-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Foot in the Door for Dermatophyte Research
article has not abstract
Published in the journal: . PLoS Pathog 8(3): e32767. doi:10.1371/journal.ppat.1002564
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1002564Summary
article has not abstract
What Diseases Do Dermatophytes Cause?
Dermatophytes are a group of filamentous fungi that cause infections of the skin (see Figure 1 for diseases, Figure 2 for typical dermatophyte species). Diseases caused by dermatophytes include athlete's foot, ringworm, jock itch, and nail infections (onychomycosis). The medical terminology for dermatophyte infections is to use the word tinea (to denote a fungal infection of the skin) followed by a word that describes the site of infection. For example, tinea pedis refers to athlete's foot and tinea capitis is scalp ringworm. In general, dermatophytes remain localized to keratinized surfaces such as skin, hair, and nails and do not invade deeper tissues. That said, dermatophyte infections in immunocompromised patients can be quite severe.
Fig. 1. Tineas throughout the body. 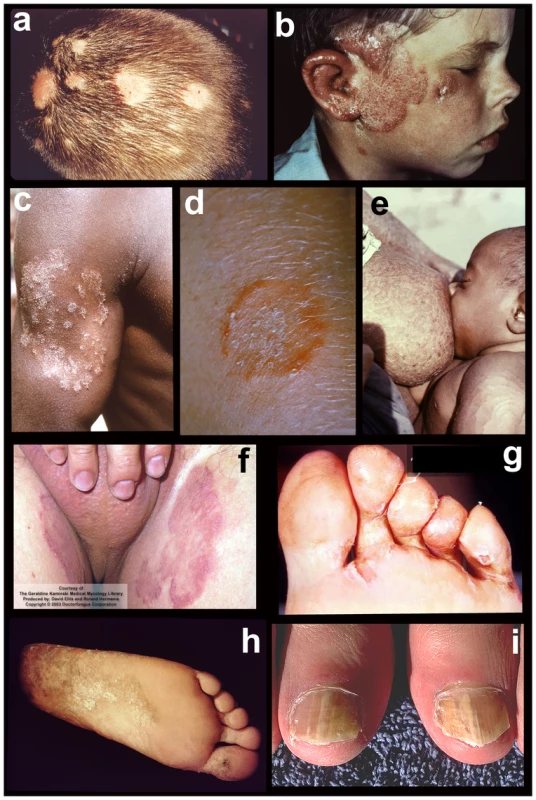
Tineas are dermatophyte infections of the skin. From top to bottom, left to right: fungal infections of a) hair (tinea capitis); b) face (tinea capitis / ringworm); c) arm (tinea corporis); d) close-up of ringworm; e) torso with concentric rings (tinea imbricata / tinea corporis); f) groin (tinea cruris); g) toe webbing (tinea pedis); h) foot (tinea pedis / “moccasin” type); and i) nails (onycomycosis). Photos courtesy of Doctor Fungus (http://www.doctorfungus.org) and the Public Health Image Library (PHIL, http://phil.cdc.gov/phil/home.asp). Fig. 2. Major dermatophyte species—appearance in the laboratory. 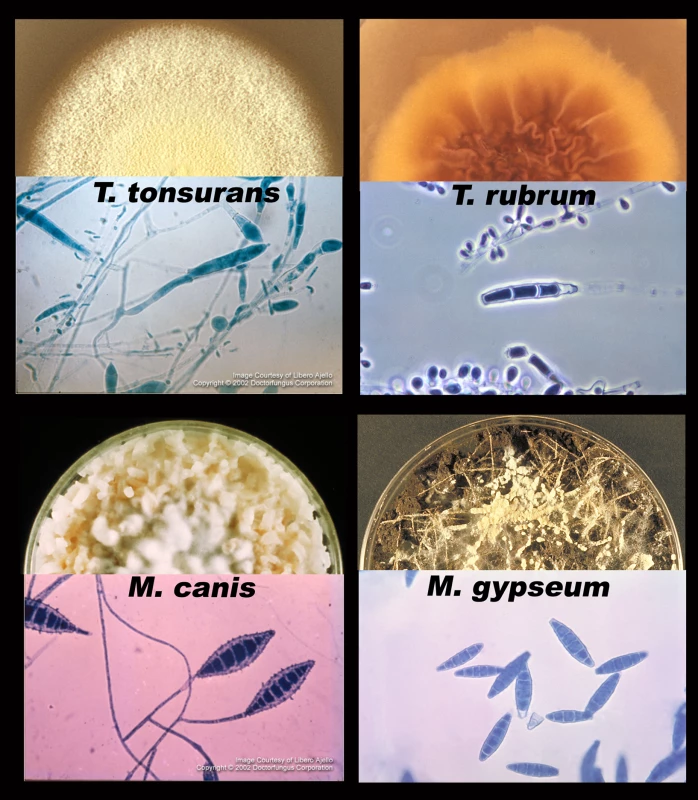
Each section shows one of four species of dermatophyte, including a semicircle of fungus growing on an agar plate (top) and a microscopic picture of the asexual spores (macroconidia and microconidia, bottom). Photos courtesy of Doctor Fungus (http://www.doctorfungus.org) and the Public Health Image Library (PHIL, http://phil.cdc.gov/phil/home.asp). Dermatophytes are grouped into three general categories based on their natural environment: anthropophilic (live exclusively on humans), zoophilic (live on an animal host), and geophilic (live in the soil) [1]. The majority of human infections are caused by anthropophilic species; however, species from all three groups have been associated with human disease. For example, pets with ringworm can transmit the infection to their owners, and stray cats carrying dermatophytes are considered to be a vector for infection in several eastern and southern European countries [2].
Is the Most Prevalent Disease the Same from Country to Country?
Although dermatophytes are found throughout the world, the most prevalent strains and the most common sites of infection vary by region [2], [3]. Hot, humid climates and overcrowding predispose populations to skin diseases, including tinea infections [4]. Developing countries have high rates of tinea capitis, while developed countries have high rates of tinea pedis and onychomycosis [2].
Low socioeconomic conditions are strongly linked to higher prevalence rates for skin infections, including tinea infections. A review of 18 studies representing large geographical areas determined that tinea capitis is present in up to 19.7% of the general population in developing countries [4]. A recent study found tinea capitis present in more than 30% of children at certain grade levels in some urban areas of the United States [5].
High prevalence rates of tinea pedis and onychomycosis have been linked to increased urbanization, community showers, sports, and the use of occlusive footwear [2], [3], [6]. These factors are thought to contribute to the high prevalence of tinea pedis in certain occupational groups, including marathon runners (22%–31% prevalence), miners (21%–72.9% prevalence), and soldiers (16.4%–58% prevalence) [2]. Several of these studies also found high rates of onychomycosis presenting with tinea pedis. Although tropical and subtropical climates have a higher overall prevalence of skin mycoses, tinea pedis and onychomycosis are rare in India and rural Africa [6].
Why Can People Get Athlete's Foot (and Other Dermatophyte Diseases) More Than Once?
Dermatophyte diseases recur at a high rate following treatment with an antifungal [7]. It is currently unknown whether this is due to insufficient clearing of the fungus during treatment and reemergence of disease, and thus an example of relapse, or if these represent new infections (Figure 3). The high false-negative culture rate from clinical samples contributes to this problem. The advent of molecular biology tools may provide a means by which clinicians can more accurately determine the presence (or absence) of dermatophytes [7]. Certainly, such tools will help determine whether a new infection is indeed caused by the same strain as a previous infection in the same patient.
Fig. 3. Relationship between fungus, disease, the immune system, and drugs. 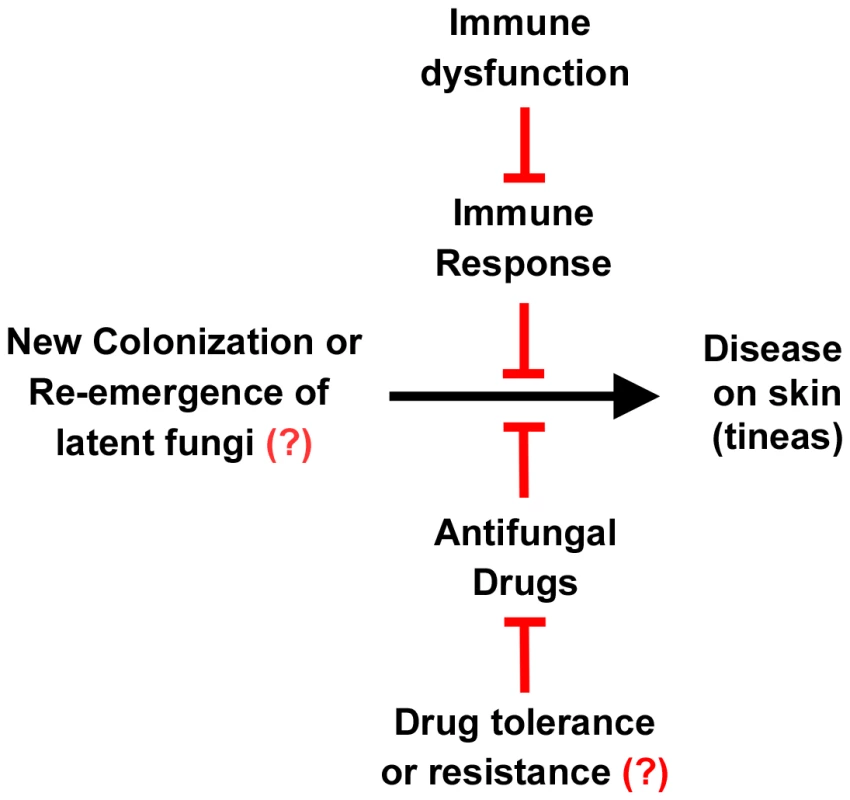
The left signifies the presence of dermatophytes on the skin, either by new infection or relapse of a previous infection. The right signifies tineas caused by these infections. This progression to disease can be inhibited by the immune system and antifungal drugs. However, immune dysfunction can reduce the immune response to these infections, and drug resistance or tolerance can overcome the action of the drugs. Resistance or tolerance to antifungal drugs is implied but is not documented in dermatophytes. The roles of the immune system, immune dysfunction, drug response, and drug resistance are all areas of active research. Treatment of dermatophyte infections represents a significant cost burden. It has been estimated that over US$500 million per year is spent worldwide on drugs to treat dermatophytoses [8]. Treatment for skin infections is generally by a topical antifungal. If hair roots or nail beds are infected, an oral antifungal agent is generally prescribed. Nail infections are often recalcitrant to treatment. Immunocompromised patients can experience disseminated dermatophyte disease, which has a particularly high treatment failure rate (30.8%) [9].
Why Aren't There More Drug-Resistant Dermatophytes?
Over-the-counter antifungals are commonly used to self-treat athlete's foot and jock itch. This might be predicted to lead to drug resistance. Surprisingly, drug resistance among dermatophytes is rare. Two large clinical studies looking at drug susceptibility in dermatophytes did not find significant increases in the minimum inhibitory concentration of several antifungal drugs used to treat dermatophytes [10], [11]. That said, occasional drug resistance has been documented. For example, a single amino acid substitution in the target enzyme was found to confer resistance to terbinafine in a clinical isolate from a patient with onychomycosis [12], [13].
The question remains, why aren't mutations conferring drug resistance a more widespread occurrence? One possibility is that dermatophytes are able to tolerate drugs without acquiring point mutations in the target enzyme. In the yeast Candida albicans, several mechanisms of resistance have been documented that do not require a point mutation in the target enzyme. More research is needed to determine if something similar is happening with dermatophytes.
Why Don't We Know More about How Dermatophytes Cause Disease?
Despite the prevalence of dermatophyte infections worldwide, a sophisticated understanding of how they cause disease is lacking [14]. The historic difficulties in working with dermatophytes have been two-fold: technical difficulties due to poor virulence models and a lack of genetic tools, and an under-appreciation of the need to study these organisms.
There have been several recent advances that minimize the technical difficulties of working with these organisms. Performing genetics in dermatophytes has historically posed a challenge; however, recent advances in the field have provided a foundation for genetic manipulation of several species of dermatophytes [15], [16].
Currently, the most common animal model for studying dermatophyte virulence is the guinea pig. Although this has been useful for zoophiles, guinea pigs and other dermatophyte animal models do not provide accurate infection models for most anthropophilic species [15]. Other virulence models include determining the ability of the organism to grow on keratinized surfaces such as sterilized nail fragments, which is a non-quantitative model. Recently, skin explants have been used to study dermatophyte adherence and invasion. Human epidermal tissues are commercially available and represent a possible virulence model to study the initial stages of dermatophyte infection [15].
One other reason that we don't know more about dermatophyte disease is that most scientists, including many mycologists, do not consider dermatophytes as important as other infectious diseases. Therefore, there are a limited number of researchers working on the problem and there are limited funding opportunities. However, dermatophyte infections are likely to infect every person at least once in their lifetime [8]. While mortality due to dermatophytes is very low, there is significant morbidity associated with these infections, particularly in the armed forces and active adults. These infections are likely the most common fungal infection worldwide, with high rates of incidence and prevalence in most countries. In addition, the worldwide cost of dermatophyte treatment each year is over US$500 million [8].
What's Next for Dermatophyte Research?
Dermatophyte research is poised to take off. The sequencing of seven dermatophyte genomes was recently completed, and the sequence information is now publically available [17], [18]. Analysis of the genome sequences demonstrates that a group of proteinases necessary for degradation of keratin is increased in number in the dermatophytes compared to closely related fungal species. These genome sequences, combined with better genetic tools and a promising model in which to study virulence, provide an optimistic outlook. Sequence information can be used to make informed hypotheses about which gene products, such as the proteinases, are important to virulence, and these genes can be deleted and tested in virulence models. These experiments will contribute to our understanding of how dermatophytes interact with human cells and cause disease. Knowing the fungal factors involved will allow development of better therapeutics and will inform preventative treatments.
Zdroje
1. WeitzmanISummerbellRC 1995 The dermatophytes. Clin Microbiol Rev 8 240 259
2. SeebacherCBoucharaJPMignonB 2008 Updates on the epidemiology of dermatophyte infections. Mycopathologia 166 335 352
3. HavlickovaBCzaikaVAFriedrichM 2008 Epidemiological trends in skin mycoses worldwide. Mycoses 51 Suppl 4 2 15
4. World Health Organization 2005 Epidemiology and management of common skin diseases in children in developing countries. Department of Child and Adolescent Health and Development. WHO reference number WHO/FCH/CAH/05.12 54
5. Abdel-RahmanSMFarrandNSchuenemannESteringTKPreuettB 2010 The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics 125 966 973
6. AmeenM 2010 Epidemiology of superficial fungal infections. Clin Dermatol 28 197 201
7. GuptaAKCooperEA 2008 Update in antifungal therapy of dermatophytosis. Mycopathologia 166 353 367
8. GraserYScottJSummerbellR 2008 The new species concept in dermatophytes-a polyphasic approach. Mycopathologia 166 239 256
9. MarconiVCKradinRMartyFMHospenthalDRKottonCN 2010 Disseminated dermatophytosis in a patient with hereditary hemochromatosis and hepatic cirrhosis: case report and review of the literature. Med Mycol 48 518 527
10. GhannoumMIshamNSheehanD 2006 Voriconazole susceptibilities of dermatophyte isolates obtained from a worldwide tinea capitis clinical trial. J Clin Microbiol 44 2579 2580
11. GhannoumMAWraithLACaiBNyiradyJIshamN 2008 Susceptibility of dermatophyte isolates obtained from a large worldwide terbinafine tinea capitis clinical trial. Br J Dermatol 159 711 713
12. MukherjeePKLeidichSDIshamNLeitnerIRyderNS 2003 Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob Agents Chemother 47 82 86
13. OsborneCSLeitnerIFavreBRyderNS 2005 Amino acid substitution in Trichophyton rubrum squalene epoxidase associated with resistance to terbinafine. Antimicrob Agents Chemother 49 2840 2844
14. WhiteTCOliverBGGraserYHennMR 2008 Generating and testing molecular hypotheses in the dermatophytes. Eukaryot Cell 7 1238 1245
15. AchtermanRRWhiteTC 2012 Dermatophyte virulence factors: identifying and analyzing genes that may contribute to chronic or acute skin infections. Int J Microbiol 2012 358305
16. GrumbtMMonodMStaibP 2011 Genetic advances in dermatophytes. FEMS Microbiol Lett 320 79 86
17. BurmesterAShelestEGlocknerGHeddergottCSchindlerS 2011 Comparative and functional genomics provide insights into the pathogenicity of dermatophytic fungi. Genome Biol 12 R7
18. The Broad Institute 2011 Dermatophyte Comparative Database. Available: http://www.broadinstitute.org/annotation/genome/dermatophyte_comparative/MultiHome.html. Accessed 2 March 2012
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2012 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- An Entomopathogenic Nematode by Any Other Name
- Induced Release of a Plant-Defense Volatile ‘Deceptively’ Attracts Insect Vectors to Plants Infected with a Bacterial Pathogen
- A Foot in the Door for Dermatophyte Research
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
- Indifferent, Affectionate, or Deceitful: Lifestyles and Secretomes of Fungi
- Mutation and Selection of Prions
- Sleeping with the Enemy: How Intracellular Pathogens Cope with a Macrophage Lifestyle
- Taste for Blood: Hemoglobin as a Nutrient Source for Pathogens
- Direct Recognition of by the NK Cell Natural Cytotoxicity Receptor NKp46 Aggravates Periodontal Disease
- A20 () Deficiency in Myeloid Cells Protects against Influenza A Virus Infection
- Anthrax Lethal Factor Cleavage of Nlrp1 Is Required for Activation of the Inflammasome
- Differential Function of Lip Residues in the Mechanism and Biology of an Anthrax Hemophore
- PK-sensitive PrP Is Infectious and Shares Basic Structural Features with PK-resistant PrP
- A Peptidoglycan Fragment Triggers β-lactam Resistance in
- Capsule Type of Determines Growth Phenotype
- Additive Function of MARTX and VvhA Cytolysins Promotes Rapid Growth and Epithelial Tissue Necrosis During Intestinal Infection
- A Novel Mouse Model of Egg-Induced Immunopathology
- Redundant Notch1 and Notch2 Signaling Is Necessary for IFNγ Secretion by T Helper 1 Cells During Infection with
- The Novel Transporter Dur31 Is a Multi-Stage Pathogenicity Factor
- Short ORF-Dependent Ribosome Shunting Operates in an RNA Picorna-Like Virus and a DNA Pararetrovirus that Cause Rice Tungro Disease
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Foot in the Door for Dermatophyte Research
- Structural Insights into a Unique Effector LidA Recognizing Both GDP and GTP Bound Rab1 in Their Active State
- An Entomopathogenic Nematode by Any Other Name
- New Insights into spp.: A Potential Link with Irritable Bowel Syndrome
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



