-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Medical Consequences of Chernobyl with Focus on the Endocrine System – Part 2
Zdravotní dopady černobylské katastrofy se zaměřením na endokrinní systém: část 2
Atomové katastrofy se udály v posledních 70 letech několikrát. Výbuch nukleárního zařízení v roce 1986 v severní částí střední Ukrajiny byl mimořádnou zkušeností proto, že radiační zátěž dopadla na všechny věkové kategorie populace. Následné studie pak přinesly velké množství informací o účinku záření na lidský organismus. Vzhledem k tomu, že se globální bezpečnost postupně zhoršuje, získávají znalosti o biologickém dopadu ionizujícího záření i preventivní opatření k omezeni jeho zhoubných účinků novy rozměr a týkají se nás všech. Tento přehled se zaměřuje na dlouhodobé důsledky černobylské katastrofy, zvláště pak na dopad na endokrinní systém u dětí a dospělých. Přehled zahrnuje souhrn preventivních opatření pro případ atomové katastrofy.
Klíčová slova:
černobylská atomová katastrofa – ionizující záření – endokrinní systém – štítná žláza – rakovina – mamma – těhotenství – děti
Authors: Thomas P. Foley 1; Zdeňka Límanová 2; Eliška Potluková 3
Authors place of work: Division of Endocrinology & Metabolism, School of Medicine, Graduate School of Public Health, University of Pittsburgh, Children’s Hospital of Pittsburgh, USA 1; Third Department of Medicine, First Faculty of Medicine, Charles University and General University Hospital in Prague, Czech Republic 2; Division of Medicine, University Hospital Basel, Switzerland 3
Published in the journal: Čas. Lék. čes. 2015; 154: 287-291
Category: Přehledový článek
Summary
In the last 70 years, atomic disasters have occurred several times. The nuclear power plant accident at Chernobyl in 1986 in North-Central Ukraine was a unique experience in population exposures to radiation by all ages, and ongoing studies have brought a large amount of information effects of radiation on human organism. Concerning the deteriorating global security situation and the strong rhetoric of some of the world leaders, the knowledge on the biological effects of ionizing radiation and the preventive measures designed to decrease the detrimental effects of radiation gains a new dimension, and involves all of us. This review focuses on the long-term effects of Chernobyl catastrophe especially on the endocrine system in children and in adults, and includes a summary of preventive measures in case of an atomic disaster.
Keywords:
Chernobyl atomic disaster – ionizing radiation – endocrine system – thyroid – cancer – breast – pregnancy – childrenDIABETES MELLITUS ASSOCIATED WITH RADIATION EXPOSURE
Residential survivors of the atomic bomb detonation at Hiroshima were found to have an increased incidence of Type 1 diabetes mellitus [Type 1 DM] (70). The average increase/incidence ratio of Type 1 DM in Gomel Region in the period 1987–1999 when compared to the period 1976–1986 was 8.9% (p < 0.001). The crude rate of incidence increased significantly after 1986 compared to an essentially a flat rate before 1987 (15). Subsequent studies have confirmed the incidence of Type 1 DM in children and adolescents after Chernobyl when compared to children and adolescents from 1980 to 1986 (72). Subsequent comments disagree with the conclusions of the study in Gomel, Belarus, as the study in northern Poland did not show an increase in Type I DM after Chernobyl (73). However, the latter opinion was countered by a summary of studies in humans, including atomic bomb survivors (71), and experimental animals (74). The authors recommend regular investigations for early markers of diabetes after exposure to radiation, especially in children (74).
However, in the offspring of women or men exposed to variable radiation doses from atomic bomb detonations, there is no evidence that exposure was associated with any increased risk for multifactorial diseases (75). One modification of this conclusion is the fact that the mean age of the study population is 48.6 years such that the incidence of multifactorial diseases may not be fully expressed until sometime in the future, necessitating continued evaluations of the study populations (75). There are several reports of associations between radiation exposure and breast cancer (13–14, 17, 19, 62, 67) and thyroid cancer (8–11).
Although the debate about the association between exposure to ionizing radiation from the Chernobyl accident and the subsequent development of diabetes mellitus in children and adults remains inconclusive, there is sufficient evidence that justifies continued monitoring of those individuals exposed during fetal life, infancy, childhood and adolescence for markers of subclinical and overt diabetes mellitus.
RELATIONSHIPS BETWEEN BREAST CANCER AND AUTOIMMUNE THYROID DISEASES
The association between thyroid disease and breast cancer has been known for more than six decades (46, 75–79) with contradictory results for positive and negative correlations. There are a variety of thyroid disorders, especially autoimmune thyroiditis among iodine-sufficient populations (78). Despite advances in the molecular studies of both diseases, there remains no common pathogenesis to link the associations (46, 75–79). Studies have shown more linkage between breast cancer and thyroid disorders in women of younger ages. Those women who are affected with either benign or malignant thyroid diseases are reported to have a greater risk of breast cancer. The effect of thyroid autoantibodies on the course of disease and overall survival remains controversial (79). The PTEN Hamartoma Tumor Syndrome (PHTS) is an interesting combination of syndromes that include benign and malignant tumors of the thyroid, breast and endometrium (80). The lifetime risk of breast cancer is 85%, and usually develops between ages 38 and 46 years. There seems to be no association of breast or thyroid cancer with pre-natal or post-natal exposure to ionizing radiation. The diagnosis is based on the presence of the pathogenic variants of the PTEN gene (80).
PREVENTIVE MEASURES IN CASE OF AN ATOMIC DISASTER
Prevention of radiation-induced endocrine diseases is directed primarily toward evacuation from the contaminated environment and using inorganic iodine as potassium iodide* (KI) treatment to block radioiodine exposure to tissues from the radioisotopes of iodine that cause external and internal radiation exposure (1–5, 58–59, 63, 65), and to a lesser extent, external radiation from 137Cs and 60Co-cobalt. The greater radioiodine dose to the thyroid of a child and the biological factors that influence the increased sensitivity of the thyroid to radiation increase the risks of thyroid diseases in children (5, 61). The fetus in utero, neonates, and young infants under age 1 year who are breast fed are among the most sensitive of any population exposed to radiation and, therefore, have the greatest risk for thyroid carcinoma (5, 59–60).
Similar observations are reported as relevant to the hyperplastic state of the female breast at the time of exposure to radioiodine. The highest risk for breast cancer would be expected to occur in utero, during transient neonatal breast hyperplasia in male and female neonates, and in females during puberty, pregnancy and lactation (5, 13–14, 62–63).
With these observations in mind, the highest priority of management for those living within 100 km of the radiation source is an immediate evacuation of pregnant women and children. The safe distance would be at least 100 km, and vehicles with women and children should have an external identification such as a banner on an antenna or sticker on the front of the vehicle. Predetermined priority evacuation routes should have been established so that no priority vehicle is delayed.
At the same time when the news of evacuation is broadcast, individuals of all ages should take their initial dose of stable iodine as recommended by radiation health authorities (Table 2). The use of oral stable iodine to block radioiodine uptake by sensitive tissues was reported from the iodine prophylaxis program in Poland within hours after the Chernobyl accident (64) and remains the most important early mode of preventive medicine, especially in children whose benefit has been confirmed (64). The administration of potassium iodide is recommended if the estimated dose to the thyroid is 100 mGy or higher. Iodine in the form of KI or SSKI* (Saturated Solution of KI and Lugol’s Iodine*) should be administered early (within 36 hours of radioiodine release) to reduce the radiation dose to the thyroid and breast. The potassium Iodide (KI) dose varies with age and exposure (Table 2). KI causes a ~ 40% reduction in rem dose to the thyroid and with early prophylaxis there is a ~ 60–70% reduction in the rem dose primarily because inhaled 131I is blocked (1, 65). In some countries, e.g. Switzerland, potassium iodide has been regularly distributed among the general population with detailed instruction how to use it in case of an atomic disaster.
There is an incidence of 0.2% for medically significant side effects, but no serious side effects were observed although KI toxicity has been reported as very rare cases of iodinism (64, 66). For exposure to the radioiodines, evacuation and KI are the top priority, and the dose and duration of therapy should be based on the advice of physicians (Table 2). If persistent exposure to radioiodines occurs and more than a single dose of KI is advised, infants and young children should be monitored with TSH levels to assure that iodine-induced hypothyroidism does not develop. L-thyroxine therapy should be initiated when TSH levels are elevated so that hypothyroidism does not persist and iodine therapy can be discontinued.
Tab. 1. Guidelines for Administration of Potassium Iodide* 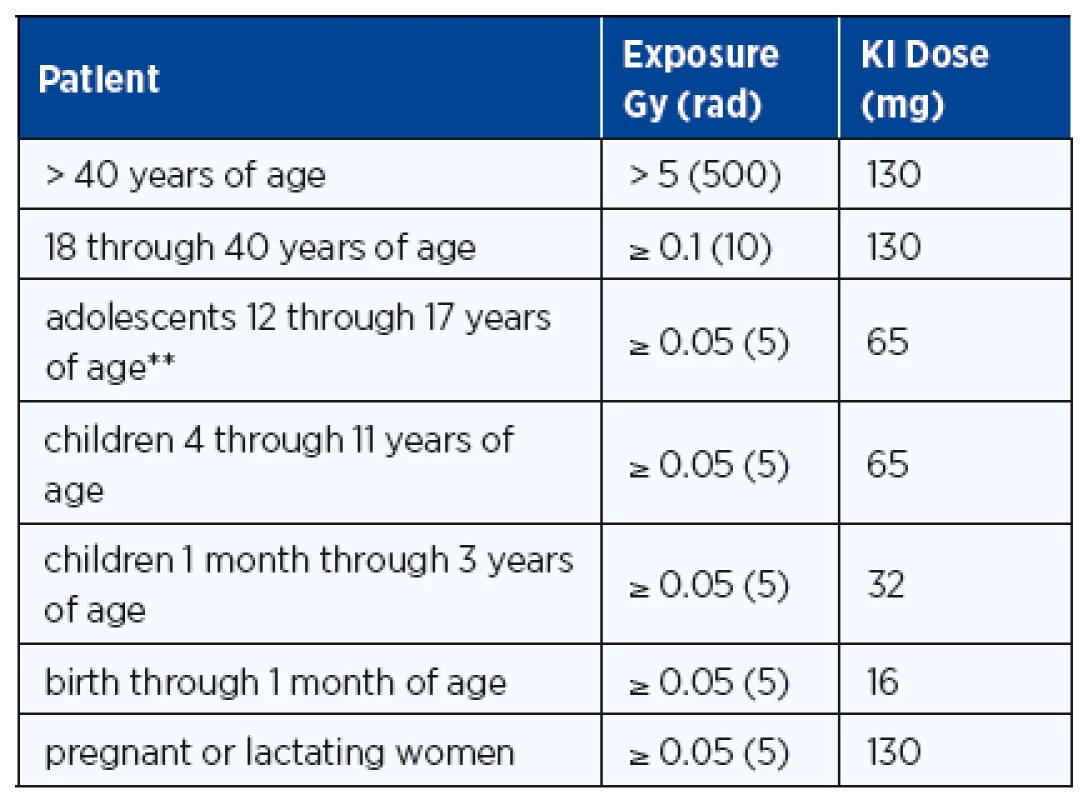
* Lugol’s Iodine (5% solution) contains 126.5 mg iodine in one ml. ** adolescents weighing more than 70 kg should receive the adult dose (130 mg) Source: www.fda.gov/downloads/Drugs/.../Guidances/ucm080549.pdf Exposure from other radionuclides is not affected by KI, and for proper clinical management, the assessment of the magnitude of radiation exposure is very important (Table 3).
Tab. 2. Guidelines for Administration of Potassium Iodide* 
* A radiation safety officer or other authority should be consulted in all aspects of management. Adapted from Jarrett DG. Medical Management of Radiological Casualties. Bethesda, MD: Armed Forces Radiobiology Research Institute; 1999. Source: Ref. # 1. General measures for reduction of radioactivity exposure in case of a nuclear disaster are crucial and international guidelines have been developed accordingly (4, 17). These actions include controlling access to the site, recommending people to stay indoors or evacuating them, removing contaminated clothing, providing respiratory protection, administering potassium iodide, restricting certain foods, and decontaminating property. People should stay indoors for up to two days if a dose of 1 rem is likely to be reached, with evacuation for up to one week if the likely dose is 5 rem or higher (4).
Temporary relocation is recommended at a likely dose of 3 rem in the first month or 1 rem in a subsequent month. Permanent resettlement is recommended if the lifetime dose is expected to be 100 rem (4).
CONCLUSIONS
In summary, the thyroid gland is one of the most vulnerable organs during a nuclear disaster. The youngest are the most susceptible and have the highest risk for thyroid carcinogenesis, transient thyroiditis and primary hypothyroidism. Radioactive iodine and cesium are volatile and thus readily inhaled and absorbed from nutrition. The exposition of the thyroid is potentiated through iodine deficiency, leading to an increased accumulation of radioactive iodine in the thyroid gland.
The Chernobyl catastrophe has ongoing effects not only on the thyroid, but probably also on other organs (breast, pancreas-islet cells). These long-term effects are subtle when regarding an individual, but become epidemiologically important when whole populations are analyzed. Further studies are needed to evaluate the late effects of the Chernobyl catastrophe especially on the thyroid and breast tissues.
The incidence of breast cancer among women exposed to the higher doses of radiation could be greater than currently considered. Genetic susceptibility data, frequent monitoring of women at increased risk and fiscal support educational programs are essential for the coming decades in order to detect breast cancer at the very earliest stage. Recommendation to regular investigations for early markers of diabetes mellitus after exposure to radiation, especially in children, should be taken to an account as well.
Concerning the number of atomic power plants worldwide and the deteriorating security situation with continuing risk of terrorist attacks it is important for everybody to be aware of the preventive measures in case of another atomic disaster.
ADRESA PRO KORESPONDENCI:
Eliška Potluková, MD, Ph.D.
Division of Medicine
University Hospital Basel
Petersgraben 4, 4056 Basel, Switzerland
e-mail: eliska.potlukova@usb.ch
Zdroje
1. American Academy of Pediatrics Committee on Environmental Health. Radiation disasters and children. Pediatrics 2003; 111 : 1455–1466.
2. Burnham JW, et al. Radiation. Review. Crit Care Clin 2005; 21 : 785–813.
3. Leikin JB, et al. A primer for nuclear terrorism. Dis Mon 2003; 49 : 485–516.
4. Mettler FA Jr, et al. Major radiation exposure – what to expect and how to respond. Review. N Engl J Med 2002; 346 : 1554–1561.
5. Williams D. Radiation carcinogenesis: lessons from Chernobyl. Review. Oncogene 2009; 27: S9–18.
6. Hatch M, et al. The Chernobyl disaster: cancer following the accident at the Chernobyl nuclear power plant. Review. Epidemiol Rev 2005; 27 : 56–66.
7. Little JB. Radiation carcinogenesis. Review. Carcinogenesis 2000; 21 : 397–404.
8. Cardis E, et al. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst 2005; 97 : 724–732.
9. Boice JD, Jr. Radiation-induced thyroid cancer – what’s new? Editorial. J Natl Cancer Inst 2005; 97 : 703–705.
10. Caudill CM, et al. Dose-dependent generation of RET/PTC in human thyroid cells after in vitro exposure to gamma-radiation: a model of carcinogenic chromosomal rearrangement induced by ionizing radiation. J Clin Endocrinol Metab 2005; 90 : 2364–2369.
11. Pacini F, et al. Thyroid consequences of the Chernobyl nuclear accident. Acta Paediatr Suppl 1999; 88; 23–27.
12. Pacini F, et al. Prevalence of thyroid autoantibodies in children and adolescents from Belarus exposed to the Chernobyl radioactive fallout. Lancet 1998; 352 : 763–766.
13. Ogrodnik A, et al. Radiation exposure and breast cancer: lessons from Chernobyl. Review. Conn Med 2013; 77 : 227–234.
14. Ronckers CM, et al. Radiation and breast cancer: a review of current evidence. Breast Cancer Res 2005; 7 : 21–32.
15. Martinucci ME, et al. Incidence of childhood type 1 diabetes mellitus in Gomel, Belarus. J Pediatr Endocrinol Metab 2002; 15 : 53–57.
16. Time: Disasters that Shook the World. New York City: Time Home Entertainment 2012.
17. Cardis E, et al. Cancer consequences of the Chernobyl accident: 20 years on. J Radiol Prot 2006; 26 : 127–140.
18. Jacob P, et al. Thyroid cancer risk in areas of Ukraine and Belarus affected by the Chernobyl accident. Radiat Res 2006; 165 : 1–8.
19. Land CE, et al. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950–1990. Radiat Res 2003; 160 : 707–717.
20. Kim IG, et al. Radiation-induced tumorigenesis. Review. J Biochem Mol Biol 2003; 36 : 144–148.
21. Calaf GM, et al. Ionizing radiation induces alterations in cellular proliferation and c-myc, c-jun and c-fos protein expression in breast epithelial cells. Int J Oncol 2004; 25 : 1859–1866.
22. Smith TR, et al. DNA damage and breast cancer risk. Carcinogenesis 2003; 24 : 883–889.
23. Thompson LH, et al. Recombinational DNA repair and human disease. Review. Mutat Res 2002; 509 : 49–78.
24. Hu JJ, et al. Genetic regulation of ionizing radiation sensitivity and breast cancer risk. Environ Mol Mutagen 2002; 39 : 208–215.
25. Pierotti MA, et al. Cytogenetics and molecular genetics of carcinomas arising from thyroid epithelial follicular cells. Genes Chromosomes Cancer 1996; 16 : 1–14.
26. Santora M, et al. Molecular defects in thyroid carcinomas: role of the RET oncogene in thyroid neoplastic transformation. Eur J Endocrinol 1995; 133 : 513–522
27. Basolo F, et al. Potent mitogenicity of the RET/PTC3 oncogene correlates with its prevalence in tall-cell variant of papillary thyroid carcinoma. Am J Pathol 2002; 160 : 247–254.
28. Nikiforov YE, et al. Distinct pattern of RET oncogene rearrangements in morphological variants of radiation induced and sporadic thyroid papillary carcinomas in children. Cancer Res 1997; 57 : 1690–1694.
29. Thomas GA, et al. High prevalence of RET-PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET-PTC3 and the solid follicular variant. J Clin Endocrinol Metab 1999; 84 : 4232–4238.
30. Santoro M, et al. Gene rearrangement and Chernobyl related thyroid cancers. Br J Cancer 2000; 82 : 315–322.
31. Maenhaut C, et al. Gene expression profiles for radiation-induced thyroid cancer. Clin Oncol (R Coll Radiol) 2011; 23 : 282–288.
32. Hall EJ. Lessons we have learned from our children: cancer risks from diagnostic radiology. Pediatr Radiol 2002; 32 : 700–706.
33. Ernst M, et al. Health hazards of radiation exposure in the context of brain imaging research: special consideration for children. J Nucl Med 1998; 39 : 689–698.
34. Ron E, et al. Benign and malignant thyroid neoplasms after childhood irradiation for tinea capitis. J Natl Cancer Inst 1980; 65 : 7–11.
35. Lundell M, et al. Mortality from leukaemia after irradiation in infancy for skin haemangioma. Radiat Res 1996; 145 : 595–601.
36. Auvinen A, et al. Fallout from Chernobyl and incidence of childhood leukaemia in Finland. Br Med J 1994; 309 : 151–154.
37. Hjalmars U, et al. Risk of acute childhood leukaemia in Sweden after the Chernobyl reactor accident. Br Med J 1994; 309 : 154–157.
38. Rallison M, et al. Thyroid nodularity in children. JAMA 1975; 233 : 1069–1072.
39. Holm L, et al. Thyroid cancer after diagnostic doses of iodine-131: a retrospective cohort study. Natl Cancer Inst 1988; 80 : 1132–1138.
40. Amsel J, et al. Relationship of site-specific cancer mortality rates to altitude. Carcinogenesis 1982; 3 : 461–465.
41. Frigerio NA, et al. Carcinogenic and genetic hazard from background radiation. In: Biological and environmental effects of low-level radiation. Vienna: International Atomic Energy Agency 1976.
42. Blot W, et al. Indoor radon and lung cancer in China. J Natl Cancer Inst 1990; 82 : 1025–1030.
43. Saenger EL, et al. Incidence of leukaemia following treatment of hyperthyroidism. JAMA 1968; 205 : 855–862.
44. Hall P, et al. Leukaemia incidence after 131I exposure. Lancet 1992; 340 : 1–4.
45. Foley TP Jr., Charron M, Linkov F, Krenzelok EP. Radiation Terrorism. In Lifshitz F (ed.) Pediatric Endocrinology, ed 5. New York: Informa Healthcare, Inc., 2007, Volume 2, Section VI: Special Considerations and Resources, Chapter 31; 705 –727.
46. Muller I, et al. Does thyroid peroxidase provide an antigenic link between thyroid autoimmunity and breast cancer? Int J Cancer 2014; 134 : 1706–1714.
47. Portulano C, et al. The Na+/I– symporter (NIS): mechanism and medical impact. Review. Endocr Rev 2014; 35 : 106–149.
48. Brenner AV, et al. I-131 dose response for incident thyroid cancers in Ukraine related to the Chernobyl accident. Environ Health Perspect 2011; 119 : 933–939.
49. Ostroumova E, et al. Measures of thyroid function among belarusian children and adolescents exposed to iodine-131 from the accident at the Chernobyl nuclear plant. Environ Health Perspect 2013; 121 : 865–871.
50. Tronko MD, et al. Autoimmune thyroiditis and exposure to iodine 131 in the Ukrainian cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: results from the first screening cycle (1998–2000). J Clin Endocrinol Metab 2006; 91 : 4344–4351.
51. Hatch M, et al. Prevalence of hyperthyroidism after exposure during childhood or adolescence to radioiodines from the Chornobyl nuclear accident: dose-response results from the Ukrainian American cohort study. Radiat Res 2010; 174 : 763–772.
52. Kazakov VS, et al. Thyroid cancer after Chernobyl. Nature 1992; 359 : 21.
53. Baverstock K, et al. Thyroid cancer after Chernobyl. Nature 1992; 359 : 21–22.
54. Evdokimova V, et al. Formation of carcinogenic chromosomal rearrangements in human thyroid cells after induction of double-strand DNA breaks by restriction endonucleases. Endocr Relat Cancer 2012; 19 : 271–281.
55. Jargin SV. On the RET rearrangements in Chernobyl-related thyroid cancer. J Thyroid Res 2012; 2012 : 373879.
56. Agate L, et al. Thyroid autoantibodies and thyroid function in subjects exposed to Chernobyl fallout during childhood: evidence for a transient radiation-induced elevation of serum thyroid antibodies without an increase in thyroid autoimmune disease. J Clin Endocrinol Metab 2008; 93 : 2729–2736.
57. McConnell RJ, et al. Factors associated with elevated serum concentrations of anti-TPO antibodies in subjects with and without diffuse goitre. Results from the Ukrainian-American Cohort Study of thyroid cancer and other thyroid diseases following the Chornobyl accident. Clin Endocrinol (Oxf) 2007; 67 : 879–890.
58. Schneider AB, et al. Potassium iodide prophylaxis: what have we learned and questions raised by the accident at the Fukushima Daiichi Nuclear Power Plant. Thyroid 2012; 22 : 344–346.
59. Reiners C, et al. Potassium iodide (KI) to block the thyroid from exposure to I-131: current questions and answers to be discussed. Radiat Environ Biophys 2013; 52 : 189–193.
60. Williams ED. Radiation-induced thyroid cancer. Histopathology 1993; 23 : 387–389.
61. Travis CC, et al. 131I ablation treatment in young females after the Chernobyl accident. J Nucl Med 2006; 47 : 1723–1727. Erratum in: J Nucl Med 2007; 48 : 7.
62. Pukkala E, et al. Breast cancer in Belarus and Ukraine after the Chernobyl accident. Int J Cancer 2006; 119 : 651–658.
63. Nauman J, et al. Iodine prophylaxis in Poland after the Chernobyl Reactor Accident: Benefits and risks. Am J Med 1993; 94 : 524–532.
64. Lincoln TA. Importance of initial management of persons internally contaminated with radionuclides. Am Ind Hyg Assoc J 1976; 37 : 16–21.
65. Law RK, et al. National surveillance for radiological exposures and intentional potassium iodide and iodine product ingestions in the United States associated with the 2011 Japan radiological incident. Clin Toxicol (Phila) 2013; 51 : 41–46.
66. Hatch MC, et al. Cancer near the Three Mile Island nuclear plant: radiation emissions. Am J Epidemiol 1990; 132 : 397–412; discussion 413–417.
67. Dardynskaia I, et al. Breast cancer trends in two oblasts of Belarus and the Chernobyl accident. Int J Occup Environ Health 2006; 12 : 415–422.
68. Okeanov AE, et al. National cancer registry to assess trends after the Chernobyl accident. Swiss Med Wkly 2004; 134 : 645–649.
69. Copson E, et al. Prospective Observational Study of Breast Cancer Treatment Outcomes for UK Women Aged 18–40 Years at Diagnosis: The POSH Study. J Natl Cancer Inst 2013; 105 : 978–988.
70. Ito C. Trends in the prevalence of diabetes mellitus among Hiroshima atomic bomb survivors. Diabetes Res Clin Pract 1994; 24: S29–S35.
71. Zalutskaya A, et al. Did the Chernobyl incident cause an increase in Type 1 diabetes mellitus incidence in children and adolescents? Diabetologia 2004; 47 : 147–148.
72. Bandurska-Stankiewicz E, et al. In Zalutskaya A, et al. (2003) Did the Chernobyl incident cause an increase in type 1 diabetes mellitus incidence in children and adolescents? Diabetologia 2004; 47 : 147–148.
73. Lorini R, et al. Comment to: Zalutskaya A, et al. (2004) Did the Chernobyl incident cause an increase in type 1 diabetes mellitus incidence in children and adolescents? Diabetologia 47 : 147–148 (Letter). Diabetologia 2005; 48 : 2193–2194.
74. Tatsukawa Y, et al. Radiation risk of individual multifactorial diseases in offspring of the atomic-bomb survivors: a clinical health study. J Radiol Prot 2013; 33 : 281–293.
75. Moossa AR, et al. Thyroid status and breast cancer. Reappraisal of an old relationship. Ann R Coll Surg Engl 1973; 53 : 178–188.
76. Ito K, et al. Breast cancer in patients with Hashimoto’s thyroiditis. Lancet 1975; 2 : 1119–1121.
77. Turken O, et al. Breast cancer in association with thyroid disorders. Breast Cancer Res 2003; 5: R110–113.
78. Prinzi N, et al. Prevalence of breast cancer in thyroid diseases: results of a cross-sectional study of 3,921 patients. Breast Cancer Res Treat 2014; 144 : 683–688.
79. Jiskra J, et al. Thyroid autoimmunity occurs more frequently in women with breast cancer compared to women with colorectal cancer and controls but it has no impact on relapse-free and overall survival. Oncol Rep 2007; 18 : 1603–1611.
80. Eng C. PTEN Hamartoma Tumor Syndrome (PHTS). In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. GeneReviews® (Internet). Seattle (WA): University of Washington, Seattle; 1993–2014. 2001 Nov 29 (updated 2014 Jan 23).
Štítky
Adiktologie Alergologie a imunologie Angiologie Audiologie a foniatrie Biochemie Dermatologie Dětská gastroenterologie Dětská chirurgie Dětská kardiologie Dětská neurologie Dětská otorinolaryngologie Dětská psychiatrie Dětská revmatologie Diabetologie Farmacie Chirurgie cévní Algeziologie Dentální hygienistka
Článek ÚvodníkČlánek Chronic heart failureČlánek Ladislav Syllaba (1868–1930)Článek GEORGE DAVIS SNELLČlánek Spolek českých lékařůČlánek Mountain sicknessČlánek Diabetologie 2015Článek JubilantiČlánek Rejstříky
Článek vyšel v časopiseČasopis lékařů českých
Nejčtenější tento týden
- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Horní limit denní dávky vitaminu D: Jaké množství je ještě bezpečné?
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- Úvodník
- Chronic heart failure
- Study PARADIGM-HF – a paradigm shift in the treatment of chronic heart failure
- Non-cardiogenic pulmonary edema, acute respiratory distress syndrome
- Mountain sickness
- Medical Consequences of Chernobyl with Focus on the Endocrine System – Part 2
- Diabetologie 2015
- Analysis of the relationship of heavy/light chain pairs of immunoglobulin (Hevylite™) to the results of gel electrophoresis and nefelometric examination of serum proteins at the time of multiple myeloma diagnosis
- Ladislav Syllaba (1868–1930)
- GEORGE DAVIS SNELL
- Komentář k práci Výplach žaludku při perorální intoxikaci – sporné pohledy na problematiku autorů Večeřa R, Ondra P, Jezdinský J, Adamus M. (Čas. Lék. čes. 2015; 154(4): 174–175)
- Nové složení výborů odborných společností a spolků lékařů ČLS JEP – 2015
- Elektronické zdravotnictví nelze zavádět bez účasti lékařů
- Tradiční setkání spolků lékařů
- Spolek českých lékařů
- Plánované akce složek ČLS JEP
- Doc. MUDr. Milan Špála, CSc. – 85 let (*20. listopadu 1930)
- Jubilanti
- Rejstříky
- Časopis lékařů českých
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Mountain sickness
- Non-cardiogenic pulmonary edema, acute respiratory distress syndrome
- Chronic heart failure
- Analysis of the relationship of heavy/light chain pairs of immunoglobulin (Hevylite™) to the results of gel electrophoresis and nefelometric examination of serum proteins at the time of multiple myeloma diagnosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



