-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Influenza in seasons 2009-2013 in the Faculty Hospital Hradec Kralove, East Bohemia
Chřipka v sezonách 2009-2013 ve Fakultní nemocnici Hradec Králové, východní Čechy
Cíle:
Porovnat čtyři postpandemické chřipkové sezony 2009–2013 ve Fakultní nemocnici Hradec Králové a porovnat používané rychlé testy detekce antigenů (RAT) s výsledky z real time RT-PCR.Materiál a metody:
V období mezi listopadem 2009 a červnem 2013 bylo vyšetřeno celkem 3845 vzorků od pacientů s respiračními obtížemi za použití rychlých testů detekce antigenů (Influenza A/B 2 Panel Test (GECKO® Pharma, Germany), Rapid VIDITEST Influenza A + B Card (VIDIA®, Czech Republic) a BinaxNOW Influenza A&B Test (ALERE®, USA) nebo za použití real time RT-PCR (RTR InfA/H1N1 Detection Set (Roche®), RealStar® Influenza S a T RT-PCR Kit 3.0 (Altona®, Germany).Výsledky:
Celkem 1059 vzorků bylo současně vyšetřeno metodou RAT a real time RT-PCR. Celková senzitivita a specificita použitých RAT v porovnání s PCR byla 32,2 % a 98,1 % pro chřipku A a 17,6 % a 99,4 % pro chřipku B. Vyšší senzitivita RAT byla zaznamenána u dětí (66,6 %) než u dospělých (14,3–40,0 %). V prvních třech postpandemických sezonách byl zaznamenán pozvolný pokles pozitivních vzorků z 23,5 % v sezoně 2009–2010 k 3,3 % v sezoně 2011–2012. Ale v poslední chřipkové sezoně 2012–2013 došlo k rapidnímu nárůstu pozitivních záchytů na 31,5 %, navíc s vysokým procentem podílu pandemického kmene chřipky A/H1N1/2009 (79,6 %).Závěr:
Naše výsledky potvrdily nedostatečnou senzitivitu všech používaných testů rychlé detekce antigen, a potvrdily tak nezbytnost rutinního používání konfirmačních metod, jako např. real time RT-PCR. Taktéž byl v naší studii zaznamenán rapidní nárůst případů chřipky v poslední sezoně, který nebyl doposud nijak vysvětlen.Klíčová slova:
chřipka – metoda PCR – immunochromatografická assays
Authors: M. Fajfr 1,2; V. Štěpánová 2; L. Plíšková 3
Authors place of work: Faculty of Military Health Sciences, University of Defence, Hradec Králové 1; Institute of Clinical Microbiology, University Hospital in Hradec Králové, Hradec Králové 2; Institute of Clinical Biochemistry and Diagnostics, Department of Molecular Biology, University Hospital Hradec Králové, Hradec Králové 3
Published in the journal: Epidemiol. Mikrobiol. Imunol. 63, 2014, č. 1, s. 10-16
Category: Souhrnná sdělení, původní práce, kazuistiky
Summary
Purpose:
The evaluation of four post-pandemic influenza seasons 2009–2013 in the Faculty hospital Hradec Králové and comparison of used rapid antigen tests (RATs) with real time RT-PCR.Material and methods:
Between November 2009 and June 2013 were examined 3845 samples from patients with respiratory tract infections by RATs (Influenza A/B 2 Panel Test (GECKO® Pharma, Germany), Rapid VIDITEST Influenza A+B Card (VIDIA®, Czech Republic) and BinaxNOW Influenza A&B Test (ALERE®, USA) or real time RT-PCR (RTR InfA/H1N1 Detection Set (Roche®), RealStar® Influenza S and T RT-PCR Kit 3.0 (Altona ®, Germany).Results:
A totally 1059 samples were examined simultaneously by RAT and real time RT-PCR. The overall sensitivity and specificity of RATs compared with real time RT-PCR were 32,2 % and 98,1 % for influenza A and 17,6 % and 99,4 % for influenza B. Higher sensitivity of RATs were in children (66,6 %) compared with adults (14,3 – 40,0 %). In the first three post-pandemic seasons were continuously decrease of positive samples from 23,5 % in season 2009–2010 to 3,3% in season 2011–2012, but in season 2012–2013 were rapidly increase of positive results, to 31,5%, with high share of influenza A/H1N1/2009 (79,6%).Conclusion:
Our results shown insufficient sensitivity of all used RATs and necessity of having other confirmatory test, like RT-PCR. It was also shown unexplained increase of case and influenza severity in season 2012–2013.Keywords:
influenza – PCR – immunochromatographic assaysINTRODUCTION
Influenza viruses belong to the most important respiratory pathogens worldwide. Seasonal influenza annually result in about three to five million severe cases and about 0,25 to 0,5 millions cases ending fatal [1]. Seasonal influenza is mainly dangerous for elder, very young, chronically or critically ill patients. In variously long periods pandemic influenza appears which caused worldwide pandemic with rapid increase of deaths. The last pandemic influenza virus was appeared in 2009 in Mexico (Influenza A/H1N1/2009). During 2009 this virus was identified in more than 213 countries and caused 17,700 deaths with relation of confirm Influenza A/H1N1/2009 infection [2]. But the real numbers of deaths could be much higher, Dawood et al. assumed worldwide about 201,200 respiratory deaths and about 83,300 cardiovascular deaths with direct association with pandemic influenza during first 12 month of pandemic [3]. In 2010 WHO decided, due to decreasing incidence of pandemic influenza strain in population and milder course of infection, that pandemic passed into post-pandemic phase, which remain until now [4, 5]. Pandemic influenza appeared to be very important nosocomial infection which rapidly worsened morbidity and mortality of severe hospitalised patients, like transplant patients or patient on ICUs [6, 7].
Rapid diagnosis of influenza is essential for adequate anti-epidemic counter-measure and antiviral therapy initiate. Rapid antigen detection tests (RATs), EIA or immunochromatographic, are widely used in most clinical laboratories for its simplicity and rapidity – results available in 15 min [7, 8]. In some studies was confirmed low sensitivity of these assays for pandemic influenza detection which limited their application for clinical use [9, 10]. Shell vial culture in combination with fluorescent antibodies can be also useful diagnostic method with rapid result in 24 to 48 hours, but in routine clinical laboratories is this method used sporadically [11]. Relatively common methods for seasonal influenza virus detection are Reverse transcription polymerase chain reaction (RT-PCR) assays. For their high sensitivity and specificity are these methods used as a gold standard in influenza virus detection. However RT-PCR needs special equipment and expertise (especially for in-house RT-PCR protocols) and examinations are more expensive compared with rapid antigen detection assays and especially in conventional RT-PCR also more time-consuming [2]. In short period after pandemic influenza outbreak starting, Real time RT-PCR assays for new pandemic influenza A virus were developed [9, 11].
In this retrospective unicentric study was evaluated four influenza seasons 2009/2010, 2010/11, 2011/12 and 2012/13. The main aims were to compare of several rapid antigen detection assays with RT--PCR used in laboratories of Faculty hospital Hradec Králové. We also try to compare the different, in severity and percentage proportion of influenza A/H1N1/2009 from detected influenza viruses, last season with previous ones.
MATERIAL AND METHODS
Clinical materials – to our study were admitted 3845 samples from patients with respiratory tract infection. The most of study samples (96.6%, n = 3 715) were from patient hospitalised or cured in Faculty hospital Hradec Králové, only small part (3.4%, n = 130) were from hospitals in Eastern Bohemia. The evaluation season for the study condition was determined the wide period from the first of November to the end of June next year. Examinations for influenza virus outside these periods were only sporadically and also positive results were very unusual. Samples used for immunochromatographic tests were nasal, nasopharyngeal swabs and laryngeal swabs. Materials used for PCR detections were in addition to this also fluids from broncho-alveolar lavage, tracheal aspirates and also section materials.
Immunochromatographic tests (ICT) – During study period were used three immunochromatographic tests: Influenza A/B 2 Panel Test (GECKO® Pharma, Germany), used in seasons 2009–2010 and 2010–2011; Rapid VIDITEST Influenza A + B Card (VIDIA®, Czech Republic) used shortly in season 2011–2012 and BinaxNOW™ Influenza A&B Test (ALERE®, USA), used from 11/2011. Immunochromatographic assays detected HA (hem-agglutinin) antigens (VIDIA) or NP (nuclear proteins) antigens (GECKO, ALERE). All used tests were certificated only for nasal or nasopharyngeal swabs or lavage. All immunochromatographic tests were used in accordance with manufacture manuals.
PCR assays – For PCR detection samples nasopharyngeal swabs, broncho-alveolar lavage and tracheal aspirates, rarely tracheal tissue (post mortem) were used. RNA was from samples isolated by QIAamp Viral RNA Mini Kit (Qiagene®, Germany) according to manufacturer instructions. Up to September 2012 were used real time RT-PCR kit RTR InfA/H1N1 Detection Set (Roche®), able to detect influenza A virus (gene for M2 protein) and in second reaction influenza A/H1N1/2009 (gene for A/H1N1/2009 specific hemaglutinin HA1). From September was used real time RT-PCR kit RealStar® Influenza S and T RT-PCR Kit 3.0 (Altona®, Germany), which used three primer/probe sets with different fluorophores (FAM, Cy5 and ROX) and which was able to determine seasonal influenza A, influenza A/H1N1/2009 and influenza B viruses. All PCR reaction ran in LightCycler 2.0 (Roche®).
RESULTS
In the study were examined 3845 samples from 2421 patients, 52,3% (1266/2421) from men and 47,7% (1155/2421) women, with respiratory tract infection symptoms. Generally were 54.3% (2086/3845) samples examined by immunochromatographic test (ICT), with 8.1% of positive results for influenza A or B (170/2086) and for 45.7% (1759/3845) of samples were used PCR, with 23.3% of positive results for influenza A or B (410/1759) (Table 1). In total 1059 samples were examined by immunochromatographic test and PCR simultaneously. Overall sensitivity of used antigen detection test compared with PCR was calculated in 33.5% for influenza A and 17.6 % for influenza B detection with specificity 98.2 %, respectively 99.4 %. The most positive results (48.2% of all positivities) were obtained from weeks no. 3 to 7. Overall 40.1% (1535/3842) samples were from ICUs, 33.8% (1292/3824) samples were from standard wards and 26.1% (997/3824) samples were from ambulances. Positive samples came 34% from ICU and 33 % from standard wards and ambulances. The group of samples examined simultaneously by PCR and RAT were divided into age groups (0–9, 10–19, etc.). The sensitivity of RATs in children’s and teenager’s group were significantly higher than in adults groups, 66.6% against 14.3–40.0% for influenza A (Table 2).
Tab. 1. Samples split up according to the season and used diagnostic method – polymerase chain reaction (PCR) and rapid antigen test (RAT) Tabulka 1. Rozdělení vzorků podle sezony a použitých metod – polymerázová řetězová reakce (PCR) a testy detekce antigenů (RAT) 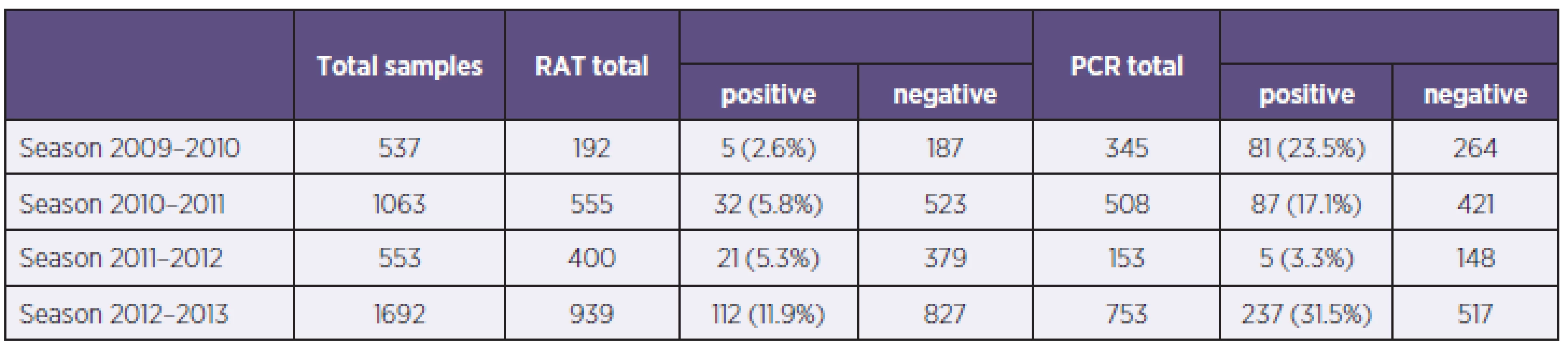
Tab. 2. Sensitivity and specificity of RATs divided according to age groups (NA – data not applicable for any positivity) Tabulka 2. Senzitivita a specificita RAT s rozdělením podle věkových kategorií (NA – data nehodnotitelná pro záchyt pouze negativních vzorků) 0–9 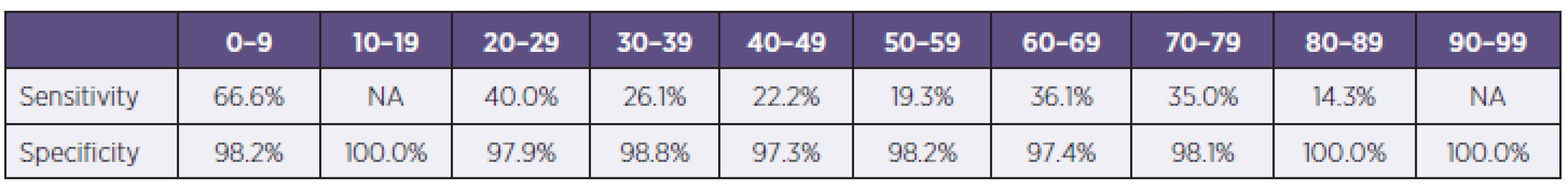
In the season 2009–2010 were examined 537 samples, 345 (64.2%) by PCR and 192 (35.8%) by ICT. There were 5 samples positive by ICT (2.6%), 81 samples were positive by PCR (23.5%) and all positive samples were influenza A, 75 PCR positive samples were positive for influenza A/H1N1/2009 (92.6%, 75/81). The most PCR positive samples came from weeks no. 47 to 52 (Graph 1). Generally were 86 patients hospitalised and 65 of them had severe course with complications, 8 patients finally died with association of influenza infection. Calculated sensitivity of ICT for influenza A was 33.3% with specificity 97.8% compared with PCR (Table 3).
Graph 1. PCR positive samples according to seasons and weeks number Graf 1. PCR pozitivní vzorky s rozdělením podle sezon a týdne záchytu 
Tab. 3. Sensitivity and specificity of used RATs divided according to seasons (NA – data not applicable for any positivity, FLUA – all influenza A strains, FLUB – influenza B virus) Tabulka 3. Senzitivita a specificita použitých RAT s rozdělením podle jednotlivých sezon (NA – data nehodnotitelná pro záchyt pouze negativních vzorků, FLUA – chřipka A, FLUB – chřipka B) 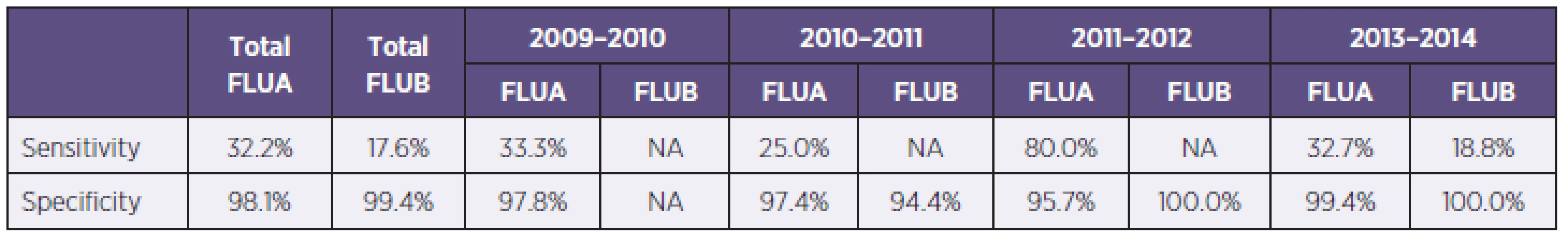
In season 2010–2011 there were examined 1063 samples, 555 (52.2%) by ICT, with 30 (5.4%) of influenza A and 2 (0.4%) of influenza B positive results, and 508 (47.8%) by PCR, with 16.9% (n = 86) of positivity for influenza A and 0.2% (n = 1) for influenza B. The most PCR positive results were from weeks no. 6 to 8 and 11. Determined sensitivity and specificity of ICT were for influenza A 25.0% resp. 97.4% (see Table 3).
In season 2011–12 there were examined 553 samples, 400 (72.3%) by ICT and 153 (27.7%) by PCR. There were 21 (5.3%) samples positive by ICT and 5 (3.3%) positivities by PCR. In this season were 2 cases of severe influenza hospitalised in Faculty hospital Hradec Králové and no death in association with influenza. There were none Influenza virus A/H1N1/2009 captured. Determined sensitivity of ICT for influenza A was 80.0 % with 95.7% specificity (see Table 3).
In season 2012–2013 were examined 1692 samples, 939 (55.5%) by ICT with 112 (11.9%) positive results, 86 for influenza A and 26 for influenza B. 753 samples (44.6%) were examined by PCR with 237 (31.5%) positive results, 201 for influenza A and 36 for influenza B. (Figure 2).The most PCR samples were from weeks 3 to 8. Generally were 117 patients with influenza hospitalized, 58 of them were severe cases. In this season were in our hospital 10 deaths associated with influenza infection. From severe influenza cases were 12 (20.7%) nosocomial. For influenza virus A/H1N1/2009 were examined 167 samples and 133 (79.6%) had positive results for pandemic strain and 34 samples (20.4 %) for other Influenza A strains (H1N1/non pandemic or H3N2). Determined sensitivity of ICT was for influenza A 32.7% and for influenza B 18.8%, specificity of these tests were 99.4% resp. 100.0 % (see Table 3).
Graph 2. Overall samples split-up according to hospital ward Graf 2. Celkové počty vzorků s rozdělením podle typu zasílajícího oddělení 
DISCUSSION
The Faculty hospital Hradec Králové is the highest health care centre in East Bohemia region with approximately 1500 beds with wide catchment area of health care facilities in eastern part of Bohemia. Diagnostic of influenza infections are made in two laboratory departments – RATs are made in Microbiology department and PCR in Department of molecular biology but all results are assessed by Microbiology department M. D. staff. Despite relatively typical symptoms of flu could be onset of cough and high fever according to published data highly sensitive (87.9%) for A/H1N1/2009 infection but the influenza virus infections specificity of these clinical marks were low (51.3%), there were other diagnostic tests needed for certain diagnosis [2]. Frequently used immunochromatographic assays had reported low sensitivity, from 11% to 83.7% according to used tests and patients groups [12]. In our study was confirmed low sensitivity of commercial immunochromatographic assays, overall sensitivity for influenza A virus was 33.5% and for influenza B poorly 17,6%. The sensitivity of used RATs varied from 25.0% to 80.0% in accordance of used test and seasons. The highest sensitivity of ICT was in season 2011–2012, when was in our region influenza epidemic mild and no A/H1N1/2009 captured in our laboratory, but the same test (BinaxNOW®) had in season 2012–2013, when was in our facility the majority of influenza A caused by pandemic strain, the lowest sensitivity – only 32.7%. This finding correlate with published data, BinaxNOW RAT for seasonal influenza had reported sensitivity from 47.2–80.0%, but for A/H1N1/2009 was the sensitivity from 9.6–60.3%, which can indicate the changing of NP in pandemic influenza [2, 11].
A comparison of all four influenza seasons shown gradual decrease of positive samples from 23.5% in season 2009–2010 to 3.3% in season 2011–2012, which was in accord with WHO and ECDC data of influenza post-pandemic phase. Results from season 2009–2010 shown high prevalence of pandemic influenza (92.6% of positive samples), the same data was captured from other European countries e. g. 91.2% of all positive samples from May 2009 to June 2010 from the Slovak Republic were pandemic strains [13]. In the last influenza season 2012–13 there were captured rapid increase of positivities – 31.5% of all samples and 76.9% of them were influenza A/H1N1/2009 strains. The reason for this discrepancy is still not clear. One of them could be in very low percentage of vaccinated population in our country and also of health care personnel, which could be reason of our nosocomial cases in the last season. Other reason could be in small genetic changes of pandemic influenza, which could allow this new mutation to escape from immune respond. Results from our region correspond with situation in the Czech Republic. Data from National Institute of Public Health (NIPH) confirmed gradual decrease of influenza cases in seasons 2009–2012, from 1938 cases with 86.7% (1681/1938) of influenza A/H1N1/2009 in season 2009–2010 to 302 cases with 4.9% (15/302) of Influenza A/H1N1/2009 in season 2011–2012. The NIPH influenza surveillance system also monitored rapid increase of influenza during season 2012–2013, 3205 cases, with 32.8% (1053/3205) of influenza A/H1N1/2009 [14].
Some data shown possible influence of samples type and patients age for sensitivity of RATs. Raynders et al. shown lower sensitivity in throat swabs in comparison with nasopharyngeal swabs (13% against 34–41.9%) and lower sensitivity was in samples from adults than from paediatrics patients (11.4–20% against 34–41.9%) [9]. In another publication from paediatric population authors shown lower sensitivity of RATs in patients group older than 2 years comparison with children younger than 2 years (43.4% against 57.6%) [15]. But the other authors did not confirmed this age influence [10]. In our study there were no significant differences in sensitivity of RATs among sample kind (nasopharyngeal swabs, tracheal aspirates and bronchoalveolar lavage), but we had significantly higher sensitivity in younger patients groups (66.6%) against adult patients (14.3–40.0%). Data from meta-analysis of 159 studies of influenza infection diagnosis had shown very similar results as was from our study. Pooled sensitivity of RATs was 62.3% with 98.2% specificity. Sensitivity of RATs were highly heterogeneous in relation with age, lower tests sensitivity were in adults (53.9%) than in children (66.6%) and higher sensitivity of RATs for influenza A (64.6%) than for influenza B (52.3%) [16]. The fact of lower sensitivity of used ICT for influenza B than for influenza A was confirmed also in our study, in season 2012–2013 were sensitivity of BinaxNOW test for influenza A 32.7%, but for influenza B poorly 18.8%. This low percentage could be caused by relatively small number of evaluated samples, but the differences between influenza A and B was well noticeable. The influence of correct sampling for sensitivity of RATs for influenza detection was also discussed. In all manufacture manuals of RATs test was emphasized the dilution of swab in small diluents volume, 0.25–0.5 ml. This requirement was also mentioned in our national guideline for influenza diagnosis [17]. It is well known, that using of larger volume rapidly decreases the sensitivity of RATs due to decrease of antigen amount in volume added into test well.
CONCLUSION
Our results showed an insufficient sensitivity of all used RATs and necessity of having other confirmatory test, like RT-PCR. This fact decreases the cost effect of rapid antigen tests wide using. Our results also confirm the dependency of RATs on patients’ age.
Do redakce došlo dne 29. 8. 2013.
Corresponding author:
MUDr. Miroslav Fajfr
Ústav klinické mikrobiologie
Fakultní nemocnice Hradec Králové
Sokolská 581
500 05 Hradec Králové
e-mail: fajfrmiroslav@seznam.cz
Zdroje
1. World Health Organisation[online]. Programmes and projects. Influenza – FluNet [cit. 2013-08-04]. Dostupné na www: http://www.who.int/influenza/gisrs_laboratory/flunet/en/.
2. Choi YJ, Nam HS, Park JS, et al. Comparative analysis of the multiple test methods for the detection of pandemic influenza A/H1N1 2009 virus. J Microbiol Biotechnol, 2010;20 : 1450–1456.
3. Dawood FS, Iuliano AD, Meltzer MI, et al. Estimated global mortality associated with the first 12 month of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis, 2012;12(9):687–695.
4. World Health Organisation[online]. Influenza – Health topic [cit. 2013-08-04]. Dostupné na www: http://www.who.int/topics/influenza/en/.
5. European Centre for Disease Prevention and Control [online]. ECDC Annual Epidemiological reports on communicable diseases in Europe 2009 – 2012[cit. 2013-08-04]. Dostupné na www: http://ecdc.europa.eu/en/publications/surveillance_reports/Pages/index.aspx.
6. Moss RB, Steigbigel RT, Sanders RL, Fang F. Perspective: Emerging challenges in the treatment of influenza and parainfluenza in transplant patients. Adv Virol, 2011;910930.
7. Genzenmueller T, Kluba J, Hilfrich B, et al. Comparison of the performance of direct fluorescent antibody staining, a point-of-care rapid antigen test and virus isolation with that of RT-PCR for the detection of novel 2009 influeza A (H1N1) virus in respiratory specimens. J Med Microbiol, 2010;59 : 713–717.
8. Leonardi GP, Mitrache I, Pigal A, Freedman L. Publichospital-based laboratory experience during an outbreak of pandemic influenza A (H1N1) virus infection. J Clin Microbiol, 2010;48 : 1189–1194.
9. Reynders M, De Foor M, Maaroufi Y et al. Prospective evaluation of Coris Influ-A&B Respi-Strip and of BinaxNOW Influenza A&B assay against viral culture and real-time PCR assay for detection of 2009 pandemic influenza A/H1N1v in Belgian patients. Acta Clin Belg, 2012;67 : 94–98.
10. Self WH, MacNaughton CD, Grijalva CG, et al. Diagnostic performance of the BinaxNow Influenza A&B rapid antigen test in ED patients. Am J Emerg Med, 2012;30(9):1955–1961.
11. Ginocchio C, Zhang F, Manji R et al. Evaluation of multiple test methods for the detection of novel influenza A (H1N1) during the New York City outbreak. J Clin Virol, 2009;45 : 191–195.
12. Cheng VCC, To KKW, Tse H, Hung IFN et Yuen K-Y. Two years after pandemic influenza A/2009/H1N1: What have we learned? Clin Microbiol Rev, 2012;25(2):223–263.
13. Kissová R, Maďarová L, Klement C. Laboratórna diagnostika pandemickej chrípky na Odbore lekárskej mikrobiológie Regionálneho úradu verejného zdravotníctva so sídlom v Banskej Bystrici v sezóne 2009/2010. Epidemiol Mikrobiol Imunol, 2011;60(1):32–37.
14. Státní zdravotní ústav[online]. Chřipka. Zpráva o chřipkové aktivitě, hlášení a výsledky laboratorních vyšetření [cit. 2013-08-26]. Dostupné na: http://www.szu.cz/tema/prevence/hlaseni-a-vysledky.
15. Cruz AT, Demmler-Harrison GJ, Caviness AC, Buffone GJ, Revell PA. Performance of a rapid influenza test in children during the H1N1 2009 influenza A outbreak. Pediatrics, 2010;125;e645.
16. Chatrand C, Leeflang MM, Minion J, Brewer T, Paj M. Accurancy of rapid influenza diagnostic tests: meta-analysis. Ann Intern Med, 2012;156(7):500-511.
17. Státní zdravotní ústav[online]. Národní referenční laboratoř pro chřipku, Surveillance [cit. 2013-08-26]. Dostupné na www: http://www.szu.cz/surveillance-nrl-pro-chripku
Štítky
Hygiena a epidemiologie Infekční lékařství Mikrobiologie
Článek Úvodník
Článek vyšel v časopiseEpidemiologie, mikrobiologie, imunologie
Nejčtenější tento týden
2014 Číslo 1- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
-
Všechny články tohoto čísla
- Influenza in seasons 2009-2013 in the Faculty Hospital Hradec Kralove, East Bohemia
- Bacteria of the Burkholderia cepacia complex: epidemiology and diagnosis of infection in patients with cystic fibrosis
- Epidemiological factors influencing the development of relapsing and severe Clostridium difficile infection
- Úvodník
- Genotyping results, laboratory diagnosis, and epidemiology of the mumps virus circulating in the Czech Republic in 2012
- Clinical and epidemiological characteristics of patients hospitalized with severe influenza in the season 2012–2013
- Legionella infection – a neglected problem
- Evaluation of prophylactic precautions after exposure to biological materials
- Epidemiological investigation in five dental offices of the Clinic of Dentistry, Faculty of Medicine, Palacký University, Olomouc and of the Olomouc University Hospital
- Sequencing analysis of the antigens included in the four-component vaccine against serogroup B meningococcus in Czech isolates of Neisseria meningitidis from 2007–2013
- Epidemiology of Cronobacter spp. isolates from patients admitted to the Olomouc University Hospital (Czech Republic)
-
Biologické a chemické zbrane
Pripravenosť a odpoveď - Významné životné jubileum RNDr. Ing. Josefa Janoška
- Zemřel prof. MUDr. Vladislav Potužník, DrSc. (*6. 1. 1924–†9. 12. 2013)
- Zemřel MUDr. Vladimír Plesník (*6. 11. 1931–†4. 2. 2014)
- Epidemiologie, mikrobiologie, imunologie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Legionella infection – a neglected problem
- Bacteria of the Burkholderia cepacia complex: epidemiology and diagnosis of infection in patients with cystic fibrosis
- Epidemiological investigation in five dental offices of the Clinic of Dentistry, Faculty of Medicine, Palacký University, Olomouc and of the Olomouc University Hospital
- Clinical and epidemiological characteristics of patients hospitalized with severe influenza in the season 2012–2013
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



