-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Effectiveness of Electronic Reminders to Improve Medication Adherence in Tuberculosis Patients: A Cluster-Randomised Trial
In a cluster-randomized controlled trial, Katherine Fielding and colleagues examine the effectiveness of electronic reminders for improving tuberculosis medication adherence.
Published in the journal: . PLoS Med 12(9): e32767. doi:10.1371/journal.pmed.1001876
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001876Summary
In a cluster-randomized controlled trial, Katherine Fielding and colleagues examine the effectiveness of electronic reminders for improving tuberculosis medication adherence.
Introduction
In 2013, China ranked second in the world in number of tuberculosis (TB) cases, accounting for 11% of the estimated 9 million global cases [1]. Implementation of the Directly Observed Treatment, Short Course (DOTS) strategy started in 1992 and covered the entire country by 2005 [2]. Initially, the use of directly observed treatment (DOT) by health care workers was the primary approach to ensure TB treatment adherence. Over time, because of difficulties in carrying out DOT in many parts of the country, national TB control policies also permitted self-administered treatment and treatment monitored by family members. Over half of TB patients now receive self-administered treatment [3]. In the 2010 National Tuberculosis Prevalence Survey, 20% of TB patients treated by the public health system—using national TB case-management approaches—were lost to follow-up or were not taking their medications regularly [4]. Thus, more effective case-management approaches are needed in China.
Electronic reminders and monitoring have been used in several disease conditions to improve medication adherence. The potential of mobile phone technology to improve the quality and delivery of health care, including diagnosis, treatment adherence, and data collection, has been recognised [5,6]. Mobile phone text messaging has been shown to improve adherence to antiretroviral treatment and outcomes in HIV-positive patients [7]. However, aside from in a small-scale pilot study [8], the use of text messaging has not been rigorously evaluated in TB patients.
Electronic medication packaging (EMP) devices can remind patients to take medications on time, monitor time of drug intake, and alert health care workers to patients who have missed doses [9,10]. The current evidence supporting the use of these devices is limited [10]. In fact, no study to our knowledge has properly evaluated the use of EMP devices in TB patients. Using adherence data from medication monitor boxes (medication monitors) to select less adherent patients for counselling or more intensive forms of case management has been suggested but not studied [11].
To evaluate the use of electronic reminders to improve medication adherence in TB patients, we conducted a cluster-randomised controlled trial to assess the effectiveness of three case-management approaches—using reminders via text messaging, a medication monitor, or both—compared to the standard of care in China.
Methods
Ethical Approval
The study was approved by the ethics committees of the Chinese Center for Disease Control and Prevention (201008) and the London School of Hygiene & Tropical Medicine (5704). All patients provided written consent prior to inclusion in the study.
Study Design
This study was a pragmatic cluster-randomised trial with one control and three intervention arms. New pulmonary TB patients, starting on standard 6-mo short-course chemotherapy and managed as outpatients, were recruited into the study. Those in the control arm were managed according to the standard of care of the National Tuberculosis Control Program. Those in the three intervention arms also received reminders to take their medications from text messages via short message service (SMS), a medication monitor, or both. If adherence problems were subsequently detected, more intensive management was recommended. For logistical simplicity, randomisation was conducted at the cluster level.
Cluster Selection
Clusters were defined as rural counties or urban districts within the provinces of Heilongjiang, Jiangsu, Hunan, and Chongqing—located in northern, eastern, central, and western China, respectively. Each cluster had at least 300 active pulmonary TB patients registered in 2009 (S3 Text). Nine clusters, with a rural to urban ratio of 2 : 1, were selected from two cities in each province.
Patient Recruitment
In each cluster, consecutive pulmonary TB patients newly registered at the public health TB clinic were screened for study eligibility. Inclusion criteria included the following: no communication impairment (mental, visual, auditory, or speech), patient at least 18 y old, and patient or family member able to use mobile phone to read SMS text messages and use the medication monitor after training. Because of the nature of the study, interventions were not masked to study staff and patients.
Randomisation
The 36 clusters were randomised to the four arms by rural/urban stratum and restricted such that each province had at least two clusters in each arm. From 5,000 randomly generated acceptable allocations, one was chosen at random as the final allocation using Stata version 12.0. See S3 Text for further details.
All Arms
All patients were treated according to National Tuberculosis Control Program guidelines including the use of isoniazid, rifampin, ethambutol, and pyrazinamide for 2 mo, followed by isoniazid and rifampin for 4 mo; the programme used every other day dosing for the entire treatment course. Patients received their blister-pack medications in a medication monitor box that electronically collected the date and time of each opening. In the control and text messaging arms, the medication monitor box was in silent mode and was not used as a reminder tool for patients. At each monthly visit, patients were dispensed enough medications for a 1-mo period.
Control Arm
At the start of treatment, the doctor and the patient selected one of three treatment monitoring approaches as per National Tuberculosis Control Program protocol: self-administered treatment, treatment supervised by family members, or treatment supervised by health care workers. The local doctor monitoring treatment at the township or village/community level was given 60 renminbi (RMB; equivalent to US$10) for each patient.
Intervention Arms
As in the control arm, patients and their doctors in the intervention arms selected one of the three treatment monitoring approaches as per National Tuberculosis Control Program protocol. The interventions had three common components: reminders for timely drug intake, reminders for monthly follow-up visits, and a recommendation for doctors to switch patients from self-administered treatment to a more intensive treatment monitoring approach when patients were found to have adherence problems based on data available to the managing doctor (Table 1).
Tab. 1. Description of the three intervention arms. 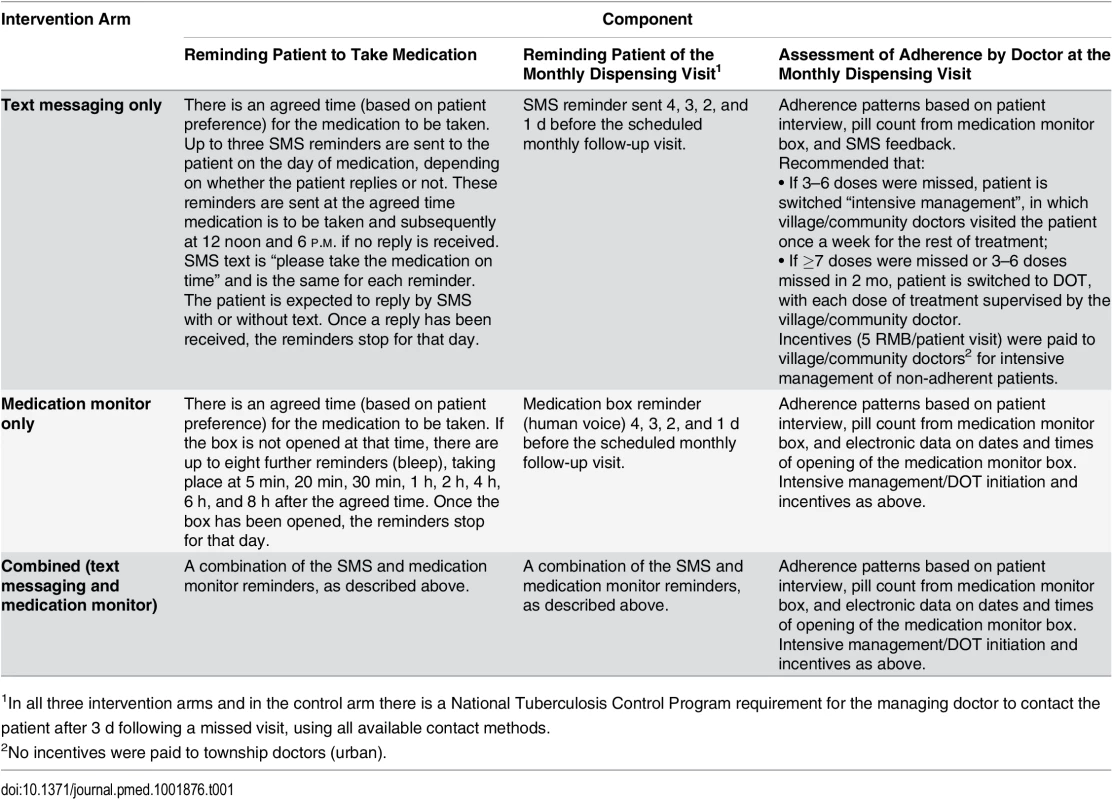
1In all three intervention arms and in the control arm there is a National Tuberculosis Control Program requirement for the managing doctor to contact the patient after 3 d following a missed visit, using all available contact methods. In the text messaging and combined arms, a text message reminded patients to take their medication at the time previously agreed on with the patient. If patient did not reply to the text message, another two text messages would be sent later in the day. Once the patient replied to the SMS reminder, with or without text, the reminders were stopped for that day. Similarly, in the medication monitor and combined arms, an audio reminder from the medication monitor box reminded patients to take their medication. If the patient did not open the medication monitor by a pre-specified time, up to eight additional reminders sounded. Once the box was opened, the reminders were stopped for that day. In all three intervention arms, patients received four reminders to attend the monthly dispensing visit (Table 1).
At each monthly follow-up visit, the managing doctor evaluated adherence patterns. Missed doses were defined as the larger of (1) missed doses based on pill count or (2) missed doses from missing SMS reply (in the text messaging only arm) or from failure to open the medication monitor box (in the other two intervention arms). If the patient reported any equipment malfunction or operation error during the previous month, the number of missed doses was based on pill count only.
If 1–2 doses were missed in the previous month, we recommended the doctor counsel the patient on the importance of adherence to medication but allowed self-administered treatment to continue. If 3–6 doses were missed, we recommended the doctor switch the patient to “intensive management”, in which township or village/community doctors visited the patient twice a month or once a week, respectively, for the rest of treatment. If seven or more doses were missed the previous month or if 3–6 doses were missed in two prior months, we recommended the doctor switch the patient to DOT, with each dose of treatment supervised by the township or village/community doctor. The local doctors monitoring treatment at the township or village/community level were given 5 RMB (US$0.8) every time they made a visit to a patient as part of the intensive management or DOT, in addition to the 60 RMB (US$10) they received for every patient.
Study Endpoints
All study endpoints were measured at the individual level. The primary study endpoint for treatment adherence was the percentage of patient-months where at least 20% of doses (equivalent to missing three of 15 doses) were missed (“poor adherence”). The secondary treatment adherence endpoints were (1) percentage of patient-months where at least 47% of doses (equivalent to seven of 15 doses) were missed, (2) percentage of total doses missed over the 6 mo of treatment, (3) binary categorisation of secondary endpoint 2 as <10% versus ≥10% of total doses missed (National Tuberculosis Control Program definition of non-adherent), and (4) percentage of patient-months on TB treatment where at least 20% of doses were missed based on pill count only.
Measurement of the adherence endpoints utilised the same data for all four study arms and included data from the medication monitor box, downloaded into a database when patients returned for their monthly medication refill. All adherence endpoints, except secondary endpoint 4, measured the number of missed doses per month as the larger of the number of missed doses from pill count or the number of failures to open the medication monitor box. A month was defined as the number of days between successive appointments, typically 30 d, during which 15 doses should have been taken, but this was adjusted for early/late or missed visits and reduced by the number of days a patient was hospitalised or temporarily discontinued treatment on doctor’s recommendation. Data were censored when a patient died, moved, or permanently discontinued treatment based on a doctor’s decision. For those who were lost to follow-up during treatment, we assumed no drug intake (100% non-adherence) for the period from the date of being lost to follow-up to the date when they should have completed treatment. We also conducted a post hoc sensitivity analysis censoring adherence measurement at the time of loss to follow-up.
The secondary TB treatment outcome endpoints, following standard WHO definitions, were (1) poor treatment outcome, defined as death, treatment failure, or patient loss to follow-up and (2) patient loss to follow-up (S3 Text). Routinely recorded data reported to the National Tuberculosis Control Program were used to define TB treatment outcomes. We also included as a secondary endpoint 2-mo smear conversion among those smear-positive at enrolment.
Sample Size
Sample size calculations were based on a binary endpoint of non-adherence and took into account the study design [12]. Assuming nine clusters per arm, a two-sided type I error of 5%, and a percentage with non-adherence in the control arm of 30%, 110 TB patients per cluster would be required to detect a 40% reduction in the endpoint in the intervention arm with power of 90% and coefficient of variation of 0.25. The sample size was adjusted to 116 per cluster assuming 5% missing endpoint data. An additional power calculation is summarised in S3 Text.
Analysis
Analysis of all endpoints used standard methods for a small number of clusters [12], accounting for the stratified design and giving each cluster equal weight (S3 Text). Patients who were reassessed as not having TB by the managing doctor or who were switched to a different treatment model within the first month (due to hospitalisation or travel) were excluded from all analyses. Pre-specified sub-group analyses for the primary endpoint were by age group, literacy, gender, and rural/urban setting.
There were problems with loose batteries in some of the medication monitors, resulting in a power outage during which data on box openings were not captured. The problem could be easily fixed by the patient or the doctor when they became aware of the problem We conducted a post hoc stratified analysis separating patient-months into those that had a record of a medication monitor problem and those that did not (S3 Text).
Analysis was conducted using Stata version 13.
Results
Study Population
From 1 June 2011 to 7 March 2012, 6,203 pulmonary TB patients were screened in the 36 clusters, and 5,057 (81.5%) met enrolment criteria, of whom 4,292 (84.9%) gave informed consent. Of these, 61 (1.4%) were reassessed as not having TB by their managing doctor, and 58 (1.4%) were withdrawn from the study as they had switched to a different treatment model within the first month (due to hospitalisation or travel) and were therefore excluded from all analyses (Fig 1; S1 Table). Therefore, 4,173 patients were included in the analysis (Fig 1). There was some variation between arms in the percentages analysed among those screened: 72.5%, 68.2%, 59.4%, and 69.8% in the control, text messaging, medication monitor, and combined arms, respectively (Fig 1).
Fig. 1. Cluster-level CONSORT diagram. 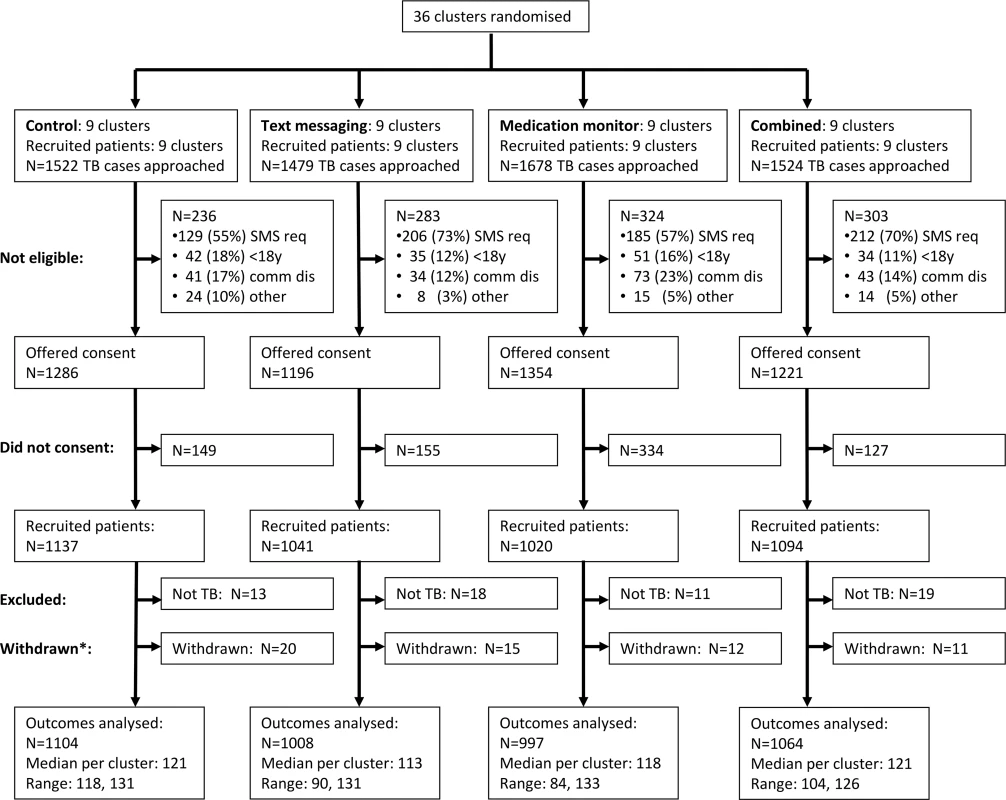
Reasons for non-eligibility: SMS req = unable to use mobile phone after training; <18y = less than 18 y of age; comm dis = communication disability. *Withdrew from the study but continued treatment in the local Center for Disease Control and Prevention. Overall, 71.0% of participants were male, median age was 43 y (inter-quartile range [IQR] 29 to 56 y), 56.0% were farmers, 7.9% were illiterate, median household income was 20,000 RMB (IQR 10,000 to 30,000 RMB), and 36.3% were smear positive (Table 2). There was some baseline imbalance by study arm for occupation, education level, income, and local residency.
Tab. 2. Characteristics at start of tuberculosis treatment for patients enrolled in the four study arms of the study (n = 4,173). 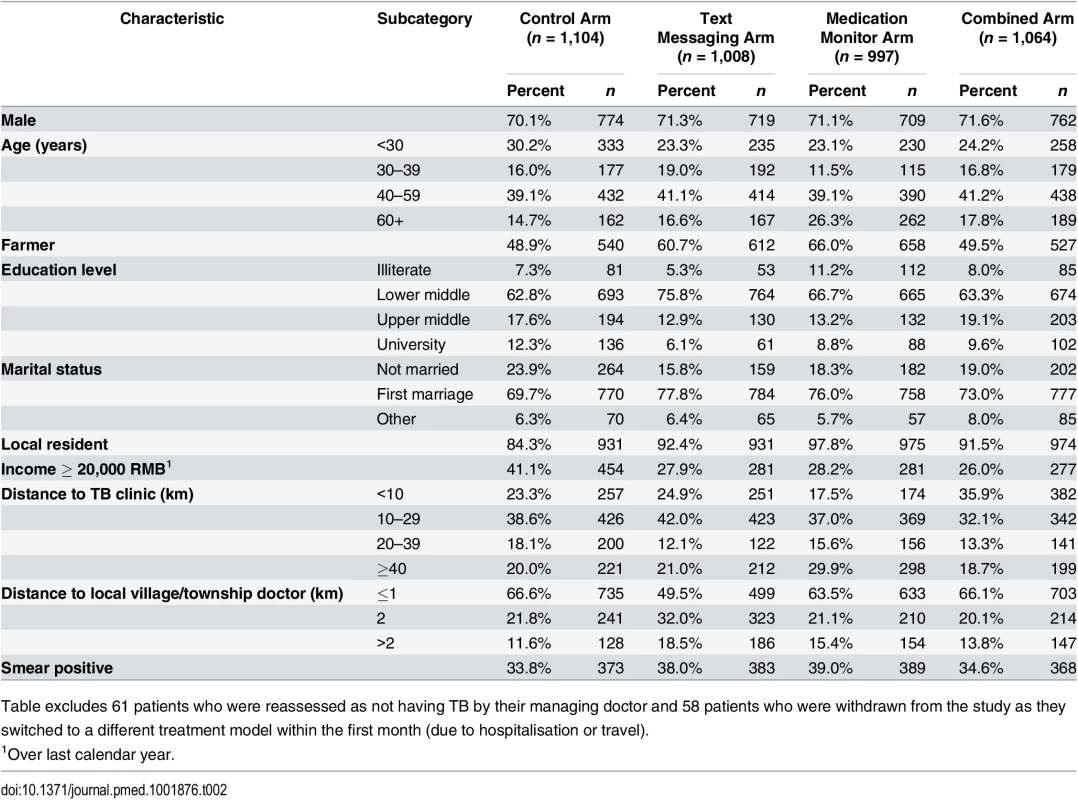
Table excludes 61 patients who were reassessed as not having TB by their managing doctor and 58 patients who were withdrawn from the study as they switched to a different treatment model within the first month (due to hospitalisation or travel). Endpoints
Primary study endpoint
The cluster geometric mean of the percentage of patient-months on TB treatment where at least 20% of doses were missed was 29.9% in the control arm (range 16.0%–48.1%; Fig 2; S2 Table); in comparison, this percentage was 27.3% in the text messaging arm (adjusted mean ratio [aMR] 0.94, 95% CI 0.71, 1.24; Table 3), 17.0% in the medication monitor arm (aMR 0.58, 95% CI 0.42, 0.79), and 13.9% in the combined arm (aMR 0.49, 95% CI 0.27, 0.88). There were no differences in the mean ratios (MRs) for the primary endpoint when stratifying by age, literacy, or gender (S3 Table). There was an indication that the reduction in poor adherence seen in the medication monitor arm compared to the control arm was only in the rural stratum (MR 0.43 for rural and 1.06 for urban, p-value for effect modification 0.011). The coefficient of variation for the primary endpoint among control clusters was 0.24.
Fig. 2. Primary endpoint of poor tuberculosis treatment adherence by study arm. 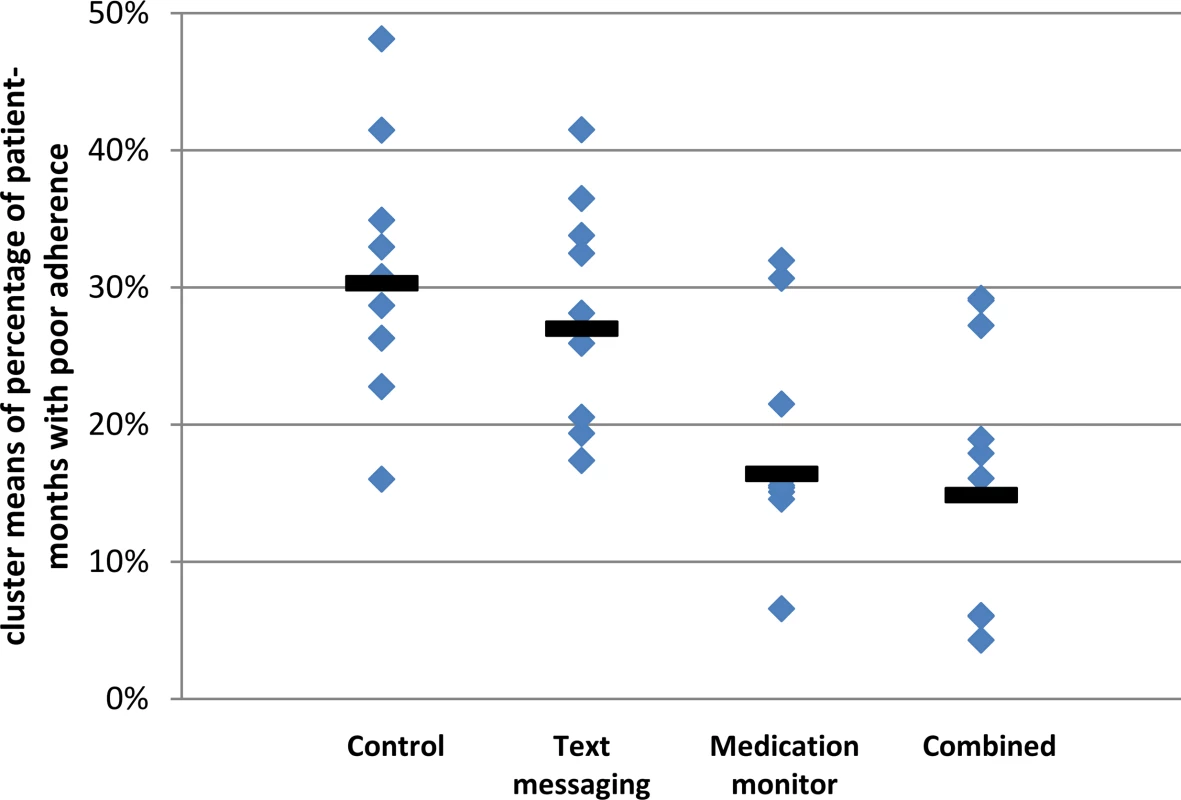
Solid bars represent geometric means of cluster-level proportions. Tab. 3. Effectiveness of interventions on tuberculosis treatment adherence and treatment outcomes endpoints. 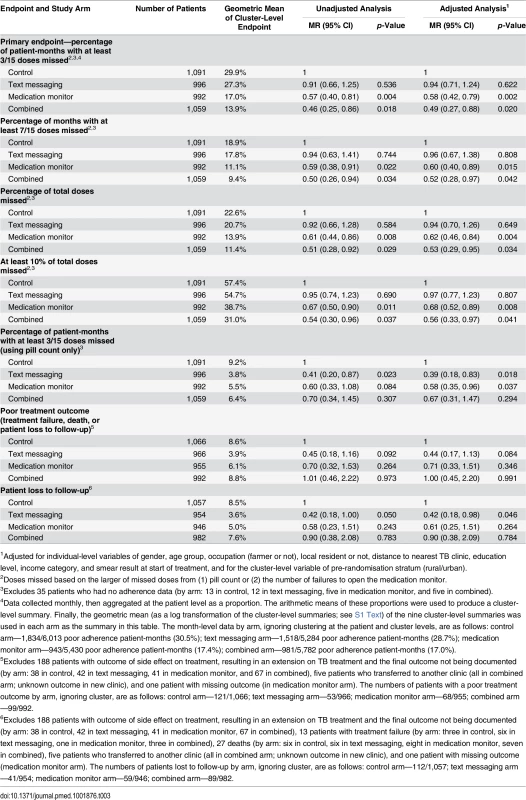
1Adjusted for individual-level variables of gender, age group, occupation (farmer or not), local resident or not, distance to nearest TB clinic, education level, income category, and smear result at start of treatment, and for the cluster-level variable of pre-randomisation stratum (rural/urban). Secondary study endpoints
There were similar reductions in the intervention arms versus the control arm in the percentage of months with at least 47% of doses missed (equivalent to 7/15 doses), the percentage of doses missed over the whole treatment period, and the percentage of patients who missed at least 10% of their doses, in both unadjusted and adjusted analyses (Table 3). The percentage of person-months with at least 20% of doses missed as judged by pill count only was much lower than that judged by both pill count and medication monitor data. This secondary endpoint was reduced by 33%–61% in the three intervention arms compared to the control arm, but this reduction was mostly driven by the imputation of months with 100% non-adherence following loss to follow-up (Table 3).
The text messaging arm had a lower patient loss to follow-up and occurrence of poor treatment outcome than the control arm. Modest reductions in patient loss to follow-up were also seen for the medication monitor and combined arms, though confidence intervals for the effect estimates included one. A post hoc sensitivity analysis that censored adherence measurement at the time of loss to follow-up showed a strengthening of the evidence for a reduction in poor adherence as measured by pill count in the three intervention arms, but otherwise very similar results (S4 Table). There were too few patients with data on sputum conversion at 2 mo for a formal analysis (summary data in S3 Text).
Problems with the medication monitor box, recorded either by the doctor at the monthly visit or by the medication monitor as power interruption, were more common in the medication monitor (49.4% of patients; Table 4) and combined arms (48.0%) than in the control (17.8%) or text messaging arms (16.7%). Stratified analysis of the primary endpoint by noted medication monitor problems showed that the reduction in poor adherence persisted in the medication monitor and combined arms regardless of whether there were power problems (S5 Table).
Tab. 4. Intervention process data and medication monitoring data by study arm. 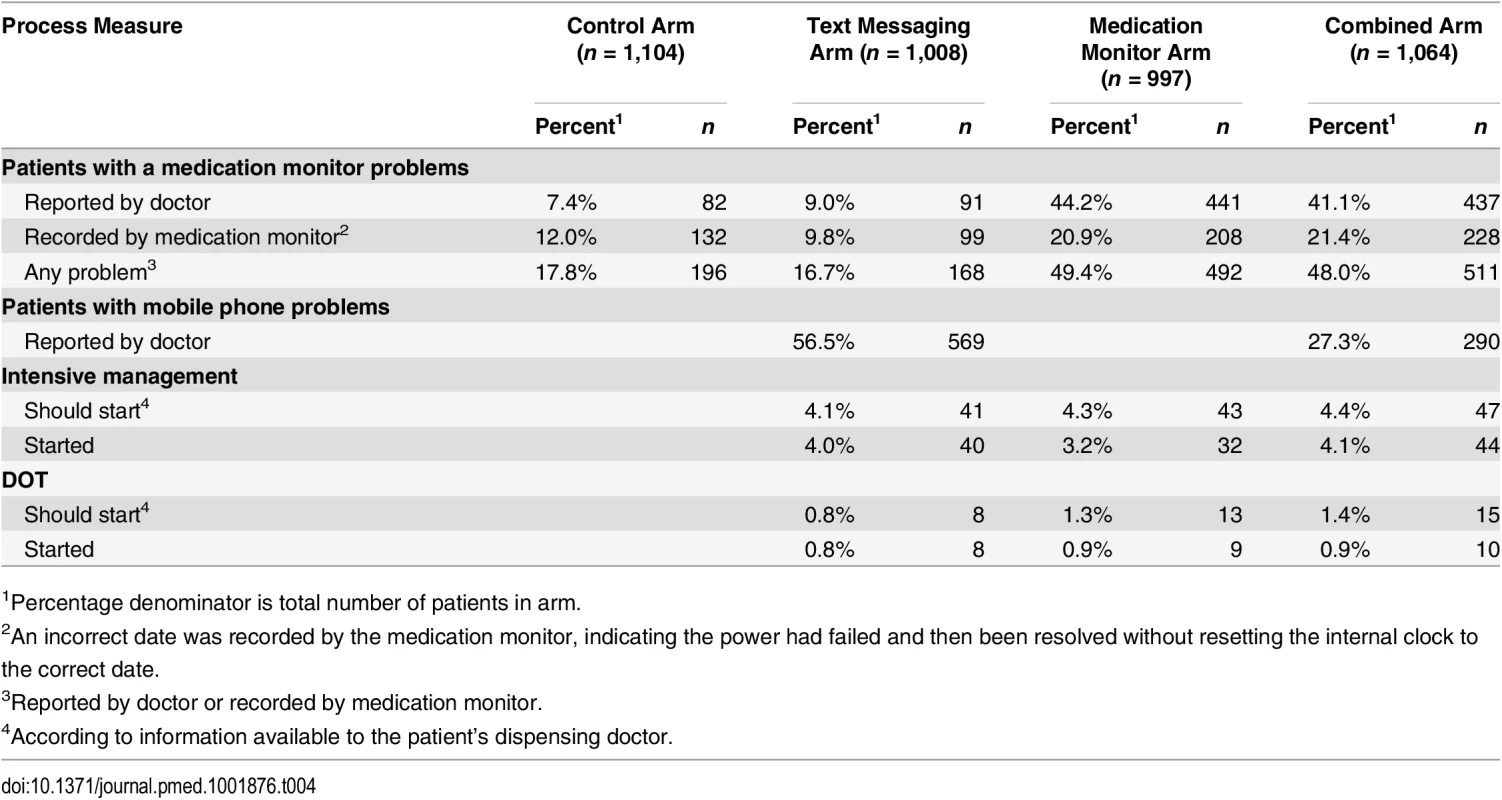
1Percentage denominator is total number of patients in arm. Process Measures
Similar percentages of patients in the three intervention arms were switched to intensive management (3.2%–4.1%) and DOT (0.8%–0.9%) (Table 4). Based on combining the data from pill counts and the medication monitor, the percentages of patients who should have been switched to intensive management or DOT, were 26.3% and 35.4%, respectively, in the text messaging arm, 16.2% and 24.1% in the medication monitor arm and 16.0% and 20.1% in the combined arm.
Minor problems with the mobile phones used to receive text messages were also common and were reported by 56.5% of those in the text messaging arm and 27.3% of those in the combined arm (Table 4). These problems included incorrect usage of the phone by the patient (42.0%), network failure (21.1%), and no money on the phone account (14.9%). Problems with the medication monitor or phone were resolved in 88.7% of occurrences (S6 Table).
Discussion
Our study found that the use of a medication monitor to remind TB patients to take their drugs reduced poor medication adherence by 40%–50% compared to the standard of care in China’s National Tuberculosis Control Program. This reduction was seen for all TB treatment adherence measures in this study. The use of text messaging did not reduce poor medication adherence but did reduce patient loss to follow-up by 58%. The use of a medication monitor alone resulted in a smaller, and not statistically significant, reduction in patient loss to follow-up compared to control; however, the study was not powered for this treatment outcome.
Even though many types of EMP devices exist and have been used for different disease conditions, a recent systematic review concluded that there were limited data supporting their use in improving adherence [9]. This study is the first to our knowledge to utilise a randomised trial design to demonstrate the effectiveness of an EMP device in improving medication adherence in TB patients. The use of our medication monitor box was integrated into the public health management of TB treatment. Such integration seems to be more frequently associated with improved medication adherence [9,10]. As the largest study to date, to our knowledge, to evaluate an EMP device for any disease condition, this study provides important evidence supporting the use of EMP to improve medication adherence.
Our results demonstrate that text messaging did not reduce poor medication adherence among TB patients. This contrasts with available evidence supporting the use of text messaging among HIV patients on antiretroviral therapy [7], which led to a strong recommendation from WHO for its use [13]. However, not all text messages are effective. There was a trend for greater effects of an intervention with texts that were less frequent than daily and with more personalised messages [7]. Frequent medication reminders using text messages can result in user fatigue. And some experts suggest that the most important factor is whether patients feel cared for, not the length or frequency of the text messages. Perhaps the lack of a more personalised engagement, the didactic nature of our messages, multiple messages per day, and the SMS message being received when the patient was not in close proximity to his/her medication all contributed to the failure to reduce poor adherence. There has been recent interest in using mobile phone technology to improve adherence to TB medication [5,14] and TB treatment outcomes [15], though, as yet, few studies have reported their findings [16].
In our intervention arms, we recommended that doctors switch patients to intensive patient management or DOT when adherence problems were documented. However, this rarely happened, despite data suggesting that a substantial percentage of patients should have switched. The trial was designed to be pragmatic, and so we did not enforce the initiation of more intensive management or DOT. Because problems with medication monitors, mobile phones, or their use were frequently reported, it is possible that doctors largely chose to ignore the electronic adherence data when deciding whether to switch patients to more intensive case-management approaches. In addition, doctors may not have had sufficient financial incentives to carry out more intensive case management.
Even though more intensive case-management approaches were underutilised in the presence of recorded treatment non-adherence, we still observed better adherence in the medication monitor and combined arms. This suggests that the use of a medication monitor to remind patients to take their medications can improve treatment adherence by itself. If information on poor treatment adherence had been used by providers to switch patients to more intensive case-management approaches, as intended, it is likely we would have seen an even greater reduction in poor treatment adherence with the use of medication monitors.
Interestingly, text messaging reduced the risk of patient loss to follow-up. Perhaps text messaging is an effective approach to remind patients of follow-up visits and resulted in better attendance at monthly visits. However, a recent meta-analysis suggests that the effectiveness of SMS reminders for appointments is modest at best and not more effective than other types of reminders [6,17].
The differences in the effects of the interventions in terms of adherence and treatment outcome endpoints suggest these do not correlate well. However, adherence is complex, and a recent taxonomy divides it into three constructs—initiation (patient takes the first dose), implementation (measure of how patient’s actual dosing history corresponds to the prescribed dosing regimen from initiation until the last dose is taken), and discontinuation (patient stops taking the prescribed medication) [18]. Given that our primary adherence endpoint is predominantly “implementation”, the seemingly discrepant results are not surprising; the evidence did not change when the primary endpoint was restricted to “implementation” only, in a post hoc sensitivity analysis. We defined poor adherence based on a threshold of 20% missed doses, a threshold commonly used in other disease areas [19].
Our study had several limitations. First, the battery problems with the medication monitors in our study led to loss of data in some patients, potentially resulting in an over-estimation of poor adherence. However, when we performed a stratified analysis using patient-months with or without this problem, we found similar reductions in poor treatment adherence. Second, more intensive case-management approaches were underutilised, possibly because doctors disregarded information from the medication monitor or SMS feedback. In addition, the financial incentives given to the doctors to perform more intensive management may have been inadequate. Third, for the adherence endpoints, we assumed that opening the medication monitor box was synonymous with drug intake, which may not have been the case, though our measure of poor adherence using a combination of this and pill count is arguably more robust than pill count alone. Pill counts have often been shown to under-report poor adherence or non-adherence [18], as is also shown in our study, where the geometric mean for the adherence endpoint measured using pill count only was lower than that of the primary endpoint. Further, data from a separate study indicated high correlation between adherence measured by medication monitor and rifampicin detected in urine [20]. Other limitations included differences in percentages enrolled by study arm, baseline imbalance of some factors, the unmasked nature of the trial, and the study not being powered for the treatment outcome endpoints. In spite of these limitations, this is the largest study to date, to our knowledge, to evaluate the use of text messaging or medication monitors to improve medication adherence in TB patients. As a pragmatic trial, implemented by the National Tuberculosis Control Program and mimicking real-world conditions, this study has produced useful lessons for future study designs.
The use of a medication monitor as a reminder for drug intake in combination with the identification of patients requiring more intensive management has been suggested as an approach for improving TB treatment adherence [11]. This is the first study to our knowledge to rigorously evaluate such an approach. Based on our results, the use of a medication monitor shows great promise. In a setting such as China, where universal use of DOT is not feasible, innovative approaches that help patients adhere to TB treatment are needed. The development of a low-cost and reliable medication monitor, as well as evidence that its use can improve clinical outcomes, could enable widespread use of medication monitors in national TB control programmes.
Supporting Information
Zdroje
1. World Health Organization. Global tuberculosis report 2014. Geneva: World Health Organization; 2014.
2. Wang L, Liu J, Chin DP. Progress in tuberculosis control and the evolving public-health system in China. Lancet. 2007;369 : 691–696. 17321314
3. Hou WL, Song FJ, Zhang NX, Dong XX, Cao SY, Yin XX, et al. Implementation and community involvement in DOTS strategy: a systematic review of studies in China. Int J Tuberc Lung Dis. 2012;16 : 1433–1440. doi: 10.5588/ijtld.12.0080 23044444
4. Wang L, Zhang H, Ruan Y, Chin DP, Xia Y, Cheng S, et al. Tuberculosis prevalence in China, 1990–2010; a longitudinal analysis of national survey data. Lancet. 2014;383 : 2057–2064. doi: 10.1016/S0140-6736(13)62639-2 24650955
5. Denkinger CM, Grenier J, Stratis AK, Akkihal A, Pant-Pai N, Pai M. Mobile health to improve tuberculosis care and control: a call worth making. Int J Tuberc Lung Dis. 2013;17 : 719–727. doi: 10.5588/ijtld.12.0638 23541232
6. Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, et al. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10:e1001362. doi: 10.1371/journal.pmed.1001362 23349621
7. Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS ONE. 2014;9:e88166. doi: 10.1371/journal.pone.0088166 24505411
8. Albino S, Tabb KM, Requena D, Egoavil M, Pineros-Leano MF, Zunt JR, et al. Perceptions and acceptability of short message services technology to improve treatment adherence amongst tuberculosis patients in Peru: a focus group study. PLoS ONE. 2014;9:e95770. doi: 10.1371/journal.pone.0095770 24828031
9. Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS. Electronic medication packaging devices and medication adherence: a systematic review. JAMA. 2014;312 : 1237–1247. doi: 10.1001/jama.2014.10059 25247520
10. Demonceau J, Ruppar T, Kristanto P, Hughes DA, Fargher E, Kardas P, et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. Drugs. 2013;73 : 545–562. doi: 10.1007/s40265-013-0041-3 23588595
11. Moulding TS. Viewpoint: adapting to new international tuberculosis treatment standards with medication monitors and DOT given selectively. Trop Med Int Health. 2007;12 : 1302–1308. 17949402
12. Hayes RJ, Moulton LH. Cluster randomised trials. Boca Raton (Florida): Chapman & Hall/CRC Press; 2009.
13. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2013.
14. Barclay E. Text messages could hasten tuberculosis drug compliance. Lancet. 2009;373 : 15–16. 19125443
15. Bediang G, Stoll B, Elia N, Abena JL, Nolna D, Chastonay P, et al. SMS reminders to improve the tuberculosis cure rate in developing countries (TB-SMS Cameroon): a protocol of a randomised control study. Trials. 2014;15 : 35. doi: 10.1186/1745-6215-15-35 24460827
16. Nglazi MD, Bekker LG, Wood R, Hussey GD, Wiysonge CS. Mobile phone text messaging for promoting adherence to anti-tuberculosis treatment: a systematic review. BMC Infect Dis. 2013;13 : 566. doi: 10.1186/1471-2334-13-566 24295439
17. Gurol-Urganci I, de Jongh T, Vodopivec-Jamsek V, Atun R, Car J. Mobile phone messaging reminders for attendance at healthcare appointments. Cochrane Database Syst Rev. 2013;12:CD007458. doi: 10.1002/14651858.CD007458.pub3 24310741
18. Vrijens B, Urquhart J, White D. Electronically monitored dosing histories can be used to develop a medication-taking habit and manage patient adherence. Expert Rev Clin Pharmacol. 2014;7 : 633–644. doi: 10.1586/17512433.2014.940896 25088004
19. Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133 : 21–30. 10877736
20. Huan S, Chen R, Liu X, Ou X, Jiang S, Zhao Y, et al. Operational feasibility of medication monitors in monitoring treatment adherence among TB patients. Chin J Antituberculosis. 2012;34 : 419–424.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 9- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Magnosolv a jeho využití v neurologii
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- The Impact of Company-Level ART Provision to a Mining Workforce in South Africa: A Cost–Benefit Analysis
- Evolution of Extensively Drug-Resistant Tuberculosis over Four Decades: Whole Genome Sequencing and Dating Analysis of Isolates from KwaZulu-Natal
- Simplified HIV Testing and Treatment in China: Analysis of Mortality Rates Before and After a Structural Intervention
- Asporin Is a Fibroblast-Derived TGF-β1 Inhibitor and a Tumor Suppressor Associated with Good Prognosis in Breast Cancer
- Four Proposals to Help Improve the Medical Research Literature
- Adding Spice to the Slog: Humanities in Medical Training
- Equity and Noncommunicable Disease Reduction under the Sustainable Development Goals
- Moving Beyond Directly Observed Therapy for Tuberculosis
- Effectiveness of Electronic Reminders to Improve Medication Adherence in Tuberculosis Patients: A Cluster-Randomised Trial
- Changes in Intake of Fruits and Vegetables and Weight Change in United States Men and Women Followed for Up to 24 Years: Analysis from Three Prospective Cohort Studies
- Value of Information: A Tool to Improve Research Prioritization and Reduce Waste
- Uptake, Accuracy, Safety, and Linkage into Care over Two Years of Promoting Annual Self-Testing for HIV in Blantyre, Malawi: A Community-Based Prospective Study
- Effectiveness of a Home-Based Counselling Strategy on Neonatal Care and Survival: A Cluster-Randomised Trial in Six Districts of Rural Southern Tanzania
- Selective Serotonin Reuptake Inhibitors and Violent Crime: A Cohort Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Asporin Is a Fibroblast-Derived TGF-β1 Inhibitor and a Tumor Suppressor Associated with Good Prognosis in Breast Cancer
- Simplified HIV Testing and Treatment in China: Analysis of Mortality Rates Before and After a Structural Intervention
- Effectiveness of Electronic Reminders to Improve Medication Adherence in Tuberculosis Patients: A Cluster-Randomised Trial
- Changes in Intake of Fruits and Vegetables and Weight Change in United States Men and Women Followed for Up to 24 Years: Analysis from Three Prospective Cohort Studies
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



