-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Frameless and fiducial-less method for deep brain stimulation
Bezrámová a bezpinová metoda pro provedení hluboké mozkové stimulace
Cíl: Hluboká mozková stimulace (deep brain stimulation; DBS) je velmi efektivní metoda léčby idiopatické Parkinsonovy choroby (Parkinson’s disease; PD), esenciálního třesu a dystonie. Autoři popisují metodu DBS bez rámu i bez pinů, s využitim systému Nexframe (Medtronic), navigace S8 (Medtronic) a O-arm (Medtronic), kterou využili u čtyř pacientů (8 elektrod) jako druhé centrum na světě, co je nám známo.
Metody: Dva dospělí pacienti léčení pro PD a dva pro esenciální třes byli indikováni k provedení bilaterální DBS. Základní neurologický status a jeho zlepšení v motorických funkcích na základě léčby DBS byly měřeny pomocí pacientského deníku, Unifikované škály pro hodnocení Parkinsonovy choroby (UPDRS) a testu Clinical Global Improvement. Implantace DBS elektrod byla provedena na základě plánovací MR, předoperačního vyšetření CT a jejich fúze s peroperačním zobrazením pomoci O-arm. Přesnost byla kontrolována pomocí stejné metody jako u systému Nexframe. Vyhodnotili jsme také průměrný čas u operací provedených s Lekselovým rámem, Nexframem a metodou bez použití rámu a pinů.
Výsledky: U našich prvních čtyř pacientů byly přesnost a klinický efekt vynikající s celkovou odchylkou 2,49 mm a bez komplikaci. Průměrný operační čas při použiti Lekselova rámu byl 290 min, u systému Nexframe 222 min a u posledního postupu 201 min.
Závěr: Zavedení elektrod při DBS bez rámu i pinů je velmi užitečným a technicky nenáročným postupem s vynikající snášenlivostí u pacientů. Bude nutné výrazně větší množství pacientů operovat tímto způsobem abychom potvrdili účinnost této metody, ale z našeho pohledu se zdá být tato metoda velmi slibná.
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Klíčová slova:
Parkinsonova choroba – esenciální třes – hluboká mozková stimulace
Authors: D. Krahulík 1
; M. Nevrlý 2; P. Otruba 2; L. Hrabálek 1; M. Vaverka 1; P. Kaňovský 2
Authors place of work: Department of Neurosurgery, University Hospital Olomouc 1; Department of Neurology, University Hospital Olomouc 2
Published in the journal: Cesk Slov Neurol N 2019; 82(3): 342-344
Category: Krátké sdělení
doi: https://doi.org/10.14735/amcsnn2019342Summary
Aim: Deep brain stimulation (DBS) is a very effective procedure for the treatment of idiopathic Parkinson‘s disease (PD), essential tremor and dystonia. The authors describe a method of DBS using frameless and fiducial-less system Nexframe (Medtronic), S8 navigation (Medtronic) and O-arm (Medtronic) for placing DBS electrodes in four patients (8 electrodes). To our knowledge, this is only the second centre in the world to have used this method.
Methods: Two adult patients with PD and two with essential tremor were indicated to bilateral DBS. Baseline neurological status and DBS-related improvement in motor function were measured using patients‘ diaries, Unified Parkinson‘s Disease Rating Scale and Clinical Global Improvement tests. The implantation of DBS leads was performed using MRI, preoperative CT examination and their fusion with perioperative O-arm imaging. The accuracy was checked using the same methodology as the Nexframe system. We also evaluated average time of surgery for Leksell frame-based surgery, Nexframe procedure and fiducial-less procedure.
Results: The accuracy and patient outcome were excellent, with a total error of 2.49 mm, without any complication. Average times of surgeries were: Leksell frame 290 min, Nexframe system 222 min and last procedure 201 min.
Conclusion: Implantation of DBS electrodes using frameless and fiducial-less system is a very useful and technically feasible procedure with excellent patient toleration. It will be necessary to operate in this way on many more patients to prove efficacy of this method, but from our point of view this method appears very promising
无框架、无基准的深部脑刺激方法
目的:
脑深部刺激(DBS)是治疗特发性帕金森病(PD)、特发性震颤和肌张力障碍的一种非常有效的方法。作者描述了一种使用无框架无基准系统Nexframe(美敦力)、S8导航(美敦力)和O-型臂(美敦力)在4例患者(8个电极)中放置DBS电极的方法。据我们所知,这是世界上第二个使用这种方法的中心。
方法:
两例成人PD患者和两例特发性震颤患者行双侧DBS检查。采用患者日记、帕金森病统一评定量表和临床整体改善试验,测量基线神经状态和运动功能改善程度。采用MRI、术前CT检查及与围手术期o形臂成像融合的方法进行DBS导联植入。使用与Nexframe系统相同的方法检查精度。我们还评估了基于Leksell框架的手术、Nexframe手术和无基准手术的平均手术时间。
结果:
准确性和患者预后良好,总误差2.49 mm,无任何并发症。平均手术时间为:Leksell frame 290 min, Nexframe system 222 min, last procedure 201 min。
结论:
采用无框架无基准系统植入DBS电极是一种非常有用且技术上可行的方法,具有良好的患者耐受性。为了证明这种方法的有效性,有必要对更多的患者进行这种手术,但从我们的角度来看,这种方法似乎很有前景。
关键词:
帕金森病-特发性震颤-脑深部刺激
Keywords:
Parkinson’s disease – essential tremor – deep brain stimulation
Deep brain stimulation (DBS) is a widely used technique for modulation of subcortical brain structures in patients with Parkinson’s disease (PD) [1,2], essential tremor [3], dystonia [4,5] and some other movement disorders. Class I evidence supports its use in PD, in comparison with best medical treatment [6]. The DBS is now being more frequently indicated also during earlier stages of PD [7,8]. DBS electrodes have conventionally been placed using frame-based stereotaxy with micro-electrode recording (MER) and physiological mapping of target structures. Frameless neuronavigation-guided implantation technique using skull-mounted aiming devices: Nexframe© (Medtronic, Dublin, Ireland), STarFix© (FHC Inc., Bowdoin, ME, USA), Clearpoint© (MRI Interventions Inc., Irvine, CA, USA) is used in some centres in conjunction with bone-implanted fiducial markers. Holloway (Minneapolis, USA) recently started to implant electrodes using the Nexframe© system without fiducials with perioperative O-arm imaging. In this technique, brain images used for targeting CT and MRI are obtained preoperatively. The O-arm picture is taken at the beginning of the surgical procedure and S8 planning software and navigation is used to register brain targets and planned trajectories. The correct position of electrodes is confirmed by micro recording, macrostimulation and perioperative O-arm control.
Methods
Four patients (eight electrodes) were implanted using the frameless and fiducial-less technique in October 2018. Two patients were treated for PD and the other two for tremor. PD patients met the Movement Disorder Society Clinical Diagnostic Criteria for Parkinson‘s Disease [9] and patients treated for tremor had pharmacoresistant essential tremor. All patients were fully informed about the procedure and the procedure was performed by a single surgeon (D. K.) and neurologists (M. N., P. O.)
Imaging
Two MRI sets were obtained a few days before the surgery for PD patient: 1. volumetric 3D Gd-enhanced gradient echo MRI sequence covering the whole brain in 1 mm axial slices, mainly for trajectory planning and 2. T2 images turbo spin echo 2 mm slices for the borders of subthalamic nucleus (STN). For tremor patients, we used tractography and segmentation of thalamic nuclei according to 1. high resolution inversion recovery T1 covering the whole brain and 2. diffusion tensor imaging sequence: non-diffusion weighted data set, 30 – 60 diffusion gradient, as high a resolution as possible.
CT scan covering whole head was obtained for the best fusion with perioperative O-arm
imaging (Medtronic, Dublin, Ireland).Surgical technique
At the beginning of the surgery, the 3D O-arm scan was obtained and fused with preoperative MRI and CT image in the S8 stereotactic navigation planning software (Medtronic). The target points for the tips of the electrodes were selected using a combination of direct (visualized) and indirect targeting in PD and with indirect targeting in tremor combined with MRI tractography and segmentation of thalamic nuclei. The trajectories were visualized on the volumetric MRI images using “navigation” views. Small adjustments were then made to avoid traversing the cortical veins and dural venous lakes (easily seen on Gd-enhanced images) and lateral ventricles. Surgical procedures were carried out in two stages during the same day. The first stage, implantation of the DBS electrodes was carried out on the patient whilst awake, and the second stage was implantation of the internal pulse generator, performed under general anaesthesia.
Using a passive planar blunt probe and active S8 navigation, the burr hole entry point of the predetermined electrode trajectory was then marked on the skin, and a small hole was drilled to mark that point on the skull. After we performed appropriate sterile preparation and draping, linear skin incisions were made, and burr holes centered on the pilot hole were completed. The lead anchoring device (Stimlock©, Medtronic) and the Nexframe© base were attached to the skull and the navigated O-arm picture was taken and fused. The sterile registration was performed with target registration error < 0.5 mm. The Nexframe© tower was then attached and aligned to the corresponding target using S8 navigation® software (Medtronic). Target depth was then calculated and set on the microTargetingTM Drive System positioning device. The dura was opened and closed by fibrin glue to prevent CSF leak or pneumocephalus (Fig. 1).
Fig. 1. Intraoperative picture of navigated O-arm system.
Obr. 1. Peroperační foto navigovaného systému O-arm.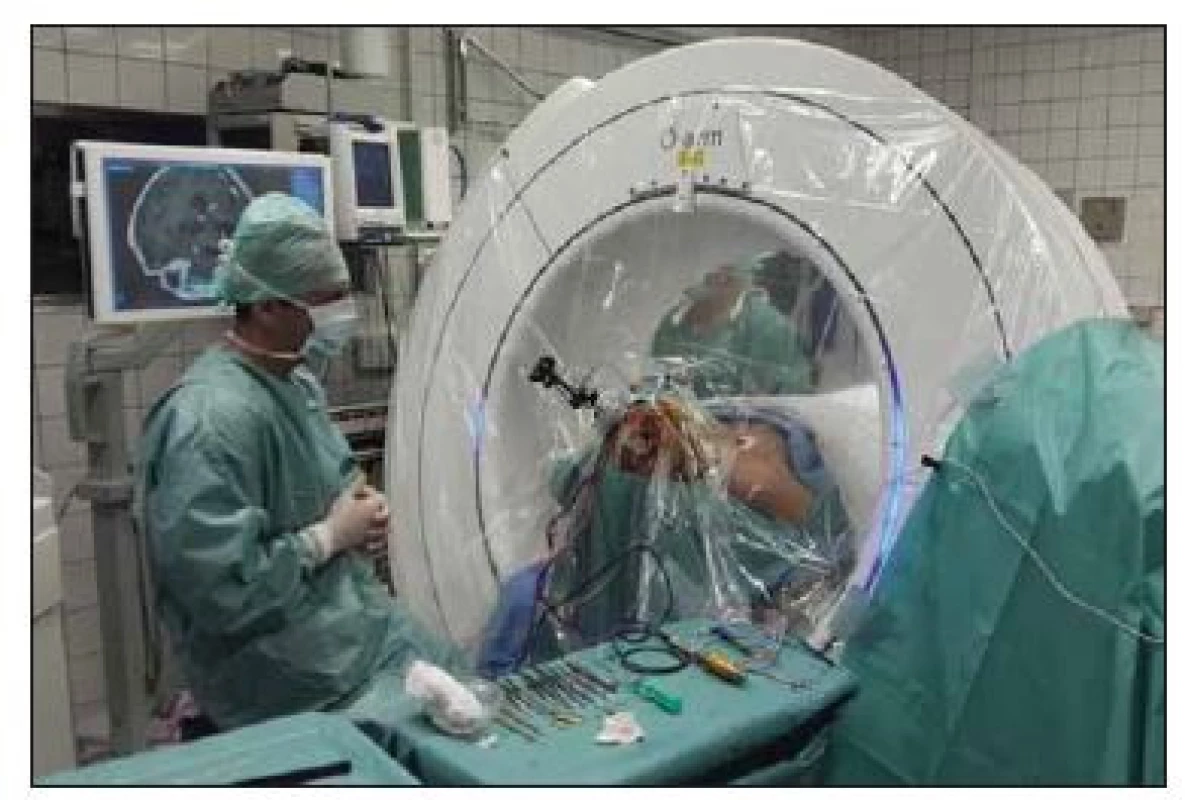
Intraoperative microelectrode registration
To perform MER in STN-DBS, four MER/ macrostimulation needles were placed in an array
with central, lateral, anterior and posterior to delineate the borders of the nucleus. A starting point for the STN 10 mm above the MRI-based target was set and the microelectrodes were advanced in steps of 500 μm towards the target by an electric microdrive.Macro-test stimulation
After MER, the tip of the microelectrode was retracted. Channels that showed significant multi-unit activity over a length longer than 3 mm were selected for intraoperative test stimulation (60 μs pulse-duration; 130 Hz pulse frequency for PD and 145 Hz frequency for tremor). The complete electrode with the macro-tip was then advanced to be used for macro-test stimulation, and this was performed by an experienced neurologist (M. N., P. O.). After evaluating the selected channels by macro-test stimulation, the one with the largest therapeutic window, i.e. the lowest current threshold for improvement of symptoms and the highest threshold for side effects, was chosen for permanent electrode implantation and the final control 3D O-arm scan was performed after insertion of final lead to confirm its accurate position. 3D O-arm scan can be used during the surgery several times to confirm accurate position of the microelectrode or the lead. It takes just a few minutes to transfer pictures from O-arm into the planning station and to fuse images with CT and MRI. Final control of the position of the electrodes is managed by the Suretune© software (Medtronic) (Fig. 2).
Fig. 2. Postoperative control of deep brain stimulation in the subthalamic nucleus using the SureTune® software (Medtronic, Dublin, Ireland).
Obr. 2. Pooperační kontrola uložení elektrod hluboké mozkové stimulace v subtalamickém jádru pomocí systému SureTune® (Medtronic, Dublin, Irsko).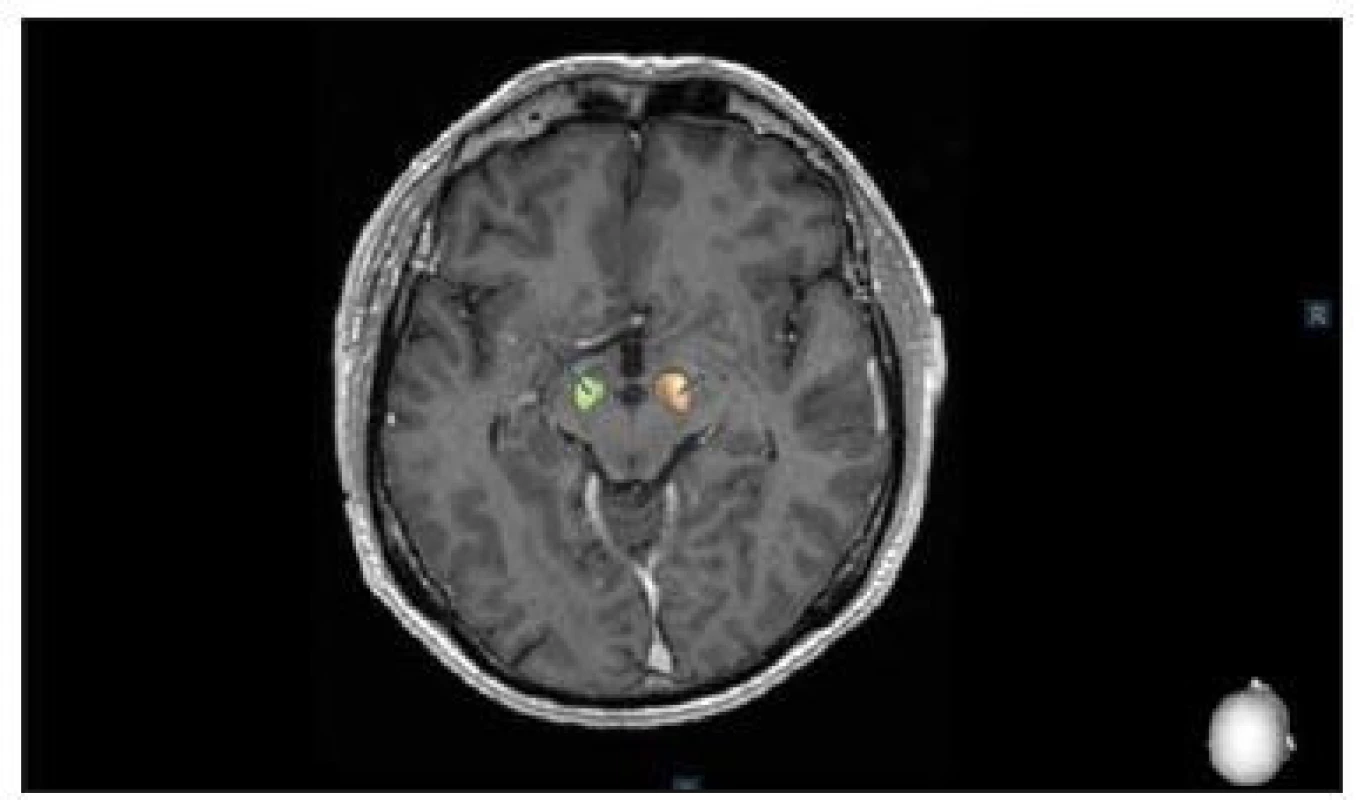
Lead anchoring and implantable pulse generator placement
Leads were anchored to the skull with a lead anchoring device (Stimlock®, Medtronic). After scalp closure, the surgery continued under the general anaesthesia and the lead extenders and pulse generators were placed.
Results
One month after surgery, all four patients had an excellent clinical outcome and there were no complications so far. Both tremor patients have improvement at Clinical Global Improvement scale + 3 (very much improved) and PD patients have 52% and 56%, respectively, reduction of OFF state and 59% and 53%, respectively, reduction of dopaminergic medication. The accuracy of this procedure was measured using the same methodology as the Nexframe© system [10]. The total error was 2.49 mm (Tab. 1) and it is comparable with the Nexframe© and the frame-based systems [10].
Tab. 1. Accuracy of the fiducial-less procedure. 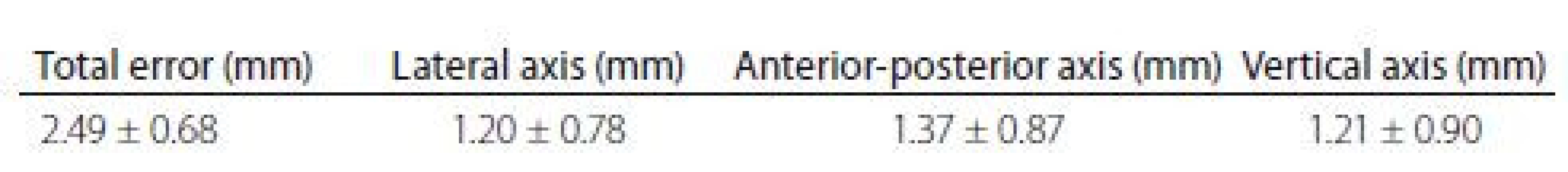
We also evaluated average time of surgery in 129 patients treated with DBS in Olomouc. Average time for Leksell frame surgery (59 patients) was 290 min. DBS using Nexframe© system (66 patients) average time of surgery was 222 min and average time with the fiducial-less procedure (4 patients) was 201 min.
Discussion
Deep brain stimulation is basically performed by two methods, one using any stereotactic frame and the other using any frameless system with small fiducials attached to the skull. This new method excludes fiducials and uses perioperative O-arm imaging and an online navigation system. None of the systems are strictly accurate and average error is between 1 – 2 mm. There are few weak points in the method that can lead to inaccuracy such as fusion between MRI, CT and O-arm, but the newest navigation system has an error of about 1 – 2 imaging voxels [11]. Urgosik et al analyzed accuracy of DBS placement using the Leksell frame according to intraoperative monitoring with very good results and minimum complications [12]. Rohlfing et al found reduced accuracy of stereotactic frames because of torque introduced by the effect of weight bearing on the frame [13]. Krahulík et al and Holloway et al confirmed comparable accuracy of frameless systems to the frame-based systems [10,14].
Conclusion
Frameless and fiducial-less method using the Nexframe© system is an accurate and safe procedure and the best tolerated by our four patients. The total error is not worse than with the Nexframe© system and frame-based systems and average surgery time for the fiducial-less procedure is shorter than with other methods used for the DBS procedure. It will be necessary for more patients to undergo this method in order to conclude its routine use.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Accepted for review: 16. 1. 2019
Accepted for print: 25. 3. 2019
doc. MUDr. David Krahulík, Ph.D., MBA
Neurochirurgická klinika
FN Olomouc
I. P. Pavlova 185/6, Nová ulice
779 00 Olomouc
e-mail: david.krahulik@fnol.cz
Zdroje
1. Deuschl G, Paschen S, Witt K. Clinical outcome of deep brain stimulation for Parkinson‘s disease. Handb Clin Neurol 2013; 116 : 107– 128. doi: 10.1016/ B978-0-444-53497-2.00010-3.
2. Weaver FM, Follett K, Stern M et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 2009; 301(1): 63– 73. doi: 10.1001/ jama.2008. 929.
3. Benabid AL, Pollak P, Gervason C et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 1991; 337(8738): 403– 406.
4. Kupsch A, Benecke R, Müller J et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355(19): 1978– 1990. doi: 10.1056/ NEJMoa063618.
5. Vidailhet M, Vercueil L, Houeto JL et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005; 352(5): 459– 467. doi: 10.1056/ NEJMoa042187.
6. Benabid AL, Chabardes S, Mitrofanis J et al. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson‘s disease. Lancet Neurol 2009; 8(1): 67– 81. doi: 10.1016/ S1474-4422(08)70291-6.
7. Schüpbach WM, Rau J, Knudsen K et al. EARLYSTIM Study Group. Neurostimulation for Parkinson‘s disease with early motor complications. N Engl J Med 2013; 368(7): 610– 622. doi: 10.1056/ NEJMoa1205 158.
8. Moro E, Schüpbach WM, Wächter T et al. Refer-
ring Parkinson‘s disease patients for deep brain stimulation: a RAND/ UCLA appropriateness study. J Neurol 2016; 263(1): 112– 119. doi: 10.1007/ s00415-015-7942-x.9. Postuma RB, Berg D, Stern M et al. MDS clinical diagnostic criteria for Parkinson‘s disease. Mov Disord 2015; 30(12): 1591– 1601. doi: 10.1002/ mds.26424.
10. Krahulík D, Nevrlý M, Otruba P. Placement accuracy of DBS stimulation using the Nexframe system. Cesk Slov Neurol N 2017; 80/ 113(2): 208– 212. doi: 10.14735/ amcsnn2017208.
11. Hemler PF, Sumanaweera TS, van den Elsen PA et al. A versatile system for multimodality image fusion. J Image Guid Surg 1995; 1(1): 35 – 45. doi: 10.1002/ (SICI)1522-712X(1995)1 : 1<35::AID-IGS6>3.0.CO;2-N.
12. Urgošík D, Jech R, Růžička E. Hluboká mozková stimulace u nemocných s extrapyramidovými poruchami pohybu – stereotaktická procedura a intraoperační nálezy. Cesk Slov Neurol N 2011; 74/ 107(2): 175– 186.
13. Rohlfing T, Maurer CR Jr, Dean D et al. Effect of changing patient position from supine to prone on the accuracy of a Brown-Roberts-Wells stereotactic head frame system. Neurosurgery 2003; 52(3): 610– 618.
14. Holloway KL, Gaede SE, Starr PA et al. Frameless stereotaxy using bone fiducial markers for deep brain stimulation. J Neurosurg 2005; 103(3): 404– 413. doi: 10.3171/ jns.2005.103.3.0404.
Štítky
Dětská neurologie Neurochirurgie Neurologie
Článek vyšel v časopiseČeská a slovenská neurologie a neurochirurgie
Nejčtenější tento týden
2019 Číslo 3- Magnosolv a jeho využití v neurologii
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Zolpidem může mít širší spektrum účinků, než jsme se doposud domnívali, a mnohdy i překvapivé
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- Editorial
- Neuromuscular diseases and pregnancy
- Are late complications of Parkinson’s disease really late? YES
- Are late complications of Parkinson’s disease really late? NO
- Are late complications of Parkinson’s disease really late? COMMENT
- Obstructive sleep apnea and cerebral blood flow
- Brief analysis of the frequency of use and spectrum of animal models in stroke research
- Factors affecting the school life of children with epilepsy
- Can endarterectomy of the external carotid artery be beneficial? A critical overview
- Circadian system disturbances in Huntington’s disease – implications for light therapy
- Experiences with an electrophysiological diagnosis of occupational ulnar nerve lesions at elbow
- Inpatient multidisciplinary rehabilitation programme for postural and gait stability in Huntington’s disease – a pilot study
- Optical coherence tomography measurements of the optic nerve head and retina in newly diagnosed idiopathic intracranial hypertension without loss of vision
- Coin in the Hand Test for detection of malingering memory impairment in comparison with mild cognitive impairment and mild dementia in Alzheimer‘s disease
- Neuropathic pain component in patients with myotonic dystrophy type 2 – a pilot study
- Equivalence of Montreal Cognitive Assessment alternate forms
- Frameless and fiducial-less method for deep brain stimulation
- Effect of vacuum-compression therapy for carpal tunnel syndrome as a part of physiotherapy – pilot study
- Anterior choroidal artery aneurysm
- Analýza dat v neurologii LXXV. Příklady chybné korelační analýzy
- Recenze knih
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Coin in the Hand Test for detection of malingering memory impairment in comparison with mild cognitive impairment and mild dementia in Alzheimer‘s disease
- Neuromuscular diseases and pregnancy
- Optical coherence tomography measurements of the optic nerve head and retina in newly diagnosed idiopathic intracranial hypertension without loss of vision
- Effect of vacuum-compression therapy for carpal tunnel syndrome as a part of physiotherapy – pilot study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



