-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
LED Fluorescence Microscopy for the Diagnosis of Pulmonary Tuberculosis: A Multi-Country Cross-Sectional Evaluation
Background:
The diagnosis of tuberculosis (TB) in resource-limited settings relies on Ziehl-Neelsen (ZN) smear microscopy. LED fluorescence microscopy (LED-FM) has many potential advantages over ZN smear microscopy, but requires evaluation in the field. The aim of this study was to assess the sensitivity/specificity of LED-FM for the diagnosis of pulmonary TB and whether its performance varies with the timing of specimen collection.Methods and Findings:
Adults with cough ≥2 wk were enrolled consecutively in Ethiopia, Nepal, Nigeria, and Yemen. Sputum specimens were examined by ZN smear microscopy and LED-FM and compared with culture as the reference standard. Specimens were collected using a spot-morning-spot (SMS) or spot-spot-morning (SSM) scheme to explore whether the collection of the first two smears at the health care facility (i.e., “on the spot”) the first day of consultation followed by a morning sample the next day (SSM) would identify similar numbers of smear-positive patients as smears collected via the SMS scheme (i.e., one on-the-spot-smear the first day, followed by a morning specimen collected at home and a second on-the-spot sample the second day). In total, 529 (21.6%) culture-positive and 1,826 (74.6%) culture-negative patients were enrolled, of which 1,156 (49%) submitted SSM specimens and 1,199 (51%) submitted SMS specimens. Single LED-FM smears had higher sensitivity but lower specificity than single ZN smears. Using two LED-FM or two ZN smears per patient was 72.8% (385/529, 95% CI 68.8%–76.5%) and 65.8% (348/529, 95% CI 61.6%–69.8%) sensitive (p<0.001) and 90.9% (1,660/1,826, 95% CI 89.5%–92.2%) and 98% (1,790/1,826, 95% CI 97.3%–98.6%) specific (p<0.001). Using three LED-FM or three ZN smears per patient was 77% (408/529, 95% CI 73.3%–80.6%) and 70.5% (373/529, 95% CI 66.4%–74.4%, p<0.001) sensitive and 88.1% (95% CI 86.5%–89.6%) and 96.5% (95% CI 96.8%–98.2%, p<0.001) specific. The sensitivity/specificity of ZN smear microscopy and LED-FM did not vary between SMS and SSM.Conclusions:
LED-FM had higher sensitivity but, in this study, lower specificity than ZN smear microscopy for diagnosis of pulmonary TB. Performance was independent of the scheme used for collecting specimens. The introduction of LED-FM needs to be accompanied by appropriate training, quality management, and monitoring of performance in the field.
Trial Registration: Current Controlled Trials ISRCTN53339491
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 8(7): e32767. doi:10.1371/journal.pmed.1001057
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001057Summary
Background:
The diagnosis of tuberculosis (TB) in resource-limited settings relies on Ziehl-Neelsen (ZN) smear microscopy. LED fluorescence microscopy (LED-FM) has many potential advantages over ZN smear microscopy, but requires evaluation in the field. The aim of this study was to assess the sensitivity/specificity of LED-FM for the diagnosis of pulmonary TB and whether its performance varies with the timing of specimen collection.Methods and Findings:
Adults with cough ≥2 wk were enrolled consecutively in Ethiopia, Nepal, Nigeria, and Yemen. Sputum specimens were examined by ZN smear microscopy and LED-FM and compared with culture as the reference standard. Specimens were collected using a spot-morning-spot (SMS) or spot-spot-morning (SSM) scheme to explore whether the collection of the first two smears at the health care facility (i.e., “on the spot”) the first day of consultation followed by a morning sample the next day (SSM) would identify similar numbers of smear-positive patients as smears collected via the SMS scheme (i.e., one on-the-spot-smear the first day, followed by a morning specimen collected at home and a second on-the-spot sample the second day). In total, 529 (21.6%) culture-positive and 1,826 (74.6%) culture-negative patients were enrolled, of which 1,156 (49%) submitted SSM specimens and 1,199 (51%) submitted SMS specimens. Single LED-FM smears had higher sensitivity but lower specificity than single ZN smears. Using two LED-FM or two ZN smears per patient was 72.8% (385/529, 95% CI 68.8%–76.5%) and 65.8% (348/529, 95% CI 61.6%–69.8%) sensitive (p<0.001) and 90.9% (1,660/1,826, 95% CI 89.5%–92.2%) and 98% (1,790/1,826, 95% CI 97.3%–98.6%) specific (p<0.001). Using three LED-FM or three ZN smears per patient was 77% (408/529, 95% CI 73.3%–80.6%) and 70.5% (373/529, 95% CI 66.4%–74.4%, p<0.001) sensitive and 88.1% (95% CI 86.5%–89.6%) and 96.5% (95% CI 96.8%–98.2%, p<0.001) specific. The sensitivity/specificity of ZN smear microscopy and LED-FM did not vary between SMS and SSM.Conclusions:
LED-FM had higher sensitivity but, in this study, lower specificity than ZN smear microscopy for diagnosis of pulmonary TB. Performance was independent of the scheme used for collecting specimens. The introduction of LED-FM needs to be accompanied by appropriate training, quality management, and monitoring of performance in the field.
Trial Registration: Current Controlled Trials ISRCTN53339491
: Please see later in the article for the Editors' SummaryIntroduction
Tuberculosis (TB) is a major public health problem: there are 9.3 million new cases and 1.7 million deaths per year [1], with 90% of cases occurring in low - and middle-income countries (LMICs) [1]. Most patients suspected of having pulmonary TB (PTB) in LMICs are investigated by examining three sputum specimens collected over a minimum of 2 d. Sputum smears are stained with Ziehl-Neelsen (ZN), and each smear examination requires on average 5–10 min, creating considerable workloads for laboratories with limited resources. An alternative technique to ZN smear microscopy, fluorescence microscopy (FM), is reported to be 10% more sensitive than ZN smear microscopy [2] and, since fluorescent acid fast bacilli (AFB) can be seen at lower magnification than ZN-stained AFB, FM smears can be examined in a fraction (about 25%) of the time needed for ZN smears [2]. Although FM can reduce laboratory workloads [3], it has been difficult to implement it widely in LMICs due to the high cost and complexity of the microscope and mercury vapour lamp lighting system, the need for a dark room, and perceived health risks associated with ultraviolet light exposure [4].
Recent technical developments have the potential to improve some of the shortcomings of the smear diagnosis of TB. These include the development of illumination systems based on LEDs (light-emitting diodes), which resulted in LED fluorescence microscopy (LED-FM) becoming commercially available [5]. Furthermore, the World Health Organization (WHO) recently modified its guidelines for the diagnosis of PTB [6] and reduced the number of AFB required to declare a smear as positive (from 10 to 1 AFB), the minimum number of specimens needed for diagnosis (from three to two), and the number of positive smears required to classify a patient as having smear-positive TB (from two to one smear). In a separate publication (also reported in PLoS Medicine [7]), we report that the ZN smear microscopy examination of two sputum specimens collected on the spot the first day of consultation, followed by the examination of a third specimen collected the following morning (i.e., spot-spot-morning [SSM]) identifies the same number of patients with smear-positive PTB as the frequently used spot-morning-spot (SMS) scheme (i.e., examination of one sputum specimen collected on the spot the first day of consultation, followed by the examination of a second specimen collected the following morning and a third specimen collected on the spot when the patient brings the morning specimen to the health centre) [8]. As most smear-positive PTB patients are identified by the first two specimens [9], a SSM scheme could detect most smear-positive patients on the first day of consultation. It is thus conceivable that a service that examines SSM specimens using LED-FM and classifies patients using the revised WHO guidelines could ease workloads and speed up the diagnostic process in LMICs, with significant benefits for patients and health systems [3].
We therefore conducted a study, nested within a clinical trial, assessing the sensitivity and specificity of LED-FM using both SSM and SMS sputum collection schemes for the diagnosis of PTB.
Methods
The protocol for the trial was registered (ISRCTN53339491), and ethical approval was obtained from the Liverpool School of Tropical Medicine and WHO ethics review committees, the institutional review boards, and respective national research ethics committees of the participating centres (see Text S1). Translated consent forms and patient information sheets were used for each site. Informed written or oral consent in front of a witness were obtained from all patients. Patients unable to provide informed consent and those who had received anti-TB treatment in the last month were excluded.
This was a prospective cross-sectional study carried out in Ethiopia, Nepal, Nigeria, and Yemen over a 15-mo period from 3 January 2008 to 30 March 2009 (see Text S2). The study was nested within a non-inferiority clinical trial assessing whether the sensitivity and specificity of ZN smear microscopy of sputum specimens collected under a SSM scheme were non-inferior to those of ZN smear microscopy following the standard SMS collection scheme for the diagnosis of PTB, which is also published in this issue [7]. The study sites were selected to obtain a wide geographical representation of LMIC settings and took advantage of a larger clinical trial on smear microscopy being undertaken at the same time. This facilitated training, as well as the use of protocols and standard operating procedures across locations.
All consecutive patients ≥18 y old with cough ≥2 wk duration presenting at participating health facilities were screened and invited to participate in the clinical trial. Of these, only the first 7–10 patients attending the health care centres each day were invited to participate in the LED-FM evaluation to avoid overloading the laboratories (see Figure 1). It is recognised that selecting the first patients may have introduced systematic biases (e.g., residents of nearby households with mild illnesses or those from remote locations with advanced disease stages). Random selection, however, was logistically difficult. The data obtained for smear microscopy (ZN and LED-FM) were paired and therefore relatively independent of the actual representativeness of the participants in relation to the reference population.
Fig. 1. Depiction of flow of participants. 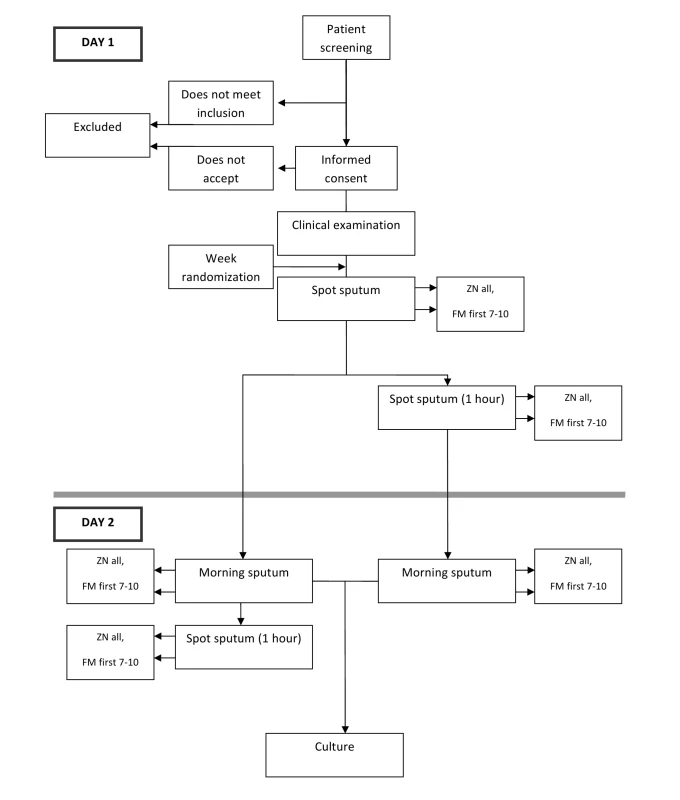
Patients enrolled in Ethiopia were attending Bushullo Major and Awassa Health Centres. These are the main health service providers for Awassa District in the Southern Region. Smear microscopy was conducted in the health centres' laboratories, and sputum specimens were cultured at the Armauer Hansen Institute, Addis Ababa. Patients in Abuja, Nigeria, were enrolled in Wuse District Hospital, and sputum specimens were processed in the Zankli Medical Centre laboratory, a private laboratory acting as a diagnostic centre for the National Tuberculosis Programme. In Nepal, patients were enrolled from the TB DOTS centre of Tribhuvan University Teaching Hospital and the Dirgh Jeevan Health Care and Research Centre, both in Kathmandu. Sputum specimens collected in both centres were processed in Tribhuvan University Health Research Laboratory. In Yemen, patients were enrolled at the Tuberculosis Institute, which is the reference centre and headquarters of the National Tuberculosis Programme and which provides diagnostic services to the surrounding population and referred patients.
All patients were requested to submit three sputum specimens following one of the two schemes. One scheme required patients to provide a sputum sample on first attendance at the health facility, a second sample (which was produced at home) the following morning, and a third sample collected on the spot when the patient brought the morning specimen to the laboratory (SMS). The second scheme required patients to provide a specimen on the spot upon first attendance at the health care facility, a second specimen one hour after the first specimen, and a third specimen that was produced at home the following morning (SSM). The schemes were allocated by week using a randomization list that assigned the scheme to be used in a given week. The scheme was disclosed at the start of the week by opening a sealed envelope. Patients were provided with pre-labelled sputum collection pots and instructed on how to produce a good quality sputum specimen following a standard operating procedure [10].
Specimens were assessed macroscopically, and smears were prepared in duplicate for staining by the hot ZN technique or with Auramine O, counterstained with potassium permanganate, for FM [11]. Slides were labelled with study numbers. These labels were then covered with wrap-around stickers before mixing and reading to ensure laboratory technicians were unaware of the grades assigned to previous smears. Grading was conducted by laboratory technicians, and the stickers were removed when another technician entered the results into the study logbooks. LED-FM was conducted on standard microscopes fitted with adaptors consisting of a specialised objective with an attached LED light source (LUMIN and the QBC Paralens Fluorescence Microscopy System). ZN smears were examined at 1,000× magnification, and LED-FM smears (in accordance with advice from the International Union Against Tuberculosis and Lung Disease Working Group on Smear Microscopy) at 200×, with confirmation of positive smears at 400× magnification. Smears were classified as positive when ≥1 AFB was detected per 100 fields, and patients were considered smear-positive if they had ≥1 positive smear [6]. Patients with missing results were classified according to the results of available smears (e.g., patients with two negative smears but missing the third specimen were classified as negative). The morning specimen, or, if not available, a spot specimen, was concentrated (Petroff's method) and cultured on solid medium. Identification of Mycobacterium tuberculosis complex was confirmed by standard biochemical tests [11].
The study was implemented at each site after training and a pilot phase, and was conducted in compliance with good clinical practice/good clinical laboratory practice. A lot quality assurance sampling scheme was used to determine the sample size for ZN smear microscopy external quality assessment (EQA) to assure a sensitivity of 90% relative to the controller, with a maximum of two errors [12]. Sampling for ZN smear microscopy EQA was performed before, during, and at the end of the study and was conducted by WHO/International Union Against Tuberculosis and Lung Disease Supranational Reference Laboratories. A random selection of 20% LED-FM smears from all sites was also selected for EQA. In addition, all LED-FM smears graded as scanty (<10 AFB per 100 fields) were sent to Supranational Reference Laboratories and the German-Nepal Tuberculosis Project in Nepal at the end of the study, where smears were re-stained and re-checked using conventional FM. The main outcomes for comparison were the sensitivity and specificity of LED-FM compared with the sensitivity and specificity of ZN smear microscopy. Culture was used as the reference standard, and the analysis was stratified by scheme. As LED-FM smears with scanty AFB (<10 AFB) were often reported negative by conventional FM, both results are presented, stratified by culture to assign true-/false-positive results. It is however acknowledged that this partial verification could result in an inaccurate estimate of test performance and that this latter sub-analysis needs to be interpreted with caution.
Data are presented using summary statistics with 95% CI. Comparisons of the characteristics of the patients studied in the four participating countries were by one-way analyses of variance for continuous measures and Fisher exact tests for categorical measures. Comparisons of sensitivity and specificity values where both methods were applied to the same samples (i.e., the observations were paired) were done using the McNemar test; comparisons where samples were taken from two different (independent) cohorts were done using the Fisher exact test. The sample size calculations for the study assumed that ZN smear microscopy would identify 50% of culture-positive patients and that LED-FM would be 10% more sensitive than ZN smear microscopy [2]. The target sample size of 1,605 was assumed to be an overestimate of the number required because the calculations presumed that specimens were not paired. Although studies evaluating paired samples have lower minimum sample sizes required than studies using independent samples, the overestimation was unavoidable without prior knowledge of the expected test discordance.
Results
A total of 2,445 patients (37% of 6,628 patients enrolled for the larger trial [7]) were enrolled for the LED-FM evaluation. Of these, 468 (19%) were enrolled in Ethiopia, 526 (22%) in Nepal, 685 (28%) in Nigeria, and 766 (31%) in Yemen. The characteristics of the participants are summarised in Table 1. More participants (1,294, 53%) were male, and the majority were married (1,679, 69%). Participants in Nepal and Yemen were older (mean [standard deviation] of 43.6 [17.7] and 41 [17.9] y) than participants in Ethiopia and Nigeria (34.9 [14.3] and 34.3 [11.1] y, respectively, p<0.001). Patients in Ethiopia and Yemen were more likely to come from rural areas (58% and 51%) than patients in Nepal and Nigeria (7% and 13%, p<0.001), and patients in Nepal presented with longer cough duration than patients in the other three countries. The most frequent complaints were cough, chest pain, weight loss, anorexia, fever, and night sweating. Of the 2,445 patients enrolled, 529 (21.6%) were culture-positive, 1,826 (74.6%) were culture-negative, and 90 (3.7%) had invalid culture results (57 [2.3%] contaminated and 33 [1.4%] missing). Among patients with positive or negative cultures, 1,156 (48.9%) were screened using the SSM scheme and 1,199 (51.1%) the SMS scheme.
Tab. 1. Characteristics of the patients on enrolment. 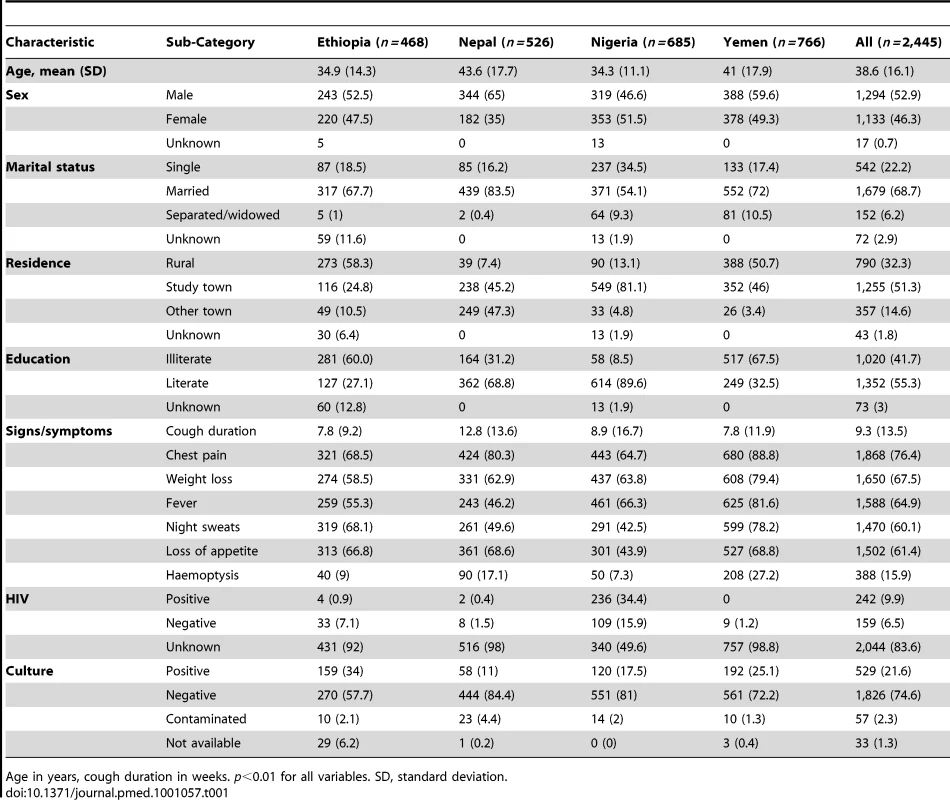
Age in years, cough duration in weeks. p<0.01 for all variables. SD, standard deviation. Individual ZN and LED-FM smear results by type of specimen are described in Text S3. Smears prepared from the morning specimens were more likely to be positive than smears prepared from spot specimens. Among culture-positive patients, ZN smears were less likely to be positive than LED-FM smears (937/1,568 [59.8%, 95% CI 57.3%–62.2%] and 1,051/1,531 [68.7%, 95% CI 66.3%–71.0%], respectively, p<0.001). Among culture-negative patients, ZN smears were more likely to be negative than the LED-FM smears (5,263/5,349 [98.4%, 95% CI 98%–98.7%] and 4,816/5,118 [94.1%, 95% CI 86.4%–88.1%], respectively, p<0.001). Single LED-FM smears therefore had higher sensitivity but lower specificity than single ZN smears. Out of 283 LED-FM smears with scanty grades that had paired conventional FM readings, 136 (48.1%) were culture-positive and 147 (51.9%) culture-negative according to conventional FM. Ninety-six (70.6%) of the culture-positive specimens were graded positive and 77 (52.3%) of the 147 culture-negative specimens were graded negative by conventional FM. LED-FM smears therefore were more sensitive but less specific than conventional FM smears for classifying smears with low AFB numbers. The sensitivity/specificity obtained after incorporating the revised scanty smears in the analysis is shown in Table 2 for illustration.
Tab. 2. Sensitivity and specificity of using two or three ZN and LED-FM smears per patient. 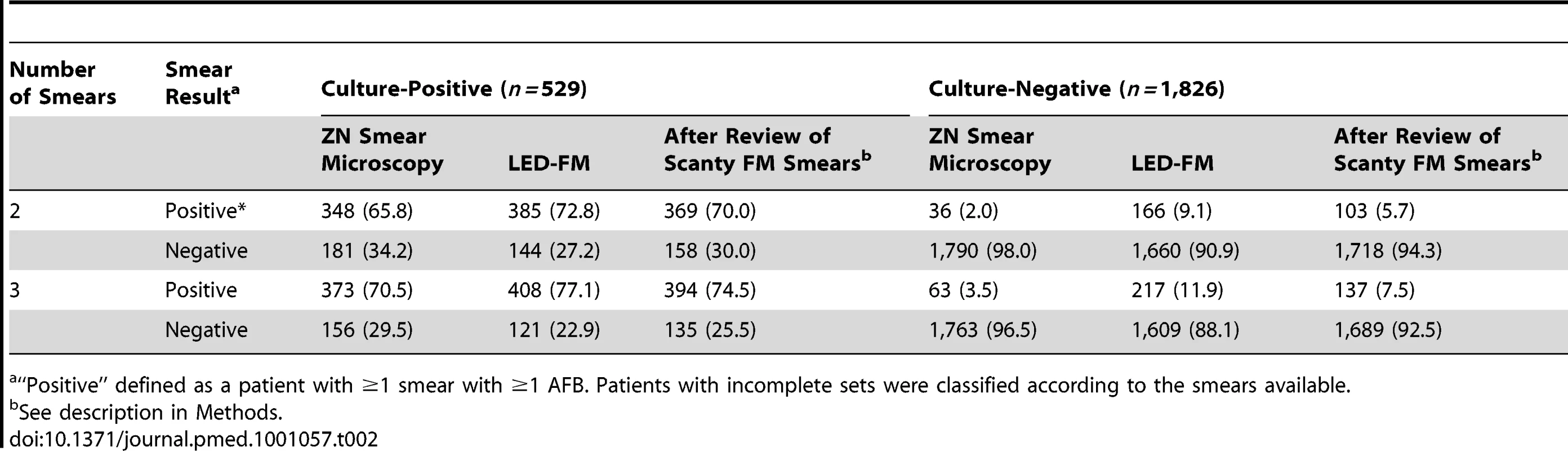
“Positive” defined as a patient with ≥1 smear with ≥1 AFB. Patients with incomplete sets were classified according to the smears available. Using two or three LED-FM smears per patient resulted in sensitivities (95% CI) of 72.8% (68.8%–76.5%) and 77.1% (73.3%–80.6%), compared to 65.8% (61.6%–69.8%) and 70.5% (66.4%–74.4%) when using two or three ZN smears (p<0.001 for both). Using two or three LED-FM smears per patient produced specificities (95% CI) of 90.9% (89.5%–92.2%) and 88.1% (86.5%–89.6%), respectively, compared to 98% (97.3%–98.6%) and 96.5% (96.8%–98.2%) when using two or three ZN smears (p<0.001 for both). The accuracy of using three LED-FM smear examinations per patient was 85% (2,017 of 2,355 patients correctly classified, 95% CI 84.2%–87%), which was lower than the 91.8% accuracy obtained when using three ZN smear examinations (2,136 of 2,355 patients, 95% CI 89.5%–91.8%, p<0.001). An illustration of the proportion of true - and false-positive and -negative results obtained with ZN smear microscopy and LED-FM using two and three smears is included in Table 3 for illustration. The use of two ZN smears would correctly classify the highest number of patients (91%), but would also miss the highest number of cases with TB, and, conversely, using three LED-FM smears would have the lowest number of cases classified correctly (85%), but would miss the lowest number of cases with TB, thus the increased sensitivity is at the expense of lower specificity. The sensitivity and specificity of the SSM and SMS schemes for both LED-FM and ZN smear microscopy are shown in Text S3. There were no statistical differences between the schemes.
Tab. 3. Absolute difference the use of two- or three-smear ZN smear microscopy and LED-FM schemes would make per 1,000 persons tested in situations with similar TB prevalence. 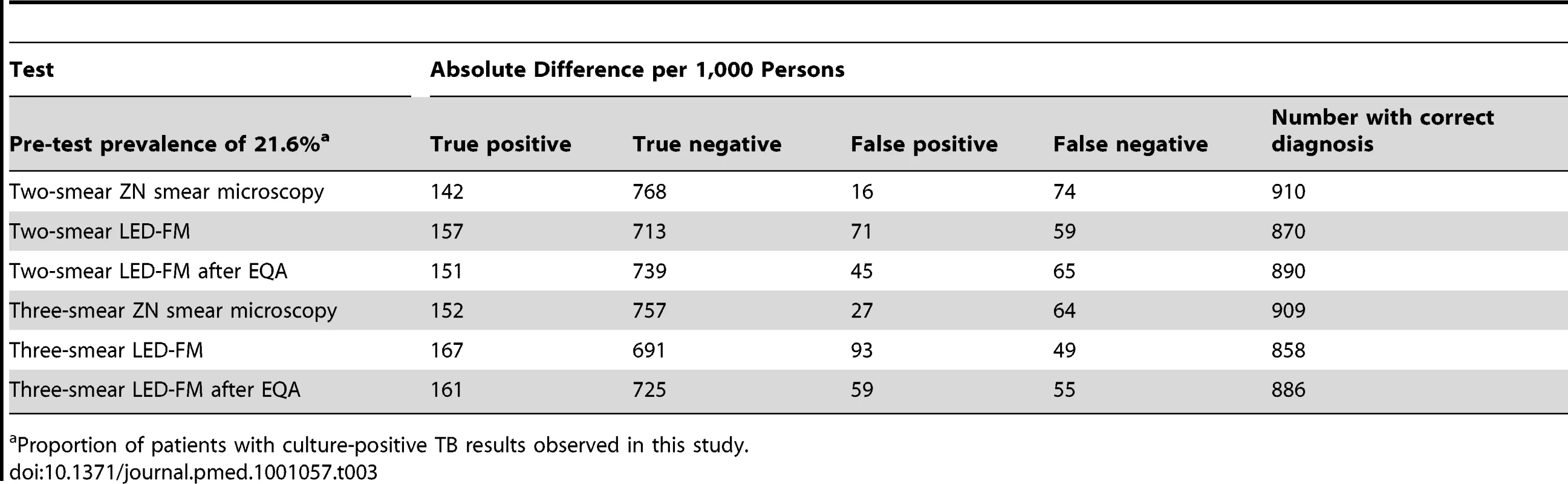
Proportion of patients with culture-positive TB results observed in this study. Discussion
The number of cases of PTB continues to increase worldwide despite major international control initiatives [1], and at least 50 million individuals with chronic cough are screened for TB each year. Although it is widely accepted that the early identification of cases through inexpensive point-of-care diagnostics is pivotal for effective control of TB, the diagnosis of PTB often relies on sputum smear microscopy, especially in resource-limited settings.
Sputum smear microscopy services are the most decentralized of the TB diagnostic services, allowing patients to be screened for tuberculosis at relatively basic health care facilities. In most LMICs, however, these centres are less numerous than treatment centres, and many patients still need to travel to submit sputum specimens for diagnosis, incurring considerable personal costs, and this often leads patients to abandon the process. The diagnosis of TB therefore is particularly difficult for poor and marginalised patients, who face multiple challenges to accessing a TB diagnosis [13], and the improvement of smear microscopy services remains necessary to increase patients' access to treatment [14]. Furthermore, smear microscopy is known to be less sensitive in HIV-associated tuberculosis, and interventions which could increase this sensitivity are needed.
LED-FM holds multiple advantages over both the conventional mercury vapour lamp FM and standard light microscopy. LEDs are relatively inexpensive, can be powered by mains or batteries, and have an effective lifespan of thousands of hours [5]. The longer lifespan of the LED systems, as well as facilitating their implementation, may result in better quality FM than conventional systems since mercury vapour and halogen lamps are often used after their effective lifespan, with diminished power, to cause fluorescence. The lower capital and maintenance costs of LED-based systems, the faster grading of smears than with ZN smear microscopy, and FM's reputed higher sensitivity makes LED-FM devices potentially more suitable for LMIC laboratories. Despite this potential, there have been very few well-designed field evaluations using culture as the reference standard [2],[15] to assess whether LED-FM performance varies in locations with no or limited experience with FM.
LED-FM in this study had higher sensitivity but lower specificity than ZN smear microscopy, which resulted in a significantly higher number of patients with PTB being treated, but lower overall diagnostic accuracy, as illustrated in Text S3. Although most previous studies are small [2],[15],[16], they have reported increased sensitivity and similar specificity of LED-FM compared with ZN smear microscopy. Recently, however, Cattamanchi et al. suggested that LED-FM had lower specificity than conventional ZN smear microscopy in patients co-infected with HIV in Uganda [17], suggesting that the loss of specificity was more evident in patients who had scanty AFB in sputum. The investigators, however, had processed different specimens for ZN smear microscopy and FM; thus, the interpretation of this study is difficult [18], and further studies are needed. If these findings are corroborated by further studies, however, it is still important to consider that if a trade-off has to be made between sensitivity and specificity, it is preferable to err on the side of increased sensitivity and to treat a small number of patients who do not have TB. There are real consequences to a false-positive result: patients incorrectly diagnosed with TB have the cost and inconvenience of taking a six-month course of treatment, and troublesome side effects are common, but life-threatening treatment-related events (hepatitis and Stevens-Johnson syndrome) are infrequent (<5% incidence). It is also necessary to discuss whether solid culture, or liquid culture, can function as an adequate comparator, as neither is a perfect reference standard, and the underlying assumption that either culture system has higher specificity than smear microscopy might be debatable. The WHO manual for laboratory services in TB control states that the probability of obtaining a positive culture is related to the number of AFB in the specimen, with only about 50% of cultures of specimens with 1–2 AFB per 100 fields being identified as positive, increasing to 80% and 96.7% for specimens with “scanty” (1–9 AFB per 100 fields) and “+” AFB grades, respectively. The analysis of our smear-positive, culture-negative results indicated that more than 98% of these were scanty smears with a median of one AFB per smear, and 23.7% had a second positive smear by either ZN smear microscopy or LED-FM. It is thus highly likely that these patients either had paucibacillary tuberculosis in which M. tuberculosis failed to grow in culture, or were infected/colonised by non-tuberculous mycobacteria. This is unfortunately an inherent weakness of solid culture (and, to a lesser extent, liquid culture) as a diagnostic reference standard. Judgements on the performance of new diagnostic tests must be done with considerations of these limitations. The “false positive” LED-FM results are thus most likely due to technician error, but we cannot discount the possibility that these were cases with TB or non-tuberculous mycobacteria with a false-negative solid medium result that would have been detected using liquid medium. There might also be cases of TB that would have been missed by both culture technologies (because of delayed processing/destruction of marginally viable bacilli during decontamination or bacterial overgrowth).
Unfortunately, HIV testing was not done systematically as part of this study. Overall, the vast majority of patients across the sites did not know their HIV status. The exception was the Nigerian site, where around half of patients reported knowing their HIV status. Only patients who volunteered this information were categorised as HIV positive or negative. Any sub-analysis of the results by HIV status would be underpowered, subject to self-selection bias, and confounded by the duration since last test and the methods used for testing.
Clinical laboratories in our study had considerable experience with ZN smear microscopy but limited experience with mercury vapour lamp FM, and their conventional FM microscopes were neither functioning nor in recent use at the time of the study. Although technicians received 2-d refresher training at the bench, were supervised for a further day to monitor performance, and had photographic bench-aids available, ZN smear microscopy had consistently higher specificity.
Interestingly, the rechecking of scanty smears and comparison against culture suggests that LED-FM also had higher sensitivity but lower specificity than conventional FM. These differences could have been due to the devices' performance characteristics, as reference laboratories are equipped with high-quality microscopes with greatly improved optics, and to the novelty of LED-FM, with laboratory technicians conducting more careful examinations and spending more time reading the smears to identify paucibacillary smears, while lacking experience to distinguish fluorescing artefacts from scanty AFB. Furthermore, technicians in the reference laboratories routinely use dark rooms, which facilitate visualizing the AFB in conventional FM but are not required with LED-FM, and may have been more cautious to declare smears with one or two bacilli as positive. Also, the smears may have faded, and AFB could have been washed off during re-staining.
Although the comparison of LED-FM and conventional FM was based on the verification of a partial selection of smears and needs to be replicated, national TB control programmes considering introducing LED-FM devices may wish to bear in mind that their performance could vary with the service setting, staff experience, and the type of devices used. In contrast to the findings of this study, other studies conducted at established research centres have found similar specificity in conventional FM and LED-FM. Our lower specificity may thus be the upside of the trade-off implicit in the larger amount of operational data collected in this study, at the cost of a less-than-ideal reference standard. The LED-FM technology, however, is likely to be widely implemented, and the study still gives valuable insights into operational issues. The performance of LED-FM, and indeed any diagnostic test, depends critically on proficiency of test operators. Adequate and appropriate training is thus required. Current expert opinion is that only moderate training (3 d) is required to make laboratory technicians already proficient in ZN smear microscopy proficient in LED-FM. This training was provided to study staff, involved instruction in specimen processing and staining techniques, and included 2 d of supervised routine microscopy during which an expert helped resolve any doubtful results in order to instil confidence in workers. All staff reported familiarity and confidence in their ability to perform the technique. We believe that this kind of training is most likely sufficient for the induction of staff in laboratories where the technique is well established and there are experienced staff who can provide continued mentoring. However, as stand-alone training, after which laboratory staff are expected to maintain performance without access to a second opinion, it is most likely inadequate, and the development of structured and standardised training packages and supervisory schemes is needed to support scale up. The introduction of LED-FM therefore needs to be accompanied by careful training, appropriate quality control, and monitoring of the performance of LED-FM in the field. Implementers may need to consider whether trade offs between higher sensitivity and lower specificity are acceptable.
The study also indicates that the sensitivity and specificity of LED-FM is independent of whether the sputum specimens are collected as SSM or SMS. There is hardly any evidence that a particular sequence of sputum collection increases the probability of finding TB bacilli, and we have recently demonstrated that this is not so [7]. Although morning specimens have a higher yield (and single-smear sensitivity), examining two spot specimens identifies equivalent numbers of smear-positive patients as examining one spot specimen followed by one morning specimen. SMS requires that all patients return to provide the morning sample, while the SSM would identify most smear-positive cases in one day. Thus, what is crucial is the potential shortening of the process. Although many clinicians are reluctant to rely on spot specimens due to the higher sensitivity of the morning specimens, the 10% higher sensitivity of a single morning specimen becomes redundant once multiple specimens are used. The WHO has recently recommended using spot specimens (indeed spot-spot) to allow the screening of patients in one day. However, the change in policy was based on the evaluation of the method using ZN smear microscopy. We now provide evidence that a frontloaded LED-FM approach that uses two spot specimens results in the same yield as approaches using a conventional SMS LED-FM scheme. This seemingly small change may hold the key to improving current microscopy services. If two smears are collected and examined on the first day and one of them is positive, as was the case in the majority of smear-positive cases in this study, patients would not have to return the next day to complete the sputum submission process. If health systems are modified to provide results on the same day, patients could save the costs of one more visit in the diagnostic process, the majority of smear-positive patients could be referred for treatment on the day of consultation, and dropout rates may be reduced.
Moreover, it is important to consider the role that LED-FM can play in the recent WHO endorsement of the new rapid, automated nucleic acid amplification test, Xpert. It is unlikely, in the short term, that Xpert can be scaled up and decentralized sufficiently to replace smear microscopy as the initial diagnostic test worldwide, even in areas with high rates of multi-drug-resistant TB or HIV-associated TB, where Xpert is recommended as the initial screening test. Furthermore, the WHO recommends continuing to use smear microscopy for treatment monitoring, even in areas served by new technologies such as TB culture, line probe assays, and the Xpert assay. In areas without high rates of multi-drug-resistant TB or HIV-associated TB, cost-effectiveness considerations favour the use of smear microscopy as the initial diagnostic tool, and thus the improved performance of direct smear microscopy (and indeed a technique that facilitates faster examination of smears) is an important step for improving diagnostic services in LMICs, one that would reduce laboratory workloads and facilitate the faster identification of patients with negative smear microscopy who could undergo further tests.
Despite the reduced specificity of LED-FM, this approach still has operational advantages that make it an attractive tool for TB laboratory diagnosis. National control programmes introducing new LED-FM services should monitor the performance of the method under operational conditions, as training needs of staff may be greater than anticipated. This study has shown that LED-FM can play a key role in reaching WHO targets for TB detection, reducing laboratory workloads, and ensuring poor patients' access to TB diagnosis and prompt treatment.
Supporting Information
Zdroje
1. World Health Organization 2009 Global tuberculosis control: epidemiology, strategy, financing. Geneva: World Health Organization. WHO/HTM/TB/ 2009411
2. MaraisBJBrittleWPainczykKHesselingACBeyersN 2008 Use of light-emitting diode fluorescence microscopy to detect acid-fast bacilli in sputum. Clin Infect Dis 47 203 207
3. RamsayACuevasLEMundyCJNathansonCMChiramboP 2009 New policies, new technologies: modelling the potential for improved smear microscopy services in Malawi. PLoS ONE 4 e7760 doi:10.1371/journal.pone.0007760
4. HanscheidT 2008 The future looks bright: low-cost fluorescent microscopes for detection of Mycobacterium tuberculosis and Coccidiae. Trans R Soc Trop Med Hyg 102 520 521
5. MinionJSohnHPaiM 2009 Light-emitting diode technologies for TB diagnosis: what is on the market? Expert Rev Med Devices 6 341 345
6. World Health Organization 2007 New WHO policies: definition of smear-positive TB case. Geneva World Health Organization
7. CuevasLEYassinMAAl-SonboliNLawsonLArbideI 2011 A multi-country non-inferiority cluster randomized trial of frontloaded smear microscopy for the diagnosis of pulmonary tuberculosis. PLoS Med 8 e443 doi:10.1371/journal.pmed.1000443
8. RamsayAYassinMACambanisAHiraoSAlmotawaA 2009 Front-loading sputum microscopy services: an opportunity to optimise smear-based case detection of tuberculosis in high prevalence countries. J Trop Med 2009 398767 398767
9. MaseSRRamsayANgVHenryMHopewellPC 2007 Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis 11 485 495
10. KhanMSDarOSismanidisCShahKGodfrey-FaussettP 2007 Improvement of tuberculosis case detection and reduction of discrepancies between men and women by simple sputum-submission instructions: a pragmatic randomised controlled trial. Lancet 369 1955 1960
11. International Union Against Tuberculosis and Lung Disease 2000 Technical guide. Sputum examination for tuberculosis by direct microscopy in low-income countries Paris International Union Against Tuberculosis and Lung Disease
12. AzizMABaFBecx-BleuminkMBretzelGHumesR 2002 External quality assessment for AFB smear microscopy. RidderhofJHumesRBoulahbalF Washington (District of Columbia) Association of Public Health Laboratories
13. Nhlema SimwakaBBelloGBandaHChimziziRSquireSB 2007 The Malawi National Tuberculosis Programme: an equity analysis. Int J Equity Health 6 1 9
14. KeelerEPerkinsMDSmallPHansonCReedS 2006 Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature 444 (Suppl 1) 49 57
15. MizunoKChikamatsuKAonoAAzumaYYamadaH 2009 [Clinical evaluation of acid-fast smear examination with light emitting diode fluorescent microscopy.] Kekkaku 84 627 629
16. TurusovAAValievRChesnokovaRV 2009 [Comparative study of microscopy by the Ziehl Neelsen method, routine fluorescence microscopy, and fluorescence microscopy using a lumin attachment in the diagnosis of acid-resistant mycobacteria.] Probl Tuberk Bolezn Legk 4 41 45
17. CattamanchiADavisJLWorodriaWden BoonSYooS 2009 Sensitivity and specificity of fluorescence microscopy for diagnosing pulmonary tuberculosis in a high HIV prevalence setting. Int J Tuberc Lung Dis 13 1130 1136
18. KlatserPAnthonyRden HertogALeeflangMBoerK 2010 Misleading conclusion. Int J Tuberc Lung Dis 14 377; author reply 377-378
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 7- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Retention in HIV Care between Testing and Treatment in Sub-Saharan Africa: A Systematic Review
- Health Care Systems and Conflict: A Fragile State of Affairs
- Simplified ART Delivery Models Are Needed for the Next Phase of Scale Up
- Individualized Cost-Effectiveness Analysis
- Is Scale-Up Worth It? Challenges in Economic Analysis of Diagnostic Tests for Tuberculosis
- Treatment Outcomes and Cost-Effectiveness of Shifting Management of Stable ART Patients to Nurses in South Africa: An Observational Cohort
- Risk Factors for Severe Outcomes following 2009 Influenza A (H1N1) Infection: A Global Pooled Analysis
- Predicting the Epidemic Sizes of Influenza A/H1N1, A/H3N2, and B: A Statistical Method
- GeneXpert—A Game-Changer for Tuberculosis Control?
- LED Fluorescence Microscopy for the Diagnosis of Pulmonary Tuberculosis: A Multi-Country Cross-Sectional Evaluation
- Configuring Balanced Scorecards for Measuring Health System Performance: Evidence from 5 Years' Evaluation in Afghanistan
- A Multi-Country Non-Inferiority Cluster Randomized Trial of Frontloaded Smear Microscopy for the Diagnosis of Pulmonary Tuberculosis
- Comparison of Xpert MTB/RIF with Other Nucleic Acid Technologies for Diagnosing Pulmonary Tuberculosis in a High HIV Prevalence Setting: A Prospective Study
- Global Pharmacovigilance for Antiretroviral Drugs: Overcoming Contrasting Priorities
- Evidence-Based African First Aid Guidelines and Training Materials
- Screening for HIV-Associated Tuberculosis and Rifampicin Resistance before Antiretroviral Therapy Using the Xpert MTB/RIF Assay: A Prospective Study
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Individualized Cost-Effectiveness Analysis
- GeneXpert—A Game-Changer for Tuberculosis Control?
- Screening for HIV-Associated Tuberculosis and Rifampicin Resistance before Antiretroviral Therapy Using the Xpert MTB/RIF Assay: A Prospective Study
- Treatment Outcomes and Cost-Effectiveness of Shifting Management of Stable ART Patients to Nurses in South Africa: An Observational Cohort
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



