-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Chromosome Mechanics and Meiotic Engine Maintenance
article has not abstract
Published in the journal: . PLoS Genet 4(9): e32767. doi:10.1371/journal.pgen.1000210
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1000210Summary
article has not abstract
The behavior of chromosomes during meiosis has been likened to a middle school dance, where partners find one another, form couples that move about and trade information, and then separate to opposite sides of the dance hall. With chromosomes, as with the dancers, forming exclusive couples often is difficult—individuals can be attracted to more than one partner or find themselves trapped behind or between other couples—and, failing to form a couple effectively, end up on the wrong side of the dance hall. For chromosomes, this failure of pairing and segregation leads to an unbalanced chromosome complement (aneuploidy), with its attendant problems of sterility and genetic disease. Two papers in this issue of PLoS Genetics [1],[2] demonstrate that telomere-promoted movements influence nearly every step in chromosome pairing and meiotic recombination, opening a new avenue to address questions that have intrigued biologists and vexed clinicians for over a hundred years.
Chromosome movement is implicit in the classically recognized stages of meiotic prophase, but descriptions of directly visualized movements have been rare (see [3]). Early in prophase, chromosomes transition from having their centromeres clustered near the spindle pole (the Rabl orientation) to having their telomeres clustered at the nuclear periphery adjacent to the spindle pole (the bouquet orientation; see Figure 1). The bouquet stage ends with dispersal of telomeres across the inner nuclear envelope as chromosomes finalize their intimate pairing by forming synaptonemal complexes (SCs) that link chromosome pairs closely along their lengths. The formation of these intimate, exclusive partnerships would seem to finish the task at hand and to end the need for active, whole-chromosome movements, but this turns out not to be the case.
Fig. 1. Chromosomes Pass through Distinct Organizational Phases as They Negotiate Meiotic Prophase. 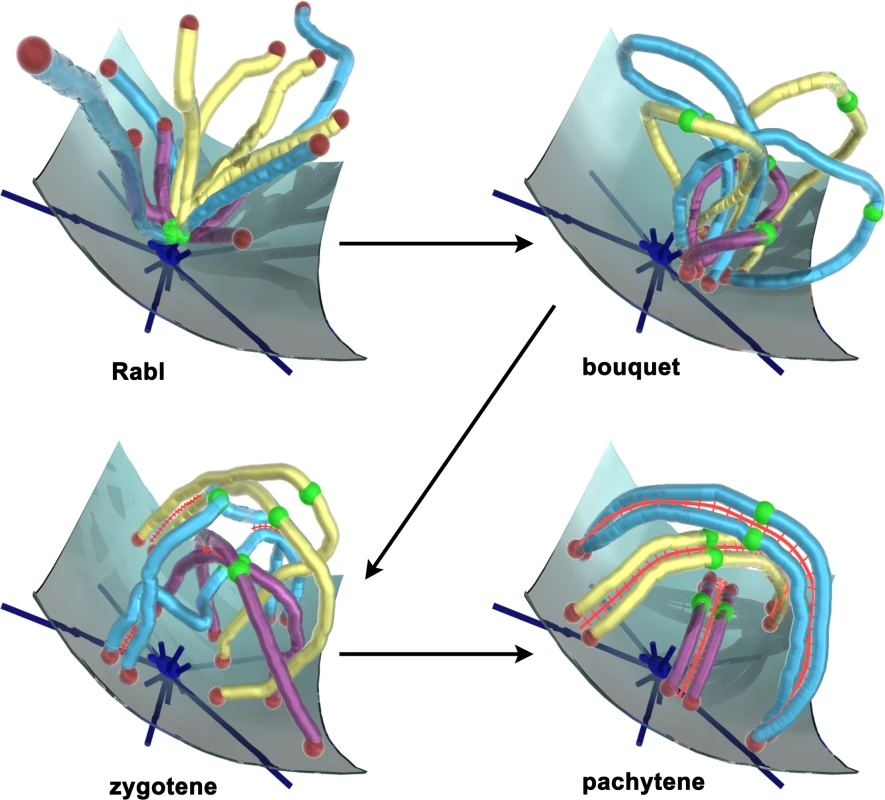
The Rabl orientation is established in the prior division, where centromeres (green balls) are pulled to the spindle pole (dark blue structure with emanating microtubules, anchored in the nuclear membrane), with the telomeres (red balls) trailing. Early in prophase, centromeres move away from the pole while telomeres attach to the nuclear membrane and move to a small area adjacent to the spindle pole, forming the bouquet. Chromosomes move out of the bouquet as the synaptonemal complex forms (red ladder-like structure), marking entry into zygotene and generating interlocks, where chromosomes are trapped between synapsing pairs. Completion of synapsis and resolution of interlocks marks pachytene, where chromosome pairs appear well separated. Movements that persist throughout meiotic prophase were first described by Hiraoka's group in the fission yeast Schizosaccharomyces pombe where, following bouquet formation, telomeres remain at the spindle pole while it leads the nucleus along microtubules, back and forth through the cell, until just before the first meiotic division [4]. Meiotic prophase is noncanonical in S. pombe in that synaptonemal complexes are not formed and recombination is not regulated to avoid forming crossovers near one another (i.e., there is no positive crossover interference). This has led some to question the generality of persistent movements. Recently, however, similarly persistent rapid prophase movements (RPMs) have been described in the budding yeast Saccharomyces cerevisiae [5]–[7]. Although these movements are of individual chromosomes rather than of the whole genomic complement, and although they appear to be promoted by actin rather than by microtubules, each system involves SUN domain–containing proteins that are known to mediate transnuclear envelope linkages, in the present case tethering telomeres to the cytoskeleton [8]–[10]. Such linkages also are present in mammalian meiotic nuclei [11],[12], indicating a widely conserved mechanism and suggesting conserved function(s).
Before its role in these movements was recognized, the budding yeast Ndj1 protein was known to promote bouquet formation, the normal kinetics of SC formation, and the usual pattern of meiotic recombination; to maintain low levels of ectopic recombination (genetic exchanges between homologous DNA sequences in nonallelic locations); and, ultimately, to reduce the frequency of aneuploidy [13]–[17]. Ndj1 also plays a role in anchoring telomeres to the inner nuclear envelope [15], apparently by stabilizing the association of telomeres with the transmembrane SUN protein, Mps3 [10]. Reports that the meiosis-specific budding yeast protein Csm4 is similar to Ndj1 in being required to prevent aneuploidy [18] led the authors of the two current papers to ask, in remarkable molecular detail, whether the meiotic requirements for Csm4 are similar to those for Mps3 and Ndj1. The simple answer is “Yes,” but the angel is in the details.
The authors find that Csm4, unlike Mps3 and Ndj1, is not required to anchor telomeres to the nuclear envelope but is required for telomeres to engage in the RPMs (see [7],[8]). Nevertheless, the impact on the progress of recombination, in all its currently understood molecular intricacies, is similar—delays in the appearances of recombination intermediates begin very early in prophase and persist or lengthen as prophase progresses. The implication of these observations is that the RPMs are the critical factor rather than telomere tethering to the nuclear envelope per se. So then, what is the role of the RPMs? Here, the authors diverge somewhat in their answers. The paper from the Shinohara lab proposes that RPMs promote the biochemistry of recombination more or less directly, perhaps by affecting chromosome structure [1]. The paper from the Alani and Kleckner labs proposes that RPMs function during an early phase when the cell determines which early recombination intermediates will become crossovers. They suggest that delays in this phase, perhaps due to a requirement for Ndj1 and Csm4 to resolve chromosome interlocks (at zygotene, see Figure 1), generates the subsequent defects [2]. Tests of these hypotheses will require considerable ingenuity in experimental design.
A simple and striking conclusion from these papers is that mechanical energy, pumped into the nucleus via the telomeres, contributes critically to the work of genetic recombination. Identification of the MNC complex (Mps3, Ndj1, Csm4) in Sa. cerevisiae and of related structures and pathways in other organisms is only the beginning to understanding how these transnuclear envelope tethers are constructed and regulated. Understanding how these connections and the movements they foster contribute to the faithful segregation of chromosomes in meiosis will be challenging and rewarding, like the middle school dance.
Zdroje
1. KosakaH
ShinoharaM
ShinoharaA
2008 Csm4-dependent telomere movement on nuclear envelope promotes meiotic recombination. PLoS Genet 4(9) e1000196doi:10.1371/journal.pgen.1000196
2. WanatJJ
KimKP
KoszulR
ZandersS
WeinerB
2008 Csm4, in collaboration with Ndj1, mediates telomere-led chromosome dynamics and recombination during yeast meiosis. PLoS Genet 4(9) e1000188doi:10.1371/journal.pgen.1000188
3. ZicklerD
KlecknerN
1998 The leptotene-zygotene transition of meiosis. Annu Rev Genet 32 619 697
4. ChikashigeY
DingDQ
FunabikiH
HaraguchiT
MashikoS
1994 Telomere-led premeiotic chromosome movement in fission yeast. Science 264 270 273
5. Trelles-StickenE
AdelfalkC
LoidlJ
ScherthanH
2005 Meiotic telomere clustering requires actin for its formation and cohesin for its resolution. J Cell Biol 170 213 223
6. ScherthanH
WangH
AdelfalkC
WhiteEJ
CowanC
2007 Chromosome mobility during meiotic prophase in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 104 16934 16939
7. KoszulR
KimKP
PrentissM
KlecknerN
KameokaS
2008 Meiotic chromosomes move by linkage to dynamic actin cables with transduction of force through the nuclear envelope. Cell 133 1188 1201
8. ConradMN
LeeCY
ChaoG
ShinoharaM
KosakaH
2008 Rapid telomere movement in meiotic prophase is promoted by NDJ1, MPS3, and CSM4 and is modulated by recombination. Cell 133 1175 1187
9. ChikashigeY
HaraguchiT
HiraokaY
2007 Another way to move chromosomes. Chromosoma 116 497 505
10. ConradMN
LeeCY
WilkersonJL
DresserME
2007 MPS3 mediates meiotic bouquet formation in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 104 8863 8868
11. SchmittJ
BenaventeR
HodzicD
HoogC
StewartCL
2007 Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proc Natl Acad Sci U S A 104 7426 7431
12. DingX
XuR
YuJ
XuT
ZhuangY
2007 SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev Cell 12 863 72
13. ChuaPR
RoederGS
1997 Tam1, a telomere-associated meiotic protein, functions in chromosome synapsis and crossover interference. Genes Dev 11 1786 1800
14. ConradMN
DominguezAM
DresserME
1997 Ndj1p, a meiotic telomere protein required for normal chromosome synapsis and segregation in yeast [see comments]. Science 276 1252 1255
15. Trelles-StickenE
DresserME
ScherthanH
2000 Meiotic telomere protein Ndj1p is required for meiosis-specific telomere distribution, bouquet formation and efficient homologue pairing. J Cell Biol 151 95 106
16. GoldmanAS
LichtenM
2000 Restriction of ectopic recombination by interhomolog interactions during Saccharomyces cerevisiae meiosis. Proc Natl Acad Sci U S A 97 9537 9542
17. WuHY
BurgessSM
2006 Ndj1, a telomere-associated protein, promotes meiotic recombination in budding yeast. Mol Cell Biol 26 3683 3694
18. RabitschKP
TothA
GalovaM
SchleifferA
SchaffnerG
2001 A screen for genes required for meiosis and spore formation based on whole-genome expression. Curr Biol 11 1001 1009
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2008 Číslo 9- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Hypogonadotropní hypogonadismus u žen a vliv na výsledky reprodukce po IVF
- Molekulární vyšetření pro stanovení prognózy pacientů s chronickou lymfocytární leukémií
-
Všechny články tohoto čísla
- Novel Mutations in (TDP-43) in Patients with Familial Amyotrophic Lateral Sclerosis
- Sex-Specific Genetic Structure and Social Organization in Central Asia: Insights from a Multi-Locus Study
- Missense Mutation in Exon 2 of SLC36A1 Responsible for Champagne Dilution in Horses
- Chromosome Mechanics and Meiotic Engine Maintenance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Missense Mutation in Exon 2 of SLC36A1 Responsible for Champagne Dilution in Horses
- Novel Mutations in (TDP-43) in Patients with Familial Amyotrophic Lateral Sclerosis
- Sex-Specific Genetic Structure and Social Organization in Central Asia: Insights from a Multi-Locus Study
- Chromosome Mechanics and Meiotic Engine Maintenance
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



