-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Safety and immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine in adults and children in Lambaréné, Gabon: A phase I randomised trial
Sanjeev Krishna and colleagues present a phase I randomised trial for safety and immunogenicity of an Ebola vaccine in both adults and children in Gabon.
Published in the journal: . PLoS Med 14(10): e32767. doi:10.1371/journal.pmed.1002402
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002402Summary
Sanjeev Krishna and colleagues present a phase I randomised trial for safety and immunogenicity of an Ebola vaccine in both adults and children in Gabon.
Introduction
The western African Ebola virus disease (EVD) public health emergency of international concern ended in June 2016 [1], after infecting approximately 28,650 individuals, of whom 11,323 died [2,3]. Global commitment led to landmarks in vaccine development against EVD, with 8 candidates out of 15 undergoing evaluation in phase I–III clinical trials worldwide by the end of 2015 [4–6]. A live-attenuated recombinant vaccine consisting of the vesicular stomatitis virus (VSV), strain Indiana, with the gene for the Kikwit-95 Zaire Ebola virus (ZEBOV) glycoprotein (GP) replacing the VSV glycoprotein (G) had given acceptable results in non-human primate challenge models and was selected for accelerated clinical development. In European and African populations, the VEBCON Consortium (VSV-EBola CONsortium) carried out parallel dose-escalation phase I trials of the recombinant VSV (rVSV)–ZEBOV candidate vaccine in Germany (NCT02283099), Kenya (NCT02296983), and Gabon (PACTR2014000089322) and a double-blind phase I/II randomised controlled trial in Switzerland (NCT02287480). Three further phase II/III trials were later launched in Guinea, Sierra Leone, and Liberia. Results from phase I trials in the US [7] and preclinical data supported selection of the 2 × 107 plaque-forming units (PFU) dose as the most immunogenic for phase IIb/III trials in Guinea, Sierra Leone, and Liberia. A final analysis of the Guinea trial showed that a single dose of 2 × 107 PFU given immediately after contact with an index case was 100% (95% CI: 70%–100%, P = 0.0045) efficacious in preventing EVD in individuals, and protected the population through a ring vaccination strategy 10 days or more post-vaccination [8].
Detailed dose-ranging studies (3 × 105, 3 × 106, 1 × 107, 2 × 107, and 5 × 107 PFU) at the 4 VEBCON sites showed acceptable safety, dose-dependent reactogenicity [9], and high seroconversion rates among all participants on day 28 after vaccination [9,10].
In Gabon, 2 seroprevalence studies in epidemic and non-epidemic regions showed varying proportions of participants with pre-vaccination ZEBOV-specific IgG antibodies [11,12]. In Lambaréné, with no reported EVD outbreak, ZEBOV-GP-specific antibody responses after vaccination were similar at 2 tested doses (3 × 105 and 3 × 106 PFU) [9]. This finding contrasted with that in vaccinees in Geneva, where antibody titres at 3 × 105 PFU were significantly lower than responses to higher vaccine doses, including 1 × 107 PFU and 5 × 107 PFU [10]. Additionally, irrespective of vaccine dose, delayed oligoarthritis and skin and mucous membrane lesions emerged as vaccine-related adverse events in a proportion of recipients more than 1 week after vaccination in Geneva [10]. These delayed complications were not observed in Gabon, despite the fact that the same vaccine batch and similar doses were used [9].
Because of these divergent site-specific observations, we need further assessments of the vaccine in Ebola virus endemic areas as well as in children. We present a comparison of safety and immunogenicity outcomes in participants vaccinated with (1) 2 × 107 PFU, the dose used in the efficacy trial; (2) 2 previously reported doses, 3 × 106 and 3 × 105 PFU [9]; and (3) 2 lower doses, 3 × 104 PFU and 3 × 103 PFU, in African adults. Furthermore, we report, to our knowledge for the first time, on children and adolescents aged 6 to 17 years vaccinated with 2 × 107 PFU.
Methods
Study design and participants
The trial protocol was approved by the Scientific Review Committee of Centre de Recherches Médicales de Lambaréné (CERMEL), the Institutional Ethics Committee of CERMEL, the National Ethics Committee of Gabon, the World Health Organization (WHO) Ethics Committee, and the Institutional Ethics Committee of the Universitätsklinikum Tübingen. The trial was registered with the Pan African Clinical Trials Registry (PACTR201411000919191).
The study was a randomised, open-label, dose-escalation phase I trial at CERMEL in Gabon. The trial was initially designed to escalate doses to 3 × 105, 3 × 106, and 2 × 107 PFU in 60 adults. After successive protocol amendments, a total of 115 adults (18–50 years), 20 adolescents (13–17 years), and 20 children (6–12 years) were enrolled, between 17 November 2014 and 7 July 2015. Written informed consent was obtained from adults and parents/guardians of adolescents/children, and written assent from minors aged 11–17 years, prior to study-related procedures (details are in S3 Text).
Healthy consenting volunteers who were aged 6–50 years and resident in the study area—which had no history of an Ebola outbreak—and willing to minimise blood/body fluid exposure to their relatives for 5 days post-vaccination were included. Field workers used the door-to-door approach to invite individuals from the Lambaréné community to screen for the study. After screening, individuals with a history of severe local or systemic allergic reaction to vaccination, known allergy to constituents of the rVSVΔG-ZEBOV-GP vaccine, or any acute or chronic clinically significant medical or psychiatric condition were excluded. All pregnant and lactating women were excluded. Volunteers who received a licensed vaccine within 14 days (or 30 days for a live vaccine), had a history of blood donation within 60 days prior to vaccination, were positive for HIV and/or hepatitis B or C virus infection, or had an immunocompromised member in the family were also excluded from the study.
Randomisation and treatment allocation
Randomisation and allocation was performed by an independent investigator from 17 November 2014 until 13 April 2015 using a web-generated sequence. Randomisation in permuted blocks was performed in 2 stages. In the first, participants were assigned in a ratio of 1 : 1:1 to 3 × 105, 3 × 106, and 2 × 107 PFU, and in the second stage, in a ratio of 1 : 1 to 3 × 103 and 3 × 104 PFU. On 9 December 2014, a temporary consortium-wide safety hold was placed on doses above 1 × 107 PFU due to adverse events reported at the Swiss site with doses of 1 × 107 and 5 × 107 PFU. In Gabon, only 1 participant had been allocated to the 2 × 107 PFU dose. In all, 20, 20, 1, 20, and 20 adults were randomised to a vaccine dose of 3 × 105, 3 × 106, 2 × 107, 3 × 103, and 3 × 104 PFU, respectively. Preliminary data from the 20 participants vaccinated with 3 × 105 PFU and the initial 19 vaccinated with 3 × 106 PFU were previously reported [9].
An unblinded safety review of VEBCON Consortium trials by the data and safety monitoring board lifted the safety hold on 5 January 2015. After this and during the third stage of the study, 19 adults were vaccinated with 3 × 106 PFU without randomisation. In addition, Merck Sharp & Dohme selected the 2 × 107 PFU dose for further development, as being the most immunogenic dose with an acceptable safety profile (S. Gupta, oral presentation at the WHO Ebola Research and Development Summit, 11–12 May 2015, Geneva) [13,14]. A subsequent amendment included 20 adolescents and 20 children aged 13 to 17 years and 6 to 12 years, respectively, to be vaccinated with 2 × 107 PFU. The National Ethics Committee of Gabon recommended that adults from this population should be vaccinated with the intended dose before administration to the paediatric cohorts, so an additional 15 adults were included in the study (S3 Text).
Vaccine and vaccination procedures
The rVSVΔG-ZEBOV-GP vaccine, developed by the Canadian National Laboratory under the patent number WO2004011488 A2 and licensed to BioProtection Systems (NewLink Genetics), was the unique intervention in this trial. The vaccine was subsequently sublicensed to Merck and was manufactured at IDT Biologika (Dessau-Rosslau, Germany). WHO supplied single-dose vials of 1 × 108 PFU (lot no 0030513) to conduct the trial at CERMEL, from a donation of rVSVΔG-ZEBOV-GP by the Canadian government to WHO. The dispensed vials were reconstituted in serial dilutions for vaccination. A single injection of 1 ml of the reconstituted vaccine for the required dose was administered intramuscularly into the deltoid muscle of volunteers at vaccination (S1 Fig).
Safety assessments
The nature, frequency, and severity of adverse events constituted the primary safety endpoint of the trial. Local and systemic reactogenicity symptoms and signs (solicited adverse events) were recorded for 14 days post-injection. Unsolicited adverse events, including laboratory anomalies, were recorded up to 28 days post-injection. Detailed descriptions of all serious adverse events were recorded throughout the study follow-up visits, as a secondary safety endpoint.
Solicited adverse events (pain, swelling, redness) were obtained by direct examination of the injection site, or direct questioning when follow-up occurred by telephone. Arthralgia and arthritic symptoms were later added as a solicited adverse event upon the request of the data and safety monitoring board. Participants were asked specifically if they were experiencing these symptoms.
rVSVΔG-ZEBOV-GP viraemia and shedding
Plasma, saliva, and urine samples (at screening and days 1, 2, and 7 post-vaccination) were processed and stored in Trizol LS at the study site, until rVSVΔG-ZEBOV-GP viral load determinations were performed by reverse transcriptase quantitative PCR as a secondary outcome. The lower limit of detection for rVSVΔG-ZEBOV-GP RNA was 30 copies/ml, and the lower level of quantification was 100 copies/ml [9].
Immunological assessments
As a secondary objective, enzyme-linked immunosorbent assays (ELISAs) were performed on days 0, 28, 56, 84, and 180 after injection. ZEBOV-specific antibody assays were conducted at the Institute for Virology, Marburg. Antibodies were detected using an antibody capture ELISA based on inactivated Ebola Zaire Makona virus particles [15]. ELISA for ZEBOV-GP-specific antibodies was performed at the US Army Medical Research Institute of Infectious Diseases (USAMRIID) using the Kikwit-95 ZEBOV strain GP (standard operating procedure AP-03-35-00). Antibodies were reported as geometric mean titres (GMTs), or geometric mean concentrations, of arbitrary ELISA units (AEU) per millilitre with 95% confidence intervals, as indicated.
Neutralising antibodies (Nabs) were detected using either particles of Ebola virus (Zaire isolate Mayinga, AF086833), with the assays being performed in a BSL4 laboratory (Institute for Virology, Marburg), or VSV pseudovirions expressing the luciferase reporter gene complemented by GP from the Kikwit-95 ZEBOV strain, with assays being performed at USAMRIID.
All 4 assays were previously reported by our team [9,15] and other researchers working on this candidate vaccine in the US [7].
Statistical analysis
WHO estimated that a sample size of 74–124 participants would be needed across the VEBCON Consortium sites to show a 2-fold change in ZEBOV-specific antibody titres between vaccine doses and proposed a target sample size of approximately 250 participants for all sites [10]. We described the frequency and intensity of adverse events using counts and percentages, means and standard deviations, or medians and interquartile ranges (IQRs), for skewed continuous variables. Chi-squared test or Fisher’s exact test was used to compare pairwise proportions. Seropositivity rates were defined as the percentage of participants having AEU above a cutoff per vaccine group. Seroconversion rates were defined as the percentage of converted participants in each group. McNemar’s test was used to compare the seropositivity between day 0 and other days. We used Fisher’s test to perform inter-group comparisons and to determine the association between the seroconversion rate and seropositivity rate at each time point. Antibody concentrations or units were normalised using log transformations, and responses are reported as GMTs with 95% confidence intervals or geometric mean of AEU per millilitre with 95% confidence intervals. Student’s test or Wilcoxon’s paired test was used to compare magnitudes of antibody induced between day 0 and other days. All statistical analyses were conducted in R statistical software version 3.1.2 [16], except for viraemias (copies/millilitre of plasma), which were analysed with a Kruskal–Wallis test combined with Dunn’s multiple comparison test using GraphPad Prism version 6.
Results
From 21 November 2014 to 13 April 2015, 115 adults were vaccinated with a single injection of rVSVΔG-ZEBOV-GP at 5 different doses. Twenty adolescents and 20 children were vaccinated between 8 May and 7 July 2015 (Fig 1).
Fig. 1. Participant flow diagram. 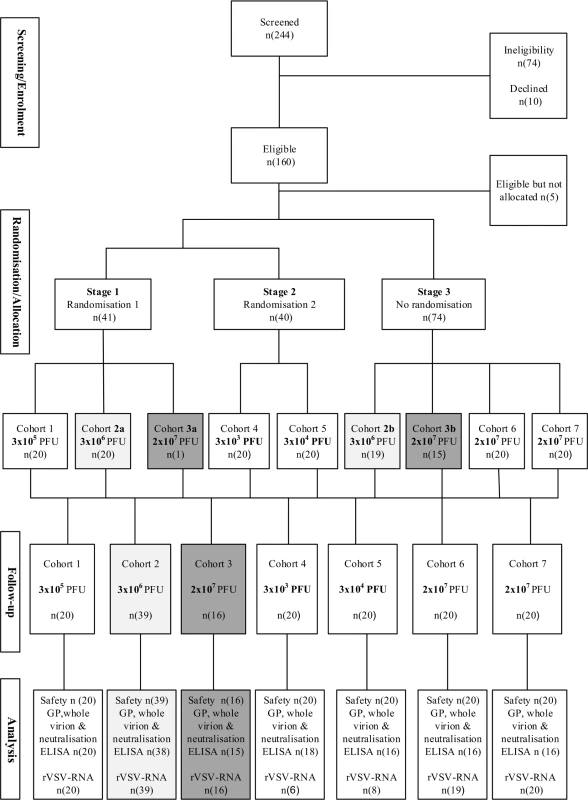
Randomisation and flow of participants over a period of 6 months for adults (cohorts 1 to 5), adolescents (cohort 6; 13–17 years), and children (cohort 7; 6–12 years). Similar dose groups are matched with shading (light grey, 3 × 106 PFU; dark grey, 2 × 107 PFU). GP, glycoprotein; ELISA, enzyme-linked immunosorbent assay; PFU, plaque-forming units; rVSV, recombinant vesicular stomatitis virus. Safety and immunogenicity data are reported until month 6 for adults, adolescents, and children for all 155 participants. In all, 108 (93%) adults and 36 (90%) adolescents and children attended all planned immunogenicity visits. Mean age and body mass index were similar among the 5 adult cohorts, with 21 adult women enrolled in the trial (Table 1).
Tab. 1. Baseline characteristics of study participants. 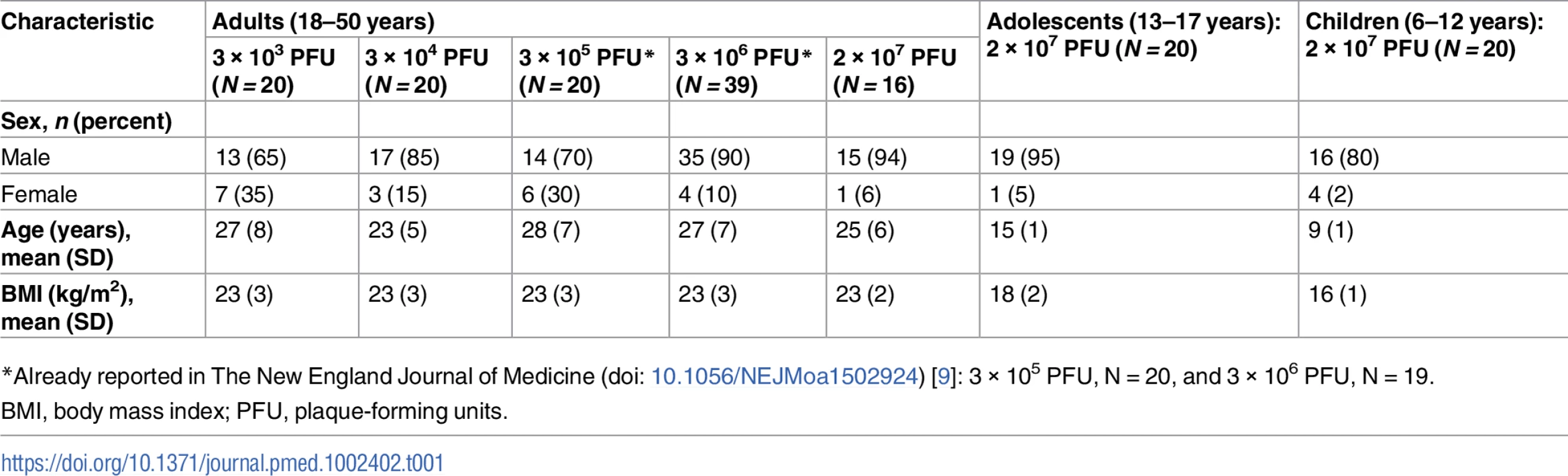
*Already reported in The New England Journal of Medicine (doi: 10.1056/NEJMoa1502924) [9]: 3 × 105 PFU, N = 20, and 3 × 106 PFU, N = 19. Assessment of 5 vaccine doses in adult volunteers
Reactogenicity and tolerability
Headaches, fatigue, pain at injection site, gastrointestinal symptoms, and subjective fever were the most frequent symptoms. Of these, 68% were mild and 32% moderately intense, with similar frequencies up to day 28 across cohorts in adults. There were no vaccine-related severe adverse events (Tables 2 and S1).
Tab. 2. Reactogenicity to rVSVΔG-ZEBOV-GP vaccine until day 28 post-vaccination. 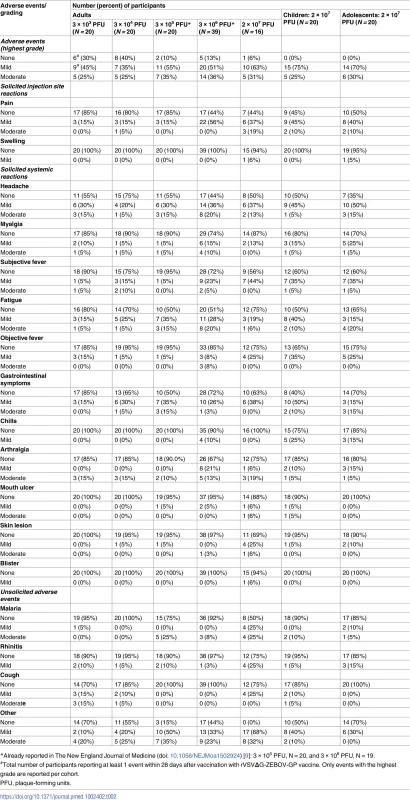
*Already reported in The New England Journal of Medicine (doi: 10.1056/NEJMoa1502924) [9]: 3 × 105 PFU, N = 20, and 3 × 106 PFU, N = 19. Monocytes increased and lymphocytes decreased in the first week after vaccination in a dose-dependent fashion (S5 Table).
Mild-to-moderate symptoms reported at days 56, 84, and 180 and during unscheduled visits were considered unrelated to the study vaccine (S4 Table). Few haematological or biochemical changes of clinical significance were captured as adverse events, and these were followed up until resolution without sequelae.
A total of 11 adult participants experienced a serious adverse event. Six participants had malaria requiring hospitalisation, 2 underwent surgery due to appendicitis, and 1 was diagnosed with glaucoma. The last probably had the condition prior to enrolment after a detailed history was obtained, and is now receiving specialised care. Two individuals were hospitalised for bleeding after dental surgery and gastritis, respectively. All of these events were judged unrelated to the vaccine. Three women became pregnant after vaccination; they were monitored until delivery. Their neonates had no safety complications.
Immunogenicity
Eleven adults were excluded from either sampling and/or analysis of immunogenicity data: a male participant was HIV positive, 3 women became pregnant beyond day 28 after vaccination, and 7 participants received anti-tetanus vaccine/immunoglobulin. These participants were not sampled on subsequent visits (days 56, 84, and 180).
ZEBOV-GP-specific and ZEBOV antibodies
In all, 70%–100% of adult participants vaccinated with all doses ≥3 × 104 PFU reached a greater than 4.0-fold increase of ZEBOV-GP-specific GMT at day 28. ZEBOV-GP antibody GMTs peaked at day 56, with antibody levels persistently higher than baseline up to 6 months post-vaccination (Table 3).
Tab. 3. Endpoint geometric mean titres, seropositivity rates, and proportions of seroresponders to rVSVΔG-ZEBOV-GP measured by ZEBOV-GP ELISA in adults. 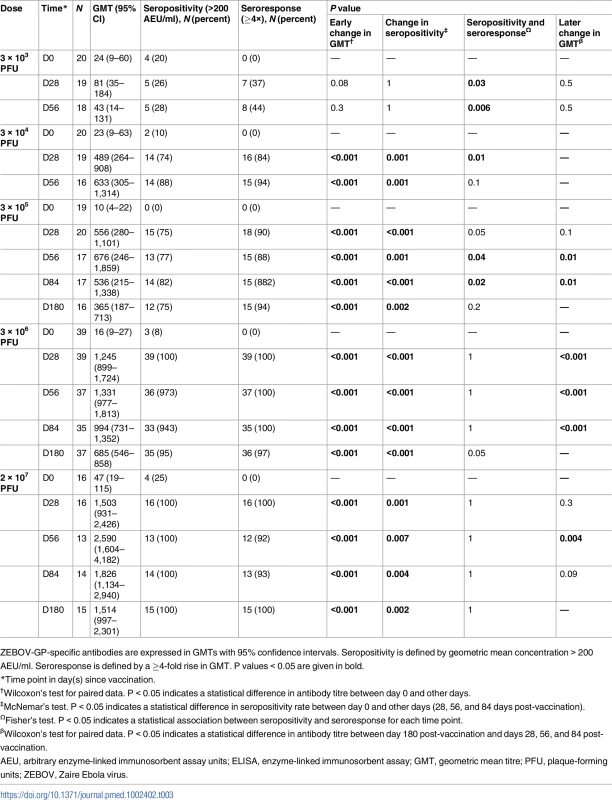
ZEBOV-GP-specific antibodies are expressed in GMTs with 95% confidence intervals. Seropositivity is defined by geometric mean concentration > 200 AEU/ml. Seroresponse is defined by a ≥4-fold rise in GMT. P values < 0.05 are given in bold. About 11% (13/114) of adult participants had ZEBOV-GP-specific ELISA antibody concentrations > 200 AEU/ml at baseline. The proportions of individuals with high concentrations at baseline were inconsistent across vaccine groups and ranged from 0% to 25%. Antibody concentrations were significantly higher at day 56 post-vaccination in individuals with prior antibodies following vaccination with doses of 3 × 103, 3 × 104, and 3 × 106 PFU (Table 4).
Tab. 4. Endpoint geometric mean titres measured by USAMRIID ZEBOV-GP ELISA in adults with and without baseline specific antibodies. 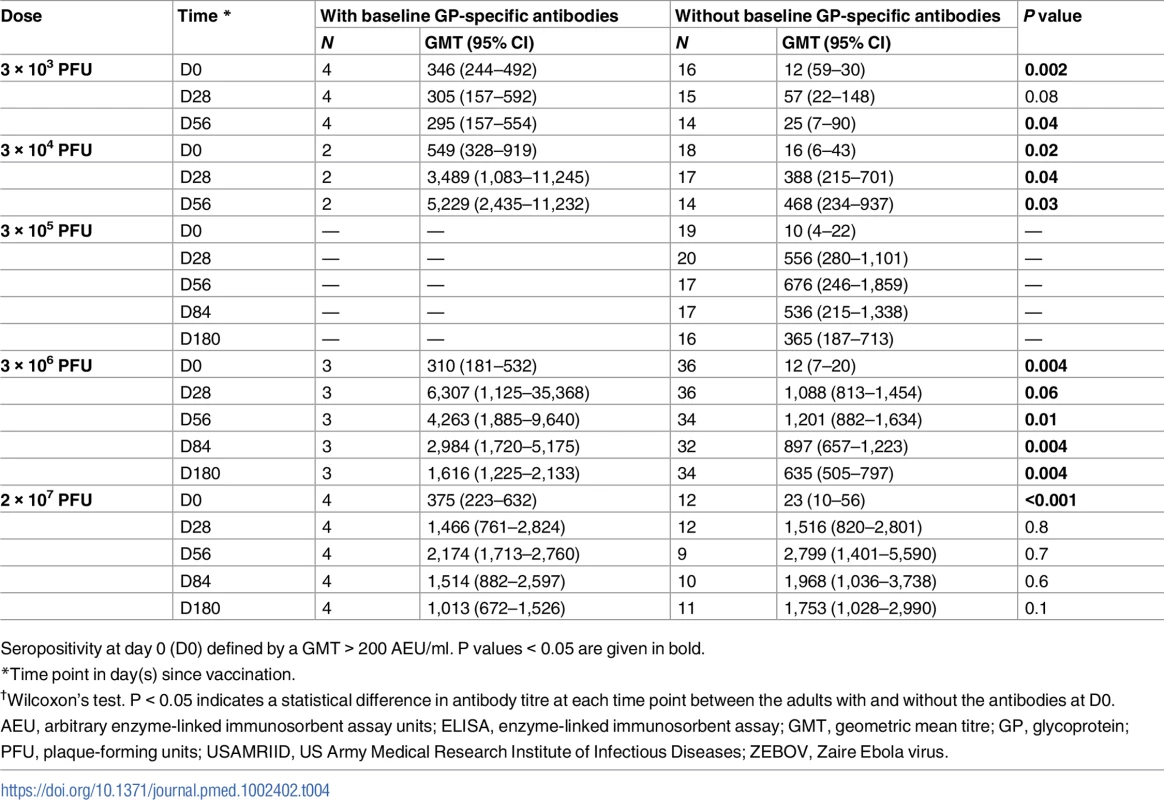
Seropositivity at day 0 (D0) defined by a GMT > 200 AEU/ml. P values < 0.05 are given in bold. The whole-virion assay is a less sensitive method to detect vaccine-induced antibody responses, which are directed against GP; 34% and 63% of vaccinees who received a dose equal to or more than 3 × 105 PFU reached a greater than 2.0-fold increase in ZEBOV antibody GMT at day 28 and 56, respectively. Only 40% (30/74) of participants in the dose groups 3 × 105, 3 × 106, and 2 × 107 PFU had a greater than 2.0-fold increase in antibody persisting up to 6 months post-injection (Table 5).
Tab. 5. Geometric mean titres, seropositivity rates, and proportions of seroresponders to rVSVΔG-ZEBOV-GP measured by whole-virion ELISA in adults. 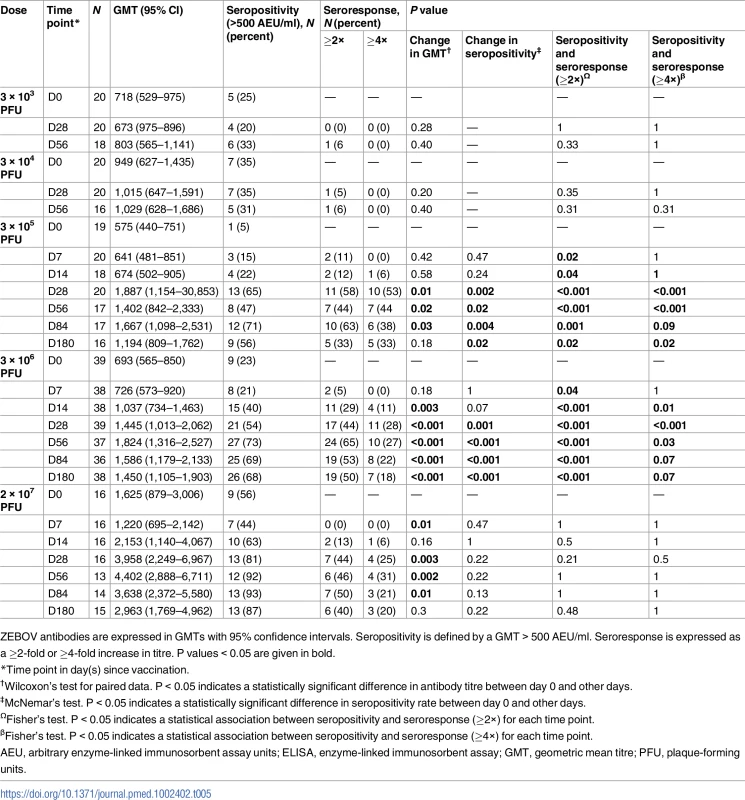
ZEBOV antibodies are expressed in GMTs with 95% confidence intervals. Seropositivity is defined by a GMT > 500 AEU/ml. Seroresponse is expressed as a ≥2-fold or ≥4-fold increase in titre. P values < 0.05 are given in bold. About 27% (31/115) of adults had ZEBOV antibody concentrations > 500 AEU/ml at baseline, with inconsistent frequencies (5% to 56%) across dose levels. In adults with pre-vaccination antibodies, a dose as low as 3 × 104 PFU yielded a 2-fold increase in ZEBOV antibodies post-injection. Regardless of baseline status, the highest antibody titres were observed with the 2 × 107 PFU dose (Table 6).
Tab. 6. Geometric mean titres of ZEBOV antibodies in adults by baseline antibody status measured by whole-virion ELISA. 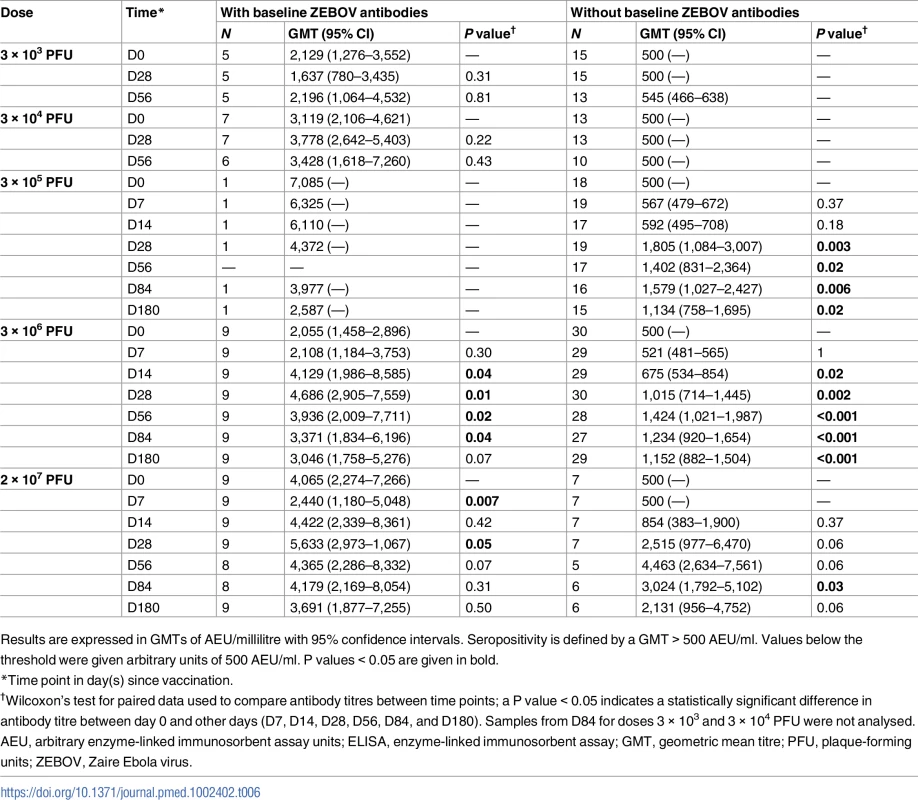
Results are expressed in GMTs of AEU/millilitre with 95% confidence intervals. Seropositivity is defined by a GMT > 500 AEU/ml. Values below the threshold were given arbitrary units of 500 AEU/ml. P values < 0.05 are given in bold. Neutralising antibodies
Nabs for doses of 3 × 104, 3 × 105, 3 × 106, and 2 × 107 were detected in 52%, 55%, 82%, and 62% of recipients against VSV-based Ebola pseudovirions (pseudovirion neutralisation assay 50% [PsVNA50]), respectively, and in 70%, 84%, and 56% of recipients against ZEBOV virus particles. The highest Nab GMTs were observed in recipients of 2 × 107 PFU. About 35%, 13%, and 25% of participants in the dose groups 3 × 105, 3 × 106, and 2 × 107 PFU, respectively, had baseline Nabs against ZEBOV virus particles (defined as GMT > GMT + SD at D0). Higher Nab GMTs were observed at day 28 regardless of baseline antibody status (Tables 7, S8 and S9).
Tab. 7. Geometric mean titres, seropositivity rates, and proportions of seroresponders to rVSVΔG-ZEBOV-GP measured by ZEBOV PsVNA50 in adults. 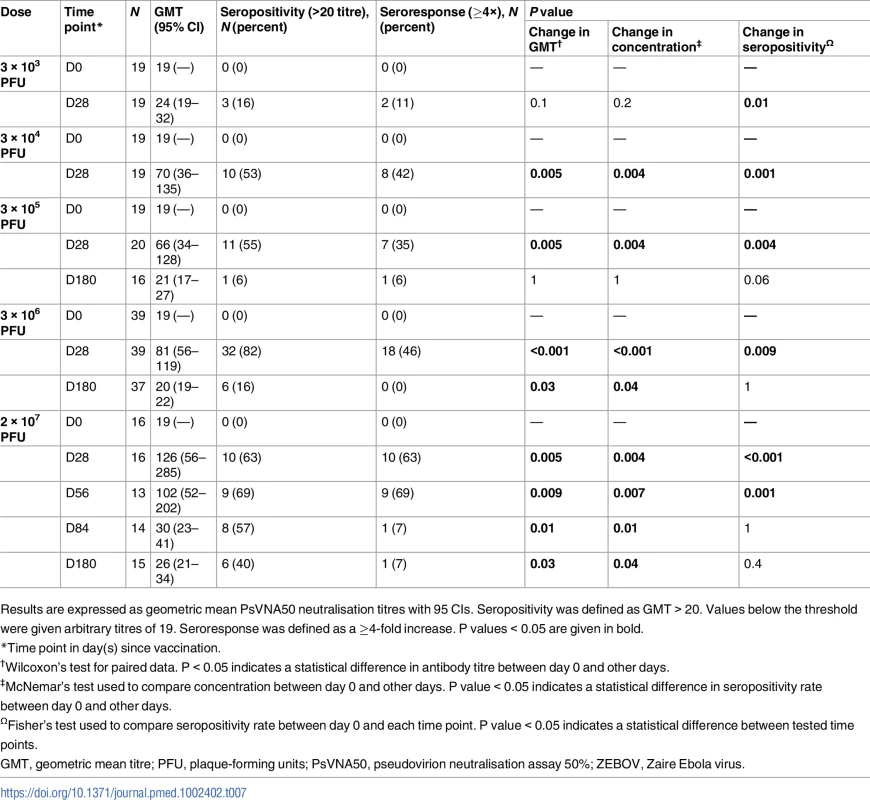
Results are expressed as geometric mean PsVNA50 neutralisation titres with 95 CIs. Seropositivity was defined as GMT > 20. Values below the threshold were given arbitrary titres of 19. Seroresponse was defined as a ≥4-fold increase. P values < 0.05 are given in bold. The vaccine dose of 2 × 107 PFU in adult, adolescent, and child volunteers
Reactogenicity and tolerability
Twenty adolescents aged 13–17 years and 20 children aged 6–12 years were vaccinated with 1 intramuscular dose of 2 × 107 PFU. Adolescents and children reported mostly headaches, fatigue, pain at injection site, gastrointestinal symptoms, and subjective fever. All reported symptoms were of mild (81% adolescents, 82% children) to moderate (19% adolescents, 18% children) intensity (Table 2).
As in adults, a general reduction in leukocyte counts was observed in adolescents and children within the first 2 days post-injection; leukocytes gradually restored to baseline values by day 28. An increase in monocyte and lymphocyte counts was observed between days 2 and 7, with lymphocytes rapidly restoring to baseline values by day 7 (S5 Table). No vaccine-related serious or severe adverse events occurred. One child was hospitalised for malaria.
rVSVΔG-ZEBOV-GP viraemia and shedding
Compared to adults vaccinated with 2 × 107 PFU, rVSVΔG-ZEBOV-GP RNA copy numbers in both adolescents and children were significantly higher at 1,592 (IQR 1,019–2,704) and 1,109 (IQR 663–1,963), respectively, versus 532 (IQR 373–898) in adults (P = 0.001) at day 2 post-injection (Table 8).
Tab. 8. Description of viraemia by dose and age. 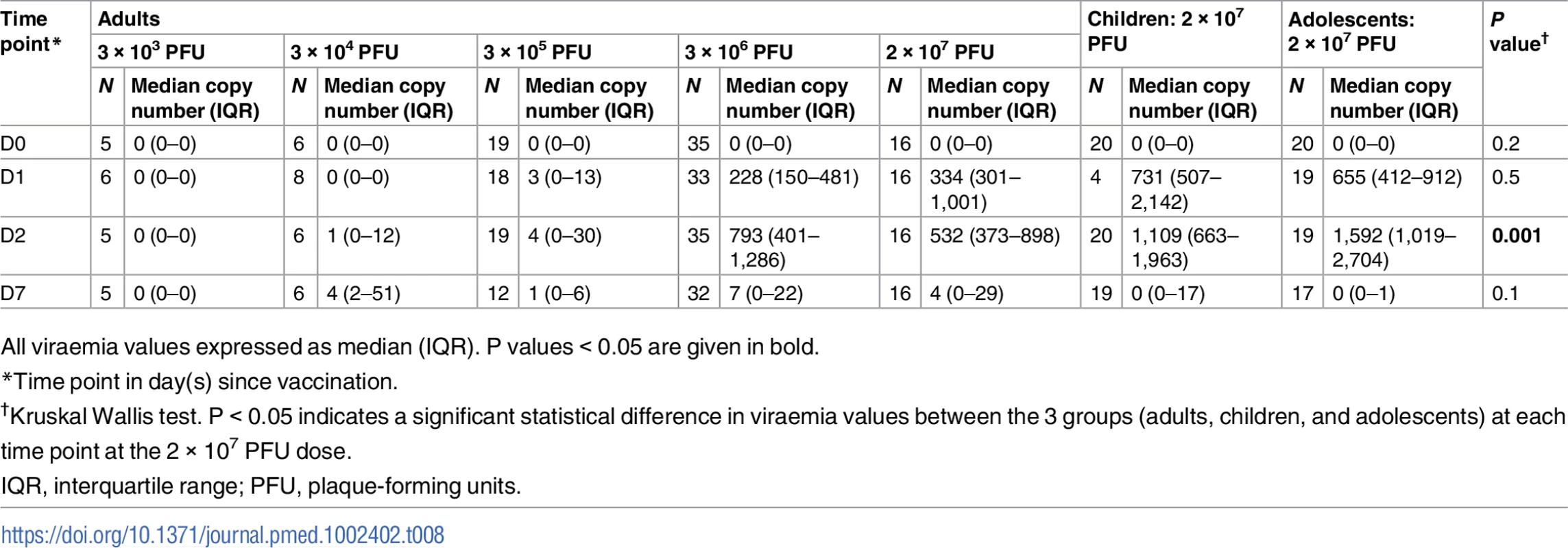
All viraemia values expressed as median (IQR). P values < 0.05 are given in bold. For viral shedding, there was a low percentage of positive samples at day 2, albeit below the level of quantification. At day 7, there was 1 child and 1 adolescent who had quantifiable RNA in urine. Saliva investigations showed that 42% and 30%, respectively, of adolescents and children had detectable RNA, corresponding with peak viraemia at day 2. At day 7, a considerably higher proportion of adolescents and children, 78% and 35% respectively, had RNA-positive saliva, with most samples being quantifiable (Figs 2 and S2; S13–S15 Tables).
Fig. 2. Viral load in saliva for children and adolescents. 
rVSVΔG-ZEBOV-GP (rVSV) RNA copy numbers in saliva presented as log10 rVSV RNA copies/ml from day 2 and 7 (d2 and d7) post-injection in adolescents and children vaccinated with 2 × 107 PFU. The broken line denotes the limit of quantitation, and the dotted line denotes the limit of detection. About 67% (12/18) and 30% (6/20) adolescents and children, respectively, had samples above the limit of quantification at day 7. *P < 0.05; **P < 0.01.PFU, plaque-forming units. Immunogenicity
ZEBOV-GP-specific and ZEBOV antibodies
In all, 90% and 100% of children and adolescents, respectively, receiving 2 × 107 PFU had ZEBOV-GP-specific antibodies at day 28. Antibody titres were similar between adolescents, adults, and children using GP ELISA regardless of baseline antibody status (Figs 3, 4, S3 and S4).
Fig. 3. Glycoprotein antibody distribution by age group: Comparison of distribution of ZEBOV-GP IgG antibodies (AEU/ml) measured by USAMRIID ZEBOV-GP ELISA for dose 2 × 107 PFU administered to children, adolescents, and adults from day 0, 28, 56, 84, and 180. 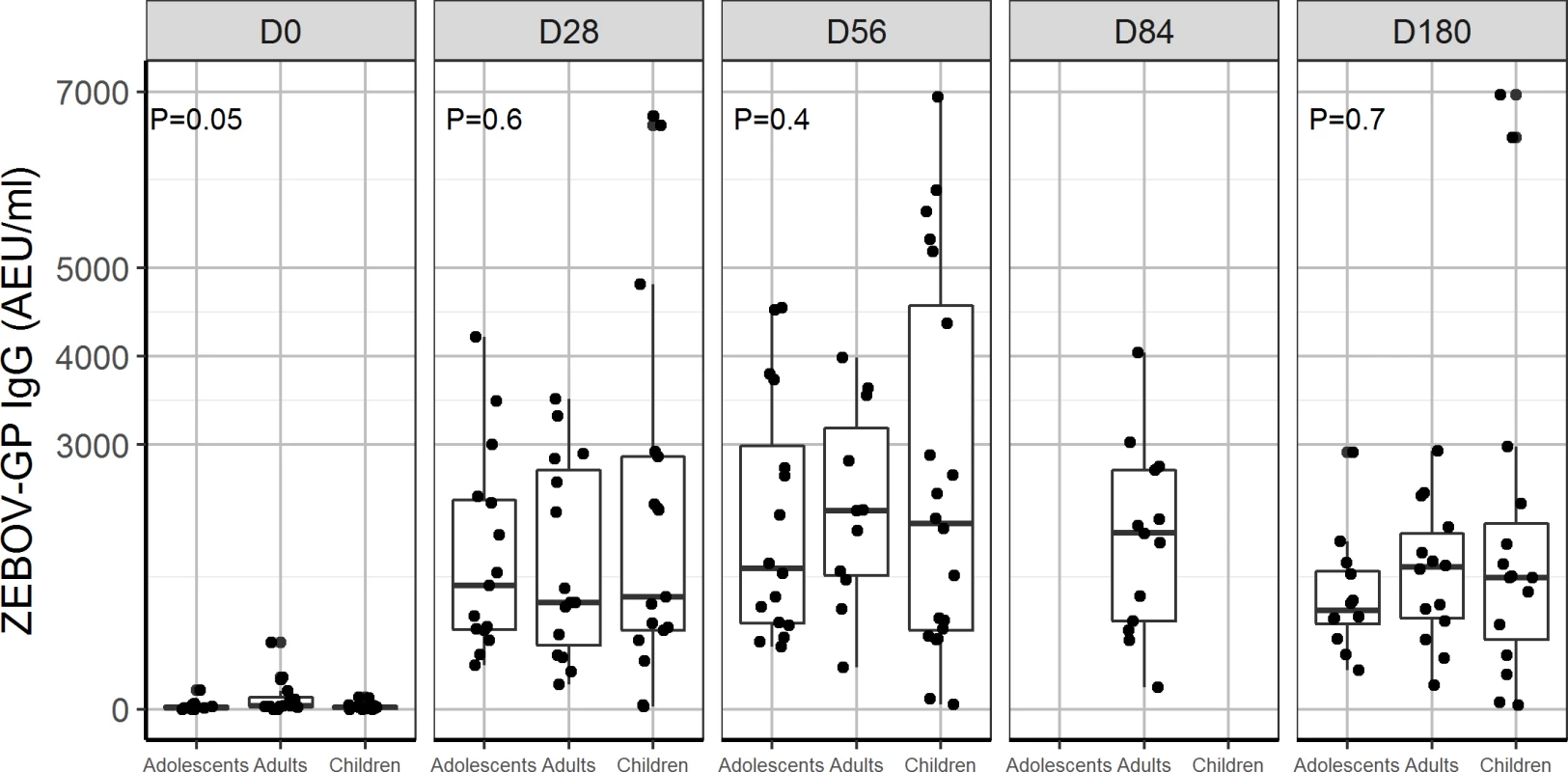
Data were not available for children and adolescents at D84. P < 0.05 indicates a statistical difference in ZEBOV-GP IgG between children, adolescents, and adults. AEU, arbitrary enzyme-linked immunosorbent assay units; ELISA, enzyme-linked immunosorbent assay; GP, glycoprotein; PFU, plaque-forming units; USAMRIID, US Army Medical Research Institute of Infectious Diseases; ZEBOV, Zaire Ebola virus. Fig. 4. Antibody responses to whole-virion ELISA (AEU/ml) by age group: Comparison of geometric mean concentration of IgG antibodies for children, adolescents, and adults vaccinated with the 2 × 107 PFU dose at day 0, 28, and 56. 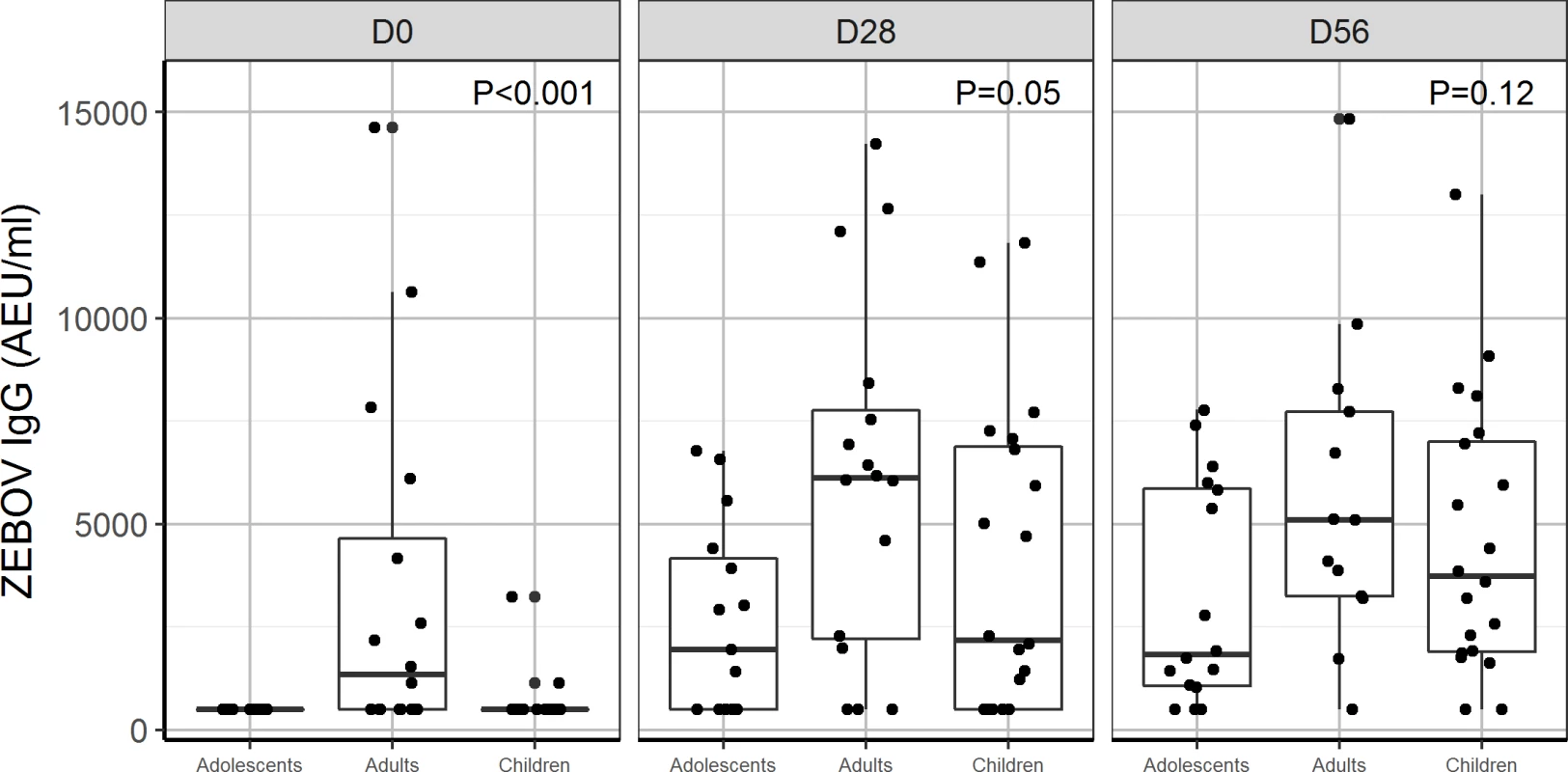
P < 0.05 indicates a statistical difference in antibody concentrations between age groups at the measured time points. AEU, arbitrary enzyme-linked immunosorbent assay units; ELISA, enzyme-linked immunosorbent assay; PFU, plaque-forming units; ZEBOV, Zaire Ebola virus. By day 28, 70% and 60% of adolescents and children, respectively, were seropositive with whole-virion ELISA, compared to 81% of adults injected with 2 × 107 PFU. Using a more sensitive GP ELISA, we obtained higher seropositivity rates, 100%, 100%, and 90% for adults, adolescents, and children, respectively, at day 28. We observed a ≥4-fold increase in ZEBOV-GP-specific antibody titres in about 90%–100% of adults, adolescents, and children consistently from day 28 to 180 post-injection. However, ZEBOV-GP-specific antibodies increased up to day 180 in children and adolescents (Table 9), while in adults, it peaked at day 56, and there was a decline until day 180 (Tables 3 and 5).
Tab. 9. Geometric mean titres, seropositivity rates, and proportions of seroresponders to rVSVΔG-ZEBOV-GP measured by ZEBOV-GP ELISA in children. 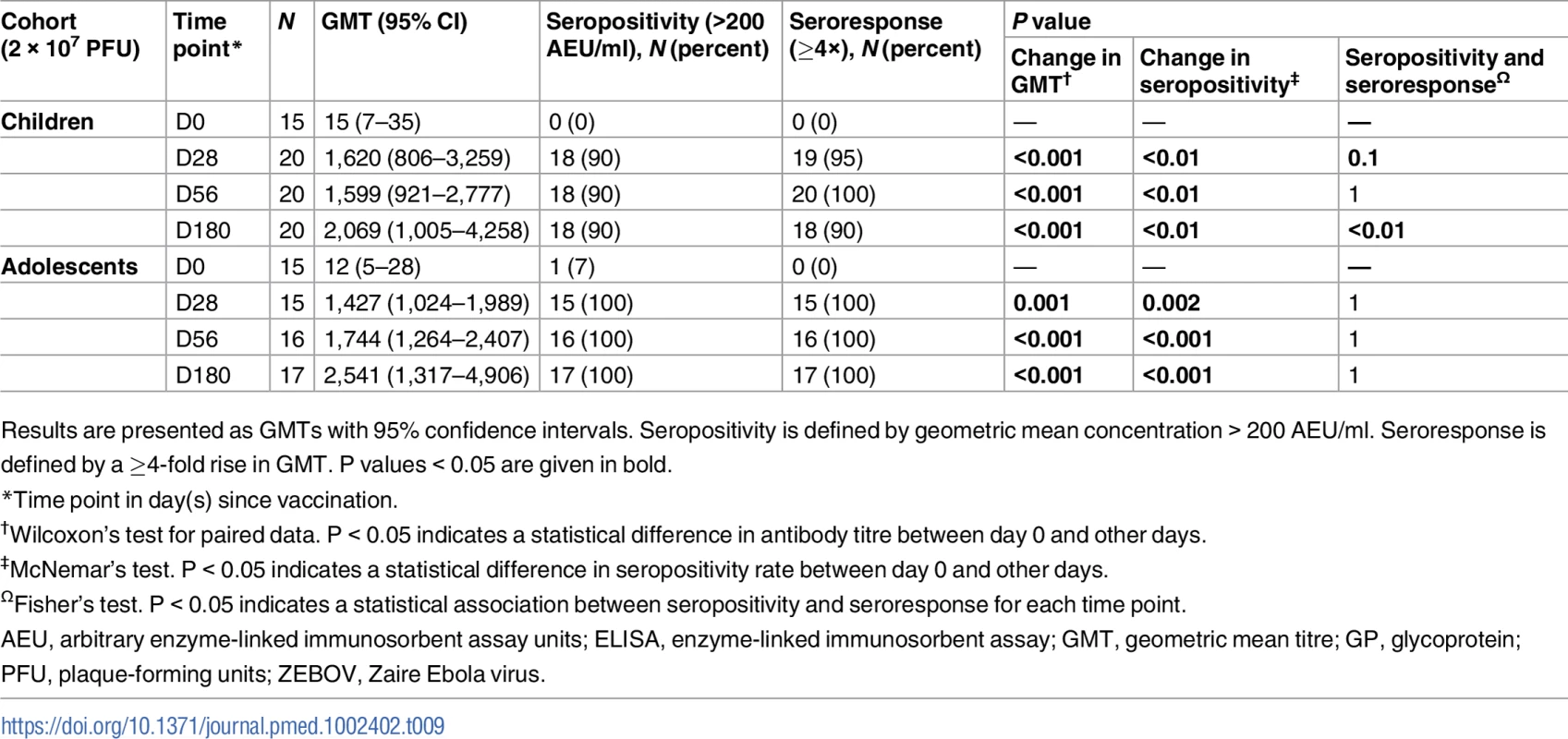
Results are presented as GMTs with 95% confidence intervals. Seropositivity is defined by geometric mean concentration > 200 AEU/ml. Seroresponse is defined by a ≥4-fold rise in GMT. P values < 0.05 are given in bold. Lower proportions of adults, adolescents, and children had a ≥4-fold increase in ZEBOV antibodies with whole-virion ELISA. Considering a ≥2-fold increase for this less sensitive ELISA, the yielded proportions were still much lower than those seen with GP ELISA. However, the proportion of adolescents and children with a ≥2 - or ≥4-fold increase in ZEBOV antibodies increased from day 28 to 56, in contrast to the lack of difference between these time points in adults (Tables 5, 6 and S6). Thirteen percent of the children, but none of the adolescents, were seropositive for ZEBOV antibodies at baseline. None of the children and 7% of the adolescents were seropositive for ZEBOV-GP antibodies at baseline. As in adults, children with ZEBOV antibodies at baseline had higher GMTs at days 28 and 56 compared to those without baseline antibodies (Tables 6 and S7).
Neutralising antibodies
Against VSV pseudovirions, about 73% of children and adolescents elicited Nabs, with higher GMTs occurring at day 56 compared to day 28. In all, 95% and 80% of children and adolescents, respectively, had ZEBOV Nabs at day 28. Overall, children produced significantly higher GMTs of Nabs against ZEBOV particles (20 [95% CI: 13–32] compared to adolescents and adults, 10 [95% CI: 8–14] and 10 [95% CI: 6–14], respectively, P = 0.04) (S10–S12 Tables).
Discussion
Although the 2014–2016 EVD emergency in western Africa has ended, the increasing mobility of people between remote and urban areas and the weak health systems in Ebolavirus endemic countries suggest that a future outbreak could reassert itself as a major international threat [17,18]. Risks include increased human-to-human secondary transmission as in the recent epidemic [19] as well as continuing transmission after recovery. Halting transmission by vaccination will be key in curbing future outbreaks [20]. The rVSVΔG-ZEBOV-GP and ChAd3-ZEBOV vaccine candidates were selected by WHO in August 2014 for fast track clinical evaluation [6]. As part of these efforts, we examined a range of doses for rVSVΔG-ZEBOV-GP in adults as well as safety and immunogenicity in children.
As reported earlier, rVSVΔG-ZEBOV-GP doses of 3 × 105 and 3 × 106 PFU were well tolerated by 39 Lambaréné participants until day 28 and were safe up to 6 months [9]. Comparable to studies in Guinea [21] and US adults [7], transient cases of arthralgia were reported after vaccination [9,21,22], but no case of arthritis. In Kilifi, Kenya, there were 2 self-limiting, low-severity, and short-duration cases of arthritis [9,23]. This contrasts with a higher frequency of vaccine-induced arthritis (24%), dermatitis (9.8%), and vasculitis (2%) in Geneva [9,10,24] and more recently in Canada, the US, and Spain [25]. There may be similarities between rVSVΔG-ZEBOV-GP vaccine and rubella vaccine, which also causes transient arthritides in some populations [26–28].
Ongoing studies are investigating the potential mechanisms by which rVSVΔG-ZEBOV-GP vaccine might disseminate into peripheral tissues and induce arthritides in specific hosts. The magnitude of innate immune responses to rVSVΔG-ZEBOV-GP vaccine correlated with the peak of rVSV RNA at day 1 in vaccinees of both Geneva and Lambaréné cohorts [29]. Importantly, high-dose vaccinees who experienced arthritis in Geneva had a significantly lower magnitude of early immune response compared to high-dose vaccinees who did not experience arthritis. These findings suggest that early and appropriate (in nature and magnitude) innate immune responses play a key role in limiting viral replication and dissemination to tissues and thus prevent the risk of arthritis. With lower vaccine dose (3 × 105 PFU), the strength of early innate immune responses was similar in cases both with and without arthritis. Thus, rVSV-ZEBOV-induced arthritis may occur through mechanisms related to either vaccine dose or underlying factors that influence immune responses in vaccinees [29].
We observed higher and persistent viraemia in children and adolescents as well as shedding in saliva and urine, in contrast to the very low proportions or no shedding previously reported in the saliva of American and European adults vaccinated with 3 × 106 to 5 × 107 PFU [7,9,10]. The shedding in saliva did not correlate with oral symptoms. Although no alarming symptoms have been detected so far, our finding suggests that a vaccine dose of 2 × 107 PFU exposed the paediatric population to prolonged or uncontrolled viraemia, with potential to disseminate to peripheral tissues. Specific studies are needed to elucidate the underlying mechanisms prolonging viraemia and causing shedding, such as differences in innate responses to vaccine between adults and younger participants. It is also necessary to assess any potential dissemination of rVSV-ZEBOV among household members of vaccinated children.
We observed dose-dependent antibody responses to the rVSVΔG-ZEBOV-GP vaccine. A very low dose (≤3 × 103 PFU) did not generate antibodies measured with either whole-virion or ZEBOV-GP-specific ELISA. In all individuals vaccinated with 3 × 104, 3 × 105, 3 × 106, and 2 × 107 PFU, the vaccine induced significant increases in ZEBOV-GP-specific antibodies measured by ZEBOV-GP ELISA alone for 3 × 104 PFU and by both whole-virion and GP ELISAs for the other vaccine doses. The highest GMTs were observed with 2 × 107 PFU irrespective of the ELISA method used.
As previously reported [9,11,12], our participants harboured naturally acquired antibodies against ZEBOV, or possibly related viruses. Western blot analysis of sub-samples showed that these antibodies were directed more often against nucleocapsid and matrix proteins of ZEBOV and not against GP. Nonetheless, 11% of our adults had ZEBOV-GP-specific antibodies before vaccination using the GP-specific ELISA. Individuals with baseline antibodies developed higher antibody titres with a dose as low as 3 × 104 PFU compared to those without. The vaccine may have elicited higher titres of antibodies in the presence of natural GP-specific antibodies but also in the presence of antibodies directed against other viral components including nucleocapsid and matrix proteins (detected in baseline sera of some study participants) [9].
In adults, vaccine-induced antibodies peaked at day 56 and declined slowly by day 180. In children and adolescents, who showed high viraemia at day 2 and shed the vaccine until day 7, antibody titres increased until day 180. The kinetics of antibodies after vaccination may be affected by the specificity of pre-existing antibodies, and persistent vaccine replication may enhance immunogenicity. Also, the highest titres of Nabs against Ebola virus, which paralleled those against VSV pseudovirions, were generated at day 28 post-injection, regardless of baseline seropositivity. The relative roles of neutralising, GP, and non-GP antibodies in protection against EVD remain undefined, so it is difficult to draw conclusions on the clinical significance of correlations between GP-binding and neutralising antibodies produced after vaccinations.
The vaccine dose of 2 × 107 PFU showed the optimal safety versus immunogenicity balance in our adult cohorts as well as in the Geneva and Hamburg cohorts [29,30]. These findings support the choice to use this dose in the context of outbreaks [8]. However, our data cannot explain the protection induced by the vaccine within 10 days observed in a phase III trial in Guinea [8] as the seroconversion rates and antibody titres were very weak before day 28 irrespective of the vaccine dose. Innate immune components induced immediately after vaccination may have played an important role in early protection. A recent study demonstrated the direct influence of innate immune responses on this vaccine’s safety and immunogenicity [29], a finding which supports the interest in assessing the efficacy of this vaccine beyond Zaire ebolavirus spp. as innate mechanisms can be cross-reactive.
Lower doses could be considered in vaccination strategies for children and individuals with impaired innate immune responses to control early rVSV replication. The dose of 3 × 105 PFU generated significantly fewer rVSV RNA copies and shorter rVSV replication cycles but high antibody titres, so is of interest. As an incidental finding in our area, where Ebola virus transmission is endemic, a proportion of participants had antibodies directed against the whole-virus or GP-specific antigen before vaccination [11,12,31]. In those participants, a vaccine dose as low as 3 × 104 PFU induced high antibody titres, suggesting lower vaccine doses should be considered in boosting strategies.
There are some limitations of our observations. For example, we cannot relate viral shedding in saliva with the oral symptoms reported by adolescents and children, suggesting that further studies are needed to evaluate this finding. We did not stratify participants based on antibody status at enrolment; future studies in Ebola virus endemic areas where such stratification is inherent in the design will provide insights into the relationships between naturally acquired antibodies and vaccine-induced immune responses and safety. We enrolled very few women across cohorts, leading to imbalances in the male/female ratio in our trial, which may reflect the general reluctance of women to enrol in phase I studies.
Our study confirms the acceptable safety and immunogenicity profile of the 2 × 107 PFU dose in adults. However, considering the persistent replication of the rVSVΔGP-ZEBOV-GP vaccine in children and adolescents, further studies investigating lower doses in this population are warranted. In addition, lower vaccine doses should be considered when boosting individuals with pre-existing antibodies.
Supporting Information
Zdroje
1. World Health Organization Regional Office for Africa. WHO declares the end of the most recent Ebola virus disease outbreak in Liberia. Brazzaville (Republic of the Congo): World Health Organization Regional Office for Africa; 2016 Jun 9 [cited 2017 Sep 12]. Available from: http://www.afro.who.int/news/who-declares-end-most-recent-ebola-virus-disease-outbreak-liberia-0.
2. World Health Organization. Ebola situation report—30 March 2016. Geneva: World Health Organization; 2016 [cited 2016 Apr 29]. Available from: http://apps.who.int/ebola/current-situation/ebola-situation-report-30-march-2016.
3. World Health Organization. Ebola outbreak 2014–2015. Geneva: World Health Organization; 2016 [cited 2017 Mar 1]. Available from: http://who.int/csr/disease/ebola/en/.
4. Sridhar S. Clinical development of Ebola vaccines. Ther Adv vaccines. 2015;3(5–6):125–38. doi: 10.1177/2051013615611017 26668751
5. Wang Y, Li J, Hu Y, Liang Q, Wei M, Zhu F. Ebola vaccines in clinical trial: the promising candidates. Hum Vaccin Immunother. 2017;13(1):153–68. doi: 10.1080/21645515.2016.1225637 27764560
6. World Health Organization. Essential medicines and health products: Ebola vaccines, therapies, and diagnostics. Geneva: World Health Organization; 2015 [cited 2017 Sep 12]. Available from: http://www.who.int/medicines/emp_ebola_q_as/en.
7. Regules JA, Beigel JH, Paolino KM, Voell J, Castellano AR, Munoz P, et al. A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med. 2017;376(4):330–41. doi: 10.1056/NEJMoa1414216 25830322
8. Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet. 2017;389(10068):505–18. doi: 10.1016/S0140-6736(16)32621-6 28017403
9. Agnandji ST, Huttner A, Zinser ME, Njuguna P, Dahlke C, Fernandes JF, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med. 2016;374(17):1647–60. doi: 10.1056/NEJMoa1502924 25830326
10. Huttner A, Dayer JA, Yerly S, Combescure C, Auderset F, Desmeules J, et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: a randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect Dis. 2015;15(10):1156–66. doi: 10.1016/S1473-3099(15)00154-1 26248510
11. Becquart P, Wauquier N, Mahlakoiv T, Nkoghe D, Padilla C, Souris M, et al. High prevalence of both humoral and cellular immunity to Zaire ebolavirus among rural populations in Gabon. PLoS ONE. 2010;5(2):e9126. doi: 10.1371/journal.pone.0009126 20161740
12. Heffernan RT, Pambo B, Hatchett RJ, Leman PA, Swanepoel R, Ryder RW. Low seroprevalence of IgG antibodies to Ebola virus in an epidemic zone: Ogooue-Ivindo region, Northeastern Gabon, 1997. J Infect Dis. 2005;191(6):964–8. doi: 10.1086/427994 15717273
13. Merck. Merck statement on rVSV-EBOV (V920) inclusion in Guinea study. Kenilworth (New Jersey): Merck; 2015 Mar 13 [cited 2017 Sep 12]. Available from: http://www.mrknewsroom.com/news/ebola-newsroom/merck-statement-rvsv-ebov-v920-inclusion-guinea-study.
14. Merck. Merck statement on rVSV-EBOV (V920) inclusion in Sierra Leone Ebola trial. Kenilworth (New Jersey): Merck; 2015 Feb 16 [cited 2017 Sep 12]. Available from: http://www.mrknewsroom.com/news/ebola-newsroom/merck-statement-rvsv-ebov-v920-inclusion-sierra-leone-ebola-trial.
15. Krahling V, Becker D, Rohde C, Eickmann M, Eroglu Y, Herwig A, et al. Development of an antibody capture ELISA using inactivated Ebola Zaire Makona virus. Med Microbiol Immunol. 2016;205(2):173–83. doi: 10.1007/s00430-015-0438-6 26475282
16. R: a language and environment for statistical computing. Vienna: R Foundation; 2015 [cited 2016 Feb 2]. Available from: https://www.R-project.org.
17. Heymann DL, Chen L, Takemi K, Fidler DP, Tappero JW, Thomas MJ, et al. Global health security: the wider lessons from the west African Ebola virus disease epidemic. Lancet. 2015;385(9980):1884–901. doi: 10.1016/S0140-6736(15)60858-3 25987157
18. Moon S, Sridhar D, Pate MA, Jha AK, Clinton C, Delaunay S, et al. Will Ebola change the game? Ten essential reforms before the next pandemic. The report of the Harvard-LSHTM Independent Panel on the Global Response to Ebola. Lancet. 2015;386(10009):2204–21. doi: 10.1016/S0140-6736(15)00946-0 26615326
19. Pigott DM, Golding N, Mylne A, Huang Z, Henry AJ, Weiss DJ, et al. Mapping the zoonotic niche of Ebola virus disease in Africa. eLife. 2014;3:e04395. doi: 10.7554/eLife.04395 25201877
20. Kanapathipillai R, Henao Restrepo AM, Fast P, Wood D, Dye C, Kieny MP, et al. Ebola vaccine—an urgent international priority. N Engl J Med. 2014;371(24):2249–51. doi: 10.1056/NEJMp1412166 25289888
21. Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;386(9996):857–66. doi: 10.1016/S0140-6736(15)61117-5 26248676
22. Tapia MD, Sow SO, Lyke KE, Haidara FC, Diallo F, Doumbia M, et al. Use of ChAd3-EBO-Z Ebola virus vaccine in Malian and US adults, and boosting of Malian adults with MVA-BN-Filo: a phase 1, single-blind, randomised trial, a phase 1b, open-label and double-blind, dose-escalation trial, and a nested, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2016;16(1):31–42. doi: 10.1016/S1473-3099(15)00362-X 26546548
23. Medaglini D, Harandi AM, Ottenhoff TH, Siegrist CA, VSV-Ebovac Consortium. Ebola vaccine R&D: filling the knowledge gaps. Sci Transl Med. 2015;7(317):317ps24. doi: 10.1126/scitranslmed.aad3106 26659569
24. Ledgerwood JE. Use of low dose rVSV-ZEBOV: safety issues in a Swiss cohort. Lancet Infect Dis. 2015;15(10):1117–9. doi: 10.1016/S1473-3099(15)00222-4 26248511
25. Halperin SA, Arribas JR, Rupp R, Andrews CP, Chu L, Das R, et al. Six-month safety data of recombinant vesicular stomatitis virus-Zaire Ebola virus envelope glycoprotein vaccine in a phase 3 double-blind, placebo-controlled randomized study in healthy adults. J Infect Dis. 2017;215(12):1789–98. doi: 10.1093/infdis/jix189 28549145
26. Institute of Medicine, Committee to Review the Adverse Consequences of Pertussis and Rubella Vaccines, Howard C, Howe C, Fineberg H, editors. Adverse effects of pertussis and rubella vaccines. Washington (DC): National Academies Press; 1994.
27. Tingle AJ, Mitchell LA, Grace M, Middleton P, Mathias R, MacWilliam L, et al. Randomised double-blind placebo-controlled study on adverse effects of rubella immunisation in seronegative women. Lancet. 1997;349(9061):1277–81. doi: 10.1016/S0140-6736(96)12031-6 9142061
28. Weibel RE, Stokes J Jr, Buynak EB, Hilleman MR. Rubella vaccination in adult females. N Engl J Med. 1969;280(13):682–5. doi: 10.1056/NEJM196903272801302 5250184
29. Huttner A, Combescure C, Grillet S, Haks MC, Quinten E, Modoux C, et al. A dose-dependent plasma signature of the safety and immunogenicity of the rVSV-Ebola vaccine in Europe and Africa. Sci Transl Med. 2017;9(385):eaaj1701. doi: 10.1126/scitranslmed.aaj1701 28404856
30. Dahlke C, Kasonta R, Lunemann S, Krahling V, Zinser ME, Biedenkopf N, et al. Dose-dependent T-cell dynamics and cytokine cascade following rVSV-ZEBOV immunization. EBioMedicine. 2017;19 : 107–18. doi: 10.1016/j.ebiom.2017.03.045 28434944
31. Miranda ME, Miranda NL. Reston ebolavirus in humans and animals in the Philippines: a review. J Infect Dis. 2011;204(Suppl 3):S757–60. doi: 10.1093/infdis/jir296 21987747
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2017 Číslo 10- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Improving tuberculosis diagnosis: Better tests or better healthcare?
- Fitness cost and benefit of antimicrobial resistance in : Multidisciplinary approaches are needed
- When cost-effective interventions are unaffordable: Integrating cost-effectiveness and budget impact in priority setting for global health programs
- Regional initiatives for malaria elimination: Building and maintaining partnerships
- Firearm-Related Injury and Death: A U.S. Health Care Crisis in Need of Health Care Professionals
- Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case–control study
- Decommissioning care: The need for rigorous multifaceted evaluations of decisions to withdraw health services
- Tuberculosis detection and the challenges of integrated care in rural China: A cross-sectional standardized patient study
- Estimating the fitness cost and benefit of cefixime resistance in to inform prescription policy: A modelling study
- Elevated blood pressure and risk of mitral regurgitation: A longitudinal cohort study of 5.5 million United Kingdom adults
- A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: The EPIC-InterAct case-cohort study
- The clinical utility and cost impact of cystatin C measurement in the diagnosis and management of chronic kidney disease: A primary care cohort study
- Quantifying underreporting of law-enforcement-related deaths in United States vital statistics and news-media-based data sources: A capture–recapture analysis
- Impact of disinvestment from weekend allied health services across acute medical and surgical wards: 2 stepped-wedge cluster randomised controlled trials
- Benefit and harm of intensive blood pressure treatment: Derivation and validation of risk models using data from the SPRINT and ACCORD trials
- Safety and immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine in adults and children in Lambaréné, Gabon: A phase I randomised trial
- Intergenerational diabetes and obesity—A cycle to break?
- Associations between an IgG3 polymorphism in the binding domain for FcRn, transplacental transfer of malaria-specific IgG3, and protection against malaria during infancy: A birth cohort study in Benin
- Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta-analysis
- A novel electronic algorithm using host biomarker point-of-care tests for the management of febrile illnesses in Tanzanian children (e-POCT): A randomized, controlled non-inferiority trial
- Childhood stunting in relation to the pre- and postnatal environment during the first 2 years of life: The MAL-ED longitudinal birth cohort study
- Effectiveness of cervical screening after age 60 years according to screening history: Nationwide cohort study in Sweden
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta-analysis
- Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case–control study
- A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: The EPIC-InterAct case-cohort study
- Quantifying underreporting of law-enforcement-related deaths in United States vital statistics and news-media-based data sources: A capture–recapture analysis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



