-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Uveal Melanoma Biopsy. A Review
Authors: Š. Rusňák 1; L. Hecová 1; Z. Kasl 1; M. Sobotová 1; L. Hauer 2
Authors place of work: Oční klinika, Fakultní nemocnice v Plzni 1; Stomatologická klinika, Fakultní nemocnice v Plzni 2
Published in the journal: Čes. a slov. Oftal., 76, 2020, No. 6, p. 247-252
Category: Přehledová práce
doi: https://doi.org/10.31348/2020/9Summary
In intraocular tumors, diagnosis is usually based on clinical examination and imaging without the need for invasive surgery or tissue sampling. The diagnosis can be confirmed by biopsy, however, in the case of intraocular malignancy, the biopsy is considered controversial. Due to the development of uveal melanoma cytogenetic prognostics and the progression in generalised uveal melanoma treatment, intraocular melanoma biopsy is becoming increasingly important. Diagnostic biopsy of intraocular tumors is indicated in cases of diagnostic uncertainty for findings with conflicting non-invasive test results and for small melanocyte lesions. Tumor prognostic biopsy is performed to obtain a tissue sample for tumor cytogenetic testing, which can help to determine the prognosis and specific metastatic risk of the patient.
For anterior segment tumors, anterior chamber fluid sampling, thin-needle iris biopsy, punch biopsy, surgical biopsy or biopsy using vitrectomy may be used. For posterior segment tumors, procedures include transscleral or transretinal thin-needle biopsy, vitrectomy-assisted biopsy, punch biopsy, endoresection or transscleral exoresection.
Complications of intraocular melanoma biopsy include too small or non-valuable sample collection, intra-tumoral heterogeneity, intra-ocular trauma and induction of intraocular or extraocular tumor dissemination.
Keywords:
uveal melanoma – diagnostic biopsy – prognostic biopsy – thin-needle biopsy – punch biopsy – vitrectomy-assisted biopsy
INTRODUCTION
In clinical oncology, the treatment of malignant tumours as a rule requires histological confirmation of the diagnosis. In intraocular tumours, diagnosis is based as standard on clinical examination and examination with the aid of imaging methods (ultrasonography, ultrasonic biomicroscopy, optical coherence tomography, autofluoresence, fluorescein and indocyanine green angiography, PET/CT or PET/MR), usually without the necessity of an invasive procedure or taking tissue samples [1,2,3]. The diagnosis can be confirmed by biopsy, but in the case of an intraocular malignant tumour biopsy is considered controversial. The main fears predominate due to the risk of dissemination of the tumour as a consequence of the invasive procedure, other potential complications include above all taking of a small or invalid sample, intratumour heterogeneity and perioperative damage to the intraocular structures. However, with regard to new observations in cytogenetic prognostics of uveal melanoma and advancing knowledge in the field of treatment of generalised uveal melanoma, intraocular melanoma biopsy is becoming increasingly important [4].
Indication of biopsy
The purpose of diagnostic biopsy of an intraocular tumour is to confirm or exclude clinical suspicion of malignancy. In total approximately 1–2 % of intraocular tumours require the performance of a diagnostic biopsy [5].
The main indication for a diagnostic intraocular biopsy of a tumour remains diagnostic uncertainty, with contradictory results of non-invasive tests. Another separate problem is the small size of uveal melanocytic lesions (maximum height up to 3 mm). The usual strategy in the case of small choroidal melanocytic lesions (covering atypical nevi and small melanomas) is regular observation, while therapy is commenced only upon identification of progression of the lesion. However, clinical oncological practice considers earlier diagnosis and treatment to represent a step toward the improvement of the prognosis for survival of the patient [6,7]. With reference to the small size and frequent posterior location of lesions in these cases, however, there is an increased risk on the one hand of taking an insufficiently large sample, and on the other of the development of sight-threatening ocular complications.
Despite improvements of local treatment of uveal melanoma, thanks to which it is possible to achieve local control over the tumour in the majority of patients while preserving the eyeball, the prevention and treatment of a metastatic disease remain a serious problem. Metastases of the liver develop in almost 50 % of patients with uveal melanoma. Current knowledge indicates that tumour cells are present in the body and already spread at the time of primary diagnosis, and remain inactive until the conditions become favourable for the formation of macro-metastases [8]. The constant search for new molecular targets of systemic therapy in patients with metastatic affliction, and their inclusion in therapeutic programmes within the framework of clinical trials on the basis of the molecular profile of the tumour require reliable genetic and molecular characterisation of the tumour [4]. For this reason, the significance of “prognostic biopsy” of the tumour is increasing, i.e. a sample is taken not in order to confirm the diagnosis but for the purpose of obtaining a sample of tissue for a cytogenetic examination of the tumour [9]. This enables determination of the prognosis, assessment of the specific metastatic risk for the patient and individual configuration of the timing of follow-up and screening examinations (laboratory checks, sonography of abdomen, X-ray of lungs) for patients with uveal melanoma. The genetic and molecular characteristics of a melanoma are more reliable in the prediction of the prognosis than classic clinical and pathological signs (such as dimensions of tumour, localisation of tumour, presence of exudative retinal detachment, and results of histopathological examination) [4,10].
Biopsy techniques
A suitable technique for biopsy of uveal melanoma is chosen primarily according to the location and size of the lesion. In the case of tumours of the anterior segment it is possible to use sampling of the anterior chamber fluid, thin-needle biopsy of the iris, punch biopsy, surgical biopsy or vitrectomy-assisted biopsy. In the case of tumours of the posterior segment, the procedures include thin-needle transscleral or transretinal biopsy, vitrectomy-assisted biopsy, punch biopsy, endoresection or transscleral exoresection.
TUMOURS OF THE ANTERIOR SEGMENT OF THE EYE
Sample of chamber fluid
Examination of the chamber fluid enables identification of cellular infiltration in the anterior chamber. This technique is used primarily in the case of selected lesions of the iris (melanomas of the iris, above all diffuse, or metastases of the iris) with apparent dissemination into the anterior chamber [4]. The subject of the study is the use of this technique in the diagnosis of melanoma of the ciliary body and/ or choroid – in comparison with benign lesions, in the case of melanoma there are higher levels of angiogenin, IL-8 and MCP-1 [11]. The main limitation of this technique consists in the fact that samples are “paucicellular” (i.e. the amount of cells in the sampled material is minimal), and it is very difficult to obtain valid results of the examination on this basis.
Thin-needle biopsy of iris
The standard technique for thin-needle biopsy of the iris consists in a limbal incision of 1 mm in the clear cornea and injection of viscoelastic material into the anterior chamber. A thin needle is then inserted into the tumour of the iris via the corneal incision and through the anterior chamber (according to the size of the lesion 25-gauge, 27-gauge or 30-gauge). The needle is inserted through the cornea under an angle of approximately 20–30° to the iris, in the anterior chamber the needle should be inserted parallel to the iris. The most suitable place for biopsy is approximately 90° from the meridian of the tumour. A specific procedure is recommended for inserting the needle into the tumour tissue – delicate movement of the needle back and forth into the lesion in order to maintain underpressure, thus enabling the release of tumour cells, which are then manually extracted by the assisting doctor [12,13]
In the case of thin-needle iris biopsy it is also possible to obtain a sample only of a limited size, with a small quantity of cells, as a result of which it may be difficult to gain a valid interpretation of the sample.
Vitrectomy-assisted biopsy of the iris
In this technique, a 21-gauge infusion is inserted into the anterior chamber, and intraocular pressure is increased to 70 mmHg. Subsequently a second limbal incision is made, through which a 20-gauge vitrectome is inserted towards the surface of the tumour, in such a manner that its mouth is covered by the tumour tissue. At a high aspiration value (400 mmHg) and low cutting frequency (80/min), a sample is then obtained from the surface of the tumour by a single cut [4,14].
Surgical biopsy of cornea
Surgical iridectomy provides a sufficient quantity of tissue for histopathological, immunohistochemical and cytogenetic analysis. In clinical practice it is used primarily as an “excision biopsy”, with the removal of all neoplastic tissue. The disadvantage of this procedure is extensive iatrogenic defect of the cornea or sclera [15]. An alternative is a minimally invasive method with the performance of a multifocal surgical biopsy of the cornea. At the beginning of the procedure, a 1.0 mm long corneal incision is made, and the anterior chamber is filled with 1 % sodium hyaluronate. A 25G aspiration port is then inserted into the cornea, and samples of the cornea are then taken in a number of places in full and partial thickness, at a high frequency of aspiration (600 mmHg) and cutting frequency (300/min) [4,16].
TUMOURS OF THE POSTERIOR SEGMENT OF THE EYE
Thin-needle biopsy
In the case of tumours of the posterior segment, it is possible to perform a thin-needle biopsy by a transretinal or transscleral approach.
A transscleral thin-needle biopsy is performed in the case of ciliochoroidal tumours and tumours of the choroid localised anteriorly or close to the equator of the eye. Upon transscleral, “direct” approach, a sample of the tumour tissue is taken via the sclera in the case of the base of the tumour, while preserving the retina intact. After localisation of the tumour, a lamellar scleral flap is created (approx. 80 % of the thickness of the sclera) in the shape of an equilateral triangle. A needle (most often 25-gauge or 30-gauge) is inserted into the tissue of the tumour via a scleral incision with a size of 300 µm, and 2–3 samples are progressively taken. The scleral incision is then sutured immediately after the sample is taken [17,18,19]. A modified technique which improves the yield of the samples for cytological and genetic analysis is the use of Essen biopsy forceps, with sealing of the scleral flap using histoacryl glue [4]. In the case of taking a sample before brachytherapy, an applicator with radionuclide is sutured to the sclera above the base of the tumour immediately after the performance of the biopsy.
In the case of lesions located more posteriorly, a transretinal approach is more appropriate due to better visualisation of the tumour. The transretinal or indirect method means an anterior approach via the pars plana (sclerotomy is localised against the tumour), and subsequently a needle is inserted via the vitreous body and through the retina into the choroidal tumour in the subretinal space. In this approach, it is necessary to bend the point of the needle (usually a 25-gauge needle, some authors recommend 27-gauge) approximately 2–3 mm from the point to an angle of 60–90° towards the cone of the needle. This enables entrance into the neoplasm with the point of the needle concurrently with the retina, thus reducing the risk of perforation of the sclera, in which 2–3 samples are taken. Careful localisation by indirect ophthalmoscopy is recommended, it is also possible to use a surgical microscope and wide-angle display system. Whereas a transscleral approach usually requires the tumour to be at least 3 mm high, a transretinal approach, thanks to transpupillary visualisation during the procedure, enables the performance of a biopsy also on small tumours. The procedure may be combined with prior vitrectomy – a combined procedure reduces the risk of haemorrhage into the vitreous body or the development of vitreous traction in the place of retinotomy (in the place of taking of the sample), which usually closes spontaneously. After suturing by sclerotomy it is possible to perform cryopexy [4,17,18,20].
According to Singh, the diagnostic yield correlates significantly with the choice of biopsy approach (transscleral 96 %, transretinal 86 %; p = 0.029) and size of tumour (diameter of base up to 5.00 mm and height up to 2.5 mm). Shields examined the potential of the technique of thin-needle biopsy for small melanomas (with maximum height of up to 3 mm) – he took samples in the place of the highest prominence of choroidal tumours using a 27-gauge needle, with the use of a transretinal approach, in 97 % of cases he succeeded in taking a representative sample of a tumour suitable for a histopathological and cytogenetic analysis [18].
Vitrectomy-assisted biopsy
Vitrectomy can be used in transretinal biopsy directly for taking a sample, or it is possible also to use combined methods: vitrectomy-assisted biopsy with a sample taken using Essen biopsy forceps or incision biopsy.
In the case of transvitreal retinochoroidal biopsy, a sample is taken for cytogenetic and histopathological examination by vitrectomy, according to the size of the lesion by a 25-gauge or 27-gauge vitrectome. After the insertion of 3 standard pars plana ports, the vitrectome is inserted under direct transpupillary visual control through the vitreous body (without the performance of vitrectomy) to the choroidal tumour. In the area of the intended biopsy, separation of the vitreous body is induced and a thorough local vitrectomy is performed, in order to prevent vitreoretinal incarceration into the vitrectome during the course of the biopsy. Intraocular pressure is increased in order to prevent haemorrhage. After the performance of retinotomy, the vitrectome is inserted into the tumour and a sample is taken at a high aspiration value (400–600 mmHg) and low cutting frequency (80–300/min). The sample is obtained through continual suction and cutting, until a sufficient quantity of sampled material appears in the aspiration tube. It is not usually necessary to treat the area of the retinotomy, but suture and cryopexy are performed by sclerotomy [4,14,21].
In intraocular biopsy of uveal melanoma, there is the potential possibility of dissemination of the tumour cells during the procedure; this risk was significantly reduced by the use of smaller cannulas and precise surgical techniques [14]. A variant which increases patient safety is the performance of a biopsy following prior irradiation of the tumour. Irradiation generates random lesions in DNA, and as long as 6 months after irradiation there is no significant change of the cytognetics of the tumour tissue, while shortly after irradiation the genetic prognosis should therefore not be influenced [22].
Transretinal biopsy with the use of Essen biopsy forceps is a sutureless method using three-port 23-gauge pars plana vitrectomy (PPV), with subsequent performance of a 0.6 mm long retinal incision, which enables the insertion of open Essen forceps into the tumour. The sample is grasped by the forceps and extracted via the vitreous space through a scleral port. The main advantage of the use of Esson biopsy forceps is the larger size of the obtained sample. In the case of samples larger than 0.6 mm, however, this technique represents a potentially higher risk of dissemination of the tumour, because the sample or parts thereof may become embedded in the scleral port [4,23].
PPV assisted incision biopsy incorporates complete three-port 23-gauge or 20-gauge PPV. Retinotomy is then performed with a diamond knife, followed by excision of 1 mm3 of tumour tissue. The tumour sample is extracted by sclerotomy with the aid of forceps. An increase of intraocular pressure and retinal diathermy are used to minimise haemorrhage, at the end of the procedure 20 % SF6 is applied to the eye. This procedure enables the taking of larger samples, which are suitable for histopathological and cytogenetic examination, but it is linked with an increased risk of retinal detachment [24].
Risks of biopsy
The risks of intraocular biopsy include above all taking of an invalid or insufficiently large sample, iatrogenic damage to the eye (hyphaema, haemophthalmos, subretinal haemorrhage, retinal detachment, cataract, endophthalmitis) and the risk of intraocular or extraocular dissemination of the tumour [4,5,12]. Another problem may be intratumour heterogeneity, in which different parts of the lesion may manifest different histopathological and cytogenetic characteristics. An examination of a small sample of tissue may therefore produce misleading false negative results [25].
CONCLUSION
Uveal melanoma biopsy incorporates a whole range of techniques, which are constantly developing, together with knowledge of the pathogenesis of uveal melanoma. In clinical practice, biopsy may be significant in terms of diagnosis and prognosis. With the progressive improvement of non-invasive techniques of clinical diagnosis, biopsy for diagnostic purposes is reserved for select cases (clinically unclear lesions, small borderline lesions). On the other hand, in connection with constant improvements in systemic therapy, biopsy is increasing in importance for the purposes of cytogenetic analysis with determination of the risk of development of metastatic pathology and individualised therapy for a specific patient (Fig. 1 and 2). With reference to the limited size of the sampled material, close co-operation with an experienced histopathologist and cytogeneticist is essential.
Fig. 1. 30-year-old woman with advanced choroidal melanoma of the left eye, diagnosed in July 2010. After treatment with Leksell gamma knife in August 2010 the tumour progressed, and as a result enucleation of the eyeball was performed in October 2010. The histological (mixed type melanoma, pleomorphism) and cytogenetic (monosomy of chromosome 3 – positive, gain in chromosome 6p, i.e. iscochromosome 6p – negative, gain in chromosome 8q – negative) examination indicated an unfavourable prognosis. Within the framework of regular monitoring, metastatic affliction of the liver was determined in April 2014. Chemotherapy was commenced, due to progressing generalisation with multiple organ affliction biological therapy was also subsequently applied. In October 2014 the patient died from the consequences of generalisation of uveal melanoma.
A: Advanced choroidal melanoma of the left eye visible on MR image from July 2010.
B: Histological finding – solid alveolar type growth, tumorous proliferation of epitheloid and spindle-cell elements (formed by spindle-cells type B), isolated with deposits of brown melanin pigment.
C: Loss of chromosome 3 detected by FISH method with the use of a fluorescence probe VHL/CEN 3 (Zytovision, Germany). The locus of VHL (3p25.3) is indicated by green colour, the control centromeric area (CEN 3) is indicated by orange colour, the nuclei are tinged with blue DAPI. In normal nuclei with two chromosomes, 2 green and 2 orange signals are expected. Loss of chromosome 3 is visible in the image (only 1 green and 1 orange signal).
D: Metastasis in liver visible on PET/CT examination.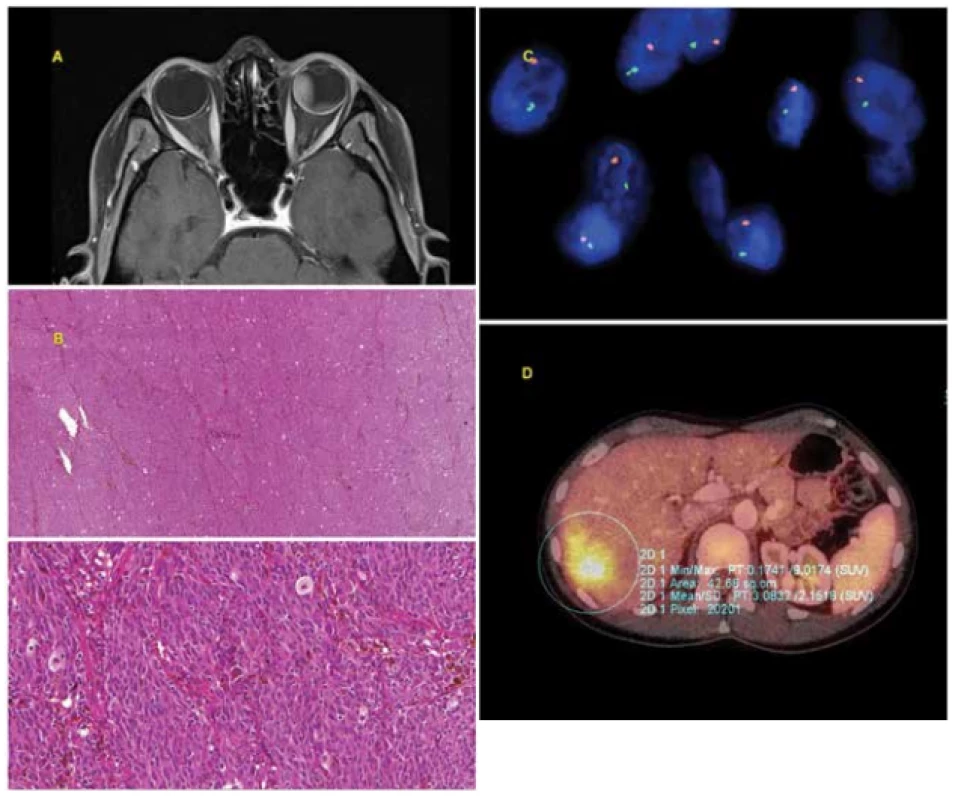
Fig. 2. 42-year-old man with advanced choroidal melanoma of the left eye, diagnosed in August 2009. In September 2009 he was treated with Leksell gamma knife, due to the size of the tumour with the risk of development of toxic tumour syndrome, exoresection of the tumour (sclerouvectotomy) was performed in October 2009. The cytogenetic examination was prognostically favourable (monosomy of chromosome 3 – negative, isochromosome 6p – positive, gain in chromosome 8q – negative). The patient is being regularly monitored (sonography of abdomen, X-ray of lungs, laboratory examination) – so far without signs of generalisation of the pathology.
A: Advanced choroidal melanoma of left eye visible on MR image from August 2009.
B: Histological finding – solid compact tumorous matter formed by spindle-cell elements and large quantity of melanin.
C: Loss in area of short arm of chromosome 6 (6q) together with gain of long arm (6p) detected by FISH method with the aid of fluorescence set Vysis Melanoma FISH Probe Kit (Vysis, Abbot Molecular, USA). The locus of MYB (6q23) is indicated by yellow colour, the locus of RREB1 (6p25) by red colour, the control centromeric region by light blue (aqua) colour, the nuclei are tinged blue by DAPI. In normal nuclei 2 red, 2 yellow and 2 aqua signals are expected. The loss of 1 copy of locus MYB (1 yellow signal) and gain of locus RREB1 (3 red signals) is visible in the image.
D: Finding on ocular fundus 10 years after uveal melanoma therapy (treatment by LGK, subsequent exoresection of tumour).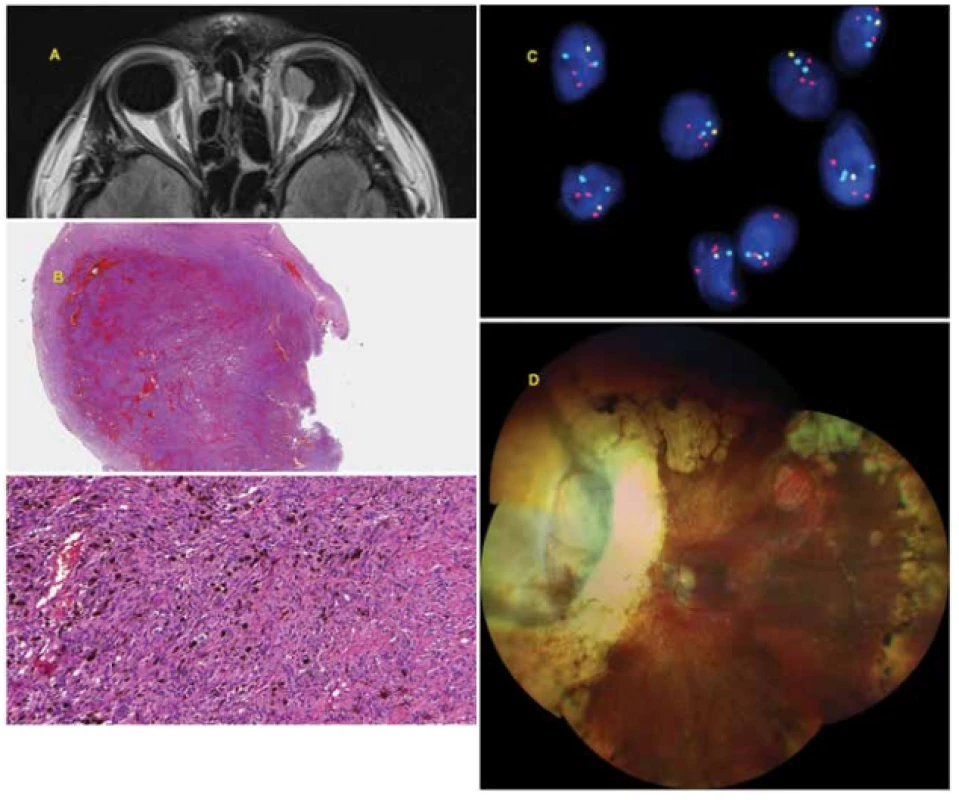
Zdroje
1. Kivelä T. Diagnosis of uveal melanoma. Dev Ophthalmol. 2012; 49 : 1–15.
2. Tarlan B, Kıratlı H. Uveal Melanoma: Current Trends in Diagnosis and Management. Turk J Ophthalmol. 2016 Jun;46(3):123 – 137.
3. Sayanagi K, Pelayes DE, Kaiser PK, Singh AD. 3D Spectral domain optical coherence tomography findings in choroidal tumors. Eur J Ophthalmol. 2011 May-Jun;21(3):271–275.
4. Frizziero L, Midena E, Trainiti S et al. Uveal Melanoma Biopsy: A Review. Cancers. 2019 Aug;11(8):1075.
5. Aronow ME, Biscotti CV, Chan CC, Singh AD. The Role of Biopsy in the Assessment of Uveal Melanoma. Retinal Physician, 2012 Jun; 9,13–17.
6. Boldt HC, Binkley E. Treating Small Choroidal Melanoma: Smaller Is Better. JAMA Ophthalmol. 2018 Dec;136(12):1333 – 1334.
7. The Collaborative Ocular Melanoma Study Group. Mortality in patients with small choroidal melanoma. COMS report no. 4. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol. 1997 Jul;115(7):886–893.
8. Singh AD. Uveal melanoma: implications of tumor doubling time. Ophthalmology. 2001 May;108(5):829–831.
9. Bagger MM. Intraocular biopsy of uveal melanoma Risk assessment and identification of genetic prognostic markers. Acta Ophthalmol. 2018 Jul;96 : 1–28.
10. Onken MD, Worley LA, Char DH et al. Collaborative Ocular Oncology Group report number 1: Prospective validation of a multigene prognostic assay in uveal melanoma. Ophthalmology. 2012 Aug;119(8):1596–1603.
11. Usui Y, Tsubota K, Agawa T, et al. Aqueous immune mediators in malignant uveal melanomas in comparison to benign pigmented intraocular tumors. Graefes Arch Clin Exp Ophthalmol. 2017 Feb;255(2):393–399.
12. Shields CL, Manquez ME, Ehya H, Mashayekhi A, Danzig CJ, Shields JA. Fine-needle aspiration biopsy of iris tumors in 100 consecutive cases: Technique and complications. Ophthalmology. 2006 Nov;113(11):2080–2086.
13. Singh AD, Biscotti CV. Fine needle aspiration biopsy of ophthalmic tumors. Saudi J Ophthalmol. 2012 Apr;26(2):117–123.
14. Bechrakis NE, Foerster MH, Bornfeld N. Biopsy in indeterminate intraocular tumors. Ophthalmology. 2002 Feb;109(2):235–242.
15. Klauber S, Jensen PK, Prause JU, Kessing SV. Surgical treatment of iris and ciliary body melanoma: follow-up of a 25-year series of patients. Acta Ophthalmol. 2012 Mar;90(2):122–126.
16. Finger PT, Milman T. Microincision, aspiration cutter-assisted multifocal iris biopsy for melanoma. Eur J Ophthalmol. 2017 Jan;27(1):62–66.
17. Shields CL, Materin MA, Teixeira L, Mashayekhi A, Ganguly A, Shields J.A. Small choroidal melanoma with chromosome 3 monosomy on fine-needle aspiration biopsy. Ophthalmology. 2007 Oct;114(10):1919–1924.
18. Singh AD, Medina CA, Singh N, Aronow ME, Biscotti CV, Triozzi PL. Fine-needle aspiration biopsy of uveal melanoma: Outcomes and complications. Br. J. Ophthalmol. 2016 Apr;100(4):456–462.
19. Midena E, Bonaldi L, Parrozzani R, Tebaldi E, Boccassini B, Vujosevic S. In vivo detection of monosomy 3 in eyes with medium-sized uveal melanoma using transscleral fine needle aspiration biopsy. Eur. J. Ophthalmol. 2006 May-Jun;16(3):422–425.
20. Rishi P, Dhami A, Biswas J. Biopsy techniques for intraocular tumors. Indian J Ophthalmol. 2016 Jun;64(6):415–421.
21. Tang PH, Shields RA, Schefler AC, Mruthyunjaya P. Biopsy of a Choroidal Melanoma Using Transvitreal Pars Plana Vitrectomy. Ophthalmic Surg Lasers Imaging Retin. 2018 Aug;49(8):645–647.
22. Hussain RN, Kalirai H, Groenewald C et al. Prognostic Biopsy of Choroidal Melanoma after Proton Beam Radiation Therapy. Ophthalmology. 2016 Oct;123(10):2264–2265.
23. Akgul H, Otterbach F, Bornfeld N, Jurklies B. Intraocular biopsy using special forceps: A new instrument and refined surgical technique. Br J Ophthalmol. 2011 Jan;95(1):79–82.
24. Seregard S, All-Ericsson C, Hjelmqvist L, Berglin L, Kvanta A. Diagnostic incisional biopsies in clinically indeterminate choroidal tumours. Eye. 2013 Feb;27(2):115–118.
25. Marigo FA, Finger PT. Anterior segment tumors: Current concepts and in
Štítky
Oftalmologie
Článek vyšel v časopiseČeská a slovenská oftalmologie
Nejčtenější tento týden
2020 Číslo 6- Stillova choroba: vzácné a závažné systémové onemocnění
- Familiární středomořská horečka
- První schválený léčivý přípravek pro terapii Leberovy hereditární optické neuropatie dostupný rovněž v ČR
- Selektivní laserová trabekuloplastika nesnižuje nitroční tlak více než argonová laserová trabekuloplastika
- Léčba chronické blefaritidy vyžaduje dlouhodobou péči
-
Všechny články tohoto čísla
- Uveal Melanoma Biopsy. A Review
- VISUAL FUNCTIONS AFTER IMPLANTATION OF ACRYSOF MONOFOCAL INTRAOCULAR LENSES.
- Evaluation of Patients Presenting to the Ophthalmology Department of a Tertiary Hospital for Nonemergency Reasons During the Covid-19 Pandemic
- Changed Eye Functions and Quality of Life of Seniors with Diabetic Retinopathy
- Can Visual Function Be Affected by an Open Foramen Ovale?
- Late Functional and Morphological Findings after Methylalcohol Poisoning
- Česká a slovenská oftalmologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Can Visual Function Be Affected by an Open Foramen Ovale?
- VISUAL FUNCTIONS AFTER IMPLANTATION OF ACRYSOF MONOFOCAL INTRAOCULAR LENSES.
- Late Functional and Morphological Findings after Methylalcohol Poisoning
- Uveal Melanoma Biopsy. A Review
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



