-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Protecting against Pneumococcal Disease: Critical Interactions between Probiotics and the Airway Microbiome
article has not abstract
Published in the journal: . PLoS Pathog 8(6): e32767. doi:10.1371/journal.ppat.1002652
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1002652Summary
article has not abstract
Streptococcus pneumoniae (the pneumococcus) is a predominant cause of pneumonia, meningitis, and bacteremia. It is a leading killer of children under 5 years of age, responsible for the deaths of up to 2 million children annually [1]. Most deaths occur in African and Asian developing countries; however, pneumococcal disease is also a significant problem in particular populations of developed countries, such as the North American Indians, and indigenous Alaskans and Australians [1]–[3]. Although vaccination is the most cost-effective method of protection against pneumococcal disease, cost remains a barrier, as does vaccine delivery and efficacy. In this opinion piece, we discuss the potential complementary role of probiotics to vaccines in preventing pneumococcal disease through targeting the microbiome of the upper respiratory tract.
A prerequisite for pneumococcal disease is adherence of the bacterium to host nasopharyngeal epithelium leading to colonization (carriage). The mucosal surface and the microbiome of the nasopharynx are thought to protect against carriage [4]. Vaccination with pneumococcal vaccines reduces carriage of the organism, and the risk of invasive disease caused by vaccine serotypes and some cross-reactive non-vaccine serotypes. Moreover, vaccines generate herd immunity that may protect unvaccinated individuals against infection [5].
In North America and other developed regions, >80% of pediatric invasive pneumococcal disease (IPD) is accounted for by serotypes contained within the first-generation seven serotype conjugate vaccine (PCV7, Prevnar, Wyeth/Pfizer, United States). In high-risk populations, several factors diminish the efficacy of pneumococcal vaccines. For example, PCV7 protects against only ∼50% of serotypes causing IPD in developing countries of Africa and Asia [6]. Pneumococcal conjugate vaccines are also too expensive for resource-poor countries that experience the overwhelming burden of disease globally. The GAVI Alliance has made significant inroads to this problem, providing access to these and other life-saving vaccines to children most in need at a cost of US$1 billion per year [7]. Nevertheless, complete vaccine delivery is another major public health challenge. While GAVI is planning to implement pneumococcal conjugate vaccines in 19 developing countries over the next 2 years [8], vaccine uptake may be more difficult in certain populations. Amongst indigenous Australians, <50% of infants aged 7 months have received the full three-dose schedule (at 2, 4, and 6 months) [9], providing suboptimal protection against colonization and disease. In many countries, the first PCV7 dose is received after colonization has occurred—usually within the first 6 weeks of life—which may further limit the efficacy of pneumococcal vaccination.
Furthermore, serotype replacement is considered the most significant problem in the post-PCV7 era. Elimination of vaccine-serotype carriage has provided new niches for colonization and subsequent rises in invasive disease with non-PCV7 serotypes [10]. Although licensure of higher valency PCVs containing ten or 13 serotypes would be expected to reduce serotype replacement, the emergence of other invasive serotypes is likely.
Other early life strategies to prevent pneumococcal disease are needed, particularly for resource-poor settings. Maternal and neonatal immunization approaches are currently under investigation for their impact on disease during the first weeks of life. Targeting the microbiome to modulate colonization has been postulated as one mechanism to improve the efficacy of a range of vaccines against multiple pathogens [11]. It has now been demonstrated that in early infancy, colonization with pneumococci prior to conjugate vaccination causes impaired immune responses to the carried serotype [12], [13]. Exploiting the beneficial effects of probiotics on microbial colonization and immunity represents a novel approach to prevent or reduce pneumococcal colonization and disease.
The World Health Organization (WHO) defines probiotics as live micro-organisms that confer a health benefit to the host and are generally regarded as safe in humans [14]. Moreover, clinical studies have confirmed the safety and feasibility of oral administration of probiotics in infancy [15], [16]. Lactobacillus and Bifidobacterium are the two most widely studied genera of probiotic bacteria [17]. Probiotic activity is highly species - and strain-specific [18], [19]. Principal amongst their pleiotropic effects is the capacity to counteract microbiome disturbances, suggesting the potential to modulate pneumococcal colonization [20]. Indeed, experimental data suggest that probiotics can influence the profile of microbial species in the nasopharynx to reduce pneumococcal colonization [21]–[24]. Probiotics also maintain epithelial barrier integrity and modulate systemic and mucosal immune responses [14]. Furthermore, probiotic-microbiome crosstalk is important, as intestinal microbiota can shape immune responses by controlling the relative activity of regulatory T cells and Th17 cells [25], [26]. A paradigm for the effects of probiotics in modulating host responses in the nasopharynx to protect against pneumococcal infection is proposed in Figure 1. Importantly, while the mechanisms of action proposed are largely supported by animal studies, more research is needed to confirm these effects in humans.
Fig. 1. Paradigm for the proposed biological effects of probiotic bacteria in protection against pneumococcal infection. 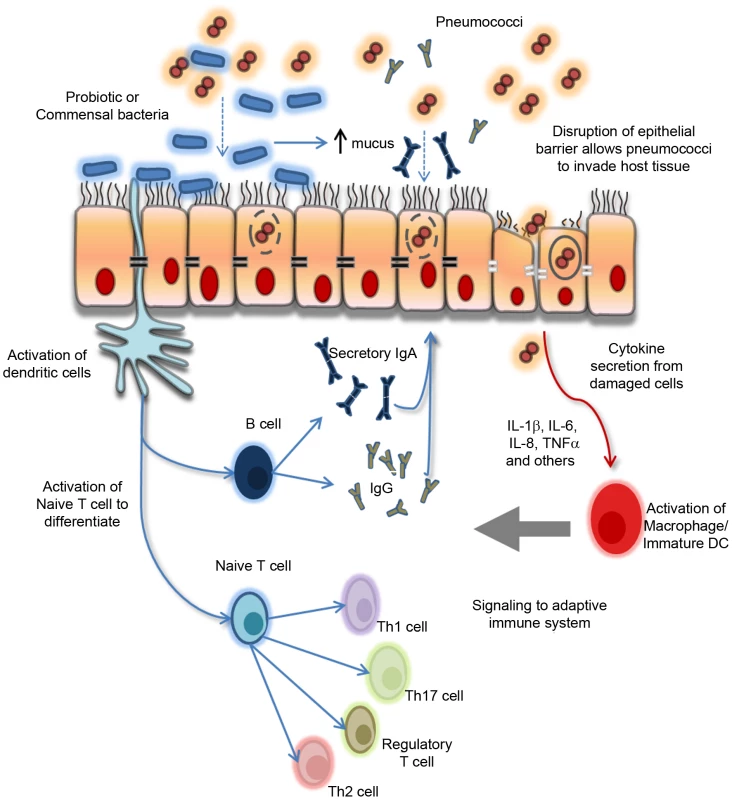
Commensal and/or probiotic bacteria can prevent pathogens (pneumococci) from attaching to and colonizing the respiratory epithelium by associating with specific cell surface receptors and by enhancing mucus secretion and the production of secretory IgA. Probiotic bacteria interact with underlying dendritic cells (DCs) which signal to the adaptive immune system to trigger a variety of effector cell types, including Th1, Th2, and Th17 as well as regulatory T cells and B cells depending on the local cytokine/chemokine microenvironment. Furthermore, probiotic bacteria also maintain the epithelial barrier integrity by upregulating the expression of specific tight junction proteins on damaged epithelium as a result of localized inflammatory responses following pathogen (pneumococcal) encounter and invasion. Refer to references [49]–[52] for more detail on probiotic–host effects. Th, T helper cell. Probiotics show specificity in their effect on microbial patterns in the nasopharynx. Most of the available data is based on animal models of colonization or disease. For example, in a mouse model of pneumococcal pneumonia, Lactobacillus lactis lowered lung colonization and increased specific IgG and IgA levels in bronchoalveolar secretions after challenge with pneumococcus serotype 14 [21], while Lactobacillus fermentum reduced nasopharyngeal colonization after challenge with pneumococcal serotype 6A [22]. In humans, the potential for probiotics to have an impact on airway microbial colonization is less clear. In 108 adult volunteers given a probiotic yogurt containing Lactobacillus rhamnosus GG (LGG), Bifidobacterium sp. B420, Lactobacillus acidophilus 145, and Streptococcus thermophilus, a significant reduction in pathogenic bacteria (including Staphylococcus aureus, S. pneumoniae, beta-hemolytic streptococci, and Haemophilus influenzae) was observed compared to a standard yogurt [24]. Streptococcus salivarius is suggested to be an appropriate probiotic species given that it is a known colonizer of the upper respiratory tract in humans [27]. It has been shown to produce bacteriocin-like substances with inhibitory activities against a number of important airway pathogens in vitro and in vivo [27], [28] as well as possess immunomodulatory properties in vitro [29], [30]. In otitis media-prone children given antibiotics prior to oral treatment with a powdered S. salivarius K12 formula, 33% were newly colonized with K12 while two of 19 children were shown to expand the pre-existing S. salivarius population [31]. No impact on clinical outcomes was reported in this study, and the small sample size used makes it difficult to draw meaningful conclusions. In contrast, when otitis-prone children (n = 155) were given a daily probiotic mix containing LGG, L. rhamnosus LC705, B. breve 99, and Propionibacterium freudenreichii JS for 24 weeks, no effect on nasopharyngeal carriage of otitis pathogens was observed. Furthermore, this probiotic formula did not prevent the occurrence of otitis media in these children, although there was a trend of reduced recurrent respiratory infections [32]. Taken together, the evidence of probiotic effects in human studies is more limited compared to animal models and justifies the continued investigation of candidate probiotic species such as S. salivarius and lactobacilli on airway microbial colonization and their mechanisms of action.
To date, the effect of probiotics on the gastrointestinal microbiome have provided the best evidence for host–microbe interactions such as pathogen exclusion, enhanced mucus secretion, production of anti-bacterial factors, and modulation of host immunity [14]. Probiotics can restore aberrant microbiota patterns associated with inflammatory diseases such as Crohn's Disease [33] and allergy [17]. Several clinical studies have shown that infants who later develop atopic dermatitis have altered microbiota, with greater numbers of pathogenic clostridial and staphylococcal species and fewer beneficial bifidobacteria [34], [35]. Importantly, dysbiosis precedes clinical symptoms of allergy [36], indicating a causal relationship between altered microbiota and disease. Administration of LGG modulates the composition of the intestinal microbiota in allergic infants, and reduced by half the incidence of atopic dermatitis in high-risk infants by age 2 [36], [37]. LGG also corrected dysbiosis and reduced disease severity in a mouse model of colitis [38].
These data have implications for pneumococcal disease. Importantly, lung immunity is affected by the intestinal microbiome, which induces Th1 and IgA responses via specific inflammasomes [39]. Therefore, modulation of inflammasome activity by probiotics represents a key biological target. The balance between microbiome status and health are also linked to the production of potent anti-inflammatory short-chain fatty acids such as butyrate and acetate [40]. Probiotics restore short-chain fatty acid levels, and the protective effects of Bifidobacteria species against enterohemorrhagic E. coli infection was shown to be dependent on acetate production [41].
Probiotics also appear to play an important role in facilitating mucosal immunity against infection [42]. Specifically, probiotics are demonstrated to be effective vaccine adjuvants, enhancing IgG - and IgA-specific responses to parenteral and mucosal vaccines such as influenza [43], H. influenzae type b (Hib) [44], polio [45], rotavirus [46], and Salmonella typhi [47] in humans. More studies on the adjuvant properties of probiotics in humans are needed, as the effects reported are often variable and have been based on clinical trials involving small sample sizes. For example, in the study by Fang et al. [47], treatment with LGG or L. lactis did not significantly enhance the IgG or IgA response to an oral S. typhi Ty21a vaccine despite LGG increasing S. typhi–specific IgA antibody secreting cells in a greater number of subjects than L. lactis or placebo. Similarly, while supplementation with a Bifidobacterium longum BL999 and L. rhamnosus LPR mix to infants doubled the anti-HBsAg IgG levels following vaccination compared to placebo, this was not statistically significant [48]. In a study by Kukkonen et al. [44], daily administration of a LGG, L. rhamnosus LC705, B. breve Bbi99, and Propionibacterium freudenreichii combination to mothers in the last 4 weeks of pregnancy, and to their infants for the first 6 months of life, increased the Hib-specific IgG response in infants. However, no change in diphtheria toxoid or tetanus toxoid IgG levels was observed, suggesting that the effects of probiotics may vary depending on the vaccine antigen used. Recently, Lactobacillus casei was reported to significantly enhance the pneumococcal protective protein A (PppA)-specific IgG and IgA response in the serum and mucosa following nasal vaccination with PppA and was associated with a significantly reduced pathogen load in the nasal lavage by day 42 post-immunization [42]. Despite this, the adjuvant activity of probiotics following pneumococcal vaccination in humans is unknown and remains an intriguing prospect for further research.
The promising findings of these studies has made it increasingly clear that significant research emphasis on reducing pneumococcal colonization during the neonatal period is warranted, ideally involving human clinical trials. Novel early life strategies that reduce infection with S. pneumoniae may have important health benefits, especially in high-risk populations. The combined effects of modulating the nasopharyngeal microbiome and enhanced mucosal immunity justify the continued investigation of probiotics for protection against pneumococcal infection.
Zdroje
1. O'BrienKLWolfsonLJWattJPHenkleEDeloria-KnollM 2009 Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374 893 902
2. SaidMAO'BrienKLNuortiJPSingletonRWhitneyCG 2011 The epidemiologic evidence underlying recommendations for use of pneumococcal polysaccharide vaccine among American Indian and Alaska Native populations. Vaccine 29 5355 5362
3. JacupsSPChengA 2011 The epidemiology of community acquired bacteremic pneumonia, due to Streptococcus pneumoniae, in the Top End of the Northern Territory, Australia–over 22 years. Vaccine 29 5386 5392
4. MalleyR 2010 Antibody and cell-mediated immunity to Streptococcus pneumoniae: implications for vaccine development. J Mol Med (Berl) 88 135 142
5. IsaacmanDJStruttonDRKalpasEAHorowicz-MehlerNSternLS 2008 The impact of indirect (herd) protection on the cost-effectiveness of pneumococcal conjugate vaccine. Clin Ther 30 341 357
6. JohnsonHLDeloria-KnollMLevineOSStoszekSKFreimanis HanceL 2010 Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med 7: e1000348 doi:10.1371/journal.pmed.1000348
7. Lob-LevytJ 2011 Contribution of the GAVI Alliance to improving health and reducing poverty. Philos Trans R Soc Lond B Biol Sci 366 2743 2747
8. NossalGJ 2011 Vaccines and future global health needs. Philos Trans R Soc Lond B Biol Sci 366 2833 2840
9. O'GradyKAKrauseVAndrewsR 2009 Immunisation coverage in Australian Indigenous children: time to move the goal posts. Vaccine 27 307 312
10. MelegaroAChoiYHGeorgeREdmundsWJMillerE 2010 Dynamic models of pneumococcal carriage and the impact of the Heptavalent Pneumococcal Conjugate Vaccine on invasive pneumococcal disease. BMC Infect Dis 10 90
11. FerreiraRBAntunesLCFinlayBB 2010 Should the human microbiome be considered when developing vaccines? PLoS Pathog 6: e1001190 doi:10.1371/journal.ppat.1001190.
12. DaganRGivon-LaviNGreenbergDFritzellBSiegristCA 2010 Nasopharyngeal carriage of Streptococcus pneumoniae shortly before vaccination with a pneumococcal conjugate vaccine causes serotype-specific hyporesponsiveness in early infancy. J Infect Dis 201 1570 1579
13. RodenburgGDvan GilsEJVeenhovenRHBogaertDvan den DobbelsteenGP 2011 Lower immunoglobulin G antibody responses to pneumococcal conjugate vaccination at the age of 2 years after previous nasopharyngeal carriage of Streptococcus pneumoniae. J Pediatr 159 965 970
14. GareauMGShermanPMWalkerWA 2010 Probiotics and the gut microbiota in intestinal health and disease. Nat Rev Gastroenterol Hepatol 7 503 514
15. BraeggerCChmielewskaADecsiTKolacekSMihatschW 2011 Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr 52 238 250
16. BoyleRJRobins-BrowneRMTangML 2006 Probiotic use in clinical practice: what are the risks? Am J Clin Nutr 83 1256 1264; quiz 1446–1257
17. TangMLLahtinenSJBoyleRJ 2010 Probiotics and prebiotics: clinical effects in allergic disease. Curr Opin Pediatr 22 626 634
18. BronPAvan BaarlenPKleerebezemM 2011 Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol 10 66 78
19. YanFPolkDB 2011 Probiotics and immune health. Curr Opin Gastroenterol 27 496 501
20. ReidGYounesJAVan der MeiHCGloorGBKnightR 2011 Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 9 27 38
21. MedinaMVillenaJSalvaSVintiniELangellaP 2008 Nasal administration of Lactococcus lactis improves local and systemic immune responses against Streptococcus pneumoniae. Microbiol Immunol 52 399 409
22. Cangemi de GutierrezRSantosVNader-MaciasME 2001 Protective effect of intranasally inoculated Lactobacillus fermentum against Streptococcus pneumoniae challenge on the mouse respiratory tract. FEMS Immunol Med Microbiol 31 187 195
23. RacedoSVillenaJMedinaMAgueroGRodriguezV 2006 Lactobacillus casei administration reduces lung injuries in a Streptococcus pneumoniae infection in mice. Microbes Infect 8 2359 2366
24. GluckUGebbersJO 2003 Ingested probiotics reduce nasal colonization with pathogenic bacteria (Staphylococcus aureus, Streptococcus pneumoniae, and beta-hemolytic streptococci). Am J Clin Nutr 77 517 520
25. NiessJHLeithauserFAdlerGReimannJ 2008 Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol 180 559 568
26. O'MahonyCScullyPO'MahonyDMurphySO'BrienF 2008 Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappaB activation. PLoS Pathog 4: e1000112 doi:10.1371/journal.ppat.1000112.
27. WallsTPowerDTaggJ 2003 Bacteriocin-like inhibitory substance (BLIS) production by the normal flora of the nasopharynx: potential to protect against otitis media? J Med Microbiol 52 829 833
28. SantagatiMScillatoMPatanèFAielloCStefaniS 2012 Bacteriocin-producing oral streptococci and inhibition of respiratory pathogens. FEMS Immunol Med Microbiol 65 23 31
29. GuglielmettiSTavernitiVMinuzzoMArioliSStuknyteM 2010 Oral bacteria as potential probiotics for the pharyngeal mucosa. Appl Environ Microbiol 76 3948 3958
30. CosseauCDevineDADullaghanEGardyJLChikatamarlaA 2008 The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect Immun 76 4163 4175
31. PowerDABurtonJPChilcottCNDawesPJTaggJR 2008 Preliminary investigations of the colonisation of upper respiratory tract tissues of infants using a paediatric formulation of the oral probiotic Streptococcus salivarius K12. Eur J Clin Microbiol Infect Dis 27 1261 1263
32. HatakkaKBlomgrenKPohjavuoriSKaijalainenTPoussaT 2007 Treatment of acute otitis media with probiotics in otitis-prone children-a double-blind, placebo-controlled randomised study. Clin Nutr 26 314 321
33. ThomasDWGreerFR 2010 Probiotics and prebiotics in pediatrics. Pediatrics 126 1217 1231
34. KalliomakiMKirjavainenPEerolaEKeroPSalminenS 2001 Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J Allergy Clin Immunol 107 129 134
35. WatanabeSNarisawaYAraseSOkamatsuHIkenagaT 2003 Differences in fecal microflora between patients with atopic dermatitis and healthy control subjects. J Allergy Clin Immunol 111 587 591
36. KalliomakiMSalminenSArvilommiHKeroPKoskinenP 2001 Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 357 1076 1079
37. LahtinenSJBoyleRJKivivuoriSOppedisanoFSmithKR 2009 Prenatal probiotic administration can influence Bifidobacterium microbiota development in infants at high risk of allergy. J Allergy Clin Immunol 123 499 501
38. SokolHPigneurBWatterlotLLakhdariOBermudez-HumaranLG 2008 Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 105 16731 16736
39. IchinoheTPangIKKumamotoYPeaperDRHoJH 2011 Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A 108 5354 5359
40. MaslowskiKMVieiraATNgAKranichJSierroF 2009 Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461 1282 1286
41. FukudaSTohHHaseKOshimaKNakanishiY 2011 Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469 543 547
42. VintiniEVillenaJAlvarezSMedinaM 2010 Administration of a probiotic associated with nasal vaccination with inactivated Lactococcus lactis-PppA induces effective protection against pneumoccocal infection in young mice. Clin Exp Immunol 159 351 362
43. ElinavEStrowigTKauALHenao-MejiaJThaissCA 2011 NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145 745 757
44. KukkonenKNieminenTPoussaTSavilahtiEKuitunenM 2006 Effect of probiotics on vaccine antibody responses in infancy–a randomized placebo-controlled double-blind trial. Pediatr Allergy Immunol 17 416 421
45. MullieCYazourhAThibaultHOdouMFSingerE 2004 Increased poliovirus-specific intestinal antibody response coincides with promotion of Bifidobacterium longum-infantis and Bifidobacterium breve in infants: a randomized, double-blind, placebo-controlled trial. Pediatr Res 56 791 795
46. IsolauriEJoensuuJSuomalainenHLuomalaMVesikariT 1995 Improved immunogenicity of oral D×RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine 13 310 312
47. FangHElinaTHeikkiASeppoS 2000 Modulation of humoral immune response through probiotic intake. FEMS Immunol Med Microbiol 29 47 52
48. SohSEOngDQGerezIZhangXChollateP 2010 Effect of probiotic supplementation in the first 6 months of life on specific antibody responses to infant hepatitis B vaccination. Vaccine 28 2577 2579
49. RemusDMKleerebezemMBronPA 2011 An intimate tête-à-tête - how probiotic lactobacilli communicate with the host. Eur J Pharmacol 668 S33 42
50. RescignoM 2011 The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol 32 256 264
51. WellsJM 2011 Immunomodulatory mechanisms of lactobacilli. Microb Cell Fact 10 S17
52. MarcoMLPavanSKleerebezemM 2006 Towards understanding molecular modes of probiotic action. Curr Opin Biotechnol 17 204 210
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 6- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- A Highly Intensified ART Regimen Induces Long-Term Viral Suppression and Restriction of the Viral Reservoir in a Simian AIDS Model
- An Endogenous Foamy-like Viral Element in the Coelacanth Genome
- Evidence for Induction of Integron-Based Antibiotic Resistance by the SOS Response in a Clinical Setting
- Manipulation of Costimulatory Molecules by Intracellular Pathogens: Veni, Vidi, Vici!!
- Highly Efficient Prion Transmission by Blood Transfusion
- Scavenges Host Zinc via Pra1 during Endothelial Invasion
- Protecting against Pneumococcal Disease: Critical Interactions between Probiotics and the Airway Microbiome
- How Do Viruses Interact with Stress-Associated RNA Granules?
- The Interdomain Linker of AAV-2 Rep68 Is an Integral Part of Its Oligomerization Domain: Role of a Conserved SF3 Helicase Residue in Oligomerization
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Protecting against Pneumococcal Disease: Critical Interactions between Probiotics and the Airway Microbiome
- Manipulation of Costimulatory Molecules by Intracellular Pathogens: Veni, Vidi, Vici!!
- A Highly Intensified ART Regimen Induces Long-Term Viral Suppression and Restriction of the Viral Reservoir in a Simian AIDS Model
- An Endogenous Foamy-like Viral Element in the Coelacanth Genome
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



