-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Studies Needed to Address Public Health Challenges of the 2009 H1N1 Influenza Pandemic: Insights from Modeling
article has not abstract
Published in the journal: . PLoS Med 7(6): e32767. doi:10.1371/journal.pmed.1000275
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1000275Summary
article has not abstract
Summary Points
-
As the global epidemiology of the pandemic (H1N1) 2009 influenza (H1N1pdm) virus strain unfolds into 2010, substantial policy challenges will continue to present themselves for the next 12 to 18 months.
-
Here, we anticipate six public health challenges and identify data that are required for public health decision making: Measuring age-specific immunity to infection; accurately quantifying severity; improving treatment outcomes for severe cases; quantifying the effectiveness of interventions; capturing the full impact of the pandemic on mortality; and rapidly identifying and responding to antigenic variants.
-
Representative serological surveys stand out as a critical source of data with which to reduce uncertainty around policy choices for both pharmaceutical and nonpharmaceutical interventions after the initial wave has passed.
-
Continuing to monitor the time course of incidence of severe H1N1pdm cases will give a clear picture of variability in underlying transmissibility of the virus during population-wide changes in behavior such as school vacations and other nonpharmaceutical interventions.
Introduction
The emergence and global spread of a novel strain of human influenza A/H1N1 during 2009 (pandemic [H1N1] 2009 influenza, or H1N1pdm) has highlighted the importance of data from both detailed outbreak investigations and population surveillance for the support of public health decision making. For example, public health organizations in several countries undertook detailed case investigations to build databases of the first few hundred cases, which include laboratory confirmation status, age, relative severity, exposure history, onset of symptoms, and contact history (for example, the UK First Few Hundred project [1]). Descriptive analyses of such data allowed decision-makers to conclude rapidly that the disease caused by the novel strain was relatively mild for the majority of confirmed cases and that it was being transmitted efficiently between children. Therefore, most countries decided that stringent interventions at the community level (such as proactive school closures) were not appropriate, because their benefits were limited when compared with the high overall cost to society. Population surveillance was also crucial in the early stages of the pandemic. Indeed, the two independent influenza cases [2] that provided the viral isolates used to discern the presence of a novel strain were obtained through a sentinel surveillance system designed for exactly that purpose [3].
In recent years, it has become common to use mathematical modeling to analyze the underlying disease dynamics of outbreaks. Parameters such as the reproductive number R [4], which can be estimated from outbreak investigation data [5], give insight into how underlying transmission dynamics will influence the likely impact of possible interventions. For example, if the underlying basic reproductive number, R0, is low, the impact of community-based mitigation strategies against a severe influenza pandemic might be substantial [6]. However, the use of specific mathematical models to explicitly support particular policy decisions masks a more general aspect of decision making, namely, the inclusion of “modelers” in the policy advice process to ensure that quantitative insights into epidemic dynamics are available. This article is the result of the first meeting of an informal network convened by the World Health Organization (WHO) for the modeling of H1N1pdm. The network is made up of public health professionals, policy makers, and scientists with expertise in the transmission dynamics, epidemiology, ecology, and evolution of human infectious diseases [7].
The H1N1pdm pandemic will continue to generate novel challenges for public health decision makers over the next one to two years. In this article, we suggest likely challenges and consider how uncertainties over the disease dynamics may affect policy formulation. The main objective of this exercise is not to provide evidence to support specific policy alternatives. Rather, we try to anticipate and prioritize the needed nonroutine data that should be planned for and funded in the short term to be of significant value to policy makers in the medium and longer term.
Public Health Challenges
Measuring Age-Specific Immunity to Infection
To be able to estimate the susceptibility of a population to future similar strains, it is important to understand the reason that initial epidemics of a new strain fade out. Using routinely collected data, it will be difficult to know with confidence why any particular local epidemic of H1N1pdm ends (Figure 1). It may be that the number of susceptible individuals has been depleted by the development of immunity, that a population-wide public health response to the epidemic has occurred (and was sustained), or that transmissibility dropped for seasonal reasons. Most likely, local fade-outs are due to a combination of these factors. Routinely collected data such as influenza-like-illness (ILI) reporting from sentinel networks and reported hospitalizations suffer from a number of frailties: They discount a potentially large unobserved subclinical population, they suffer from age-specific biases in health care–seeking patterns, and they cannot differentiate among upper respiratory viruses with similar presentations. Representative serological surveys provide the only viable means to infer population-level susceptibility with any accuracy, especially if there is a substantial proportion of asymptomatic infections.
Fig. 1. Epidemic curves based on surveillance data could mask quite different underlying transmission dynamics. 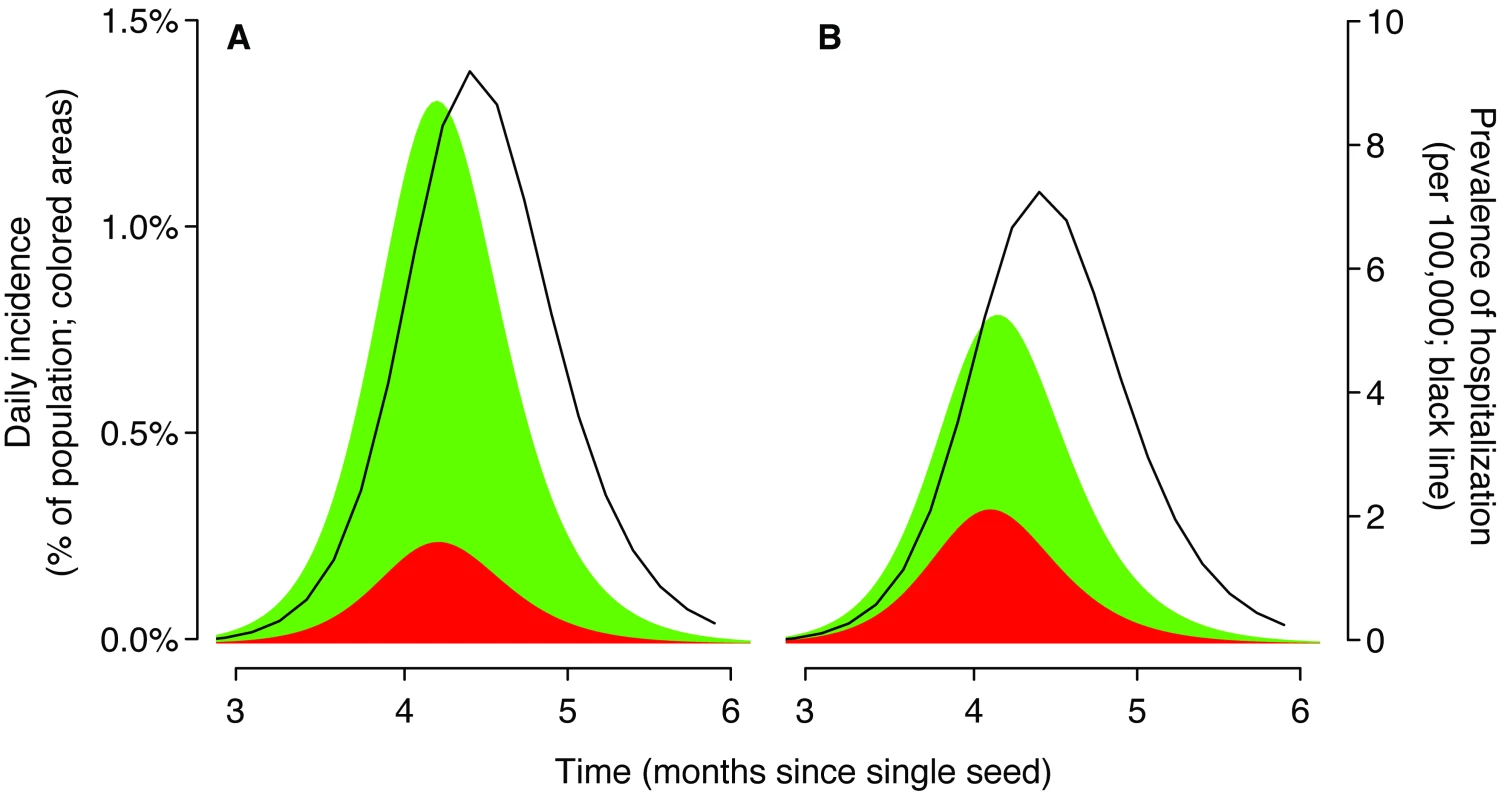
We used a deterministic SIR (susceptible–infected–recovered) model [27] with two age classes: children (20% of the population, a typical proportion for ages 0–18 years in a developed population) and adults (80%). The initial doubling time was set to 5 days with a 2.6 day generation time. These parameters imply a basic reproductive number of 1.4 (for this model [5]). The seed was equivalent to one infectious individual in a population of 7 million at time 0, and mixing between age groups was consistent with contact diary data for the UK (children defined as aged<20 y) [28]. The shaded regions show daily incidence of symptomatic cases for children (red) and adults (green). We assumed that 86% of infections were symptomatic [8]. The black line is the estimated number of hospital beds required at a given time. The susceptibility of children relative to adults was parameterized using the ratio of child cases to adult cases during the exponential phase of epidemic growth. (A) Baseline scenario. The ratio of early cases was proportionate to the population (20∶80, children∶adults) and all ages were equally likely to require hospitalization. (B) A scenario likely to be closer to current nH1N1 dynamics. The ratio of early cases was 50∶50 and adults were much more likely to require hospitalization. Early available data suggested that children are more likely to become infected than adults [8],[9],[10]. With a relatively low overall transmissibility, these characteristics are likely to lead to a lower attack rate among adults and a higher attack rate among children. Therefore, one option for H1N1pdm to evolve to maintain fitness after an initial wave of infection would be to improve its ability to infect adults, with or without a substantial antigenic change (for instance, a shift in the transmission efficiency of the virus between droplet and aerosol could affect children and adults in different ways). Should H1N1pdm evolve to be more infectious to adults, knowing with confidence the proportion of the population exposed during the initial wave would be of substantial public health value, because it would allow robust upper bounds to be placed on the size of subsequent waves. Also, it would be important to capture any such change because the severity of illness for confirmed cases seems to be greater in adults than in children [9],[11].
Accurately Quantifying Severity
Accurate estimates of the per-person risk of severe outcomes, such as the case hospitalization ratio (i.e., the number of hospitalized cases divided by total number of infections), the hospitalization ICU ratio (the total number of cases requiring intensive care divided by total number of hospitalized cases), and the case-fatality ratio (the total number of deaths that are caused by H1N1pdm infection divided by the total number of infections), are required for planning purposes and also to provide at-risk individuals with the best possible information. Unfortunately, reporting biases for both the numerator and the denominator in hospitalization ratio calculations make accurate estimates difficult. For example, mild infections in young children are much more likely to be reported than mild infections in adults, whereas deaths attributable to H1N1pdm depend on testing capacity and policy. In addition, some countries have hospitalized patients for isolation purposes, rather than because they were suffering from severe illness. Therefore, quantifying the overall exposure of the population using a time series of representative, age-stratified serological surveys will greatly improve the accuracy of our estimates of risk, by giving definitive denominator information.
Recent vaccination programs targeting those at higher risk of severe clinical outcomes will further complicate the accurate assessment of severity, because many individuals were infected before being vaccinated. Although it is unlikely that serological assays can distinguish accurately between natural infection and vaccination at the individual level, surveys should continue to include vaccinated individuals and to record symptoms and vaccination status where possible. At the population level, it is likely that the vaccine-induced immune response will have a substantially different distribution of antibody levels than the immune response generated by natural infection.
Improving Treatment Outcomes for Severe Cases
We suggest that, although they are not directly linked to epidemic dynamics, hospital-based cohort studies of H1N1pdm cases are needed to assess the pathogenicity of H1N1pdm infection and to help clarify estimates of relative risk of severe disease and death among routinely reported clinical cases. These studies should collect detailed information on the clinical spectrum of disease including onset and duration of symptoms, prevalence of underlying conditions (such as pregnancy, chronic respiratory disease, immunosuppression, smoking, obesity, chronic respiratory conditions, diabetes, and neurologic disorders), duration of hospital/ICU stay, complications from infection including bacterial superinfection, antiviral/antibiotic treatment including when administered, the efficacy of other adjunctive measures (such as immune modulation, novel oxygenation, or ventilation strategies), and serial blood and respiratory samples for RT-PCR and virus culture to determine the extent and duration of viral shedding and antiviral treatment failure. The WHO, US Centers for Disease Control and Prevention, Canadian Clinical Trials Group, and South East Asian Infectious Disease Clinical Research Network have developed clinical data collection forms for such studies [12],[13] that could be adapted to be context specific and implemented in a representative group of hospitals in each participating country. Although these studies will not capture the mild spectrum of illness (as discussed above), they will fill the current data gaps about pathogenicity and the clinical course of illness including prognostic information. The use of propensity scores could yield valuable insights into the relative efficacy of different treatment strategies in the short term, while awaiting results of prospective trials.
Quantifying the Effectiveness of Interventions
After an initial establishment phase, changes in the growth rate of a novel infectious disease can provide an accurate measure of changes in transmission rates. If the doubling time of the number of new cases is constant in the early stages, then significant changes in underlying transmissibility are unlikely to have occurred. However, if the doubling time appears to slow down during school vacations/holidays/closures or during other widespread changes in mixing, then this likely indicates a genuine shift in the rate of disease transmission [14]. Also, in populations with high vaccine coverage in children during the early stages of the epidemic, we would hope that the coarse time series of incidence would have been affected. Accurate measures of changes in the growth rate—and possibly also the age-composition of reported cases—are required to quantify the population-wide effect of changes in behavior, such as the start of school vacations and restrictions in mass gatherings. Countries with hospital-based respiratory surveillance systems, which are often not optimized in its data specification and collection, could be enhanced to collect more detailed clinical and laboratory data (described above) from ILI, ARI (acute respiratory illness), and SARI (severe acute respiratory illness) patients [15]. In addition, clinical information of ILI, ARI, and SARI patients paired with laboratory testing could provide estimates of the burden of seasonal influenza compared with that of pandemic influenza.
Capturing the Full Impact of the Pandemic on Mortality
We should aim to monitor excess mortality due to H1N1pdm in the timeliest way possible. The number of deaths attributable to seasonal and previous pandemic influenza is considerably higher than the number certified by vital statistics registration as due to influenza or by the number of influenza deaths reported through surveillance schemes [16]; the total number estimated depends strongly on whether the excess above baseline is confined to deaths from pneumonia and influenza, or whether all respiratory and circulatory deaths or all-cause deaths are considered [17]. Often, a number of causes contribute to individual mortality. Influenza-associated mortality has traditionally been estimated as the excess pneumonia and influenza (P & I) mortality above a baseline of deaths during seasonal influenza epidemic periods. Excess P & I mortality estimates are often not timely, as data compilation can take months. To monitor influenza excess mortality in a more timely fashion, several countries have set up sentinel systems that they integrate in their routine influenza surveillance (e.g., European monitoring of excess mortality for public health action—EuroMoMo, http://www.euromomo.eu). The US CDC established a sentinel system in 121 US cities several decades ago. More recently, several European countries have developed real-time monitoring schemes of mortality in which number of deaths by age are transmitted electronically from all or a subset of municipalities to a central database. These schemes allow much more rapid assessment of overall mortality trends and are being utilized in near-real–time during the 2009/2010 Northern Hemisphere influenza season to ensure that policy makers are continuously kept abreast of how excess mortality for the pandemic will compare with similar statistics often quoted for seasonal influenza.
Rapidly Identifying and Responding to Antigenic Variants
It will be useful to isolate virus from infected individuals for whom there is also a serum sample. Although doing this systematically from all cases would place an intolerable burden on supporting laboratory services, there will be value in developing substudies amongst larger serological surveys. Obtaining such paired virological and serological data from vaccinated individuals will be particularly useful because it will allow the investigation of antiviral resistance and vaccine failure. In some instances, vaccine failure could be due to an antigenic variant that is not protected by immunity raised against the vaccine strain. Therefore, active sampling of symptomatic vaccinated individuals could help to provide early warning of vaccine-escape mutants, which, if they are rare initially, might take longer to be detected by routine surveillance.
Meeting the Challenges
While there have been recommendations focusing on how to maintain and enhance population-level surveillance when in most countries case numbers have far exceeded routine testing capacity [12],[18], here we suggest specific nonroutine data that will help public health policy makers to address six public health challenges that we anticipate will continue for the next 12 to 18 months. Because of inherent biases in the routine reporting of cases of differing levels of severity, sufficiently powered representative serological surveys will be useful in the short and medium term to help quantify the degree of susceptibility in the population and to help characterize individual-level severity. Systematic reporting of the incidence of ARI and SARI will help to characterize the speed of growth of the epidemic and hence allow the detection of significant changes in underlying transmissibility. Specific data gathering processes are also required to accurately define the clinical spectrum of severe disease, measure excess mortality in a timely fashion, and help to rapidly detect possible vaccine escape, antiviral resistant strains, and other mutant strains.
The need for rapid serological studies stands out among these impending knowledge gaps. Historically, the best information on circulating seasonal influenza has come from prospective community studies based on households. Two important such studies in the US were the Tecumseh community study [19],[20] and the Seattle virus watch [21]. Study participants provided periodic serological samples every 4 to 6 months over several years. Bracketing sera were used to detect infections through significant antibody titer rises. In addition, the serologic data provided estimates of the degree of partial immunity in the populations under study at any point in time. Finally, influenza symptom data coupled with virological identification provided valuable information of infection and illness attack rates by age and other demographic characteristics, as well as the pathogenicity and virulence of the identified circulating strains of influenza. Even today, these studies provide the most complete description of the epidemiology of influenza circulating in the community. It is therefore encouraging that such detailed prospective community serologic studies are underway or planned in the many countries including Argentina, Australia, Bangladesh, Canada, Chile, China, France, Finland, Germany, Hong Kong SAR, India, Italy, Japan, Mexico, the Philippines, Singapore, Sweden, Taiwan, Thailand, Turkey, the UK, and the US.
Observing the serological attack rate across countries gives us a standardized measure of the risk of infection across countries. Such a standardized measure facilitates international comparisons that are essential to assess the effectiveness of interventions against influenza in different countries. It is difficult to compare doctor consultations, hospitalizations, and even deaths, because of differences in reporting systems; by relating the number of doctor consultations, hospitalizations, and deaths to the serological attack rate we can assess country-specific biases in the reporting systems.
Although H1N1pdm is antigenically distinct from other currently circulating, seasonal human influenza strains [22], work is ongoing to validate reliable serological assays. Current standard techniques that have been used to quantify antigenic distance between strains depend on antibodies raised in animal models [23]. However, it is reasonable to expect that unpaired assays using human sera will give a good indication of prior exposure to the pandemic strain in most age groups [24], especially once cross-sectional data have been calibrated using paired sera. Despite these potential issues, it seems reasonable to assume that unpaired serological surveys will give an informative snapshot of exposure history at a population level and that it will be straightforward to characterize the degree of uncertainty associated with any single titration.
Pharmaceutical interventions will likely play a minor role in middle - and low-income countries for the 2009/2010 pandemic, nor would they have for a more severe strain. Both the epidemiology and options for interventions are clearly different for less-developed countries compared with highly industrialized countries. Population density, mobility, household structure, and school attendance patterns all differ significantly between and within regions. Therefore, it is not safe to assume that patterns of infection well-described in one population will be widely representative of the world's population. In particular, after the initial Northern and Southern Hemisphere waves of infection, it will not be wise to assume that all other populations have experienced similar infection attack rates. In particular, there may be substantial differences between urban and rural populations, with their different mobility and mixing patterns. Empirical studies should be conducted in multiple representative populations.
Building on existing demographic surveillance or influenza surveillance systems provides an option for many countries. Where possible, samples can be obtained from cross-sectional serological surveys. Where available, samples from national blood supply systems can provide real-time monitoring of infection incidence or cross-reactive antibody responses to H1N1pdm, as can residual blood samples taken from patients for diagnostic laboratory testing (although these samples will not necessarily represent the entire population). In low-resource countries without such systems in place, surveillance systems for diseases such as dengue and polio could be adapted for H1N1pdm. For example, in several countries in South East Asia and Central and South America, community-based surveillance studies were established to assess the burden of dengue among children and adults. Similar surveys have proved extremely useful during outbreaks of chikungunya in the Indian Ocean in 2006/2007 [25],[26]. Polio surveillance, which aims to identify all acute flaccid paralysis cases among children through reporting and laboratory testing, has wide geographic coverage in Africa and Asia. In addition, such systems, which routinely collect blood, could be used to evaluate antibody levels. Lastly, seroprevalence studies that are currently planned or underway for highly pathogenic avian influenza (HPAI)/H5N1 in several African and Asian countries could also test for anti-H1N1pdm antibodies.
Preparedness plans will be revised by many nations in the medium term to incorporate lessons learned from the 2009 pandemic. A thorough assessment of the value of data from all sources will be crucial if the quality of information available for decision-makers during future pandemics is to be improved. We suggest that some of the most valuable data, such as estimates of age-specific serological attack rates, have not become available until far after the time when it would have been needed to support decision making. The establishment of a preapproved ethical review status for key field studies is a priority. Also, if such studies are to be initiated in a short time, investigators may choose to design and pilot them in association with nonacademic partners.
Supporting Information
Zdroje
1. Novel Swine-Origin Influenza A Virus Investigation Team 2009 Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans. N Engl J Med 360 2605 2615
2. Centers for Disease Control and Prevention (CDC) 2009 Swine influenza A (H1N1) infection in two children—Southern California, March-April 2009. MMWR Morb Mortal Wkly Rep 58 400 402
3. FaixDJ
ShermanSS
WatermanSH
2009 Rapid-test sensitivity for novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 361 728 729
4. HeesterbeekJAP
2002 Heesterbeek JAP (2002) A brief history of R0 and a recipe for its calculation. Acta Biotheor 50 189 204
5. WallingaJ
LipsitchM
2007 How generation intervals shape the relationship between growth rates and reproductive numbers. Proc R Soc Lond B Biol Sci 274 599 604
6. WuJT
RileyS
FraserC
LeungGM
2006 Reducing the Impact of the Next Influenza Pandemic Using Household-Based Public Health Interventions. PLoS Med 3 e361 doi:10.1371/journal.pmed.0030361
7. World Health Organization (WHO) 2009 Mathematical modelling of the pandemic H1N1 2009. Wkly Epidemiol Rec 84 341 352
8. FraserC
DonnellyCA
CauchemezS
HanageWP
Van KerkhoveMD
2009 Pandemic Potential of a Strain of Influenza A (H1N1): Early Findings. Science 324 1557 1561
9. WHO 2009 Preliminary information important for understanding the evolving situation. Pandemic (H1N1) 2009 briefing note 4. Available: http://www.who.int/csr/disease/swineflu/notes/h1n1_situation_20090724/en/index.html. Accessed 10 July 2009
10. CauchemezS
DonnellyC
ReedC
GhaniA
FraserC
2009 Household Transmission of 2009 Pandemic Influenza A (H1N1) Virus in the United States. NEJM 361 2619 2627
11. VaillantL
La RucheG
TarantolaA
BarbozaP
epidemic intelligence team at InVS 2009 Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Eurosurveillance 14 pii:19309
12. WHO 2009 Human infection with pandemic (H1N1) 2009 virus: updated interim WHO guidance on global surveillance. Available http://www.who.int/csr/resources/publications/swineflu/interim_guidance/en/index.html. World Health Organization. Accessed 10 July 2009
13. CDC 2009 CDC H1N1 Flu: Clinical Data Collection Forms and Templates for Collection of Information on Patients Infected with Novel Influenza A (H1N1). Available: http://www.cdc.gov/h1n1flu/clinicians/clinician_forms_templates.htm. Accessed 10 July 2009
14. WuJ
CowlingB
LauE
IpD
HoL
2009 Reduced transmissibility of pandemic influenza A (H1N1) associated with school closure and summer vacation, Hong Kong, 2009. EID In press
15. OrtizJR
SotomayorV
UezOC
OlivaO
BettelsD
2009 Strategy to Enhance Influenza Surveillance Worldwide. Emerging Infect Dis 15 1271 1278
16. ThompsonW
WeintraubE
DhankharP
Cheung P-Y, BrammerL
2009 Estimates of US influenza-associated deaths made using four different methods. Influenza Other Respi Viruses 3 37 49
17. ThompsonW
ShayD
WeintraubE
BrammerL
CoxN
2003 Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289 179 186
18. LipsitchM
HaydenF
CowlingB
LeungG
2009 How to maintain surveillance for novel influenza A H1N1 when there are too many cases to count. Lancet 374 1209 1211
19. LonginiI
KoopmanJ
HaberM
CotsonisG
1988 Statistical inference for infectious diseases. Risk-specific household and community transmission parameters. Am J Epidemiol 128 845 859
20. MontoA
KoopmanJ
LonginiI
1985 Tecumseh study of illness. XIII. Influenza infection and disease, 1976-1981. Am J Epidemiol 121 811 822
21. FoxJP
HallCE
CooneyMK
FoyHM
1982 Influenzavirus infections in Seattle families, 1975-1979. I. Study design, methods and the occurrence of infections by time and age. Am J Epidemiol 116 212 227
22. GartenRJ
DavisCT
RussellCA
ShuB
LindstromS
2009 Antigenic and Genetic Characteristics of Swine-Origin 2009 A(H1N1) Influenza Viruses Circulating in Humans. Science 325 197 201
23. SmithDJ
LapedesAS
de JongJC
BestebroerTM
RimmelzwaanGF
2004 Mapping the Antigenic and Genetic Evolution of Influenza Virus. Science 305 371 376
24. HancockK
VeguillaV
LuX
ZhongW
ButlerEN
2009 Cross-Reactive Antibody Responses to the 2009 Pandemic H1N1 Influenza Virus. N Engl J Med 361 1945 1952
25. GérardinP
GuernierV
PerrauJ
FianuA
Le RouxK
2008 Estimating Chikungunya prevalence in La Réunion Island outbreak by serosurveys: two methods for two critical times of the epidemic. BMC Infect Dis 8 99
26. SissokoD
MoendandzeA
MalvyD
GiryC
EzzedineK
2008 Seroprevalence and risk factors of chikungunya virus infection in Mayotte, Indian Ocean, 2005-2006: a population-based survey. PLoS One 3 e3066 doi:10.1371/journal.pone.0003066
27. HethcoteHW
2000 The mathematics of infectious diseases. SIAM Rev 42 599 653
28. MossongJ
HensN
JitM
BeutelsP
AuranenK
2008 Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med 5 e74 doi:10.1371/journal.pmed.0050074
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2010 Číslo 6- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Studies Needed to Address Public Health Challenges of the 2009 H1N1 Influenza Pandemic: Insights from Modeling
- The Association of Factor V Leiden and Prothrombin Gene Mutation and Placenta-Mediated Pregnancy Complications: A Systematic Review and Meta-analysis of Prospective Cohort Studies
- Evaluating the Quality of Research into a Single Prognostic Biomarker: A Systematic Review and Meta-analysis of 83 Studies of C-Reactive Protein in Stable Coronary Artery Disease
- Gestational Age at Delivery and Special Educational Need: Retrospective Cohort Study of 407,503 Schoolchildren
- Closing the Gaps: From Science to Action in Maternal, Newborn, and Child Health in Africa
- Sub-Saharan Africa's Mothers, Newborns, and Children: How Many Lives Could Be Saved with Targeted Health Interventions?
- Secondary Prevention of Suicide
- The Prevalence and Drug Sensitivity of Tuberculosis among Patients Dying in Hospital in KwaZulu-Natal, South Africa: A Postmortem Study
- Estimating the Global Clinical Burden of Malaria in 2007
- Long-Term Biological and Behavioural Impact of an Adolescent Sexual Health Intervention in Tanzania: Follow-up Survey of the Community-Based MEMA kwa Vijana Trial
- Where to for Sexual Health Education for Adolescents in Sub-Saharan Africa?
- Incidence and Reproduction Numbers of Pertussis: Estimates from Serological and Social Contact Data in Five European Countries
- Hospital Performance, the Local Economy, and the Local Workforce: Findings from a US National Longitudinal Study
- Sub-Saharan Africa's Mothers, Newborns, and Children: Where and Why Do They Die?
- Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): Extending the CONSORT Statement
- Developing ANDI: A Novel Approach to Health Product R&D in Africa
- Maternal Health: Time to Deliver
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Gestational Age at Delivery and Special Educational Need: Retrospective Cohort Study of 407,503 Schoolchildren
- Evaluating the Quality of Research into a Single Prognostic Biomarker: A Systematic Review and Meta-analysis of 83 Studies of C-Reactive Protein in Stable Coronary Artery Disease
- Closing the Gaps: From Science to Action in Maternal, Newborn, and Child Health in Africa
- Secondary Prevention of Suicide
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



