-
Články
- Vzdělávání
- Časopisy
Top články
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Virus Control Goes Epigenetic
article has not abstract
Published in the journal: . PLoS Pathog 10(9): e32767. doi:10.1371/journal.ppat.1004370
Category: Viewpoints
doi: https://doi.org/10.1371/journal.ppat.1004370Summary
article has not abstract
Cells have developed a number of mechanisms to detect and suppress microbial infections. The use of pattern recognition receptors (PRRs) is one such mechanism. PRRs may be expressed on the cell surface, in the cytosol, or in the endosomes, to detect pathogen-associated molecular patterns (PAMPs) or the danger signals released by damaged cells (i.e., damage-associated molecular patterns; DAMPs). PRR activation triggers a signaling cascade, resulting in the production of antimicrobial cytokines, including type I interferons. These cytokines will in turn induce the expression of genes, such as interferon-stimulated genes (ISGs), that exhibit antimicrobial activities. In addition to PRRs, autophagy is another intracellular defense mechanism. Autophagy is used by the cells to remove intracellular pathogens in a process known as xenophagy [1]. Many viruses have developed different mechanisms to suppress these innate immune responses for their survival. A notable example is the cleavage of the mitochondrial antiviral signaling protein (MAVS) by the hepatitis C virus (HCV) NS3 protease [2]. MAVS is an important downstream adaptor molecule of RIG-I, which is a cytosolic PRR that can be activated by the HCV genomic RNA. Another example is the influenza virus NS1 protein, which binds to the E3 ubiquitin ligase TRIM25 to prevent it from activating RIG-I [3]. Similarly, the autophagy pathway may also be suppressed by viruses or even exploited by viruses to benefit their own replication. The former is typified by the herpes simplex virus-1 (HSV-1) ICP34.5 protein, which binds to Beclin-1 to prevent it from binding to and activating the class III phosphatidylinositol-3-kinase, an enzyme important for the initiation of autophagy. An example of the latter is HCV, which uses autophagy to enhance its replication [4].
In the report by Ducroux et al. [5], the authors presented yet another type of cellular control of viral infections. By using a tandem-affinity purification approach, the authors identified Spindlin1 as a binding partner of the hepatitis B virus (HBV) X protein (HBx). They subsequently discovered that Spindlin1 could suppress the replication of HBV, as its overexpression reduced the levels of HBV RNA and DNA replicative intermediates. On the contrary, depletion of Spindlin1 by RNA interference enhanced HBV replication. They then demonstrated that the suppressive effect of Spindlin1 on HBV was at the transcriptional level, as the overexpression of Spindlin1 reduced the transcriptional activity of HBV DNA by 80% in a nuclear run-on experiment. This effect of Spindlin1 on HBV was specific, as it was not observed with the cellular gene cyclin A2.
Spindlin1 contains three Tudor-like domains and can bind to trimethylated lysine 4 (H3K4me3) and asymmetric dimethylated arginine 8 (H3R8me2a) of histone H3 [6]. In the chromatin immunoprecipitation (ChIP) assay, Ducroux et al. demonstrated that Spindlin1 could bind to the HBV covalently closed circular DNA (cccDNA) genome. Interestingly, this binding was enhanced if the expression of HBx from the viral genome was abolished, suggesting an inhibitory role of HBx in the binding of Spindlin1 to the HBV genome. It does not appear likely that Spindlin1 binds to HBV cccDNA via H3K4me3, though, as the depletion of Spindlin1 actually led to a significant increase of the level of H3K4me3 in the HBV cccDNA. Their results suggested that Spindlin1, by binding to the HBV cccDNA, inhibits this post-translational modification of histone H3, which is associated with actively transcribed genes (Figure 1) [7].
Fig. 1. Model for epigenetic regulation of HBV gene expression by Spindlin1. 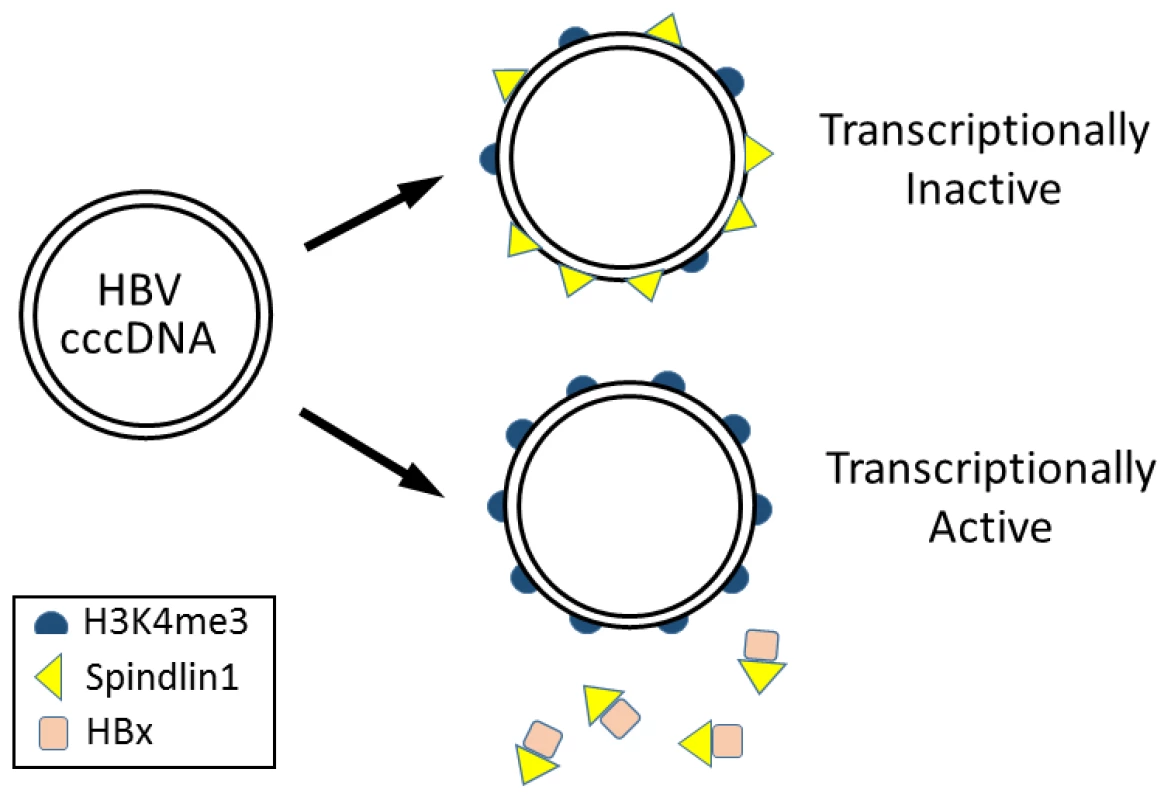
HBV cccDNA in the nucleus is associated with nucleosomes, which may be modified by trimethylation at lysine 4 of histone H3 (H3K4me3). Spindlin1 binds the HBV cccDNA to reduce the H3K4me3 level, resulting in the suppression of HBV gene expression. In the presence of HBx, Spindlin1 is sequestered from the cccDNA and the level of H3K4me3 increases, leading to the activation of HBV gene expression. A similar suppressive effect of Spindlin1 on the transcription of HSV-1 RNA was also observed by the authors. They found that the depletion of Spindlin1 also increased the ICP27 RNA level of HSV-1. In contrast, Spindlin1 had no effect on the replication of HCV, an RNA virus. Their results raise the possibility that Spindlin1, either by itself or by interacting with an unknown partner or partners, may represent a new class of PRRs that detect and suppress gene expression of DNA viruses by reducing the H3K4me3 level on the viral genome. If Spindlin1 is indeed a new PRR, then it will be interesting to determine how Spindlin1 differentiates episomal viral DNA from self DNA and what PAMPs it recognizes on the viral DNA. In their studies on HSV-1, Ducroux et al. did not demonstrate whether Spindlin1 could indeed reduce the H3K4me3 level associated with the HSV-1 genome, which must be demonstrated if the ability of Spindlin1 to exert epigenetic control on gene expression of DNA viruses is to be generalized. It will also be interesting in the future to determine whether Spindlin1 exhibits the same suppressive effect on other DNA viruses such as papillomaviruses and polyomaviruses and whether these DNA viruses also express gene products that can antagonize the suppressive effect of Spindlin1.
Zdroje
1. DongX, LevineB (2013) Autophagy and viruses: adversaries or allies? J Innate Immun 5 : 480–493.
2. HornerSM, GaleMJr (2013) Regulation of hepatic innate immunity by hepatitis C virus. Nat Med 19 : 879–888.
3. GackMU, AlbrechtRA, UranoT, InnKS, HuangIC, et al. (2009) Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5 : 439–449.
4. SirD, OuJH (2010) Autophagy in viral replication and pathogenesis. Mol Cells 29 : 1–7.
5. DucrouxA, BenhendaS, RiviereL, SemmesOJ, BenkiraneM, et al. (2014) The Tudor domain protein Spindlin1 is involved in intrinsic antiviral defense against incoming hepatitis B virus and herpes simplex virus type 1. PLoS Pathog 10: e1004343.
6. SuX, ZhuG, DingX, LeeSY, DouY, et al. (2014) Molecular basis underlying histone H3 lysine-arginine methylation pattern readout by Spin/Ssty repeats of Spindlin1. Genes Dev 28 : 622–636.
7. Santos-RosaH, SchneiderR, BannisterAJ, SherriffJ, BernsteinBE, et al. (2002) Active genes are tri-methylated at K4 of histone H3. Nature 419 : 407–411.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell ResponsesČlánek RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct MechanismsČlánek Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
-
Všechny články tohoto čísla
- Virus Control Goes Epigenetic
- The Role of Iron in Prion Disease and Other Neurodegenerative Diseases
- The Ins and Outs of Rust Haustoria
- Prion Strains and Amyloid Polymorphism Influence Phenotypic Variation
- Teaching Fido New ModiFICation Tricks
- Can Enhance Infection in Mosquitoes: Implications for Malaria Control?
- MIF Contributes to Associated Immunopathogenicity Development
- Persistence of Virus Reservoirs in ART-Treated SHIV-Infected Rhesus Macaques after Autologous Hematopoietic Stem Cell Transplant
- Bacillus Calmette-Guerin Infection in NADPH Oxidase Deficiency: Defective Mycobacterial Sequestration and Granuloma Formation
- EhCoactosin Stabilizes Actin Filaments in the Protist Parasite
- Molecular Insights Into the Evolutionary Pathway of O1 Atypical El Tor Variants
- LprG-Mediated Surface Expression of Lipoarabinomannan Is Essential for Virulence of
- Structural Correlates of Rotavirus Cell Entry
- Multivalent Adhesion Molecule 7 Clusters Act as Signaling Platform for Host Cellular GTPase Activation and Facilitate Epithelial Barrier Dysfunction
- The Effects of Vaccination and Immunity on Bacterial Infection Dynamics
- Myeloid Derived Hypoxia Inducible Factor 1-alpha Is Required for Protection against Pulmonary Infection
- Functional Characterisation of Germinant Receptors in and Presents Novel Insights into Spore Germination Systems
- Global Analysis of Neutrophil Responses to Reveals a Self-Propagating Inflammatory Program
- Host Cell Invasion by Apicomplexan Parasites: The Junction Conundrum
- Comparative Phenotypic Analysis of the Major Fungal Pathogens and
- Unravelling the Multiple Functions of the Architecturally Intricate β-galactosidase, BgaA
- Sialylation of Prion Protein Controls the Rate of Prion Amplification, the Cross-Species Barrier, the Ratio of PrP Glycoform and Prion Infectivity
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- Ontogeny of Recognition Specificity and Functionality for the Broadly Neutralizing Anti-HIV Antibody 4E10
- Identification and Characterisation of a Hyper-Variable Apoplastic Effector Gene Family of the Potato Cyst Nematodes
- Crimean-Congo Hemorrhagic Fever Virus Entry into Host Cells Occurs through the Multivesicular Body and Requires ESCRT Regulators
- Age-Dependent Enterocyte Invasion and Microcolony Formation by
- CD160-Associated CD8 T-Cell Functional Impairment Is Independent of PD-1 Expression
- Functional Fluorescent Protein Insertions in Herpes Simplex Virus gB Report on gB Conformation before and after Execution of Membrane Fusion
- The Tudor Domain Protein Spindlin1 Is Involved in Intrinsic Antiviral Defense against Incoming Hepatitis B Virus and Herpes Simplex Virus Type 1
- Transgenic Analysis of the MAP Kinase MPK10 Reveals an Auto-inhibitory Mechanism Crucial for Stage-Regulated Activity and Parasite Viability
- Evidence for a Transketolase-Mediated Metabolic Checkpoint Governing Biotrophic Growth in Rice Cells by the Blast Fungus
- Incomplete Deletion of IL-4Rα by LysM Reveals Distinct Subsets of M2 Macrophages Controlling Inflammation and Fibrosis in Chronic Schistosomiasis
- Identification and Functional Expression of a Glutamate- and Avermectin-Gated Chloride Channel from , a Southern Hemisphere Sea Louse Affecting Farmed Fish
- Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell Responses
- Strong Epistatic Selection on the RNA Secondary Structure of HIV
- Hematopoietic but Not Endothelial Cell MyD88 Contributes to Host Defense during Gram-negative Pneumonia Derived Sepsis
- Delineation of Interfaces on Human Alpha-Defensins Critical for Human Adenovirus and Human Papillomavirus Inhibition
- Exploitation of Reporter Strains to Probe the Impact of Vaccination at Sites of Infection
- RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct Mechanisms
- Helminth Infections Coincident with Active Pulmonary Tuberculosis Inhibit Mono- and Multifunctional CD4 and CD8 T Cell Responses in a Process Dependent on IL-10
- MHC Class II Restricted Innate-Like Double Negative T Cells Contribute to Optimal Primary and Secondary Immunity to
- Reactive Oxygen Species Regulate Caspase-11 Expression and Activation of the Non-canonical NLRP3 Inflammasome during Enteric Pathogen Infection
- Evolution of Plastic Transmission Strategies in Avian Malaria
- A New Human 3D-Liver Model Unravels the Role of Galectins in Liver Infection by the Parasite
- Translocates into the Myocardium and Forms Unique Microlesions That Disrupt Cardiac Function
- Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
- The Cofilin Phosphatase Slingshot Homolog 1 (SSH1) Links NOD1 Signaling to Actin Remodeling
- Kaposi's Sarcoma Herpesvirus MicroRNAs Induce Metabolic Transformation of Infected Cells
- Reorganization of the Endosomal System in -Infected Cells: The Ultrastructure of -Induced Tubular Compartments
- Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways
- Exploitation of the Complement System by Oncogenic Kaposi's Sarcoma-Associated Herpesvirus for Cell Survival and Persistent Infection
- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Structural Insight into Host Recognition by Aggregative Adherence Fimbriae of Enteroaggregative
- The CD14CD16 Inflammatory Monocyte Subset Displays Increased Mitochondrial Activity and Effector Function During Acute Malaria
- Infection Induces Expression of a Mosquito Salivary Protein (Agaphelin) That Targets Neutrophil Function and Inhibits Thrombosis without Impairing Hemostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- MIF Contributes to Associated Immunopathogenicity Development
- The Ins and Outs of Rust Haustoria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



