-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Minimum inhibitory concentrations of erythromycin and other antibiotics for Czech strains of Bordetella pertussis
Minimální inhibiční koncentrace erytromycinu a dalších antibiotik u českých kmenů Bordetella pertussis
Cíl práce:
Vyšetření citlivosti k antibiotikům volby a alternativám u 70 kmenů Bordetella pertussis (B. pertussis) izolovaných od pacientů s dávivým kašlem v letech 1967 až 2010 v rámci národní surveillance pertuse v České republice (ČR).Materiál a metodika:
Minimální inhibiční koncentrace (MIC) erytromycinu, klaritromycinu, azitromycinu, ciprofloxacinu a kotrimoxazolu byla vyšetřena referenční diluční agarovou metodou, na Bordet-Gengou agaru s 15 % defibrinované ovčí krve.Výsledky:
Všech 70 kmenů vyšetřovaného souboru bylo inhibováno dvěma koncentracemi erytromycinu a azitromycinu (0,06 a 0,12 mg/l), klaritromycin inhiboval kmeny ve třech koncentracích (0,03; 0,06 a 0,12 mg/l), nejvyšší koncentrace v rozmezí MIC byla u těchto tří podobných antibiotik identicky 0,12 mg/l. Koncentrace erytromycinu inhibující 90 % kmenů byla o jeden stupeň ředění dvojnásobně geometrické řady nižší (0,06 mg/l) než byla MIC90 u klaritromycinu a azitromycinu (0,12 mg/l). Všechny kmeny inhibovala jediná koncentrace 0,06 mg/l ciprofloxacinu a dvě koncentrace kotrimoxazolu (0,12 a 0,25 mg/l).Závěr:
Soubor 70 českých kmenů B. pertussis se podle výsledků jeví jako homogenní co se týče MIC antibiotik, neboť všechny kmeny byly inhibovány dvěma/třemi nízkými koncentracemi. Nebyl tudíž potvrzen žádný kmen inhibovaný vyšší koncentrací erytromycinu, klaritromycinu, azitromycinu, ciprofloxacinu ani kotrimoxazolu.KLÍČOVÁ SLOVA:
Bordetella pertussis – minimální inhibiční koncentrace (MIC) – antibiotika
Authors: V. Jakubů 1; J. Zavadilová 2; K. Fabiánová 3,4
; P. Urbášková 1
Authors place of work: National Reference Laboratory for Antibiotics, Centre for Epidemiology and Microbiology, The National Institute of Public Health, Prague 1; National Reference Laboratory for Pertussis and Diphtheria, Centre for Epidemiology and Microbiology, The National Institute of Public Health, Prague 2; Department of Infectious Disease Epidemiology, Centre for Epidemiology and Microbiology, The National Institute of Public Health, Prague 3; Department of Epidemiology, Third Faculty of Medicine, Charles University in Prague 4
Published in the journal: Epidemiol. Mikrobiol. Imunol. 64, 2015, č. 1, s. 12-15
Category: Souhrnná sdělení, původní práce, kazuistiky
Summary
Aim of the study:
To test the susceptibility to first-line and alternative antibiotics of 70 Bordetella pertussis (B. pertussis) strains recovered from patients with whooping cough through national pertussis surveillance in the Czech Republic (CR) in 1967–2010.Material and methods:
The minimum inhibitory concentrations (MICs) of erythromycin, clarithromycin, azithromycin, ciprofloxacin, and co-trimoxazole were tested by the reference agar dilution method on Bordet-Gengou agar with 15 % defibrinated sheep blood.Results:
Each of the 70 study strains was inhibited by two concentrations of erythromycin and azithromycin (0.06 and 0.12 mg/l) and by three concentrations of clarithromycin (0.03, 0.06, and 0.12 mg/l), with the highest concentration of the MIC range being 0.12 mg/l for all these similar antibiotics. Tested in a 2-fold geometric dilution series, the concentration of erythromycin required to inhibit 90 % of the study strains (MIC90) was one dilution step lower (0.06 mg/l) than those of clarithromycin and azithromycin (0.12 mg/l). All study strains were inhibited by a single concentration of ciprofloxacin (0.06 mg/l) and two concentrations of co-trimoxazole (0.12 and 0.25 mg/l).Conclusion:
The panel of 70 Czech strains of B. pertussis appears to be homogeneous in terms of the MICs of the antibiotics tested, with two to three low concentrations being effective against all strains. To be inhibited, no strain required a higher concentration of erythromycin, clarithromycin, azithromycin, ciprofloxacin, or co-trimoxazole.KEYWORDS:
Bordetella pertussis – minimum inhibitory concentration (MIC) – antibioticsINTRODUCTION
Erythromycin, a newer macrolide clarithromycin, or the azalide azithromycin are standard first-line therapies in patients with infection caused by Bordetella pertussis (B. pertussis) [1, 2, 3]. Co-trimoxazole [1, 3, 4] or one of the fluoroquinolones [2, 3] are alternatives in intolerant patients. Given the global rise in laboratory confirmed cases of B. pertussis infection and so far sporadic reports of erythromycin resistant strains appearing since the 1990s [5, 6, 7,8], it is vital to pay attention to the epidemiology and biological properties of this species and its resistance to the first-line antibiotics and their alternatives at the national level.
MATERIAL AND METHODS
Bacteria
The minimum inhibitory concentrations (MICs) of the relevant antibiotics were determined for 70 clinical isolates of B. pertussis referred to the National Reference Laboratory for Pertussis and Diphtheria within the national pertussis surveillance programme in 1967–2010. These strains were recovered from patients with clinical pertussis in Prague (22 strains) and the following administrative regions: South Bohemian (27 strains), South Moravian (4 strains), Central Bohemian (1 strain), Liberec (1 strain), North Moravian (1 strain), and East Bohemian (1 strain). The geographical origin of 13 strains could not be identified. The age of the patients ranged from one month to 47 years but remained unknown for 10 samples. B. pertussis isolates from 1967 to 2004 were stored lyophilised while those recovered since 2007 were kept frozen at -70 °C. The strains of B. pertussis were cultured on Charcoal agar (OXOID CZ s.r.o.) plates and incubated under normal air conditions at 35 ± 1 °C for 96 hours. To confirm the species identification, the diagnostic serum for Bordetella pertussis (Remel Ltd, USA) was used in accordance with the manufacturer’s instructions.
Minimum inhibitory concentration (MIC) determination
The MIC was determined using the agar dilution method [9, 10]. The substances of erythromycin, clarithromycin, azithromycin, ciprofloxacin as a representative of the fluoroquinolones, trimethoprim, and sulfamethoxazole with known activity were purchased from the Sigma – Aldrich company (Czech Republic). Using the respective diluents and solvents [10] (for clarithromycin, in accordance with the manufacturer’s instructions), the stock concentrations of 19000 mg/l and 1000 mg/l were obtained from the substances of sulfamethoxazole and the other five antibiotics, respectively. The 1000 mg/l stock concentration of co-trimoxazole containing trimethoprim and sulfamethoxazole at a ratio of 1 : 19 was obtained by mixing 1.5 ml of the 1000 mg/l concentration of trimethoprim and 1.5 ml of the 19000 mg/l concentration of sulfamethoxazole.
Medium
Bordet-Gengou Agar (BGA) (Difco) was prepared in accordance with the manufacturer’s instructions on the day of MIC determination. BGA was cooled to ca 52 °C and added with defibrinated sheep blood (LabMediaServis, Ltd) to a final concentration of 15%. To remain liquefied, the medium was maintained on a water bath at 52 °C before the plates with antibiotics were prepared.
Preparation of plates with antibiotics
On the day when the plates with antibiotics were prepared, 12 working concentrations were obtained from the stock concentrations in a geometric dilution series involving two-fold dilution steps for each antibiotic in a range of 0.02 to 40 mg/l, i.e. 10 times higher than the final concentrations required. The final plate concentrations in a range from 0.002 to 4 mg/l for each antibiotic were obtained by mixing 2 ml of the working concentration with 18 ml of BGA with 15% of sheep blood. The plates were shortly pre-dried before seeding.
Inoculum
Strains of B. pertussis were seeded onto Charcoal agar plates and incubated under normal air conditions at 35 ± 1 °C for 72 hours. The inocula for susceptibility testing were prepared by suspending the colonies in Mueller-Hinton (MH) broth thermostated to 35 °C. The turbidity of the inoculum was adjusted to that of a 0.5 McFarland standard, which corresponds to a bacterial concentration of about 5 x 108 cells/ml.
Seeding and incubation of plates
The surface of each of 12 plates with antibiotics arranged from the lowest to highest concentration was seeded with the inocula of multiple strains using a 36-pin inoculator. Two plates containing BGA with 15% of sheep blood without antibiotics were added to each series as growth controls (one was seeded before and the other after seeding the plates with antibiotics). The final concentration of the inoculum at the pin print on the test medium was about 5 x 105 cells/ml. Thirty-five test strains and one control strain were used in each series. The seeded plates were incubated upside down under normal air conditions at 35 °C ± 1 °C for 72 hours.
Quality kontrol
For the purpose of antibiotic dilution quality control, Streptococcus pneumoniae (S. pneumoniae) ATCC 49619 with the known MICs of the antibiotics tested was used [11]. The MIC of this control strain was determined using the same protocol as was used for the test strains, except for the preparation of the inoculum from the Columbia agar plate.
Evaluation
After incubation, the antibiotic concentration which clearly inhibited the growth of the strain tested was read. This concentration was considered as the MIC.
RESULTS
All 70 strains tested for antibiotic susceptibility showed good growth on the control medium BGA with 15% of sheep blood. The MICs of four antibiotics tested for the control strain S. pneumoniae ATCC 49619 were in the required range reported for this strain [11]: erythromycin 0.06 mg/l, azithromycin 0.06 mg/l, clarithromycin 0.03 mg/l, and co-trimoxazole 0.5 mg/l. The MIC of ciprofloxacin, which has not been indicated for this control strain, was 0.5 mg/l. It means that the concentrations of antibiotics in the test media were in accordance with those stated.
On the test medium, BGA with 15% of defibrinated sheep blood, the strains investigated were clearly inhibited by the respective concentrations of the antibiotics and therefore, the MICs were easily read.
The MIC ranges and MICs90 of erythromycin, clarithromycin, azithromycin, ciprofloxacin, and co-trimoxazole for 70 strains of B. pertussis are given in Table 1. All strains of the panel investigated were inhibited by two concentrations of erythromycin and azithromycin (0.06 and 0.12 mg/l) and by three concentrations of clarithromycin (0.03, 0.06, and 0.12 mg/l), with the highest MICs of these three similar antibiotics reaching equally 0.12 mg/l. The concentration of erythromycin inhibiting 90 % of the strains was one dilution step of the two-fold geometric series lower (0.06 mg/l) than the MICs90 of clarithromycin and azithromycin (0.12 mg/l). All strains were inhibited by a single concentration of ciprofloxacin (0.06 mg/l) and two concentrations of co-trimoxazole (0.12 and 0.25 mg/l).
Tab. 1. Rozmezí minimálních inhibičních koncentrací (MIC) a MIC90 u 70 kmenů Bordetella pertussis Table 1. MIC ranges and MICs90 for 70 strains of Bordetella pertussis 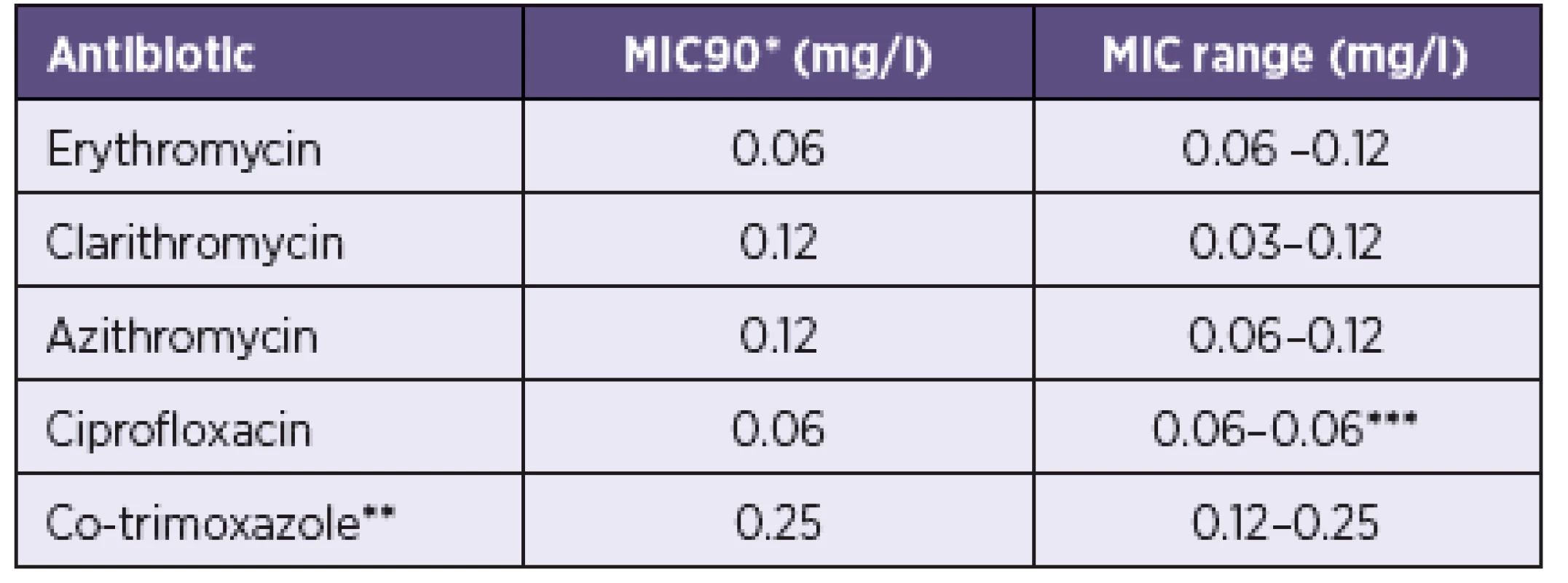
*MIC90: concentration inhibiting 90 % of the strains **trimethoprim and sulfamethoxazole at a ratio of 1:19 ***all strains were inhibited by a single concentration
*MIC90 koncentrace, která inhibuje 90 % kmenů **trimetoprim a sulfamethoxazol v poměru 1 : 19 ***všechny kmeny inhibovala jediná koncentraceDISCUSSION
Despite a generally good response of patients with B. pertussis infection to erythromycin therapy, which was later replaced, due to side effects, by better tolerated clarithromycin or azithromycin therapy [2, 3, 4, 12], sporadic cases of treatment failure have been reported. A detailed analysis showed that the most probable cause of treatment failure was mostly low immunocompetence of the patients infected by a B. pertussis strain that was inhibited in vitro by low concentrations of the clinically relevant antibiotics used in the treatment of this infection [4, 13]. Nevertheless, since 1982, sporadic strains of B. pertussis resistant to erythromycin have been reported [6, 7, 8, 14, 15, 16]. As such resistant strains result from mutation in the 23S rRNA gene [5], their epidemic spread is unlikely, in contrast to when the resistance is confer-red by plasmid-bound genes. Nevertheless, given the heterogeneous nature of their resistance, such strains may escape attention.
Considering the good clinical effect of the first-line antibiotics and expected susceptibility of the causative agent to these and many other antibiotics, it is not recommended to perform routine antibiotic susceptibility testing, since a standard method has not yet been available and the results are subject to inherent errors and suffer from low reproducibility. The reason lies in low nutritional requirements and slow growth of B. pertussis. No general guidelines are available concerning the method to be used for B. pertussis susceptibility testing, culture medium suitable for this purpose, type and concentration of blood to be added to the medium, and concentration of the inoculum [12, 17, 18]. Despite the discordances, it is recommended that the surveillance of antibiotic susceptibility of B. pertussis strains recovered from a geographically defined area be performed by a laboratory, which is experienced and skilled in MIC determination of fastidious bacteria. The culture medium and blood type and its quantity to be added to the medium should be selected to allow for adequate growth of strains and, at the same time, not to interfere with the efficacy of the antimicrobial tested. Such approach makes it possible to obtain results indicative of the dynamics of antimicrobial activity in a defined bacterial population and to detect the strains that are inhibited by higher concentrations of antibiotics.
Of the three culture media most often used for B. pertussis susceptibility testing, the most suitable appears to be MH agar with 5% of blood [1, 18, 19, 20] whose composition does not affect the activity of antimicrobials, thus being the medium of choice for fastidious bacteria in accordance with both the European [21] and US authorities for [10] standardization of laboratory methods. Nevertheless, despite meeting all conditions required, only half of our panel of 70 strains yielded adequate and reliable growth when seeded onto this culture medium. The failure to grow on MH agar with blood may have been related to the age of strains recovered between 1967 and 2010. Therefore, BGA with 15% of sheep blood was used for B. pertussis susceptibility testing where all panel strains showed good growth. The MICs of antimicrobials were determined by the agar dilution method in compliance with the standard ČSN [9] using the globally recognized standard protocol [10]. This is the reference method for MIC determination which provides the basis for all other susceptibility testing methods. Our results of MIC ranges and MICs90 of erythromycin, clarithromycin, azithromycin, ciprofloxacin, and co-trimoxazole are identical to those reported by others on the same culture medium [5, 8, 17, 18, 22] and are also in accordance with those obtained on MH agar [17, 18, 19, 20]. Based on our results, the panel of 70 Czech strains of B. pertussis appears to be homogeneous in terms of MICs, as all strains were inhibited by one to three low concentrations of antibiotics. None of the panel strains was inhibited by a higher concentration of erythromycin or any of the other four antibiotics tested.
Acknowledgement
The work was supported by research grant NT/14058-3 of the Internal Grant Agency of the Ministry of Health of the Czech Republic and by grant of the Ministry of Health, Czech Republic – conceptual development of research organization („The National Institute of Public Health – NIPH, 75010330“). The authors wish to express their thanks to Iveta Vrbová and Michaela Horáková from the National Reference Laboratory for Antibiotics, NIPH for their outstanding technical assistance.
Do redakce došlo dne 17. 12. 2014.
Corresponding author:
Vladislav Jakubů, MS
National Reference Laboratory for Antibiotics
Centre for Epidemiology and Microbiology
The National Institute of Public Health
100 42, Prague 10, Šrobárova 48
Czech Republic
E-mail: vjakubu@szu.cz
Zdroje
1. Hoppe JE. State of art in antibacterial susceptibility of Bordetella pertussis and antibiotic treatment of pertussis. Infection, 1998;26(4):242-246.
2. Mandell, Douglas, and Bennett‘s. Principles and Practice of Infectious Diseases. 7th edition. Mandell GL, Bennet JE, Dolin R (eds). Churchill Livingstone, Elsevier 2010.
3. John Hopkins Poc-it Guides. [online] [cit. 2014-11-11] Dostupné na: http://www.hopkinsguides.com/hopkins/ub/index/Johns_Hopkins_ABX_Guide/.
4. Hoppe JE. Update of epidemiology, diagnosis, and treatment of pertussis. Eur J Clin Microbiol Infect Dis, 1996;15(3)189–193.
5. Bartkus JM, Juni BA, Ehresmann K et al. Identification of a mutation associated with erythromycin resistance in Bordetella pertussis: implications for surveillance of antimicrobial resistance. J Clin Microbiol, 2003;41(3):1167–72.
6. Lewis K, Saubolle MA, Tenover FC et al. Pertussis caused by an erythromycin resistant strain of Bordetella pertussis. Pediatr Infect Dis J, 1995;14(5):388–91.
7. Korgenski K, Daly JA. Surveillance and detection of erythromycin resistance in Bordetella pertussis isolates recovered from a pediatric population in the Intermountain West Region of the United States. J Clin Microbiol, 1997;35(11):2989–2991.
8. Yao SM, Liaw GJ, Chen YY et al. Antimicrobial susceptibility testing of Bordetella pertussis in Taiwan prompted by a case of pertussis in a paediatric patient. J Med Microbiol, 2008;57(12):1577–1580.
9. Česká technická norma ČSN EN ISO 20776-1. Klinické a laboratorní zkoušky a zkušební systémy pro diagnostiku in vitro-Zkoušení citlivosti původců infekcí a hodnocení účinnosti prostředků pro stanovení antimikrobiální citlivosti-Část 1: referenční metody pro zkoušení aktivity antimikrobiálních činidel in vitro proti rychle rostoucím aerobním bakteriím způsobujícím infekční nemoci. Český normalizační institut 2007. (Clinical laboratory testing and in vitro diagnostic test systems - Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices - Part 1: Reference methods for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. Czech Standardization Institute 2007.)
10. Clinical and Laboratory Standards Institute. M07-A9. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically;approved standard-ninth edition (2012). Clinical and Laboratory Standards Institute, Wayne, Pa.
11. European Committee on Antimicrobial Susceptibility Testing. EUCAST Quality Control. EUCAST routine QC tables v 4.0, valid from 2014-09-05. [online] [cit. 2014-10-25] Dostupné na www: http://www.eucast.org/antimicrobial_susceptibility_testing/qc_tables/
12. Mertsola J, He Q. Bordetella pertussis (Whooping Cough) and other species. Infec Dis Antimicrobial Agents. [online] [cit. 2014-11-07] Dostupné na www: http://www.antimicrobe.org/b83.asp
13. Halsey N, Welling MA, Lehman RM. Nosocomial pertussis: a failure of erythromycin treatment and prophylaxis. Am J Dis Child, 1980;134(5):521–522.
14. Bannatyne RM, Cheung R. Antibiotic resistance of degraded strains of Bordetella pertussis. Antimicrob Agents Chemother, 1984;25(4):537-8.
15. Bannatyne RM, Cheung R. Antimicrobial susceptibility of Bordetella pertussis strains isolated from 1960 to 1981. Antimicrob Agents Chemother, 1982;21(4):666–667.
16. Hill BC, Baker CN, Tenover FC. A simplified method for testing Bordetella pertussis for resistance to erythromycin and other antimicrobial agents. J Clin Microbiol, 2000;38(3):1151–1155.
17. Hoppe JE, Tschirner T. Comparison of media for agar dilution susceptibility testing of Bordetella pertussis and Bordetella parapertus-sis. Eur J Clin Microbiol Infect Dis, 1995;14(9):775–779.
18. Hoppe JE, Paulus T. Comparison of three media for agar dilution susceptibility testing of Bordetella pertussis using six antibiotics. Eur J Microbiol Infect Dis, 1998;17(6):391–393.
19. Bourgeois N, Ghnassia JC, Doucet-Populaire F. In vitro activity of fluoroquinolones against erythromycin-susceptible and – resistant Bordetella pertussis. J Antimicrob Chemother, 2003;51(3):742–743.
20. Fry NK, Duncan J, Vaghji L et al. Antimicrobial susceptibility testing of historical and recent clinical isolates of Bordetella pertussis in the United Kingdom using the Etest method. Eur J Microbiol Infect Dis, 2010;29(9):1183–1185.
21. European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 4.0, valid from 2014-01-01. [online] [cit. 2014-10-15] Available at http://www.eucast.org/antimicrobial_susceptibility_testing/breakpoints/.
22. Kurzynski TA, Boehm DM, Rott-Petri JA et al. Antimicrobial susceptibilities of Bordetella species isolated in a Multicenter Pertussis Surveillance Project. Antimicrob Agents Chemother, 1988;32(1):137-40.
Štítky
Hygiena a epidemiologie Infekční lékařství Mikrobiologie
Článek vyšel v časopiseEpidemiologie, mikrobiologie, imunologie
Nejčtenější tento týden
2015 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
-
Všechny články tohoto čísla
- Minimum inhibitory concentrations of erythromycin and other antibiotics for Czech strains of Bordetella pertussis
- Contribution of the detection of IgA antibodies to the laboratory diagnosis of mumps in the population with a high vaccination coverage
- A case of tuberculous meningitis associated with persistently reduced CD4+ T lymphocyte counts
- Impact of climate changes on the incidence of tick-borne encephalitis in the Czech Republic in 1982–2011
- Editorial
- A multifactor epidemiological analysis of risk factors for pancreatic cancer in women
- Macrolide resistance in Treponema pallidum subsp. pallidum in the Czech Republic and in other countries
- The population’s attitudes to colorectal cancer screening in the Czech Republic
- Prevalence of selected congenital anomalies in the Czech Republic: congenital anomalies of the central nervous system and gastrointestinal tract
- Prof. MUDr. Jan Šejda, DrSc. – malé připomenutí životního jubilea
- XXIV. Tomáškovy dny mladých mikrobiologů
-
Procházka Bohumír
STRUČNÁ BIOSTATISTIKA PRO LÉKA
- Epidemiologie, mikrobiologie, imunologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Macrolide resistance in Treponema pallidum subsp. pallidum in the Czech Republic and in other countries
- Contribution of the detection of IgA antibodies to the laboratory diagnosis of mumps in the population with a high vaccination coverage
- Minimum inhibitory concentrations of erythromycin and other antibiotics for Czech strains of Bordetella pertussis
- A case of tuberculous meningitis associated with persistently reduced CD4+ T lymphocyte counts
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



