnosis is very frequent in children. Grisby et al. [19] reported a 29% incidence of cervical adenopathy clinically, but pathologically, 73% of patients had cervical lymph node disease. Similar results were observed by other authors [21,22]. Another commonpresenting sign is pulmonary metastases. The incidence of lung metastasis at presentation is reported to range from 6% to 33% [18,19,23]. It is noteworthy that no child with thyroid carcinoma reported in the literature has had bone metastasis at diagnosis. This is in contrast to the frequent occurrence of bone metastasis in adults [19]. Additionally, recurrence rates tend to be higher in children. Long-term follow-up data shows up to 30% of children with differentiated thyroid carcinoma have recurrent disease [24]. Significant prognostic factors associated with the development of recurrent thyroid carcinoma, such as capsular invasion, soft tissue invasion, positive margins, and tumor location at diagnosis (thyroid only vs. thyroid and lymph nodes vs. thyroid, lymph nodes, and lung metastasis) were identified. In the study by Grisby et al. [19], none of the patients developed recurrent disease when the initial disease was confined to the thyroid. This was in contrast to a 50% recurrence rate in patients with thyroid and lymph node disease at diagnosis and a 29% recurrence rate in patients with thyroid, lymph node, and lung metastasis at diagnosis.
Taken together, thyroid carcinoma in pediatric patients tends to present with disease at a more advanced stage than adults. But, paradoxically, their overall survival rate is excellent.
Long-term follow-up data show 20-year survival rates of 90–99% for children with thyroid carcinoma [25,26]. The discrepancy between aggressive tumor presentation and excellent prognosis in children is not well explained. One suggestion is that this observation may be related to the nondiploid DNA content of the tumor. Zimmerman et al. [21] found that 25% of tumors in adults had nondiploid DNA compared with only 10% of tumors in children. Another suggestion for the difference in prognosis between children and adults is that thyroid tumors in children have a larger dependence on TSH. Therefore, TSH suppression with thyroid hormone replacement is a more effective treatment in children. In adults, over time, well differentiated thyroid carcinoma may dedifferentiate to poorly differentiated thyroid carcinoma, especially in the course of the development of metastatic disease. Children may have a better prognosis because of a lack of progression from well to poorly differentiated neoplasms [27].
Management of thyroid nodules in children
Diagnosis
Although thyroid cancer in children usually has very indolent course, even with pulmonary metastases, early diagnosis is important to identify patients as soon as possible who need to undergo surgery. The diagnostic steps for thyroid nodules in children and adolescents are similar to adults. In children, the traditional diagnostic approach to thyroid nodules consists of clinical, laboratory, and imaging evaluations. Risk factors for malignancy of thyroid nodules are a fast growing nodule, family history of thyroid carcinoma, previous neck irradiation, hoarseness, a very firm nodule, fixation of the nodule to adjacent structures, and cervical lymphadenopathy [1,3,28].
In the evaluation and management of nodular thyroid diseases, ultrasonography and fine needle aspiration biopsy (FNAB) are most important. Thyroid ultrasonography is the imaging method of choice for the evaluation of thyroid gland structure. Several ultrasound characteristics have been studied as potential predictors of thyroid malignancy. These characteristics are solid lesions, subcapsular nodule location, hypoechogenecity, microcalcifications, irregular margins, no halo, intranodular vascularity, and lesions of more height rather than width [29,30]. Sonographic features of associated lymph nodes also potentially increase the likelihood of malignancy, thus suggesting further diagnostic investigation. The ratio between the longitudinal and transversal axes of the lymph nodes below 1.5 (normally greater than 2), rounded profile of a lymph node, absence of hilum, presence of eccentric cortical thickening, nonhomogeneous pattern, and the increased vascular flow are other parameters commonly included as indicators of malignancy [31,32].
Furthermore, regular ultrasound screening should be performed in survivors of malignancy in childhood who received neck irradiation. After a first tumor in childhood, thyroid cancers are most likely to arise 6–7 years or more after the primary cancer, and the radiation-associated risk for thyroid cancer remains elevated for at least 20 years [33]. With these premises and considering that an early diagnosis might improve the outcome, it has recently been suggested to screen the population of childhood cancer survivors who had previously undergone radiotherapy involving the head, neck or upper thorax by ultrasound [3].
In the last 30 years, FNAB has become a cornerstone in the evaluation of solitary thyroid nodules, cysts, and dominant nodules within multinodular goiters in adults. Fewer data are available in pediatrics given the consistently lower occurrence of the disease. Studies dealing with this issue recently [2,3] estimated its diagnostic accuracy as ranging from 75 to 95%, a value approximating that reported for adults.
The important question is whether FNAB is the most useful procedure to detect malignancy. In our experience, a biopsy is not always necessary as its diagnostic value is somehow limited due to false negative biopsy results, especially in the case of follicular histology [34,35], and might be too incriminatory in young children. From our experiences with adult and pediatric thyroidectomies, the indications for surgical revision were a dynamic change of a thyroid nodule, increase in size over time and signs of activation in laboratory values independent of histological confirmation.
In this case, a preoperative fine needle aspiration biopsy was not performed, and surgery was indicated according to the clinical, laboratory and ultrasonography findings.
Treatment
The primary treatment of thyroid nodules should be surgical. Thyroidectomy (total or hemithyroidectomy) is the most effective treatment modality of thyroid nodules in children and adults [28,36]. In management of solitary thyroid nodules, peroperative histopathologic investigation of thyroid nodule is a useful procedure that helps to distinguish benign from malignant pathology and modify the extent of the surgery – hemithyroidectomy vs. total thyroidectomy. Nonetheless, difficulties remain in distinguishing thyroid carcinoma from benign lesions, particularly in the case of follicular thyroid carcinoma vs. follicular adenoma or the follicular variant of PTC [37].
In our case report, according to results of peroperative histology that did not show structures of papillary carcinoma, hemithyroidectomy was indicated. Definitive histopathological investigation showed follicular variant of PTC; therefore, total thyroidectomy and selective lymph node dissection was performed.
The optimal management of children and adolescents is not well defined. Low treatment morbidity is a critical requirement of any strategy for this group of patients with an excellent prognosis and long life expectancy. Some investigators suggest that limited surgery that has lower incidence of complications, mainly permanent hypoparathyroidism, and subsequent thyroid hormone suppressive therapy are the most effective means of controlling this disease [21,24,38]. The majority of investigators recommend a combination of total thyroidectomy, selective lymph node dissection, postoperative 131I therapy, and thyroid hormone suppressive therapy [18,38]. Total thyroidectomy is a safe operation with low incidence of permanent nerve palsies or hypoparathyroidism when performed by experienced surgeon [19,28,36]. No complication was observed in our patient. Moreover, subsequent regular screening of thyroglobulin levels after total thyroidectomy helps to detect early recurrence of thyroid carcinoma.
Conclusion
Thyroid cancer comprises 0.5–3% of all childhood tumors and represents the most common head and neck malignant tumor in young people. Risk factors for developing thyroid nodules in children are female sex, post-pubertal age, previous or co-existing thyroid disease, previous irradiation of the neck, and a family history of thyroid disease. In children younger than 10 years, when no risk factors are present, the incidence rates are practically negligible.
We report a very rare case of PTC in a two-year-old child with no risk factors. The child was treated by total thyroidectomy and selective lymph node dissection. Postoperative 131I therapy and thyroid hormone suppressive therapy was administered. Our treatment recommendations are standard for all children and adolescents. Recurrence is common and lifelong follow-up is essential. Follow-up evaluation should rely on periodic physical examination, thyroglobulin measurements, and total body 131I scintigrams.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE recommendation for biomedical papers.
Submitted: 3. 11. 2015
Accepted: 7. 12. 2015
Barbora Uhliarova, MD, PhD.
Department of Otorhinolaryngology
FD Roosevelt Faculty Hospital
Námestie L. Svobodu 1
974 01 Banska Bystrica
Slovak Republic
e-mail: b.uhliarova@gmail.com
Zdroje
1. Uhliarová B, Bugová G, Hajtman A. Malignant tumors of thyroid gland. Klin Onkol 2015; 28(2): 121 – 129. doi: 10.14735/amko2015121.
2. Wiersinga WM. Management of thyroid nodules in children and adolescents. Hormones (Athens) 2007; 6(3): 194 – 199.
3. Corrias A, Mussa A. Thyroid nodules in pediatrics: which ones can be left alone, which ones must be investigated, when and how. J Clin Res Pediatr Endocrinol 2013; 5 (Suppl 1): 57 – 69.
4. Halac I, Zimmerman D. Thyroid nodules and cancers in children. Endocrinol Metab Clin North Am 2005; 34(3): 725 – 744.
5. Dean DS, Gharib H. Epidemiology of thyroid nodules. Best Pract Res Clin Endocrinol Metab 2008; 22(6): 901 – 911. doi: 10.1016/j.beem.2008.09.019.
6. Bajčiová V, Onderčová Z, Kodýtková D. Cancer in adolescents. Klin Onkol 2015; 28 (Suppl 2): 2S81 – 2S90. doi: 10.14735/amko20152S81.
7. Corrias A, Mussa A, Baronio F et al. Diagnostic features of thyroid nodules in pediatrics. Arch Pediatr Adolesc Med 2010; 164(8): 714 – 719. doi: 10.1001/archpediatrics.2010.114.
8. Jarzab B, Handkiewicz-Junak D, Wloch I. Juvenile differentiated carcinoma and the role of radioiodine in its treatment: a qualitative review. Endocr Relat Cancer 2005; 12(4): 773 – 803.
9. Kubeš J, Vítek P, Dědečková K et al. Very late effects of radiotherapy – limiting factor of current radiotherapy techniques. Klin Onkol 2014; 27(3): 161 – 165. doi: 10.14735/amko2014161.
10. Sigurdson AJ, Ronckers CM, Mertens AC et al. Primary thyroid cancer after a first tumors in childhood (the Childhood Cancer Survivor Study): a nested case control study. Lancet 2005; 365(9476): 2014 – 2023.
11. Duffy BJ Jr, Fitzgerald PJ. Thyroid cancer in childhood and adolescence; a report on 28 cases. Cancer 1950; 3(6): 1018 – 1032.
12. Rabes HM, Demidchik EP, Sidorow JD et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin Cancer Res 2000; 6(3): 1093 – 1103.
13. Nikiforov Y, Gnepp DR. Pediatric thyroid cancer after the Chernobyl disaster. Pathomorphological study of 84 cases (1991 – 1992) from the Republic of Belarus. Cancer 1994; 74 : 748 – 766.
14. Pacini F, Vorontsova T, Demidchik EP et al. Post-Chernobyl thyroid carcinoma in Belarus children and adolescents: comparison with naturally occurring thyroid carcinoma in Italy and France. J Clin Endocrinol Metab 1997; 82(11): 3563 – 3569.
15. Cohen A, Rovelli A, Merlo DF et al. Risk for secondary thyroid carcinoma after hematopoietic stem-cell transplantation: an EBMT Late Effects Working Party Study. J Clin Oncol 2007; 25(17): 2449 – 2454.
16. Ricarte-Filho JC, Li S, Garcia-Rendueles ME et al. Identification of kinase fusion oncogenes in post--Chernobyl radiation-induced thyroid cancers. J Clin Invest 2013; 123(11): 4935 – 4944. doi: 10.1172/JCI69766.
17. Cordioli MI, Moraes L, Cury AN et al. Are we really at the dawn of understanding sporadic pediatric thyroid carcinoma? Endocr Relat Cancer 2015; 22(6): R311 – R324. doi: 10.1530/ERC-15-0381.
18. Piciu D, Piciu A, Irimie A. Thyroid cancer in children: a 20-year study at a Romanian oncology institute. Endocr J2012; 59(6): 489 – 496.
19. Grigsby PW, Gal-or A, Michalski JM et al. Childhood and adolescent thyroid carcinoma. Cancer 2002; 95(4): 724 – 729.
20. Hogan AR, Zhuge Y, Perez EA et al. Pediatric thyroid carcinoma: incidence and outcomes in 1,753 patients. J Surg Res 2009; 156(1): 167 – 172. doi: 10.1016/j.jss.2009.03.098.
21. Zimmerman D, Hay ID, Gough IR et al. Papillary thyroid carcinoma in children and adults: long-term follow-up of 1,039 patients conservatively treated at one institution during three decades. Surgery 1988; 104(6): 1157 – 1166.
22. McHenry C, Smith M, Lawrence AM et al. Nodular thyroid disease in children and adolescents: a high incidence of cacinoma. Am Surg 1988; 54(7): 444 – 447.
23. Biko J, Reiners C, Kreissl MC et al. Favourable course of disease after incomplete remission on (131) I therapy in children with pulmonary metastases of papillary thyroid carcinoma: 10 years follow-up. Eur J Nucl Med Mol Imaging 2011; 38(4): 651 – 655. doi: 10.1007/s00259-010-1669-9.
24. La Quaglia MP, Corbally MT, Heller G et al. Recurrence and morbidity in differentiated thyroid carcinoma in children. Surgery 1988; 104(6): 1149 – 1156.
25. Thompson GB, Hay ID. Current strategies for surgical management and adjuvant treatment of childhood papillary thyroid carcinoma. World J Surg 2004; 28(12): 1187 – 1198.
26. Landau D, Vini L, A’Hern R et al. Thyroid cancer in children: the Royal Marsden Hospital experience. Eur J Cancer 2000; 36(2): 214 – 220.
27. De Keyser LF, Van Herle AJ. Differentiated thyroid cancer in children. Head Neck Surg 1985; 8(2): 1001 – 1114.
28. Calkovky V, Hajtman A. Thyroid diseases in children and adolescents. Bratisl Lek Listy 2009; 110(1): 31 – 34.
29. Frates MC, Benson CB, Charboneau JW et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology 2005; 237(3): 794 – 800.
30. Varverakis E, Neonakis E. Contribution of highresolution ultrasonography in the differential diag-nosis of benign from malignant thyroid nodules. Hormones (Athens) 2002; 1(1): 51 – 56.
31. Babcock DS. Thyroid disease in the pediatric patient: emphasizing imaging with sonography. Pediatr Radiol 2006; 36(4): 299 – 308.
32. Rago T, Vitti P. Role of thyroid ultrasound in the diagnostic evaluation of thyroid nodules. Best Pract Res Clin Endocrinol Metab 2008; 22(6): 913 – 928. doi: 10.1016/j.beem.2008.09.016.
33. Acharya S, Sarafoglou K, LaQuaglia M et al. Thyroid neoplasms after therapeutic radiation for malignancies during childhood or adolescence. Cancer 2003; 97(10): 2397 – 2403.
34. Peli M, Capalbo E, Lovisatti M et al. Ultrasound guided fine-needle aspiration biopsy of thyroid nodules: guidelines and recommendations vs clinical practice; a 12-month study of 89 patients. J Ultrasound 2012; 15(2): 102 – 107. doi: 10.1016/j.jus.2011.12.004.
35. Coorough N, Hudak K, Jaume JC et al. Nondiagnostic fine-needle aspirations of the thyroid: is the risk of malignancy higher? J Surg Res 2013; 184(2): 746 – 750. doi: 10.1016/j.jss.2013.02.018.
36. Fahrner R, Übersax L, Mettler A et al. Paediatric thyroid surgery is safe – experiences at a tertiary surgical centre. Swiss Med Wkly 2014; 144: w13939. doi: 10.4414/smw.2014.13939.
37. Hamming JF, Vriens MR, Goslings BM et al. Role of fine-needle aspiration biopsy and frozen section examination in determining the extent of thyroidectomy. World J Surg 1998; 22 (6): 575 – 579.
38. Massimino M, Gasparini M, Ballerini E et al. Primary thyroid carcinoma in children: a retrospective study of 20 patients. Med Pediatr Oncol 1995; 24(1): 13 – 17.
Štítky
Dětská onkologie Chirurgie všeobecná Onkologie
Článek vyšel v časopiseKlinická onkologie
Nejčtenější tento týden
2016 Číslo 2- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejlepší kůže je zdravá kůže: 3 úrovně ochrany v moderní péči o stomii
- Nejasný stín na plicích – kazuistika
- Metamizol v léčbě různých bolestivých stavů – kazuistiky
-
Všechny články tohoto čísla
- Anticancer Effect of Fish Oil – a Fable or the Truth?
- News in Adjuvant Therapy of Non-seminomatous Germ Cell Testicular Tumors of Stage I
- Comment – Active Surveillance vs. Adjuvant Therapy in Clinical Stage I Non-seminomatous Germ Cell Testicular Cancer
- Change in Quality of Life Measured over Time in Czech Women with Breast Cancer
- Discordance between Clinical and Pathological TNM Classifications in Patients with Oropharyngeal Cancer – Influence on Treatment and Prognosis
- Enzalutamide and Abiraterone in the Treatment of Metastatic Castration-resistant Prostate Cancer after Chemotherapy
- The Role of BRAF/MEK Inhibition in Metastatic Malignant Melanoma – a Case Study
- Papillary Carcinoma of Thyroid Gland in a Two-year-old Child
- Informace z České onkologické společnosti
-
Průvodce mladého onkologa infuzní terapií a výživou
Díl 2 – Hyponatremie. Energie. Kazuistika 2 - Aktuality z odborného tisku
- SOUTĚŽ NA PODPORU AUTORSKÝCH TÝMŮ PUBLIKUJÍCÍCH V ZAHRANIČNÍCH ODBORNÝCH TITULECH
-
Sekvence adenom - karcinom v polypu tlustého střeva s mutací
p53 - SOUTĚŽ O NEJLEPŠÍ PRÁCI
- Máme se bát predátorských časopisů?
- Proč Klinická onkologie nikdy nebyla a nebude predátorský časopis?
- Vyhlášení výsledků soutěže O nejlepší práci v roce 2015
- Extravasation of Cytostatic Drugs – Prevention and Best Practices
- Klinická onkologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Extravasation of Cytostatic Drugs – Prevention and Best Practices
- Anticancer Effect of Fish Oil – a Fable or the Truth?
- Enzalutamide and Abiraterone in the Treatment of Metastatic Castration-resistant Prostate Cancer after Chemotherapy
-
Sekvence adenom - karcinom v polypu tlustého střeva s mutací
p53
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



