-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAssociations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study
Robert A.
Scott and colleagues use genetic instruments to identify causal associations between known risk factors and Alzheimer's disease.
Published in the journal: . PLoS Med 12(6): e32767. doi:10.1371/journal.pmed.1001841
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001841Summary
Robert A.
Scott and colleagues use genetic instruments to identify causal associations between known risk factors and Alzheimer's disease.Introduction
Alzheimer disease (AD) prevalence is rising [1], further increasing the social and economic burden of this disease [2]. Epidemiological studies have aimed to identify potentially modifiable risk factors that could be targeted in preventive measures to reduce the incidence of AD. These include type 2 diabetes (T2D) and glycemic traits [3,4], hypertension [5], obesity [6], dyslipidemia [7], smoking [8], physical inactivity [5], depression [9], and low educational attainment [5]. It has been reported that approximately one-third of AD cases worldwide may be attributable to these risk factors [9]. However, this suggestion is predicated on these risk factors having causal effects on AD risk, which is currently uncertain [9]. Given the difficulties in implementing large-scale randomized trials of risk factor modification, alternative approaches are required to investigate the causality of associations and to prioritize the targets for which interventions may be most fruitful [10].
One method for estimating the causal effects of risk factors with known genetic determinants is Mendelian randomization (MR) [11]. The MR approach exploits the fact that genotypes are randomly assorted at meiosis, and are thus independent of conventional confounding factors and the disease process. Therefore, genetic variants associated with intermediate traits can be used to provide an unconfounded estimate of the causal association between the intermediate trait and disease outcome, unaffected by reverse causality. This is akin to a “genetically randomized trial.” For example, if body mass index (BMI) is causally associated with AD, genetic variants causing higher BMI should also be associated with higher risk of AD. However, if an observed BMI—AD association is not causal but is due to confounding or reverse causation, genetic variants causing higher BMI would not result in higher risk of AD. Here, we sought to estimate the causal effects of potentially modifiable risk factors on risk of AD using MR to inform the etiology of AD and the extent to which AD may be preventable by interventions targeting potentially modifiable risk factors.
Methods
Study Design
We performed MR analyses using single nucleotide polymorphisms (SNPs) with known associations with potentially modifiable AD risk factors. We used summary statistics from the International Genomics of Alzheimer’s Project (IGAP) [12], the largest genome-wide meta-analysis of AD reported to date, and individual genotype data from a large subset of IGAP to estimate the unconfounded association between each risk factor and AD risk. S1 Fig illustrates the study design.
SNPs Associated with Alzheimer Disease Risk Factors
We identified SNPs that had genome-wide significant (p < 5 × 10−8) associations with each risk factor using the largest published genome-wide meta-analysis available in individuals of European ancestry. We identified 49 SNPs associated with T2D [13], 36 with fasting glucose [14], and ten with insulin resistance [14,15]. We identified 32 SNPs associated with BMI [16] and 25 associated with systolic blood pressure (SBP) [17]. Given the overlap of SNPs associated with systolic, diastolic, mean arterial, and pulse pressures, we focused on SBP, which had the largest number of associated SNPs [17,18]. We identified 74 SNPs associated with total cholesterol, 71 with high-density lipoprotein (HDL)–cholesterol, 58 with low-density lipoprotein (LDL)–cholesterol, and 40 with triglycerides [19]. We identified one SNP associated with smoking initiation (rs6265 in BDNF; r2 = 0.74 with the BDNF BMI-associated variant), and three associated with smoking quantity in smokers [20]. We identified two SNPs associated with the probability of completing university and one associated with the number of years of education [21]. We show the SNPs and their associations with their relative traits in S1 Table. Where lead SNPs were not available, we selected a suitable proxy (r2 > 0.8; except for rs4420638, where the best available proxy was rs6857 [r2 = 0.46]), as detailed in S1 Table. Within each trait, no SNPs were in linkage disequilibrium (LD) (r2 < 0.01). No SNPs have been reported to be associated with physical activity levels or depression at p < 5 × 10−8.
Alzheimer Disease Genetic Data
IGAP is a large two-stage study based upon genome-wide association studies (GWASs) of AD in individuals of European ancestry [12]. In stage 1, IGAP used genotyped and imputed data on 7,055,881 SNPs to meta-analyze four previously published GWAS datasets consisting of 17,008 AD cases and 37,154 controls (full details in S1 Text). Further details on the original genetic discovery analyses, including information regarding recruitment and diagnostic assessment as well as analytical approaches to adjust for population structure, are provided in S1 Text or described in detail elsewhere [12]. We extracted individual SNP associations with AD from IGAP’s stage 1 results. Three SNPs (rs850303 for SBP; rs3177928 for total and LDL-cholesterol; rs645040 for triglycerides) were not available (S1 Table), so were excluded from analyses.
Mendelian Randomization Analyses
We used estimated SNP—risk factor and SNP—AD associations to calculate estimates of each risk factor—AD association using an inverse-variance weighted combination of estimates from each SNP [22]. For continuous exposures (BMI, fasting glucose, insulin resistance, lipids, and SBP), we scaled MR estimates per standard deviation (SD) difference of the risk factor. Effect sizes on log-fasting insulin were used as weights for the insulin-resistance-associated variants. SDs were estimated from up to 10,445 (Nmin = 9,963) middle-aged adults from the UK population-based Fenland study [23]. Causal estimates are thus presented per genetically predicted SD, and a log-linear association with odds of AD is implicit across the range of intermediate risk factor values. We scaled smoking quantity per ten cigarettes per day and scaled educational attainment per year of education. For binary exposures (T2D, smoking initiation, completing university), MR estimates are odds ratios (ORs) per genetically predicted unit difference in log-odds of having the relevant exposure. Overall, we included 302 non-overlapping SNPs. To minimize the possibility of pleiotropic associations influencing results, we performed sensitivity analyses excluding SNPs with a more significant association with AD than expected by chance (p < 0.05/302 = 0.00017), which excluded only four variants in total (S1 Table). Furthermore, we investigated the association of each variant with the risk factor relative to the magnitude of association with AD risk to further identify variants that appeared to be outliers and were candidates to be pleiotropic. As a further sensitivity analysis, for risk factors that showed evidence of a causal association with AD (p < 3.8 × 10−3), we also performed a “leave one out” analysis to further investigate the possibility that the causal association was driven by a single SNP.
We also performed MR analyses of risk factors that showed evidence of a causal association with AD (p < 3.8 × 10−3) using individual-level SNP data from studies in the Alzheimer’s Disease Genetics Consortium (ADGC) (cases = 10,079; controls = 9,613) [24] and the Genetic and Environmental Risk in AD (GERAD1) Consortium (cases = 3,146; controls = 1,224) [25], which account for 51% of the IGAP effective sample size (see S1 Text for a description of the ADGC and GERAD1 samples). We performed logistic regression analyses of the SNP-predicted AD association adjusting for study site, population substructure, age, and sex, again scaled per 1-SD difference in risk factor.
We created unweighted genetic scores based on the number of risk alleles for each SNP—risk factor association and investigated the association of these scores with a range of traits in up to 16,554 individuals from the EPIC-InterAct study [26] to check the assumption that the SNPs used in the MR analyses are not associated with potential confounders of exposure—AD associations. We standardized outcomes and included scores in linear regression models adjusted for age, sex, recruitment center, and subcohort status. We natural-log-transformed triglyceride levels before standardization. We investigated the association of the SBP-associated variants with both SBP and diastolic blood pressure (DBP). We did not adjust observed blood pressure values for antihypertensive usage. We used logistic regression to determine associations with the probability of being physically active, being a smoker, or taking antihypertensive medications, and included covariates as above. The distribution of the SBP risk score in the EPIC-InterAct study [26] is shown in S2 Fig.
Results
Table 1 shows the estimated associations of each genetically predicted risk factor with AD from MR analysis using a large-scale international investigation of the genetic basis of AD risk in 17,008 individuals with AD and 37,154 controls. We observed evidence for a causal association between genetically predicted SBP and AD risk. A genetically predicted 1-SD (15.4 mm Hg) higher SBP was associated with lower risk of AD (OR [95% CI]: 0.75 [0.62–0.91]; p = 3.4 × 10−3). We examined each of the SBP SNPs to investigate if particular SNPs were driving the association with AD, but observed no obvious outliers (S3 Fig). Furthermore, when we performed all 24 permutations of the “leave one out” analysis, all SNP sets showed consistent evidence of causality (OR per SD of SBP [95% CI] ranged from 0.72 [0.59–0.87] to 0.78 [0.64–0.95]). Individual SNP associations with AD are shown in S1 Table. We also performed analyses on a subset of the overall sample using individual-level SNP data from ADGC and GERAD1, which showed results similar to those observed using the inverse-variance weighted approach (OR [95% CI]: 0.69 [0.55–0.85]; p = 2.0 × 10−3; Fig 1). We saw no evidence of heterogeneity between individual studies (p = 0.33).
Tab. 1. Estimated associations of each genetically predicted risk factor with Alzheimer disease. 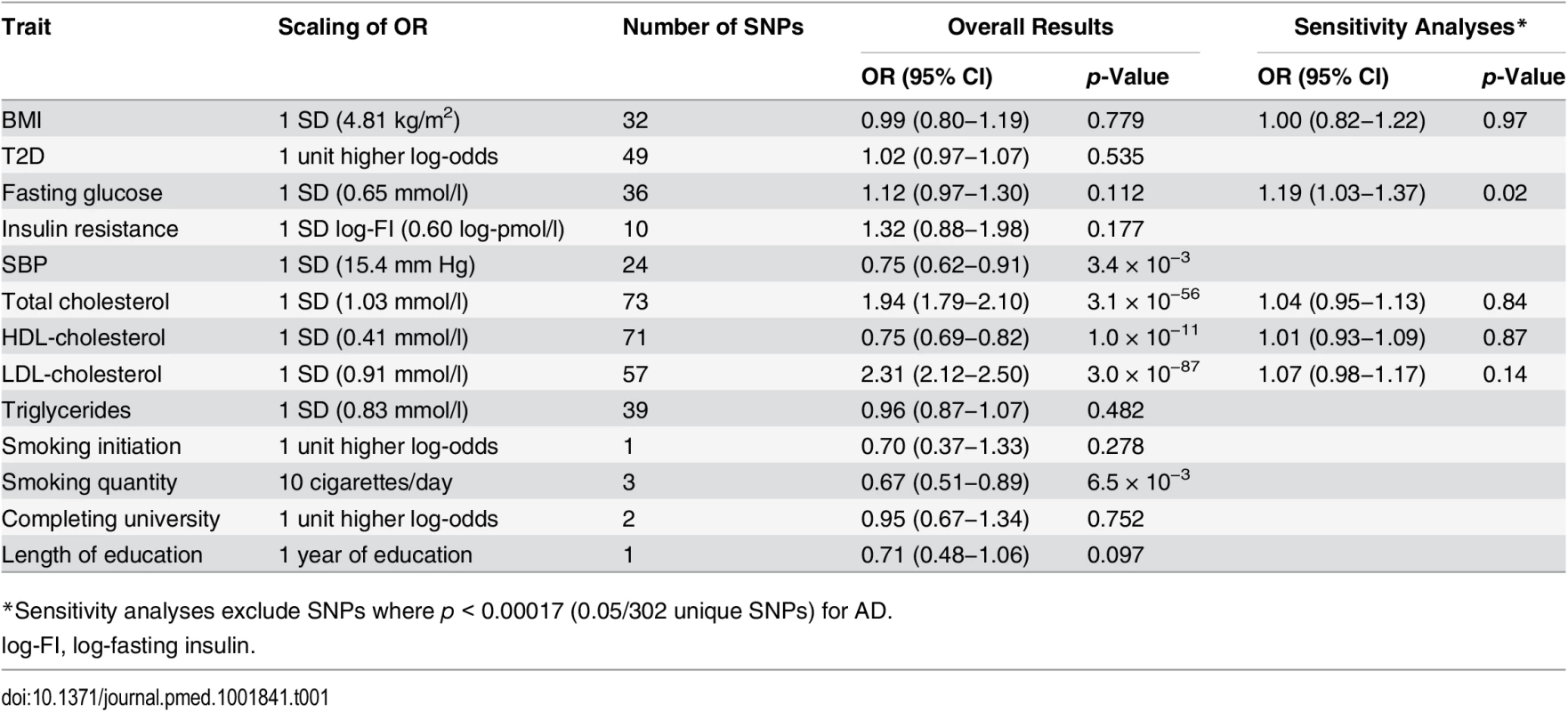
*Sensitivity analyses exclude SNPs where p < 0.00017 (0.05/302 unique SNPs) for AD. Fig. 1. Mendelian randomization estimates of the association of systolic blood pressure with AD in individual ADGC studies and overall in ADGC, GERAD1, and IGAP. 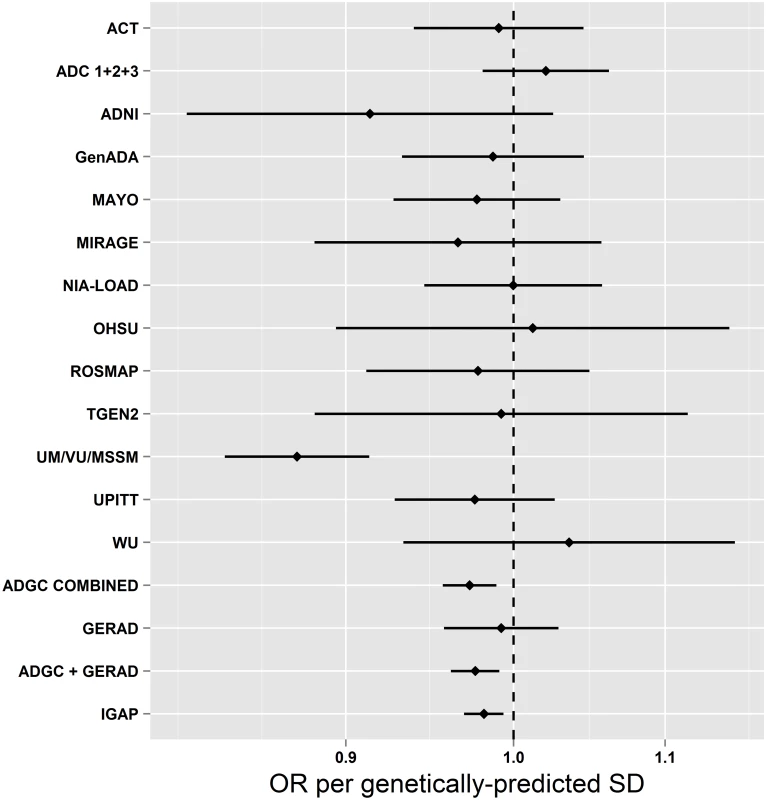
This figure shows MR estimates for the association of SBP-associated variants with AD in each of the participant studies in ADGC [24] and in GERAD1 [25] using individual SNP-level data compared to that observed in IGAP [12] using summary-level data. See S1 Text (supplemental results) for individual study name abbreviations. In the EPIC-InterAct study, the unweighted SBP genetic score was strongly associated with SBP and DBP overall (Fig 2) and in all age groups (Fig 3) (p < 0.015 for SBP; p < 0.002 for DBP). We did not observe associations of the SBP score with other potentially confounding variables in the EPIC-InterAct study (Fig 2). The unweighted SBP genetic score was associated with a higher probability of taking antihypertensive medication (OR [95% CI]: 1.05 [1.03–1.08]; p = 6.7 × 10−8) but not with the probability of being physically active or being a smoker (Fig 4). Forty-nine percent of the individuals in the highest quartile of the unweighted SBP genetic score reported taking antihypertensive medication compared to 39% in the lowest quartile.
Fig. 2. Associations of the systolic blood pressure genetic score with quantitative traits in the EPIC-InterAct study. 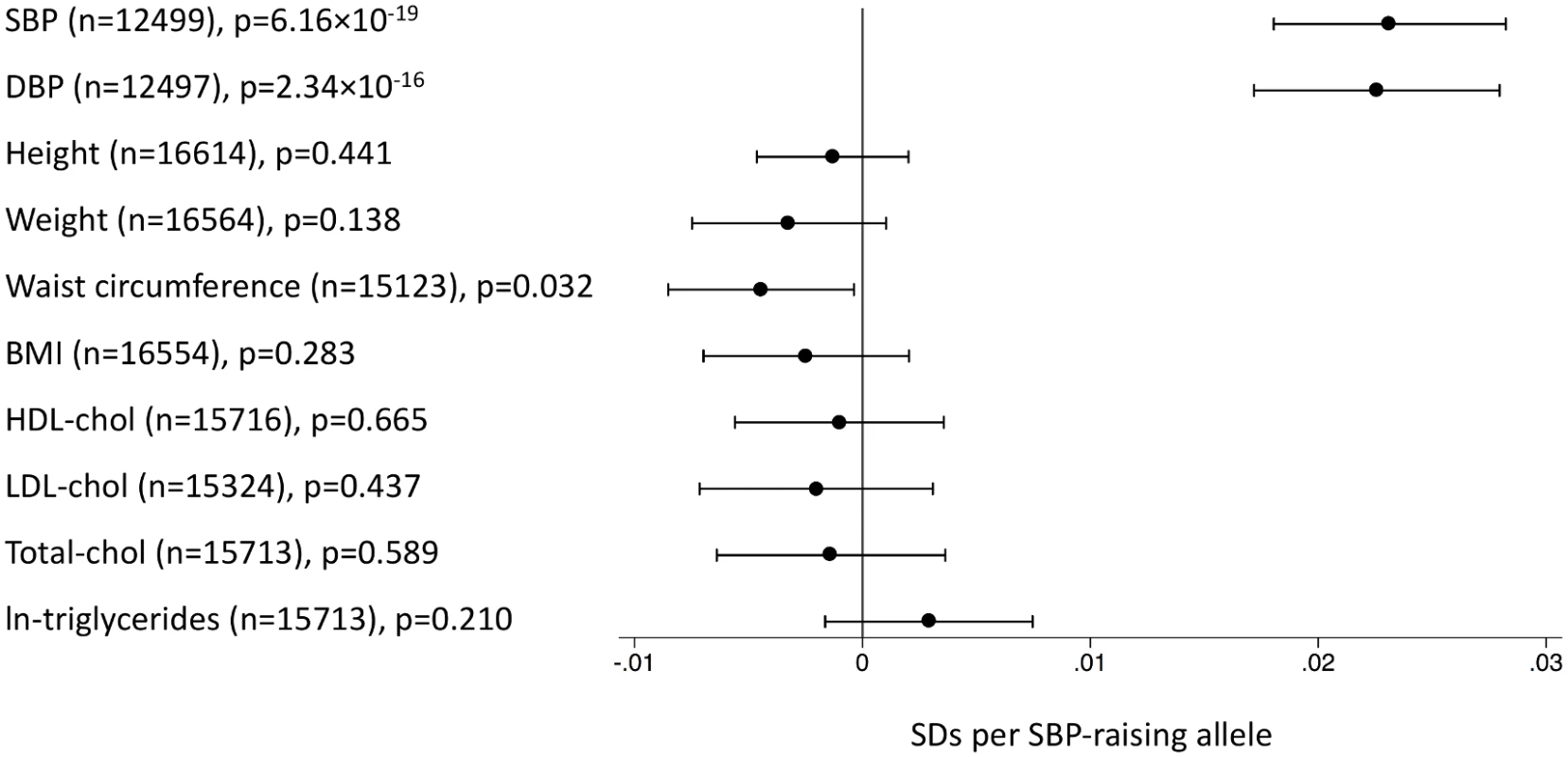
This figure shows the investigation of pleiotropic associations of genetic score for SBP with quantitative traits in the EPIC-InterAct study [26]. Effect sizes are expressed in SDs per SBP-raising allele. Analyses were adjusted for age, sex, center of recruitment, and subcohort status. Fig. 3. Association of the systolic blood pressure genetic score with systolic blood pressure by age stratum in the EPIC-InterAct subcohort. 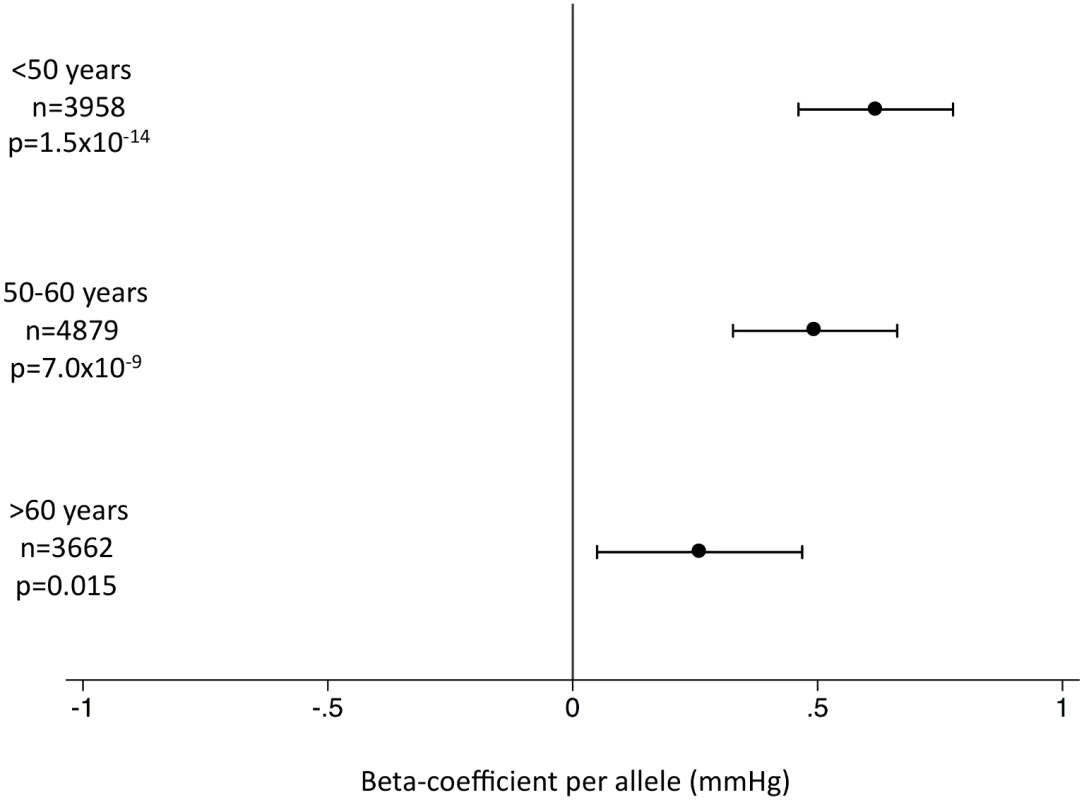
This figure shows the association between the genetic score for SBP and SBP in the EPIC-InterAct study by age stratum [26]. Analyses were adjusted for sex, center of recruitment, and subcohort status. Fig. 4. Associations of the systolic blood pressure genetic score with binary outcomes in the EPIC-InterAct study. 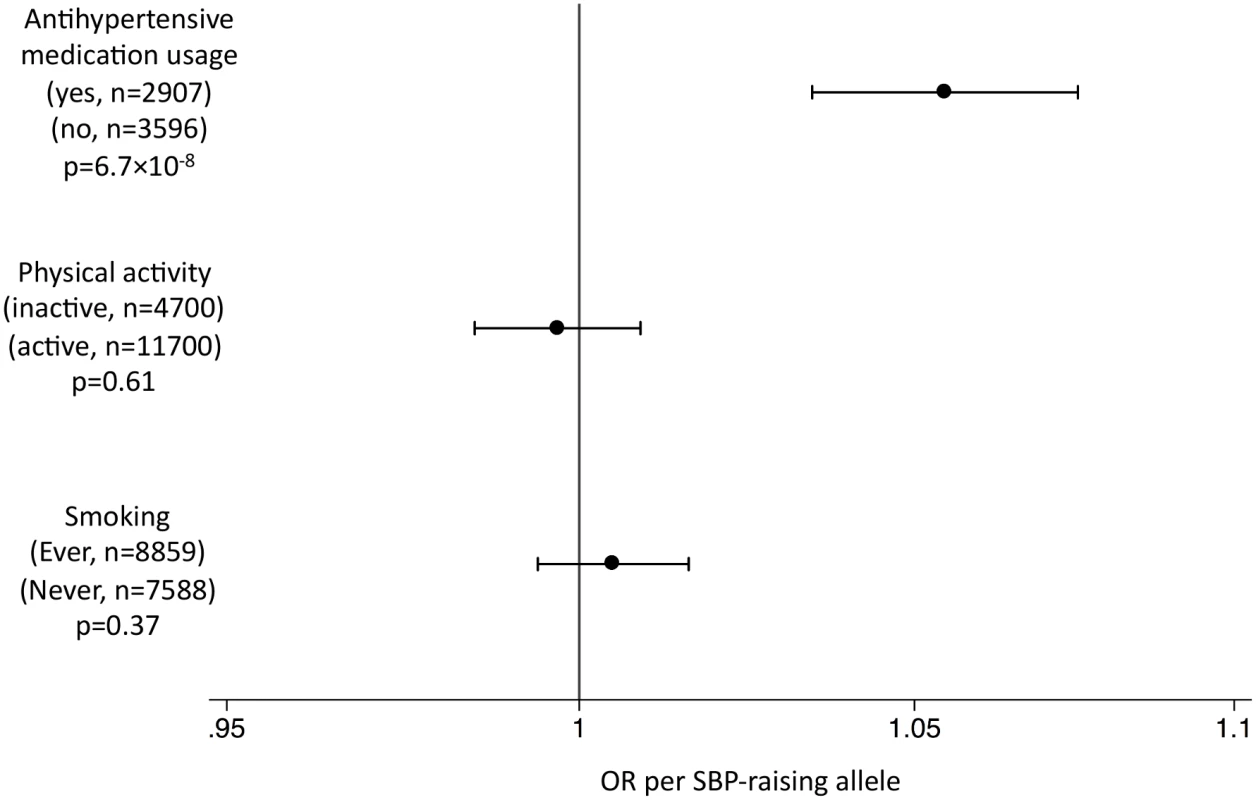
This figure shows the investigation of pleiotropic associations of the genetic score for SBP with binary outcomes in the EPIC-InterAct study [26]. The OR per SBP-raising allele is shown. We found strong associations between genetically predicted total, LDL-, and HDL-cholesterol and AD (Table 1). Each of these SNP sets included rs6857 near APOE, which is strongly associated with AD risk (OR: 3.2; p = 2.5 × 10−575) [12] (S4 and S5 Figs) and which was a very clear outlier when we compared effect sizes on lipids against effect sizes on AD (S6–S11 Figs). Two of these SNP sets also included rs1883025 from ABCA1, which was associated with AD at a significance level beyond that expected by chance (OR: 1.07; p = 1.0 × 10−4) [12] and which is in a gene previously implicated in association with AD [27]. After sensitivity analyses excluding these potentially pleiotropic SNPs, we saw no evidence for causal associations between lipid fractions and AD risk (Table 1).
We found no evidence to support causal associations between BMI and AD (OR per SD of BMI [95% CI]: 0.99 [0.80–1.19]; p = 0.78), fasting glucose (OR [95% CI]:1.12 [0.97–1.30]; p = 0.11), insulin resistance (OR [95% CI]: 1.32 [0.89–1.97]; p = 0.17), or T2D (OR [95% CI]: 1.01 [0.96–1.07]; p = 0.57) (Table 1). S4 Fig shows the associations with AD of the SNPs included in all genetic analyses compared to those expected by chance. Other than rs6857 near APOE, the most significant associations with AD were observed for rs11039149 (p = 3.7 × 10−6) in the fasting glucose SNPs and rs3817334 (p = 9.3 × 10−5) in the BMI SNPs (see S1 Table and S5 Fig). These SNPs are both in LD (r2 = 0.58 and r2 = 0.33, respectively) with a genome-wide significant association signal for AD in CELF1 [12]. After excluding these variants from their respective SNP sets, BMI results were unchanged (Table 1). However, for fasting glucose, following the removal of rs11039149 near MADD, there was a suggestion of an association between higher glucose and higher AD risk (OR [95% CI]: 1.19 [1.03–1.37]; p = 0.02).
We found no evidence to support causal associations between smoking initiation and AD (OR [95% CI]: 0.70 [0.37–1.33]; p = 0.28). We did find an association between genetically predicted higher smoking quantity and lower AD (OR per ten cigarettes/day [95% CI]: 0.67 [0.51–0.89]; p = 6.5 × 10−3). We did not have smoking behavior data for IGAP to obtain estimates for the association with AD among smokers and non-smokers. The SNP with the strongest association with smoking quantity [20] was nominally associated with AD risk (rs1051730: OR of AD per smoking-quantity-raising allele [95% CI]: 0.96 [0.93–0.99]; p = 0.01), while the others were not (S12 Fig). We saw no association between AD risk and either university completion (OR [95% CI]: 0.95 [0.67–1.34]; p = 0.75) or years of education (OR [95% CI]: 0.71 [0.48–1.06]; p = 0.10) (Table 1).
Discussion
The potential of risk factor modifications to impact upon AD incidence depends entirely on causal links between the risk factors and AD. Using genetic variants associated with risk factors for AD in a very large consortium of well-characterized research participants, we found evidence for an association between genetically inherited higher levels of blood pressure and lower AD risk.
Hypertension has been implicated as a risk factor for AD [5]. However, uncertainties remain over the nature of the association, perhaps complicated by misclassification of AD with other forms of dementia, or the age of study participants [28]. While previous studies have suggested that high blood pressure in midlife is associated with higher AD risk [29,30], other studies have indicated that high blood pressure in late life may be protective against AD [31,32]. We found that genetically inherited higher SBP levels are associated with lower risk of AD (Table 1). Previous studies have suggested that hypotension may indeed be a risk factor for AD, particularly in the elderly [33], potentially via resultant cerebral hypoperfusion [34]. The unweighted SBP gene score was associated with higher SBP levels across the adult lifespan (Fig 3). It should be noted that the SNPs associated with SBP overlap extensively with those associated with DBP [17] as well as with pulse pressure [18], so we were unable to distinguish between individual components of blood pressure. A recent meta-analysis of prospective studies suggested that a 10-mm Hg higher SBP was associated with a protective relative risk of 0.95 (95% CI: 0.91–1.00) for AD [28]. Scaling our results to a genetically predicted 10-mm Hg difference in SBP would result in an OR of 0.83 (95% CI: 0.73–0.94) for AD. Clearly, given that blood pressure is a major risk factor for cardiovascular disease [35], one would not advocate raising blood pressure as a preventive strategy, yet these findings offer intriguing etiological insight.
We also found that genetically predicted higher SBP was associated with a higher probability of being on antihypertensive medication (Fig 4). There is considerable interest in the role of antihypertensives in dementia, and while findings are equivocal [36], recent studies have suggested a possible protective effect of antihypertensive therapy on AD risk [37], potentially with heterogeneity of effect by therapeutic class [38], suggesting that any effect on AD risk may not be entirely attributable to the lowering of blood pressure, but potentially to other mechanisms. The unweighted SBP gene score was strongly associated with observed SBP in the EPIC-InterAct study, ignoring any SBP-lowering effects of antihypertensive medications. Thus, if antihypertensive medications are indeed protective and confound the association between genetically predicted SBP and AD, their effect on AD risk is likely to be independent of their effect on SBP, as the SBP-associated variants have a strong association with SBP regardless of the higher prevalence of treatment with antihypertensive medication. While the null association between genetically predicted lipid levels and AD risk reflects the equivocal findings from trials of statins and cognitive decline [39], our results suggest the imperative need for further investigation of the possibility that antihypertensive medications may reduce AD risk independently of their effects on blood pressure. Future MR analyses stratified by antihypertensive treatments would be desirable to more precisely estimate the magnitude of the causal effect of higher BP on AD risk, but will be difficult to carry out using existing data due to the time-varying nature of antihypertensive treatments across the life course, and the non-availability of data on lifetime medication usage in most studies.
We also observed an association between AD and smoking quantity (Table 1). Early reports implicated smoking as protective for AD [40], potentially via a neuroprotective effect of nicotine [41]. However, this association may be due to differential survival bias [42], and a recent meta-analysis of prospective studies implicates smoking as a risk factor for AD, showing current smokers as being at higher risk of AD than never smokers [8]. One smoking-quantity-related SNP was associated with AD (p = 0.01). This SNP is in the gene CHRNA3 from the nicotinic receptor gene cluster CHRNA5-CHRNA3-CHRNB4. Given the putative actions of nicotine, variants in this locus may confer neuroprotective effects by influencing nicotinic receptor function [41,43]. Thus, altered nicotinic receptor function may underlie the MR association between smoking quantity and AD risk. The ideal study would perform MR analyses stratified by smoking status [44], particularly if sensitivity analyses could exclude variants in nicotinic receptor genes. Such analyses would address the causality of smoking as a risk factor, and offer valuable insight into nicotine’s role in the etiology of AD [41,43]. Since smoking is a major cause of global disease burden [45], increasing knowledge of the role of nicotine in the etiology of AD may prove to be the more actionable insight.
Our findings for total, LDL-, and HDL-cholesterol are not consistent with a causal effect of major lipid fractions on AD risk, as previously suggested in a smaller study [46]. Rather, the well-established association of APOE haplotypes with AD risk [47] implicates APOE itself as a key causal factor in the etiology of AD. Indeed a recent GWAS of plasma APOE levels identified only genetic variants in APOE, and not those in other lipid loci, as being associated with APOE levels at genome-wide significance [48]. When we compared the effect sizes for the effects of SNPs on major lipids relative to the magnitude of their association with AD, the APOE variant was a very clear outlier (S6–S11 Figs).
We did not find evidence consistent with a causal role for the other potentially modifiable risk factors we evaluated (Table 1). In our sensitivity analysis that excluded the potentially pleiotropic variant near MADD, genetically predicted higher fasting glucose was nominally associated with higher AD risk. While these results are consistent with the notion that higher blood glucose may be causally related to AD risk [4], the borderline significance warrants a cautious interpretation.
A limitation of the MR approach is the limited strength of the SNPs to explain variation in the intermediate traits, restricting statistical power. This is particularly true when findings are null, where narrow confidence intervals are important to aid robust inference. For example, while we saw no evidence to support causal roles for BMI, fasting glucose, or insulin resistance in AD (all p > 0.1), confidence intervals allow for an almost 20% higher AD risk per 1-SD difference in BMI, a 30% higher AD risk per 1-SD difference in fasting glucose, and an almost 100% higher AD risk per 1-SD difference in log-fasting insulin (Table 1). Thus, improving the intermediate trait variance explained by the instrumental variables by further genetic discovery efforts will improve the precision of MR analyses. Likewise, ever larger AD GWASs will further narrow confidence intervals around MR estimates. The association of genetically predicted blood pressure with AD risk remained after Bonferroni correction for the 13 individual SNP sets we tested (0.05/13 = 3.8 × 10−3), although the association of the smoking-associated variants did not. However, we consider this a conservative correction, given the correlation between the intermediate risk factors. We cannot exclude the possibility that the protective associations of blood pressure with AD arise as a result of differential survival bias, but the consistency of the observations across both prospective and cross-sectional studies of AD makes this less likely (Fig 1), as does the absence of similar MR associations for other major vascular risk factors (Table 1).
The main data source for this study is the summary statistics from IGAP, the largest genome-wide meta-analysis of AD reported to date [12]. Since all participants in IGAP are of European ancestry, the results of this study are not necessarily valid for other ethnic groups.
In conclusion, we found associations between genetically predicted higher SBP and lower AD risk. This finding is contrary to the notion that societal interventions to lower blood pressure will reduce the incidence of AD. However, since there is a strong association between higher SBP gene scores and exposure to antihypertensive treatments, there is a need to evaluate the possible protective role of some of these substances against AD, independent of their effects on blood pressure.
Supporting Information
Zdroje
1. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9 : 63–75.e2. doi: 10.1016/j.jalz.2012.11.007 23305823
2. Reitz C, Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol. 2014;88 : 640–651. doi: 10.1016/j.bcp.2013.12.024 24398425
3. Vagelatos NT, Eslick GD. Type 2 diabetes as a risk factor for Alzheimer’s disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev. 2013;35 : 152–160. doi: 10.1093/epirev/mxs012 23314404
4. Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, et al. Glucose levels and risk of dementia. N Engl J Med. 2013;369 : 540–548. doi: 10.1056/NEJMoa1215740 23924004
5. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10 : 819–828. doi: 10.1016/S1474-4422(11)70072-2 21775213
6. Anstey KJ, Cherbuin N, Herath PM. Development of a new method for assessing global risk of Alzheimer’s disease for use in population health approaches to prevention. Prev Sci. 2013;14 : 411–421. doi: 10.1007/s11121-012-0313-2 23319292
7. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64 : 277–281. 15668425
8. Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8 : 36. doi: 10.1186/1471-2318-8-36 19105840
9. Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13 : 788–794. doi: 10.1016/S1474-4422(14)70136-X 25030513
10. Burgess S, Butterworth A, Malarstig A, Thompson SG. Use of Mendelian randomisation to assess potential benefit of clinical intervention. BMJ. 2012;345:e7325. doi: 10.1136/bmj.e7325 23131671
11. Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32 : 1–22. 12689998
12. Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45 : 1452–1458. doi: 10.1038/ng.2802 24162737
13. Morris AP, Voight BF, Teslovich TM, Ferreira T, Segre AV, Steinthorsdottir V, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44 : 981–990. doi: 10.1038/ng.2383 22885922
14. Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44 : 991–1005. doi: 10.1038/ng.2385 22885924
15. Scott RA, Fall T, Pasko D, Barker A, Sharp SJ, Arriola L, et al. Common genetic variants highlight the role of insulin resistance and body fat distribution in type 2 diabetes, independently of obesity. Diabetes. 2014;63 : 4378–4387. doi: 10.2337/db14-0319 24947364
16. Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42 : 937–948. doi: 10.1038/ng.686 20935630
17. International Consortium for Blood Pressure Genome-Wide Association Studies, Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478 : 103–109. doi: 10.1038/nature10405 21909115
18. Wain LV, Verwoert GC, O’Reilly PF, Shi G, Johnson T, Johnson AD, et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43 : 1005–1011. doi: 10.1038/ng.922 21909110
19. Global Lipids Genetics Consortium, Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, et al. Discovery and refinement of loci associated with lipid levels. Nat Genet. 2013;45 : 1274–1283. doi: 10.1038/ng.2797 24097068
20. Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42 : 441–447. doi: 10.1038/ng.571 20418890
21. Rietveld CA, Medland SE, Derringer J, Yang J, Esko T, Martin NW, et al. GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science. 2013;340 : 1467–1471. doi: 10.1126/science.1235488 23722424
22. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37 : 658–665. doi: 10.1002/gepi.21758 24114802
23. Rolfe Ede L, Loos RJ, Druet C, Stolk RP, Ekelund U, Griffin SJ, et al. Association between birth weight and visceral fat in adults. Am J Clin Nutr. 2010;92 : 347–352. doi: 10.3945/ajcn.2010.29247 20519560
24. Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43 : 436–441. doi: 10.1038/ng.801 21460841
25. Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41 : 1088–1093. doi: 10.1038/ng.440 19734902
26. InterAct Consortium, Langenberg C, Sharp S, Forouhi NG, Franks PW, Schulze MB, et al. Design and cohort description of the InterAct project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC study. Diabetologia. 2011;54 : 2272–2282. doi: 10.1007/s00125-011-2182-9 21717116
27. Katzov H, Chalmers K, Palmgren J, Andreasen N, Johansson B, Cairns NJ, et al. Genetic variants of ABCA1 modify Alzheimer disease risk and quantitative traits related to beta-amyloid metabolism. Hum Mutat. 2004;23 : 358–367. 15024730
28. Power MC, Weuve J, Gagne JJ, McQueen MB, Viswanathan A, Blacker D. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology. 2011;22 : 646–659. doi: 10.1097/EDE.0b013e31822708b5 21705906
29. Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, et al. Midlife blood pressure and dementia:the Honolulu-Asia aging study. Neurobiol Aging. 2000;21 : 49–55. 10794848
30. Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322 : 1447–1451. 11408299
31. Qiu C, Winblad B, Fratiglioni L. Low diastolic pressure and risk of dementia in very old people: a longitudinal study. Dement Geriatr Cogn Disord. 2009;28 : 213–219. doi: 10.1159/000236913 19752556
32. Albanese E, Lombardo FL, Prince MJ, Stewart R. Dementia and lower blood pressure in Latin America, India, and China: a 10/66 cross-cohort study. Neurology. 2013;81 : 228–235. doi: 10.1212/WNL.0b013e31829bfe66 23771488
33. Guo Z, Viitanen M, Fratiglioni L, Winblad B. Low blood pressure and dementia in elderly people: the Kungsholmen project. BMJ. 1996;312 : 805–808. 8608286
34. de la Torre JC. Cardiovascular risk factors promote brain hypoperfusion leading to cognitive decline and dementia. Cardiovasc Psychiatry Neurol. 2012;2012 : 367516. doi: 10.1155/2012/367516 23243502
35. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360 : 1903–1913. 12493255
36. Forette F, Seux ML, Staessen JA, Thijs L, Birkenhager WH, Babarskiene MR, et al. Prevention of dementia in randomised double-blind placebo-controlled systolic hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352 : 1347–1351. 9802273
37. Yasar S, Xia J, Yao W, Furberg CD, Xue QL, Mercado CI, et al. Antihypertensive drugs decrease risk of Alzheimer disease: Ginkgo Evaluation of Memory Study. Neurology. 2013;81 : 896–903. doi: 10.1212/WNL.0b013e3182a35228 23911756
38. Forette F, Seux ML, Staessen JA, Thijs L, Babarskiene MR, Babeanu S, et al. The prevention of dementia with antihypertensive treatment: new evidence from the systolic hypertension in Europe (Syst-Eur) study. Arch Intern Med. 2002;162 : 2046–2052. 12374512
39. Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360 : 1623–1630. 12457784
40. Graves AB, van Duijn CM, Chandra V, Fratiglioni L, Heyman A, Jorm AF, et al. Alcohol and tobacco consumption as risk factors for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(Suppl 2):S48–S57. 1833354
41. Salomon AR, Marcinowski KJ, Friedland RP, Zagorski MG. Nicotine inhibits amyloid formation by the beta-peptide. Biochemistry. 1996;35 : 13568–13578. 8885836
42. Riggs JE. Smoking and Alzheimer’s disease: protective effect or differential survival bias? Lancet. 1993;342 : 793–794. 8103882
43. Alkadhi KA. Chronic stress and Alzheimer’s disease-like pathogenesis in a rat model: prevention by nicotine. Curr Neuropharmacol. 2011;9 : 587–597. doi: 10.2174/157015911798376307 22654719
44. Freathy RM, Kazeem GR, Morris RW, Johnson PC, Paternoster L, Ebrahim S, et al. Genetic variation at CHRNA5-CHRNA3-CHRNB4 interacts with smoking status to influence body mass index. Int J Epidemiol. 2011;40 : 1617–1628. doi: 10.1093/ije/dyr077 21593077
45. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380 : 2224–2260. doi: 10.1016/S0140-6736(12)61766-8 23245609
46. Proitsi P, Lupton MK, Velayudhan L, Newhouse S, Fogh I, Tsolaki M, et al. Genetic predisposition to increased blood cholesterol and triglyceride lipid levels and risk of Alzheimer disease: a Mendelian randomization analysis. PLoS Med. 2014;11:e1001713. doi: 10.1371/journal.pmed.1001713 25226301
47. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261 : 921–923. 8346443
48. Mannila MN, Mahdessian H, Franco-Cereceda A, Eggertsen G, de Faire U, Syvanen AC, et al. Identification of a functional apolipoprotein E promoter polymorphism regulating plasma apolipoprotein E concentration. Arterioscler Thromb Vasc Biol. 2013;33 : 1063–1069. doi: 10.1161/ATVBAHA.112.300353 23430611
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 6- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Magnosolv a jeho využití v neurologii
- Ferinject: správně indikovat, správně podat, správně vykázat
- Optimální dávkování apixabanu v léčbě fibrilace síní
-
Všechny články tohoto čísla
- Inequitable and Ineffective: Exclusion of Mental Health from the Post-2015 Development Agenda
- Mistreatment of Women in Childbirth: Time for Action on This Important Dimension of Violence against Women
- HIV Programs for Sex Workers: Lessons and Challenges for Developing and Delivering Programs
- Efficacy of Handwashing with Soap and Nail Clipping on Intestinal Parasitic Infections in School-Aged Children: A Factorial Cluster Randomized Controlled Trial
- Assessing Development Assistance for Mental Health in Developing Countries: 2007–2013
- Achieving Systemic and Scalable Private Sector Engagement in Tuberculosis Care and Prevention in Asia
- Maximizing the Impact of Training Initiatives for Health Professionals in Low-Income Countries: Frameworks, Challenges, and Best Practices
- Shifts in the Antibiotic Susceptibility, Serogroups, and Clonal Complexes of in Shanghai, China: A Time Trend Analysis of the Pre-Quinolone and Quinolone Eras
- Transmission of Multidrug-Resistant and Drug-Susceptible Tuberculosis within Households: A Prospective Cohort Study
- The Potential for Reducing the Number of Pneumococcal Conjugate Vaccine Doses While Sustaining Herd Immunity in High-Income Countries
- Effects of Hormone Therapy on Cognition and Mood in Recently Postmenopausal Women: Findings from the Randomized, Controlled KEEPS–Cognitive and Affective Study
- The Mistreatment of Women during Childbirth in Health Facilities Globally: A Mixed-Methods Systematic Review
- Exposure to Second-Hand Smoke and the Risk of Tuberculosis in Children and Adults: A Systematic Review and Meta-Analysis of 18 Observational Studies
- Associations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Mistreatment of Women during Childbirth in Health Facilities Globally: A Mixed-Methods Systematic Review
- The Potential for Reducing the Number of Pneumococcal Conjugate Vaccine Doses While Sustaining Herd Immunity in High-Income Countries
- Mistreatment of Women in Childbirth: Time for Action on This Important Dimension of Violence against Women
- Associations between Potentially Modifiable Risk Factors and Alzheimer Disease: A Mendelian Randomization Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



