-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Involvement of glucocorticoid receptor on hyperpyrexia induced by methamphetamine administration
Vliv glukokortikoidového receptoru na hyperpyrexii navozenou metamfetaminem
Byl zkoumán vliv glukokortikoidu na metamfetaminem (MA) navozenou hyperpyrexii
s užitím bio-telemetrického systému. Prokazatelná úroveň hyperpyrexie byla pozorována u krys, kterým byl podáván MA. Naproti tomu vzestup tělesné teploty byl potlačen adrenalektomií nebo podáváním RU-486, tj. antagonisty glukokortikoidového receptoru. Tyto údaje nasvědčují, že glukokortokoidový receptor může být odpovědný za hyperpyrexii navozenou metamfetaminem.Klíčová slova:
metamfetamin – hyperpyrexie – glukokortikoidy – kortikosteron
Authors: S. Yoshida 1; H. Kinoshita 2; T. Tatara 1; C. Tashiro 1; M. Nishiguchi 3; H. Ouchi 3; T. Minami 3; S. Hishida 3
Authors place of work: Department of Anesthesia, Hyogo College of Medicine, 1-1, Mukogawa-cho, Nishinomiya, Hyogo, 66 -8501, Japan 1; Department of Forensic Medicine, Faculty of Medicine, Kagawa University, 1750-1, Ikenobe, Miki, Kita, Kagawa, 761-079 , Japan 2; Department of Legal Medicine, Hyogo College of Medicine, 1-1, Mukogawa-cho, Nishinomiya, Hyogo, 663-8501, Japan 3
Published in the journal: Soud Lék., 57, 2012, No. 4, p. 66-68
Category: Původní práce
Summary
We have investigated the involvement of glucocorticoid on methamphetamine (MA) induced hyperpyrexia using a bio-telemetric system. A significant level of hyperpyrexia was observed in MA administered rats. In contrast, increase of body temperature was suppressed by adrenalectomy or by the administration of RU-486, an antagonist of the glucocorticoid receptor. These data suggest that the glucocorticoid receptor may be involved in hyperpyrexia induced by MA.
Keywords:
methamphetamine – hyperpyrexia – glucocorticoid – corticosteroneMethamphetamine (MA) is a potent central nervous system systemic stimulant, which cause restlessness, euphoria, dizziness, dyskinesia, tremor, dysphoria and insomnia (1). Personality changes including irritability, hyperactivity and psychosis may result from chronic use (1). In Japan, the third epidemic of MA abuse has been continued since 1995, and it is a serious social problem (2).
Hyperpyrexia is sometimes observed in cases of MA poisoning or MA-related death (3,4). Hyperpyrexia induced by MA administration may involve both central and peripheral nervous system. MA releases dopamine and other biogenic amines, and inhibit monoamine transporters (5). It has been reported that the activation of dopaminergic and serotonergic neurons in central nervous system contributes to hyperpyrexia (6,7). On the other hand, it has been believed that hyperpyrexia may be due to metabolic hyperactivity in skeletal muscles (8). MA also contributes to the hypermetabolism of the skeletal muscle through the stimulation of the sympathetic nervous system under the permissive action of glucocorticoid (9). It is well known that acute administration of MA or amphetamine (a metabolite of MA, which has similar pharmacological properties as MA), causes corticosterone secretion from the adrenal cortex, as the final stage of the stimulation of the hypothalamo-pituitary-adrenal (HPA) axis (10,11,12,13). Glucocorticoid has an influence on thermoregulation (14). However, contribution of the glucocorticoid in MA induced hyperpyrexia is not well understood. In the present study, we have investigated the involvement of glucocorticoid on hyperpyrexia induced by MA administration.
MATERIALS AND METHODS
Adult male Wistar rats (n=41) with a weight range of 290–420 g were used. All animals were housed in a temperature and humidity controlled environment and maintained on a 12h light : 12h darkness cycle. All rats had free access to food and water. Animals were handled several times prior to the experiments to familiarize the animals with investigator and experimental procedures. All parts of this study were approved by the Animal Investigation Committee, Hyogo College of Medicine.
Body temperature measurement
The body temperature of rat was measured by a biotelemetric system (Star Medical, Tokyo, Japan) (15). This system consisted of a transmitter (model IMT-10T-T), a receiving board (model IMT-10RT) and a computer. A battery-operated transmitter containing transducers to measure body temperature was implanted intraperitoneally by aseptic procedure under the pentobarbital anesthesia (50mg/kg) into each rat seven days before the experiments. The body temperature was monitored every 5 minutes for 4 hrs. The results are presented as temperature changes from the body temperature before MA administration.
Experiment 1:
Rats were housed three or four to a cage. The animals were divided into following four groups: MA administered non-operated group (n=6), MA administered bilateral adrenalectomy (ADX) group (n=10), MA administered Sham-ADX group (n=5) and control group (n=7). The surgery of ADX was performed by aseptic procedure using the dorsal approach under the sodium pentobarbital anaesthesia (50mg/kg). ADX rats were given 0.9% NaCl solution instead of water to drink. The sham-operated (Sham-ADX) rats underwent the same procedure, except that the adrenal glands were left in situ. Following surgery, animals were housed in individual cages. Seven days after surgery, the experiment was performed.
On the day of the experiment, rats were moved to the experimental cage. Following a one hour of the equilibrium period, rats received MA by i.p. injection (2mg/kg of body weight), and the control group received the same volume of sterilized saline. The body temperature was measured non-invasively for 4 hrs using telemetric system. Rats were decapitated 4 hours following the MA administration and trunk blood was collected into ice-cold heparinized tubes. Blood samples were centrifuged and the plasma stored at -40 °C prior to assay for corticosterone.
Experiment 2:
The animals were divided into following two groups: RU-486 administered group (n=7), and control group (n=6). The rat received an i.p. injection of RU-486 (LKT laboratories, Inc., St. Paul, MN, USA) at a dose of 25mg/kg. The protocol for RU-486 administration was in accordance with a previous report (16). In brief, animals were administered RU-486 in morning (0900h) and afternoon (1600h) for two days, on the day of experiment, rats received a final injection at 0900h, and moved to the experimental cage. Control animals received the same volume of sterilized saline. Following a one hour of the equilibrium period, all rats received MA by i.p. injection (2mg/kg of body weight). The body temperature was measured non-invasively using telemetric system. Rats were decapitated 4 hours following the MA administration and trunk blood was collected, and the plasma stored at -40 °C.
Corticosterone Assay
Total plasma corticosterone was measured by enzyme immunoassaay kit (Assay Designs Inc, Ann Arbor, MI, USA), and the protocol was followed in accordance with the manufacturer’s specifications (17).
Drug and Chemicals
Methamphetamine hydrochloride was purchased from Dainippon Sumitomo Pharmaceutical Co., Ltd. (Osaka, Japan), RU-486 was purchased from LKT laboratories Inc, (MN, USA) and all other reagents were analytical grade.
Statistics
The data are expressed as mean ± SEM. Statistical analysis was performed by one-way analysis of variance followed by the Fisher PLSD test. A value of p<0.05 was considered significant.
RESULTS
The time course of temperature changes of each group following MA administration in experiment 1 is shown in Figure 1. MA administration significantly increased body temperature, peaking at 60 min, and decreased gradually. In contrast, the body temperature of the ADX group did not increase following MA administration compared with the sham-ADX rats.
Figure 1. Time course of temperature changes in rats given saline (Control), methamphetamine (MA), ADX with MA (ADX+MA) or sham operation with MA (Sham ADX+MA). Values are mean ± SEM. 
Table 1 shows the plasma corticosterone concentration 4hours following MA administration. There were significantly elevated levels of plasma corticosterone concentrations in the MA administrated group (p<0.05) compared to those in the control group. However, a trace concentration of corticosterone was detected in the ADX group.
Tab. 1. Plasma concentration of corticosterone 4 hours following MA administration <em>(Experiment 1)</em>. 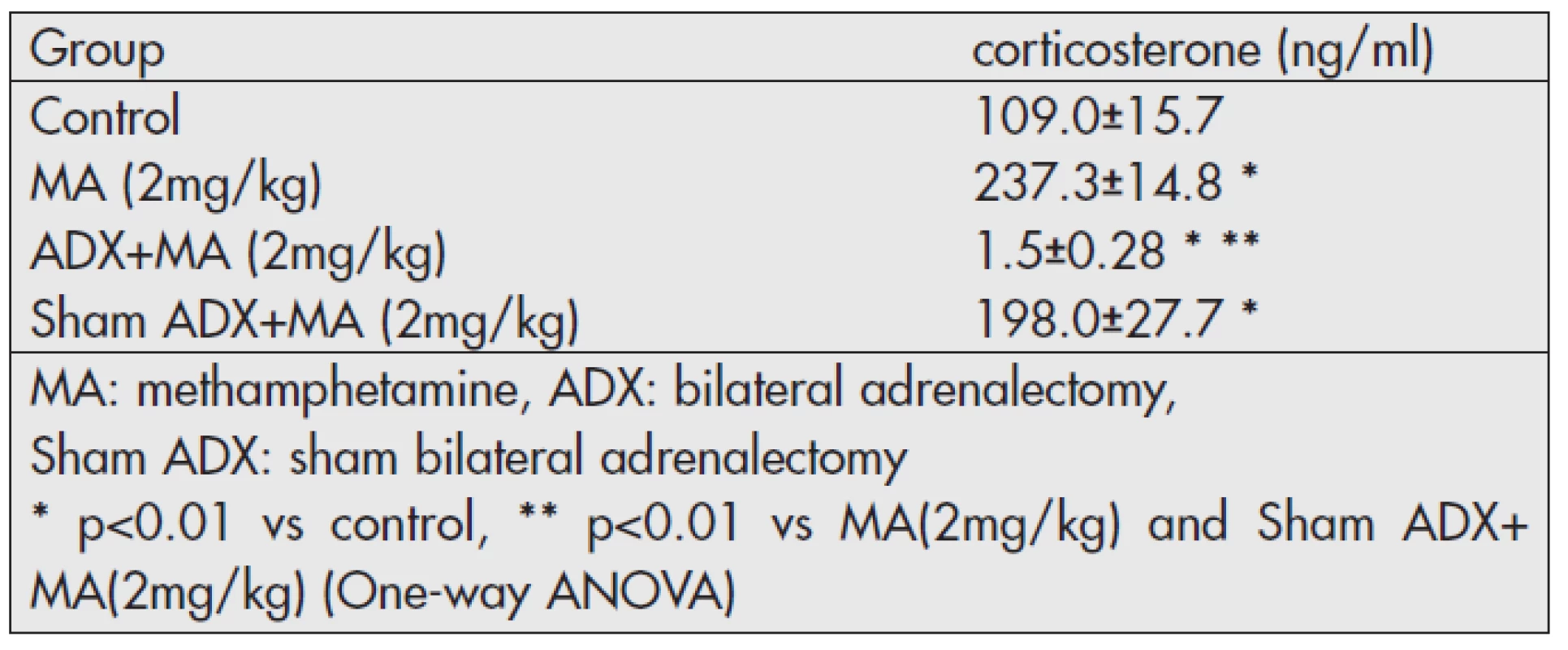
The temperature changes of each group in Experiment 2 following MA administration are shown in Figure 2. The increase of body temperature following MA administration was significantly attenuated by RU-486 pretreatment. The plasma concentration of corticosterone, however, showed no significant difference between two groups (Table 2).
Tab. 2. Plasma concentration of corticosterone 4 hours following MA administration (<em>Experiment 2</em>). 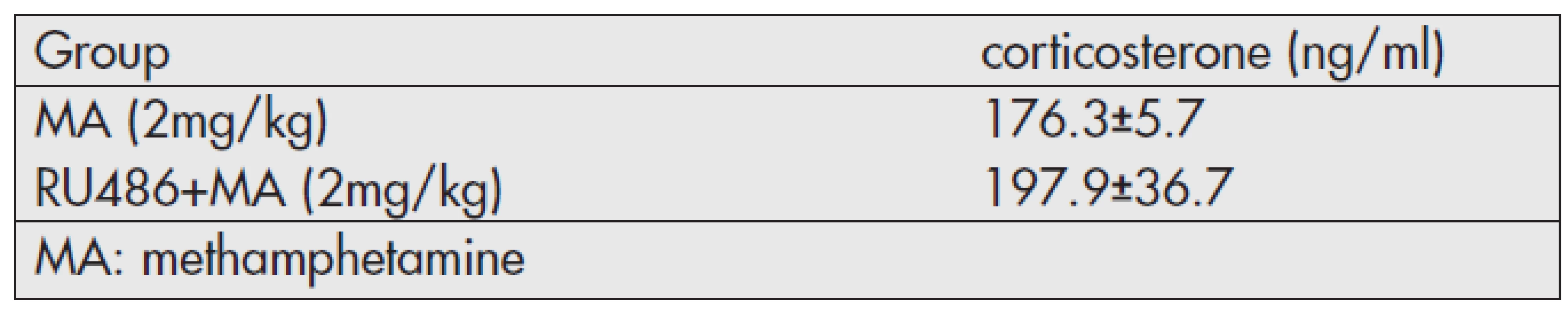
Figure 2. Time course of temperature changes in rats given methamphetamine (MA; 2 mg/kg) or RU-486 with MA (RU+MA). Values are mean ± SEM. 
DISCUSSION
Hyperpyrexia is an important feature of MA intoxication, which is one of the life-threatening response, associated with rhabdomyolysis, renal failure and intravascular coagulation (3,4,8). We have focused on the regulatory mechanism of endogenous glucocorticoid on MA-induced hyperpyrexia. In experiment 1, there was a significant increase of body temperature following MA administration, as reported previously (8,9). Increase of plasma corticosterone concentration was also observed following MA administration in both of non-operated and sham-ADX group. In contrast, ADX significant attenuated hyperpyrexia induced by 2mg/kg MA administration. Depletion of corticosterone, by the surgical removal of the bilateral adrenal glands, eliminates the febrile response following MA administration. Our data shows that endogenous glucocorticoid play an important role in the regulation of temperature response by MA.
Glucocorticoid, secreted from the adrenal cortex, regulates various kinds of biological function, are essential for life and the maintenance of homeostasis. It influences the activity of almost every cell in the body, and modulate the expression of the gene (18). The biological actions of glucocorticoid are mediated through two types of intracellular corticosteroid receptors: Type I or mineralcorticoid receptors (MR) and Type II or glucocorticoid receptors (GR). The MR have high affinity for corticosterone and saturated at low concentration, and its distrubution is restricted. The GR is widely distributed in the body with low affinity for corticosterone. When the concentration of corticosterone is raised, GR is responsible for mediating the biological effects (18). In the present study, we used RU-486, a potent GR antagonist, attenuated hyperpyrexia, despite the elevation of the plasma corticosterone concentration following MA administration. These results suggest that MA-induced hyperpyrexia is regulated by glucocorticoid through GR.
It has been confirmed that glucocorticoid act as endogenous antipyretics (14,19), and corticosterone depress the febrile response of LPS induced experimental fever (19). This response is regulated by pro-inflamatory cytokines such as IL-1β, IL-6 and TNF-α (14). Glucocorticoids inhibit the transcription of these pyrogenic cytokines at various molecular levels of the signal cascades, and blunt the formation of endogenous pyrogen (14). It plays an important role in thermoregulation of the body. We observed opposite effects that lack of glucocorticoid causes loss of hyperthermic response. The present results indicate that MA-induced febrile responses may be less contribution of the pro-inflamatory cytokine, and other mechanism may be involved.
Recent studies have been reported that uncoupling protein 3 (UCP3) and thyroid hormone are involved in MA-induced hyperpyrexia (20). Uncoupling proteins (UCPs) are particular mitochondrial transporters of the inner membrane of mitochondria, appear to be controlling the level of respiration coupling (21,22). The UCP3, a member of UCP family is highly expressed in skeletal muscle. It is an effector of mitochondrial thermogenesis and involves thyroid hormone-induced thermogenesis in skeletal muscle (21,22,23). It has been recognized that skeletal muscle thermogeneration contributes to MA-induced hyperpyrexia (8,9). As the UCP3 is also upregulated by glucocorticoid (23,24), it is speculated that the elevation of plasma corticosterone concentration by the MA lead to upregulation of UCP3 and cause hyperpyrexia. Although, we have focused on the endocrinological mechanism of MA induced hyperpyrexia, sympatho-adrenal involvement of MA-induced hyperpyrexia have been reported (9). Since the UCP3 also upregulated by beta3-adrenergic agonist (21), contribution of sympatho-adrenal-axis should also be considered.
The present results clearly indicate that the biological action of glucocorticoid through GR may be involved in hyperpyrexia induced by MA. The role of glucocorticoid could represent an interesting target for the study of mechanism of MA induced hyperpyrexia. The detailed mechanism is not clear and additional studies will be required to clarify its regulatory mechanisms.
Correspondence address:
Dr. H. Kinoshita
Department of Forensic Medicine
Faculty of Medicine, Kagawa University
1750-1, Ikenobe, Miki, Kita, Kagawa, 761-0793, Japan
tel.: +81-87-891-2140
fax: +81-87-891-2141
e-mail: kinochin@med.kagawa-u.ac.jp
Zdroje
1. Baselt RC. Methamphetamine. In: Drug effects on psychomotor performance. Foster City, CA: Biomedical Publications, 2001, pp. 244–246.
2. Wada K, Ozaki S, Kondo A. Current situation of drug abuse/dependence and political task against it. Nihon Arukoru Yakubutsu Igakkai Zasshi 2008; 43 : 120–131.
3. Karch SB. The pathology of drug abuse (2nd ed). Boca raton: CRC press; 1996 : 199–230.
4. Kojima T, Une I, Yashiki M, Noda J, Sakai K, Yamamoto K. A fatal methamphetamine poisoning associated with hyperpyrexia. Forensic Sci Int 1984; 24 : 87–93.
5. Westfall TC, Westfall DP. Adrenergic agonists and antagonists. In: Brunton LL, Lazo JS, Parker KL. eds. Goodman & Gilman’s The pharmacological basis of therapeutics (11th ed). New York: McGraw-Hill; 2006 : 237–295.
6. Matsumoto C, Griffin W. Antagonism of (+)-amphetamine-induced hyperthermia in rats by pimozide. J Pharm Pharmac 1971; 23 : 710.
7. Yamawaki S, Lai H, Horira A. Dopaminergic and Serotonergic mechanisms of thermoregulation: mediation of thermal effects of apomorphine and dopamine. J Pharmacol Exp Ther 1983; 227 : 383–388.
8. Uchima E. Physiologic stidies on acute methamphetamine poisoning. Nihon Hoigaku Zasshi 1984; 38 : 814–826.
9. Makisumi T, Yoshida K, Watanabe T, Tan N, Murakami N, Morimoto A. Sympatho-adrenal involvement in methamphetamine-induced hyperthermia through skeletal muscle hypermetabolism. Eur J Pharmacol 1998; 363 : 107–112.
10. Harbuz MS, Lightman SL. Stress and the hypothalamo-pituitary-adrenal axis: acute, chronic and immunological activation. J Endocrinol 1992; 134 : 327–339.
11. Knych ET, Eisenberg RM. Effect of amphetamine on plasma corticosterone in the conscious rat. Neuroendocrinology 1979; 29 : 110–118.
12. Swerdlow NR, Koob GF, Cador M, Lorang M, Hauger RL. Pituitary-adrenal axis response to acute amphetamine in the rat. Pharmacol Biochem Behav 1993; 45 : 629–637.
13. Williams MT, Inman-Wood SL, Morford LL, et al. Preweaning treatment with methamphetamine induces increase in both corticosterone and ACTH in rats. Neurotoxicol Teratol 2000; 22 : 751–759.
14. Roth J. Endogenous antipyretics. Clin Chim Acta 2006; 371 : 13–24.
15. Takahashi A, Ishimaru H, Ikarashi Y, Kishi E, Maruyama Y. Opposite regulation of body temperature by cholinergic input to the paraventricular nucleus and supraoptic nucleus in rats. Brain Res 2001; 909 : 102–111.
16. McCullers DL, Sullivan PG, Scheff SW, Herman JP. Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res 2002; 947 : 41–49.
17. Kinoshita H, Harbuz MS, Nishiguchi M, et al. High alcohol preferring (HAP) and low alcohol preferring (LAP) rats show altered proopiomelanocortin (POMC) messenger RNA expression in the arcuate nucleus. Alcohol Alcohol 2004; 39 : 406–409.
18. Buckingham JC. Glucocorticoids: exemplars of multi-tasking. Br J Pharmacol 2006; 147: S258–S268.
19. Coelho MM, Souza GEP, Pela IR. Endotoxin-induced fever is modulated by endogenous glucocorticoids in rats. Am J Physiol 1992; 263: R423–427.
20. Sprague JE, Mallett NM, Rusyniak DE, Mills E. UCP3 and thyroid hormone involvement in methamphetamine-induced hyperthermia. Biochem Pharmacol 2004; 68 : 1339–1343.
21. Gong DW, He Y, Karas M, Reitman M. Uncoupling protein-3 is a mediator of thermogenesis regulated by thyroid hormone, beta3-adrenergic agonists and leptin. J Biol Chem 1997; 272 : 24129–24132.
22. Rousset S, Alves-Guerra M-C, Mozo J, et al. The biology of mitochondrial uncoupling proteins. Diabetes 2004; 53 (Suppl.1): S130–S135.
23. Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem J 2000; 345 : 161–179.
24. Sun X, Wray C, Tian X, Hasselgren PO, Lu J. Expression of uncoupling protein 3 is upregulated in skeletal muscle during sepsis. Am J Physiol Endocrinol Metab 2003; 285: E512–E520.
Štítky
Patologie Soudní lékařství Toxikologie
Článek vyšel v časopiseSoudní lékařství

2012 Číslo 4-
Všechny články tohoto čísla
- Death due to peripheral vascular injury following blunt trauma
- Sudden death due to a rare brain tumor: An autopsy case
- Cranial injury caused by penetrating non-missile foreign body: An autopsy case
- Unusual head and neck injury in elevator: autopsy study
- Involvement of glucocorticoid receptor on hyperpyrexia induced by methamphetamine administration
- A fatal case due to cough syrup abuse
- 5th International Symposium of the Osteuropaverein on Legal Medicine
- Fatal and survived motorcycle accidents: a selected topics for medicolegal evaluation
- Effects of alcohol on the brain biomembranes: A review
- Two anniversaries in Czech forensic medicine
- 21st International Meeting on Forensic Medicine Alpe – Adria – Pannonia
- XXII. Congress of International Academy of Legal Medicine.
- Soudní lékařství
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A fatal case due to cough syrup abuse
- Sudden death due to a rare brain tumor: An autopsy case
- Two anniversaries in Czech forensic medicine
- Unusual head and neck injury in elevator: autopsy study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



