-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaGenetic Testing and Prevention of Hereditary Cancer at the MMCI – Over 10 Years of Experience
Genetické testování a prevence hereditárních nádorů v MOÚ – více než desetiletá zkušenost
Dědičné nádorové syndromy jsou často přítomny u mladých pacientů a pacientů s rodinným výskytem onemocnění Genetické testování je důležité pro identifikaci rizikového jednice a pro časný začátek specializované preventivní péče nebo pro indikaci profylaktických operací Vysoce rizikové tumor supresorové geny ( a ) a DNA reparační geny (, a ) jsou zodpovědné za významnou část hereditárních nádorů prsu, ovaria a kolorekta Jiné hereditární nádory se objevují méně často Genetické testování pro specifické typy dědičných nádorů nebo nádorové syndromy se rozšiřuje Genetická centra a molekulárně genetické laboratoře jsou většinou součástí univerzitních nebo místních nemocnic, některá centra jsou soukromá Společnost lékařské genetiky ČLS doporučuje, aby všechny laboratoře měly akreditaci dle ISO 15 189 a dále aby indikace k testování nádorových syndromů prováděli lékařští genetici Indikační kritéria a preventivní postupy byly publikovány v supplementu 22 Klinické onkologie 2009 Preventivní péče o rizikové jedince je organizována ve třinácti onkologických centrech, která provádějí většinu onkologické péče v ČR Genetické testování a preventivní péče jsou hrazeny z veřejného zdravotního pojištění Molekulárně genetická laboratoř Masarykova onkologického ústavu poskytuje testování , , genů pro hereditární syndrom nádorů prsu/ ovaria, , a genů pro Lynchův syndrom, pro Li ‑ Fraumeni syndrom, pro familiární maligní melanom a pro hereditární difuzní karcinom žaludku Jiné syndromy jsou vyšetřovány ve spolupracujících laboratořích Využívání genetického testování se zvyšuje kvůli narůstajícímu počtu odeslaných pacientů onkology a jinými specialisty na genetické vyšetření, ale i kvůli zvětšujícímu se spektru testovaných genů Nicméně stále se u mnoha pacientů na genetické vyšetření zapomíná a jejich příbuzní umírají kvůli pozdní diagnóze hereditárního syndromu Je důležité větší povědomí lékařů o úloze genetického testování v onkologii
Klíčová slova:
dědičný nádor – syndromy – geny – genetické testování – prevence
Authors: L. Foretová 1; K. Petrakova 2; M. Palacova 2; R. Kalabova 2; M. Svoboda 1,2
; M. Navratilova 1; M. Schneiderová 4; K. Bolcak 3; E. Krejci 5; L. Drazan 6; M. Mikova 1; J. Hazova 1; P. Vasickova 1; E. Machackova 1
Authors place of work: Department of Cancer Epidemiology and Genetics, Masaryk Memorial Cancer Institute, Brno, Czech Republic 1; Department of Comprehensive Cancer Care, Masaryk Memorial Cancer Institute, Brno, Czech Republic 2; Department of Nuclear Medicine, Masaryk Memorial Cancer Institute, Brno, Czech Republic 3; Department of Radiology, Masaryk Memorial Cancer Institute, Brno, Czech Republic 4; Oncological and Experimental Pathology, Masaryk Memorial Cancer Institute, Brno, Czech Republic 5; Department of Plastic and Aesthetic Surgery, St. Anne’s University Hospital, Brno, Czech Republic 6
Published in the journal: Klin Onkol 2010; 23(6): 388-400
Category: Přehledy
Summary
Hereditary cancer syndromes are frequently seen in young cancer patients and patients with a positive family history Genetic testing is important for the identification of high-risk individuals, and for the early introduction of specialized preventive care or prophylactic surgeries High-risk tumour suppressor genes ( and ) and DNA repair genes ( and ) are responsible for a substantial part of hereditary breast, ovarian and colorectal cancer Other hereditary cancers are seen less frequently, but genetic testing has increased for many other site-specific cancers and complex syndromes Genetic centres and molecular genetic laboratories are located mostly within university or regional hospitals Some genetic centres are private It is highly recommended (Czech Society for Medical Genetics) that all laboratories are accredited according to ISO 15,189 and that genetic testing of hereditary cancer syndromes is indicated by medical geneticists The indication criteria and prevention strategies were published in Supplement 22 of Clinical Oncology 2009 (in Czech) Preventive care for high-risk individuals is organized by thirteen Oncology Centres, which provide most of the oncology care in the Czech Republic Genetic testing and preventive care for high-risk individuals and mutation carriers is covered by health insurance The molecular genetic laboratory at the MMCI provides molecular genetic testing of for hereditary breast/ovarian cancer, for Lynch syndrome, TP53 for Li-Fraumeni syndrome, for familial malignant melanoma syndrome and CDH1 gene for hereditary diffuse gastric cancer Other syndromes are tested in specialized laboratories elsewhere The use of genetic testing is increasing because of more frequent referrals from oncologists and other specialists and the increasing variety of genes tested However, in some patients the testing is not recommended and other family members are dying because of the late diagnosis of hereditary syndrome Greater awareness of the importance of genetic testing in oncology is needed
Key words:
hereditary cancer – syndromes – genes – genetic testing – preventionIntroduction
Breast cancer is a very common malignancy all over the world, especially in developed countries. In the Czech Republic, there were 5 533 women diagnosed with breast cancer in 2005 and the incidence is slowly growing (2,1% annually). The crude incidence in 2005 was 105,4 cases per 100 000 inhabitants [1]. The cumulative risk of breast cancer for Czech women (0 – 74 years) is 6 – 7%, which is still less than in other Western European countries. The other frequent malignancy is colorectal cancer with 4 746 and 3 236 newly diagnosed cases in males and females in 2005, respectively (94,9 males and 61,7 females per 100 000 inhabitants), which is the highest incidence rate for males in the world. The cumulative risk (0 – 74 years) of colorectal cancer is 7,32% for males and 3,6% for females (Cancer Incidence in Five Continents Vol. IX, IARC 2007).
In 1992 and 1993 first DNA repair genes for hereditary nonpolyposis colorectal cancer, and were found [2 – 5]. In 1994 and 1995, tumor suppressor genes and , which are responsible for a high proportion of hereditary breast and ovarian cancer, were discovered [6,7]. Since that time the genetic counseling and testing for these most frequent hereditary cancers could be introduced. Some other genes causing less frequent syndromes were already known before, for example for Li Fraumeni syndrome, gene for hereditary retinoblastoma [8 – 10]. The spectrum of hereditary cancer syndromes, which can be tested, and the location of genetic centers and laboratories within the Czech Republic were published [11 – 13].
Hereditary breast and ovarian cancer syndrome
Breast cancer may be repeatedly seen in families. There are many genes, which can more or less predispose women to breast cancer (Fig. 1). Mutations in tumor suppressor genes (OMIM#113705, Online Mendelian Inheritance in Man) and (OMIM#6001856) predispose to breast and ovarian/ fallopian tube cancers and some other cancerous diseases (colorectal, prostate, gastric, hepatobiliary, melanoma, pancreatic) and are considered the most frequent cause of hereditary breast or ovarian cancer [14]. The frequency of mutations in general population is estimated to be 1 : 300 to 1 : 800, but in more recent study in Canada the frequency is estimated to be higher, 1 : 140 to 1 : 300 [15]. The frequency of these mutations in the Czech population is not known.
Fig. 1. Breast cancer susceptibility genes [58]. ![Fig. 1. Breast cancer susceptibility genes [58].](https://pl-master.mdcdn.cz/media/image/aa80ea55c8624a2652c62029bfde10ab.jpeg?version=1537790292)
BRCA1/ 2 biology
and proteins are essential in the homologous recombination (HR) process. This mechanism is central in the repair of DNA double strand breaks that can lead to chromosome translocation and genomic instability. Double strand DNA damage can be induced by many chemotherapy agents such as topoisomerase inhibitors, alkylating agents and platinum drugs [16].
The main pathway for repair of such lesions is HR in a process that uses a region of DNA with high sequence identity, often the identical sister chromatid, to copy and replace the damaged DNA sequence. HR is conservative and potentially error free. Cells that lack either or are unable to repair DNA double strand breaks by HR. This defect results in the repair of these DNA lesions by non conservative, potentially mutagenic mechanisms that in turn favor genomic instability, and these tumors should also develop alterations in genes that control these check points in order to progress [16].
Triple Negative and/ or Basal Like Breast Cancers
Breast cancer is a heterogeneous disease with different morphologies, molecular profiles, clinical behavior and response to therapy. A triple negative breast cancer (TNBC) is a particular type of breast cancer characterized by an absence of estrogen (ER), progesterone (PR) and HER 2 receptors’ expression. TNBC comprised about 11 to 17% of Caucasian and up to 30% of black and Hispanic women with breast cancer [17,18].
The triple negative group of breast cancer is not a homogeneous disease entity, but encompass other molecular subtypes of breast cancer. A substantial fraction (70 – 80%) of triple negative tumors displays a basal like phenotype as defined by gene expression profiling or immunohistochemical studies (the expression of basal cytokeratins (e. g. cytokeratins 5, 14, and 17), and/ or the expression of EGFR). This molecular subtype of TNBC is called triple negative basal like breast cancer (TN BLBC). On the other hand, not all basal like breast cancer (BLBC) is triple negative. Up to 20% of BLBC express ER or over express HER2 [18].
There is a link between the pathway and TNBC. More than 75% of tumors arising in women carrying a germline mutation have morphologic features and gene expression profiles very similar to those of nonhereditary TNBC and often display a basal like phenotype. These findings support the hypothesis that loss of function may play a major role in TNBC development. Clinically, the triple negative or the basal like phenotype indicates the possible presence of a germline mutation. However, the additional usefulness of assays that measure the expression of cytokeratins and other “basal associated” markers in determining mutation status remains unclear given the substantial overlap between basal like and triple negative cancers [18]. Since not all TNBC harbor mutations in , it appears that it is not structural mutations alone to be necessary for the development of TNBC. The low expression could also be the result of gene regulatory mechanisms, such as DNA methylation or an expression of inhibitor of DNA binding 4 (ID4), representing a negative regulator of [19].
Triple negative and basal like breast cancers are usually high grade, invasive ductal carcinomas, characterized by an unusually attenuated relationship between the size of the primary tumor and the probability of survival. Their rapid growth and frequent occurrence in young women can make mammography detection difficult. They are more likely than other types of breast cancer to metastasize to viscera, particularly to the lungs and brain, and are less likely to metastasize to bones. Multiple studies have indicated that triple negative and basal like breast cancers, as a group, are associated with an adverse prognosis. There is a sharp decrease in survival during the first 3 to 5 years after diagnosis, but distant relapse after this time is much less common than among patients with ER positive cancers. Thus, although as a group triple negative and basal like breast cancers are biologically aggressive; many are potentially curable, reflecting their heterogeneity [18,19].
Chemotherapy nevertheless improves the outcome to a greater extent when used in patients with TNBC than when used in patients with the much more common ER positive subtype. Neoadjuvant studies suggest that there is a subgroup of women with TNBC whose tumors are extremely sensitive to chemotherapy, but there are many women for whom chemotherapy is of uncertain benefit. Currently, there is no preferred standard form of chemotherapy for TNBC, retrospective analyses suggest that the addition of docetaxel or paclitaxel to anthracycline containing adjuvant regimens may be of greater benefit for the treatment of TNBC [18].
The biology of triple negative breast cancer is studied at MMCI with the support of the grant from Ministry of Health of the CR NS/ 10357.
Genetic testing
Since 1999 almost three thousand probands (patients) and two thousands of family members were tested at MMCI.
The indication criteria for testing were published [20, 21]:
Sporadic cases:
- sporadic breast or ovarian cancer diagnosed before the age of 40
- sporadic bilateral breast/ ovarian cancer before the age of 50
- sporadic medullary breast cancer or triple negative breast cancer (ER, PR and HER2 negative) before 50
- duplication of breast and ovarian cancer at any age
- men with breast cancer at any age
Familial cases
- families with two breast or ovarian cancer in close relatives (at least one before 50)
- families with three or more breast or ovarian cancers at any age
By the testing of unselected breast cancer patients, using methods identifying about 80% of detectable mutations, it was estimated that there is about 2,4% frequency of BRCA1/ 2 mutations [22].
Gene (OMIM#604373) is considered to be a gene causing a moderate increase of breast cancer risk (2 – 5×) and may also predispose to some other cancers like prostate, brain, sarcomas, thyroid or lung [23,24]. In some families clinically resembling Li Fraumeni (or LFS like) syndrome, mutations in gene may be found. We were testing predominantly two mutations, del 9 – 10 (del 5567 bp) and c.1100delC, in some of the breast/ ovarian cancer families.
Deletion of exon 9 and 10 (genomic deletion of 5 567 bp) was disclosed in two USA patients having breast or ovarian cancer. Both patients were of Czechoslovakian ancestry. This deletion was subsequently found in 8/ 631 (1,3%) breast cancer patients in CR and Slovakia, and in no control women. All patients were sharing the same haplotype indicating that this mutation had a single source [25].
Mutation detection rate
According to our testing results, the overall mutation detection rate in 2 100 tested patients was around 26%. The detection rate differed according to the inclusion criteria and the results can be found in the Tab. 1 [26]. The / 2 mutations were most frequently found in ovarian or breast/ ovarian cancer families (HOC or HBOC) where the frequency of mutation detection was reaching 61% (three or more cases in a family) and 57,5% (two cases). The detection rate was much lower in families with only breast cancer cases, 32,5% with three and more cases and 23,1% with only two cases. It is very important to offer the genetic testing to all women with bilateral breast cancer bellow 50 and women with the breast and ovarian cancer (detection rate 31% and 73,7%, respectively). In a sporadic early onset breast cancer, the mutation was discovered in about 10% of tested young women. Male breast cancer is frequently hereditary, with mutation found in 37,5% of tested male patients. The occurrence of ovarian cancer in a family is a high predictor of possible heritability.
Tab. 1. Detection rate (in %) of pathogenic BRCA mutations in different risk categories of patients [26]. ![Detection rate (in %) of pathogenic BRCA mutations in different risk categories of patients [26].](https://www.prolekare.cz/media/cache/resolve/media_object_image_small/media/image/d9d707b64314fe3ad931d76c8b993183.jpeg)
Abbreviations: HOC – hereditary ovarian cancer syndrome; HBOC – hereditary breast and ovarian cancer syndrome; HBC – hereditary breast cancer only syndrome. I. – Three and more cases; II. Two cases in a family. VI. – families, where no patient with cancer can be tested. Testing is starting in a healthy relative. Testing methods
In 2007 a new methods were implemented in the testing protocol (Tab. 2). First of all the heteroduplex analysis and protein truncation tests were exchanged for more reliable and sensitive method, high resolution melting analysis (HRM), which can detect more variants and missense mutations in both genes with the sensitivity reaching 98% [27]. By using this method new previously undetectable mutations in gene p.Glu1413X and cryptic splice site c.213 – 12A>G were found.
Tab. 2. Laboratory methods used for BRCA1 and BRCA2 analysis at MMCI. 
Testing results
BRCA1/ 2 genes
There is a broad spectrum of mutations found in and genes in the Czech patients tested. mutation was found in 392 families (78 different mutations), mutation in 159 families (61 different mutations). Altogether pathogenic mutation was found in 551 out of 2 100 families tested (26,2%). Mutations are scattered all over the coding sequences of both genes and many families have their private mutation. Several mutations are seen more frequently in our population, specifically c.5266dupC, c.3700_3704del5 and p.Cys61Gly in gene and c.7913_7917del5 and c.8537_8538del2 in gene [26, 28]. Altogether with other 5 frequent mutations they represent about 54,5% of all detected mutations found (Tab. 3). But the testing of these 10 mutations is insufficient in our population and the screening of all coding regions of both genes is necessary.
Tab. 3. Ten most frequent causal mutations found in the BRCA1 and BRCA2 gene in the Czech patients tested at MMCI, responsible for 54,5% of all detected mutations. 2 100 families were tested during period 1999-2009, BRCA1 mutation was found in 392 families (78 diff erent mutations), BRCA2 mutation was found in 159 families (61 diff erent mutations). Altogether pathogenic mutation was found in 551 families (26,24%). 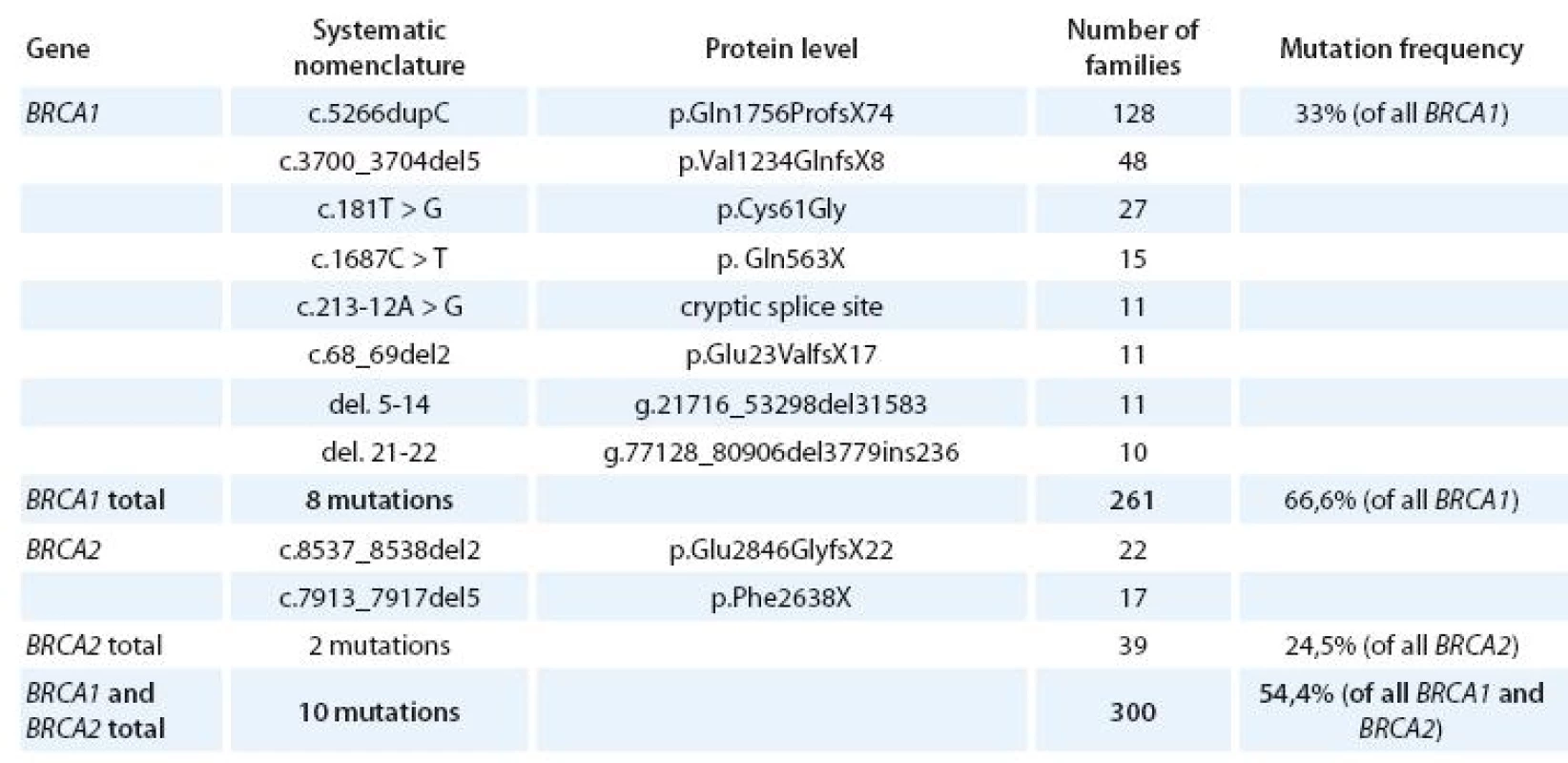
In our population there is a high frequency of large genomic rearrangements in gene, which can be detected by MLPA – multiplex ligation dependent probe amplification (Tab. 4). This method may detect additional mutations in about 6% of patients previously tested negative [29]. No large deletions or duplications in gene were found in 1 000 patients tested and this method is not used for regular testing in our laboratory.
Tab. 4. Large genomic rearrangements in BRCA1 gene. 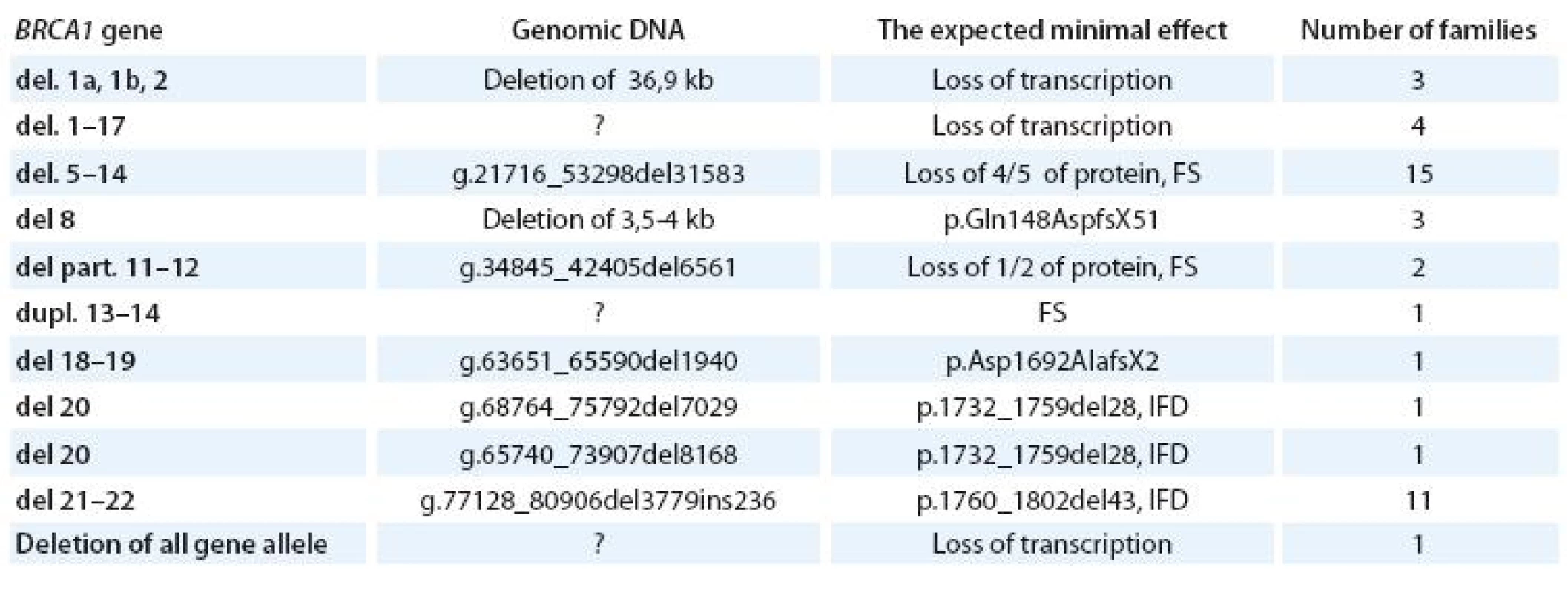
FS – frame shift; IFD – in frame deletion Variants with unknown clinical significance
In about 13% of tested families only a variant with unknown clinical significance (UV) is found; the functional tests are mostly not available. In that case we offer predictive testing only for research purposes and preventive care is offered to both carriers and non carriers.
Together with the research team at the Institute of Experimental and Clinical Medicine in Prague some detected unknown variants located in an important part of the gene (exon 18) and segregating with the disease are examined by functional test which may improve our knowledge of the biological significance of the DNA change [30] with the support of the grant from Ministry of Health of the CR NS/ 10536 – 3/ 2009.
CHEK2 gene
mutations were found in 17 / 2 negative families, in eight cases del 9 – 10, in nine cases c.1100delC. The frequency of mutation carriers among unselected breast cancer Czech patients was estimated to be about 1,3% for del 9 – 10 [25] and 0,44% for c.1100delC, with control frequencies 0% and 0,27%, respectively [31]. It is considered to be low frequency gene in our population causing moderate increase of breast cancer risk (2 – 4×).
mutations were tested only in families, where no mutations in or genes were discovered. In one family both (deletion of exon 20) and mutations (c.1100delC) in proband were detected, both by MLPA analysis (Fig. 2).
Fig. 2. Family with both BRCA1 (del of exon 20) and CHEK2 (c.1100delC) mutation in a proband with breast cancer at 44 years. 
Predictive testing
Predictive testing of known familial mutation was done in 1 796 relatives. Mutation was found and carrier status of / 2 was confirmed in 806 cases, in 12 cases. In 978 cases predictive testing was negative and carrier status of / 2 was excluded. If predictive testing of is offered, carriers and non carriers are recommended to have preventive screening as women with moderate risk of breast cancer [24].
Preventive care
The protocol for the and carriers follow up and for other individuals with high risk of breast cancer was published in 2001 and 2009 [20,21]. The main purpose of these publications was to standardize the prevention within oncology centers and provide to the carriers the most updated preventive procedures. MRI is introduced to the breast cancer screening at the age 25 or earlier, if the youngest breast cancer occurred in the family before the age of 35.
Within four years (2005 – 2008) the preventive MRI examination of breasts was done in 284 high risk women (488 examinations) and six carcinomas were detected. All tumors were with no positive lymphonodes (N0). Mammography was negative in all cases, ultrasound was negative twice, positive four times in a secondary examination after positive MRI [32,33].
Predictive testing is offered to relatives starting at the age of 18. If the woman is not a carrier of the familial mutation, she is advised to have prevention as a woman with moderate risk of breast cancer (2 – 3×) and have yearly breast check up by ultrasound and latter by mammography [34,22].
Prophylactic surgeries are explained to all carriers and information brochures are provided. Oophorectomy is recommended between the ages 35 to 40, prophylactic mastectomy at any age [35 – 40]. The youngest woman, who decided for preventive mastectomy and reconstruction of both breasts, was a 22 years old woman a carrier of mutation, whose mother died because of breast cancer at 32, right after she was born.
Surgical prevention of breast cancer in BRCA carriers – 10 years of experience
The carriers of / 2 mutation are consulted by geneticists at MMCI and the information about possibilities of prophylactic mastectomy, the reconstruction methods and the importance for cancer prevention are provided to all of them. As a consequence of these consultations 84 women, carriers of or mutation, underwent prophylactic mastectomy with immediate reconstruction. From those 84 women forty were healthy carriers without any previous surgery for breast cancer. Eighty prophylactic mastectomies were performed, 46 skin sparing and 34 subcutaneous mastectomies. The other women were patients treated before for unilateral (40 patients) or bilateral (4 patients) breast cancer. Among those patients 34 prophylactic skin sparing mastectomies and 6 prophylactic subcutaneous mastectomies on healthy breasts were performed. On the other previously treated breast mastectomy was completed in 15 cases and the scar excision and reconstruction in 25 cases.
I all 83 cases the reconstruction was performed in one time with prophylactic mastectomy. In 63 patients 126 DIEP with own tissue reconstruction was used, in 20 patients the reconstruction with the use of silicon implants was done (Fig. 3). One woman decided for the delay of the reconstruction after the prophylactic surgery.
Fig. 3. A. Healthy carrier of BRCA1 mutation, before surgery, scars after biopsies; B. Skin sparing mastectomies; C. The result of bilateral reconstruction with DIEP, reconstruction of nipples and tattoo. 
According to previous investigation [41] 88% of patients were evaluating the result of prophylactic mastectomy and tissue reconstruction as nice and satisfactory, 96% would be willing to undergo the surgery again on the basis of their own experience.
The efficacy of the prophylactic mastectomy with reconstruction and the psychological significance is the issue of the grant project of the Ministry of Health of the Czech Republic NS/ 10401 - 3.
Hereditary nonpolyposis colorectal cancer, Lynch syndrome
Highly penetrant DNA repair genes (OMIM #120436), (OMIM #609309), and (OMIM#600678) are responsible for the majority of hereditary colorectal cancer in families with several cases of colorectal and/ or endometrial cancer. These three genes are tested when there is a very young patient with colorectal cancer (bellow 40), or a family with at least two cases of colorectal cancer in close relatives, one younger than 50. Predictive testing is offered to all relatives at risk starting at the age of 18 [42,43]).
In all patients with colorectal cancer bellow 50 MLH1, MSH2, MSH6 and PMS2 proteins are tested at MMCI by immunohistochemistry in the tumor. In case of a pathology result, the genetic counseling is recommended in the pathology report. But oncologists, gastroenterologists or other physicians refer most of the patients.
In 310 tested families (at 2nd MF of CHU, Prague or MMCI) the pathogenic mutation was found in 39 (12,6%). In 24 families the mutation was in gene (14 different mutations), in 13 families in gene (10 mutations) and in 2 families in gene (2 mutations). The most frequent mutation was / c.1489dup.C, which was seen in 8 families. By the use of MLPA four different intragenic rearrangements were found in five families, three large deletions causing loss of at least half of the coding regions of the gene (, del 1 – 13, del 1 – 8 and 9 – 16), and one large duplication. No large rearrangement was found in gene. In 29 families variants of unknown clinical significance (UVs) were found. If the predictive testing is offered in these families with UV, it is for segregation analysis and research purposes. In those families both carriers and non carriers of UVs are advised to have colonoscopy every 2 – 3 years together with other preventions.
Prevention
The lifetime risk of different cancers in carriers of pathogenic mutations is described in Tab. 5. The complex prevention is offered mostly in the oncology centers and the oncologist is seeing the carrier with Lynch syndrome regularly checking all the results of examinations they have to go through. The oncologist and a specialized nurse are keeping these individuals within the prevention system by telephone calls and invitations for visits. The colonoscopy is starting at the age of 20 (or earlier if very young family members had colorectal cancer). The whole spectrum of examinations was published [44]. Early prevention may be very successful in people with Lynch syndrome. Unfortunately we are still counseling families, where the Lynch syndrome was clinically detectable, but no clinician was referring the patient for testing early enough.
Tab. 5. Different types of cancer and potential lifetime risks of the disease in Lynch syndrome. 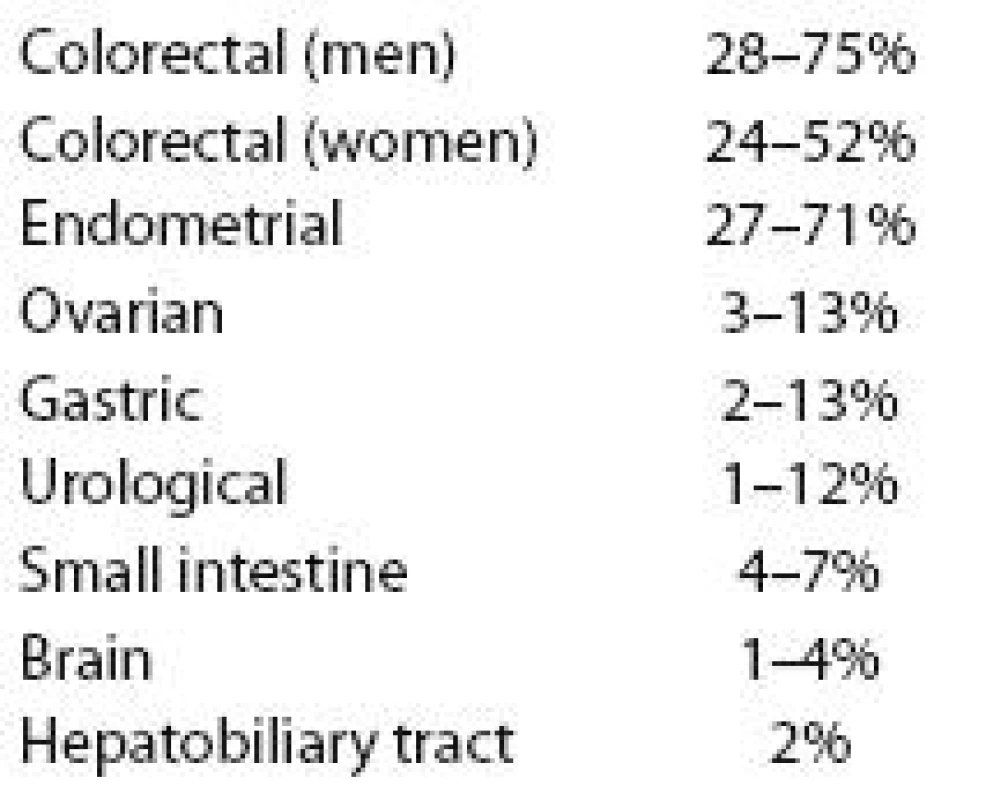
Li Fraumeni syndrome – LFS
Li Fraumeni syndrome is caused in many families by (OMIM #191170) germline mutations. LFS is considered to be one of the most severe hereditary cancer syndromes where cancer may occur in young individuals and spectrum of cancers is very broad, mostly adrenocortical cancer, breast cancer, leukemia, brain tumors, sarcomas [45 – 47]. The prevention of cancer is very complicated and predictive testing is not offered to children until the age of 18. Children who have a parent with LFS should be followed regularly by oncologist or informed pediatrician. There are several reasons for not providing predictive testing to healthy children, predominantly because the prevention of cancers related to LFS is not satisfactory.
Genetic testing
Genetic testing is done by direct sequencing of all coding exons of gene and by MLPA for large deletions and duplications. So far 85 families with certain probability of having LFS were tested (50 in MMCI, 35 in Prague 2nd MF CHU) and in 8 of them mutation was detected. In 7 families LFS was caused by 6 different missense mutations (p.Gly245Ser, p.Arg248Trp, p.Ile254Val, p.Arg267Gln, p.Cys275Phe, p.Glu286Lys,) and one splice site mutation c.375G>A. In one family large deletion encompassing exon 2 – 12 was discovered. The proband is a patient with malignant melanoma at 24, bilateral breast cancer at 31, her daughter had brain tumor at 3, her brother had lymphoma at 18, his daughter histiocytoma, her father liposarcoma at 39, her grandmother bilateral breast cancer and died at 46 (Fig. 4). She is followed regularly as a clinical LFS. She had both breasts completely removed and reconstructed by implants. She was offered to have yearly PET examination but refused.
Fig. 4. Family with TP 53 large deletion of exon 2-12. The same mutation was seen in a proband and her niece. 
In seven families with clinical suspicion to LFS germline mutation in gene was found, del 9 – 10 three times, p.Thr387Asn, p.Ile157Thr four times, but no c.1100delC mutation.
Prevention
The prevention should be complex, including ultrasound of breasts, stomach, MRI of breasts and brain, colonoscopy, gastroscopy, regular gynecological exam with transvaginal ultrasound, tumor markers, blood and urine analysis etc. [48]. Since 2007 the regular examination by positron emission tomography PET/ LD CT (the whole body and brain) is used at MMCI yearly not only for follow up of cancer patients with LFS, but also for healthy adult carriers starting at 18. In one patient there was a gastric cancer diagnosed early by PET examination (Fig. 5). It was estimated by radiologists that the radiation exposure is around 7mSv from 18F-fluoro deoxy - glucose (FDG) and 1 mSv from LD CT (for comparison the yearly exposure limit for medical professionals is 50 mSv and there is no limit for patient exposure). The use of PET/ CT may be of a great importance for early detection of many cancers in different body sites, but the use of other detection methods without radiation exposure is preferred.
Fig. 5. Family with Li-Fraumeni syndrome, missense mutation in TP53 p.Cys275Phe. By the regular use of PET gastric cancer was diagnosed at the early stage. 
Familial (atypical multiple mole) melanoma syndrome FAMMM
In some families the risk of melanoma is very high, melanomas occur in family members at a young age, with or without multiple moles. Germline mutations in (OMIM# 600160) gene may be responsible for some of the familial melanoma cases. So far we have tested 34 families with early onset or familial occurrence of melanomas and discovered pathogenic mutation, c.15 – 20del6insC, in one family. Both the mother and daughter had malignant melanoma at the age of 31 and 38, respectively. The mothers’ sister is also a carrier and is healthy at the age of 65 without any sign of melanoma or multiple moles. Her daughter is a healthy carrier too at the age of 41 (Fig. 6). There may be a high variability of clinical symptoms within a family. In some families higher risk of breast or pancreatic cancer can be seen in carriers. In this syndrome the primary prevention is very important and all of the carriers should be well informed. Clinical examinations should start early, from the age of 10, be done regularly with the fotodocumentation of risk moles [49].
Fig. 6. Family with hereditary malignant melanoma syndrome, CDKN2A mutation. The aunt and her daughter are also carriers, the aunt is healthy at the age of 65. The penetrance of the mutation may be variable within the family. 
Hereditary diffuse gastric cancer
Diffuse gastric cancer is not so frequently seen as intestinal type. In young patients with diffuse gastric cancer or patients with a positive family history of gastric cancer the genetic testing of (OMIM #192090) gene should be offered.
Eighteen young patients with sporadic diffuse gastric cancer or patients with other family members with gastric cancer were tested at MMCI and the pathogenic mutation was not found yet. Only a variant of unknown significance was detected in one family.
It is expected that about 30% of patients with diffuse gastric cancer and positive family history may have a germline mutation in gene but it is very rare in sporadic cases.
In carriers the risk of gastric cancer is very high, reaching 67% and 83% in men and women respectively at the age of 80 [50].
Gastric cancer screening is problematic with the need of chromoendoscopic methods. In some cases prophylactic gastrectomy should be offered. In women carriers of mutation the risk of lobular carcinoma of breast may be increased. Gastric cancer can be frequently seen also as part of the Lynch syndrome, Li Fraumeni syndrome and in carriers.
Differential diagnoses of polyposes
Familial adenomatous polyposis coli (FAP) is a severe syndrome in many cases with thousands of polyps in colon, small intestine but also in stomach. The situation may be complicated by desmoids, benign tumors that are frequently growing very progressively threatening the patient’s life. According to the mutation location at the gene (OMIM# 175100) the type of polyposis, severity and possible complications can be predicted (genotype phenotype correlation) [51,52]. In many cases the polyposis is not seen in any parent and the mutation in gene occurs de novo (in a germ cell).
So far 35 patients with polyposis were tested (1st MF CHU Prague) and the mutation in gene was found in 27 patients.
In many cases the pathology report may help us to differentiate between FAP and other polyposis syndromes. In case of hamartomas other syndromes should be tested. Hamartomas are seen in Cowden syndrome ( gene OMIM #601728), juvenile polyposis (genes, OMIM ) or Peutz-Jeghers syndrome ( gene, OMIM ) [53,54]. Genetic testing may diagnose the right syndrome and specify the potential risk of cancerous diseases and other complications. Biallelic recessive germline mutations in gene (OMIM #604933) are causing mild polyposis in latter age [55]. This type of genetic predisposition is only rarely seen in the Czech population.
Other syndromes are tested in specialized laboratories like neurofibromatosis 1/ 2, MEN1/ 2, von Hippel Lindau syndrome, MEN1/ 2, tuberous sclerosis, Gorlin syndrome, Birt Hogg Dubé syndrome and other [50].
Discussion
Genetic testing is highly recommended to patients and families with a possibility of inherited predisposition to cancer. The developments of positional cloning enabled the discoveries of several cancer predisposition genes for common diseases, especially for breast, ovarian and colorectal cancer. This was a great success of cancer genetics, which provided a lot of information on biology of hereditary cancer.
In 1997 genetic clinic was established at MMCI and since that time more than 6 000 probands and their relatives were counseled. Molecular genetic laboratory at MMCI covers the need for genetic testing of substantial part of oncology patients and their relatives not only from Brno, but also from other parts of the Czech Republic. Methods used for mutation detection improved moving from heteroduplex analysis, protein truncation test to high resolution melting analysis, MLPA and much more reliable sequencing. The laboratory completes international quality control tests (EMQN) yearly for / 2 genes, , and with an excellent result, which is an important quality assurance of the laboratory work.
The clinical usefulness and limitations of genetic testing depend on many factors. The clinical utility may be characterized as an additional value, which can be used by the patient and doctors in the management of cancer. In some cancer syndromes the additional value does not have to be high enough in order to justify the testing itself.
So far the additional value of and genetic testing for hereditary breast and ovarian cancer, or , and for Lynch syndrome is considered to be very high and the testing should be highly recommended in all families which fulfill the testing criteria. Clinical geneticist should counsel all tested individuals before and after the testing. In those families’ not only healthy carriers but also patients should be managed differently for example by the use of more radical surgical therapy.
Genetic testing of germline mutations is important mostly for prevention of disease but not for the evaluation of prognosis or the response to treatment. Some clinical studies are evaluating new options of treatment in hereditary cancer.
The use of cisplatin, carboplatin and targeted agents to treat triple negative breast cancers carrying dysfunction of or pathways is currently being assessed in clinical trials. At this time, the most interesting clinical target in triple negative breast cancer is the enzyme poly(adenosine diphosphate ribose) polymerase (PARP), which is involved in base excision repair after DNA damage [18].
PARP1 is an enzyme that has an important function in the repair of DNA single strand breaks (SSB) as a part of the base excision repair pathway [18,19]. In this pathway, PARP1 binds to the exposed ends of the corrupted DNA strand and recruits essential enzymes needed to repair SSBs. When PARP1 is inhibited, the base excision repair pathway fails, which leads to accumulation of SSBs. In a replicating cell entering the S phase, replication is arrested at a SSB site, leading to a DNA double strand break (DSB). In the absence of , DSBs cannot be repaired by homologous recombination, and cells activate an alternative repair pathway termed non homologous (see above). Thus, in – deficient cells, the damage executed by PARP inhibitors leads to accumulation of structural DNA lesions, which results in genomic instability and finally apoptotic cell death. Since operates in the same pathway like , deficiency of this protein renders the cell vulnerable to PARP inhibitors as well [19].
PARP inhibitors (olaparib, iniparib) have recently shown very encouraging clinical activity in early trials of tumors arising in BRCA mutation carriers and in sporadic triple negative cancers [18,19]. One of these inhibitors, iniparib (BSI 201), was recently used in a randomized phase 2 trial involving patients with triple negative cancer. When the inhibitor was added to a chemotherapy combination of gemcitabine and carboplatin, there were significant improvements in the rate of tumor regression (48 % vs 16%, P = 0.002), median progression free survival (6.9 months vs 3.3 months; hazard ratio, 0.34; P < 0.001), and median overall survival (9.2 months vs 5.7 months; hazard ratio, 0.35; P < 0.001). An updated analysis showed a median overall survival rate of 12.2 months versus 7.2 months (hazard ratio, 0.5; P = 0.005). PARP inhibitors and other targeted agents are now at the forefront of clinical research on the treatment of triple negative breast cancer [18].
Rare highly penetrant genes are the cause of the predisposition to cancer in a small but a significant proportion of cases. The polygenic inheritance may be more frequently characteristic for the cancer heritability. Genome wide association studies are discovering multiple germline variants in susceptibility loci for different cancer types. The effect of these variants on cancer prediction is mostly low, not exceeding 1,5, and the biological role of them is usually unknown. Over 100 low penetrance cancer susceptibility loci causing mild increase of cancer risk have been identified [56]. These common variants may explain only about 8% of breast, 20% of prostate and 6% of colorectal cancer predisposition. The role of less frequent (frequencies less than 10%) low penetrance variants starts to be investigated. Other genetic variants such as large insertions, deletions, copy number variations, translocations and inversions should are also explored. The use of new technologies like whole genome/ exome sequencing will help in discovering more moderate or high risk predisposition loci [57].
The genetic research is supported by grant of the Ministry of Health MZ0 MOU 2005, the research of UVs is supported by Internal Grant Agency of the Ministry of Health of the CR NS/ 10536 - 3, the research of special preventive care is supported by Internal Grant Agency of the Ministry of Health of the CR NS/ 10401 - 3, the research of TNBC by Internal Grant Agency of the Ministry of Health of the CR NS/ 10357-3.
The authors declare they have no potential conflicts of interest concerning drugs, pro-ducts, or services used in the study.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.The Editorial Board declares that the ma-nuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bi omedicínských časopisů.Lenka Foretova MD., Ph.D.
Department of Cancer Epidemiology and Genetics
Masaryk Memorial Cancer Institute
Zluty kopec 7
656 53 Brno
Czech Republic
e-mail: foretova@mou.cz
Zdroje
1. Dusek L et al. Czech Cancer Care in Numbers 2009 – 2009. Praha: Grada 2009 : 153.
2. Lynch HT, de la Chapelle A. Genetic susceptibility to non‑polyposis colorectal cancer. J Med Genet 1999; 36(11): 801 – 818.
3. Lynch HT, Lynch J. Lynch syndrome: genetics, natural history, genetic counseling, and prevention. J Clin Oncol 2000; 18 (Suppl): 19S – 31S.
4. Lynch HT, Smyrk TC, Watson P et al. Genetics, natural history, tumor spectrum and patology of hereditary non‑polyposis colorectal cancer: an updated review. Gastroenterology 1993; 104 : 1535 – 1549.
5. Lynch HT, Chapelle A. Hereditary colorectal cancer. N Eng J Med 2003; 348(10): 919 – 932.
6. Miki Y, Swensen J, Shattuck ‑ Eidens D et al. A strong candidate for the breast and ovarian cancer susceptibility gene. Science 1994; 266(5182): 66 – 71.
7. Wooster R, Bignell G, Lancaster J et al. Identification of the breast cancer susceptibility gene BRCA2. Nature 1995; 378(6559): 789 – 792.
8. Friend SH, Bernards R, Rogelj S. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarkoma. Nature 1986; 323(6089): 643 – 646.
9. Li FP, Correa P, Fraumeni JF jr. Testing for germline p53 mutations in cancer families. Cancer Epidemiol Biomark Prev 1991; 1 : 91 – 94.
10. Srivastava S, Zou Z, Pirrollo K et al. Germ‑line transmission of a mutated p53 gene in a cancer ‑ prone family with Li ‑ Fraumeni syndrome. Nature 1990; 348(6303): 747 – 749.
11. Goetz P, Foretova L, Puchmajerova A. Hereditary aetiology of cancer diseases and the importance of genetic counselling and testing in oncology. Klin Onkol 2006; 19 (Suppl): 44 – 48.
12. Garber JE, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol 2005; 23(2): 276 – 292
13. Foretová L, Petráková K, Palácová M et al. Genetic and preventive services for hereditary breast and ovarian cancer in the Czech Republic. Hereditary Cancer in Clinical Practice 2006; 4(1): 3 – 6.
14. Ford D, Easton DF, Stratton M et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. Am J Hum Genet 1998; 62(3): 676 – 689.
15. Shulman LP. Hereditary breast and ovarian cancer (HBOC): Clinical features and Counseling for BRCA1 and BRCA2, Lynch syndrome, Cowden syndrome, and Li.-Fraumeni. Obstet Gynecol Clin N Am 2010; 37(1): 109 – 133.
16. Bosch A, Eroles P, Zaragoza R et al. Triple ‑ negative breast cancer: Molecular features, pathogenesis, treatment and current lines of research. Cancer Treatment Reviews 2010; 36(3): 206 – 215.
17. Stead LA, Lash TL, Sobieraj JE et al. Triple ‑ negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Research 2009; 11: R18.
18. Foulkes WD, Smith IE, Reis ‑ Filho JS. Triple ‑ Negative Breast Cancer. N Engl J Med 2010; 363(20): 1938 – 1948.
19. de Ruijter TC, Veeck J, de Hoon JP et al. Characteristics of triple ‑ negative breast cancer. J Cancer Res Clin Oncol 2010 [Epub ahead of print].
20. Bartoňková H, Foretová L, Helmichová E et al. The recommendation of clinical care for patients with breast and ovarian cancer and for healthy people with germline mutations in BRCA1 and BRCA2 genes. Klin Onkol 2003; 16(1): 28 – 34.
21. Plevová P, Novotný J, Petráková K et al. Syndrom hereditárního karcinomu prsu a ovarií. Klin Onkol 2009 (Suppl S1): S6 – S11.
22. Mateju J, Stribrna M, Zikan M et al. Population based study of BRCA1/ 2 mutations: family history based criteria identify minority of mutation carriers. Neoplasma 2010; 57(3): 280 – 285.
23. Cybulski C, Górski T, Huzarski T et al. CHEK2 is a multiorgan cancer susceptibility gene. Am J Hum Genet 2004; 75(6): 1131 – 1135.
24. The CHEK2 Breast Cancer Case ‑ Control Consortium. CHEK2 1100delC and susceptibility to breast cancer: a collaborative analysis involving 10,860 breast cancer cases and 9,065 controls from 10 studies. Am J Hum Genet 2004; 74(6): 1175 – 1182.
25. Walsh T, Casadei S, Coats KH et al. Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer. JAMA 2006; 295(12): 1379 – 1388.
26. Machackova E, Foretova L, Lukesova M et al. Spectrum and characterisation of BRCA1 and BRCA2 deleterious mutations in high‑risk Czech patients with breast and/ or ovarian cancer. BMC Cancer 2008; 8(140): 12.
27. Stoep N, Paridon Ch, Janssens T et al. Diagnostic guidelines for High‑Resolution Melting curve (HRM) analysis: A validation of BRCA1 mutation scanning using the 96 - well LightScanner. Human Mutation 2009; 30(6): 899 – 909.
28. Foretova L, Machackova E, Navratilova M et al. BRCA1 and BRCA2 mutations in women with familial or early ‑ onset breast/ ovarian cancer in the Czech Republic. Human Mutation 2004; 23(4): 397 – 398.
29. Vasickova P, Machackova E, Lukesova M et al. High occurrence of BRCA1 intragenic rearrangements in hereditary breast and ovarian cancer syndrome in the Czech Republic. BMC Med Genet 2007; 8 : 32.
30. Hucl T, Rago C, Gallmeier E et al. A syngeneic variance library for functional annotation of human variation: application to BRCA2. Cancer Res 2008; 68(13): 5023 – 5030.
31. Kleibl Z, Novotny J, Bezdickova D et al. The CHEK2 c.1100delC germline mutation rarely contributes to breast cancer development in the Czech Republic. Breast Cancer Research and Treatment 2005; 90(2): 165 – 167.
32. Schneiderova M, Belanova R, Lidakova J et al. Magnetic resonance of breast as a screening method in women with high risk of breast cancer. Educational Proceedings. BOD 2009 : 91 – 92.
33. Schneiderova M, Bartonkova H. Breast magnetic resonance imaging in surveillance of women at high risk for breast cancer. Klin Onkol 2006; 19 (Suppl): 91 – 96.
34. Evans DG, Shenton A, Woodward E. Penetrance estimated for BRCA1 and BRCA2 based on genetic testing in a clinical cancer genetics service setting: Risk of breast/ ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer 2008; 8 : 155.
35. Blanchard DK, Hartmann LC. Prophylactic surgery for women at high risk for breast cancer. Clin Breast Cancer 2000; 1(2): 127 – 134.
36. Eisen A, Rebbeck TR, Wood WC et al. Prophylactic Surgery in Women With a Hereditary Predisposition to Breast and Ovarian Cancer 2000; J Clin Oncol 2000; 18(9): 1980 – 1995.
37. Hughes KS, Papa MZ, Whitney T et al. Prophylactic mastectomy and inherited predisposition to breast carcinoma. Cancer Supplement 1999; 86 : 2502 – 2515.
38. Meijers ‑ Heijboer EJ, Verhoog LC, Brekelmans CTM et al.Presymptomatic DNA testing and prophylactic surgery in families with a BRCA1 or BRCA2 mutation. Lancet 2000; 355(9220): 2015 – 2020.
39. Meijers ‑ Heijboer H, van Geel B, van Putten WLJ et al. Breast cancer after prophylactic bilateral mastectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med 2001; 345 : 159 – 163.
40. Drazan L. Prophylactic mastectomy and its indications in high‑risk women Klin Onkol 2006; 19 (Suppl): 97 – 100.
41. Dražan L, Hýža P, Stupka I et al. Oboustranná rekonstrukce prsů dvěma DIEP laloky: Jak ji hodnotí pacientky? Praktický lékař 2009; 89(6): 306 – 311.
42. Ivanovich JL, Reed TE, Ciske DJ et al. A practical approach to familial and hereditary colorectal cancer. Am J Med 1999; 107(1): 68 – 77.
43. Křepelová A, Pavlíková K, Plevová P. Diagnostika Lynchova syndromu – nové geny a metody. Klin Onkol 2006; 19 (Suppl): 76 – 81.
44. Plevová P, Novotný J, Šachlová M et al. Hereditární nepolypózní kolorektální karcinom (HNPCC, Lynchův syndrom). Klin Onkol 2009; (Suppl S1): S12 – S15.
45. Hisada M, Garber JE, Fung CY et al. Multiple primary cancers in families with Li ‑ Fraumeni syndrome. J Natl Cancer Inst 1998; 90(8): 606 – 611.
46. Krutílková V, Trková M, Kodet R et al. Syndrom Li ‑ Fraumeni. Čs pediatrie 2003; 58(9): 552 – 555.
47. Foretová L, Navrátilová M, Petráková K. Kasuistiky Li ‑ Fraumeni syndromu: diagnostické a preventivní možnosti. Klin Onkol 2006; 19 (Suppl): 85 – 87.
48. Plevová P, Krutílková V, Petráková K et al. Syndrom Li ‑ Fraumeni. Klin Onkol 2009; (Suppl S1): S20 – S22.
49. Foretová L, Macháčková E, Šachlová M et al. Syndrom familiárního melanomu (s dysplastickými naevy či bez nich). Klin Onkol 2009; (Suppl S1): S32 – S33.
50. Hodgson SV, Foulkes WD, Eng CH et al. A practical guide to human cancer genetics. 3rd ed. Cambridge University Press, 2007.
51. Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Human Molecular Genetics 2001; 10(7): 721 – 733.
52. Plevová P, Štekrová J, Kohoutová M et al. Familiární adenomatózní polypóza. Klin Onkol 2009 (Suppl S1): S16 – S19.
53. Giardiello FM, Brensinger JD, Tersmett AC et al. Very high risk of cancer in familial Peutz ‑ Jeghers syndrome. Gastroenterology 2000; 119(6): 1447 – 1453.
54. Ivanovich JF, Whelan AJ. Cancer and Peutz ‑ Jeghers Syndrome: a Review. Journal of Genetic Counseling 1997; 6(2): 193 – 206.
55. Morak M, Laner A, Bacher U et al. MUTYH‑associated polyposis ‑ variability of the clinical phenotype in patients with biallelic and monoallelic MUTYH mutations and report on novel mutations. Clin Genet 2010; 78(4): 353 – 363.
56. Stadler ZK, Vijai J, Thom P et al. Genome ‑ wide association studies of cancer predisposition. Hematol Oncol Clin N Am 2010; 24 : 973 – 996.
57. Robson M, Offit K. Inherited predisposition to cancer. Introduction and overview. Hematol Oncol Clin N Am 2010; 24(5): 793 – 797.
58. Ripperger T, Gadzicki D, Meindl A et al. Breast cancer susceptibility: current knowledge and implications for genetic counselling. Eur J Hum Genet 2009; 17(6): 722 – 731.
Štítky
Dětská onkologie Chirurgie všeobecná Onkologie
Článek vyšel v časopiseKlinická onkologie
Nejčtenější tento týden
2010 Číslo 6- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejasný stín na plicích – kazuistika
- Hojení análních fisur urychlí čípky a gel
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Cinitaprid – v Česku nová účinná látka nejen pro léčbu dysmotilitní dyspepsie
-
Všechny články tohoto čísla
- New Therapeutic Options in Therapy of Glioblastoma Multiforme
- Genetic Testing and Prevention of Hereditary Cancer at the MMCI – Over 10 Years of Experience
- Acute Myeloblastic Leukaemia with Alternationsof MLL Proto-Oncogene Protein (11q23/ MLL+ AML)
- Actual Problems in Structure and Orientation of Concise National Cancer Control Programs
- The Problematic of Original and Generic Drugs and Biosimilars – Switching of Drugs Today and Tomorrow with the Main Targeting on Biotechnologies- Associated Risks
- Evaluation of Neoadjuvant Chemo- Radiotherapy with Locally Advanced Rectal Cancer by Comparing Tumour Volume before and after Treatment
- Hormonal Contraceptives and Their Relationship to Breast Cancer
- Postoperative Accelerated Partial Radiotherapy for Breast Cancer
- Economic Evaluation of Targeted Biologic Therapy in Metastatic Renal Cell Carcinoma
- Malignant Subtype of Cystosarcoma Phyllodes with Brain Metastases
- Inflammatory Skin Metastasis as a First Sign of Progression of Lung Cancer – a Case Report
- The Variance of Melphalan Doses Related to Kilogram of Body Weight and the Consequences
- Zápis ze schůze výboru České onkologické společnosti dne 26. 10. 2010 ve FN Bulovka Praha
- Klinická onkologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- New Therapeutic Options in Therapy of Glioblastoma Multiforme
- Hormonal Contraceptives and Their Relationship to Breast Cancer
- Acute Myeloblastic Leukaemia with Alternationsof MLL Proto-Oncogene Protein (11q23/ MLL+ AML)
- Postoperative Accelerated Partial Radiotherapy for Breast Cancer
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



