-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Major Radiodiagnostic Imaging in Pregnancy and the Risk of Childhood Malignancy: A Population-Based Cohort Study in Ontario
Background:
The association between fetal exposure to major radiodiagnostic testing in pregnancy—computed tomography (CT) and radionuclide imaging—and the risk of childhood cancer is not established.Methods and Findings:
We completed a population-based study of 1.8 million maternal-child pairs in the province of Ontario, from 1991 to 2008. We used Ontario's universal health care–linked administrative databases to identify all term obstetrical deliveries and newborn records, inpatient and outpatient major radiodiagnostic services, as well as all children with a malignancy after birth. There were 5,590 mothers exposed to major radiodiagnostic testing in pregnancy (3.0 per 1,000) and 1,829,927 mothers not exposed. The rate of radiodiagnostic testing increased from 1.1 to 6.3 per 1,000 pregnancies over the study period; about 73% of tests were CT scans. After a median duration of follow-up of 8.9 years, four childhood cancers arose in the exposed group (1.13 per 10,000 person-years) and 2,539 cancers in the unexposed group (1.56 per 10,000 person-years), a crude hazard ratio of 0.69 (95% confidence interval 0.26–1.82). After adjusting for maternal age, income quintile, urban status, and maternal cancer, as well as infant sex, chromosomal or congenital anomalies, and major radiodiagnostic test exposure after birth, the risk was essentially unchanged (hazard ratio 0.68, 95% confidence interval 0.25–1.80).Conclusions:
Although major radiodiagnostic testing is now performed in about 1 in 160 pregnancies in Ontario, the absolute annual risk of childhood malignancy following exposure in utero remains about 1 in 10,000. Since the upper confidence limit of the relative risk of malignancy may be as high as 1.8 times that of an unexposed pregnancy, we cannot exclude the possibility that fetal exposure to CT or radionuclide imaging is carcinogenic.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 7(9): e32767. doi:10.1371/journal.pmed.1000337
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000337Summary
Background:
The association between fetal exposure to major radiodiagnostic testing in pregnancy—computed tomography (CT) and radionuclide imaging—and the risk of childhood cancer is not established.Methods and Findings:
We completed a population-based study of 1.8 million maternal-child pairs in the province of Ontario, from 1991 to 2008. We used Ontario's universal health care–linked administrative databases to identify all term obstetrical deliveries and newborn records, inpatient and outpatient major radiodiagnostic services, as well as all children with a malignancy after birth. There were 5,590 mothers exposed to major radiodiagnostic testing in pregnancy (3.0 per 1,000) and 1,829,927 mothers not exposed. The rate of radiodiagnostic testing increased from 1.1 to 6.3 per 1,000 pregnancies over the study period; about 73% of tests were CT scans. After a median duration of follow-up of 8.9 years, four childhood cancers arose in the exposed group (1.13 per 10,000 person-years) and 2,539 cancers in the unexposed group (1.56 per 10,000 person-years), a crude hazard ratio of 0.69 (95% confidence interval 0.26–1.82). After adjusting for maternal age, income quintile, urban status, and maternal cancer, as well as infant sex, chromosomal or congenital anomalies, and major radiodiagnostic test exposure after birth, the risk was essentially unchanged (hazard ratio 0.68, 95% confidence interval 0.25–1.80).Conclusions:
Although major radiodiagnostic testing is now performed in about 1 in 160 pregnancies in Ontario, the absolute annual risk of childhood malignancy following exposure in utero remains about 1 in 10,000. Since the upper confidence limit of the relative risk of malignancy may be as high as 1.8 times that of an unexposed pregnancy, we cannot exclude the possibility that fetal exposure to CT or radionuclide imaging is carcinogenic.
: Please see later in the article for the Editors' SummaryIntroduction
Cancer remains the second leading cause of hospitalization and death among children aged 14 y and younger in industrialized nations [1]–[3]. Fewer than 10% of childhood cancers are attributable to a genetic predisposition [4]. With the exception of a few known risk factors, including exposure to ionizing radiation in utero and after birth [5],[6], the etiology of most childhood cancer remains unknown.
There has been a substantial increase in the utilization of computed tomography (CT) and radionuclide (nuclear medicine) radiodiagnostic procedures, making them a common source of radiation to patients [7]–[9]. Since these tests are often ordered under emergency circumstances [10], inadvertent exposure in early pregnancy may occur, since half of all pregnancies are unplanned [11].
There may be an association between exposure to ionizing radiation in pregnancy and childhood cancer, yet, the supporting data are somewhat conflicting. For example, studies of cancer incidence following intrauterine exposure to the atomic bomb are inconsistent [12]–[14], as have some [15], but not all [16]–[18] studies examining maternal exposure to plain radiographs in pregnancy. Less is known about the risk related to major radiodiagnostic tests, such as CT and radionuclide imaging, both of which expose the fetus to considerably higher doses of radiation than plain radiographs administered at the same anatomical level [19],[20].
We studied women exposed to major radiodiagnostic tests in pregnancy in an effort to provide clinicians and mothers with better estimates of the risk of pediatric malignancy in their offspring.
Methods
Study Design
We completed a retrospective population-based cohort study of women who delivered a liveborn infant in Ontario between April 1, 1992 and March 31, 2008. The study was approved by the Ethics Review Board of Sunnybrook Health Sciences Centre.
Participants
We included singleton and multifetal pregnancies, and for the latter we included the first infant birth record to avoid multiple counts per pregnancy. We included maternal-infant pairs comprising term liveborn infants who survived more than 30 d after date of birth. For three reasons, we restricted the sample to term infants ≥37 wk gestation, and who weighed at least 2,500 g at birth: First, preterm infants are commonly exposed to radiodiagnostic imaging procedures in hospital [21],[22], which could potentially contaminate the studied effect of prenatal exposure. Second, since term infants represent 94% of all livebirths [23], our findings would remain applicable to most pregnancies. We defined term infants by actual gestational age from 2002 onward, the time in which it was captured in our dataset; before 2002, term status was based on the absence of any diagnostic codes for prematurity according to the International Classification of Diseases, 9th (ICD-9) and 10th (ICD-10CA) revisions (Table S1). Thus, limiting our cohort to term infants enabled us to better estimate the gestational age of fetal exposure to a major radiodiagnostic test prior to the year 2002.
Exposure and Outcome
The study exposure was CT or radionuclide imaging in an index pregnancy, up to 2 d before the day of delivery (to maximize the likelihood that the fetus was in utero). Prior to the year 2002, gestational age at birth was not recorded in our databases, so the estimated gestational age at exposure was calculated by subtracting 37 wk from the delivery date. This approach has a reported sensitivity of 90% and specificity of 99% [24]. From 2002 onward, we used the recorded gestational age at delivery to determine the timing of exposure to a radiodiagnostic test.
The main study outcome was a confirmed diagnosis of any pediatric malignancy in a child born in the index pregnancy at any time 30 d or more after his/her birth. This 30-d interval was used to exclude infants who died from major morbidity, such as neonatal sepsis or severe anomalies.
Database Sources
We used anonymized databases for the entire province of Ontario, where universal health care, including prenatal care and radiodiagnostic testing, is available to all residents. Databases were linked using unique encrypted identifiers, which enabled us to link radiation exposure in an index pregnancy to a diagnosed malignancy in a child.
Individual obstetrical deliveries were identified in the Canadian Institute for Health Information Discharge Abstract Database (DAD). The DAD contains age and sex, dates of admission, and up to 16 diagnoses coded by ICD-9 up to March 31, 2002, and up to 25 diagnoses coded by ICD-10-CA thereafter. Up to 2002, inpatient admission records for mothers and newborns are probabilistically linked using delivery/birth dates in the same hospital, same residential postal codes, and diagnostic information to create each mother-child pair. This approach leads to successful matching in 95% of cases. Validation studies of similar maternal-newborn linkages have shown that this approach yields high degrees of successful matching, with a sensitivity of about 95% and a specificity of nearly 90% [25]–[27]. In a Canadian study linking newborn birth records and infant death records, over 99% of records were successfully linked [28]. Starting in the fiscal year 2002/2003, a common identifying number between a delivering mother's chart number and her newborn's chart number permitted a deterministic linkage between the two from 2002 and onward. The DAD was also used to define study covariates (Table S1).
For the study exposure—a major radiodiagnostic test performed on the mother up to one day before her delivery date—we used the Ontario Health Insurance Plan (OHIP) database, which captures all billing information for physician services, including inpatient and outpatient major radiodiagnostic services [29],[30]. In an audit of a representative sample of 11,824 outpatient CT scans and 11,867 outpatient magnetic resonance imaging (MRI) scans identified from 29 randomly selected hospitals across Ontario, 95% of the procedures studied in the chart audit had a corresponding claim in the OHIP database, indicating that these administrative databases have high accuracy in identifying imaging procedures [30]. In the current study, major radiodiagnostic testing included all forms of CT and radionuclide imaging (Table S2). Because the OHIP database does not capture all billings for inpatient radionuclide imaging before 2006, the DAD was used to identify inpatient radionuclide tests performed throughout the study period (Table S2).
For the study outcome—pediatric malignancy—each child record was linked to the Ontario Cancer Registry (OCR), a computerized database of information on all Ontario residents who have been newly diagnosed with cancer [31]. All new cases of cancer, except for nonmelanoma skin cancer, are registered, and about 80% have the tissue pathological diagnosis recorded within the OCR. Validation studies have shown the OCR to be effective in ascertaining cancer cases in the province, with a sensitivity of 98% [32]. Close to 400,000 records are submitted to the OCR annually, and coded using ICD-9 diagnostic codes (Table S1). Incident cases of pediatric malignancy were captured according to a date of diagnosis between May 1, 1991 (a minimum of 30 d after the earliest birth in the study cohort) and March 31, 2009 (a maximum of 18 y after the earliest birth in the cohort).
Mortality data were retrieved from the Registered Persons Database (RPDB), which contains demographic information for all OHIP-eligible individuals. Income quintile and rurality were defined using Statistics Canada census data. The OHIP database was also used to identify outpatient claims for prenatal ultrasonography at any point in pregnancy (Table S2) [33].
Statistical Analysis
We measured the absolute number of radiodiagnostic tests performed in each livebirth pregnancy in a given year and calculated the annual test rate per 1,000 livebirths, from 1992 to 2008. Changes over time in the rates for radionuclide tests, CT, and both were analyzed using a Cochran-Armitage test for trend.
Time-to-event analyses were conducted using Cox proportional hazards regression models, to derive a hazard ratio (HR) and 95% confidence interval (CI) for pediatric malignancy among children born to mothers exposed versus not exposed to major radiodiagnostic imaging in pregnancy.
The period of observation for each child started 31 d after birth, to ensure that he or she survived long enough to develop, or to be diagnosed with, a malignancy. A child was censored (i.e., determined to have not had a study outcome event) at the point in time in which he or she either reached the end of the period of March 31, 2009 without having experienced a study outcome event or died during the period of study. While emigration from the province could not be ascertained, the annual rate is less than 1% (http://www.statcan.gc.ca/pub/75-001-x/2008110/t/10711/5800473-eng.htm); such persons were classified as being event-free up to March 31, 2009.
The HR was adjusted for maternal age at delivery (in years), income quintile, urban status, and maternal cancer diagnosed in the index pregnancy or up to 6 mo thereafter, as well as infant sex, a chromosomal or congenital anomaly documented at the time of birth or up to 365 d thereafter (present or absent), and exposure to CT or radionuclide imaging (as present or absent) starting 31 d after birth and up to 365 d before either the date that he/she experienced a study outcome event or the date that he/she was censored. The latter considered postnatal exposure to the same ionizing radiation sources as in pregnancy. Information about maternal parity, prior pregnancy loss, smoking, and medication use were not available.
All p-values were two-sided, at a significance level of 0.05. All statistical analyses were performed using SAS for UNIX, Version 9.1 (SAS Institute).
Results
There were 2,018,924 maternal-child pairs initially generated, of which 183,442 (9.1%) were excluded for the following reasons: newborn weight <2,500 g or >6,000 g (n = 113,718); preterm delivery <37 wk gestation (n = 50,881); multiple births subsequent to the first birth (n = 10,116); maternal age <16 y or >50 y (n = 4,091); nonresident of Ontario (n = 2,310); infant death ≤30 d after birth (n = 1,514); stillbirth (n = 780); extreme post-term birth >43 wk gestation (n = 29); and incorrect coding of birth date (n = 3). Among those excluded, the rate of stillbirth was not significantly different among mothers exposed (0.65%) and not exposed (0.84%) to major radiodiagnostic testing in pregnancy. Among all mothers exposed to major radiodiagnostic testing in pregnancy, the characteristics of those who were included and excluded are listed in Table S3.
The final cohort comprised 1,835,517 maternal-child pairs. The demographic characteristics of the 5,590 mothers exposed to major radiodiagnostic testing in pregnancy were generally similar to the unexposed cohort of 1,829,927 women (Table 1). However, the rate of diagnosed cancer during or soon after pregnancy was higher in the exposed mothers (0.63% versus 0.050%), as was the rate of early prenatal ultrasonography (33.0% versus 19.0%).
Tab. 1. Characteristics of mothers and their infants who were and who were not exposed to a major radiodiagnostic testing in pregnancy. 
All data are presented as a number (%) unless otherwise indicated. Among the liveborn infants of mothers exposed and not exposed to a major radiodiagnostic test, just under half were female, and the mean (standard deviation [SD]) gestational ages at birth were 39.1 (1.2) and 39.3 (1.1) wk, respectively (Table 1). The rates of chromosomal (0.13% versus 0.11%) and congenital (3.9% versus 3.7%) anomalies were about the same in both groups, as were the rates of CT or radionuclide imaging after birth (4.4% versus 4.4%).
The overall rate of exposure to major radiodiagnostic testing in pregnancy was 3.0 per 1,000, which increased significantly over time, peaking at 6.3 per 1,000 livebirth pregnancies in 2008 (trend p<0.001) (Figure 1). Exposure to major radiodiagnostic testing in pregnancy occurred at an estimated mean (SD) gestational age of 15.7 (12.8) wk, and about half were completed before 14 wk gestation (Table 1). 15% of women had two or more tests performed in pregnancy. About 73% of all major radiodiagnostic tests were CT scans, of which 68% were of an extremity or head, nearly 10% of the thorax, and 22.6% of the abdomen, spine, or pelvis (Table 1).
Fig. 1. Annual rate of major radiodiagnostic testing in pregnancy in Ontario over time. 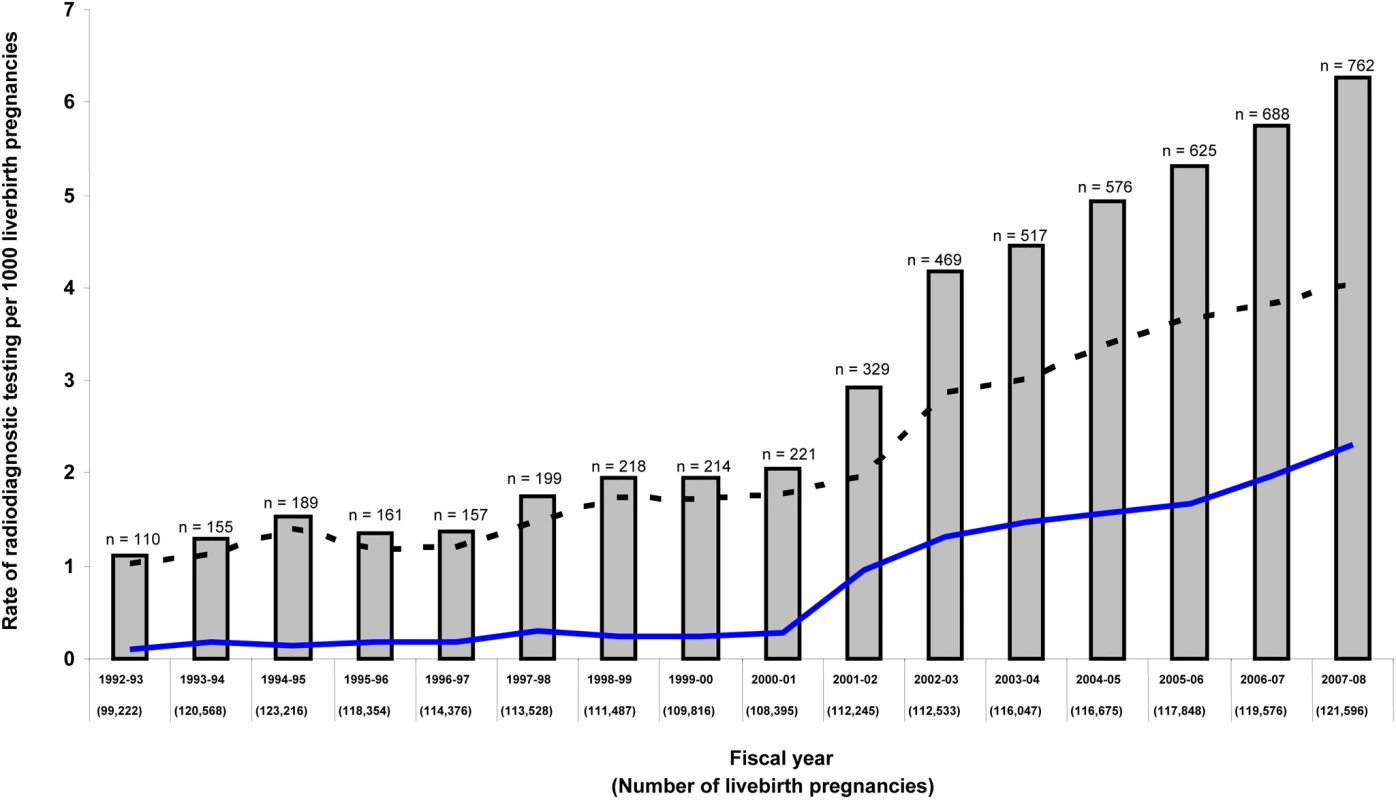
Data are presented for radionuclide testing (lower solid line), CT scan (upper dashed line), and both (solid bars with total number per year). There were 35,487 and 16,326,410 persons-years of follow-up in the exposed and unexposed groups, respectively. Among the entire cohort of 1,835,517 children, the median (interquartile range [IQR]) duration of follow-up was 8.91 (4.83–13.00) y. A total of 1,015,789 (55.3%) children were followed to age 8 y, 797,940 (43.5%) to age 10 y, 573,445 (31.2%) to age 12 y, and 341,432 (18.6%) to age 14 y.
A total of four childhood cancers occurred in the exposed group (1.13 per 10,000 person-years) and 2,539 cancers in the unexposed group (1.56 per 10,000 person-years), corresponding to a crude HR of 0.69 (95% CI 0.26–1.82) (Figure 2; Table 2). After adjusting for potential confounders, the risk was not significantly higher among exposed versus unexposed maternal-child pairs (HR 0.68, 95% CI 0.25–1.80). Upon adding into the sample previously excluded infants at extremes of gestational age and birthweight, the crude (0.71, 95% CI 0.29–1.70) and adjusted (0.69, 95% CI 0.29–1.66) HR remained unchanged. We could not specify the types of cancers arising in the exposed group because of a strict privacy policy at our institution about the reporting of specific information for cell sizes under the number 5.
Fig. 2. Risk of childhood cancer in the offspring of women exposed (lower solid) and not exposed (upper dashed) to a major radiodiagnostic test in pregnancy. 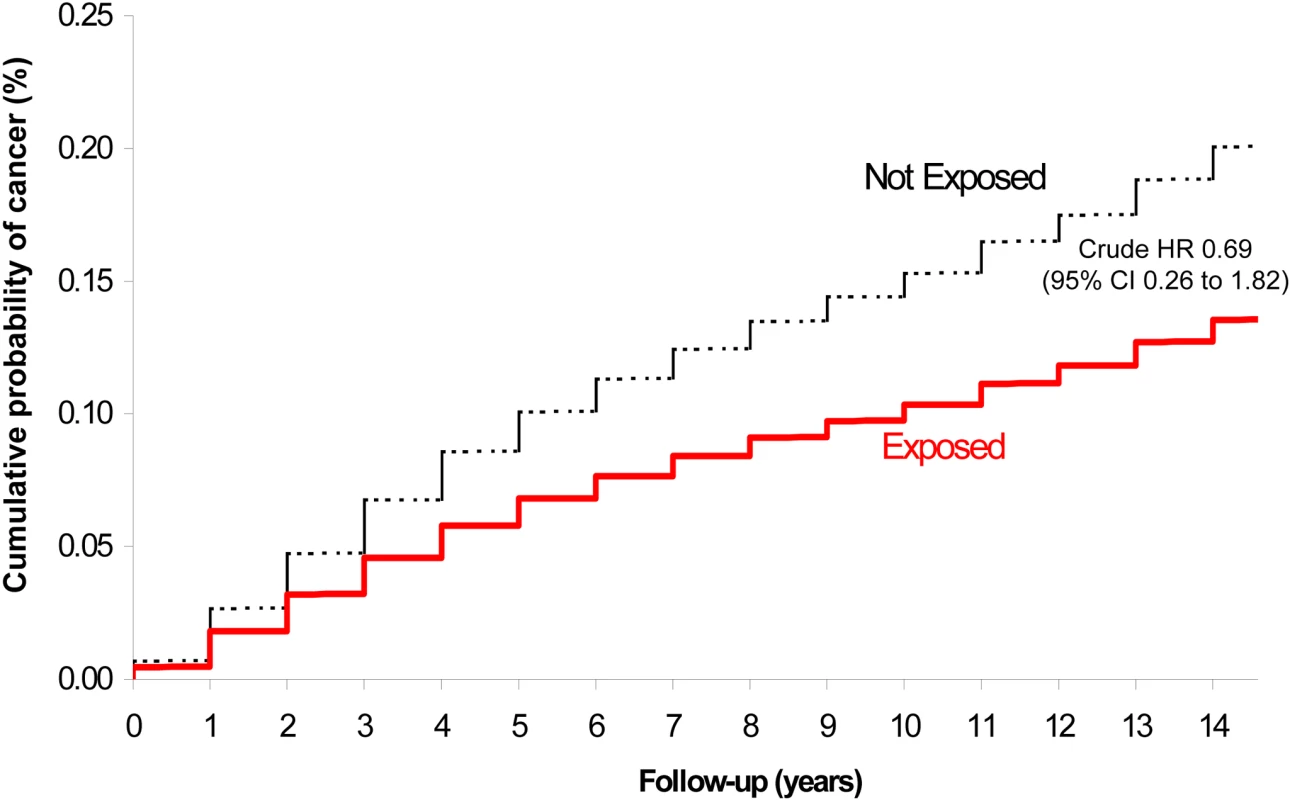
Tab. 2. Risk of childhood malignancy in the offspring of women exposed to major radiodiagnostic testing in pregnancy compared to unexposed women. 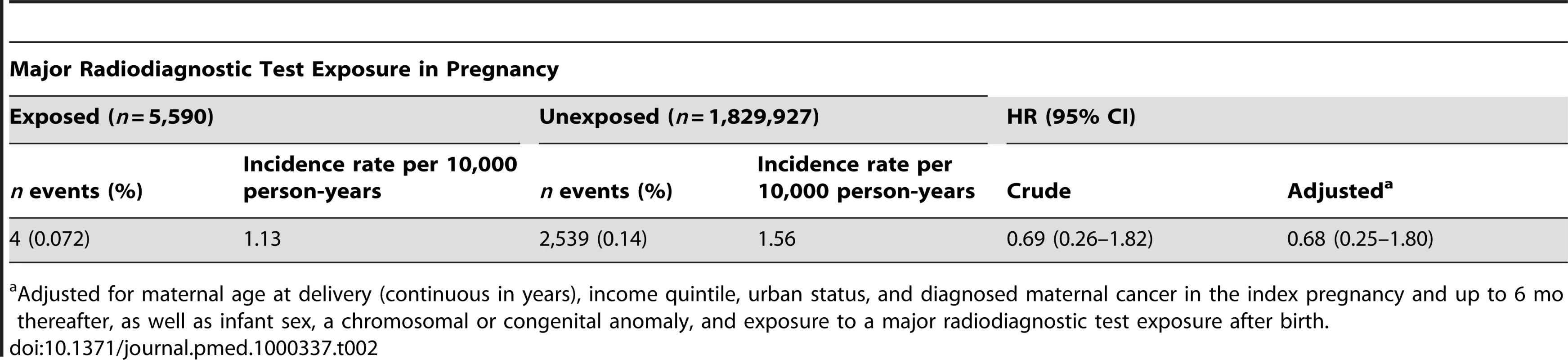
Adjusted for maternal age at delivery (continuous in years), income quintile, urban status, and diagnosed maternal cancer in the index pregnancy and up to 6 mo thereafter, as well as infant sex, a chromosomal or congenital anomaly, and exposure to a major radiodiagnostic test exposure after birth. Discussion
About 1 in 160 term pregnancies now appear to be exposed to a major radiodiagnostic test. The offspring of women exposed to major radiodiagnostic testing in pregnancy do not appear to be at higher risk of childhood malignancy than the children of unexposed mothers. Moreover, the overall prevalence of pediatric malignancy following exposure to CT or radionuclide imaging in pregnancy was under 0.07%, an incidence rate of 1.13 per 10,000 person-years.
Study Strengths and Limitations
Among those exposed to major radiodiagnostic testing, totaling nearly 5,600 pregnancies and 35,000 persons-years of follow-up, just four cases of childhood malignancy were identified. The incidence rate of 1.56 cancers per 10,000 person-years, based on 2,539 events arising among 1,829,995 unexposed pregnancies, is highly concordant with population studies from Canada, the United States, Ireland, and Europe [1]–[3]. Post hoc, considering the number of participants enrolled in the current study, at a conventional p-value of 0.05, we had just under 20% statistical power to detect the observed difference of 0.40 per 10,000 person-years in the incidence rate of childhood malignancy between exposed and unexposed mothers. To achieve a power of 80%, approximately 60 and 19,613 cancers would be needed in the exposed and unexposed groups, respectively—about eight times higher than the current number of events. That so few cases of childhood malignancy were identified among the exposed is a testament to the difficulty of conducting research in this area, and why previous data may have been lacking. Nonetheless, the wide confidence limits of our relative risk estimates do not exclude the possibility that fetal exposure to CT or radionuclide imaging in pregnancy is carcinogenic, ranging from as low as one-quarter, to as high as 1.8 times, that of an unexposed pregnancy.
We did not include women who had a radiodiagnostic test in a pregnancy that ended in a spontaneous or therapeutic termination before 20-wk gestation. However, this study's focus was on childhood malignancy after 30 d of life, rather than on pregnancy loss, and it is unlikely that including that group of women would have systematically biased the association between the study exposure and outcome. Moreover, the rates of stillbirth after 20 wk among excluded women exposed (0.65%) and not exposed (0.84%) to major radiodiagnostic testing in pregnancy did not differ significantly. By limiting our sample to term deliveries between 37 and 43 wk gestation and restricting the period of exposure up to 2 d before a woman's date of delivery, we improved the accuracy of our determination of the timing of exposure to radiodiagnostic testing. At the same time, our findings may not apply to preterm infants, who both have higher rates of congenital and chromosomal anomalies, as well as major radiodiagnostic testing soon after birth (Table S3) [21],[22].
We did not have on record whether a woman's pregnancy was known at the time of exposure, or if she received lead apron shielding of the abdominal-pelvic area. Assuming that shielding occurred in most cases, as is routine practice, then this may partly explain the low rate of childhood malignancy in the exposed group. Moreover, two-thirds of CT scans were of an extremity or head, with the remaining sites in closer proximity to the fetus, but only 23% were of the abdomen, spine, or pelvis. Hence, much of our CT data reflects “low-risk” scans, in terms of fetal radiation exposure.
We also do not possess information about the generation(s) of CT scans that were used, or the measured dose of ionizing radiation that a woman and her fetus were exposed to. The cited average effective maternal radiation dose of CT of the head or neck is about 2 millisievert (mSv); for a CT of the chest, spine, abdomen, or pelvis it ranges from 6 to 8 mSv [9],[34]; and for a ventilation-perfusion lung scan it is about 2.5 mSv [34]. While these doses are between 10 to 100 times more than that of an equivalent single plain X-ray [34], the effective radiation dose to the fetus remains lower than that to the mother [18],[35]. The rate of major radiodiagnostic testing in pregnancy in our study is certainly lower than when plain X-rays were performed in pregnancy for maternal pelvimetry [36]. In the era of the current study, pelvimetry was not routine, but we would have missed plain X-rays performed for other reasons.
Mothers, as well as clinicians of all types—internists, surgeons, radiologists, obstetricians and midwives, pediatricians, and family and emergency medicine physicians—need data to better estimate the risk of exposure to radiodiagnostic testing in pregnancy. Our findings, though statistically underpowered, inform this process in a manner that has been lacking to date, especially using population-based data over a long period of follow-up, and within a health care system that provides universal coverage of radiological testing and health care.
Clinical Implications
Our findings can help inform clinicians and mothers about the risk of childhood malignancy following major radiodiagnostic testing in pregnancy. First, the absolute risk appears to be low (about 0.07% or 1 per 10,000 person-years), while the relative risk is not statistically significantly higher than unexposed controls, notwithstanding the wide confidence limits. However potentially small that risk may be, beta hCG testing should continue to be done in all potentially pregnant women before undergoing major radiodiagnostic testing, and lead apron shielding used in all women of reproductive age, whether or not pregnant [6],[37]. Furthermore, nonradiation-emitting imaging (e.g., MRI and ultrasonography) should be considered first, when appropriate.
Some pregnant women will nevertheless be faced with the decision to undergo CT or nuclear imaging because the test is clinically warranted. Unfortunately, some health care providers may be unwilling to counsel a woman about the related fetal risks or may provide misinformation [37],[38]. This issue seems more pressing than ever, given that we and others [9] have noted that CT testing is on the rise, and has become the mainstay for the investigation of pulmonary embolism [39], stroke [40], appendicitis [41], and other common conditions encountered in emergency situations [10]. Both physiological and anatomical changes in pregnancy may obscure the clinical diagnosis of pulmonary embolism [42], for example, such that CT or radionuclide testing may be especially warranted. Delaying the diagnosis of such conditions may postpone therapy, in turn, jeopardizing both mother and child [43].
When indicated, major radiodiagnostic testing in pregnancy should be carried out, along with brief counseling [37]. The latter will hopefully lessen the level of anxiety experienced by an expectant mother (and her family), not only at the time of illness, but after her child is born.
Supporting Information
Zdroje
1. Leading Causes of Death and Hospitalization in Canada Ottawa Public Health Agency of Canada Available: http://www.phac-aspc.gc.ca/publicat/lcd-pcd97/pdf/lcd-pcd-t1-eng.pdf. Accessed 10 July 2010
2. LinaberyAM
RossJA
2008 Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer 112 416 432
3. StackM
WalshPM
ComberH
RyanCA
O'LorcainP
2007 Childhood cancer in Ireland: a population-based study. Arch Dis Child 92 890 897
4. PlonSE
NathansonK
2005 Inherited susceptibility for pediatric cancer. Cancer J 11 255 267
5. DollR
WakefordR
1997 Risk of childhood cancer from fetal irradiation. Br J Radiol 70 130 139
6. PatelSJ
ReedeDL
KatzDS
SubramaniamR
AmorosaJK
2007 Imaging the pregnant patient for nonobstetric conditions: algorithms and radiation dose considerations. Radiographics 27 1705 1722
7. LevinDC
RaoVM
ParkerL
FrangosAJ
SunshineJH
2008 Recent trends in utilization rates of abdominal imaging: the relative roles of radiologists and nonradiologist physicians. J Am Coll Radiol 5 744 747
8. BhargavanM
2008 Trends in the utilization of medical procedures that use ionizing radiation. Health Phys 95 612 627
9. FazelR
KrumholzHM
WangY
RossJS
ChenJ
2009 Exposure to low-dose ionizing radiation from medical imaging procedures. N Engl J Med 361 849 857
10. BroderJ
WarshauerDM
2006 Increasing utilization of computed tomography in the adult emergency department, 2000–2005. Emerg Radiol 13 25 30
11. RayJG
SinghG
BurrowsRF
2004 Evidence for suboptimal use of periconceptional folic acid supplements globally. BJOG 111 399 408
12. YoshimotoY
DelongchampR
MabuchiK
1994 In-utero exposed atomic bomb survivors: cancer risk update. Lancet 344 345 346
13. MillerRW
BoiceJDJr
1997 Cancer after intrauterine exposure to the atomic bomb. Radiat Res 147 396 397
14. PrestonDL
CullingsH
SuyamaA
FunamotoS
NishiN
2008 Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst 100 428 436
15. NaumburgE
BelloccoR
CnattingiusS
HallP
BoiceJDJr
2001 Intrauterine exposure to diagnostic X rays and risk of childhood leukemia subtypes. Radiat Res 156 718 723
16. StewartA
KnealeGW
1970 Radiation dose effects in relation to obstetric x-rays and childhood cancers. Lancet i 1185 1188
17. BithellJF
1989 Epidemiological studies of children irradiated in utero.
BaverstockKF
StatherJW
Low dose radiation: biological bases of risk assessment London Taylor and Francis 77 87
18. LinetMS
KimKP
RajaramanP
2009 Children's exposure to diagnostic medical radiation and cancer risk: epidemiologic and dosimetric considerations. Pediatr Radiol 39 Suppl 1 S4 S26
19. ChenMM
CoakleyFV
KaimalA
LarosRKJr
2008 Guidelines for computed tomography and magnetic resonance imaging use during pregnancy and lactation. Obstet Gynecol 112 333 340
20. WakefordR
2008 Childhood leukaemia following medical diagnostic exposure to ionizing radiation in utero or after birth. Radiat Prot Dosimetry 132 166 174
21. SmansK
StruelensL
SmetM
BosmansH
VanhavereF
2008 Patient dose in neonatal units. Radiat Prot Dosimetry 131 143 147
22. DougeniED
DelisHB
KaratzaAA
KalogeropoulouCP
SkiadopoulosSG
2007 Dose and image quality optimization in neonatal radiography. Br J Radiol 80 807 815
23. JosephKS
KramerMS
MarcouxS
OhlssonA
WenSW
1998 Determinants of preterm birth rates in Canada from 1981 through 1983 and from 1992 through 1994. N Engl J Med 339 1434 1439
24. TohS
MitchellAA
WerlerMM
Hernandez-DiazS
2008 Sensitivity and specificity of computerized algorithms to classify gestational periods in the absence of information on date of conception. Am J Epidemiol 167 633 640
25. QuantinC
GouyonB
AvillachP
FerdynusC
SagotP
2009 Using discharge abstracts to evaluate a regional perinatal network: assessment of the linkage procedure of anonymous data. Int J Telemed Appl 2009 181842
26. MérayN
ReitsmaJB
RavelliAC
BonselGJ
2007 Probabilistic record linkage is a valid and transparent tool to combine databases without a patient identification number. J Clin Epidemiol 60 883 891
27. HermanAA
McCarthyBJ
BakewellJM
WardRH
MuellerBA
1997 Data linkage methods used in maternally-linked birth and infant death surveillance data sets from the United States (Georgia, Missouri, Utah and Washington State), Israel, Norway, Scotland and Western Australia. Paediatr Perinat Epidemiol 11 Suppl 1 5 22
28. FairM
CyrM
AllenAC
WenSW
GuyonG
2000 An assessment of the validity of a computer system for probabilistic record linkage of birth and infant death records in Canada. The Fetal and Infant Health Study Group. Chronic Dis Can 21 8 13
29. YouJJ
AlterDA
IronK
SlaughterPM
KoppA
2007 Diagnostic services in Ontario: descriptive analysis and jurisdictional review Toronto Institute for Clinical Evaluative Sciences Available: http://www.ices.on.ca/file/diagnostic_services_ontario_oct16.pdf. Accessed 10 July 2010
30. YouJJ
LaupacisA
NewmanA
BellCM
2010 Non-adherence to recommendations for further testing after outpatient CT and MRI. Am J Med 123 557.e1 557.e8
31. MarrettLD
ClarkeEA
HatcherJ
WeirHK
1986 Epidemiologic research using the Ontario Cancer Registry. Can J Public Health 77 Suppl 1 79 85
32. McLaughlinJR
KreigerN
MarrettLD
HolowatyEJ
1991 Cancer incidence registration and trends in Ontario. Eur J Cancer 27 1520 1524
33. YouJJ
AlterDA
StukelTA
McDonaldSD
LaupacisA
2010 Proliferation of prenatal ultrasonography. CMAJ 182 143 151
34. MettlerFAJr
HudaW
YoshizumiTT
MaheshM
2008 Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 248 254 263
35. AngelE
WellnitzCV
GoodsittMM
YaghmaiN
DeMarcoJJ
2008 Radiation dose to the fetus for pregnant patients undergoing multidetector CT imaging: Monte Carlo simulations estimating fetal dose for a range of gestational age and patient size. Radiology 249 220 227
36. MoleRH
1990 Childhood cancer after prenatal exposure to diagnostic X-ray examinations in Britain. Br J Cancer 62 152 168
37. BrentRL
2009 Saving lives and changing family histories: appropriate counseling of pregnant women and men and women of reproductive age, concerning the risk of diagnostic radiation exposures during and before pregnancy. Am J Obstet Gynecol 200 4 24
38. RatnapalanS
BonaN
ChandraK
KorenG
2004 Physicians' perceptions of teratogenic risk associated with radiography and CT during early pregnancy. AJR 182 1107 1109
39. PrologoJD
GilkesonRC
DiazM
AsaadJ
2004 CT pulmonary angiography: a comparative analysis of the utilization patterns in emergency department and hospitalized patients between 1998 and 2003. AJR 183 1093 1096
40. KidwellCS
HsiaAW
2006 Imaging of the brain and cerebral vasculature in patients with suspected stroke: advantages and disadvantages of CT and MRI. Curr Neurol Neurosci Rep 6 9 16
41. KimK
LeeCC
SongKJ
KimW
SuhG
2008 The impact of helical computed tomography on the negative appendectomy rate: a multi-center comparison. J Emerg Med 34 3 6
42. FedulloPF
TapsonVF
2003 Clinical practice. The evaluation of suspected pulmonary embolism. N Engl J Med 349 1247 1256
43. MasonE
Rosene-MontellaK
PowrieR
1998 Medical problems during pregnancy. Med Clin North Am 82 249 269
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Examining the “Urban Advantage” in Maternal Health Care in Developing Countries
- Radiodiagnostic Imaging in Pregnancy and the Risk of Childhood Malignancy: Raising the Bar
- Does It Matter Who Writes Medical News Stories?
- Drug Companies Should Be Held More Accountable for Their Human Rights Responsibilities
- Are Drug Companies Living Up to Their Human Rights Responsibilities? The Merck Perspective
- Seventy-Five Trials and Eleven Systematic Reviews a Day: How Will We Ever Keep Up?
- Persistence with Statins and Onset of Rheumatoid Arthritis: A Population-Based Cohort Study
- Effectiveness of Chest Physiotherapy in Infants Hospitalized with Acute Bronchiolitis: A Multicenter, Randomized, Controlled Trial
- Combined Impact of Lifestyle-Related Factors on Total and Cause-Specific Mortality among Chinese Women: Prospective Cohort Study
- Are Drug Companies Living Up to Their Human Rights Responsibilities? The Perspective of the Former United Nations Special Rapporteur (2002-2008)
- AIDS Vaccine for Asia Network (AVAN): Expanding the Regional Role in Developing HIV Vaccines
- Are Drug Companies Living Up to Their Human Rights Responsibilities? Moving Toward Assessment
- The Haunting of Medical Journals: How Ghostwriting Sold “HRT”
- Major Radiodiagnostic Imaging in Pregnancy and the Risk of Childhood Malignancy: A Population-Based Cohort Study in Ontario
- A Genetic Association Study of Serum Acute-Phase C-Reactive Protein Levels in Rheumatoid Arthritis: Implications for Clinical Interpretation
- Cost-Effectiveness of Pooled Nucleic Acid Amplification Testing for Acute HIV Infection after Third-Generation HIV Antibody Screening and Rapid Testing in the United States: A Comparison of Three Public Health Settings
- Community Case Management of Fever Due to Malaria and Pneumonia in Children Under Five in Zambia: A Cluster Randomized Controlled Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Seventy-Five Trials and Eleven Systematic Reviews a Day: How Will We Ever Keep Up?
- A Genetic Association Study of Serum Acute-Phase C-Reactive Protein Levels in Rheumatoid Arthritis: Implications for Clinical Interpretation
- Persistence with Statins and Onset of Rheumatoid Arthritis: A Population-Based Cohort Study
- The Haunting of Medical Journals: How Ghostwriting Sold “HRT”
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



