-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Susceptibility of clinical isolates of Bordetella pertussis to chemicals
Citlivost klinických izolátů Bordetella pertussis na chemické látky
Cíl práce:
Cílem práce bylo u klinických izolátů kmenů Bordetella pertussis získaných z Národní referenční laboratoře pro pertusi a difterii stanovit citlivost k běžně dostupným dezinfekčním přípravkům. Kromě potvrzení citlivosti bylo naším úkolem stanovit přesnou koncentraci a dobu působení chemických látek při jejich praktickém použití, případně odhalit možný vznik rezistence celkem u 34 kmenů B. pertussis zaslaných do Národní referenční laboratoře pro pertusi a difterii v letech 2014 a 2015.
Materiál a metody:
Celkem bylo testováno 34 klinických izolátů, které byly zkoušeny třemi různými metodami na citlivost k chemickým látkám. Suspenzní mikrometoda byla použita jako prvotní screening, zkouška byla prováděna bez bílkovinného znečištění. Další testy probíhaly dle EN 14885, podle které je stanoveno testování přípravků v několika krocích. V prvním kroku jsou použity kvantitativní suspenzní metody (Fáze 2, Stupeň 1) a v druhém kroku metody pro praktické použití (Fáze 2, Stupeň 2). V prvním stupni byla použita kvantitativní suspenzní metoda modifikovaná dle EN 13727+A2, kdy byla potvrzena baktericidní účinnost daných přípravků za podmínek vyššího bílkovinného znečištění. Ve druhém stupni byly klinické izoláty testovány pomocí kvantitativní metody na nosiči modifikované dle EN 14561 v přítomnosti vyššího bílkovinného znečištění. Podle této normy jsou simulovány praktické podmínky použití daného přípravku. Celkem byly testovány čtyři různé dezinfekční přípravky s různým složením a různým určením jejich použití.
Výsledky:
Přípravek č. 1 vykazoval baktericidní účinky již při koncentraci 0,5 % po 2 minutách působení v případě ponoru a při 5% koncentraci po 2 minutách v případě otření. Druhý přípravek byl účinný v případě ponoru při koncentraci 0,1 % po 2minutovém působení a v případě otření při 1% koncentraci po časové expozici 2 minuty. Přípravek č. 3 ani ve 100% koncentraci po 5 minutách nevykazoval baktericidní účinky. Poslední přípravek účinkoval po 5 minutách při 10% koncentraci a po 2 minutách při 30% koncentraci.
Závěry:
V souboru klinických kmenů nebyl objeven žádný rezistentní kmen. Díky použitým metodám, které simulují praktické podmínky, byla stanovena přesná doba působení a koncentrace nutná k dezinfekci ploch a předmětů u přípravků č. 1 a 2 za přítomnosti bílkovinného znečištění. Poslední dva přípravky nejsou primárně určeny k dezinfekci ploch či předmětů, ale k dezinfekci kůže, respektive sliznic. Výsledky získané pro přípravky č. 3 a 4 jsou tedy zajímavé, ale nelze z nich vyvozovat konečné závěry, jelikož nebyly přesně simulovány podmínky praktického použití těchto přípravků.
KLÍČOVÁ SLOVA
Bordetella pertussis – citlivost – dezinfekční přípravky
Epidemiol. Mikrobiol. Imunol., 67, 2018, č. 3, s. 122–128
Authors: P. Uttlová 1,2; J. Urban 1; V. Melicherčíková 1; J. Zavadilová 3; K. Fabiánová 4
Authors place of work: National Reference Laboratory for Disinfection and Sterilisation, National Institute of Public Health, Prague, Czech Republic 1; National Reference Laboratory for Pertussis and Diphtheria, National Institute of Public Health, Prague, Czech Republic 3; Department of Infectious Disease Epidemiology, National Institute of Public Health, Prague, Czech Republic 4; rd Faculty of Medicine, Charles University, Prague, Czech Republic 23
Published in the journal: Epidemiol. Mikrobiol. Imunol. 67, 2018, č. 3, s. 122-128
Category: Původní práce
Summary
The aim of study:
To test clinical isolates of Bordetella pertussis from the National Reference Laboratory for Pertussis and Diphtheria for susceptibility to commonly available disinfectants. Another aim was to determine the concentration and exposure time for each chemical under real conditions of use and possibly to detect the emergence of resistance to disinfectants among 34 strains of B. pertussis referred to the National Reference Laboratory for Pertussis and Diphtheria in 2014 and 2015.
Material and methods:
A total of 34 clinical isolates of Bordetella pertussis were tested for susceptibility to chemical disinfectants by three different methods. The microsuspension method was used for the primary screening, and the tests were carried out without protein contamination. Further testing was conducted in accordance with standard EN 14885, where the test procedure consists of several steps. Step 1 involves quantitative suspension methods (Phase 2, Step 1), and step 2 uses methods designed for practice (Phase 2, Step 2). The quantitative suspension method modified according to EN 13727+A2 was used in step 1 to confirm bactericidal activity of the test products under the dirty conditions. In step 2, clinical isolates were tested using a quantitative carrier test method under the dirty conditions modified according to EN 14561. Based on this standard, the real conditions of product use are simulated. Four disinfectants differing in composition and intended use were tested.
Results:
Disinfectant No. 1 showed bactericidal activity at a concentration of 0.5% after 2 min of exposure in the case of immersion or at a concentration of 5% after 2 min of exposure when treated by wiping. Disinfectant No. 2 was active at a concentration of 0.1% after 2 min of exposure or at a concentration of 1% after 2 min of exposure, respectively. Disinfectant No. 3 did not show bactericidal activity even at a concentration of 100% after 5 min of exposure. Disinfectant No. 4 showed bactericidal activity at a concentration of 10% after 5 min of exposure or at a concentration of 30% after 2 min of exposure.
Conclusions: None of the strains tested was resistant. Using the methods that simulate the real conditions of use of disinfectants Nos. 1 and 2, it was possible to determine the concentration and exposure time needed to achieve disinfection of surfaces under the dirty conditions. Disinfectants Nos. 3 and 4 are not primarily intended for the treatment of surfaces but for the treatment of the skin and mucous membranes. The results obtained with the latter two products are interesting but inconclusive as the real conditions of their use were not simulated accurately.
KEYWORDS
Bordetella pertussis – susceptibility – disinfectants
INTRODUCTION
Pertussis (whooping cough) is a highly contagious disease of the respiratory tract. It occurs worldwide, and according to the WHO data, 20–40 million cases resulting in 400 000 deaths are reported annually [1]. The causative agent is the bacterium Bordetella pertussis or Bordetella parapertussis, and possibly also B. bronchiseptica or B. holmesii. Bordetellae are very small, Gram-negative, non-motile, encapsulated, strictly aerobic, non-invasive coccobacilli with surface pili [2, 3, 4]. Some authors have reported the presence of saccharide biofilm on the surface of some Bordetellae [5]. Pertussis spreads from person to person while in close contact through respiratory droplets produced by coughing and sneezing that adhere to the mucous membrane of a susceptible individual. It is assumed that, exceptionally, indirect transmission can take place via fomites freshly contaminated by secretions from the upper and lower airways [6]. Vysoká-Buriánová has reported that bordetellae can survive in dried upper airway secretions for several hours [7]. However, some studies have shown that bordetellae can survive on dry fomite surfaces for as long as three to five days [8], on paper surfaces for two days, and on glass surfaces even for six days [9]. A recent study has found some zoopathogenic species of Bordetella can survive and grow in soil [10]. Surprisingly enough, the bacterium B. bronchiseptica has been discovered to survive and proliferate inside the cells of the amoeba Dictyostelium discoideum [11]. Therefore, it is of utmost importance to carry out thorough disinfection of surfaces. Some studies have reported bordetellae to be susceptible to glutaraldehyde [12]. Moreover, similarly to most vegetative bacteria, they are also susceptible to low concentrations of chlorine [13], 70% ethanol, phenols, and peracetic acid [14]. A widely studied phenomenon has long been antibiotic resistance, but recently, increasing attention has also been drawn to the emergence of resistance to antiseptics, disinfectants, and preservatives, collectively referred to as biocides [15]. Some bacteria such as Staphylococcus aureus have been reported to develop the mechanism of biocide-antibiotic co-resistance. In particular, co-resistance to beta-lactams and quaternary ammonium compounds has been recorded [16]. In this study, we focus on bactericidal activity of selected disinfectants on recent clinical isolates of B. pertussis.
MATERIAL AND METHODS
Bordetella pertussis strains
The test strains were 34 recent clinical isolates of B. pertussis from 2014 to 2015, referred to the National Reference Laboratory (NRL) for Pertussis and Diphtheria, National Institute of Public Health (NIPH), Prague, Czech Republic within national surveillance of pertussis.
Storage and culture of strains
Clinical isolates of B. pertussis were stored frozen at -70 °C(Kryobanka B, ITEST plus Ltd). They were inoculated on charcoal agar plates (Media Preparation Unit, Centre for Epidemiology and Microbiology (CEM), NIPH) and incubated at 36 ± 1 °C for 72 hours in normal atmosphere. To confirm the species identification, the Bordetella pertussis diagnostic serum (Remel Ltd, USA) was used in accordance with the manufacturer's instructions. After the identification to the species level by the NRL for Pertussis and Diphtheria, the isolates in pure culture on charcoal agar were submitted to the NRL for Disinfection and Sterilisation.
Disinfectants and their active ingredients
Disinfectant No. 1
The product active ingredient is sodium hypochlorite. The product has a bactericidal, virucidal, levurocidal, and fungicidal activity. It is intended for the disinfection of floors, surfaces, fomites, and hygienic tools as well as for the disinfection of drinking water and swimming pools.
Disinfectant No. 2
The product contains quaternary ammonium compounds, benzyl-C8-18-alkyldimethyl ammonium, and chlorides. It has a bactericidal, virucidal, levurocidal, and fungicidal activity. It is commonly used for the disinfection of hands, skin, wounds, and superficial injuries, but also of fomites and surfaces.
Disinfectant No. 3
The product active ingredients are ethanol, butan-2-one, glycerol 85%, tetradecan-1-ol, propan-1-ol, and purified water. It has a bactericidal, virucidal, levurocidal, fungicidal, and tuberculocidal activity. It is commonly used for hygienic and surgical hand disinfection.
Disinfectant No. 4
The product active ingredients are octenidine dihydrochloride, glycerin, PEG 40 hydrogenated ricin oil, sodium gluconate, aspartame, citric acid, and aroma. The product has a bactericidal, levurocidal, and fungicidal activity. It is used for oral cavity disinfection.
Chemicals and reagents
Hard water
Hard water was prepared by mixing solutions A and B (obtained from the Media Preparation Unit, CEM, NIPH):
- Solution A – 19.84 g of magnesium chloride (MgCl2) and 46.24 g of calcium chloride (CaCl2) dissolved in 1 litre of distilled water.
- Solution B – 35.02 g of sodium hydrogen carbonate (NaHCO3) dissolved in 1 litre of distilled water.
Solution A was sterilised in an autoclave and solution B by membrane filtration. After sterilisation, 6 ml of solution A and 8 ml of solution B were mixed, and the volume was adjusted to 1 litre with sterile distilled water.
Diluting solution (Media Preparation Unit, CEM, NIPH)
1 g of pancreatic casein hydrolysate and 8.5 g of sodium chloride (NaCl) were dissolved in distilled water, and the volume was adjusted to 1 litre. The solution was sterilised in an autoclave. After that, pH was adjusted to 7.0 ± 0.2.
Interfering substance
Three grams of bovine serum were dissolved in 97 ml of diluting solution. After that, 97 ml were taken and added with 3 ml of sheep erythrocytes. The final concentration of the bovine serum with sheep erythrocytes used in the test procedure is 3 g/l and 3 ml/l respectively.
Microsuspension method
The microsuspension method is a semiquantitative method which was modified to meet the growth requirements of clinical isolates of B. pertussis. [17]. The principle of the microsuspension method consists in the action of a substance on the microorganisms suspended in the test solution. After 2, 4, 8, 16, and 32 minutes of exposure, a part of the solution was transferred to 100 µl of Stainer-Scholte liquid medium in 96-well microtiter plates. After inoculation, the 96-well microtiter plates were placed in a thermostat at a temperature of 36 ± 1 °C for seven days. After incubation, microbial growth was evaluated depending on the concentration and contact time. The lowest concentration of the test product at which no microbial growth was observed was considered as the minimal inhibitory or bactericidal concentration. In this method, bacteria are exposed to the test products without dirty conditions.
Quantitative suspension method under the dirty conditions
In accordance with EN 14885 “Chemical disinfectants and antiseptics – Application of European Standards for Chemicals and Antiseptics” [18], the products were first tested by the quantitative suspension method in step 1. To test products Nos. 1, 2, and 4, the modified method according to EN 13727+A2 [19] was used under the dirty conditions. First of all, 1 ml of the bacterial test suspension was mixed with 1 ml of the interfering substance (3.0 g/l of bovine albumin and 3.0 ml/l of sheep erythrocytes). Two minutes after the mix was prepared, it was added with 8 ml of the test disinfectant (the concentration of the product added was 20% higher than the final concentration required). The test disinfectant was diluted with hard water. In the case of product No. 3, the modified method in accordance with EN 13727+A2 [19] was used again. This product is commonly used in a concentrated form. In the testing, 0.1 ml of the bacterial suspension was first mixed with 0.2 ml of the interfering substance. The mix was stirred and let for incubation for two minutes. After that, 9.7 ml of the test disinfectant was added (resultant concentration of 97%). The exposure times for products Nos. 1, 2, and 4 were 30 s, 60 s, 5 min, and 15 min. For product No. 3, the exposure times were only 30 s and 60 s. The exposure times for all test products were those recommended by the manufacturer. At the end of exposure, an aliquot was taken and diluted in a decimal series in Stainer-Scholte liquid medium, and the dilutions were plated onto charcoal agar plates. After that, the plates were placed in the incubator at 36 ± 1 °C for seven days. The results were read and evaluated. According to EN 13727+A2, a 5 log or higher reduction in the bacterial count is required for a disinfectant to be classified as bactericidal.
Quantitative carrier method under the dirty conditions
In accordance with EN 14885 “Chemical disinfectants and antiseptics – Application of European Standards for Chemicals and Antiseptics” [18], the test products were further analysed using the quantitative carrier method in step 2. The quantitative carrier test was modified in accordance with EN 14561 [20]. It simulates real conditions of product use in terms of drying artificially contaminated carriers, temperature, exposure time, and protein contamination. First of all, nine ml of bacterial suspension were mixed with 1 ml of the interfering substance (3.0 g/l of bovine albumin and 3.0 ml/l of sheep erythrocytes). Subsequently, 100 µl aliquots of the mix were applied onto sterile glass carriers and were let to dry. When dried, the carriers were treated with the solutions of disinfectants Nos. 1, 2, and 4 diluted with hard water and in the case of disinfectant No. 3, the carriers were exposed to a concentrated solution. The treatment was carried out in two ways – by immersion and wiping. The immersion method simulates real conditions of product use for the disinfection of instruments and other things by immersion. The wiping method simulates real conditions of product use for the disinfection of surfaces. The exposure times in both methods were 2 min and 5 min. After exposure to the test product, the contaminated carriers were placed in 20 ml of Stainer-Scholte liquid medium, covered with sterile glass beads, and vortexed to release bacteria from the surface. The suspension was diluted in a decimal series in Stainer-Scholte liquid medium and streaked on charcoal agar plates. The plates were then placed in the incubator at 36 ± 1 °C for seven days. The results were read and evaluated. According to EN 14561 [20], the minimum bactericidal concentration is the lowest concentration of an antibacterial agent which results at least in a 5 log reduction in the bacterial count over a fixed period of time.
RESULTS
Microsuspension method
When tested by the microsuspension method [17], all test products showed bactericidal activity even at very low concentrations and an exposure time of 2 min. For disinfectant No. 1, the active concentration was tested in a wide range from 0.01% to 10%. Nevertheless, the disinfectant proved to be active against all 34 B. pertussis strains even at a very low concentration of 0.37%. For disinfectant No. 2, the active concentration was tested in a range from 0.14% to 100%. Disinfectant No. 2 was found to be active at a concentration of 0.14%. Similarly, to disinfectant No. 2, disinfectant No. 3 was tested at a concentration range from 0.14% to 100%. Disinfectant No. 3 proved active already at a concentration of 3.70%. The last product, disinfectant No. 4, was also tested at concentrations ranging from 0.14% to 100% and showed bactericidal activity against all test strains at a concentration of 1.23% (Table 1).
Tab. 1. Overview of product active concentrations determined by the microsuspension method at an exposure time of 2 min 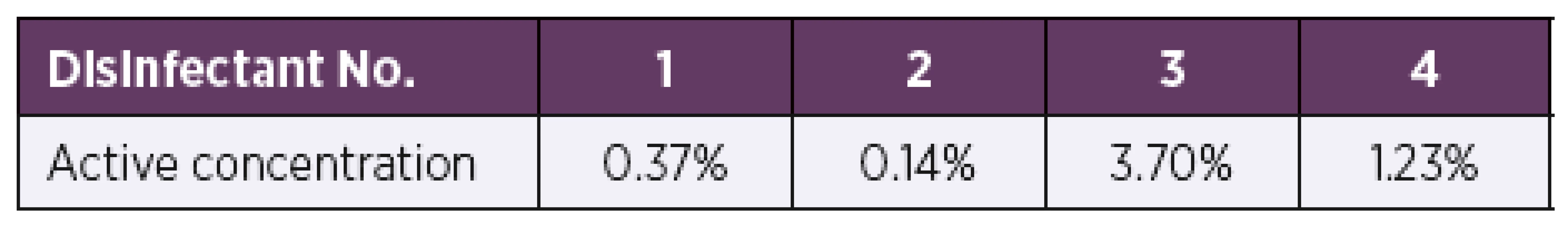
Quantitative suspension method under the dirty conditions
When tested by the quantitative suspension method under the dirty conditions modified in accordance with EN 13727+A2 [19], all four test disinfectants showed bactericidal activity at the concentrations and exposure times recommended by the manufacturer (Table 2). In all cases, a 5 log or higher reduction in the bacterial count was achieved, as required for a product to have bactericidal activity in accordance with EN 13727+A2 [19]. Disinfectant No. 1 showed bactericidal activity against all test strains at a concentration of 0.5% and exposure times of 5 min and 15 min. Disinfectant No. 2 at a concentration of 0.1% proved active against bacterial growth of all test strains as early as after 60 s of exposure. Disinfectant No. 3 showed bactericidal activity at a concentration of 97% at exposure times of 30 s and 60 s. Disinfectant No. 4 showed bactericidal activity against all test strains at a concentration of 10% and exposure times of 30 s and 60 s.
Tab. 2. Overview of the mean log reduction in the bacterial counts depending on the concentration and exposure time 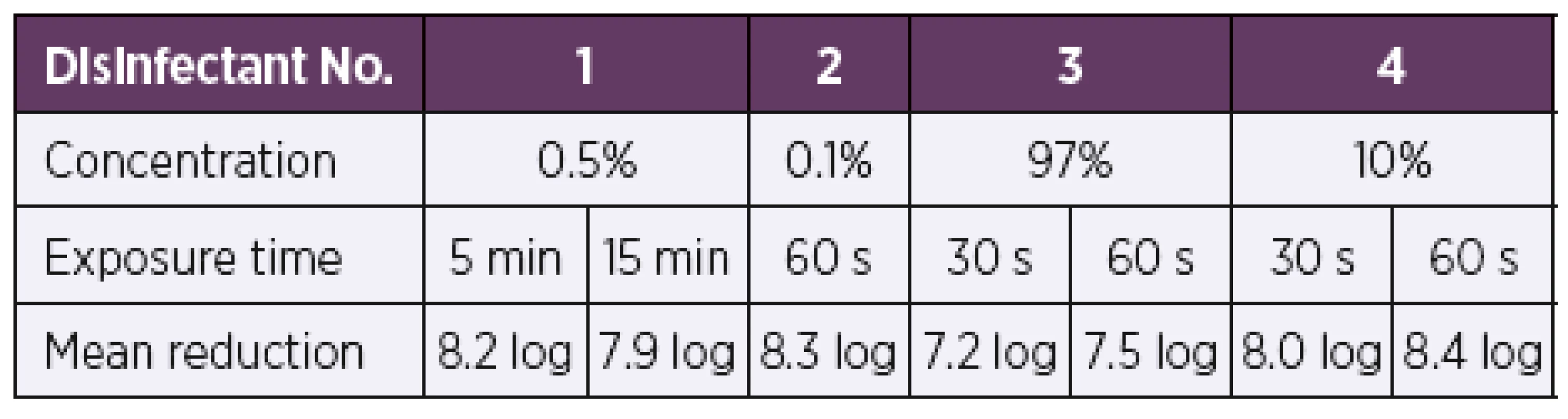
A 5 log or higher reduction in the bacterial count is required by EN 13727+A2 [19] for a product to have bactericidal activity. Quantitative carrier method under the dirty conditions
Disinfectant No. 1
Based on the results of the quantitative suspension tests, the test concentrations were selected for testing bactericidal activity by the quantitative carrier method under the dirty conditions. The initial test concentrations for disinfectant No. 1 were set to be 0.5% and 1%. First of all, the artificially contaminated carriers were treated by immersion in the disinfectant solutions at the desired concentrations. When tested by the quantitative carrier method under the dirty conditions according to modified EN 14561 [20], disinfectant No. 1 showed bactericidal activity at concentrations of 0.5% and 1% and exposure times of 2 min and 5 min (Table 3). A 5 log or higher reduction in the bacterial count was achieved against all 34 test strains of B. pertussis, as required for a product to have bactericidal activity in accordance with EN 14561 [20]. Another group of artificially contaminated carriers were treated by wiping. Disinfectant No. 1 was first tested at concentrations of 0.5% and 1% as was the case with immersion. At a concentration of 0.5% and exposure times of 2 min and 5 min, a 0.5 log reduction in the bacterial count was achieved at both exposure times. When tested at a concentration of 1%, a 0.8 log reduction in the bacterial count was achieved after 2 min of exposure while a 5 min exposure resulted in a 1.2 log reduction in the bacterial count. Therefore, the test concentration of disinfectant No. 1 solution was first increased to 2%, which after 2 min and 5 min of exposure resulted in a 2.0 log and 2.5 log reduction in the bacterial counts, respectively. When the product concentration was increased to 5%, after 2 min of exposure, a 5 log or higher reduction in the bacterial count was achieved (Table 4).
Tab. 3. Overview of the mean log reduction in the bacterial counts achieved using disinfectant No. 1 depending on the concentration and exposure time when tested by the carrier immersion method 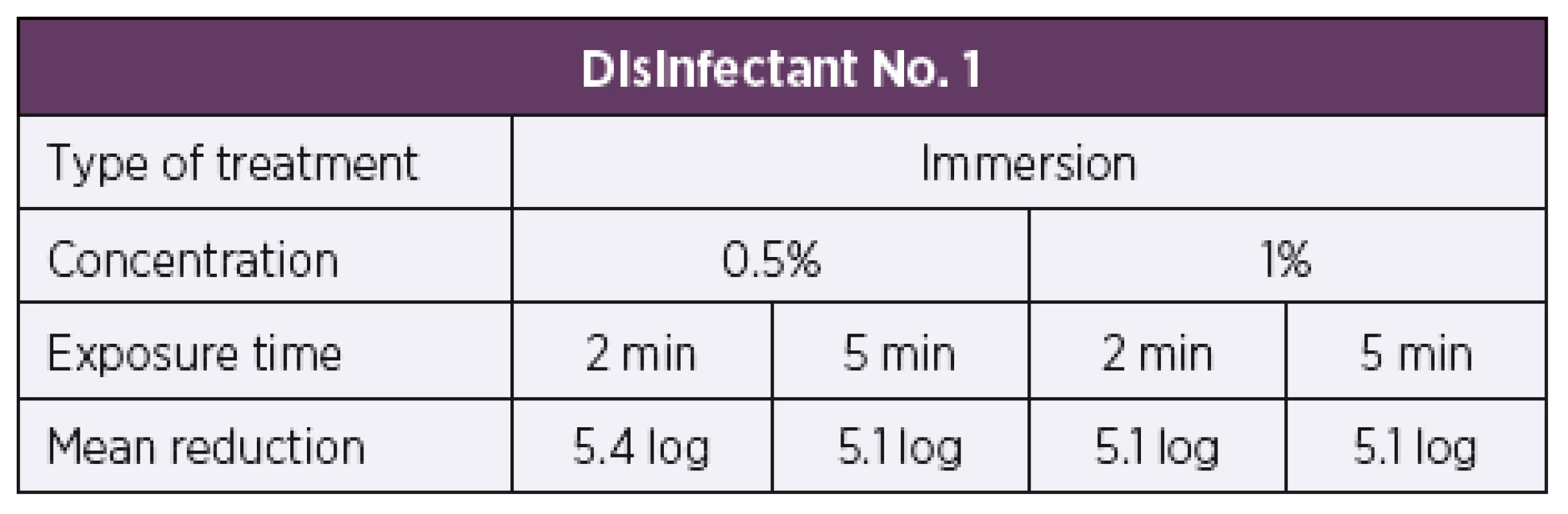
A 5 log or higher reduction in the bacterial count is required by EN 14561 [20] for a product to have bactericidal activity. Tab. 4. Overview of the mean log reduction in the bacterial counts achieved using disinfectant No. 1 depending on the concentration and exposure time when tested by the carrier wiping method 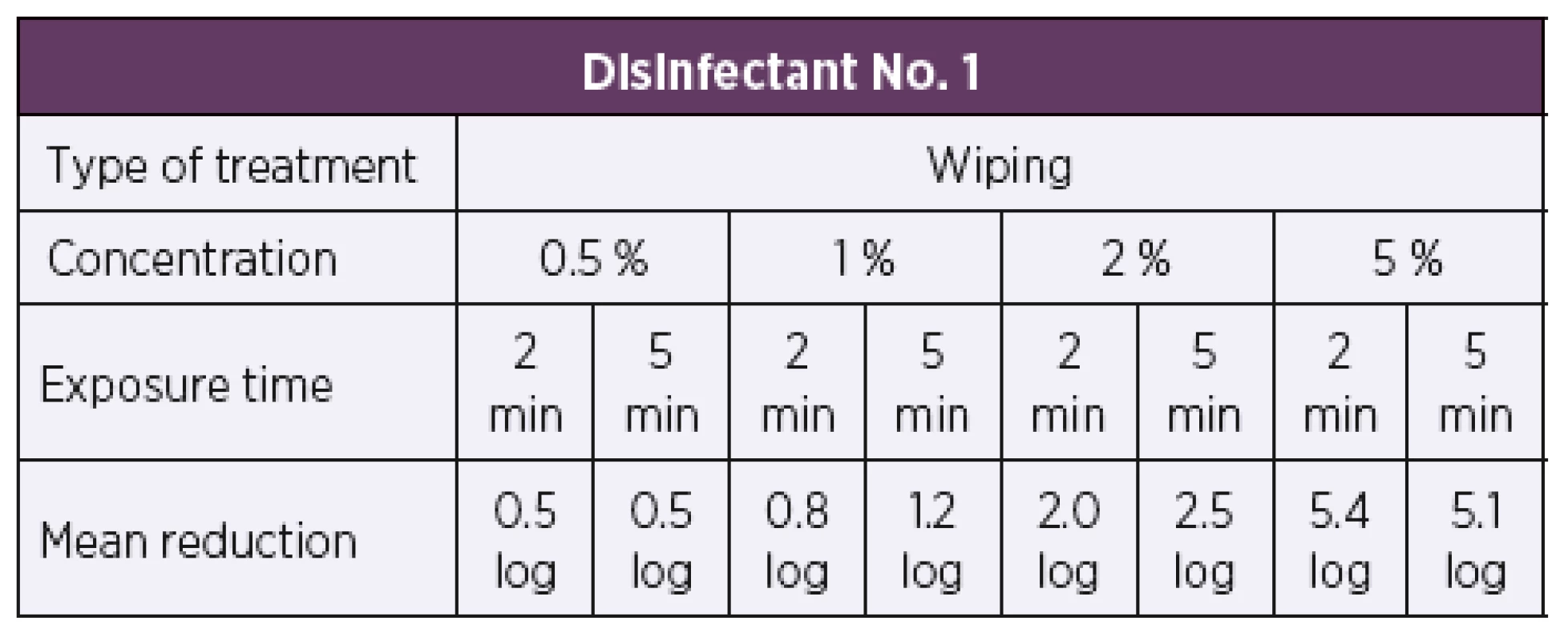
A 5 log or higher reduction in the bacterial count is required by EN 14561 [20] for a product to have bactericidal activity. Disinfectant No. 2
Based on the results of the suspension tests, the initial test concentration of disinfectant No. 2 was set to be 0.1%. First of all, the artificially contaminated carriers were treated by immersion in the disinfectant solution. At the test concentration of 0.1%, disinfectant No. 2 showed bactericidal activity according to the parameters of EN 14561 [20] against all 34 test strains of B. pertussis at exposure times of 2 min and 5 min, resulting in a 5 log or higher reduction in the bacterial counts in both cases (Table 5). Another group of artificially contaminated carriers were treated by wiping. Similarly, to the immersion method, the disinfectant 2 solution was first tested at a concentration of 0.1%. At this concentration, the disinfectant proved inactive, as after 2 min and 5 min of exposure a 0.1 log and 0.2 log reduction in the bacterial counts was only achieved, respectively. When tested at a higher concentration of 1%, a 5 log or higher reduction in the bacterial count was achieved as early as after 2 min of exposure (Table 6).
Tab. 5. Overview of the mean log reduction in the bacterial counts achieved using disinfectant No. 2 depending on the concentration and exposure time when tested by the carrier immersion method 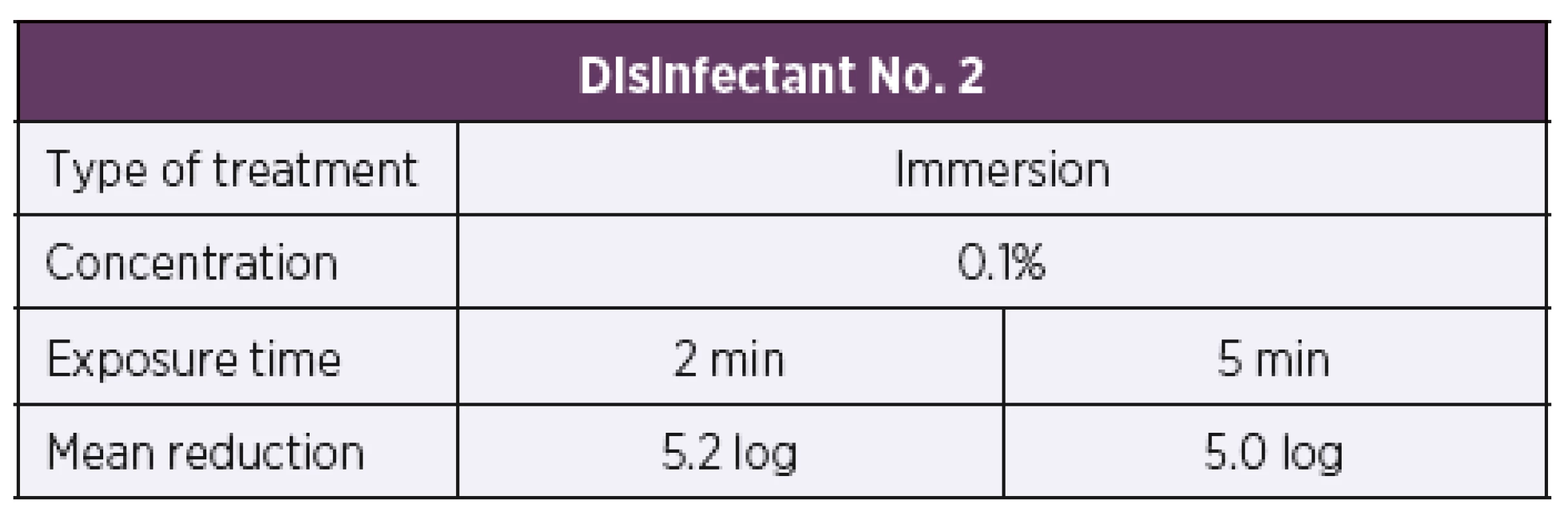
A 5 log or higher reduction in the bacterial count is required by EN 14561 [20] for a product to have bactericidal activity. Tab. 6. Overview of the mean log reduction in the bacterial counts achieved using disinfectant No. 2 depending on the concentration and exposure time when tested by the carrier wiping method 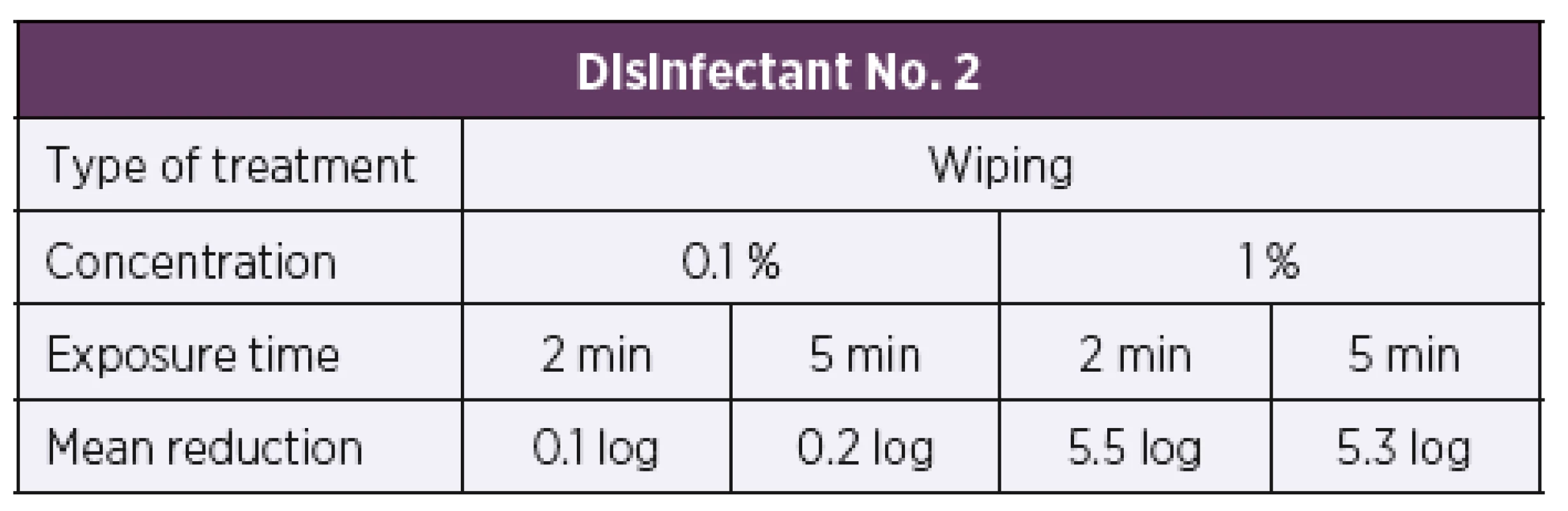
A 5 log or higher reduction in the bacterial count is required by EN 14561 [20] for a product to have bactericidal activity. Disinfectant No. 3
Disinfectant No. 3 was only tested by the immersion method as it is recommended for use in hygienic and surgical hand disinfection. Based on the results of the suspension tests, the initial test concentration of disinfectant No. 3 was set to be 10%. At this concentration, a 0.3 log reduction in the bacterial count was only achieved after both 2 min and 5 min of exposure. As this product is used concentrated, the following test concentration was set to be 100%. After 2 min and 5 min of exposure, a 1.2 log and 1.7 log reduction in the bacterial counts was only achieved, respectively (Table 7). When tested by the immersion method, disinfectant No. 3 proved to be inactive as a 5 log or higher reduction in the bacterial count as required by EN 14561 [20] for a product to have bactericidal activity was not achieved.
Tab. 7. Overview of the mean log reduction in the bacterial counts achieved with disinfectant No. 3 depending on the concentration and exposure time 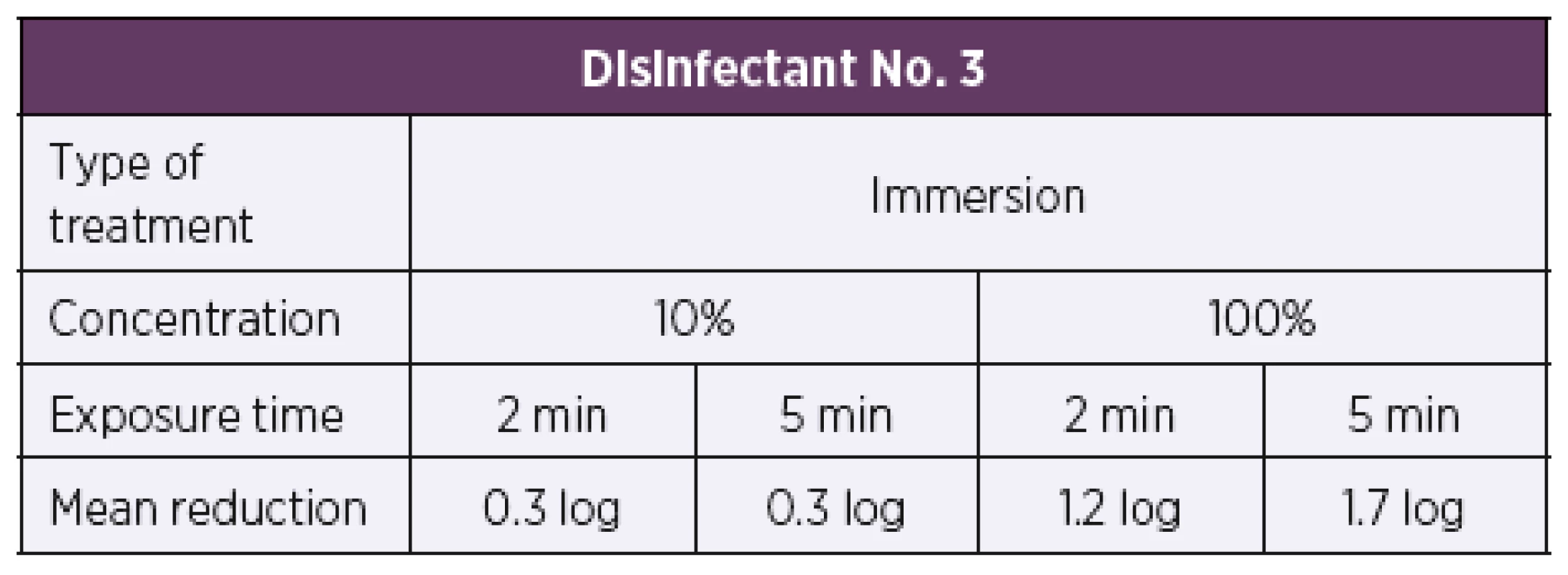
A 5 log or higher reduction in the bacterial count is required by EN 14561 [20] for a product to have bactericidal activity. Disinfectant No. 4
Based on the results of the quantitative suspension tests, the initial test concentration of disinfectant No. 4 was set to be 2%. Disinfectant No. 4 on the artificially contaminated carriers was only tested by the immersion method as it is primarily intended for oral cavity disinfection. When tested using the quantitative carrier method under the dirty conditions in accordance with modified EN 14561 [20], disinfectant No. 4 did not show bactericidal activity at the test concentration of 2% after 2 min or 5 min of exposure, resulting, respectively, in a 0.4 log and 0.6 log reduction in the bacterial counts. When tested at a higher concentration of 10%, a 2.0 log reduction in the bacterial count was achieved after 2 min of exposure and a 5 log or higher reduction in the bacterial count was observed after 5 min of exposure. At the concentration of 10%, disinfectant No. 4 only showed bactericidal activity at an exposure time of 5 min. A 5 log or higher reduction in the bacterial count after 2 min of exposure was only achieved at a higher concentration of 30%. When tested by the quantitative carrier method under the dirty conditions according to modified EN 14561 [20], disinfectant No. 4 showed bactericidal activity at a concentration of 5% and exposure time of 5 min and at a concentration of 30% and exposure time of 2 min (Table 8).
Tab. 8. Overview of the mean log reduction in the bacterial counts achieved with disinfectant No. 4 depending on the concentration and exposure time. 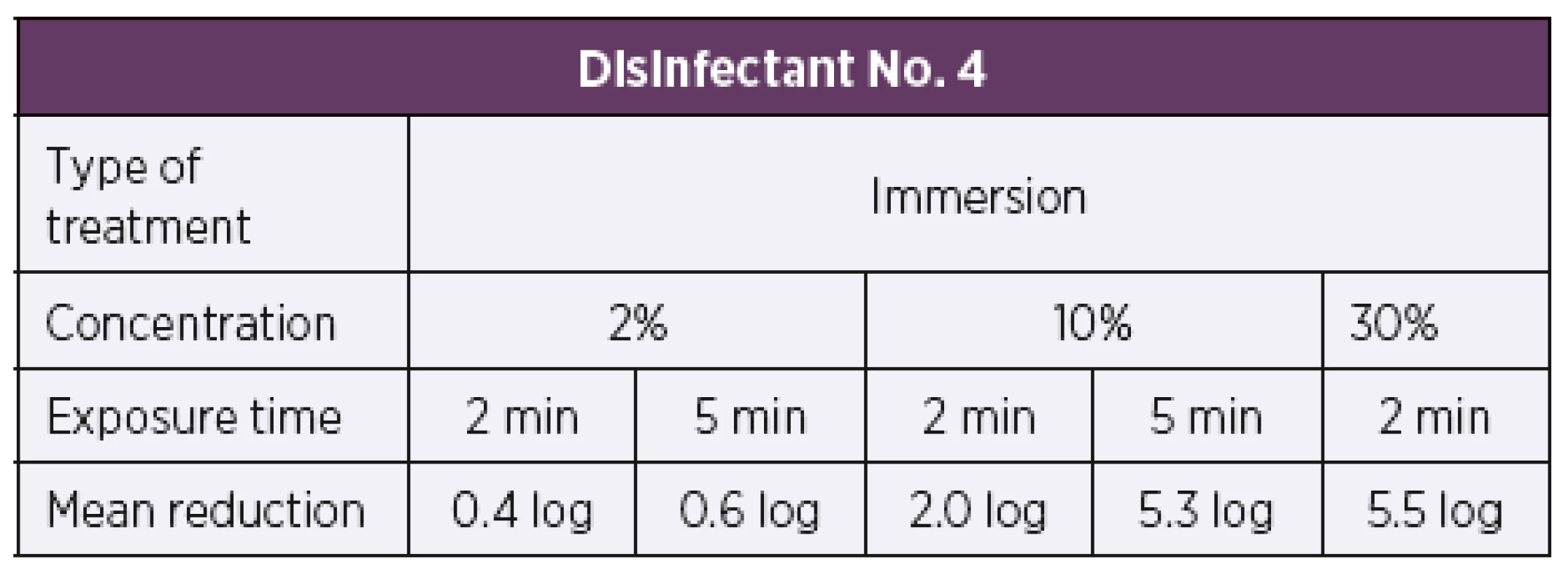
A 5 log or higher reduction in the bacterial count is required by EN 14561 [20] for a product to have bactericidal activity. DISCUSSION
Microbial resistance to antibiotics is a global problem, which is discussed and studied by countless research teams all over the world. Each antimicrobial drug targets a specific site, and the mechanisms possibly involved in the emergence of resistance are target site modification, chemical modification or inactivation of the drug, and efflux pump that reduces drug concentration [21]. Recently, increasing attention has also been paid to the widely used antiseptics and disinfectants. As a general rule, biocides have a broader spectrum of activity than antibiotics and, in particular, may have a number of target sites; therefore, microbial resistance to biocides is rather rare. However, there have been speculations that microbes might deve - lop cross-resistance to antibiotics and biocides [22]. Triclosan resistance is among the best described cases of resistance to biocides [23]. Microbial non-susceptibility or even resistance to triclosan has been reported for instance in methicillin-resistant Staphylococcus aureus [24]. In other bacterial species, such as Escherichia coli and Pseudomonas aeruginosa, triclosan resistance might lead to the selection of bacteria with the ability to resist lower or higher concentrations of a previously effective antibiotic due to cross-resistance or co-resistance [25, 26]. Another type of confirmed resistance is chlorhexidine resistance in P. aueruginosa. From the reported data, it follows that Gram-negative bacteria are generally more resistant to antibiotics and biocides than Gram-positive bacteria [27].
Bordetella pertussis, a Gram-negative bacterium, has been reported to show resistance to macrolides and quinolones [28, 29] but not to biocides. Thirty-four clinical isolates of Bordetella pertussis from 2014–2015, which have previously been proven susceptible to disinfectants commonly available in chemists' or pharmacies, were tested. Four disinfectants with different active ingredients and ways of use, resistance to which has not yet been reported in clinical bacterial isolates, were selected. Decreased bacterial susceptibility to the active ingredients of disinfectants 1 and 2 has been observed when used in water treatment and the food industry [30, 31]. Bacterial resistance to high concentrations of ethanol, which is the active ingredient of disinfectant No. 3, or to octenidine dihydrochloride, which is the active ingredient of disinfectant No. 4, has not yet been reported to occur. First of all, the microsuspension method [17] was used for primary screening of microbial resistance to the test disinfectants. All four test products showed very good bactericidal activity as early as after two minutes of exposure. The active concentrations of some disinfectants were much lower than those recommended by the manufacturer. When tested by the microsuspension method, disinfectant No. 1 showed bactericidal activity at a concentration as low as 0.37%, disinfectant No. 2 at a concentration of 0.14%, disinfectant No. 3 at a concentration of 3.70%, and disinfectant No. 4 at a concentration of 1.23% (see Table 1).
Subsequently, all 34 bacterial strains were tested using the procedures specified in EN 14885 [18]. In accordance with the above-mentioned standard, disinfectants are tested in two steps. Step 1 includes the tests modified in accordance with EN 13727+A2 [19], more precisely, the quantitative suspension method under the dirty conditions. The method was modified in terms of sample processing. During the dilution procedure, no neutralizer was used to stop the reaction since its use resulted in lysis of bacterial cells and the controls showed weak growth. Further use of the neutralizer would bias the results. Therefore, the neutralizer was no longer used and was replaced with pure Stainer-Scholte medium. Based on the screening data, disinfectant No. 1 was tested at a concentration of 0.5% and exposure times of 5 min and 15 min. Disinfectant No. 2 was tested at a concentration of 0.1% and exposure time of 60 s. The remaining two disinfectants Nos. 3 and 4 were tested at concentrations of 97% and 10%, respectively, and exposure times of 30 s and 60 s, respectively (see Table 2). When used at the above-mentioned concentrations and exposure times, all four disinfectants showed bactericidal activity since a 5 log or higher reduction in the bacterial count was achieved as specified by EN 13727+A2 [19].
Based on EN 14885 [18], the quantitative suspension tests are followed by the carrier tests which simulate real conditions of product use. Clinical isolates of Bordetella pertussis were exposed to the test disinfectants under the conditions simulating product use in real practice. The modified quantitative carrier method was used in accordance with EN 14561 [20]. Disinfectants Nos. 1 and 2 were tested by immersion and wiping. Disinfectants Nos. 3 and 4 were only tested by the immersion method, as a reference method, as they are not intended for the disinfection of surfaces. Disinfectants Nos. 1 and 2 showed concordantly ten times higher activity in the case of immersion compared to wiping. When using contaminated carriers, the disinfectant solution concentration had to be increased from 0.5% to 5% for product No. 1 and from 0.1% to 1% for product No. 2. The results obtained with disinfectants Nos. 1 and 2 revealed significant differences between the immersion and wiping methods. Although primarily intended for use in hygienic and surgical hand disinfection, disinfectant No. 3 was also tested by the immersion method. The test concentrations of 10% and 100% unfortunately did not prove active in this type of testing. Nevertheless, this way of treatment does not correspond with product use in real practice, and so it cannot be stated with certainty that the product is inactive. The last disinfectant No. 4 was also tested by the immersion method alone as it is primarily intended for oral cavity disinfection. The disinfectant was active at a concentration of 10% and longer exposure time of 5 min. At a higher concentration of 30%, bactericidal activity was achieved after 2 min of exposure.
All four test disinfectants showed clear differences between the results of the suspension tests and quantitative carrier tests. Tangible differences were also seen between the carrier test results of the immersion and wiping methods for disinfectants Nos. 1 and 2. According to EN 14561 [20], a 5 log or higher reduction in the bacterial counts was achieved under the conditions simulating product use in real practice. Such reduction is required by EN 14561 [20] for a disinfectant to have bactericidal activity.
CONCLUSION
In conclusion, it should be pointed out that none of the 34 clinical isolates of the bacterium B. pertussis showed resistance to any of the disinfectants tested. One method alone is not enough to test bactericidal activity, but the testing should be conducted in several steps. In the first step, the screening microsuspension method was used, and in step 2, the disinfectants were tested in accordance with the EU standards – by the quantitative suspension test and quantitative carrier test simulating real conditions of product use, to establish the final concentration and exposure time required to achieve bactericidal activity in real practice. Of importance, also, is to determine the level of protein contamination depending on the area and way of use of each disinfectant. Dirty conditions reduce product activity, with the bacteria becoming less susceptible to higher concentrations of the active ingredient.
Acknowledgments
Supported by Ministry of Health, Czech Republic – conceptual development of research organization (“The National Institute of Public Health – NIPH, 75010330”).
Do redakce došlo dne 29. 3. 2018.
Adresa pro korespondenci:
Mgr. Petra Uttlová
Centrum epidemiologie a mikrobiologie
Státní zdravotní ústav
Šrobárova 48
100 42 Praha 10
Zdroje
1. Tan T. Summary: Epidemiology of pertussis. Pediatric Infectious Disease Journal, 2005; 24(5):S35–S38.
2. Loeffelholz MJ, Sanden GN Bordetella. In Murray PR, Baron EJ, Jorgensen JH, Landry ML & Pfaller MA. (Eds.) Manual of Clinical Microbiology, 9th ed. Washington, DC.: ASM Press; 2007 : 803–814.
3. Ryan KJ. Haemophilus and Bordetella. In Ryan KJ & Ray CG. (Eds.) Sherris Medical Microbiology, 4th ed., USA: The McGraw-Hill Companies, Inc., 2004 : 395–420.
4. Votava M, et al. Lékařská mikrobiologie speciální. 1. vyd., Brno: Neptun, 2003 : 39–42.
5. Bosch A, Massa NE, Donolo A, et al. Molecular Characterisation by Infrared Spectroscopy of Bordetella pertussis Grown as Biofilm. Wiley Online Library, 2000;220 : 635–640.
6. Heymann DL. (ed.) Control of communicable diseases manual, 19th ed. American Public Health Association, Washington, DC., Baltimore: United Book Press, 2008 : 746.
7. Vysoká-Buriánová B. Pertusse – Parapertusse. Praha, 1961 : 122. Disertační práce na Lékařské fakultě hygienické Univerzity Karlovy. Vedoucí disertační práce Prof. MUDr. Karel Raška.
8. Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infectious Diseases, 2006;6 : 130.
9. Kirilenko NI & Army Biological Labs. Viability of Hemophilus pertussis in Air and on Some Environmental Objects No. Trans-1559 Retrieved from DTIC Online Information for the Defense Community, 1965.
10. Hamidou Soumana I, Linz B, Harvill ET. Environmental Origin of the Genus Bordetella. Frontiers in Microbiology, 2017;8 : 28.
11. Taylor-Mulneix DL, Bendor L, Linz B, et al. Bordetella bronchiseptica exploits the complex life cycle of Dictyostelium discoideum as an amplifying transmission vector. PLOS Biology, 2017;15(4).
12. Gupta RK, Saxena SN, Sharma SB, et al. Studies on the optimal conditions for inactivation of Bordetella pertussis organisms with glutaraldehyde for preparation of a safe and potent pertussis vaccine. Vaccine, 1988;6(6):491–496.
13. Burnett LAC, Lunn G, Coico R. Biosafety: Guidelines for working with pathogenic and infectious microorganisms. Current Protocols in Microbiology, 2009;13(1A.1.1–1A.1.14).
14. Rutala WA. APIC guideline for selection and use of disinfectants. American Journal of Infection Control, 1996;24(4):313–342.
15. Russell AD. Bacterial resistance to disinfectants: present knowledge and future problems. Journal of Hospital Infection, 1999;43(1):57–68.
16. Poole K. Mechanisms of bacterial biocide and antibiotic resistance. Journal of Applied Microbiology, 2002;92(1):55–64.
17. Kneiflová J. Hodnocení baktericidní účinnosti dezinfekčních prostředků suspenzní mikrometodou. Československá epidemiologie, mikrobiologie, imunologie, 1988;37(2):97–103.
18. European Committee for Standardization (CEN). Chemical disinfectants and antiseptics – Application of European Standards for chemical disinfectants and antiseptics. Brussels, Belgium: CEN, Central Secretatiat. prEN 14885, 2016.
19. European Committee for Standardization (CEN). Chemical disinfectants and antiseptics – Quantitative suspension test for the evaluation of bactericidal activity in the medical area – Test method and requirements (phase 2, step 1). Brussels, Belgium: CEN, Central Secretatiat. prEN 13727+A2, 2016.
20. European Committee for Standardization (CEN). Chemical disinfectants and antiseptics – Quantitative carrier test for the evaluation of bactericidal activity for instruments used in the medical area – Test method and requirements (phase 2, step 2). Brussels, Belgium: CEN, Central Secretatiat. prEN 14561, 2006.
21. Munita JM, Arias CA. Mechanisms of Antibiotic Resistance. Microbiology Spectrum, 2016;4(2).
22. McDonnell G, Russell AD. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clinical Microbiology Reviews, 1999;12(1):147–179.
23. Thompson A, Griffin P, Stuetz R, et al. The Fate and Removal of Triclosan during Wastewater Treatment. Water Environment Research, 2005;77(1):63–67.
24. Brenwald NP, Fraise AP. Triclosan resistance in methicillin-resistant Staphylococcus aureus (MRSA). Journal of Hospital Infection, 2003;55(2):141–144.
25. Russell AD. Biocides and pharmacologically active drugs as residues and in the environment: Is there a correlation with antibiotic resistance? American Journal of Infection Control, 2002;30(8):495–498.
26. Yazdankhan SP, Scheie AA, Høiby EA, et al. Triclosan and antimicrobial resistence in bacteria: an overview. Microbial Drug Resistance, 2006;12(2):83–90.
27. Thomas L, Maillard JY, Lambert RJ, et al. Development of resistance to chlorhexidine diacetate in Pseudomonas aeruginosa and the effect of a "residual" concentration. Journal of Hospital Infection, 2000;46(4):297–303.
28. Guillot S, Descours G, Gillet Y, et al. Macrolide-resistant Bordetella pertussis infection in newborn girl, France. Emerging Infectious Diseases, 2012;18(6):966–968.
29. Ohtsuka M, Kikuchi K, Shimizu K, et al. Emergence of quinolone-resistant Bordetella pertussis in Japan. Antimicrobial Agents and Chemotherapy, 2009;53(7):3147–3149.
30. Luddin N, Ahmed HMA. The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: A review on agar diffusion and direct contact methods. Journal of Conservative Dentistry: JCD, 2013;16(1):9–16.
31. Sundheim G, Langsrud S, Heir A, et al. Bacterial resistence to disinfectants containing quaternary ammonium compounds. International Biodeterioration & Biodegradation, 1998; 41(3–4):235–239.
Štítky
Hygiena a epidemiologie Infekční lékařství Mikrobiologie
Článek vyšel v časopiseEpidemiologie, mikrobiologie, imunologie
Nejčtenější tento týden
2018 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
-
Všechny články tohoto čísla
- Fecal bacteriotherapy in the treatment of Clostridium difficile infection
- Human rotavirus A detection: Comparison of enzymatic immunoassay and rapid chromatographic test with two quantitative RT-PCR assays
- Human Mozdok leptospirosis first diagnosed by serum agglutinin-absorption tests in the Slovak Republic
- Susceptibility of clinical isolates of Bordetella pertussis to chemicals
- Laboratory evaluation of repellency of traditional Czech homemade repellents against Aedes aegypti
- Comparison of the epidemiological patterns of Lyme borreliosis and tick-borne encephalitis in the Czech Republic in 2007–2016
- Successful rituximab treatment of granulomatous/lymphocytic interstitial lung disease in common variable immunodeficiency
- Zemřel doc. MUDr. Bohumír Kříž, CSc.
- Molecular characterization of Streptococcus pneumoniae isolates recovered from cases of pneumococcal vaccine failure in children under five years of age in the Czech Republic in 2012–2014
- Epidemiologie, mikrobiologie, imunologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Comparison of the epidemiological patterns of Lyme borreliosis and tick-borne encephalitis in the Czech Republic in 2007–2016
- Fecal bacteriotherapy in the treatment of Clostridium difficile infection
- Successful rituximab treatment of granulomatous/lymphocytic interstitial lung disease in common variable immunodeficiency
- Susceptibility of clinical isolates of Bordetella pertussis to chemicals
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



