-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInosine pranobex in the prevention and treatment of acute respiratory viral infections including COVID-19 Answers to Frequently Asked Questions from Healthcare Professionals
3. 4. 2020
In the last few days and weeks I have been asked by many colleagues (even dentists) about inosine pranobex and about its potential in prevention and treatment of Covid-19 coronavirus infection caused by SARS-CoV-2. hey appeal to me with this enquiry mainly because I have been involved in research of acute viral respiratory infections, including COVID-19 and I deal with this topic in my clinical practice on daily basis. I presented and published some results in the Czech Republic and also abroad.
Author: Professor Jiří Beran, MD.
In the last few days and weeks I have been asked by many colleagues (even dentists) about inosine pranobex and about its potential in prevention and treatment of Covid-19 coronavirus infection caused by SARS-CoV-2.
InzerceThey appeal to me with this enquiry mainly because I have been involved in research of acute viral respiratory infections, including COVID-19 and I deal with this topic in my clinical practice on daily basis. I presented and published some results in the Czech Republic and also abroad.
I tried to generalize and sort individual questions of healthcare workers, so after reading this text, it should be clear to everyone how inosine pranobex could be used in the treatment and prevention of Covid-19 and what to expect from the treatment and prevention.
The text is dedicated not only to physicians but, also to the general public, so it was simplified in explaining some immunological events or knowledge of virology. For this reason, I apologize very much to all virologists, immunologists and other colleagues in another medical specializations for the simplifications that I did in order to make the text fully comprehensible to everyone.
-
How immune system acts against a virus, which enters the human body?
Certainly, it is not worth writing down what kinds of viruses we know and what they consist of, because this information is available in many websites. Very simply, you can find this information at Wikipedia: https://en.wikipedia.org/wiki/Virus.
As soon as the virus enters the human body, it tries to reach a susceptible host cell as soon as possible to ensure its own replication. The susceptible host cell is that one which possess on its surface a receptor to which the virus binds by its glycoprotein spike and subsequently enters the cell. Within the cell, individual processes of virus reproduction and assembly take place.
Once the virus is inside, the cells of the immune system cannot “see” it and therefore they do not know that the host cell is infected. To overcome this problem, cells use the main histocompatibility system (HLA = Human Leucocyte Antigen) to display peptides from inside of the cell on the cell surface. It allows cells to present on their surface what is foreign and what is their own. If the cell is infected with a virus, fragments of the proteins and peptides produced by the virus, it will also be included and displayed on the cell surface.
White cells of natural immunity circulate in the body and check surface of cells for abnormalities. The most important class of white cells in natural immunity are so-called NK (Natural Killers) cells. Once NK cells detect a virus in any cell, they try to destroy the cell (by perforin and granzymes). (https://www.immunology.org/public-information/bitesized-immunology/pathogens-and-disease/immune-responses-viruses).
NK cells serve to slow the infection process from the start of the infection, to give enough of time to activate adaptive immunity, which finally, at healthy subjects, stops the infection process. The virus must be presented to immune system first by antigen presenting cells (APCs) together with Th1 helper cells. Thereafter, clonal proliferation occurs and new clones of specific cells, called cytotoxic T lymphocytes (Tc) are produced by immune system. They are able to destroy the virus-infected cell as well as NK cells, but specifically. Tc generated against a specific virus destroys only cells infected with this virus, while NK cells destroy any virus in the human body. Also, specific antibodies are produced that bind to the circulating virus and try to label it, aggregate, neutralize, etc.
The sufficient level of NK cells from the onset of infection is essential for protection against viral infection and further prognosis.
2. Is the penetration of SARS-CoV-2 virus into human cells and the Covid-19 infection development in any way specific, in comparison with other viral infections?
Based on current research, we know, that majority of the genetic information encoding SARS-CoV-2 virus is similar to the coronavirus found in the bat (Rhinolophus affinis). On the other hand, superficial glycoprotein spikes appear to come from coronaviruses isolated from Malayan pangolins (Manis javanica). The reason why SARS-CoV-2 virus spreads well in the human body is not so much related to the fact that they bind to the ACE2 receptor through glycoprotein (and, unfortunately, these receptors are more on the apical side than on the basal side of susceptible respiratory and gastrointestinal system), because it had previously bound also SARS-CoV. However, SARS-CoV-2 has undergone a change on the glycoprotein spike, particularly on its S1 subunit, where a new version of the receptor-binding domain (RBD) for the ACE2 receptor has been developed. The binding between ACE2 and the RBD is very strong. A second negative condition is that the SARS-CoV-2 virus and its S1 and S2 glycoprotein subunits are cleaved from each other by a human protease (furin) and thus binding to a susceptible host cell and intracellular penetration is substantially simpler. In a nearly identical way, highly pathogenic avian influenza (HPAI) viruses enter the cells of the respiratory tract and lungs. The SARS-CoV-2 virus contains the best combination for both spreading and development of the infection. For more reading please follow the reference: (https://doi.org/10.1038/s41591-020-0820-9).
Due to the strong binding between the SARS-CoV-2 glycoprotein RBD and the host cell receptor ACE2, the level of NK cells is more than essential as a "brake" for the development of the infection process until adaptive immunity, consisting of cytotoxic Tc lymphocytes and antibodies, becomes effective.
Therefore, it is useful to increase the activity and number of NK cells as the most important element of natural immunity in protection against the viral infection Covid-19 in highly exposed individuals (HCP in daily contact with Covid-19 positive patients).
3. What is inosine pranobex and is it a registered product? Is it available in the Czech Republic?
Inosine pranobex commonly known as inosine acedoben dimepranol, isoprinosine or methisoprinol was initially authorised in 1971 and is currently marketed in more than 70 countries worldwide for the treatment of viral infections. Thanks to the detailed immunological research, especially in the UK over the past 5 years, we already know how it works. The substance is effective in the treatment of various acute or chronic viral infections. Inosine pranobex affects the immune system comprehensively; however, in protection against viral infection, it modulates the cytotoxicity of adaptive immunity via T cells, and in natural immunity primarily via NK cells. NK cells are increased significantly already after 90 minutes of inosine pranobex administration and the double level is reached on the fifth day of the drug administration.
In acute infections caused by respiratory viruses, administration of inosine pranobex leads to the decrease of time with symptoms period.
Inosine pranobex is indirect antiviral drug, which acts against any virus (herpes virus, acute respiratory infection virus or measles virus), via very fast activation of natural immunity and its antiviral component - NK cells.
NK cells act against viral infection non-specifically, but quickly and consistently when they are present in the human body.
Brand names and producers of inosine pranobex

4. Is Inosin pranobex registered for the treatment of acute viral respiratory infections?
Inosine pranobex has shown in a double-blind, placebo-controlled study (https://doi.org/10.1186/s12879-016-1965-5) that it significantly reduces the time to resolution of symptoms in acute viral respiratory infections. The study has shown that even at a non-maximal dose of 3x2 tbl it can be effective against acute viral respiratory infection.
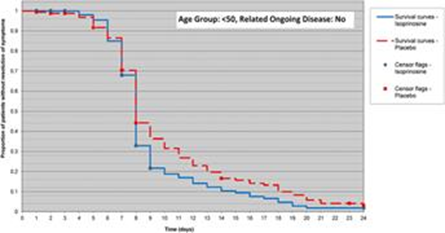
From the figure, it is evident that with the treatment of 3x2 tbl/day there is a significant effect of treatment visible on 7 and 8 days, when the number of people without any symptoms increases dramatically.
Based on this study, inosine pranobex is registered in some countries for the treatment of acute viral respiratory diseases. In countries where it is not registered for this indication, its use is off-label. (http://www.olecich.cz/encyklopedie/muze-lekar-pouzit-nejaky-lek-neschvalenym-zpusobem).
5. How inosin pranobex (IP) stimulates the immune system and its particular components?
Inosine pranobex stimulates the immune system, which is not significantly affected at the beginning of Covid-19 infection (lymphopenia is already present around the end of the first week of the disease due to the suppressive action of the virus), and so timely administration of the drug should significantly help to start the defense:
a. IP activates NK cells which number is higher as early as 90 min after the drug administration and after 5 days of administration the NK cell level is at least double (https://doi.org/10.1016/j.intimp.2016.11.023). NK cells are able to recognize virus-infected cells and destroy them with perforin and granzymes. In this way, natural immunity is significantly involved in the destruction of infected cells from the beginning of the infection and significantly affects the prognosis of viral infection
b. IP accelerates antigen presentation and triggers adaptive immune response, ie production of virus-specific antibodies and cytotoxic T-lymphocytes, which are able to specifically target virus infected cells at the later stage of the disease (https://doi.org/10.1007/s12325-019-00995 -6).
c. The last demonstrated effect of IP: inosine pranobex improves the visibility of infected cells for immune system due to the enhancement of metabolic processes inside the cell, which goal is to indicate on the surface of infected cells that the cell is indeed infected. Therefore, a virus-infected cell is better and faster recognized and destroyed by NK cells (http://dx.doi.org/10.1002/eji.201847948).
6. Why are NK cells of the immune system important in defense against viral infection, including Covid-19?
NK cells are large granular cells of the immune system that are classified as lymphocytes. They are capable to kill cells infected by viruses. They belong to the non-specific part of the immune system called natural immunity. NK cells carry two types of receptors on the surface. Lectin receptors which activate cytotoxicity (destruction of the target cell infected with virus, by using perforin and granzymes), while receptors recognizing first class MHC I (HLA I) turn this action off. If the NK cell recognizes the HLA I complex on the surface of another cell, this cell is left alive. However, if the NK cell encounters a cell that lacks HLA I or possess modification of these complexes, it is immediately destroyed.
At the beginning of viral infection, population of NK cells, via their activities, decide how the course of an infection is going to be. The adaptive immune response (especially cytotoxic T lymphocytes) may develop after a very short time and the infection may end, or the adaptive immune response may fail and more severe forms of the disease will develop.
Therefore, NK cells are extremely important in defence of human body against viral infection, because they help to gain time for the develop of adaptive immunity response. (https://www.immunology.org/public-information/bitesized-immunology/pathogens-and-disease/immune-responses-viruses).
7. May the use of inosine pranobex suppress further immune responses and will the immune protection be sufficient after treatment with inosine pranobex?
Inosine pranobex enhances elements of both natural and adaptive immunity, and as a result of this enhancement the immune response to viral infection should be more durable. Application of classic antiviral drugs in early phase of infection usually results into the termination of replication and the connected disappearance of the signals required for the robust and sustained immune response. The components of the immune response generated after infection are described here (https://doi.org/10.1038/s41591-020-0819-2).
8. Do you have any opinion about the increase in cytokines brought about by inosine pranobex, in light of the "cytokine storm" presumed to be occurring in the ARDS suffered by Covid-19 patients?
There are two "major classes" of cytokines in our immune system. The first class is related to the Th2 activity and the antibody defense and here plays the dominant role IL-6 which is responsible together with other cytokines for the so-called "cytokine storm". The second class contain cytokines related to the Th1 response and cellular immunity (cytotoxic T lymphocytes and NK cells) and here plays the dominant role IL-2. Both classes of cytokines should be in equilibrium. Inosine pranobex activates immune response via Th1 cytokines and in my view, its use should have the opposite effect than IL-6 cascade. In conclusion inosine pranobex and it uses does not support initiation or maintenance of "cytokine storm".
9. How should be inosine pranobex administered for Covid-19 and at what dose?
Inosine pranobex should start to be administered in the treatment of Covid-19 right after the detection. The best option is to take the first treatment dose immediately after the onset of the first symptoms of the disease such as fever and cough, muscle pain, joint pain, malaise. This is the only way of how to exploit its full potential in protecting against viral infection by NK cells production and stimulation of adaptive immunity.
For the treatment of Covid-19 it is recommended to use the maximum dosage of 4x2 tbl (4x1000 mg = 4g / day). The duration of treatment should be 7-10 days or two days after symptoms resolution.
For children from 1 year of age, the dosage is 50 mg / kg body weight, ie 1 tablet / 10 kg to 20 kg body weight. Furthermore, the dosage is as in adult patients.
Six-hour intervals between treatment doses is not necessary to keep strictly for example due to sleep, because shifting the interval by one or two hours cannot affect sustained stimulation of the immune system.
It may be unnecessary to administer the inosine pranobex to young and otherwise healthy persons (without any underlying chronic illness) and with mild symptoms of the disease. Inosine pranobex should be used for the initial treatment of all health care professionals, patients over 50 years of age, and all patients with chronic illness. Furthermore, inosine pranobex should be used for all those who are suspected of repeated inoculation of the infectious dose during the incubation period, such as all HCP taking care of patients, dentists for direct contact with patients and other professions where repeated inoculation could not be excluded.
Administration of inosine pranobex in very severe infections and for primary viral pneumonia would probably not be as successful. Nevertheless, in the measles pre-vaccine era, inosine pranobex has been shown to be effective in preventing death from measles complications including primary measles pneumonia tab. 1.
Table 1
Analysis of inosine pranobex treatment in 200 children hospitalized for measles or their complications.
Half of them took placebo and the other half inosine pranobex.Inosine pranobex
Placebo
Total
Lethal course of infection
6
15
21
Non-lethal course of infection
94
85
179
Total
100
100
100
Note: Inosine pranobex treatment was 100 mg/kg/body weight and showed a statistically significant difference (0.05 <p <0.025) in preventing lethal course of infection (https://www.ncbi.nlm.nih.gov/pubmed/?term=gallais+H+inosine).
10. Can inosine pranobex be used as a preventive measure and, if so, at what dose and for how long?
All who are in repeated contact with Covid-19 will be at a very high risk of developing the disease. Unfortunately, the incubation period is very long and so several additional infectious doses may be gradually inoculated during the first week of the incubation period without the health care professional knowing. The result will eventually be a very severe form of infection.
For those who directly take care of patients with Covid-19 (first-line healthcare professionals), I would recommend the so-called "interval preventive use" of inosine pranobex at a dose of 2x1 tbl (2x500 mg) for 10 days than 20 days without taking. The cycle should be repeated two more times.
There will be a significant increase and mainly maintenance of the NK cell population. A high proportion of functional NK cells among lymphocytes can greatly slow down the development of a possible Covid-19 infection in highly exposed healthcare professionals.
In the event of development of Covid-19 disease, I would advise to healthcare professionals to immediately switch from preventive dose to regular inosine pranobex treatment for acute respiratory viral disease at a dose of 4x2 tbl (4x1000 mg) for 7-10 days or two days after disappearance signs.
11. Are there any patients who cannot be recommended for the inosine pranobex treatment?
Patients who are advised to be treated or use inosine pranobex for prevention should be assessed for precautions and contraindications and country-specific SPC should be used as reference.
The drug should be used with caution with xanthine oxidase inhibitors or uricosuric agents, including diuretics. Inosine pranobex may be administered after but not concomitantly with immunosuppressive agents, as there may be a pharmacokinetic influence on the desired therapeutic effects. Concomitant use with zidovudine (AZT, azidothymidine) increases AZT nucleotide formation through multiple mechanisms involving increased plasma AZT bioavailability and increased intracellular phosphorylation in human blood monocytes. As a result, inosine pranobex increases the effect of AZT.
Inosine pranobex may cause a transient elevation of baseline serum and urinary uric acid, usually remaining within the normal range (using 8mg % as the upper limit), particularly in males and in the ageing population of both sexes. The elevation of uric acid levels is due to the catabolic metabolism of the inosine moiety of this product in humans to uric acid. It is not due to a fundamental drug-induced alteration of respective enzyme or renal clearance function. Therefore, inosine pranobex may be administered with caution in patients with a history of gout, hyperuricaemia, urolithiasis, or to patients with impaired renal function. During treatment, uric acid levels in these patients should be monitored closely.
In some people acute hypersensitivity reactions (urticaria, angioedema, anaphylaxis) may occur. Treatment with inosine pranobex should be withdrawn in these cases.
Wheat starch in this medication contains only very low levels of gluten and is considered as gluten-free. It is very unlikely to cause problems if a patient has coeliac disease. One tablet contains no more than 10.5 micrograms of gluten. If a patient has a wheat allergy (different from coeliac disease) he/she should not take this medicine.
SPC valid for UK is available here (https://www.medicines.org.uk/emc/product/2824/smpc).
CONCLUSION:
Inosine pranobex recommendations for the treatment of Covid-19
For the treatment of Covid-19 it is recommended to use the maximum dosage of 4x2 tbl (4x1000 mg = 4g / day). The duration of treatment should be 7-10 days or two days after symptoms resolution.
Six-hour intervals between treatment doses is not necessary to keep strictly for example due to sleep, because shifting the interval by one or two hours cannot affect sustained stimulation of the immune system.
Inosine pranobex should be used as soon as possible after the occurrence of first symptoms of the disease such as fever and cough, muscle pain, joint pain, malaise.
Inosine pranobex should be used for the initial treatment of all health care professionals, patients over 50 years of age, and in all patients with chronic disease. Furthermore, for all those who would be suspected of repeated inoculation of the infectious dose during the incubation period, such are doctors and all the medical personnel taking care of patients, dentists in direct contact with patients and other professions where repeated inoculation could not be excluded.
Inosine pranobex is not indicated for the treatment of severe forms of Covid-19 and their complications
Recommendations for the prevention of severe forms of Covid-19 disease
For those who take direct care of patients with Covid-19 (health care professionals in the first line of treatment), or residents of retirement houses, for them it is advised to use interval preventive use of inosine pranobex at a dose of 2x1 tbl (2x500 mg) for 10 days followed by 20 days without taking. The cycle should be repeated two more times.
Professor Jiri Beran, MD.
SCI = 3024; h-index= 29; i10-index=60
Head-Department for Tropical and Travel Medicine and Immunization
Institute of Postgraduate Medical Education in Prague, Czech Republic
Director-Vaccination and Travel Medicine Centre, Hradec Králové, Czech Republic
Email: jiri.beran@vakcinace.cz
Líbil se Vám článek? Rádi byste se k němu vyjádřili? Napište nám − Vaše názory a postřehy nás zajímají. Zveřejňovat je nebudeme, ale rádi Vám na ně odpovíme.
Štítky
Alergologie a imunologie Dermatologie Gynekologie a porodnictví Neonatologie Otorinolaryngologie Pediatrie Praktické lékařství pro dospělé
Nejnovější kurzy
Autoři: MUDr. Jiří Dvořák
Autoři: MUDr. Zuzana Humlová, MUDr. Michal Mihula
Přejít do kurzů
Nejčtenější tento týden Celý článekPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



