-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Adverse Renal, Endocrine, Hepatic, and Metabolic Events during Maintenance Mood Stabilizer Treatment for Bipolar Disorder: A Population-Based Cohort Study
In a study of data from the United Kingdom's primary care database, THIN, Joseph Hayes and colleagues report on adverse events associated with use of lithium and other maintenance treatments for bipolar disorder.
Published in the journal: . PLoS Med 13(8): e32767. doi:10.1371/journal.pmed.1002058
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002058Summary
In a study of data from the United Kingdom's primary care database, THIN, Joseph Hayes and colleagues report on adverse events associated with use of lithium and other maintenance treatments for bipolar disorder.
Introduction
Bipolar disorder (BPD) is a complex, recurrent, severe mental illness that affects over 350 million people worldwide [1]. Individuals with BPD will often require long-term drug treatment with the aim of preventing relapse or reccurrence [2]. Much of the evidence for maintenance medication comes from relatively short-term randomised controlled trials, which are then extrapolated to longer-term use [3]. However, this fails to take into account the potential longer-term adverse effects of the recommended medications. In 2014, the update of the United Kingdom National Institute for Health and Care Excellence (NICE) guidelines [3], a meta-analysis [4], and a network meta-analysis [5] all suggested that lithium should be seen as first-line monotherapy, whereas previous guidelines from around the world also recommended valproate, lamotrigine, carbamazepine, olanzapine, quetiapine, aripiprazole, oxcarbazepine, and risperidone injection [6,7]. Prescribing in the UK has reflected the previous NICE guidance for first-line treatment [8], with lithium, valproate, olanzapine, and quetiapine being the most frequently prescribed maintenance treatments [9].
A number of adverse effects of lithium have been identified since its use as a mood stabilizer became established in the 1970s [10], but it is only recently that they have begun to be characterised and quantified [11–15]. Lithium’s adverse effects include renal, thyroid, and parathyroid dysfunction. Lithium is also recognised to cause weight gain, but the risk of weight gain relative to other potential maintenance therapies has not been widely investigated [11]. Alternatives, such as second-generation antipsychotics and valproate, have been found to be obesogenic [16], especially olanzapine, which is the most commonly prescribed antipsychotic in BPD [9]. Weight gain is associated with a number of adverse events, such as hypertension, type 2 diabetes mellitus (T2DM), and cardiovascular disease (CVD) [17]. Valproate, olanzapine, and quetiapine are metabolized by the liver. Valproate has been found to be associated with a high risk of asymptomatic elevated transaminases and can cause idiosyncratic hepatic failure [15,18]. Olanzapine and quetiapine have also been associated with rare cases of hepatotoxicity [19–21]. Therefore, the balance of risks associated with maintenance mood stabilizer selection is not straightforward, and we are aware of no studies that make these comparisons across treatment options.
This study used a large electronic patient record database to compare rates of major recognised adverse outcomes amongst individuals prescribed lithium, valproate, olanzapine, or quetiapine for mood stabilization in BPD. The adverse events examined were chronic kidney disease (CKD), hypothyroidism, hyperthyroidism, hypercalcemia, weight gain, hypertension, T2DM, CVD, and hepatotoxicity [15,18].
Methods
Study Design
A population-based longitudinal cohort from January 1, 1995, to December 31, 2013.
Setting
The Health Improvement Network (THIN) is a UK primary care database that contains anonymised patient information from routine clinical consultations [16]. The National Health Service (NHS) South-East Multicentre Research Ethics Committee approved THIN’s provision of anonymous patient data to researchers in 2003. Scientific approval for this study was obtained from the data provider’s Scientific Review Committee in March 2015.
THIN contained records of over 11 million people at the time of cohort extraction [22]. Included patients are broadly representative of the UK population, and physicians contributing data are representative in terms of consultation and prescribing statistics [23,24]. Approximately 98% of the UK population is registered with a primary care physician [25]. The incidence rate of BPD in THIN has been shown to be similar to European cohorts [26], and the validity of severe mental illness diagnoses held in primary care has been established [27]. NICE guidance recommends that any patient with suspected BPD should be referred to a psychiatrist for diagnosis and treatment planning [8]. Therefore, individuals in this cohort (psychiatrist-diagnosed BPD plus appropriate mood stabilizer treatment) are considered to fulfil International Statistical Classification of Diseases and Related Health Problems (ICD)-10 criteria for BPD.
In THIN, physicians use Read codes, a hierarchical coding system, to record information [28]. These codes include diagnoses made in primary and hospital care (which map onto ICD-10 codes), symptoms, examination findings, information from specialists, and test results [29]. In the UK, primary care physicians are responsible for drug prescriptions issued within the NHS, so this information is also complete and well recorded [30]. CKD, thyroid disease, T2DM, hypertension, CVD, and other chronic health condition diagnoses have been validated in THIN [23].
Participants
Patients with a diagnosis of BPD were included if they had at least one 28-day prescription of lithium, valproate, olanzapine, or quetiapine after January 1, 1995 or after the date at which the medical records met quality assurance criteria for data entry (based on computer usage and mortality recording rates [31,32]). Patients were excluded if they were prescribed another study drug at the start of follow-up or in the month before this. Diagnosis of BPD could occur at any time in the patient record. For each outcome requiring hematological or biochemical confirmation for diagnosis (CKD, thyroid disease, hypercalcemia, hepatotoxicity), patients were excluded from the primary analysis if they did not receive a specific blood test for the outcome, to reduce surveillance bias. For the weight gain outcome, patients were excluded if they did not have a baseline or pre-treatment weight and at least one other weight measurement. For the outcome of hyperthyroidism, patients taking thyroxine were excluded, as this can result in thyroid-stimulating hormone (TSH) suppression [33]. Patients were also excluded if they had the outcome of interest at baseline (as we were interested in incident events). Therefore, each outcome has a different number of patients included.
Exposure
Date of first prescription was taken as the start of exposure time. The end of the prescription was calculated from the amount prescribed and dosage instructions coded by the physician. Patients were considered to have a period of continuous prescribing if another prescription for the drug was issued within 3 mo of the calculated end date. If this did not occur, the date of stopping the study drug was the end date of the final prescription. Three mo was added to this end date to account for late development of the adverse event or delayed recording. Each patient could only contribute exposure time to one of the study drugs (the first they received) and did not re-enter the cohort if they restarted the drug after more than 3 mo. Patients could be prescribed other psychiatric medications but not combinations of the study drugs. If they commenced another study drug, their outcomes were censored in the analysis (to ensure the outcome could be assigned to a particular drug).
Main Outcomes
All outcomes were defined by appropriate Read codes and/or lab results. Outcomes of interest were: CKD stage 3 or above (or an estimated glomerular filtration rate [eGFR] of <60 ml/min/1.73 m2), CKD stage 4 or above (or an eGFR <30 ml/min/1.73 m2) [34,35] (if eGFR was unavailable we calculated it from available creatinine blood tests using the CKD-EPI equation [36]), hypothyroidism (or a TSH of >10 mU/L), hyperthyroidism (or a TSH <0.1 mU/L) [33], hypercalcemia (adjusted calcium >2.65 mmol/L) [37], >7% and >15% weight gain from baseline [38], hypertension, T2DM (or HbA1c >48 mmol/mol) [39], CVD (defined as any ischemic heart disease [IHD], myocardial infarction [MI] or cerebrovascular event [CVE]), and hepatotoxicity (or alanine transaminase [ALT] >200 U/L, or aspartate aminotransferase [AST] >250 U/L) [40].
Patients were followed up until the earliest of (i) the first record of the adverse event of interest, (ii) the date of stopping the study drug plus 3 mo, (iii) the date of switching to another study drug, (iv) date of death or date of leaving the physician’s practice, or (v) December 31, 2013.
Propensity Score Estimation Using Observed Pre-treatment Variables
A number of baseline patient characteristics were extracted from THIN. Physical and mental health conditions were considered present if referenced in patient notes and absent if they were not. If a patient had multiple entries of the same (or similar) codes, the start date of the condition was taken as the earliest date of entry.
A propensity score (PS) for each individual was estimated using variables defined a priori, based on existing research and clinical experience of factors influencing prescribing choice [3,41,42]. The PS is the conditional probability of receiving one study drug rather than another, given the variables included in the model [42,43]. Included variables were: sex, age at start of treatment with the study drug, year of entry to the cohort, ethnicity (grouped as White, Black, Asian, Mixed, other, with missing values coded as White [44]), IHD diagnosis before baseline, history of MI, history of CVE, hypertension, CKD at baseline (defined by Read code or blood test), history of hypo - or hyperthyroidism (defined by Read code or blood test), history of liver disease or hepatotoxicity (defined by Read code or blood test), T2DM (defined by Read code or blood test), epilepsy, alcohol use (grouped as none/low, moderate, high/dependent), history of illicit drug use, smoking status (grouped as never-smoker, ex-smoker, current smoker), body mass index (BMI) (grouped as healthy weight, overweight [BMI 25 to 30], obese [BMI over 30]), anxiety symptoms or diagnosis before baseline, depressive symptoms or diagnosis, sleep disturbance before baseline, treatment with one of the study drugs at or before baseline, and clustering by practice in which the treating physician was working. The PS was checked by comparison of covariate balance across treatments, within strata. The variables in the PS excluded the outcome variable for that particular analysis. Although PS estimation cannot remove all bias, it has been postulated to also reduce confounding from unmeasured covariates, because of their association with measured variables [45–47]. In this way, use of a PS aims to replicate a randomized experiment as closely as possible by obtaining treatment groups with similar covariate distributions [48].
Statistical Analysis
Cox regression analyses were conducted, comparing the rates of adverse events in the four treatment groups. The proportional hazards model was tested formally with analysis of Schoenfeld residuals [49]. The PS was calculated using multinomial logistic regression, using drug treatment as the dependent variable and the covariates described as independent variables. The PS was then used as a linear term in a Cox regression analysis that also included age, calendar year, and clustering by practice [50]. In all cases, this model was shown to be superior to stratifying on PS using Akaike information criterion and Bayesian information criterion [51], and was a more efficient use of data than PS matching (because no patients were excluded). To account for the competing risk of each outcome with death, we plotted graphs of cumulative incidence function, adjusted for PS and age, following competing-risks regression [52,53]. We conducted sensitivity analyses in which individuals who did not receive blood tests or weight measurements were not dropped from the cohort, and in which individuals were assigned inverse probability weights (IPW) based on how likely they were to have blood test or weight records [54]. We used multiple demographic and clinical variables to predict missingness for the IPW model. All analyses were completed using Stata 14 [55].
Results
For each outcome, 6,671 individuals with BPD diagnosis were potentially included in the analysis, 2,148 prescribed lithium, 1,670 prescribed valproate, 1,477 prescribed olanzapine, and 1,376 prescribed quetiapine (see S1 Text). The median duration of drug treatment was 1.48 y (interquartile range 0.64–3.43). The characteristics of the potentially included cohort are shown in Table 1. The number of individuals included for each outcome by treatment group is shown in S1 Table.
Tab. 1. Patient characteristics. 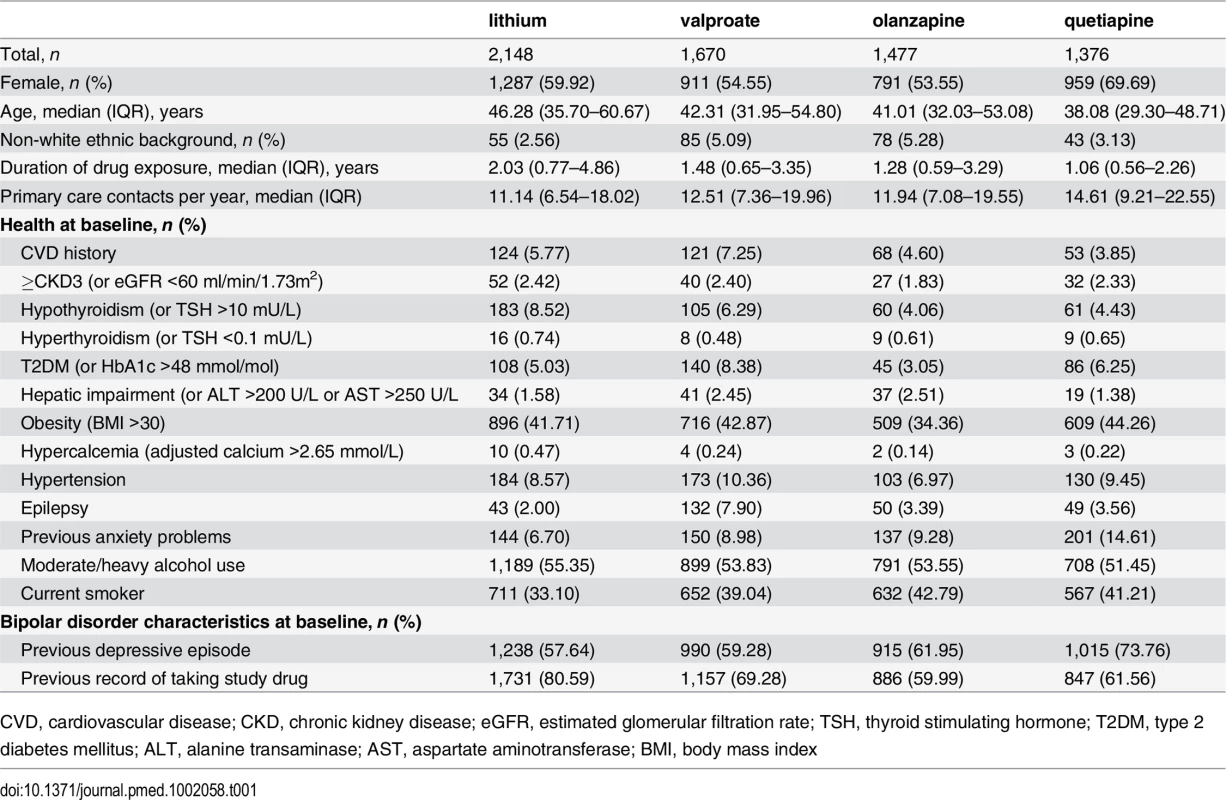
CVD, cardiovascular disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; TSH, thyroid stimulating hormone; T2DM, type 2 diabetes mellitus; ALT, alanine transaminase; AST, aspartate aminotransferase; BMI, body mass index In unadjusted analysis and after adjustment for PS, age, calendar year, and clustering by practice in which the primary care physician worked, rates of CKD stage 3 or above in individuals prescribed valproate (HR 0.56; 95% CI 0.45–0.69; p < 0.001), olanzapine (HR 0.57; 95% CI 0.45–0.71; p < 0.001), or quetiapine (HR 0.62; 95% CI 0.47–0.80; p < 0.001) were reduced compared to lithium (Table 2, Fig 1).
Fig. 1. Cumulative incidence estimates of adverse renal and hepatic event rates. 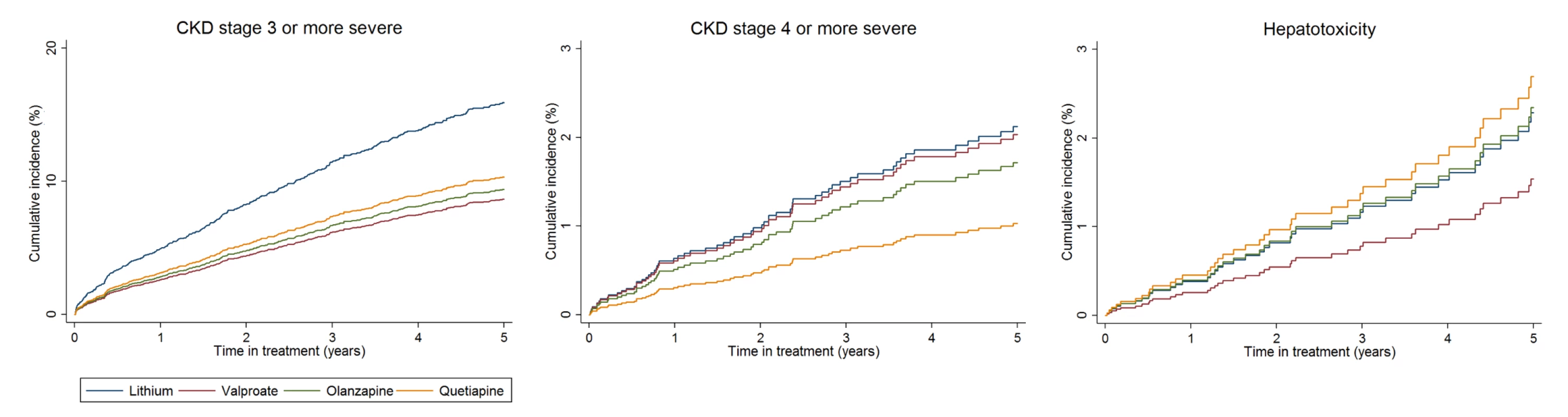
From PS and age-adjusted competing-risks regression. Note differences in scale of y-axis for each plot. Tab. 2. Adverse effects during maintenance treatment. 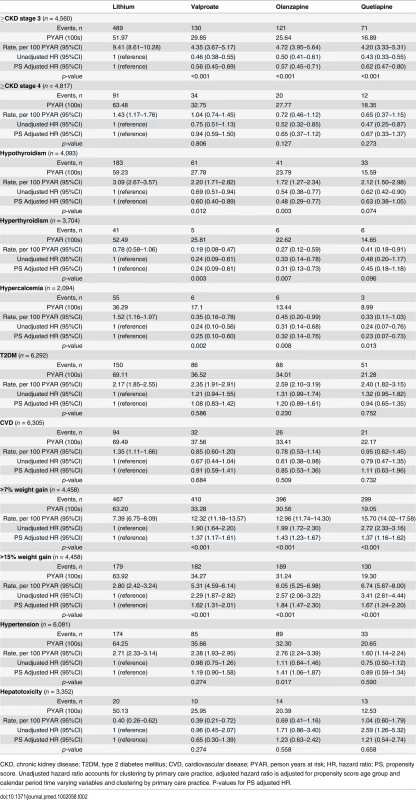
CKD, chronic kidney disease; T2DM, type 2 diabetes mellitus; CVD, cardiovascular disease; PYAR, person years at risk; HR, hazard ratio; PS, propensity score. Unadjusted hazard ratio accounts for clustering by primary care practice, adjusted hazard ratio is adjusted for propensity score age group and calendar period time varying variables and clustering by primary care practice. P-values for PS adjusted HR. Compared to lithium, rates of hypothyroidism were reduced in those prescribed valproate (HR 0.60; 95% CI 0.40–0.89; p = 0.012) or olanzapine (HR 0.48; 95% CI 0.29–0.77; p = 0.003), but not quetiapine (HR 0.63; 95% CI 0.38–1.05; p = 0.074) after adjustment. Rates of hyperthyroidism were lower in those prescribed valproate (HR 0.24; 95% CI 0.09–0.61; 0.003) and olanzapine (HR 0.31; 95% CI 0.13–0.73; 0.007), but not quetiapine (HR 0.45; 95% CI 0.18–1.18; 0.096), compared to lithium. Hypercalcemia was less common in those prescribed valproate (HR 0.25; 95% CI 0.10–0.60; p = 0.002), olanzapine (HR 0.32; 95% CI 0.14–0.76; p = 0.008), or quetiapine (HR 0.23; 95% CI 0.07–0.73; p = 0.013) compared to lithium (Table 2, Fig 2).
Fig. 2. Cumulative incidence estimates of adverse endocrine event rates. 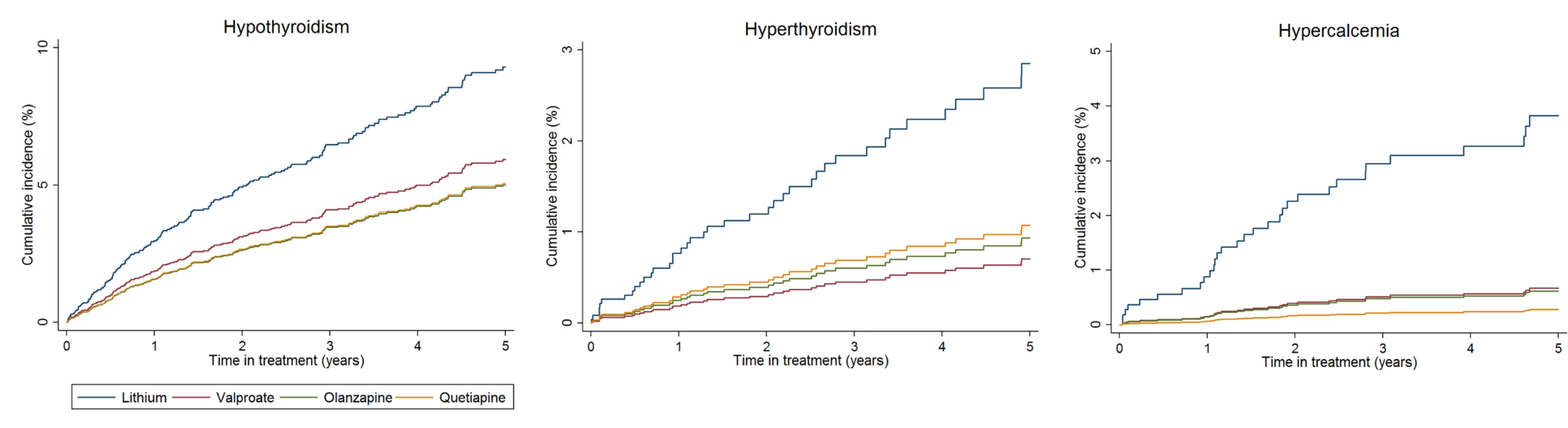
From PS and age adjusted competing-risks regression. Note differences in scale of y-axis for each plot. After adjustment, rates of weight gain were higher with valproate, olanzapine, and quetiapine than lithium (>15% weight gain: valproate HR 1.62; 95% CI 1.31–2.01; p < 0.001; olanzapine HR 1.84; 95% CI 1.47–2.30; p < 0.001; quetiapine HR 1.67; 95% CI 1.24–2.20; p < 0.001). Rates of hypertension were higher with olanzapine (HR 1.41; 95% CI 1.06–1.87; p = 0.017) than lithium (Table 2, Fig 3). We found no significant difference in rates of CKD stage 4 or above, T2DM, cardiovascular disease, or hepatotoxicity between groups (Table 2). The median number of eGFR/creatinine and TSH blood tests per year in treatment was higher in those taking lithium than the other drugs (see S2 Table). Weight measurement and blood tests for adjusted calcium and ALT/AST were less frequent in patients prescribed lithium (see S2 Table). For outcomes in which patients had been excluded because of missing tests (CKD, hypo - and hyperthyroidism, hypercalcemia, weight gain, and hepatotoxicity), sensitivity analyses including all patients resulted in reduced incident rate estimates compared to the primary analyses, but had little effect on HRs (see S3 Table). Sensitivity analyses using IPW suggest results from the primary analyses are robust (see S3 Table). From Schoenfeld residuals, there was no evidence against the assumption of proportional hazards for any outcome.
Fig. 3. Cumulative incidence estimates of adverse metabolic event rates. 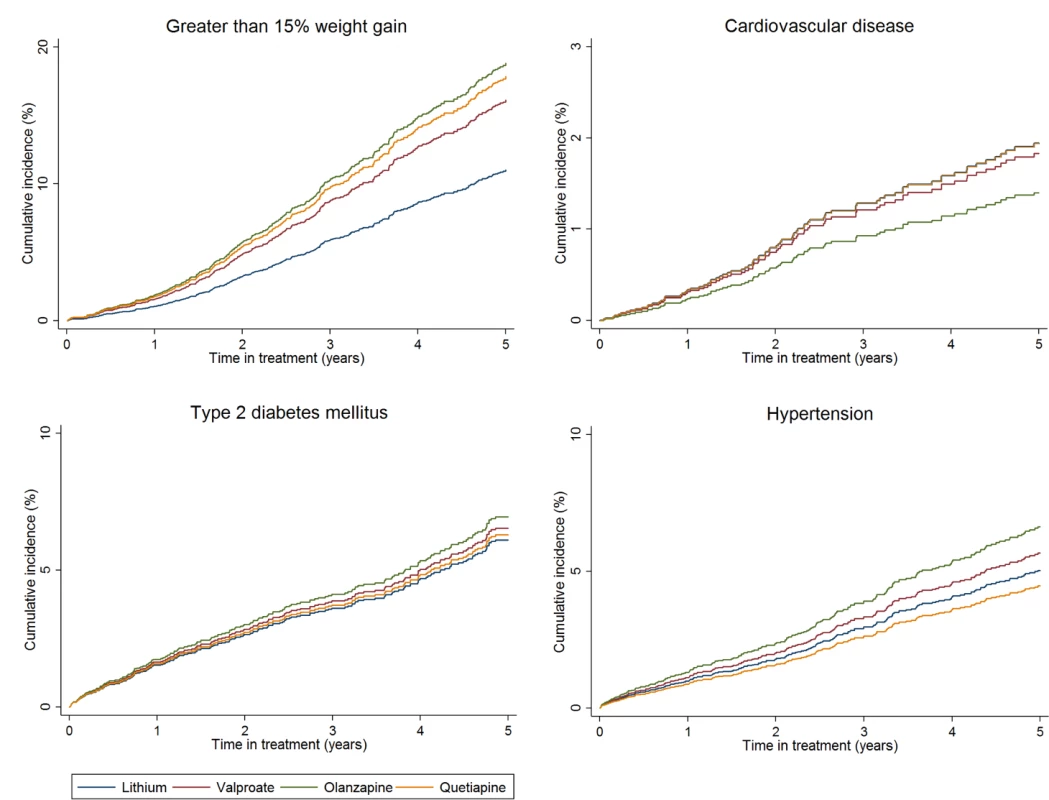
From PS and age adjusted competing-risks regression. Note differences in scale of y-axis for each plot. Discussion
In a large dataset of nearly 7,000 individuals treated for BPD with lithium, valproate, olanzapine, or quetiapine, with follow-up times of up to 17 y, we found differential rates of a number of adverse events. Those prescribed lithium were more likely to have a decline in renal function and develop hypo - or hyperthyroidism and hypercalcemia. However, they were less likely to gain significant weight. Individuals prescribed olanzapine had the highest rate of weight gain and new onset hypertension. We did not find any statistically significant differences in the rate of new T2DM, cardiovascular disease, or hepatotoxicity across drug treatment groups.
Severe CKD (stage 4 or above) was uncommon in the cohort (approximately 1 in 100 person years at risk), and we did not find differences by drug treatment, but less severe CKD (stage 3 or above) occurred most frequently in patients prescribed lithium. Whilst many of these patients (i.e., those with CKD stage 3) would not progress to a clinically relevant decline in renal function, a number of them would be at increased risk of doing so. It remains unclear if this result is due to (1) lack of power to determine a true difference in rates of severe CKD, (2) surveillance bias due to increased monitoring of renal function in those taking lithium, which would lead to apparent increased rates of asymptomatic CKD stage 3, or (3) lithium treatment truly increasing the risk of reduced renal function without increasing severe CKD risk. Previous studies have found similar results and have not been able to account for this potential bias [12–14,56]. Clos et al. found no decline in eGFR in individuals taking lithium, using a similar active comparator design, but were also limited by potential ascertainment bias [57].
Rates of both hypothyroidism and hyperthyroidism were increased in individuals prescribed lithium compared to valproate and olanzapine (but not quetiapine). Increased hypothyroidism has been shown previously [11,58], but literature on the association between lithium and hyperthyroidism is inconsistent [13], and lithium-induced hyperthyroidism is considered rare [59]. Monitoring thyroid dysfunction in BPD is vital because of evidence that abnormalities are associated with longer time to remission and more symptoms during the maintenance period [60]. It is possible that thyroid function normalises on cessation of lithium, but only one study has investigated this [61]. Hypercalcemia is also recognised to be associated with lithium prescribing [11,13,62,63]. Calcium monitoring in patients prescribed lithium was rare in our representative sample of primary care (37% had one or more calcium blood test result), despite it being recommended in the 2006 NICE guidance [8].
The rate of individuals gaining more than 7%, and more than 15% of their baseline weight, was greater in those prescribed olanzapine, quetiapine, or valproate than those prescribed lithium. This degree of weight gain represents a significant risk factor for a number of adverse physical health outcomes, including CVD and T2DM [38]. We may not have captured increased rates of CVD or T2DM because of the relatively brief median follow-up time, in relation to the time taken to develop these diseases. Olanzapine had the highest adjusted rate of greater than 15% weight gain compared to lithium, and the highest rate of new onset hypertension. This has been shown previously in comparisons of antipsychotic drugs [64] and in trials of olanzapine versus lithium or valproate [65].
Hepatotoxicity was rare in the cohort and, before PS adjustment rates, appeared to be elevated in the quetiapine group, compared to lithium. This association has been identified previously [20]. After adjustment, there was no evidence of between-group differences.
Strengths and Limitations
The major strength of this study, beyond size and length of follow-up, is the direct comparison between BPD maintenance mood stabilizer treatment options for a number of adverse effects. The use of electronic health records also means it is possible to adjust for a number of demographic and physical health characteristics that may have influenced the clinician’s decision to treat with a particular medication or potentially confound the relationship between treatment and adverse outcome. Despite including numerous variables in the PS, it is possible that residual confounding remained, especially as those prescribed lithium were older and were more likely to have taken the drug previously, perhaps reflecting a more chronic illness course. It may be that important patient or clinician features were not captured by the score, and despite the balance of observed covariates, we cannot confirm balance of unobserved covariates [66,67]. We were also unable to consider dosage differences across the different treatment groups in this analysis. Periods of lithium toxicity may be particularly important with regards to developing renal failure, and we were unable to capture this information from the available data. Missing data can be a problem in studies utilising electronic patient records, especially as there may be a clinical reason why information is missing. Because of the way outcomes were defined, T2DM, cardiovascular disease, and diagnoses of hypertension had no missing data, and no covariates in the PS had missing values.
Patients prescribed lithium had no more physician contacts than those taking other mood stabilizer medication. In individuals that ever received tests during treatment exposure, testing frequency was similar in all study drugs for adjusted calcium, liver function, and weight (see S2 Table). Frequency of testing renal and thyroid function was higher in those taking lithium, which reflects the guidance for monitoring [8]. Patients prescribed lithium were also more likely to have at least one renal function, thyroid function, calcium, or liver function test compared to patients taking other drugs. This is likely to be due to both drug-related indications for monitoring and the longer drug exposure seen in those taking lithium. IPW sensitivity analysis to account for this difference did not alter our conclusions (see S3 Table). In the primary analysis, the likely effect of this differential missingness would be to reduce the hazard ratios for lithium compared to the other drugs, relative to their true values, as blood tests in the non-lithium group are more likely to be related to clinical symptoms than monitoring guidance (for instance, this is likely to represent an underestimation of the true hypercalcemia hazard ratio for lithium versus other drugs). The median number of weight measurements was similar in each group, suggesting detection of weight gain was not related to differential monitoring. The sensitivity analyses including individuals irrespective of blood tests produced similar adjusted hazard ratios as the primary analyses for each outcome, but often with reduced incidence of the outcome in each treatment group (see S3 Table). These analyses may more accurately reflect testing occurring because of clinical indication.
Conclusions
Lithium remains an important treatment option for individuals with BPD. However, there is clear evidence that its use is associated with a number of adverse events. These risks need to be offset with the potentially superior effectiveness and anti-suicidal benefits of the drug compared to other treatment options [5,68]. It is also true that other recommended maintenance treatments can have serious side effects, often related to weight gain, and are not suitable for use in certain patient groups (such as the contraindication of valproate in women of childbearing potential [3]).
Assiduous monitoring of patients prescribed lithium should ameliorate some risk associated with effects on renal physiology and endocrine systems. Given the need to balance an array of risks and benefits, an individualised and collaborative approach to treatment choice is likely to be most appropriate. To achieve this, further research identifying patient characteristics that are risk factors for specific side effects and an understanding of the risks and benefits of stopping treatment in those who experience adverse effects is necessary.
Supporting Information
Zdroje
1. Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–86. doi: 10.1016/S0140-6736(13)61611-6 23993280
2. Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. 2016; 387(10027):1561–72. doi: 10.1016/S0140-6736(15)00241-X
3. National Institute for Health and Care Excellence. Bipolar Disorder: the Management of Bipolar Disorder in Adults, Children and Adolescents, in Primary and Secondary Care. GC185. London, UK: NICE; 2014
4. Severus E, Taylor MJ, Sauer C, Pfennig A, Ritter P, Bauer M, et al. Lithium for prevention of mood episodes in bipolar disorders: systematic review and meta-analysis. Int J Bipolar Disord. 2014;2(1):15.
5. Miura T, Noma H, Furukawa TA, Mitsuyasu H, Tanaka S, Stockton S, et al. Comparative efficacy and tolerability of pharmacological treatments in the maintenance treatment of bipolar disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2014;1(5):351–9.
6. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 Suppl):1–50.
7. Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Beaulieu S, Alda M, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar disord. 2013;15(1):1–44. doi: 10.1111/bdi.12025 23237061
8. National Institute for Health and Care Excellence. Bipolar Disorder: the Management of Bipolar Disorder in Adults, Children and Adolescents, in Primary and Secondary Care. GC38. London, UK: NICE; 2006.
9. Hayes J, Prah P, Nazareth I, King M, Walters K, Petersen I, et al. Prescribing trends in bipolar disorder: cohort study in the United Kingdom THIN primary care database 1995–2009. PLoS ONE. 2011;6(12):e28725. doi: 10.1371/journal.pone.0028725 22163329
10. Bech P. The full story of lithium. Psychother Psychosom. 2006;75(5):265–9. 16899962
11. McKnight R, Adida M, Budge K, Stockton S, Goodwin G, Geddes J. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379(9817):721–728. doi: 10.1016/S0140-6736(11)61516-X 22265699
12. Close H, Reilly J, Mason JM, et al. Renal failure in lithium-treated bipolar disorder: a retrospective cohort study. PLoS ONE. 2014;9(3):e90169. doi: 10.1371/journal.pone.0090169 24670976
13. Shine B, McKnight RF, Leaver L, Geddes JR. Long-term effects of lithium on renal, thyroid, and parathyroid function: a retrospective analysis of laboratory data. Lancet. 2015; 386(992):461–468.
14. Kessing LV, Gerds TA, Feldt-Rasmussen B, Andersen PK, Licht RW. Use of Lithium and Anticonvulsants and the Rate of Chronic Kidney Disease: A Nationwide Population-Based Study. JAMA Psychiatry. 2015; 72(12):1182–91.
15. Murru A, Popovic D, Pacchiarotti I, Hidalgo D, León-Caballero J, Vieta E. Management of Adverse Effects of Mood Stabilizers. Curr Psychiatry Rep. 2015 Aug 1;17(8):603.
16. Tarricone I, Ferrari Gozzi B, Serretti A, Grieco D, Berardi D. Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychol Med. 2010;40(02):187–200.
17. Haupt DW. Differential metabolic effects of antipsychotic treatments. Eur Neuropsychopharmacol. 2006;16:S149–S55. 16872808
18. Dols A, Sienaert P, van Gerven H, Schouws S, Stevens A, Kupka R, Stek ML. The prevalence and management of side effects of lithium and anticonvulsants as mood stabilizers in bipolar disorder from a clinical perspective: a review. Int Clin Psychopharmacol. 2013; 1;28(6):287–96. doi: 10.1097/YIC.0b013e32836435e2 23873292
19. Ozcanli T, Erdogan A, Ozdemir S, Onen B, Ozmen M, Doksat K, Sonsuz A. Severe liver enzyme elevations after three years of olanzapine treatment: a case report and review of olanzapine associated hepatotoxicity. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006;30(6):1163–6. 16632162
20. Atasoy N, Erdogan A, Yalug I, Ozturk U, Konuk N, Atik L, Ustundag Y. A review of liver function tests during treatment with atypical antipsychotic drugs: a chart review study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(6):1255–60. 17600607
21. El Hajj I, Sharara AI, Rockey DC. Subfulminant liver failure associated with quetiapine. Eur J Gastroenterol Hepatol. 2004;16(12):1415–8.
22. IMS Health. http://www.epic-uk.org/. Accessed October 28 2015.
23. Blak B, Thompson M, Bourke A, editors. National representativeness and data quality of the Health Improvement Network (THIN) database of primary care information for epidemiological research. 9th Annual Conference of the UK Federation of Primary Care Research Organisations Podium presentation Liverpool, UK; 2006.
24. Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251–5. 22828580
25. Lis Y, Mann RD. The VAMP Research multi-purpose database in the UK. J Clin Epidemiol. 1995;48(3):431–43. 7897464
26. Hardoon S, Hayes JF, Blackburn R, et al. Recording of severe mental illness in United Kingdom primary care, 2000–2010. PLoS ONE. 2013; 8(12): e82365.
27. Nazareth I, King M, Haines A, Rangel L, Myers S. Accuracy of diagnosis of psychosis on general practice computer system. BMJ. 1993;307(6895):32–4. 8343670
28. Chisholm J. The Read clinical classification. BMJ. 1990;300(6732):1092. 2344534
29. Davé S, Petersen I. Creating medical and drug code lists to identify cases in primary care databases. Pharmacoepidemiol Drug Saf. 2009;18(8):704–7. doi: 10.1002/pds.1770 19455565
30. Health and Social Care Information Centre. Prescriptions Dispensed in the Community, Statistics for England—2001–2011. 2012. Health and Social Care Information Centre, UK.
31. Horsfall L, Walters K, Petersen I. Identifying periods of acceptable computer usage in primary care research databases. Pharmacoepidemiol Drug Saf. 2013;22(1):64–9. doi: 10.1002/pds.3368 23124958
32. Maguire A, Blak BT, Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf. 2009;18(1):76–83. doi: 10.1002/pds.1688 19065600
33. Beastall G, Beckett G, Franklyn J, et al. UK Guidelines for the use of thyroid function tests. The association for Clinical Biochemistry. 2006.
34. Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the National Kidney Foundation. Am J Kidney Dis. 2007;50(2):169–80. 17660017
35. Crowe E, Halpin D, Stevens P. Guidelines: early identification and management of chronic kidney disease: summary of NICE guidance. BMJ. 2008;337:a1530
36. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–12. 19414839
37. Smellie WSA, Vanderpump M, Fraser WD, Bowley R, Shaw N. Best practice in primary care pathology: review 11. J Clin Pathol. 2008;61(4):410–8. 17965216
38. Manu P, Dima L, Shulman M, Vancampfort D, De Hert M, Correll C. Weight gain and obesity in schizophrenia: epidemiology, pathobiology, and management. Acta Psych Scand. 2015;132(2):97–108.
39. John W. Use of HbA1c in the diagnosis of diabetes mellitus in the UK. The implementation of World Health Organization guidance 2011. Diabet Med. 2012;29(11):1350–7. doi: 10.1111/j.1464-5491.2012.03762.x 22957983
40. Sabin CA. Pitfalls of assessing hepatotoxicity in trials and observational cohorts. Clin Infect Dis. 2004;38(Supplement 2):S56–64.
41. Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79(387):516–24.
42. Holmes WM. Using propensity scores in quasi-experimental designs. Thousand Oaks, California, US; SAGE Publications: 2013.
43. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55.
44. Mathur R, Bhaskaran K, Chaturvedi N, Leon DA, Grundy E, Smeeth L. Completeness and usability of ethnicity data in UK-based primary care and hospital databases. J Public Health. 2014;36(4):684–92.
45. Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150(4):327–33. 10453808
46. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. 21818162
47. Seeger JD, Kurth T, Walker AM. Use of propensity score technique to account for exposure-related covariates: an example and lesson. Med Care. 2007;45(10):S143–S8. 17909373
48. Stuart EA. Matching methods for causal inference: A review and a look forward. Stat Sci. 2010;25(1):1. 20871802
49. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69(1):239–41.
50. d’Agostino RB. Tutorial in biostatistics: propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–81.
51. Lin TH, Dayton CM. Model selection information criteria for non-nested latent class models. J Educ Behav Stat. 1997;22(3):249–64.
52. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509.
53. Noordzij M, Leffondré K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. 2013;28(11):2670–7. doi: 10.1093/ndt/gft355 23975843
54. Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–95. doi: 10.1177/0962280210395740 21220355
55. StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP: 2015.
56. Aiff H, Attman PO, Aurell M, Bendz H, Schön S, Svedlund J. End-stage renal disease associated with prophylactic lithium treatment. Eur Neuropsychopharmacol. 2014;24(4):540–4. doi: 10.1016/j.euroneuro.2014.01.002 24503277
57. Clos S, Rauchhaus P, Severn A, Cochrane L, Donnan PT. Long-term effect of lithium maintenance therapy on estimated glomerular filtration rate in patients with affective disorders: a population-based cohort study. Lancet Psychiatry. 2015;2(12):1075–83. doi: 10.1016/S2215-0366(15)00316-8 26453408
58. Kibirige D, Luzinda K, Ssekitoleko R. Spectrum of lithium induced thyroid abnormalities: a current perspective. Thyroid Res. 2013;6(1):3. doi: 10.1186/1756-6614-6-3 23391071
59. Lazarus JH. Lithium and thyroid. Best Prac Res Clin Endocrinol Metab. 2009;23(6):723–33.
60. Fagiolini A, Kupfer DJ, Scott J, Swartz HA, Cook D, Novick DM, et al. Hypothyroidism in patients with bipolar I disorder treated primarily with lithium. Epidemiol Psichiatr Soc. 2006;15(02):123–7.
61. Souza FG, Mander AJ, Foggo M, Dick H, Shearing CH, Goodwin GM. The effects of lithium discontinuation and the non-effect of oral inositol upon thyroid hormones and cortisol in patients with bipolar affective disorder. J Affect Disord. 1991; 22(3):165–70. 1918659
62. Khandwala HM, Uum SV. Reversible hypercalcemia and hyperparathyroidism associated with lithium therapy: case report and review of literature. Endocr Prac. 2006;12(1):54–8.
63. Lehmann SW, Lee J. Lithium-associated hypercalcemia and hyperparathyroidism in the elderly: What do we know? J Affect Disord. 2013;146(2):151–7. doi: 10.1016/j.jad.2012.08.028 22985484
64. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects. CNS Drugs. 2005;19(1):1–93.
65. Nashed MG, Restivo MR, Taylor VH. Olanzapine-induced weight gain in patients with bipolar I disorder: a meta-analysis. Prim Care Companion CNS Disord. 2011;13(6). doi: 10.4088/PCC.11r01174
66. Austin PC, Mamdani MM, Stukel TA, Anderson GM, Tu JV. The use of the propensity score for estimating treatment effects: administrative versus clinical data. Stat Med. 2005;24(10):1563–78. 15706581
67. Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA. 2007;297(3):278–85. 17227979
68. Cipriani A, Hawton K, Stockton S, Geddes J. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646. doi: 10.1136/bmj.f3646 23814104
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2016 Číslo 8- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- Dementia across the Lifespan and around the Globe—Pathophysiology, Prevention, Treatment, and Societal Impact: A Call for Papers
- Progress and Challenges in Scaling Up Laboratory Monitoring of HIV Treatment
- Facility-Based Delivery during the Ebola Virus Disease Epidemic in Rural Liberia: Analysis from a Cross-Sectional, Population-Based Household Survey
- Availability and Use of HIV Monitoring and Early Infant Diagnosis Technologies in WHO Member States in 2011–2013: Analysis of Annual Surveys at the Facility Level
- Duration of Adulthood Overweight, Obesity, and Cancer Risk in the Women’s Health Initiative: A Longitudinal Study from the United States
- Improving Clinical Risk Stratification at Diagnosis in Primary Prostate Cancer: A Prognostic Modelling Study
- Genetically Predicted Body Mass Index and Breast Cancer Risk: Mendelian Randomization Analyses of Data from 145,000 Women of European Descent
- Social Dancing and Incidence of Falls in Older Adults: A Cluster Randomised Controlled Trial
- Genetic and Environmental Risk for Chronic Pain and the Contribution of Risk Variants for Major Depressive Disorder: A Family-Based Mixed-Model Analysis
- Long-Term Outcomes Associated with Traumatic Brain Injury in Childhood and Adolescence: A Nationwide Swedish Cohort Study of a Wide Range of Medical and Social Outcomes
- On Risk Estimation versus Risk Stratification in Early Prostate Cancer
- Make Data Sharing Routine to Prepare for Public Health Emergencies
- Assessment of Adverse Events in Protocols, Clinical Study Reports, and Published Papers of Trials of Orlistat: A Document Analysis
- Concussions and Repercussions
- South Asia as a Reservoir for the Global Spread of Ciprofloxacin-Resistant : A Cross-Sectional Study
- Building from the HIV Response toward Universal Health Coverage
- Transitioning to Country Ownership of HIV Programs in Rwanda
- Accelerating the Uptake and Timing of Antiretroviral Therapy Initiation in Sub-Saharan Africa: An Operations Research Agenda
- Glycemic Control and the Risk of Tuberculosis: A Cohort Study
- Adjuvant Trastuzumab in HER2-Positive Early Breast Cancer by Age and Hormone Receptor Status: A Cost-Utility Analysis
- Uptake of Home-Based HIV Testing, Linkage to Care, and Community Attitudes about ART in Rural KwaZulu-Natal, South Africa: Descriptive Results from the First Phase of the ANRS 12249 TasP Cluster-Randomised Trial
- An Audit and Feedback Intervention for Reducing Antibiotic Prescribing in General Dental Practice: The RAPiD Cluster Randomised Controlled Trial
- Multidrug-Resistant Tuberculosis Treatment in North Korea: Is Scale-Up Possible?
- Associations between Mental Health and Ebola-Related Health Behaviors: A Regionally Representative Cross-sectional Survey in Post-conflict Sierra Leone
- Pancreatic Cancer Surgical Resection Margins: Molecular Assessment by Mass Spectrometry Imaging
- Integrated Delivery of Antiretroviral Treatment and Pre-exposure Prophylaxis to HIV-1–Serodiscordant Couples: A Prospective Implementation Study in Kenya and Uganda
- Measuring Burden of Unhealthy Behaviours Using a Multivariable Predictive Approach: Life Expectancy Lost in Canada Attributable to Smoking, Alcohol, Physical Inactivity, and Diet
- Comparison of Outcomes before and after Ohio's Law Mandating Use of the FDA-Approved Protocol for Medication Abortion: A Retrospective Cohort Study
- Core Outcomes for Colorectal Cancer Surgery: A Consensus Study
- Adverse Renal, Endocrine, Hepatic, and Metabolic Events during Maintenance Mood Stabilizer Treatment for Bipolar Disorder: A Population-Based Cohort Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Glycemic Control and the Risk of Tuberculosis: A Cohort Study
- Transitioning to Country Ownership of HIV Programs in Rwanda
- Dementia across the Lifespan and around the Globe—Pathophysiology, Prevention, Treatment, and Societal Impact: A Call for Papers
- Social Dancing and Incidence of Falls in Older Adults: A Cluster Randomised Controlled Trial
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



