-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaRole of Non-conventional T Lymphocytes in Respiratory Infections: The Case of the Pneumococcus
Non-conventional T lymphocytes constitute a special arm of the immune system and act as sentinels against pathogens at mucosal surfaces. These non-conventional T cells (including mucosal-associated invariant T [MAIT] cells, gamma delta [γδ] T cells, and natural killer T [NKT] cells) display several innate cell-like features and are rapidly activated by the recognition of conserved, stress-induced, self, and microbial ligands. Here, we review the role of non-conventional T cells during respiratory infections, with a particular focus on the encapsulated extracellular pathogen Streptococcus pneumoniae, the leading cause of bacterial pneumonia worldwide. We consider whether MAIT cells, γδ T cells, and NKT cells might offer opportunities for preventing and/or treating human pneumococcus infections.
Published in the journal: . PLoS Pathog 10(10): e32767. doi:10.1371/journal.ppat.1004300
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1004300Summary
Non-conventional T lymphocytes constitute a special arm of the immune system and act as sentinels against pathogens at mucosal surfaces. These non-conventional T cells (including mucosal-associated invariant T [MAIT] cells, gamma delta [γδ] T cells, and natural killer T [NKT] cells) display several innate cell-like features and are rapidly activated by the recognition of conserved, stress-induced, self, and microbial ligands. Here, we review the role of non-conventional T cells during respiratory infections, with a particular focus on the encapsulated extracellular pathogen Streptococcus pneumoniae, the leading cause of bacterial pneumonia worldwide. We consider whether MAIT cells, γδ T cells, and NKT cells might offer opportunities for preventing and/or treating human pneumococcus infections.
Introduction
Streptococcus pneumoniae (commonly referred to as “pneumococcus”) is an important human pathogen that causes severe pulmonary and invasive diseases. Over the past decades, progress has been made in (i) the understanding of host innate and acquired immunity during S. pneumoniae infection and (ii) the development of antipneumococcal vaccines. Non-conventional T lymphocytes (including mucosal-associated invariant T [MAIT] cells, gamma delta [γδ] T cells, and natural killer T [NKT] cells) are antigen (Ag)-reactive immune cells with important innate-like functions. In the present review article, we discuss recent advances in our understanding of the innate-like mechanisms underlying the activation of nonconventional T cells and consider their putative roles in pneumococcal infection and disease.
Pneumococcus: A Major Respiratory Pathogen Worldwide
Pneumococcal infection causes around two million deaths per year and is associated with a huge economic burden. Community-acquired pneumonia caused by pneumococci accounts for more than 25% of all cases of pneumonia (for reviews, [1]–[3]). S. pneumoniae is an encapsulated, gram-positive, extracellular bacterium. More than 90 serologically and biochemically distinct serotypes (based on the structure of the bacterial capsule) have been described; they differ in terms of invasiveness, virulence, and antibiotic resistance [1]–[4]. In healthy individuals, S. pneumoniae colonizes the upper respiratory tract but does not appear to have an obvious negative impact. However, in people with an immature or compromised immune system, this asymptomatic colonization can progress to mild disease (such as sinusitis and otitis media) and occasionally to pneumonia, sepsis, and meningitis [3]–[5]. The incidence of pneumococcal infections depends on a number of parameters, including bacterial virulence factors (i.e., the nature of the polysaccharide capsule and the presence or absence of the exotoxin pneumolysin) and host factors (i.e., smoking habit, immune status, and history of respiratory infections) [6]–[9]. For example, influenza infection leads to enhanced susceptibility to pneumococcal infection, a major cause of deaths during influenza epidemics and pandemics [10], [11]. The relative inefficacy of antibiotics is a major issue in pneumococcal infection post-influenza. Furthermore, an increasing number of antibiotic-resistant strains are now emerging [12], [13]. Enhanced susceptibility to pneumococcal infection also occurs during conditions with chronic lung inflammation such as chronic obstructive pulmonary disease [14], which is forecast to become the third most common cause of death worldwide by 2020 [15].
Vaccination is an efficient strategy for preventing and controlling pneumococcal infections, although currently available vaccines do have some issues (for reviews, [16]–[18]). For instance, the 23-valent vaccine that contains purified capsular polysaccharides does not adequately protect young children under two years old or the elderly ([18], [19]). The main reason is that capsular polysaccharides are T-cell independent Ags and are therefore poorly immunogenic. The 7-valent vaccine (containing polysaccharides conjugated to protein carriers, to enhance immunogenicity) is associated with a reduction in the number of invasive pneumococcal diseases, but only those involving the seven serotypes included in the formulation [20]. At present, a 13-valent vaccine is used for infants. In the future, alternative pneumococcal vaccines (directed against virulence factors shared by numerous serotypes and coupled to adequate adjuvants) are likely to be developed [17].
Nonconventional T Lymphocytes
Over the last few years, interest in understanding the role of nonconventional T lymphocytes in immune homeostasis and disease has grown tremendously. These “innate-like” T cells differ from conventional, adaptive T lymphocytes in many respects (Table 1). When nonconventional T lymphocytes emerge from the thymus, they are already capable of cytolysis and cytokine release. The ability to exert effector function soon after activation suggests that nonconventional T cells occupy a unique niche in the immune system (between innate and adaptive immunity). In contrast to the huge receptor diversity of conventional T cells, the T cell receptor (TCR) expressed on the surface of nonconventional T cells presents a limited number of rearrangements and only recognizes conserved, nonpeptide Ags. On the basis of this definition, nonconventional T lymphocytes correspond to three major cell types: MAIT cells, γδ T cells, and NKT cells (Table 1).
Tab. 1. Differences between conventional T cells and non-conventional T cells. 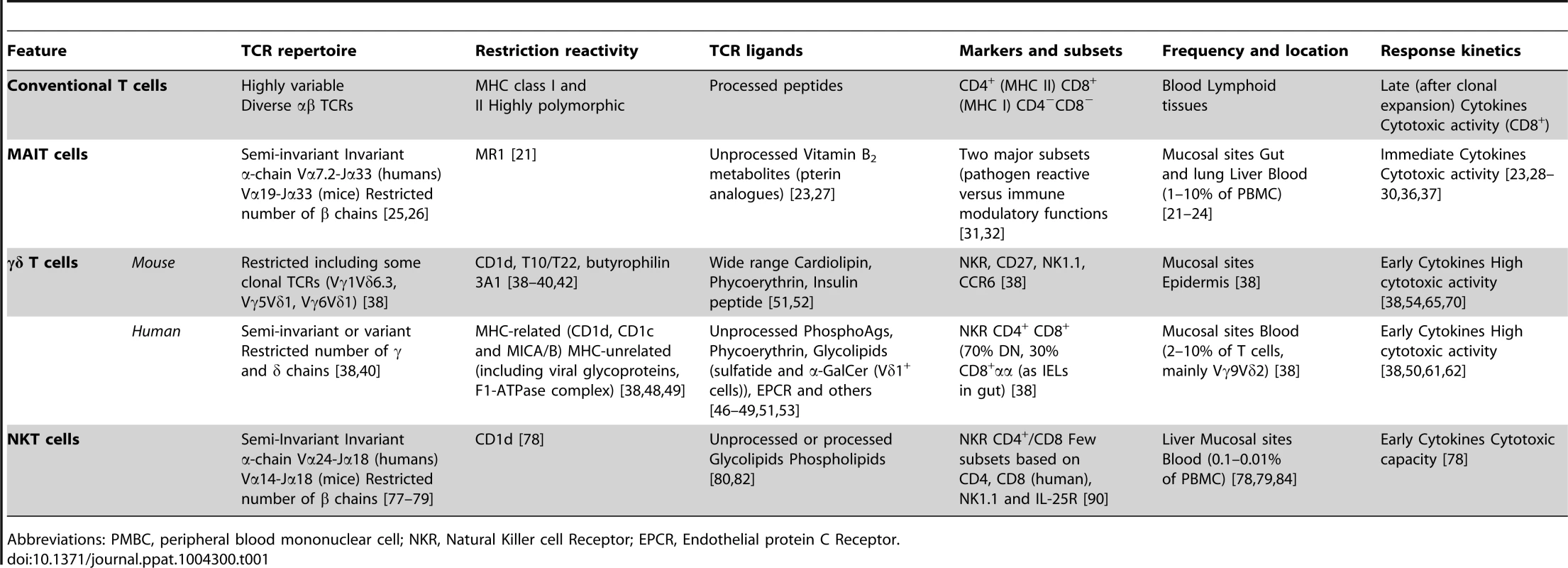
Abbreviations: PMBC, peripheral blood mononuclear cell; NKR, Natural Killer cell Receptor; EPCR, Endothelial protein C Receptor. MAIT cells
General characteristics
In humans, MAIT cells account for between 1% and 10% of T cells in the peripheral circulation [21]. They are also found in liver and mucosal tissues, including the intestine (lamina propria) and the lung [22]–[24]. Although murine MAIT cells also populate lymphoid and mucosal tissues, their frequency is much lower than in humans [25]. MAIT cells express a semi-invariant TCR, which comprises an essentially invariant TCR α-chain (Vα7.2-Jα33 in humans and Vα19-Jα33 in mice) that is preferentially paired with Vβ2, Vβ13, or Vβ22 segments in humans and Vβ6 or Vβ8 segments in mice [25], [26]. The MAIT TCR is restricted to Ags presented by the monomorphic major histocompatibility complex (MHC) class I-related molecule MR1, which is highly conserved in mammals [21]. Recent research has demonstrated that metabolites of riboflavin (also known as vitamin B2) are MR1-dependent ligands for MAIT cells [27]. Unlike certain microorganisms, mammals do not possess the enzymatic machinery needed to generate these riboflavin compounds. Hence, MAIT-cell–activating riboflavin Ags are generated by pathogenic and commensal bacteria [23], [28], the latter being essential for the development and expansion of MAIT cells [21], [22], [28]. In view of their physiological sites, this observation suggests that MAIT cells are important in the early detection of microbial infection and the subsequent elicitation of a successful immune response. Along with TCR-mediated stimulation, cytokines (IL-12, IL-1β, IL-23) are instrumental in amplifying MAIT cell activation [29], [30]. MAIT cells are able to swiftly exert their effector functions upon activation and therefore make an important contribution to the immune response. The role of MAIT cells in health and disease is becoming clearer. A growing body of evidence indicates that MAIT cells are involved in autoimmune disorders, rheumatoid arthritis, intestinal inflammation, and infection (for reviews, [31]–[34]).
Role during respiratory infections
Human and/or mouse MAIT cells are activated when host cells become infected by certain bacteria (i.e., Salmonella enterica, Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Lactobacillus acidophilus, Shigella flexneri, Mycobacteria) and yeasts (Candida and Saccharomyces) but not by viruses [23], [28], [35]. This activation is MR1-dependent and leads to the production of TNF-α and IFN-γ, cytokines known to control intracellular infection. In contrast, Enterococcus, Listeria monocytogenes, and group A Streptococcus (S. pyogenes) fail to activate MAIT cells [23], [28], suggesting a defect in the enzymatic pathway leading to the generation of riboflavin metabolites in these bacterial species. Several clinical studies have investigated the dynamics and activation status of MAIT cells in patients with pulmonary bacterial pathologies (especially in the context of tuberculosis) [23], [28]. Patients with tuberculosis displayed a significant decrease in the frequency and number of circulating MAIT cells [28]. Moreover, MAIT cells were virtually absent in the blood of patients with active tuberculosis [23]. This is probably due to their recruitment and, possibly, subsequent expansion in the lung tissue and pleural effusions of patients [23], [28]. MAIT cells recognize and kill Mycobacterium tuberculosis-infected cells, including dendritic cells (DCs) and lung epithelial cells [23], [36]. Murine MAIT cells are also important in innate immunity against mycobacteria [29]. Through IFN-γ secretion, they can efficiently inhibit the growth of Mycobacteria bovis bacillus Calmette-Guerin (BCG) in macrophages [29]. In this setting, MAIT cell activation relies on MR1 recognition and co-signals, including IL-12 released from infected macrophages. M. bovis BCG–infected Mr1−/− mice displayed a higher bacterial burden in the lung than MAIT cell–proficient animals [29]. Francisella tularensis is a highly virulent, ulcerative, gram-negative bacterium that causes tularaemia. During infection, murine MAIT cells expand gradually in the lungs; this is accompanied by the secretion of IL-17, IFN-γ, and TNF-α [37]. As observed in the murine M. bovis BCG model, MAIT cell deficiency leads to a delay in the control of bacterial growth. The role of MAIT cells in the development of the acquired immune response has yet to be defined. It is noteworthy that after F. tularensis challenge, mice lacking MAIT cells display fewer activated pulmonary CD4+ and CD8+ T cells. Although the specificity of these conventional T cells was not formally addressed, this finding suggests that MAIT cells have a role in the adaptive immune response [37].
γδ T cells
General characteristics
Several subtypes of γδ T cells have been described. They differ according to the rearrangement of the TCR during early development in the thymus (for reviews, [38]–[40]). Subsets of γδ T cells bearing specific γ and δ TCR chains are assigned to particular body sites. For example, the majority of human γδ T cells in the blood (2%–10% of peripheral T cells) carry a Vγ9Vδ2 TCR, whereas the expression of other Vδ elements (particularly Vδ1 and Vδ3 TCR) predominates at epithelial surfaces and mucosae. This infers homogeneous but distinct Ag recognition repertoires and functional specificities when comparing one tissue with another. In the mouse system, γδ T cells with distinct Vγ/Vδ usage are present in lymphoid tissues (Vγ1+ and Vγ4+ cells), skin (Vγ5+ cells), and mucosal tissues such as the intestine (Vγ7+ cells), reproductive tract (Vγ6+ cells), and lung (Vγ1+, Vγ4+ and Vγ6+). In the lung, γδ T cells are uniformly distributed in parenchymal and non-parenchymal sites [41].
Gamma delta T cells are uniquely equipped to sense cellular stress-induced ligands via both TCR-dependent and -independent pathways (Figure 1). These danger-associated molecular pattern-like structures can be expressed during infection, transformation and inflammation. Activation of γδ T cells via TCRs can be mediated by non-classical MHC molecules (i.e., T10/T22 and CD1 family members) and MHC-unrelated molecules (i.e., viral glycoproteins and butyrophilin 3A1) [38]–[40], [42]. The nature of Ags recognized by γδ TCRs is diverse and still incomplete. Human Vγ9Vδ2 T cells react to phosphoAgs derived from the mevalonate pathway [43]–[46]. Human γδ (Vδ1+) T cells recognize lipids presented by CD1 molecules [47]–[49], as well as unprocessed proteins including viral proteins, phycoerythrin, insulin peptide, and stress-induced molecules [50]–[53]. In addition to the TCR, γδ T cells express a wide array of nonclonal receptors, including pattern recognition receptors (PRRs) and natural killer (NK) cell receptors. Regarding the latter group, γδ T cells can sense stressed-induced ligands (Rae1 and MICA/B) through NKG2D engagement. Gamma delta T cells are also directly activated through Toll-like receptors (TLRs) and C-type lectins (including dectin-1) [54] and indirectly activated by microbial products via Ag-presenting cells. In this context, the release of pro-inflammatory cytokines by stressed DCs activates γδ T cells in the absence of additional TCR engagement [54]–[58]. These different modes of activation suggest that γδ T cells are involved in many disease situations. Lastly, γδ T cells have a crucial role in immune surveillance against tumours, protective immunity against pathogens, inflammation, tissue healing, and epithelial cell maintenance (for reviews, [38]–[40], [59], [60]). This multifaceted role of γδ T cells is due to (i) the existence of distinct γδ subsets endowed with specific functions and (ii) functional polarization in the periphery under stressful conditions. Gamma delta T cells produce a wide array of cytokines and display potent cytotoxic activities against infected or transformed cells, with effector functions via apoptosis-inducing receptors (FAS and TRAIL) and cytolytic proteins (perforin and granzyme) [61], [62]. In addition to their beneficial mediation of local immune surveillance, γδ T cells can also contribute to the immunopathology and progression of diseases, including autoimmune diseases [63].
Fig. 1. Mode of activation and role of γδ T cells and NKT cells during bacterial respiratory infections. 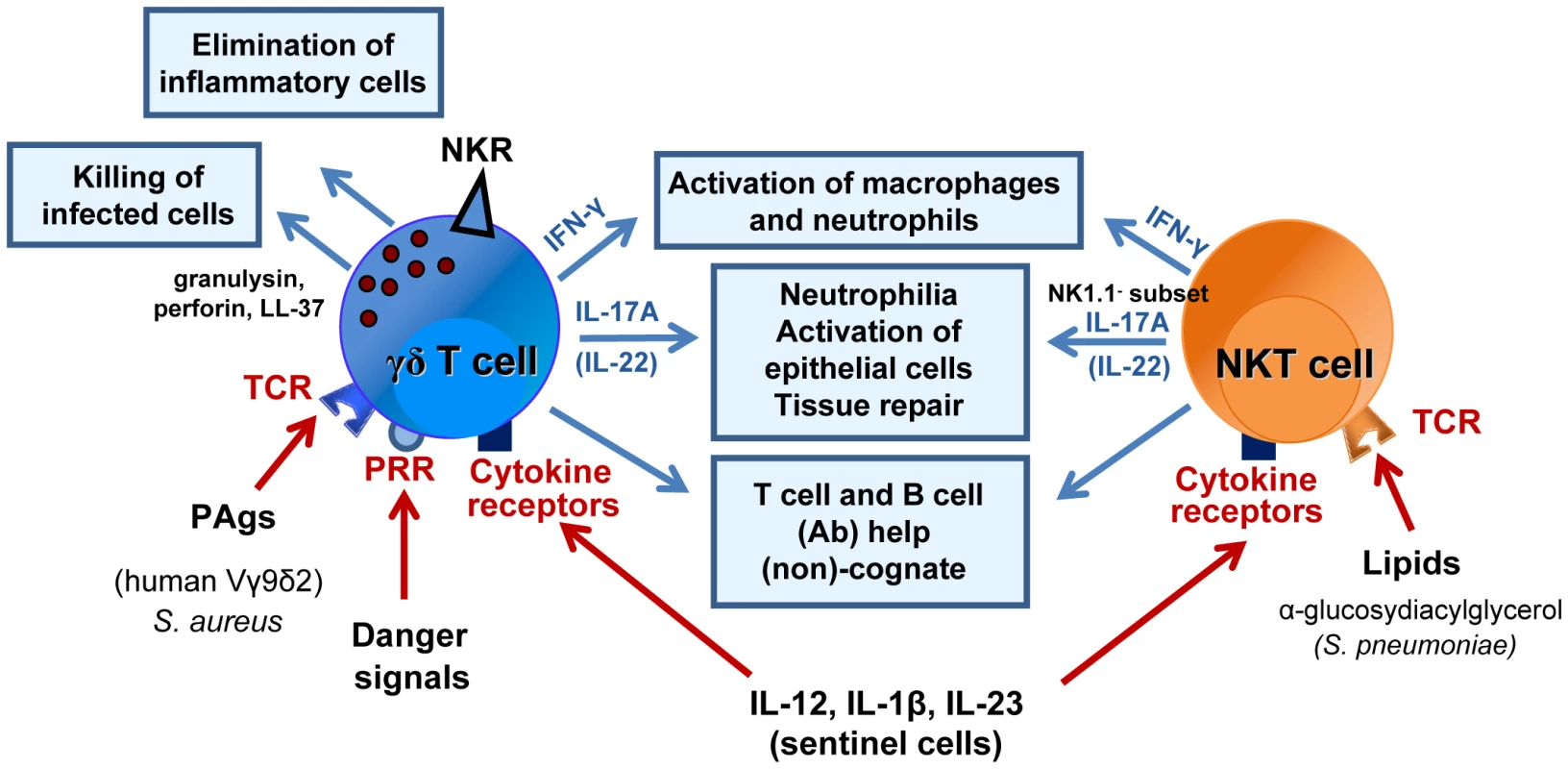
γδ T cells and NKT cells are activated through the TCR, cytokine receptors and/or PRRs (at least for γδ T cells). Their protective role during respiratory bacterial infections (S. aureus, M. tuberculosis, C. pneumoniae, S. pneumoniae) is diverse and comprises activation of innate effector cells, such as macrophages and neutrophils (IFN-γ, IL-17), and epithelial cells (IL-17, IL-22) or direct killing of infected cells (γδ T cells). At later time points, γδ T cells and NKT cells might also play a crucial role in tissue repair, for instance, by acting on epithelial cells and/or by eliminating inflammatory cells. They also promote the development of acquired immune responses. Activation and expansion of T lymphocytes and B lymphocytes (Abs) can occur in a non-cognate way through cytokine release and activation of dendritic cells or in a cognate manner (see Figure 3). Abbreviations: NKR, NK cell receptor; TCR, T cell receptor; PRR, pattern recognition receptor. Roles during respiratory infections
Systemic infections can lead to γδ T cell activation and expansion. Gamma delta T cells mediate pathogen clearance directly through the production of bacteriostatic and lytic molecules, such as granulysin and defensins [61], [64], and indirectly through cytokine-mediated activation of effector cells including macrophages and neutrophils [65]. Pulmonary γδ T cells are uniformly distributed in the lungs, where they are closely associated with Ag-presenting cells [41]. Gamma delta T cells have a role during respiratory infections. For example, the activation of human Vγ9Vδ2 T cells by endogenous mevalonate metabolites during influenza A virus (IAV) infection leads to virus clearance through NKG2D engagement and IFN-γ secretion [62], [66]–[68]. Gamma delta T cells also contribute to the clearance of intracellular and extracellular respiratory bacteria (Figure 1). During S. aureus infection, Vγ9Vδ2 T cells sense the dysregulation of the mevalonate pathway within infected cells—a mechanism that might lead to staphylococcal immunity [69]. In mice, S. aureus triggers IL-17 production by γδ T cells, thus leading to protection and airway inflammation [70]. Gamma delta T cells become activated and expand during M. tuberculosis infections in mice [71], [72]. Whereas IFN-γ and TNF-α derived from γδ T cells might be protective against M. tuberculosis infection, IL-17 production by Vγ4+ and Vγ6+ γδ T cells might induce granuloma formation [73]–[76]. In humans, peripheral blood γδ T cells are a major source of IL-17 during active pulmonary tuberculosis; however, the functional significance of this secretion is not clear [72].
NKT cells
General characteristics
Natural killer T cells recognize a broad range of endogenous and exogenous lipid Ags. These Ags are presented by the monomorphic CD1d molecule expressed by Ag-presenting cells such as DCs [77], [78]. CD1d-restricted NKT cells are divided into two major subsets on the basis of their TCR repertoire and antigenic profile (for reviews, [78]–[81]). The best-characterized NKT cell subset by far is the “type I NKT cell” population. These cells express a restricted TCR, within which an invariant α chain (Vα24-Jα18 in humans and Vα14-Jα18 in mice) is combined with a limited Vβ-chain repertoire (Vβ8.2, Vβ7, or Vβ2 in mice and Vβ11 in humans) [77]. An important feature of type I NKT cells is the rapid release of cytokines and chemokines. This phenomenon is responsible for early effector and regulatory functions. The second major subset of NKT cells (“type II”) expresses a more diverse TCR repertoire and recognize different CD1d-associated lipids [80], [82]. This population may be important in auto-immunity, infections, and cancer [83], although the current lack of specific molecular markers is hindering research in this field. In the present review, we shall focus on type I NKT cells (referred to henceforth as “NKT cells”).
Natural killer T cells are present at higher frequencies than MAIT cells and γδ T cells in mice but at lower frequencies in humans. Human NKT cells comprise a small proportion of peripheral T cells (∼0.1%–0.01% in humans) and vary in their frequency from one tissue to another [79]. In mice, NKT cells are abundant in the liver (∼30% of the T cell pool, compared with ∼1% in human) and, to a lesser extent, in the spleen and mucosal tissues. In the lung, NKT cells are resident in the blood microvasculature and extravasate to reach the parenchyma upon activation [84].
Several distinct populations of NKT cells (based on CD4, NK1.1, and IL-25 receptor expression) have been reported and differ in their tissue distribution, cytokine profile, and effector functions. Many physiological roles are fulfilled by NKT cells; they include protective immunity against pathogens, tumour surveillance, regulation of inflammatory diseases and the modulation of innate and adaptive immune responses [78], [79], [85]. These functions are mainly mediated by cytokine secretion, although NKT cells also exert cytotoxic properties. The multifunctional properties of NKT cells are primarily due to the presence of distinct NKT subsets, the cytokine environment and the nature of the Ag itself and the Ag-presenting cell.
Roles during respiratory infections
Natural killer T cells respond to a wide range of microbial pathogens (from viruses to helminth parasites) (for reviews, [85]–[90]). The cells activate directly in response to microbial (mostly bacterial) CD1d-restricted lipids and/or indirectly in response to inflammatory cytokines (particularly IL-12), in conjunction with (in some cases) self-lipids [91]–[95]). In this setting, PRRs (including TLRs) expressed by sentinel cells have a major functional role (Figure 1) [91], [96]–[98]. The apparent roles of NKT cells in host defence against respiratory pathogens and in infection-associated pulmonary disorders vary according to the model studied and the nature of the initial trigger. Recent evidence indicates that lung NKT cells contribute to pathogen clearance in acute viral infections (including respiratory syncytial virus and influenza A virus [IAV] infection) [99]–[103]. It is known that NKT cells cause pulmonary eosinophilia and fibrosis after respiratory syncytial virus infection [99] but limit inflammation in the context of IAV infection [100]–[103]. Natural killer T cells have a variety of functional roles during bacterial lung infections (Figure 1); whilst the cells protect against the intracellular bacteria Chlamydia pneumoniae, they promote susceptibility to C. muridarum lung infection (a phenomenon due to the recruitment/activation of different NKT cell subsets) [104], [105]. There is evidence for a role of NKT cells in murine tuberculosis infection, although their absence is not essential to control infection [106]–[110]. In contrast, exogenous activation of NKT cells with the superagonist α-galactosylceramide protects susceptible mice from tuberculosis [111], [112]. Moreover, early NKT cell activation (via IFN-γ) has a role in resistance to M. bovis BCG infection in mice [113], [114]. Lastly, patients with active/acute tuberculosis have fewer peripheral NKT cells than patients with latent tuberculosis; normal NKT cell counts can be restored by treating the active tuberculosis [115], [116].
Roles of Non-conventional T Lymphocytes in Pneumococcal Infection
Innate immunity in the early control of S. pneumoniae
The early control of pneumococcal colonization/infection is a dynamic process involving a wide array of host innate factors, including natural antibodies (Abs), elements of the complement pathways, nonopsonic receptors (such as the macrophage scavenger receptor MARCO and the mannose receptor), PRRs, cytokines, and many cell types (for reviews, [6], [7], [117], [118]). S. pneumoniae activates a plethora of PRRs in sentinel cells, including nucleotide-binding oligomerization domain (NOD)-like receptor 2, TLRs (TLR2, TLR4 and TLR9), the cytosolic DNA sensor AIM2, and the inflammasome-forming protein NOD-like receptor family, pyrin domain-containing 3 (NLRP3) (for a review, [118]). In turn, these stimulatory pathways induce neighbouring immune and non-immune cells to produce various inflammatory mediators. Epithelial surfaces constitute the first line of defence against S. pneumoniae [119]. Adhesion of S. pneumoniae to the epithelium of the nasopharynx or within the alveoli causes local release of pro-inflammatory mediators (including cytokines, chemokines, and antimicrobial peptides), which in turn initiate innate immunity. Macrophages have a crucial role in the early control of S. pneumoniae through phagocytic ingestion of the bacteria (a process facilitated by opsonic Abs) [6], [7], [117], [118], [120]. Through phagocytosis and the release of oxygen radicals and antimicrobial peptides, neutrophils are also essential for innate immune responses against pneumococci. Research has also highlighted the essential contribution of NK cells to the early response to pulmonary S. pneumoniae infection, although they are also involved in pathogenesis (through IFN-γ) in a model of pneumococcal meningitis [121], [122]. Interferon-γ and IL-17 are the crucial T helper-type cytokines involved in the early control of S. pneumoniae infection [120], [123]–[132]. Soon after pneumococcal colonization and/or lower respiratory tract infection, IFN-γ and IL-17 are produced by various cell types, including “innate” T cells, NK cells, neutrophils, and at later time points, conventional T lymphocytes [94], [95], [120], [124], [126]–[132]. Pneumococcal-specific CD4+ T lymphocytes (and particularly those producing IL-17) have an important role in protection against bacterial carriage and pulmonary infection [120], [129], [133]–[136]. Our recent data also suggest that IL-22 (a member of the Th17-type cytokine family) might exert an early anti-pneumococcal effect [137]. Type 3 innate lymphoid cells (ILC3) constitute an innate cell population that reacts to inflammatory cytokines (e.g., IL-1β/IL-23) and is thought to be strongly involved in antimicrobial defence and tissue repair in intestines (for a review, [138]). It is noteworthy that pulmonary ILC3 produce IL-22 during S. pneumoniae infection [137].
There is a growing body of evidence for an early-onset role of “innate” T cells in pneumococcal immunity. Elucidation of the roles of MAIT cells, γδ T cells, and NKT cells (all of which produce IL-17 and IFN-γ) in pneumococcal infection may not only provide an in-depth understanding of host defence mechanisms and disease pathogenesis but also accelerate the development of novel efficient therapies.
Potential roles of MAIT cells in pneumococcal infection
The activation status and functions of MAIT cells during pneumococcal infection have not yet been characterized. Three MR1-binding metabolites of the riboflavin pathway activate MAIT cells, namely, reduced 6-hydroxymethyl-8-d-ribityllumazine, 7-hydroxy-6-methyl-8-d-ribityllumazine, and to a lesser extent, the latter's precursor, 6,7-dimethyl-8-d-ribityllumazine [27]. It is not known whether S. pneumoniae activates MAIT cells via MR1. However, the genomic analysis of various S. pneumoniae serotypes and strains indicates that these bacteria might express enzymes involved in the synthesis of riboflavin metabolites [139], [140]. In contrast, and in line with the observation that group A streptococci fail to activate MAIT cells [28], S. pyogenes lacks 3,4-dihydroxy-2-butanone 4-phosphate synthase and 6,7-dimethyl-8-ribityllumazine synthase [141], [142], two critical enzymes involved in the conversion of ribulose 5-phosphate to MAIT cell-activating Ags. This suggests that S. pneumoniae may produce MR1 ligands able to activate MAIT cells and that the latter might play a part in the early recognition of pneumococci. Through their capacity to produce IFN-γ, as well as IL-17 [24], [143], MAIT cells might contain pneumococcal infection. This hypothesis must be tested in future studies.
Roles of γδ T cells in pneumococcal infection
The potential role of γδ T cells in pneumococcal infection has only been studied in animal models. Most experiments have used the frequently colonizing, invasive serotype 3 (Figure 1). In the mouse system, γδ (Vγ1+, Vγ4+, Vγ6+) T cells accumulate and activate in the lungs during S. pneumoniae infection [144], [145]. It is noteworthy that mice lacking γδ T cells display a higher bacterial load in the lung and have a lower survival rate than wild-type controls [130], [132], [144]. Gamma delta T cell deficiency is associated with defective secretion of MIP-2, TNF-α, and IL-17 and poor recruitment of neutrophils [130], [132], [144]. It is likely that γδ T cells contribute to neutrophilia and antipneumococcal defenses through IL-17 production [126], [130], [132]. To a lower extent [126], [132], γδ T cells also produce IFN-γ in the context of infection by S. pneumoniae serotype 3 and serotype 1 (an infrequently colonizing but invasive serotype), although the significance of this observation has yet to be established ([145] and our unpublished data). The mechanisms responsible for γδ T cell activation are still elusive and might rely on the production of self, stressed-induced, or pneumococcal ligand(s) and/or the local synthesis of inflammatory cytokines including IL-12 plus IL-18 (IFN-γ) and IL-1β plus IL-23 (IL-17) (Figure 1). Pneumolysin may synergize with pneumococcal and endogenous danger signals to trigger the secretion of inflammatory cytokines through TLR and NLRP3 activation [126]. Generation of Abs to pneumococcal capsular polysaccharides is important in the control of S. pneumoniae (i.e. opsonization and complement fixation). To date, no study has yet investigated the natural role of γδ T cells in Ab formation during the course of S. pneumoniae infection. Along with their role in early defence against S. pneumoniae, γδ T cells participate in the resolution phase of pneumococcal pneumonia by eliminating inflammatory mononuclear phagocytes [146].
Respiratory viral infections can lead to secondary pneumococcal infections following alterations in the tract's mechanical and immunological defences [10], [11], [147], [148]. Since γδ T cells are critical for host defense against S. pneumoniae [130], [132], [144], it was suggested that respiratory virus infections may inhibit γδ T cell activation and functions in the lung. Of interest, γδ T cells from influenza-experienced mice are less able to produce IL-17 upon challenge with S. pneumoniae—a phenomenon that depends on the IFN type I/IL-27 axis and leads to enhanced pneumococcal susceptibility [131], [132]. It is tempting to speculate that targeting γδ T cells cells to boost immunity against pneumococcal infection might help to limit post-influenza bacterial infection. Unfortunately, ligands capable of specifically activating γδ T cells have not yet been described in the mouse system. It is therefore not possible to study the effects of exogenous γδ T cell activation on host defence in the context of murine pneumococcal infection.
Roles of NKT cells in pneumococcal infection
Several studies have highlighted the role of lung NKT cells in host defence against S. pneumoniae (Figure 1). When infected with S. pneumoniae (serotype 3), NKT-cell-deficient mice exhibit a higher mortality rate and bacterial load in the lung than wild-type controls [149]. In the absence of NKT cells, the early recruitment of neutrophils is impaired by a lack of MIP-2 secretion. It has been suggested that NKT-cell–derived IFN-γ has a critical role in protection against pneumococcal pneumonia [125]. Exogenous administration of recombinant IFN-γ restored the ability of NKT-cell-deficient mice to eliminate bacteria from the lung. It is noteworthy that the administration of IFN-γ led to the production of MIP-2 and TNF-α, which in turn promoted neutrophil recruitment to the infected area. Two other studies have confirmed the natural protective role of NKT cells in animal models of S. pneumoniae serotype 3 infection, although the underlying mechanisms were not studied. Using S. pneumoniae serotype 1, we have also found that NKT cells are important innate effectors in the early clearance of pneumococci (our unpublished data).
Mouse NKT cells produce IFN-γ and (to a much lower extent) IL-17 in the natural course of S. pneumoniae (serotypes 1 and 3) infection ([94], [95], [126], [132], our unpublished data). The mechanism leading to NKT cell activation is now well understood (Figure 2). In S. pneumoniae serotype 3, the cell wall contains α-glucosyldiacylglycerol—a glycolipid that is structurally similar to the canonical NKT cell activator α-galactosylceramide. It is noteworthy that this glycolipid activates NKT cells in a CD1d-restricted manner [94]. Moreover, S. pneumoniae activates the release of IL-12 by DCs, a phenomenon contributing to IFN-γ production by NKT cells [95], [150]. In contrast to the situation with γδ T cells, it is possible to study the effects of exogenous NKT cell activators on host responses in mice. Prophylactic administration of α-galactosylceramide protects against lethal S. pneumoniae serotype 1 and 3 infections [125], [149], [151]. Our findings suggest a critical role for IFN-γ, IL-17, and neutrophils (but not alveolar macrophages) in pneumococcal clearance (Figure 2) [151]. No studies have yet addressed the potential role of NKT cells in human pneumococcal infections.
Fig. 2. Mechanisms of NKT cell–based antibacterial immunity in response to exogenous α-galactosylceramide activation. 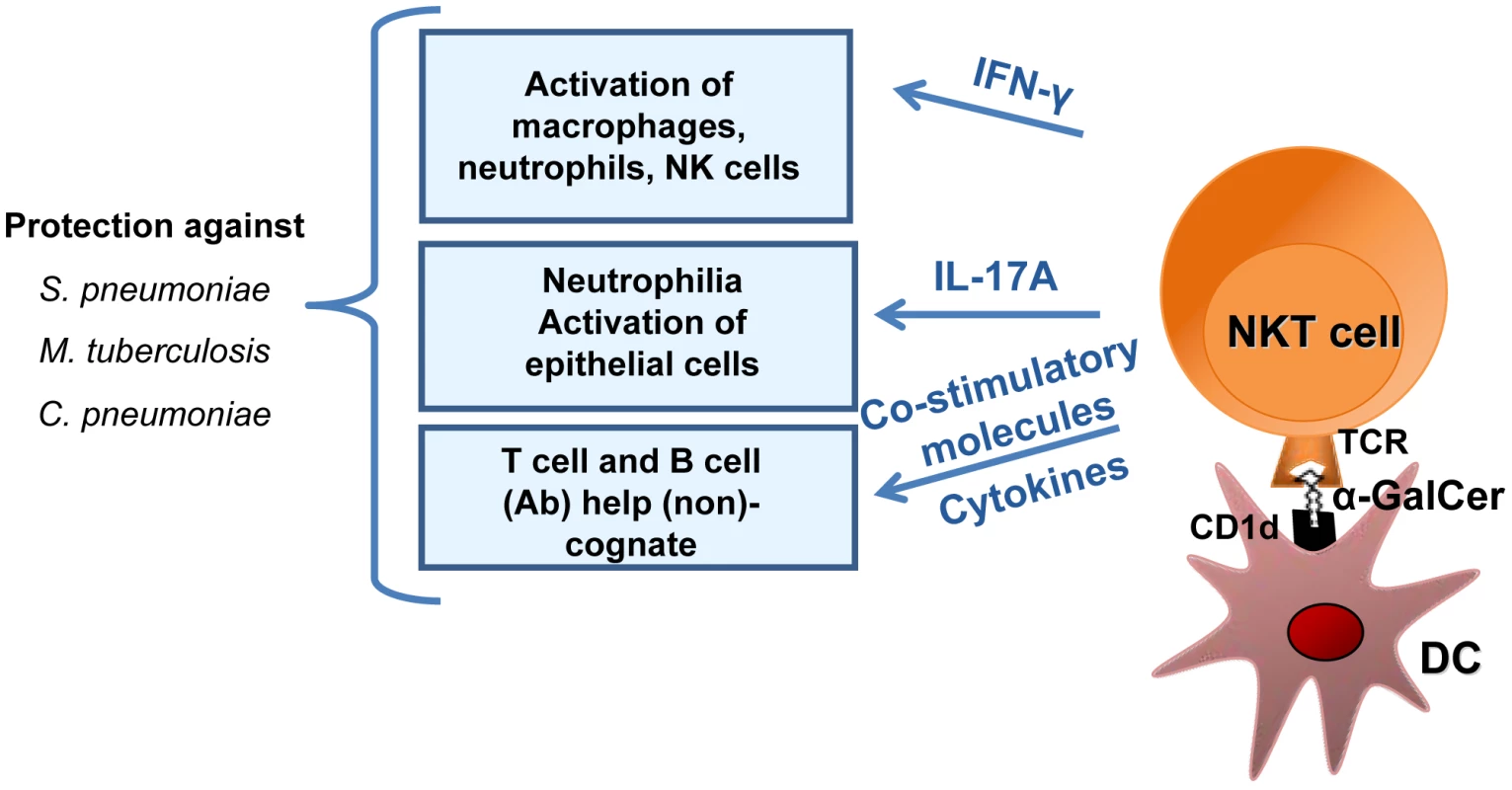
α-galactosylceramide (α-GalCer) presented by respiratory DCs in the context of CD1d activates pulmonary NKT cells to produce IFN-γ and IL-17, which in turn activate macrophages, neutrophils, and possibly NK cells and epithelial cells. Since NKT cells can provide help to conventional T lymphocytes and B lymphocytes, it is likely that NKT cell activation by α-galactosylceramide not only controls the bacterial burden early after infection but also promotes memory protective immune responses against secondary respiratory bacterial infections. Natural killer T cells can indirectly or directly help B cells to mount Ab responses [152]–[157]. Although this activity has not yet been studied in the context of pneumococcal infection, NKT cells might indirectly help B cells through DC licensing and enhanced priming of conventional CD4 T cells (as this is the case for indirect help in other infectious systems) [78], [85]. In parallel, the synthesis of NKT cell agonist by S. pneumoniae suggests the existence of direct help by NKT cells. It is noteworthy that NKT cells have a crucial function in the production of antipneumococcal Abs and class switching in response to pneumococcal polysaccharide vaccines [158], [159]. In humans, there is a positive correlation between the peripheral NKT cell count and the production of IgG after administration of the 23-valent polysaccharide vaccine [160]. Remarkably, the use of liposomal nanoparticles displaying synthetic NKT-cell-activating lipids and polysaccharides (mimicking natural pneumococcal Ags) results in the generation of long-lasting memory B cells and efficient isotype switch [161]. The mechanism leading to Ab production and class switching requires the recognition of lipid and capsular polysaccharide Ags by NKT cells and B cells, respectively, and involves cognate NKT-B cell conjugate formation (direct help, Figure 3). In the future, it might be possible to exploit this unique property (i.e., direct help) and thus optimize Ab responses against T cell-independent pneumococcal polysaccharide Ags [161], [162].
Fig. 3. Promotion of B cell responses by cognate NKT cells help. 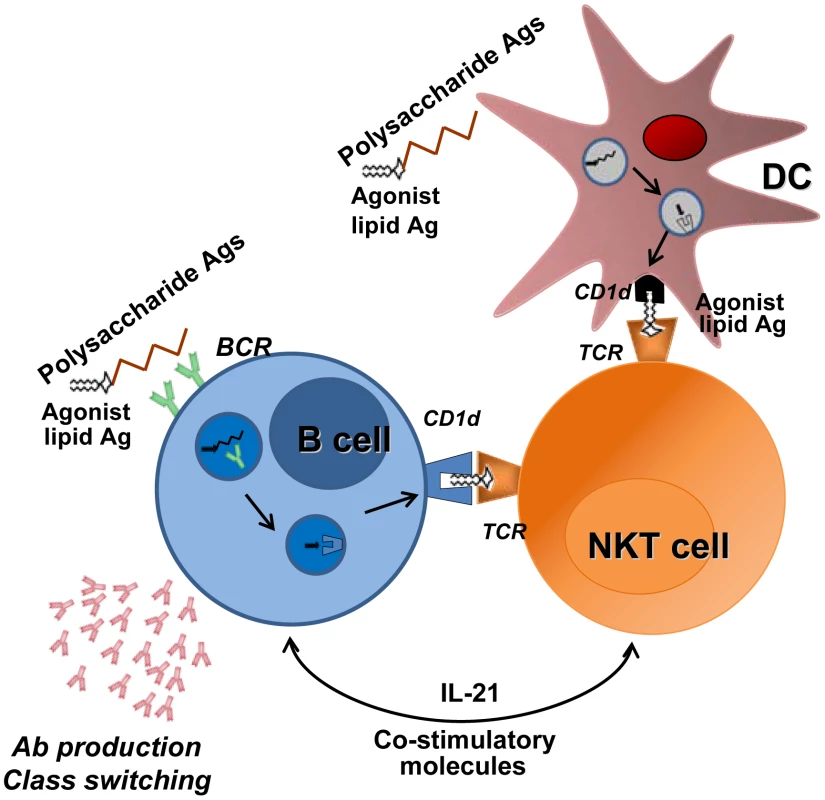
B cells recognize, through their B cell receptor (BCR), capsular polysaccharide Ags associated with lipids (e.g., formulated particles). BCR crosslinking leads to endocytosis and transport of Ags to endosomal compartment. After endosomal/lysosomal digestion, lipid Ags bind to the CD1d molecule. The cell surface CD1d-lipid complex activates NKT cells through TCR engagement, which in turn provide help to B cells. In this setting, NKT cells must be primed by DCs before interacting with B cells. NKT cells favor B cell responses (Ab production and class switching) through IL-21 secretion and co-stimulatory molecules. The ability of NKT cells to promote cognate-dependent B cell responses might be instrumental in the formulation of new vaccines against T cell–independent Ags such as pneumococcal capsular polysaccharides. Of note, during infection, natural pneumococcal NKT cell ligands might also promote Ab responses through cognate NKT cell help to B cells. Conclusions and Future Directions
There is a now a consensus on the importance of γδ T cells and NKT cells in animal models of pneumococcal infection. However, no study has yet addressed the potential role of MAIT cells during pneumococcal infection, and so further research is required. Streptococcus pneumoniae might have a functional riboflavin-biosynthesis pathway and thus the potential to activate MAIT cells. Now that MAIT Ags have been identified, the use of MR1-Ag tetramers will enable the characterization and tracking of MAIT cells during pneumococcal infections in animal models and in humans. Since a growing body of evidence suggests that MAIT cells are involved in the early recognition and containment of microbial infection, these Ag-experienced effector T cells are likely to exert important functions during pneumococcal colonization and/or infection. If so, manipulation of these cells might be of value. Although the role of MAIT cells in acquired responses has yet to be established, it might also be worth looking at whether these cells could be candidates for vaccine targeting.
In the mouse system, γδ T cells and NKT cells act as key elements in antipneumococcal immunity through the production of Th1 and/or Th17-related cytokines. However, several important questions must be answered before these cells can be exploited as potential targets for anti-pneumococcus immunotherapies. For instance, a more comprehensive understanding of their precise modes of activation and their functions is essential. Moreover, the γδ T cells' and NKT cells' respective roles in pneumococcal pneumonia are still elusive. By controlling tissue damage and/or promoting tissue repair processes (via IL-22 and growth factors), these cells may be important in the control of lung pathogenesis. Along the same lines, the potential role(s) of γδ T cells and NKT cells in pneumococcal colonization versus lung and/or systemic invasion merits further investigation. In view of their diverse and sometimes opposing functions, the respective roles of γδ T cell and NKT cell subsets have yet to be characterized. In this context, the development of relevant animal models (enabling specific depletion of γδ cell and NKT cell subsets) and the discovery of novel ligands would be important breakthroughs in this field. In parallel, the use of humanized mice and nonhuman primates is likely to (i) provide highly useful information on the role of these non-conventional T cells during pneumococcal infection and (ii) speed the design of preventive or curative γδ T cell – and NKT cell–based immunotherapies.
There are currently no data on the roles of γδ T cells and NKT cells in pneumococcal infections in humans. Large-scale clinical and genetic studies are clearly warranted, in order to (for instance) link γδ T cell and NKT cell (dys)functions to the human lung diseases associated with pneumococcal infection. Could γδ T cells and/or NKT cells be attractive prophylactic/therapeutic targets for preventing and/or treating pneumococcal infections? This question is an important one, in view of the many situations in which the (innate) immune system is compromised as a consequence of cancer, trauma, immunosuppressive drug treatment, sepsis, chronic inflammation or prior infections. Preclinical data suggest that γδ T cell functions are impaired in the context of influenza—an effect that could (at least in part) account for secondary pneumococcal infection. In this context, targeting γδ T cells via phosphoAgs (to assist immunity against pneumococcal infection) might be a useful approach (in combination with antibiotic treatment) for limiting pneumonia - and/or bacteraemia-associated mortality in patients. The same strategy might also be of value in the case of NKT cells and MAIT cells. Furthermore, the adjuvant properties of γδ T cells and/or NKT cells might be exploited in the design of more efficient antipneumococcal vaccines. Ligands for these cells present many advantages over conventional adjuvants and might conceivably be used to optimize the magnitude and duration of the adaptive immune response. Indeed, γδ T cells and NKT cells can directly activate DCs through non-PRR mechanisms; this unique interplay might not only fine-tune immune responses but also extend the magnitude and duration of the memory T and B cell responses. Lastly, optimized particulate vaccines containing conjugated pneumococcal polysaccharides and non-conventional T cell agonists might perform better than today's poorly immunogenic B cell carbohydrate vaccines.
Zdroje
1. BartlettJG, BreimanRF, MandellLA, FileTMJr (1998) Community-acquired pneumonia in adults: guidelines for management. The Infectious Diseases Society of America. Clin Infect Dis 26 : 811–838.
2. FileTM (2003) Community-acquired pneumonia. Lancet 362 : 1991–2001.
3. WroePC, FinkelsteinJA, RayGT, LinderJA, JohnsonKM, et al. (2012) Aging population and future burden of pneumococcal pneumonia in the United States. J Infect Dis 205 : 1589–1592.
4. WelteT, TorresA, NathwaniD (2012) Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 67 : 71–79.
5. ObaroS, AdegbolaR (2002) The pneumococcus: carriage, disease and conjugate vaccines. J Med Microbiol 51 : 98–104.
6. KadiogluA, WeiserJN, PatonJC, AndrewPW (2008) The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol 6 : 288–301.
7. van der PollT, OpalSM (2009) Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 374 : 1543–1556.
8. VernatterJ, PirofskiLA (2013) Current concepts in host-microbe interaction leading to pneumococcal pneumonia. Curr Opin Infect Dis 26 : 277–283.
9. MitchellAM, MitchellTJ (2010) Streptococcus pneumoniae: virulence factors and variation. Clin Microbiol Infect 16 : 411–418.
10. McCullersJA (2006) Insights into the interaction between influenza virus and pneumococcus. Clin Microbiol Rev 19 : 571–582.
11. van der SluijsKF, van der PollT, LutterR, JuffermansNP, SchultzMJ (2010) Bench-to-bedside review: bacterial pneumonia with influenza - pathogenesis and clinical implications. Crit Care 14 : 219.
12. ViasusD, Garcia-VidalC, CarratalaJ (2013) Advances in antibiotic therapy for community-acquired pneumonia. Curr Opin Pulm Med 19 : 209–215.
13. HackelM, LascolsC, BouchillonS, HiltonB, MorgensternD, et al. (2013) Serotype prevalence and antibiotic resistance in Streptococcus pneumoniae clinical isolates among global populations. Vaccine 31 : 4881–4887.
14. KydJM, McGrathJ, KrishnamurthyA (2011) Mechanisms of bacterial resistance to antibiotics in infections of COPD patients. Curr Drug Targets 12 : 521–530.
15. LopezAD, ShibuyaK, RaoC, MathersCD, HansellAL, et al. (2006) Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J 27 : 397–412.
16. LevineOS, O'BrienKL, KnollM, AdegbolaRA, BlackS, et al. (2006) Pneumococcal vaccination in developing countries. Lancet 367 : 1880–1882.
17. RodgersGL, KlugmanKP (2011) The future of pneumococcal disease prevention. Vaccine 29 Suppl 3: C43–48.
18. DavisSM, Deloria-KnollM, KassaHT, O'BrienKL (2013) Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: Review of evidence on indirect effects. Vaccine 32 : 133–145.
19. BogaertD, HermansPW, AdrianPV, RumkeHC, de GrootR (2004) Pneumococcal vaccines: an update on current strategies. Vaccine 22 : 2209–2220.
20. HausdorffWP, BryantJ, ParadisoPR, SiberGR (2000) Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis 30 : 100–121.
21. TreinerE, DubanL, BahramS, RadosavljevicM, WannerV, et al. (2003) Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422 : 164–169.
22. MartinE, TreinerE, DubanL, GuerriL, LaudeH, et al. (2009) Stepwise development of MAIT cells in mouse and human. PLoS Biol 7: e54.
23. GoldMC, CerriS, Smyk-PearsonS, CanslerME, VogtTM, et al. (2010) Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol 8: e1000407.
24. DusseauxM, MartinE, SerriariN, PeguilletI, PremelV, et al. (2011) Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117 : 1250–1259.
25. TilloyF, TreinerE, ParkSH, GarciaC, LemonnierF, et al. (1999) An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med 189 : 1907–1921.
26. PorcelliS, YockeyCE, BrennerMB, BalkSP (1993) Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8 - alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med 178 : 1–16.
27. Kjer-NielsenL, PatelO, CorbettAJ, Le NoursJ, MeehanB, et al. (2012) MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491 : 717–723.
28. Le BourhisL, MartinE, PeguilletI, GuihotA, FrouxN, et al. (2010) Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 11 : 701–708.
29. ChuaWJ, TruscottSM, EickhoffCS, BlazevicA, HoftDF, et al. (2012) Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect Immun 80 : 3256–3267.
30. ChibaA, TajimaR, TomiC, MiyazakiY, YamamuraT, et al. (2012) Mucosal-associated invariant T cells promote inflammation and exacerbate disease in murine models of arthritis. Arthritis Rheum 64 : 153–161.
31. GoldMC, LewinsohnDM (2013) Co-dependents: MR1-restricted MAIT cells and their antimicrobial function. Nat Rev Microbiol 11 : 14–19.
32. Le BourhisL, MburuYK, LantzO (2013) MAIT cells, surveyors of a new class of antigen: development and functions. Curr Opin Immunol 25 : 174–180.
33. BirkinshawRW, Kjer-NielsenL, EckleSB, McCluskeyJ, RossjohnJ (2014) MAITs, MR1 and vitamin B metabolites. Curr Opin Immunol 26 : 7–13.
34. GapinL (2014) Check MAIT. J Immunol 192 : 4475–4480.
35. Le BourhisL, DusseauxM, BohineustA, BessolesS, MartinE, et al. (2013) MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog 9: e1003681.
36. GoldMC, EidT, Smyk-PearsonS, EberlingY, SwarbrickGM, et al. (2013) Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunol 6 : 35–44.
37. MeierovicsA, YankelevichWJ, CowleySC (2013) MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc Natl Acad Sci U S A 110: E3119–3128.
38. BonnevilleM, O'BrienRL, BornWK (2010) Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol 10 : 467–478.
39. KalyanS, KabelitzD (2013) Defining the nature of human gammadelta T cells: a biographical sketch of the highly empathetic. Cell Mol Immunol 10 : 21–29.
40. VantouroutP, HaydayA (2013) Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol 13 : 88–100.
41. WandsJM, RoarkCL, AydintugMK, JinN, HahnYS, et al. (2005) Distribution and leukocyte contacts of gammadelta T cells in the lung. J Leukoc Biol 78 : 1086–1096.
42. FerreiraLM (2013) Gammadelta T cells: innately adaptive immune cells? Int Rev Immunol 32 : 223–248.
43. TanakaY, SanoS, NievesE, De LiberoG, RosaD, et al. (1994) Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci U S A 91 : 8175–8179.
44. TanakaY, MoritaCT, TanakaY, NievesE, BrennerMB, et al. (1995) Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 375 : 155–158.
45. HintzM, ReichenbergA, AltincicekB, BahrU, GschwindRM, et al. (2001) Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human gammadelta T cells in Escherichia coli. FEBS Lett 509 : 317–322.
46. GoberHJ, KistowskaM, AngmanL, JenoP, MoriL, et al. (2003) Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med 197 : 163–168.
47. SpadaFM, GrantEP, PetersPJ, SugitaM, MelianA, et al. (2000) Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med 191 : 937–948.
48. RussanoAM, BassottiG, AgeaE, BistoniO, MazzocchiA, et al. (2007) CD1-restricted recognition of exogenous and self-lipid antigens by duodenal gammadelta+ T lymphocytes. J Immunol 178 : 3620–3626.
49. UldrichAP, Le NoursJ, PellicciDG, GherardinNA, McPhersonKG, et al. (2013) CD1d-lipid antigen recognition by the gammadelta TCR. Nat Immunol 14 : 1137–1145.
50. BukowskiJF, MoritaCT, BrennerMB (1994) Recognition and destruction of virus-infected cells by human gamma delta CTL. J Immunol 153 : 5133–5140.
51. ZengX, WeiYL, HuangJ, NewellEW, YuH, et al. (2012) gammadelta T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity 37 : 524–534.
52. ZhangL, JinN, NakayamaM, O'BrienRL, EisenbarthGS, et al. (2010) Gamma delta T cell receptors confer autonomous responsiveness to the insulin-peptide B:9–23. J Autoimmun 34 : 478–484.
53. WillcoxCR, PitardV, NetzerS, CouziL, SalimM, et al. (2012) Cytomegalovirus and tumor stress surveillance by binding of a human gammadelta T cell antigen receptor to endothelial protein C receptor. Nat Immunol 13 : 872–879.
54. MartinB, HirotaK, CuaDJ, StockingerB, VeldhoenM (2009) Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 31 : 321–330.
55. ContiL, CasettiR, CardoneM, VaranoB, MartinoA, et al. (2005) Reciprocal activating interaction between dendritic cells and pamidronate-stimulated gammadelta T cells: role of CD86 and inflammatory cytokines. J Immunol 174 : 252–260.
56. DevilderMC, MailletS, Bouyge-MoreauI, DonnadieuE, BonnevilleM, et al. (2006) Potentiation of antigen-stimulated V gamma 9V delta 2 T cell cytokine production by immature dendritic cells (DC) and reciprocal effect on DC maturation. J Immunol 176 : 1386–1393.
57. DevilderMC, AllainS, DoussetC, BonnevilleM, ScotetE (2009) Early triggering of exclusive IFN-gamma responses of human Vgamma9Vdelta2 T cells by TLR-activated myeloid and plasmacytoid dendritic cells. J Immunol 183 : 3625–3633.
58. PagetC, ChowMT, DuretH, MattarolloSR, SmythMJ (2012) Role of gammadelta T cells in alpha-galactosylceramide-mediated immunity. J Immunol 188 : 3928–3939.
59. HaydayAC (2009) Gammadelta T cells and the lymphoid stress-surveillance response. Immunity 31 : 184–196.
60. ZhengJ, LiuY, LauYL, TuW (2013) gammadelta-T cells: an unpolished sword in human anti-infection immunity. Cell Mol Immunol 10 : 50–57.
61. DieliF, Troye-BlombergM, IvanyiJ, FournieJJ, KrenskyAM, et al. (2001) Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis 184 : 1082–1085.
62. QinG, MaoH, ZhengJ, SiaSF, LiuY, et al. (2009) Phosphoantigen-expanded human gammadelta T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J Infect Dis 200 : 858–865.
63. SuD, ShenM, LiX, SunL (2013) Roles of gammadelta T cells in the pathogenesis of autoimmune diseases. Clin Dev Immunol 2013 : 985753.
64. DudalS, TurriereC, BessolesS, FontesP, SanchezF, et al. (2006) Release of LL-37 by activated human Vgamma9Vdelta2 T cells: a microbicidal weapon against Brucella suis. J Immunol 177 : 5533–5539.
65. HamadaS, UmemuraM, ShionoT, HaraH, KishiharaK, et al. (2008) Importance of murine Vdelta1gammadelta T cells expressing interferon-gamma and interleukin-17A in innate protection against Listeria monocytogenes infection. Immunology 125 : 170–177.
66. TuW, ZhengJ, LiuY, SiaSF, LiuM, et al. (2011) The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a gammadelta T cell population in humanized mice. J Exp Med 208 : 1511–1522.
67. LiH, XiangZ, FengT, LiJ, LiuY, et al. (2013) Human Vgamma9Vdelta2-T cells efficiently kill influenza virus-infected lung alveolar epithelial cells. Cell Mol Immunol 10 : 159–164.
68. JamesonJM, CruzJ, CostanzoA, TerajimaM, EnnisFA (2010) A role for the mevalonate pathway in the induction of subtype cross-reactive immunity to influenza A virus by human gammadelta T lymphocytes. Cell Immunol 264 : 71–77.
69. KistowskaM, RossyE, SansanoS, GoberHJ, LandmannR, et al. (2008) Dysregulation of the host mevalonate pathway during early bacterial infection activates human TCR gamma delta cells. Eur J Immunol 38 : 2200–2209.
70. ChengP, LiuT, ZhouWY, ZhuangY, PengLS, et al. (2012) Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol 13 : 38.
71. JanisEM, KaufmannSH, SchwartzRH, PardollDM (1989) Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science 244 : 713–716.
72. PengMY, WangZH, YaoCY, JiangLN, JinQL, et al. (2008) Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell Mol Immunol 5 : 203–208.
73. LockhartE, GreenAM, FlynnJL (2006) IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 177 : 4662–4669.
74. UmemuraM, YahagiA, HamadaS, BegumMD, WatanabeH, et al. (2007) IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol 178 : 3786–3796.
75. Okamoto YoshidaY, UmemuraM, YahagiA, O'BrienRL, IkutaK, et al. (2010) Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J Immunol 184 : 4414–4422.
76. SaitohT, YanoI, KumazawaY, TakimotoH (2012) Pulmonary TCR gammadelta T cells induce the early inflammation of granuloma formation by a glycolipid trehalose 6,6′-dimycolate (TDM) isolated from Mycobacterium tuberculosis. Immunopharmacol Immunotoxicol 34 : 815–823.
77. Scott-BrowneJP, MatsudaJL, MallevaeyT, WhiteJ, BorgNA, et al. (2007) Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat Immunol 8 : 1105–1113.
78. BendelacA, SavagePB, TeytonL (2007) The biology of NKT cells. Annu Rev Immunol 25 : 297–336.
79. BerzinsSP, SmythMJ, BaxterAG (2011) Presumed guilty: natural killer T cell defects and human disease. Nat Rev Immunol 11 : 131–142.
80. RossjohnJ, PellicciDG, PatelO, GapinL, GodfreyDI (2012) Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol 12 : 845–857.
81. BrennanPJ, BriglM, BrennerMB (2013) Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol 13 : 101–117.
82. RhostS, LofbomL, RynmarkBM, PeiB, ManssonJE, et al. (2012) Identification of novel glycolipid ligands activating a sulfatide-reactive, CD1d-restricted, type II natural killer T lymphocyte. Eur J Immunol 42 : 2851–2860.
83. BerzofskyJA, TerabeM (2009) The contrasting roles of NKT cells in tumor immunity. Curr Mol Med 9 : 667–672.
84. ScanlonST, ThomasSY, FerreiraCM, BaiL, KrauszT, et al. (2011) Airborne lipid antigens mobilize resident intravascular NKT cells to induce allergic airway inflammation. J Exp Med 208 : 2113–2124.
85. CohenNR, GargS, BrennerMB (2009) Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv Immunol 102 : 1–94.
86. TupinE, KinjoY, KronenbergM (2007) The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol 5 : 405–417.
87. FaveeuwC, MallevaeyT, TrotteinF (2008) Role of natural killer T lymphocytes during helminthic infection. Parasite 15 : 384–388.
88. TessmerMS, FatimaA, PagetC, TrotteinF, BrossayL (2009) NKT cell immune responses to viral infection. Expert Opin Ther Targets 13 : 153–162.
89. BriglM, BrennerMB (2010) How invariant natural killer T cells respond to infection by recognizing microbial or endogenous lipid antigens. Semin Immunol 22 : 79–86.
90. PagetC, TrotteinF (2013) Role of type 1 natural killer T cells in pulmonary immunity. Mucosal Immunol 6 : 1054–1067.
91. MattnerJ, DebordKL, IsmailN, GoffRD, CantuC3rd, et al. (2005) Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434 : 525–529.
92. KinjoY, WuD, KimG, XingGW, PolesMA, et al. (2005) Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434 : 520–525.
93. KinjoY, TupinE, WuD, FujioM, Garcia-NavarroR, et al. (2006) Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol 7 : 978–986.
94. KinjoY, IllarionovP, VelaJL, PeiB, GirardiE, et al. (2011) Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol 12 : 966–974.
95. BriglM, TatituriRV, WattsGF, BhowruthV, LeadbetterEA, et al. (2011) Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med 208 : 1163–1177.
96. BriglM, BryL, KentSC, GumperzJE, BrennerMB (2003) Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol 4 : 1230–1237.
97. PagetC, MallevaeyT, SpeakAO, TorresD, FontaineJ, et al. (2007) Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity 27 : 597–609.
98. SalioM, SpeakAO, ShepherdD, PolzellaP, IllarionovPA, et al. (2007) Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci U S A 104 : 20490–20495.
99. JohnsonTR, HongS, Van KaerL, KoezukaY, GrahamBS (2002) NK T cells contribute to expansion of CD8(+) T cells and amplification of antiviral immune responses to respiratory syncytial virus. J Virol 76 : 4294–4303.
100. De SantoC, SalioM, MasriSH, LeeLY, DongT, et al. (2008) Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest 118 : 4036–4048.
101. PagetC, IvanovS, FontaineJ, BlancF, PichavantM, et al. (2011) Potential role of invariant NKT cells in the control of pulmonary inflammation and CD8+ T cell response during acute influenza A virus H3N2 pneumonia. J Immunol 186 : 5590–5602.
102. PagetC, IvanovS, FontaineJ, RennesonJ, BlancF, et al. (2012) Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J Biol Chem 287 : 8816–8829.
103. KokWL, DenneyL, BenamK, ColeS, ClellandC, et al. (2012) Pivotal Advance: Invariant NKT cells reduce accumulation of inflammatory monocytes in the lungs and decrease immune-pathology during severe influenza A virus infection. J Leukoc Biol 91 : 357–368.
104. BilenkiL, WangS, YangJ, FanY, JoyeeAG, et al. (2005) NK T cell activation promotes Chlamydia trachomatis infection in vivo. J Immunol 175 : 3197–3206.
105. JoyeeAG, QiuH, WangS, FanY, BilenkiL, et al. (2007) Distinct NKT cell subsets are induced by different Chlamydia species leading to differential adaptive immunity and host resistance to the infections. J Immunol 178 : 1048–1058.
106. BeharSM, DascherCC, GrusbyMJ, WangCR, BrennerMB (1999) Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med 189 : 1973–1980.
107. Sada-OvalleI, ChibaA, GonzalesA, BrennerMB, BeharSM (2008) Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog 4: e1000239.
108. SkoldM, BeharSM (2003) Role of CD1d-restricted NKT cells in microbial immunity. Infect Immun 71 : 5447–5455.
109. SousaAO, MazzaccaroRJ, RussellRG, LeeFK, TurnerOC, et al. (2000) Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc Natl Acad Sci U S A 97 : 4204–4208.
110. SzalayG, ZugelU, LadelCH, KaufmannSH (1999) Participation of group 2 CD1 molecules in the control of murine tuberculosis. Microbes Infect 1 : 1153–1157.
111. ChackerianA, AltJ, PereraV, BeharSM (2002) Activation of NKT cells protects mice from tuberculosis. Infect Immun 70 : 6302–6309.
112. Sada-OvalleI, SkoldM, TianT, BesraGS, BeharSM (2010) Alpha-galactosylceramide as a therapeutic agent for pulmonary Mycobacterium tuberculosis infection. Am J Respir Crit Care Med 182 : 841–847.
113. ChibaA, DascherCC, BesraGS, BrennerMB (2008) Rapid NKT cell responses are self-terminating during the course of microbial infection. J Immunol 181 : 2292–2302.
114. DieliF, TaniguchiM, KronenbergM, SidobreS, IvanyiJ, et al. (2003) An anti-inflammatory role for V alpha 14 NK T cells in Mycobacterium bovis bacillus Calmette-Guerin-infected mice. J Immunol 171 : 1961–1968.
115. ImJS, KangTJ, LeeSB, KimCH, LeeSH, et al. (2008) Alteration of the relative levels of iNKT cell subsets is associated with chronic mycobacterial infections. Clin Immunol 127 : 214–224.
116. VeenstraH, BaumannR, CarrollNM, LukeyPT, KiddM, et al. (2006) Changes in leucocyte and lymphocyte subsets during tuberculosis treatment; prominence of CD3dimCD56+ natural killer T cells in fast treatment responders. Clin Exp Immunol 145 : 252–260.
117. PatersonGK, MitchellTJ (2006) Innate immunity and the pneumococcus. Microbiology 152 : 285–293.
118. KoppeU, SuttorpN, OpitzB (2012) Recognition of Streptococcus pneumoniae by the innate immune system. Cell Microbiol 14 : 460–466.
119. HippenstielS, OpitzB, SchmeckB, SuttorpN (2006) Lung epithelium as a sentinel and effector system in pneumonia–molecular mechanisms of pathogen recognition and signal transduction. Respir Res 7 : 97.
120. ZhangZ, ClarkeTB, WeiserJN (2009) Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J Clin Invest 119 : 1899–1909.
121. Elhaik-GoldmanS, KafkaD, YossefR, HadadU, ElkabetsM, et al. (2011) The natural cytotoxicity receptor 1 contribution to early clearance of Streptococcus pneumoniae and to natural killer-macrophage cross talk. PLoS ONE 6: e23472.
122. MitchellAJ, YauB, McQuillanJA, BallHJ, TooLK, et al. (2012) Inflammasome-dependent IFN-gamma drives pathogenesis in Streptococcus pneumoniae meningitis. J Immunol 189 : 4970–4980.
123. YamamotoN, KawakamiK, KinjoY, MiyagiK, KinjoT, et al. (2004) Essential role for the p40 subunit of interleukin-12 in neutrophil-mediated early host defense against pulmonary infection with Streptococcus pneumoniae: involvement of interferon-gamma. Microbes Infect 6 : 1241–1249.
124. SunK, SalmonSL, LotzSA, MetzgerDW (2007) Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect Immun 75 : 1196–1202.
125. NakamatsuM, YamamotoN, HattaM, NakasoneC, KinjoT, et al. (2007) Role of interferon-gamma in Valpha14+ natural killer T cell-mediated host defense against Streptococcus pneumoniae infection in murine lungs. Microbes Infect 9 : 364–374.
126. McNeelaEA, BurkeA, NeillDR, BaxterC, FernandesVE, et al. (2010) Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog 6: e1001191.
127. YamadaM, GomezJC, ChughPE, LowellCA, DinauerMC, et al. (2011) Interferon-gamma production by neutrophils during bacterial pneumonia in mice. Am J Respir Crit Care Med 183 : 1391–1401.
128. WeberSE, TianH, PirofskiLA (2011) CD8+ cells enhance resistance to pulmonary serotype 3 Streptococcus pneumoniae infection in mice. J Immunol 186 : 432–442.
129. LuYJ, GrossJ, BogaertD, FinnA, BagradeL, et al. (2008) Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog 4: e1000159.
130. MaJ, WangJ, WanJ, CharboneauR, ChangY, et al. (2010) Morphine disrupts interleukin-23 (IL-23)/IL-17-mediated pulmonary mucosal host defense against Streptococcus pneumoniae infection. Infect Immun 78 : 830–837.
131. LiW, MoltedoB, MoranTM (2012) Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. J Virol 86 : 12304–12312.
132. CaoJ, WangD, XuF, GongY, WangH, et al. (2014) Activation of IL-27 signalling promotes development of postinfluenza pneumococcal pneumonia. EMBO Mol Med 6 : 120–140.
133. KadiogluA, CowardW, ColstonMJ, HewittCR, AndrewPW (2004) CD4-T-lymphocyte interactions with pneumolysin and pneumococci suggest a crucial protective role in the host response to pneumococcal infection. Infect Immun 72 : 2689–2697.
134. MalleyR, TrzcinskiK, SrivastavaA, ThompsonCM, AndersonPW, et al. (2005) CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A 102 : 4848–4853.
135. TrzcinskiK, ThompsonCM, SrivastavaA, BassetA, MalleyR, et al. (2008) Protection against nasopharyngeal colonization by Streptococcus pneumoniae is mediated by antigen-specific CD4+ T cells. Infect Immun 76 : 2678–2684.
136. WrightAK, BangertM, GritzfeldJF, FerreiraDM, JamboKC, et al. (2013) Experimental human pneumococcal carriage augments IL-17A-dependent T-cell defence of the lung. PLoS Pathog 9: e1003274.
137. Van MaeleL, CarnoyC, CayetD, IvanovS, PorteR, et al. (2014) Activation of Type 3 Innate Lymphoid Cells and Interleukin 22 Secretion in the Lungs During Streptococcus pneumoniae Infection. J Infect Dis 210 : 493–503.
138. SpitsH, ArtisD, ColonnaM, DiefenbachA, Di SantoJP, et al. (2013) Innate lymphoid cells–a proposal for uniform nomenclature. Nat Rev Immunol 13 : 145–149.
139. TettelinH, NelsonKE, PaulsenIT, EisenJA, ReadTD, et al. (2001) Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293 : 498–506.
140. LanieJA, NgWL, KazmierczakKM, AndrzejewskiTM, DavidsenTM, et al. (2007) Genome sequence of Avery's virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J Bacteriol 189 : 38–51.
141. FerrettiJJ, McShanWM, AjdicD, SavicDJ, SavicG, et al. (2001) Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc Natl Acad Sci U S A 98 : 4658–4663.
142. BeresSB, SylvaGL, BarbianKD, LeiB, HoffJS, et al. (2002) Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc Natl Acad Sci U S A 99 : 10078–10083.
143. SerriariNE, EocheM, LamotteL, LionJ, FumeryM, et al. (2014) Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin Exp Immunol 176 : 266–274.
144. NakasoneC, YamamotoN, NakamatsuM, KinjoT, MiyagiK, et al. (2007) Accumulation of gamma/delta T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes Infect 9 : 251–258.
145. KirbyAC, NewtonDJ, CardingSR, KayePM (2007) Evidence for the involvement of lung-specific gammadelta T cell subsets in local responses to Streptococcus pneumoniae infection. Eur J Immunol 37 : 3404–3413.
146. KirbyAC, NewtonDJ, CardingSR, KayePM (2007) Pulmonary dendritic cells and alveolar macrophages are regulated by gammadelta T cells during the resolution of S. pneumoniae-induced inflammation. J Pathol 212 : 29–37.
147. SnelgroveRJ, GodleeA, HussellT (2011) Airway immune homeostasis and implications for influenza-induced inflammation. Trends Immunol 32 : 328–334.
148. MetzgerDW, SunK (2013) Immune dysfunction and bacterial coinfections following influenza. J Immunol 191 : 2047–2052.
149. KawakamiK, YamamotoN, KinjoY, MiyagiK, NakasoneC, et al. (2003) Critical role of Valpha14+ natural killer T cells in the innate phase of host protection against Streptococcus pneumoniae infection. Eur J Immunol 33 : 3322–3330.
150. KingIL, AmielE, TigheM, MohrsK, VeerapenN, et al. (2013) The mechanism of splenic invariant NKT cell activation dictates localization in vivo. J Immunol 191 : 572–582.
151. IvanovS, FontaineJ, PagetC, Macho FernandezE, Van MaeleL, et al. (2012) Key role for respiratory CD103(+) dendritic cells, IFN-gamma, and IL-17 in protection against Streptococcus pneumoniae infection in response to alpha-galactosylceramide. J Infect Dis 206 : 723–734.
152. GalliG, NutiS, TavariniS, Galli-StampinoL, De LallaC, et al. (2003) CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med 197 : 1051–1057.
153. GalliG, PittoniP, TontiE, MalzoneC, UematsuY, et al. (2007) Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci U S A 104 : 3984–3989.
154. LeadbetterEA, BriglM, IllarionovP, CohenN, LuteranMC, et al. (2008) NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci U S A 105 : 8339–8344.
155. TontiE, FedeliM, NapolitanoA, IannaconeM, von AndrianUH, et al. (2012) Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4(+) T cell help. J Immunol 188 : 3217–3222.
156. KingIL, FortierA, TigheM, DibbleJ, WattsGF, et al. (2011) Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol 13 : 44–50.
157. ChangPP, BarralP, FitchJ, PratamaA, MaCS, et al. (2011) Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol 13 : 35–43.
158. KobrynskiLJ, SousaAO, NahmiasAJ, LeeFK (2005) Cutting edge: antibody production to pneumococcal polysaccharides requires CD1 molecules and CD8+ T cells. J Immunol 174 : 1787–1790.
159. MiyasakaT, AkahoriY, ToyamaM, MiyamuraN, IshiiK, et al. (2013) Dectin-2-dependent NKT cell activation and serotype-specific antibody production in mice immunized with pneumococcal polysaccharide vaccine. PLoS ONE 8: e78611.
160. MiyasakaT, AoyagiT, UchiyamaB, OishiK, NakayamaT, et al. (2012) A possible relationship of natural killer T cells with humoral immune response to 23-valent pneumococcal polysaccharide vaccine in clinical settings. Vaccine 30 : 3304–3310.
161. BaiL, DengS, RebouletR, MathewR, TeytonL, et al. (2013) Natural killer T (NKT)-B-cell interactions promote prolonged antibody responses and long-term memory to pneumococcal capsular polysaccharides. Proc Natl Acad Sci U S A 110 : 16097–16102.
162. DengS, BaiL, RebouletR, MatthewR, EnglerDA, et al. (2014) A peptide-free, liposome-based oligosaccharide vaccine, adjuvanted with a natural killer T cell antigen, generates robust antibody responses. Chem Sci 5 : 1437–1441.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Identification of the Microsporidian as a New Target of the IFNγ-Inducible IRG Resistance SystemČlánek Human Cytomegalovirus Drives Epigenetic Imprinting of the Locus in NKG2C Natural Killer CellsČlánek APOBEC3D and APOBEC3F Potently Promote HIV-1 Diversification and Evolution in Humanized Mouse Model
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 10- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Diagnostický algoritmus při podezření na syndrom periodické horečky
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Theory and Empiricism in Virulence Evolution
- -Related Fungi and Reptiles: A Fatal Attraction
- Adaptive Prediction As a Strategy in Microbial Infections
- Antimicrobials, Stress and Mutagenesis
- A Novel Function of Human Pumilio Proteins in Cytoplasmic Sensing of Viral Infection
- Social Motility of African Trypanosomes Is a Property of a Distinct Life-Cycle Stage That Occurs Early in Tsetse Fly Transmission
- Autophagy Controls BCG-Induced Trained Immunity and the Response to Intravesical BCG Therapy for Bladder Cancer
- Identification of the Microsporidian as a New Target of the IFNγ-Inducible IRG Resistance System
- mRNA Structural Constraints on EBNA1 Synthesis Impact on Antigen Presentation and Early Priming of CD8 T Cells
- Infection Causes Distinct Epigenetic DNA Methylation Changes in Host Macrophages
- Neutrophil Crawling in Capillaries; A Novel Immune Response to
- Live Attenuated Vaccine Protects against Pulmonary Challenge in Rats and Non-human Primates
- The ESAT-6 Protein of Interacts with Beta-2-Microglobulin (β2M) Affecting Antigen Presentation Function of Macrophage
- Characterization of Uncultivable Bat Influenza Virus Using a Replicative Synthetic Virus
- HIV Acquisition Is Associated with Increased Antimicrobial Peptides and Reduced HIV Neutralizing IgA in the Foreskin Prepuce of Uncircumcised Men
- Uses a Unique Ligand-Binding Mode for Trapping Opines and Acquiring A Competitive Advantage in the Niche Construction on Plant Host
- Involvement of a 1-Cys Peroxiredoxin in Bacterial Virulence
- Ethanol Stimulates WspR-Controlled Biofilm Formation as Part of a Cyclic Relationship Involving Phenazines
- Densovirus Is a Mutualistic Symbiont of a Global Crop Pest () and Protects against a Baculovirus and Bt Biopesticide
- Insights into Intestinal Colonization from Monitoring Fluorescently Labeled Bacteria
- Mycobacterial Antigen Driven Activation of CD14CD16 Monocytes Is a Predictor of Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome
- Lipoprotein LprG Binds Lipoarabinomannan and Determines Its Cell Envelope Localization to Control Phagolysosomal Fusion
- Dampens the DNA Damage Response
- MicroRNAs Suppress NB Domain Genes in Tomato That Confer Resistance to
- Novel Cyclic di-GMP Effectors of the YajQ Protein Family Control Bacterial Virulence
- Vaginal Challenge with an SIV-Based Dual Reporter System Reveals That Infection Can Occur throughout the Upper and Lower Female Reproductive Tract
- Detecting Differential Transmissibilities That Affect the Size of Self-Limited Outbreaks
- One Small Step for a Yeast - Microevolution within Macrophages Renders Hypervirulent Due to a Single Point Mutation
- Expression Profiling during Arabidopsis/Downy Mildew Interaction Reveals a Highly-Expressed Effector That Attenuates Responses to Salicylic Acid
- Human Cytomegalovirus Drives Epigenetic Imprinting of the Locus in NKG2C Natural Killer Cells
- Interaction with Tsg101 Is Necessary for the Efficient Transport and Release of Nucleocapsids in Marburg Virus-Infected Cells
- The N-Terminus of Murine Leukaemia Virus p12 Protein Is Required for Mature Core Stability
- Sterol Biosynthesis Is Required for Heat Resistance but Not Extracellular Survival in
- Allele-Specific Induction of IL-1β Expression by C/EBPβ and PU.1 Contributes to Increased Tuberculosis Susceptibility
- Host Cofactors and Pharmacologic Ligands Share an Essential Interface in HIV-1 Capsid That Is Lost upon Disassembly
- APOBEC3D and APOBEC3F Potently Promote HIV-1 Diversification and Evolution in Humanized Mouse Model
- Structural Basis for the Recognition of Human Cytomegalovirus Glycoprotein B by a Neutralizing Human Antibody
- Systematic Analysis of ZnCys Transcription Factors Required for Development and Pathogenicity by High-Throughput Gene Knockout in the Rice Blast Fungus
- Epstein-Barr Virus Nuclear Antigen 3A Promotes Cellular Proliferation by Repression of the Cyclin-Dependent Kinase Inhibitor p21WAF1/CIP1
- The Host Protein Calprotectin Modulates the Type IV Secretion System via Zinc Sequestration
- Cyclophilin A Associates with Enterovirus-71 Virus Capsid and Plays an Essential Role in Viral Infection as an Uncoating Regulator
- A Novel Alpha Kinase EhAK1 Phosphorylates Actin and Regulates Phagocytosis in
- The pH-Responsive PacC Transcription Factor of Governs Epithelial Entry and Tissue Invasion during Pulmonary Aspergillosis
- Sensing of Immature Particles Produced by Dengue Virus Infected Cells Induces an Antiviral Response by Plasmacytoid Dendritic Cells
- Co-opted Oxysterol-Binding ORP and VAP Proteins Channel Sterols to RNA Virus Replication Sites via Membrane Contact Sites
- Characteristics of Memory B Cells Elicited by a Highly Efficacious HPV Vaccine in Subjects with No Pre-existing Immunity
- HPV16-E7 Expression in Squamous Epithelium Creates a Local Immune Suppressive Environment via CCL2- and CCL5- Mediated Recruitment of Mast Cells
- Dengue Viruses Are Enhanced by Distinct Populations of Serotype Cross-Reactive Antibodies in Human Immune Sera
- CD4 Depletion in SIV-Infected Macaques Results in Macrophage and Microglia Infection with Rapid Turnover of Infected Cells
- A Sialic Acid Binding Site in a Human Picornavirus
- Contact Heterogeneity, Rather Than Transmission Efficiency, Limits the Emergence and Spread of Canine Influenza Virus
- Myosins VIII and XI Play Distinct Roles in Reproduction and Transport of
- HTLV-1 Tax Stabilizes MCL-1 via TRAF6-Dependent K63-Linked Polyubiquitination to Promote Cell Survival and Transformation
- Species Complex: Ecology, Phylogeny, Sexual Reproduction, and Virulence
- A Critical Role for IL-17RB Signaling in HTLV-1 Tax-Induced NF-κB Activation and T-Cell Transformation
- Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-miR122-HSP90
- Role of Non-conventional T Lymphocytes in Respiratory Infections: The Case of the Pneumococcus
- Kaposi's Sarcoma-Associated Herpesvirus Induces Nrf2 during Infection of Endothelial Cells to Create a Microenvironment Conducive to Infection
- A Relay Network of Extracellular Heme-Binding Proteins Drives Iron Acquisition from Hemoglobin
- Glutamate Secretion and Metabotropic Glutamate Receptor 1 Expression during Kaposi's Sarcoma-Associated Herpesvirus Infection Promotes Cell Proliferation
- Reduces Malaria and Dengue Infection in Vector Mosquitoes and Has Entomopathogenic and Anti-pathogen Activities
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Novel Cyclic di-GMP Effectors of the YajQ Protein Family Control Bacterial Virulence
- MicroRNAs Suppress NB Domain Genes in Tomato That Confer Resistance to
- The ESAT-6 Protein of Interacts with Beta-2-Microglobulin (β2M) Affecting Antigen Presentation Function of Macrophage
- Characterization of Uncultivable Bat Influenza Virus Using a Replicative Synthetic Virus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



