-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe acute pancreatitis severity prediction using adiponectin, adipocyte fatty acid binding protein and fibroblast growth factor 21 levels in day 4 after admission
Predikce závažnosti akutní pankreatitidy za použití sérových hladin adiponektinu, adipocytárního proteinu vázajícího mastné kyseliny a fibroblastového růstového faktoru 21 v 4 den po přijetí.
Cíle studie:
Studie byla zaměřena na vyhodnocení sérových hladin adiponektinu (ADP), adipocytárního proteinu vázajícího mastné kyseliny (A-FABP) a fibroblastového růstového faktoru 21 jako potenciálních prediktorů závažnosti akutní pankreatitidy (AP) 4. den po přijetí. Současně byly stanoveny vybrané klasické prozánětlivé markery.Název a sídlo pracoviště:
Oddělení klinické biochemie a II. interní klinika Fakultní nemocnice Olomouc, I. P. Pavlova 6, 775 20 Olomouc; Nemocnice Písek, Karla Čapka 589, 397 23 Písek.Materiál a metody:
Studie byla provedena u pacientů s akutní pankreatitidou (n = 84, 37 žen, 47 mužů). Laboratorní markery byly měřeny v podskupinách podle klasifikace lehká/ těžká AP, a částečně také v podskupinách vytvořených na základě CTSI (computed-tomography severity index) skóre.Výsledky:
Ze sledovaných adipokinů měl u pacientů s těžkou AP pouze FGF 21 tendenci k vyšším hladinám v den 4 po přijetí. Za pomoci mnohočetné regresní analýzy byla zjištěna pozitivní asociace A-FABP s prokalcitoninem (PCT), zatímco FGF 21 asocioval u těžké AP pozitivně s ADP a negativně s C-reaktivním proteinem (CRP). ROC (receiver-operator characteristics) analýza potvrdila, že pouze CRP a interleukin 6 (IL-6) jsou využitelné jako potenciální prediktory závažnosti onemocnění. Pro oba parametry byly stanoveny cut-off hodnoty: 100 mg/l pro CRP a 37 ng/l pro IL-6, s negativními prediktivními hodnotami (NPV) 96 % a 92 %, a s pozitivními prediktivními hodnotami (PPV) 39 % a 29 %.Závěr:
Ve studii jsme zjistili, že hladiny ADP, A-FABP a FGF 21 nejsou vhodnými parametry pro predikci tíže AP 4. den po přijetí. Naopak stanovení CRP a IL-6 v tento den by mohlo být vhodné zejména pro vyloučení těžké akutní pankreatitidy.Klíčová slova:
akutní pankreatitida, adiponektin, A-FABP, FGF 21, závažnost onemocnění.
Authors: D. Novotný 1; P. Malina 2; P. Krumpholcova 3; I. Tozzi 3; V. Procházka 3
Authors place of work: Department of Clinical Biochemistry, University Hospital Olomouc, I. P. Pavlova 6, 775 0 Olomouc, Czech Republic 1; Department of Clinical Biochemistry, Hospital Písek, Karla Čapka 589, 97 2 Písek, Czech Republic 2; 2nd Department of Internal Medicine, Faculty of Medicine and Dentistry, Palacky University Olomouc and University Hospital Olomouc, I. P. Pavlova 6, 775 20 Olomouc, Czech Republic 3
Published in the journal: Klin. Biochem. Metab., 23 (44), 2015, No. 1, p. 9-16
Summary
Objective:
The study aimed to evaluate plasma adiponectin (ADP), adipocyte fatty acid binding protein (A-FABP) and fibro-blast growth factor 21 (FGF 21) levels as potential predictors of severity of acute pancreatitis (AP) in day 4 after admission. Simultaneously, the classical proinflammatory makers were analysed as well.Settings:
Department of Clinical Biochemistry and 2nd Department of Internal Medicine, Faculty Hospital Olomouc, I. P. Pavlova 6, 775 20 Olomouc, Czech Republic; Hospital Písek, Karla Čapka 589, 397 23 Písek, Czech Republic.Study design:
The study was conducted in subjects with acute pancreatitis (n = 84, 37 females, 47 males). The analyses were performed in the groups according to the mild/severe classification of AP, and partly in the computed-tomography severity index (CTSI) score subgroups.Results:
From adipokines, only FGF 21 tended to be higher in the severe AP subgroup in day 4. The multiple regression analysis revealed a positive association of A-FABP with procalcitonin (PCT), whilst FGF 21 was associated positively with ADP and negatively with C-reactive protein (CRP) in patients with the severe AP. The receiver-operator characteristics (ROC) analysis confirmed that not adipokines, but only CRP and interleukin 6 (IL-6) were suitable as potential predictors of the disease severity. The cut-off values were established for both parameters: 100 mg/L for CRP and 37 ng/L for IL-6, with negative predictive values (NPV) 96 % and 92 %, and positive predictive values (PPV) 39 % and 29 %, respectively.Conclusion:
The role of ADP, A-FABP and FGF 21 has been limited in a prediction of the disease severity in day 4 after admission, while CRP and IL-6 might be useful to exclude a severe AP.Keywords:
acute pancreatitis, adiponectin, A-FABP, FGF-21, disease severity.Introduction
Acute pancreatitis (AP) is a nonbacterial inflammatory disease that varies markedly in course and severity. AP results from the uncontrolled activation of digestive proteases within pancreatic acinar cells causing pancreatic tissue damage. The inflammatory response with the recruitment of inflammatory cells and the secretion of mediators of inflammation by activated acinar and inflammatory cells are present, but the complex mechanism of disease is not fully understood [1]. Over the past two decades, a mortality rate in an early phase of AP decreased significantly, in contrast to mortality in a late stage. After the diagnosis of acute pancreatitis establishment, an early assessment of the disease severity is of great importance in order to patient’s monitoring and proper treatment, with the aim of reducing morbidity and mortality [2]. Nevertheless, the main problem still remains, that neither prognostic scores nor single predictors are not able to precisely predict the disease severity, development of pancreatic or peripancreatic necrosis, and outcomes during the first hours or days of hospitalization [3].
The proinflammatory cytokines are strong candidates to act as risk factors for AP. They play a crucial role in the pathogenesis of AP by driving the additional inflammatory response [4]. Interleukin 6 (IL-6) and other cytokines have been usually studied as markers of the AP severity [5,6], together with other parameters, such as C-reactive protein (CRP) and procalcitonin (PCT). In the last decade, an increasing interest has been extended to the secretory products of visceral tissue adipocytes [7,8], with specific emphasis on adipokines. Several human studies evaluating the prognostic values of adipokines in the early stage of AP were performed, and especially serum levels of adiponectin, visfatin and resistin were significantly different between mild and severe AP [3]. Thus, some of adipokines probably have a potential for the disease severity and the pancreatic necrosis prediction.
Adiponectin (ADP), an adipokine abundantly produced and secreted by adipose tissue, exhibits protective activity in inflammatory diseases by several molecular mechanisms. Recent clinical studies showed that plasma adiponectin levels tended to be lower in subjects with severe AP, compared to those with mild form, but the precise role of adiponectin in AP remains still unclear [9].
Adipocyte fatty acid binding protein (A-FABP) is an intracellular lipid transport protein in mature adipocytes and macrophages. A-FABP serves as a regulator of lipid and glucose metabolism, and has been shown to regulate many inflammatory cytokines in vivo and in vitro, including IL-6 [10]. Nevertheless, no information concerning the relationship of A-FABP to AP is published in recent literature.
Fibroblast growth factor 21 (FGF 21) is a member of the FGF superfamily, with relevant metabolic actions [11]. FGF 21 expression is increased in multiple major metabolic organs, including liver, pancreas, skele-tal muscle, white adipose tissue and brown adipose tissues in response to diverse stressors [12]. FGF 21 gene expression is regulated by peroxisome proliferator-activated receptor-dependent pathways, which are enhanced during pancreatitis. In experimental model, FGF 21 has been suggested as an immediate response gene during AP injury that may stimulate the reduction of tissue inflammation and fibrosis [13].
In the early phase of AP, the levels of adipokines are influenced by a hemodynamic instability and a cytokine storm [14]. An imbalance between the proinflammatory and the anti-inflammatory responses leads to localized tissue destruction and distant organ damage. Therefore, in the presented study, day 4 was chosen for evalu-ation of a prognostic value of adipokines and other proinflammatory biomarkers because the dynamics of the most of them is not affected at this stage by the above-mentioned processes. Simultaneously, the next course of AP is usually determined.
Thus, the study aimed to evaluate plasma ADP, A-FABP and FGF 21 levels as potential predictors of severity of AP in day 4 after admission. We hypothesized, that their determinations could be useful for prediction of a disease severity. Concurrently, the classical proinflammatory makers (CRP, PCT and IL-6) were analysed for the same reason.
Materials and methods
Study design and subjects
The study was performed in the subjects with acute pancreatitis (n = 84, 37 females, 47 males), diagnosed according to revised Atlanta classification from 2012. They had been examined for the first time after admission on the 2nd Department of Internal Medicine, University Hospital Olomouc, Czech Republic (n = 69), and Department of Internal Medicine, Hospital Písek, Czech Republic (n =15), during the period from June 2012 to April 2014. Diagnosis of acute pancreatitis was based on clinical, laboratory and radiological findings during computed-tomography (CT) examination. The patients with very mild pancreatitis and a short hospital stay (within 3 days) were excluded. The study was reviewed and approved by the Ethics Committee of Medical Faculty and University Hospital Olomouc, and the Ethics Committee of Hospital Písek, and written informed consent was obtained from all participants.
Laboratory analysis
Venous blood samples were drawn at admission and day 4 for laboratory examinations. After centrifugation, the serum was used for other analyses. Routine serum biochemical parameters, including CRP, were analyzed on Cobas 8000 system, module c701, on Department of Clinical Biochemistry, University Hospital Olomouc, and Cobas 6000 system, module c501, on Department of Clinical Biochemistry, Hospital Písek (both Roche, Basel, Switzerland) in the same day of the blood collection. Concentrations of adipokines were measured in the sample aliquotes stored at -80 °C, no more than 6 months. C-reactive protein was assessed by an ultrasensitive immunoturbidimetric method using the kit CRPL3 (Roche, Basel, Switzerland). Procalcitonin and interleukin 6 were determined by ECLIA technique using the identical following kits: Elecsys Brahms PCT Kit and Elecsys IL-6 Kit (both Roche, Basel, Switzerland), in the day of blood collection, on e602 module (University Hospital Olomouc), and e601 module (Hospital Písek, both Roche, Basel, Switzerland).
Total adiponectin, A-FABP and FGF 21 were measured in serum using the following imunochemical kits: Human Adiponectin ELISA, Human A-FABP ELISA and Human FGF 21 ELISA (all Biovendor Laboratory Medicine Inc., Brno, Czech Republic), according to the manufacturer’s instructions and after verification of all three methods. Both the intra - and inter-assay coefficients of variation were below 10 % for all parameters.
Scoring systems
All 84 patients underwent contrast-enhanced CT examination (University Hospital Olomouc: multislice helical CT, CT LightSpeed VCT and LightSpeed RT16, GE Healthcare, Waukesha, USA; Hospital Písek: Toshiba Aquilion 64 CT scanner, Toshiba, Tokyo, Japan) within 5 days of admission. CT scans were interpreted retrospectively for the assessment of extra - and intrapancreatic disease and the presence of pancreatic necrosis. The following scores were determined: Balthazar score [15] (scores A to E were classified as 1-5 points, maximum score of 5), and computed tomography severity index (CTSI) as the sum of Balthazar score and the score of pancreatic necrosis [16].
Statistical analysis
The statistical analyses were performed in the groups according and mild/severe classification of AP (mild AP, 0-3 points; severe AP, 4-10 points), and partly in all individuals with diagnosis of AP, and the CTSI score subgroups (CTSI A, 0-2 points; CTSI B, 3-4 points; CTSI C, 5-10 points).
All values of quantitative parameters are expressed as means ± standard deviation (SD), and as medians. The Kolmogorov-Smirnov test was used to check for normal distribution. Differences in variables between the mild AP and the severe AP subgroups were analyzed by the Mann-Whitney U test. Differences in variables between three groups divided according to CTSI score were analyzed by the Kruskal-Wallis test. For statistical evaluation of a correlation between individual parameters we used a univariate Spearman correlation analysis for variables with skewed distribution. The multiple regression analysis was performed for testing of an independent association between dependent and independent variables. For calculation of cut-off values, positive predictive values (PPV), negative predictive value (NPV), area under the curve (AUC), sensitivity and specificity, the receiver-operator characteristics (ROC) analysis was made. The statistical analysis was performed by SPSS for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA). Probability values of p<0.05 were considered as statistically significant.
Results
The study was conducted with 37 females and 47 males, with the mean age 58.3 ± 17.8 years, weight 78.6 ± 17.9 kg, and BMI 27.52 ± 6.64 kg/m2. The subgroup of patients with severe AP (n = 14) had significantly higher the mean weight (88.6 ± 28.6 kg vs. 76.6 ± 14.5 kg, p< 0.01), and the mean BMI (30.40 ± 10.16 kg/m2 vs. 26.93 ± 5.41 kg/m2, p< 0.01), compared to the mild AP participants group (n = 70).
The laboratory parameters in all individuals and the subgroups according to AP severity in day 4 after admission are introduced in Table 1. Significant elevation of CRP (p< 0.001) and IL-6 (p< 0.01) levels was observed in the severe AP subgroups compared to subjects with mild form of disease in day 4, while no significant differences in plasma adipokines and PCT concentrations were found. However, the levels of FGF 21 and PCT tended to be higher in severe AP.
Tab. 1. The laboratory parameters in all individuals and the subgroups according to the AP severity in day 4 after admission 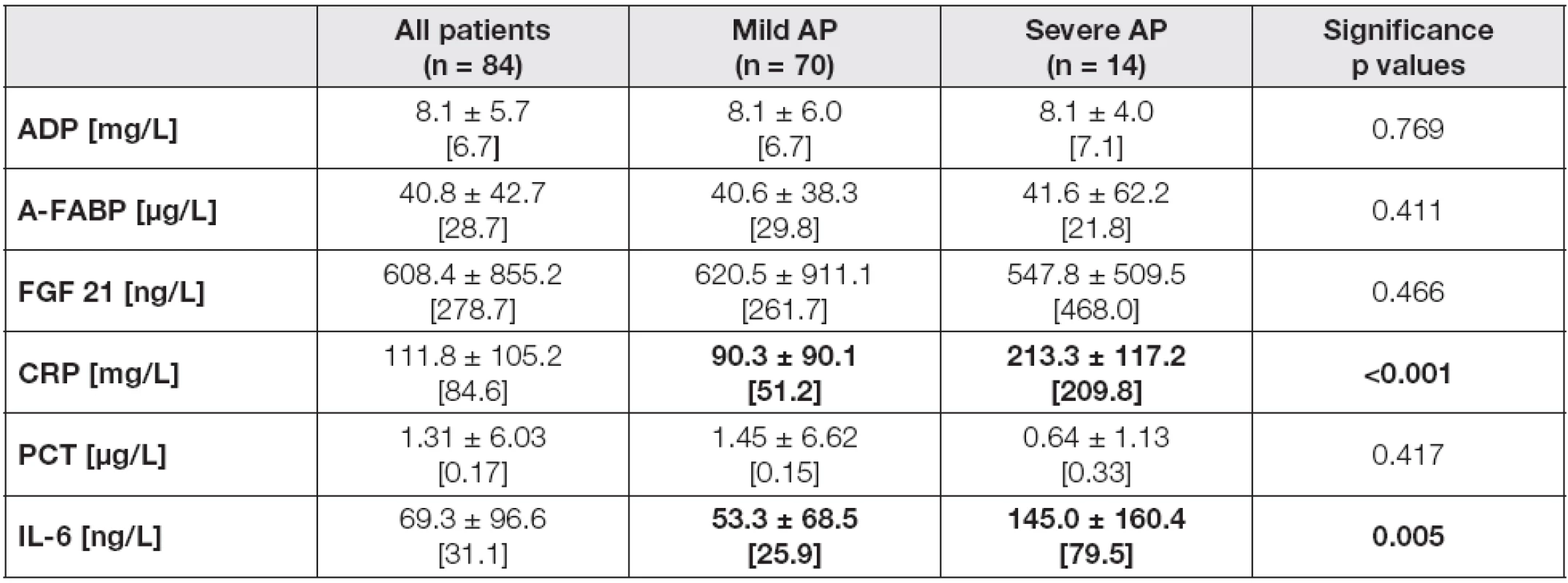
Data are expressed as means ± standard deviations, and as medians [in brackets]. Differences in variables between Mild AP and Severe AP subgroups were analyzed by the Mann-Whitney U test. Probability of p<0.05 were considered as statistically significant (bold values). Abbreviations: AP, acute pancreatitis; ADP, adiponectin; A-FABP, adipocyte fatty acid binding protein; FGF 21, fibroblast growth factor; CRP, C-reactive protein; PCT, procalcitonin; IL-6, interleukin 6. The evaluation of laboratory characteristics between three groups divided according to CTSI score in day 4 confirmed the above-mentioned trends. Unlike adipokines, CRP and IL-6 levels were significantly different between three CTSI subgroups in day 4, with the lowest concentrations in CTSI A (CRP 4 median 48.7 mg/L, IL-6 4 median 24.2 ng/L), followed by CTSI B (CRP 4 median 167.1 mg/L, IL-6 4 median 48.4 ng/L), and CTSI C subgroups (CRP 4 median 230.7 mg/L, IL-6 4 median 79.5 ng/L), with the levels of significance: p< 0.001 for CRP, and p= 0.006 for IL-6 (data not shown in table).
In the mild/severe subgroups, A-FABP levels correlated significantly with all proinflammatory parameters and, in the case of severe AP subjects, also with FGF 21 in day 4 after admission (Table 2). A positive correlation of FGF 21 with all investigated parameters was found in severe AP, whilst in the mild AP subgroup only a correlation with CRP was observed. In order to evaluate the independent association of the observed parameters with A-FABP and FGF 21, the multiple regression analysis with adipokines in day 4 as dependent variables and correlated parameters as independent predictors was performed (Table 3). A-FABP was associated positively with PCT in both mild and severe AP subgroups, and also ADP and CRP levels have predicted A-FABP concentrations in the mild AP subgroup subjects. In contrast, FGF 21 was associated positively with ADP and negatively with CRP, exclusively in the severe AP subgroup.
Tab. 2. The correlation analysis of laboratory characteristics with A-FABP and FGF 21 in the groups of patients in day 4 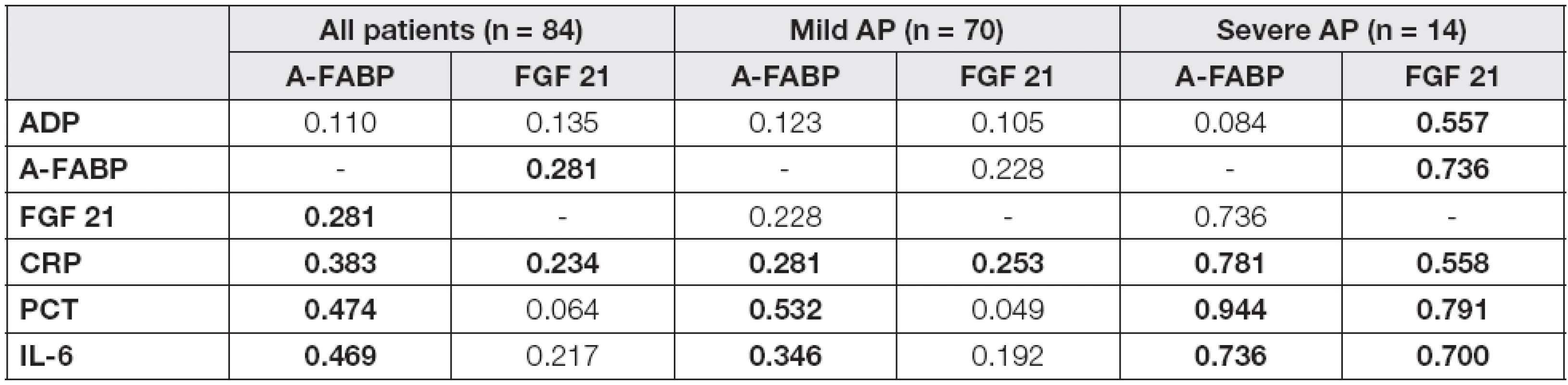
r values of correlation coefficients are introduced in Table 2 Probability of p<0.05 were considered as statistically significant (bold values). Abbreviations: AP, acute pancreatitis; ADP, adiponectin; A-FABP, adipocyte fatty acid binding protein; FGF 21, fibroblast growth factor; CRP, C-reactive protein; PCT, procalcitonin; IL-6, interleukin 6. Tab. 3. The multiple regression analysis of laboratory characteristics with A-FABP and FGF 21 as dependent variables in the groups of patients in day 4 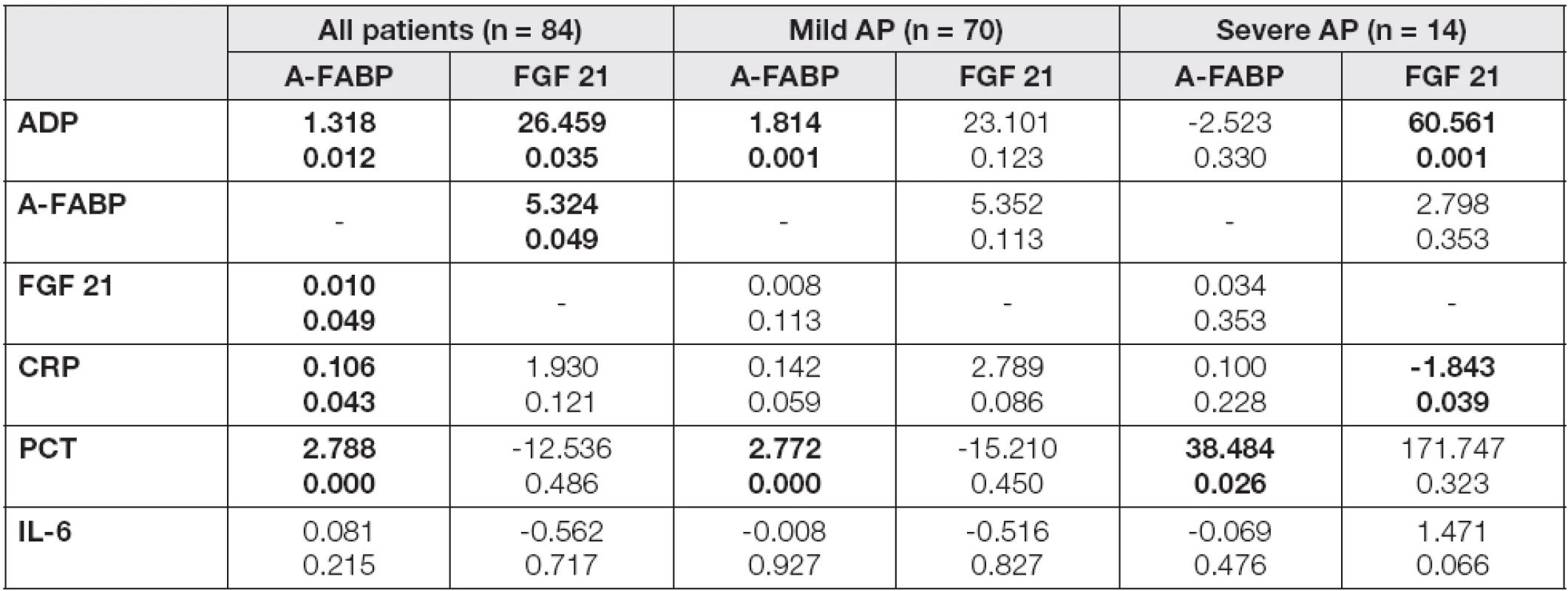
b and p values are introduced in Table 3 Probability of p<0.05 were considered as statistically significant (bold values). Abbreviations: AP, acute pancreatitis; ADP, adiponectin; A-FABP, adipocyte fatty acid binding protein; FGF 21, fibroblast growth factor; CRP, C-reactive protein; PCT, procalcitonin; IL-6, interleukin 6. As the next step of the study, a calculation of cut-off values for CRP and IL-6 in the prediction of severe AP was performed. Adipokines and proinflammatory markers were used for receiver-operator characteristics (ROC) analysis. The data are summarized in Table 4. The results of area under curve (AUC) calculations revealed that only CRP (AUC = 0.815) and IL-6 (AUC = 0.738) were suitable, but not excellent, as potential predictors of the disease severity in day 4 (Fig. 1-3). The cut-off values were established for both parameters: 100 mg/L for CRP and 37 ng/L for IL-6, with negative predictive values 96 % and 92 %, and positive predictive values 39 % and 29 %, respectively.
Tab. 4. ROC analysis for the prediction of severe AP in day 4 after admission 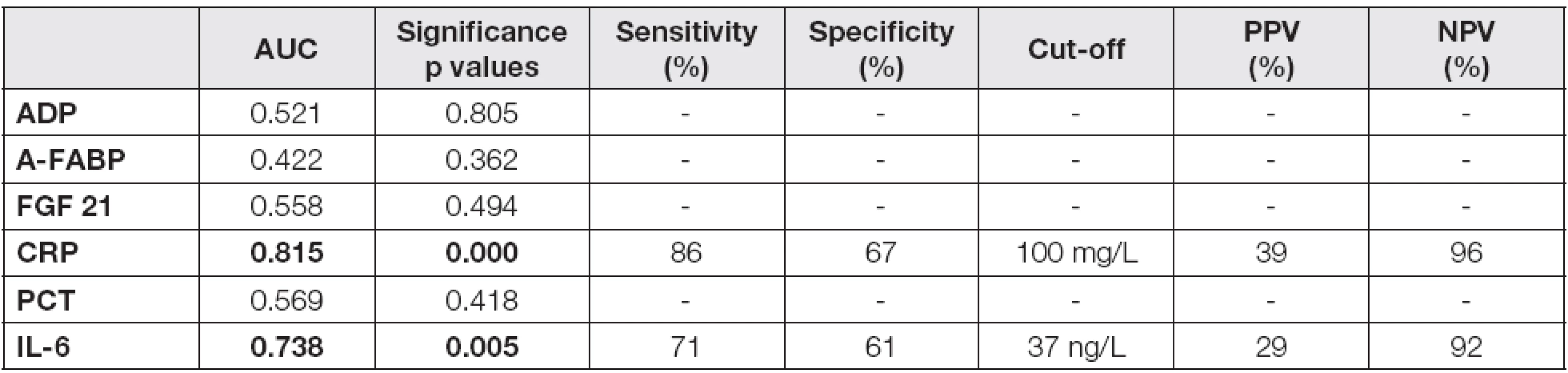
Probability of p<0.05 were considered as statistically significant (bold values). Abbreviations: AUC, area under curve; PPV, positive predictive value; NPV, negative predictive value; AP, acute pancreatitis; ADP, adiponectin; A-FABP, adipocyte fatty acid binding protein; FGF 21, fibroblast growth factor; CRP, C-reactive protein; PCT, procalcitonin; IL-6, interleukin 6. <strong>Fig. 1.</strong> ROC curve for CRP. The cut-off value is 100 mg/L. 
<strong>Fig. 2.</strong> ROC curve for IL-6. The cut-off value is 37 ng/L. 
<strong>Fig. 3.</strong> ROC curves for ADP, A-FABP and FGF 21. Non-significant results of ROC analyses. 
Discussion
Adipokines in day 4
Formerly, we have evaluated the levels of ADP, A-FABP and FGF 21 at admission in comparison to day 4 in the patients with AP (data submitted for publication). While no differences were observed in ADP, the significant decline of A-FABP and FGF 21 levels was found during the first four days. Therefore, the presen-ted work aimed to examine the potential of predictive values of especially A-FABP and FGF 21 together with proinflammatory markers in day 4 in order to differentiate between mild and severe forms of AP. The similar approach was used in several studies, but not with A-FABP and FGF 21.
The study of Sharma et al. revealed that serum ADP levels from days 4 to 7 were significantly lower in patients with severe AP than those with mild form, and could serve as an inverse marker of systemic inflammatory response to pancreatic injury [17]. In contrast, the concentrations of adiponectin and leptin did not correlate with AP severity on admission and during the first week of the disease in another study [18], and had no potential for clinical routine use in predicting both the severity of AP and the extent of peripancreatic fat cell necrosis [9]. Similarly, the presented work revealed no ADP levels difference, comparing patients with mild and severe disease in day 4. Nevertheless, the observation of an experimental model suggests a protective role of ADP in cerulein-induced AP in high-fat diet mouse model [19], and the precise role of ADP in the course of AP should be examined in further research [20].
A-FABP is considered a key proinflammatory mediator linking obesity with cardiovascular disease in humans [21]. The plasma A-FABP levels were identified as an independent risk factor for vascular inflammation [22] and correlated positively with several proinflammatory markers including CRP and IL-6, and inversely with adiponectin in experimental studies [23].
The relationship of A-FABP to AP has not been examined in both animal and human studies. Nevertheless, the evaluation of a prognostic value of A-FABP in the course of AP could be useful, because of its ability to link metabolic homeostasis with inflammation. A-FABP may be associated with the expression of Toll-like receptors, macrophages activation, synthesis and release of proinflammatory cytokines (including IL-6), activation of expression of cyclooxygenase-2 and eicosanoid synthesis. These factors may cause insulin resistance and initiation and progression of inflammation and sepsis. A-FABP serum concentrations were elevated in critically ill patients, and correlated with disease severity in the study of Huang et al. [24]. In addition, a positive correlation of circulating A-FABP with tumor necrosis factor-alpha and procalcitonin, and no association with BMI was found. In our study, A-FABP levels of subjects with severe AP did not differ markedly from those with the mild AP in day 4, suggesting their poor ability to discriminate the severity of AP. This phenomenon was confirmed by a subsequent ROC analysis where the mean A-FABP levels proved to be a non-suitable parameter for the identification of patients with severe AP. In both forms of disease, A-FABP correlated positively with PCT, and also with IL-6 and CRP, unlike the above-mentioned study [24]. The multiple regression analysis of laboratory characteristics with A-FABP as dependent variable showed a positive association with procalcitonin in both subgroups, in spite of the nonbacterial inflammatory origin of AP. Thus, we demonstrated that A-FABP is closely linked with proinflammatory factors during the early phase of AP in our study, regardless of the disease severity. The positive association with ADP in the mild AP subjects was surprising and we have no relevant explanation of this phenomenon.
FGF 21 has been suggested as a novel hepatokine, adipokine and myokine [25, 26]. Several studies described the presence of FGF 21 in the exocrine pancreas and beta cells at significantly higher levels than in liver, adipose tissue and muscles. But the precise mechanism of its expression and regulation in pancreas is unknown so far. The presence of FGF 21 in pancreatic acinar cells was described and its protective action against a damage during AP was observed as well [27]. It seems likely that the induction of FGF 21 is limited to the early phase of inflammation, unlike the expression of other FGF-family factors, which are produced during the regeneration of pancreas. New findings suggest that FGF 21 acts as an important regulator in the adaptation to stress [28], and can reduce its progression in diverse disease conditions [12]. At present, no information is available in human clinical studies concerning the role of FGF 21 in the early stage of AP. As with A-FABP, we found no significant differences in the mean plasma levels of FGF 21 between the study groups in day 4. FGF 21 tended to be higher in the patients with the severe form of AP. Nevertheless, an AUC of 0.558 was calculated and FGF 21 thus failed to provide a significant result in ROC analysis. In the next step, a correlation analysis revealed closer links of FGF 21 with all measured parameters in severe AP, but most of them have lost significance after a multiple regression analysis; FGF 21 was associated negatively with CRP and positively with ADP in patients with severe AP. In the study of Li et al. [29], serum FGF 21 levels were positively and independently associated with CRP levels in newly diagnosed patients with type 2 diabetes mellitus. Similarly, a positive correlation with CRP was found in a study of the patients with chronic kidney disease [30]. A negative association with CRP probably reflects the course of both parameters in the first four days of AP in our study. While the mean levels of FGF 21 decreased markedly in severe AP, the elevation of CRP was found in day 4, in contrast to the individuals with mild form of AP. This phenomenon could reflect the course of clinical complications in severe AP as well (data submitted for publication). In an experimental model the authors demonstrated that ADP is a mediator of the systemic effects of FGF 21 on insulin sensitivity and energy metabolism [31]. ADP possesses many functional and regulatory similarities with FGF 21. But the strong positive association of FGF 21 with ADP only in severe AP observed in our study needs to be clarified in further research.
Proinflammatory markers in day 4
CRP has been proposed as a useful severity predictor in the early stage of AP [4,32]. In the presented study, CRP was significantly elevated in the subgroups of the patients with both severe AP, and higher CTSI score in day 4. High NPV (96 %) was found for CRP cut-off value of 100 mg/L. As shown in Figure 1, the course of the curve is not ideal and a positive prediction of AP severity is troublesome. Thus, a selected value of plasma CRP could exclude the severe form of AP with a satisfying sensitivity and specificity. The similar NPV with a lower AUC value was achieved in the study of Fisic et al. [4], but for the CRP cut-off value of 214 mg/L, which was established for day 3 after admission to discriminate the patients with systemic complications of disease. In general, CRP was tested as a predictor of AP severity during the first 48 hours in recent studies [32, 33], with higher limit values for disease severity prediction.
Both IL-6 and PCT have been suggested for the differential diagnostics of patients with a risk of severe and/or necrotic form of AP [33-35]. In contrast to PCT, elevated IL-6 levels were associated with severe forms of AP in day 4 in our work. The limit value of IL-6 37 ng/L could rule out severe AP with NPV of 92 %, but PPV was only 29%. These values are similar to those in the above-mentioned study (4), where the measurements of plasma IL-6 levels were performed in day 1. Thus, our results might indicate a diagnostic utility of both CRP and IL-6 to exclude a severe form of AP with a high negative prediction in day 4 after admission.
Limitation of the study
The major limitation of the study is the small number particularly of subjects in the severe AP subgroup, which could reduce the power of significance of the observed relationships. However, it represents a common problem due to a small number of patients with severe AP in general. Further prospective studies with larger sample sizes are needed to determine the role of especially FGF 21 and A-FABP in the course of AP.
Conclusion
Using the multiple regression analysis, we demonstrated a close link of A-FABP with procalcitonin during the early phase of AP, regardless of the disease severity. A negative association of FGF 21 with CRP probably reflects the course of these parameters in the first four days in severe AP. The strong positive association of FGF 21 with ADP needs to be clarified in further research. ADP, A-FABP and FGF 21 failed to provide a significant result in ROC analysis suggesting a poor potential for the discrimination of the disease severity in day 4. This study also gave evidence about the diagnostic usefulness of CRP and IL-6 plasma concentrations to exclude the severe form of AP with a high negative prediction in the fourth day after admission. The selected cut-off value for IL-6 could be used even at admission for the prognosis of a severity and systemic complication of AP.
This study was supported by a grant from the Internal Grant Agency of the Palacky University Olomouc No. IGA_LF_2014_005
Do redakce došlo 4. 11. 2014
Adresa pro korespondenci:
Ing. Dalibor Novotný, Ph.D.
Oddělení klinické biochemie
Fakultní nemocnice Olomouc
I. P. Pavlova 6
775 20 Olomouc
E-mail: dalibor.novotny@fnol.cz
Zdroje
1. Fétaud-Lapierre, V., Pastor, C. M., Jorge-Costa, M., et al. Time-course proteomic analysis of taurocholate-induced necrotizing acute pancreatitis. J. Proteom., 2013, 85, p. 12-27.
2. Jakchairoongruang, K., Arjhansiri, K. Prognostic va-lue of contrast-enhanced computed tomography in acute pancreatitis. Asian Biomed., 2013, 3, p. 357-364.
3. Karpavicius, A., Dambrauskas, Z., Sileikis, A., Vitkus, D., Strupas, K. Value of adipokines in predicting the severity of acute pancreatitis: Comprehensive review. World J. Gastroenterol., 2012, 18(45), p. 6620-6627.
4. Fisic, E., Poropat,G., Bilic-Zulle, L., Licul, V., Milic, S., Stimac, D. The Role of IL-6, 8, and 10, sTNFr, CRP, and Pancreatic Elastase in the Prediction of Systemic Complications in Patients with Acute Pancreatitis. Gastroenterol. Res. Pract., 2013, Article ID 282645, 6 pages. http://dx.doi.org/10.1155/2013/282645.
5. Al-Bahrani, A., Ammori, Z. B. Clinical laboratory assessment of acute pancreatitis. Clin. Chim. Acta, 2005, 362(1-2), p. 26-48.
6. Matull, W. R., Pereira, S. P., O’Donohue, J. W. Biochemical markers of acute pancreatitis. J. Clin. Pathol., 2006, 59(4), p. 340-344.
7. Wozniak, S. E., Gee, L. L., Wachtel, M. S., Frezza, E. E. Adipose Tissue: The New Endocrine Organ? A Review Article. Dig. Dis. Sci., 2009, 54, p.1847-1856.
8. Franco-Pons, N., Gea-Sorlí, S., Closa, D. Release of inflammatory mediators by adipose tissue during acute pancreatitis. J. Pathol., 2010, 22, p.175-182.
9. Schaffler, A., Landfried, K., Volk, M., et al. Potential of adipocytokines in predicting peripancreatic necrosis and severity in acute pancreatitis: pilot study. J. Gastroenterol. Hepatol. 2007, 22, p. 326-334.
10. Hui, X., Li, H., Zhou, Z., et al. Adipocyte fatty acid-binding protein modulates inflammatory responses in macrophages through a positive feedback loop involving c-Jun NH2-terminal kinases and activator protein-1. J. Biol. Chem., 2010, 285, p. 10273-10280.
11. Iglesias, P., Selgas, R., Romero, S., Diez, J. Biological role, clinical significance, and therapeutic possibilities of the recently discovered metabolic hormone fibroblastic growth factor 21. Eur. J. Endocrinol., 2012, 167, p. 301-309.
12. Kim, K. H., Lee, M. S. FGF21 as a Stress Hormone: The Roles of FGF21 in Stress Adaptation and the Treatment of Metabolic Diseases. Diabetes Metab. J. 2014, 38, p. 245-251.
13. Johnson, C. L., Weston, J. Y., Chadi, S. A., et al. Fibroblast Growth Factor 21 Reduces the Severity of Cerulein-Induced Pancreatitis in Mice. Gastroenterology 2009,137, p.1795-1804.
14. Makhija, R., Kingsnorth, A. J. Cytokine storm in acute pancreatitis. J. Hepatobiliary Pancreat. Surg., 2002, 9(4), p. 401-410.
15. Balthazar, E. J. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002, 223, p. 603-13.
16. Banks, P. A., Bollen, T. L., Dervenis, C., et al. Classification of acute pancreatitis 2012: revision of the Atlanta classification and definitions by international consensus; Acute Pancreatitis Classification Working Group. Gut, 2013, 62, p.102-11.
17. Sharma, A., Muddana, V., Lamb, J., Greer, J., Papachristou, G. I., Whitcomb, D. C. Low serum adiponectin levels are associated with systemic organ failure in acute pancreatitis. Pancreas, 2009, 38, p. 907-912.
18. Tukiainen, E., Kylanpaa, M. L., Ebeling, P., Kemppainen, E., Puolakkainen, P., Repo, H. Leptin and adiponectin levels in acute pancreatitis. Pancreas, 2006, 32, p. 211-214.
19. Araki, H., Nishihara, T., Matsuda, M., et al. Adiponectin plays a protective role in caerulein-induced acute pancreatitis in mice fed a high-fat diet. Gut, 2008, 57, p. 1431-1440.
20. Kishida, K., Funahashi, T., Shimomura, I. Adiponectin as a routine clinical biomarker. Best Pract. Res. Clin. Endocrinol. Metab., 2014, 28, p.119-130.
21. Xu, A., Vanhoutte, P. M. Adiponectin and adipocyte fatty acid binding protein in the pathogenesis of cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol., 2012, 302, p. H1231-H1243.
22. Yoo, H. J., Kim, S., Park, M. S., et al. Serum adipocyte fatty acid-binding protein is associated independently with vascular inflammation: analysis with (18)F-fluorodeoxyglucose positron emission tomography. J. Clin. Endocrinol. Metab., 2011, 96, p. E488–E492.
23. Xu, A., Tso, A. W., Cheung, B. M., et al. Circulating adipocyte-fatty acid binding protein levels predict the development of the metabolic syndrome: a 5-year prospective study. Circulation, 2007, 115, p.1537-1543.
24. Huang, C. L., Wu, Y. W., Hsieh, A. R., Hung, Y. H., Chen, W. J., Yang, W. S. Serum adipocyte fatty acid-binding protein levels in patients with critical illness are associated with insulin resistance and predict mortality. Crit. Care, 2013, 17, R22.
25. Izumiya, Y., Bina, H. A., Ouchi, N., Akasaki ,Y., Kharitonenkov, A., Walsh, K. FGF21 is an Akt-regula-ted myokine. FEBS Lett., 2008, 582(27), p. 3805-3810.
26. Kharitonenkov, A., Larsen, P. FGF21 reloaded: challenges of a rapidly growing field. Trends Endocrinol. Metab., 2011, 22(3), p. 81-86.
27. Algül, H. A Novel Role for the Fibroblast Growth Factor 21 in Acute Pancreatitis. Gastroenterology, 2009, 137(5), p.1577-1579.
28. Kim K. H., Lee, M. S. FGF21 as a Stress Hormone: The Roles of FGF21 in Stress Adaptation and the Treatment of Metabolic Diseases. Diabetes Metab. J., 2014, 38, p. 245-251.
29. Li, X., Fan, X., Ren, F., et al. Serum FGF21 levels are increased in newly diagnosed type 2 diabetes with nonalcoholic fatty liver disease and associated with hsCRP levels independently. Diabetes Res. Clin. Pract., 2011, 93(1), p.10-16.
30. Lin, Z., Zhou, Z., Liu, Y., et al. Circulating FGF21 levels are progressively increased from the early to end stages of chronic kidney diseases and are associated with renal function in Chinese. PLoS One, 2011, 6(4), e18398. http://dx.doi.org/10.1371/journal.pone.0018398.
31. Lin, Z., Tian, H., Lam, K. S. L, et al. Adiponectin Media-tes the Metabolic Effects of FGF21 on Glucose Homeo-stasis and Insulin Sensitivity in Mice. Cell Metab., 2013, 17, p. 779-789.
32. Khanna, A. K., Meher, S., Prakash, S., et al. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and Procalcitonin in Predicting Severity, Organ Failure, Pancreatic Necrosis, and Mortality in Acute Pancreatitis. HPB Surg., 2013, 367581. http://dx.doi.org/10.1155/2013/367581.
33. Nieminen, A., Maksimow, M., Mentula, P., et al. Circulating cytokines in predicting development of severe acute pancreatitis. Crit. Care, 2014,18, R104. http://dx.doi.org/10.1186/cc13885.
34. Lesina, M., Wörmann, S. M., Neuhöfer, P., Song, L., Algül, H. Interleukin-6 in inflammatory and malignant diseases of the pancreas. Semin. Immunol., 2014, 26, p. 80-87.
35. Mofidi, R., Suttie, S. A., Patil, P. V., Ogston, S., Parks, R. W. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: systematic review. Surgery, 2009,146, p. 72-81.
Štítky
Biochemie Nukleární medicína Nutriční terapeut
Článek vyšel v časopiseKlinická biochemie a metabolismus
Nejčtenější tento týden
2015 Číslo 1- GLP-1RA a PCOS: Je to „jenom“ o hmotnosti?
- Moderní přístupy zvyšující efektivitu antibiotické léčby v nemocniční praxi
- Farmakologická léčba obezity u pacientek se syndromem polycystických ovarií – systematický přehled a klinická doporučení
- Zpracované masné výrobky a červené maso jako riziko rozvoje kolorektálního karcinomu u žen? Důkazy z prospektivní analýzy
- Efektivita léčby a možné indikace liraglutidu v gynekologii
-
Všechny články tohoto čísla
- Comparison of two most frequent methods for the determination of total bilirubin in newborns.
- State of standardization for determination of catalytic concentration of alkaline phosphatase as viewed by different programs of external quality control: Our view of the situation and its solution.
- Recommendation for laboratory screening of inborn defects in the first and second trimester of pregnancy
- Is science on the increase or fall?
- Problems of measuring PTH in following chronic renal disease: A minireview.
- The acute pancreatitis severity prediction using adiponectin, adipocyte fatty acid binding protein and fibroblast growth factor 21 levels in day 4 after admission
- Klinická biochemie a metabolismus
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Comparison of two most frequent methods for the determination of total bilirubin in newborns.
- Problems of measuring PTH in following chronic renal disease: A minireview.
- Recommendation for laboratory screening of inborn defects in the first and second trimester of pregnancy
- State of standardization for determination of catalytic concentration of alkaline phosphatase as viewed by different programs of external quality control: Our view of the situation and its solution.
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



