-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Self-Administered Outpatient Antimicrobial Infusion by Uninsured Patients Discharged from a Safety-Net Hospital: A Propensity-Score-Balanced Retrospective Cohort Study
In a propensity score-balanced retrospective cohort study, Kavita Bhavan and colleagues compare health outcomes for patients undergoing self-administered versus healthcare-administered outpatient parenteral antimicrobial therapy.
Published in the journal: . PLoS Med 12(12): e32767. doi:10.1371/journal.pmed.1001922
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001922Summary
In a propensity score-balanced retrospective cohort study, Kavita Bhavan and colleagues compare health outcomes for patients undergoing self-administered versus healthcare-administered outpatient parenteral antimicrobial therapy.
Introduction
A substantial source of hospital costs is the long-term administration of antimicrobial agents to patients with serious or life-threatening infections, such as osteomyelitis, endocarditis, and staphylococcal bacteremia [1–3]. A recent study estimated the number of endocarditis cases hospitalized in US hospitals in 2009 at 43,419, with an average stay of 15.3 d, generating average hospital charges of US$122,204 per case (estimated to be US$150,000 in 2014) [1]. Typically, patients with infections requiring long-term antibiotics receive intensive diagnostic and therapeutic services in the first several hospital days but thereafter remain in the hospital only to receive antimicrobial infusions. Insured patients may be discharged early to complete their antimicrobial courses at home with contracted nursing assistance or in lower-cost nursing facilities, but uninsured patients usually remain in the hospital, posing a burden particularly on safety-net hospitals [4,5].
Outpatient parenteral antimicrobial therapy (OPAT), defined as the administration of parenteral antimicrobial therapy for at least two doses on different days without intervening hospitalization [6,7], has gained wide acceptance in modern medical practice in the US [7,8]. The primary goal of an effective OPAT program is to allow patients to safely and effectively complete a planned treatment course in their home or alternate outpatient site [9]. Secondary goals of an OPAT program include avoiding the inconveniences, complications, and expense of hospitalization to complete a prescribed intravenous (IV) antibiotic course.
OPAT is generally delivered in one of the following four settings: an infusion center, a nursing home, at home with daily skilled nursing visits, or at home with self - or family-administered therapy [7]. The first three—healthcare-administered OPAT (H-OPAT)—are most commonly seen in the US and are achievable with adequate insurance funding. The fourth—self-administered OPAT (S-OPAT)—is relatively uncommon globally, with the few published studies having been conducted in England and Ireland [10–12].
Parkland Hospital is an 800-bed safety-net hospital serving the low-income patient population of Dallas County, Texas. An internal review of early efforts to treat medically stable patients requiring long courses of antibiotics outside the hospital found emergency department visits in >50% of these patients and a substantial 30-d readmission rate in May 2009. With multidisciplinary input from physicians, nurses, pharmacists, and case managers, we established an S-OPAT clinic to address this problem by centralizing services for patients discharged with S-OPAT (S1 Fig). Finding no published baseline experience in the US against which to evaluate our S-OPAT program, we undertook this study to determine whether the low-income, mostly non-English-speaking patients in our S-OPAT program could administer their own parenteral antimicrobials at home by gravity with the same or better outcomes than traditionally accepted models of outpatient care available to patients with funding for healthcare services (H-OPAT).
Methods
Ethics Statement
The University of Texas Southwestern Medical Center’s Institutional Review Board approved study procedures and waived written informed consent.
Study Design and Participating Communities
Ahead of data analysis, we hypothesized that patients in our S-OPAT program would have better clinical outcomes—because of the standardized training and weekly follow-up—than patients in the H-OPAT group, who received services of variable quality from numerous unregulated home health agencies and other sub-acute-care settings contracted by the hospital. As the primary test of this hypothesis, we identified from the Parkland Hospital electronic medical records all hospital readmissions within 30 d of discharge from the hospitalization episode in which the antimicrobial therapy was started for all patients in the S-OPAT and H-OPAT groups discharged in fiscal years 2010 (1 October 2009 to 30 September 2010) through 2013 (1 October 2012 to 30 September 2013). The fiscal year 2010 for the H-OPAT group also included patients discharged in the previous 9 mo (1 January 2009 to 30 September 2010) to achieve an adequate sample size. As a secondary test, we detected all deaths within 1 y of discharge in the two groups from the hospital’s electronic death records and by telephone follow-up of all patients not known to have died but whose electronic medical records showed no clinical contacts beyond 1 y from discharge. We excluded patients who required hospitalization for the duration of antimicrobial therapy because they were ineligible for OPAT by the set criteria given in S2 Fig, e.g., unstable home environment (homeless), history of IV drug abuse, or need for continued medical care beyond antimicrobial infusions [13]. Accurate lists of patients in the S-OPAT and H-OPAT groups were obtained from the OPAT clinic log and the social work office that arranges home health services for funded patients. The completeness of the lists was verified by a search of the electronic medical records and pharmacy data for all patients prescribed IV antimicrobial agents at discharge. The infectious disease diagnosis requiring long-term IV administration of antimicrobial agents was obtained from the electronic medical records.
Since the allocation to S-OPAT or H-OPAT was largely determined by healthcare funding status, comparison of the outcomes was expected to be affected by selection bias and confounding. Consequently, we collected the following measures at the patient level to use in multivariable analyses: age, gender, race/ethnicity, language if not English-speaking, body mass index, healthcare funding source, type of infection requiring IV antimicrobial treatment, and comorbid diagnosis of diabetes mellitus and chronic renal insufficiency. These measures were collected from the patients’ electronic medical records. Race/ethnicity was derived from patient self-identification as documented in the electronic medical records. The census tract of each patient’s residential address was determined by the SAS Geocode procedure and by manual searching of online databases, and the following characteristics were obtained from the 2012 US census data [14]: home location (central city core, suburban, or rural), distance from patient’s home address to Parkland Hospital, and family income estimated from median income of the census tract. The treatment plans for all patients being discharged to receive long-term antibiotic therapy outside the hospital were routinely reviewed by an infectious disease pharmacy specialist, with the assistance of an infectious disease physician as needed, to ensure that all patients received therapy appropriate for their infections, i.e., therapy in accordance with written clinical guidelines such as those of the Infectious Diseases Society of America [9]. The selection of antibiotics was not constrained by financial considerations because we had low outpatient pricing under the federal 340B Drug Pricing Program for the care of low-income populations in public hospitals.
Outpatient Services for the Healthcare-Administered OPAT Patients
Patients with healthcare funding (i.e., private health insurance, Medicare, or Medicaid) have several alternatives for long-term IV antimicrobial therapy outside the hospital. Those most often used in both the Parkland Hospital and private hospital settings are daily home visits by private nursing services or admission to skilled nursing facilities or sub-acute-care facilities. Patients discharged to care facilities are not necessarily more seriously ill than those discharged to home, but their insurance coverage designates care facility services as standard of care for outpatient antimicrobial therapy.
Management Plan for the Self-Administered OPAT Patients
As stipulated in the OPAT protocol (S1 Fig), patients were accepted into the S-OPAT program according to written eligibility criteria (S2 Fig) applied through an infectious diseases/OPAT consultation [15]. Before hospital discharge, S-OPAT patients received standardized training in appropriate technique for self-administration of parenteral antimicrobial therapy delivered by counting the drops of antimicrobial-containing IV fluid delivered by gravity (no infusion pumps were provided). Education materials, developed in 2009–2010 at a fourth grade literacy level, included pictures of necessary supplies, hand hygiene technique, and technique for aseptically connecting antibiotic solution to the IV catheter (S3 and S4 Figs). Instruction was delivered verbally following a pamphlet printed in both English and Spanish. A smaller group of patients who spoke only less common languages—including Vietnamese (seven patients), the Burmese dialect of Chin (two patients), Ethiopian Amharic (three patients) and 12 additional languages/dialects—were also effectively trained with the assistance of the hospital’s multilingual telephone translation services.
Most importantly, following training, competency was established before discharge through a standardized protocol, developed by a multidisciplinary team of physicians, nurses, pharmacists, and case managers, requiring patients to repeatedly demonstrate mastery of all the steps in self-administration by gravity (S5 Fig). Patients were then followed throughout treatment with weekly clinic visits for maintenance of their peripherally inserted central catheter and laboratory monitoring [16], and evaluation by an infectious disease physician or nurse practitioner every 2 wk (S1 Fig). The monthly attendance rate for scheduled clinic follow-up visits averaged approximately 85%. The total number of days a patient required IV antimicrobial therapy as an outpatient that would have otherwise been administered in the inpatient setting was determined, to reflect the number of inpatient days saved by the S-OPAT program.
Development of the Propensity Score
A propensity score [17,18] was developed by a stepwise logistic regression analysis to predict S-OPAT versus H-OPAT group membership based on all of the covariates listed in Table 1, using an entry criterion of p ≤ 0.05. Polychotomous nominal variables were included in the pool of predictors as sets of dummy variables. Ordinal measures were included as regression variables on the original scale as well as transformed by the log, square root, or square, and as sets of dummy variables. The final propensity score model (Table 2) classified group membership extremely well (area under the receiver operating characteristic curve = 0.91). Each patient’s probability of S-OPAT membership conditioned on the characteristics in the model calculated from the multivariable logistic regression model was added to the database as the propensity score, which was then arbitrarily categorized at quintiles, as is commonly done [17]. We compared the distribution of patients in the S-OPAT and H-OPAT groups across the quintiles of the propensity score to evaluate sample size adequacy within the higher quintiles (S1 Table). We found that excluding the top two quintiles from the final outcome analyses did not alter the findings.
Tab. 1. Baseline characteristics of the patients in the self-administered OPAT and healthcare-administered OPAT groups. 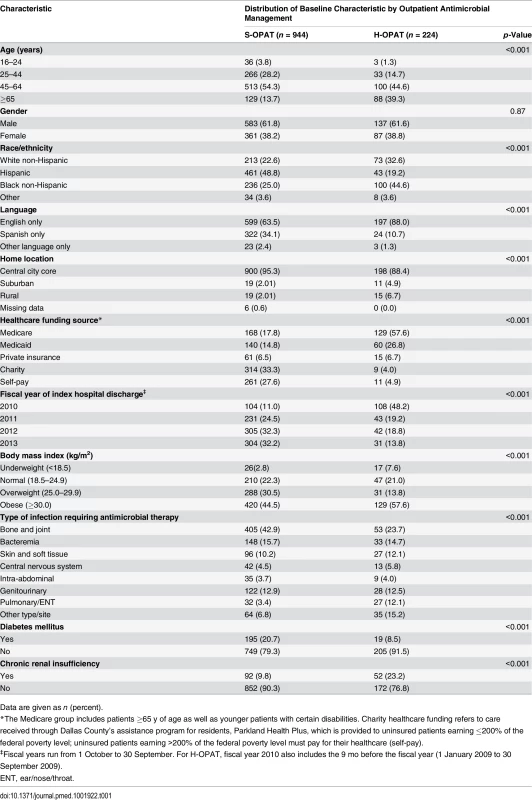
Data are given as n (percent). Tab. 2. Multivariable logistic regression model of propensity score for participation in the self-administered OPAT versus healthcare-administered OPAT program. 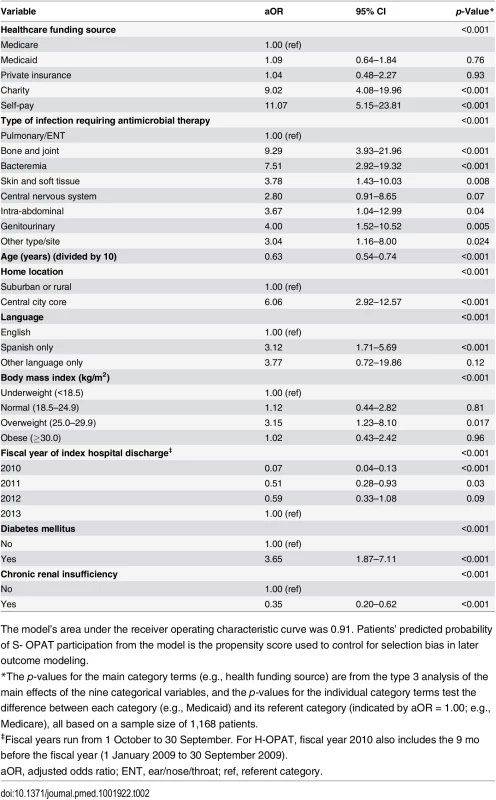
The model’s area under the receiver operating characteristic curve was 0.91. Patients’ predicted probability of S- OPAT participation from the model is the propensity score used to control for selection bias in later outcome modeling. Analyses of Outcomes
The primary study outcome was ≥1 readmission from all causes within 30 d of discharge, and the secondary outcome was death from all causes within 1 y of discharge. For each outcome, two models were developed. In model 1 the covariates included those characteristics in Table 1 that entered a stepwise proportional hazards analysis of time (in days) to readmission (or death) with p ≤ 0.05 as the entry criterion and with the indicator of S-OPAT versus H-OPAT forced in. Model 2 included the same covariates as model 1 except that, in addition, the propensity score was forced in as a categorical variable partitioned at quintiles, with the first quantile as the referent. The stability of model 2 was tested by repeating it three times with these three modifications [19]: (1) excluding from the analysis all patients in the fourth and fifth quintiles of the propensity score, (2) replacing the categorized propensity score with the continuous one as a quadratic effect in the model, and (3) using multiple logistic regression analysis in place of proportional hazards analysis. Variation in the result was tested with interaction terms for treatment group by infection type, year of index hospital discharge, race/ethnicity, and healthcare funding source. All analyses were performed with version 9.4 of SAS for Windows (SAS Institute), and all p-values are two-tailed, with p ≤ 0.05 considered statistically significant.
Results
This study includes 1,168 patients who were discharged from Parkland Hospital between 1 January 2009 and 30 September 2013 to receive IV antimicrobial therapy outside the hospital. The antimicrobial therapy of 944 (80%) of these patients was managed by the S-OPAT program, with weekly outpatient clinic follow-up, and the antimicrobial therapy of the remaining 224 (19%) of these patients was managed by a funded third party agency or institution (Fig 1). An additional 261 patients, with similar demographic characteristics but ineligible for outpatient infusion due to IV drug abuse, homelessness, or need for continued hospital care, remained in the hospital to complete their therapy and were excluded from the study (Fig 1).
Fig. 1. Summary of patient selection. 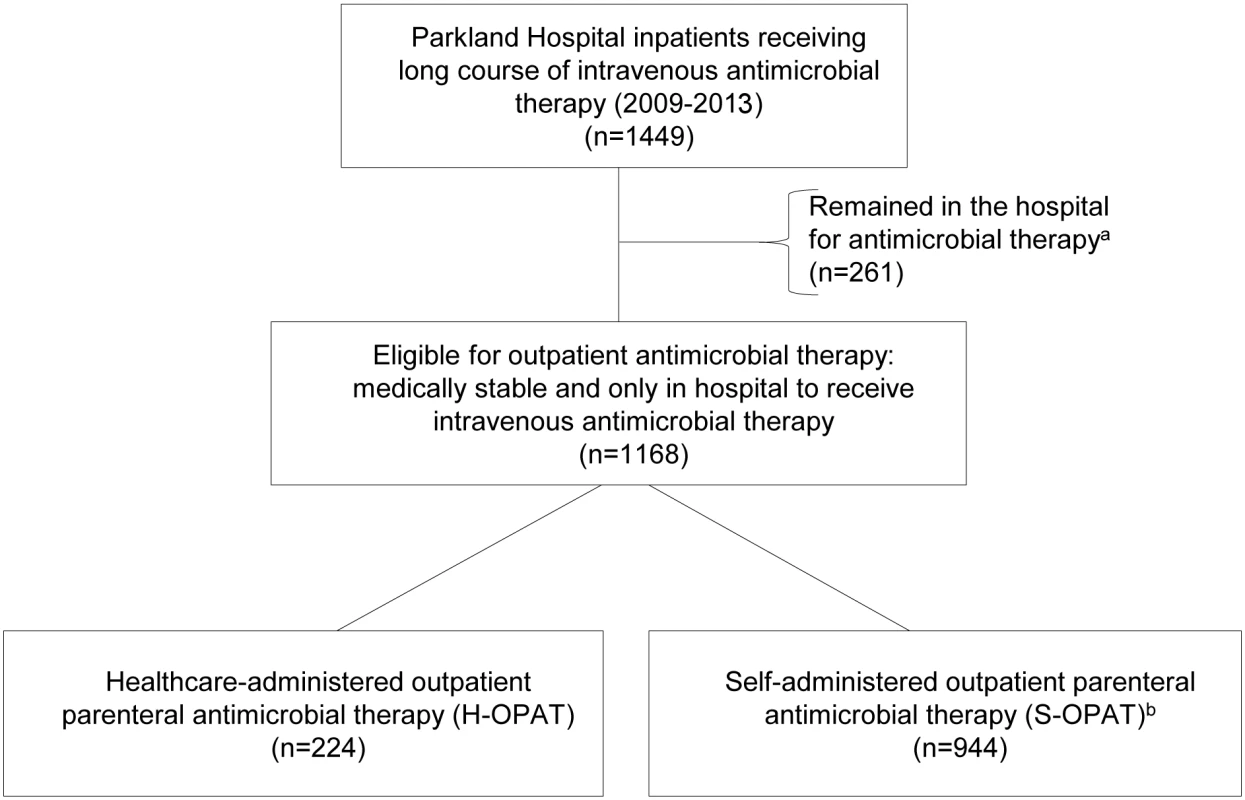
aPatients who were homeless, had a history of IV drug abuse, or were medically unstable. bThe eligibility criteria for inclusion in the S-OPAT group are given in S2 Fig. Baseline Group Differences
Compared with the H-OPAT group, the S-OPAT group had a higher percentage of patients with the following characteristics: healthcare funded by charity or self-pay, <65 y old, Hispanic, Spanish-only speaker, resident of the central city core, diabetic, and on long-term antimicrobial therapy for bone and joint infections, and a lower percentage of patients with chronic renal insufficiency (Table 1). Some S-OPAT patients had insurance funding, but their insurance plans did not extend to outpatient IV antimicrobial therapy (Table 1).
30-d All-Cause Readmission
The 30-d all-cause readmission rate was strongly associated with the 1-y all-cause mortality rate, the mortality rate for readmitted patients being 13.3% compared with 3.5% for those not readmitted (odds ratio, 4.3; 95% CI 2.5–7.3; p < 0.001; S1 Table), suggesting that complications in patients with potentially life-threatening infections that necessitate readmission are often very serious.
The 30-d all-cause readmission rate in the S-OPAT patients (16.7%) was 35% lower than that in the H-OPAT patients (23.7%) (crude hazard ratio, 0.65; 95% CI 0.47–0.88; p = 0.006; S1 Table), and this difference remained statistically significant after controlling for confounding in model 1 and for selection bias as well in model 2 (adjusted hazard ratio [aHR], 0.53; 95% CI 0.35–0.81; p = 0.003; Table 3). In model 2, the S-OPAT patients had lower readmission rates in each of the four years of the study (group-by-year interaction p = 0.31), in each of the infection type categories (group-by-infection-type interaction p = 0.99), in Hispanics versus non-Hispanics (group-by-race/ethnicity interaction p = 0.94), and in the self-pay group versus the other healthcare funding source groups (group-by-funding-source interaction p = 0.99). The repeat analysis with logistic regression gave similar results (Table 3). It is noteworthy that introducing the propensity score in model 2 yielded a lower hazard ratio than that in model 1.
Tab. 3. Multivariable proportional hazards regression models of 30-d readmission. 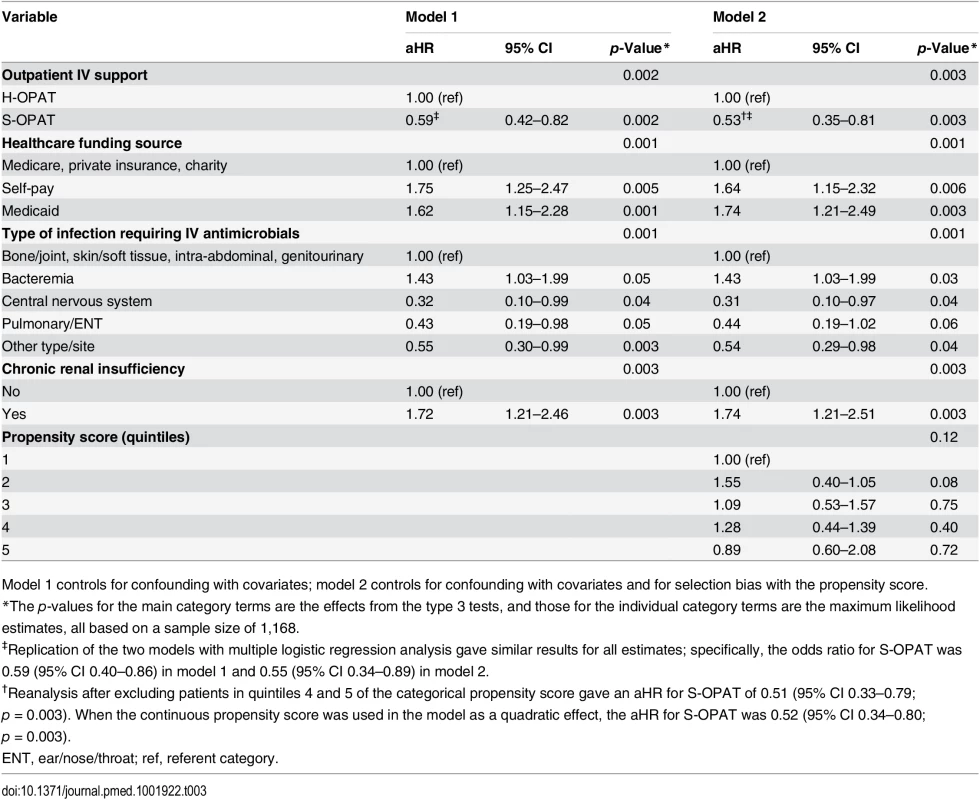
Model 1 controls for confounding with covariates; model 2 controls for confounding with covariates and for selection bias with the propensity score. Readmission for reasons directly attributable to antimicrobial infusions affected 21 of 944 (2.2%) S-OPAT patients and four of 224 (1.8%) H-OPAT patients. These included 13 with dysfunction of the peripherally inserted central catheter, ten whose underlying infection was not improving, six with renal or hepatic toxicity from the antibiotics, four with catheter-related bloodstream infection, and one with deep vein thrombosis. Readmission for reasons not directly related to the infusions occurred in 131 S-OPAT patients (13.9%) and 49 H-OPAT patients (21.9%).
1-y All-Cause Mortality
Despite the potentially life-threatening infections for which these patients were being treated, the 1-y all-cause mortality rate was low in both groups (S-OPAT, 5.4%; H-OPAT, 4.5%; p = 0.57; S1 Table). Stepwise proportional hazards analysis including the covariates and propensity score confirmed no significant difference in mortality between the treatment groups (aHR, 0.86; 95% CI, 0.37–2.00; p = 0.73; Table 4). The repeat analysis with logistic regression gave similar results (Table 4). Again, introducing the propensity score in model 2 yielded a lower hazard ratio for mortality than that in model 1.
Tab. 4. Multivariable proportional hazards regression models of 1-y mortality. 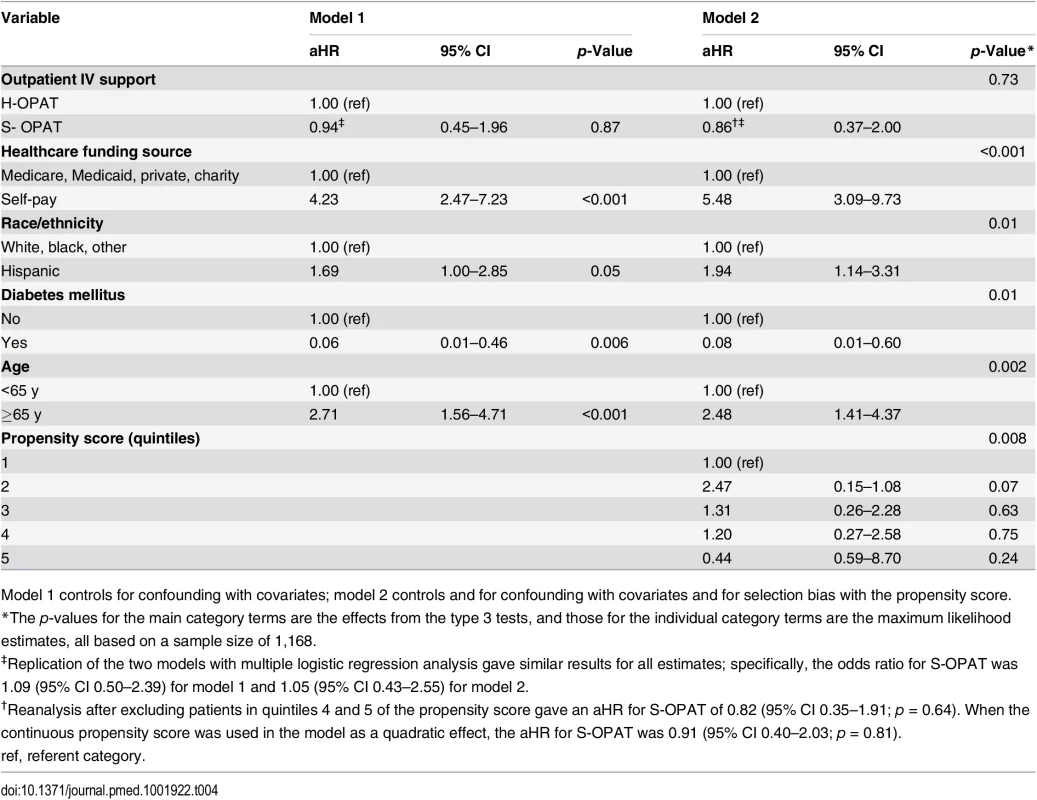
Model 1 controls for confounding with covariates; model 2 controls and for confounding with covariates and for selection bias with the propensity score. However, the analysis identified an unanticipated strong association of mortality with self-pay healthcare funding status (uninsured patients earning >200% of the poverty level, the “working poor”). Compared with 31 deaths (3.6%) in the 865 patients with Medicare, Medicaid, private insurance, or charity care (Dallas County residents earning ≤200% of the federal poverty level receive full care through the assistance program Parkland Health Plus), 30 deaths (12.4%) occurred in the 242 self-pay patients (aHR 5.48, 95% CI 3.09–9.73; p < 0.001; Table 4).
Impact on Hospital Resource Utilization
Over the study period, the 944 patients in the S-OPAT program administered their own outpatient antimicrobial infusions at home for a median 26 d, saving the hospital 27,666 patient-days of hospitalization and, by the last year, freeing up an average of 26 hospital beds each day to accommodate other patients requiring more intensive services (Table 5).
Tab. 5. Impact of the self-administered OPAT program on the hospital’s inpatient bed utilization. 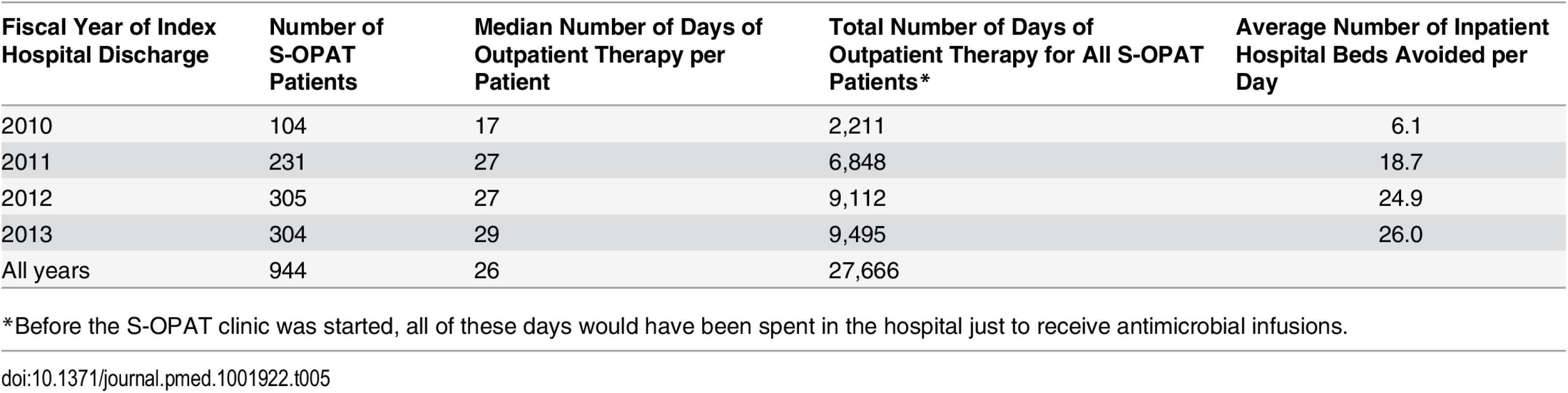
*Before the S-OPAT clinic was started, all of these days would have been spent in the hospital just to receive antimicrobial infusions. Discussion
Our findings demonstrate that—after controlling for confounding with covariates and leveling preexisting differences between the two treatment groups as measured by a propensity score—the risk of 30-d readmission was significantly lower in the S-OPAT group than in the H-OPAT group, and the risk of dying within 1 y of hospital discharge was not significantly different in the two groups. This suggests that S-OPAT was an acceptable model for a select group of uninsured, medically stable patients, many of whom spoke no English, to complete extended courses of IV antimicrobial therapy for a variety of infections at home in an urban, safety-net-hospital setting. A standardized multilingual training program requiring patients to demonstrate proficiency in management of their IV lines and antimicrobial administration, followed by weekly clinic visits, allowed this broad cross-section of patients to manage their own antimicrobial administration for prolonged periods with good results. Despite having a higher mix of disadvantaged patients, the S-OPAT group had 30-d readmission and 1-y mortality rates that were equivalent to, or better than, those of patients with healthcare funding to support commercial outpatient services such as visiting nurses, skilled nursing facilities, and sub-acute-care facilities. The structure of our S-OPAT program meets national guidelines [9] and has all the elements of the outpatient antimicrobial therapy bundle recently proposed by Muldoon et al. [8].
The advent of newer antimicrobials offers medically stable patients hospitalized for long-term IV antimicrobial administration greater opportunity for completing their treatment course outside the hospital [12,20]. This leads not only to greater patient satisfaction but also to a theoretical lowering of the risks of complications from prolonged hospitalizations such as nosocomial infection with antibiotic-resistant hospital pathogens and Clostridium difficile colitis. S-OPAT benefits hospitals by reducing length of stay. By allowing patients to manage their own antimicrobial therapy at home, the program avoided a total of 27,666 hospital days of relatively low intensity care for antimicrobial infusion over the 4-y study period and, by the end, was freeing up an average of 26 hospital beds per day.
The greatest potential weakness of our study was the high possibility of bias in testing the effects of the S-OPAT intervention with an observational study design. That a patient’s healthcare funding status is associated with many risk factors for bad outcomes could introduce selection bias and confounding. Besides including covariates in the multivariable outcome analyses to control for confounding and increase precision, we developed and included in the outcome models a propensity score to control for selection bias. A propensity score is derived by initially developing a multivariable logistic regression model predicting treatment group membership (S-OPAT versus H-OPAT) from a combination of patients’ demographic and clinical characteristics [18]. The probability of being in one of the groups (e.g., the S-OPAT group) is then calculated for each patient in the study from the logistic regression equation. In the later multivariable outcome analysis (of 30-d readmission or 1-y mortality), incorporating the propensity score in the model balances between the two treatment groups all the characteristics that were in the propensity score development model, as well as any unmeasured characteristics that are correlated with the included characteristics but not those that are uncorrelated with the included characteristics. In general, propensity scores that adequately control for selection bias can enable observational studies to “approximate randomized experiments” [21]. In our study, however, the marked differences in the characteristics of patients in the two groups raise concerns over whether the findings could be due to residual selection bias not completely controlled for by the propensity score.
We believe this concern is mitigated by the following features of the analysis. First, the initial logistic regression model that generated our propensity score contained the actual characteristics that determined assignment to the S-OPAT or H-OPAT groups, e.g., the patients’ healthcare funding status and administrative, demographic, and clinical characteristics [22]. Second, the multivariable logistic regression model predicted group assignment very accurately (area under the receiver operating characteristic curve, 0.91), and thus the resulting propensity score should have controlled for selection bias well in the outcome analyses. Third, in the findings, the significant difference in 30-d readmission rate between the two treatment groups (S-OPAT lower than H-OPAT), adjusted by potential confounding variables and the propensity score, remained after excluding the patients in the top two quintiles of the propensity score, where sparse numbers (S1 Table) could have allowed residual bias. Fourth, and most powerfully, the apparent direction of the selection bias from inherent differences between patients in the two treatment groups went in the opposite direction from the finding. That is, reducing the selection bias by introducing the propensity score in model 2 (Table 3) caused the difference in 30-d readmission rate to widen further (the aHR decreased from 0.59 [41% lower in the S-OPAT group] in model 1 to 0.53 [47% lower in the S-OPAT group] in model 2), indicating that the selection bias had been tending to obscure the difference. Thus, we would expect any residual selection bias to be causing further underestimation of the group difference. We observed the same effect of the propensity score in the outcome model of 1-y all-cause mortality (the aHR decreased from 0.94 [6% lower in the S-OPAT group] in model 1 to 0.86 [14% lower in the S-OPAT group] in model 2; Table 4).
Other limitations are that with only 61 deaths, the study was underpowered to find subtle group differences in the secondary outcome, 1-y all-cause mortality, and because of the diversity of services accessed by patients in the H-OPAT group, the exact practices of the various third party settings are unknown. Although the study was conducted in a single hospital, since Parkland Hospital shares many similarities with other public hospitals, the findings are likely to apply to other hospitals caring for large numbers of low-income, uninsured patients. Whether the model applies to private hospital settings and to classes of insured patients should be evaluated.
A most provocative incidental finding was the disproportionately high risk of death in self-pay patients (the “working poor”). Irrespective of the type of outpatient antimicrobial administration and other sources of confounding and selection bias, patients making too much income to qualify for Medicaid or county assistance but too little to afford private insurance had a much higher risk of 1-y all-cause mortality from serious infections (aHR, 5.48; 95% CI 3.09–9.73, p < 0.001), as previously found with sepsis [23], cancer [24,25], and trauma [26,27]. One possible explanation is that the need to pay full price for care may have caused these patients to delay returning to the hospital when they had life-threatening complications. Hispanic patients also had a higher risk of 1-y all-cause mortality, possibly reflecting reluctance to return for care due to undocumented resident status [28,29]. Interestingly, diabetic patients had a significantly lower mortality rate, possibly resulting from an aggressive program by Parkland Hospital to manage transition of care services for diabetics during the period of the study. Including covariates for these characteristics in the propensity score model and in the outcome models avoided bias from their effects, and we confirmed this by demonstrating the lack of interaction with the treatment group variable in the outcome models.
In making the business case for S-OPAT in the United Kingdom, the British Society for Antimicrobial Chemotherapy reflected, “The idea that IV antibiotics can be safely administered at home, by patients themselves, is one that some years ago may have caused gasps of horror amongst the medical fraternity” [30]. In an era when hospitals are actively trying to reduce the rate of 30-d readmission, particularly when this quality measure is tied to reimbursement, we have shown clinical outcomes for our S-OPAT program to be better than the standard model of reimbursed care for 30-d readmission, and no different than the standard model for 1-y all-cause mortality.
Our study did not address reasons for the S-OPAT program’s superior outcome, but we suggest that rigorous patient training and weekly clinic consultation may equip patients to administer infusions more consistently for their own well-being than for-profit clinical businesses do, and the weekly clinic visits during outpatient antibiotic administration may identify and manage developing problems before they progress to hospitalization.
We conclude that S-OPAT, a model at the intersection of patient-centered care [31] and the hospital-at-home movement [32], can be an acceptable model of treatment for uninsured, medically stable patients. By offering the choice of the home environment over a skilled nursing facility, and the freedom of scheduling infusions not available with scheduled home health services, our model may also be an attractive option for patients with funding and access to third party healthcare services. Since our model is not resource intensive, it may be readily replicated in a variety of settings. If reimbursed suitably by health insurers, the S-OPAT model for suitable patients could largely supplant third party services. Our findings thus have important implications for healthcare financing agencies and for improving resource utilization in safety-net hospitals and other resource-limited settings that care for uninsured patients.
Supporting Information
Zdroje
1. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU (2013) Infective endocarditis in the U.S., 1998–2009: a nationwide study. PLoS ONE 8: e60033. doi: 10.1371/journal.pone.0060033 23527296
2. Lipsky BA, Weigelt JA, Gupta V, Killian A, Peng MM (2007) Skin, soft tissue, bone, and joint infections in hospitalized patients: epidemiology and microbiological, clinical, and economic outcomes. Infect Control Hosp Epidemiol 28 : 1290–1298. doi: 10.1007/s00125-010-1672-5 17926281
3. Eisenberg JM, Kitz DS (1986) Savings from outpatient antibiotic therapy for osteomyelitis. Economic analysis of a therapeutic strategy. JAMA 255 : 1584–1588. doi: 10.1001/jama.1986.03370120062024 3951094
4. Institute of Medicine Committee on the Changing Market, Managed Care, and the Future Viability of Safety Net Providers (2000) America’s health care safety net: intact but endangered. Washington (District of Columbia): National Academies Press.
5. Cunningham PJ, Bazzoli GJ, Katz A (2008) Caught in the competitive crossfire: safety-net providers balance margin and mission in a profit-driven health care market. Health Aff (Millwood) 27: w374–w382. doi: 10.1377/hlthaff.27.5.w374
6. Gilbert DN, Dworkin RJ, Raber SR, Leggett JE (1997) Outpatient parenteral antimicrobial-drug therapy. N Engl J Med 337 : 829–838. doi: 10.1056/NEJM199709183371207 9297114
7. Paladino JA, Poretz D (2010) Outpatient parenteral antimicrobial therapy today. Clin Infect Dis 51 (Suppl): 198–208. doi: 10.1086/653520
8. Muldoon EG, Snydman DR, Penland EC, Allison GM (2013) Are we ready for an outpatient parenteral antimicrobial therapy bundle? A critical appraisal of the evidence. Clin Infect Dis 57 : 419–424. doi: 10.1093/cid/cit211 23572486
9. Tice AD, Rehm SJ, Dalovisio JR, Bradley JS, Martinelli LP, et al. (2004) Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis 38 : 1651–1672. doi: 10.1086/420939 15227610
10. Matthews PC, Conlon CP, Berendt AR, Kayley J, Jefferies L, et al. (2007) Outpatient parenteral antimicrobial therapy (OPAT): is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother 60 : 356–362. doi: 10.1093/jac/dkm210 17566002
11. Kieran J, O’Reilly A, Parker J, Clarke S, Bergin C (2009) Self-administered outpatient parenteral antimicrobial therapy: a report of three years experience in the Irish healthcare setting. Eur J Clin Microbiol Infect Dis 28 : 1369–1374. doi: 10.1007/s10096-009-0794-5 19697069
12. Seaton RA, Barr DA (2013) Outpatient parenteral antibiotic therapy: principles and practice. Eur J Intern Med 24 : 617–623. doi: 10.1016/j.ejim.2013.03.014 23602223
13. Brown RB (1991) Selection and training of patients for outpatient intravenous antibiotic therapy. Rev Infect Dis 13 (Suppl 2): S147–S151. 2017642
14. United States Census Bureau (2010) American FactFinder [database]. Available: http://factfinder2.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed 4 May 2015.
15. Shrestha NK, Bhaskaran A, Scalera NM, Schmitt SK, Rehm SJ, et al. (2012) Contribution of infectious disease consultation toward the care of inpatients being considered for community-based parenteral anti-infective therapy. J Hosp Med 7 : 365–369. doi: 10.1002/jhm.1902 22315151
16. Huck D, Ginsberg JP, Gordon SM, Nowacki AS, Rehm SJ, et al. (2014) Association of laboratory test result availability and rehospitalizations in an outpatient parenteral antimicrobial therapy programme. J Antimicrob Chemother 69 : 228–233. doi: 10.1093/jac/dkt303 23887864
17. Rosenbaum PR, Rubin DB (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70 : 41–55. doi: 10.1093/biomet/70.1.41
18. D’Agostino RB Jr, D’Agostino RB Sr (2007) Estimating treatment effects using observational data. JAMA 297 : 314–316. 17227985
19. Austin PC, Mamdani MM (2006) A comparison of propensity score methods: a case-control study estimating the effectiveness of post-AMI state. Stat Med 25 : 2084–2106.
20. Torok ME, Chapman AL, Lessing MP, Sanderson F, Seaton RA (2010) Outpatient parenteral antimicrobial therapy: recent developments and future prospects. Curr Opin Investig Drugs 11 : 929–939. 20721835
21. Rubin DB (2007) The design versus the analysis of observational studies for causal effects: parallels with the design of randomized trials. Stat Med 26 : 20–36. doi: 10.1002/sim.2739 17072897
22. Austin PC, Mamdani MM, Stukel TA, Anderson GM, Tu JV (2005) The use of the propensity score for estimating treatment effects: administrative versus clinical data. Stat Med 24 : 1563–1578. 15706581
23. Kumar G, Taneja A, Majumdar T, Jacobs ER, Whittle J, et al. (2014) The association of lacking insurance with outcomes of severe sepsis: retrospective analysis of an administrative database. Crit Care Med 42 : 583–591. 24152590
24. Gorey KM, Luginaah IN, Holowaty EJ, Zou G, Hamm C, et al. (2012) Effects of being uninsured or underinsured and living in extremely poor neighborhoods on colon cancer care and survival in California: historical cohort analysis, 1996–2011. BMC Pub Health 12 : 897. doi: 10.1186/1471-2458-12-897
25. Aizer AA, Falit B, Mendu ML, Chen MH, Choueiri TK, et al. (2014) Cancer-specific outcomes among young adults without health insurance. J Clin Oncol 32 : 2025–2030. doi: 10.1200/JCO.2013.54.2555 24888800
26. Greene WR, Oyetunji TA, Bowers U, Haider AH, Mellman TA, et al. (2010) Insurance status is a potent predictor of outcomes in both blunt and penetrating trauma. Am J Surg 199 : 554–557. doi: 10.1016/j.amjsurg.2009.11.005 20359573
27. Bell TM, Zarzaur BL (2013) Insurance status is a predictor of failure to rescue in trauma patients at both safety net and non-safety net hospitals. J Trauma Acute Care Surg 75 : 728–733. 24064890
28. Bustamante AV, Fang H, Garza J, Carter-Pokras O, Wallace SP, et al. (2012) Variations in healthcare access and utilization among Mexican immigrants: the role of documentation status. J Immigr Minor Health 14 : 146–155. doi: 10.1007/s10903-010-9406-9 20972853
29. Lerman-Garber I, Villa AR, Caballero E (2004) Diabetes and cardiovascular disease. Is there a true Hispanic paradox? Rev Invest Clin 56 : 282–296. 15612509
30. British Society for Antimicrobial Chemotherapy (2011) Outpatient and parenteral antimicrobial Therapy (OPAT): toolkit for developing a business case for OPAT services in the UK. Available: http://e-opat.com/wp-content/uploads/2014/11/Business_case_toolkit.pdf. Accessed 4 May 2015.
31. Rathert C, Wyrwich MD, Boren SA (2013) Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev 70 : 351–379. doi: 10.1177/1077558712465774 23169897
32. Leff B, Burton L, Mader SL, Naughton B, Burl J, et al. (2005) Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med 143 : 798–808. doi: 10.7326/0003-4819-143-11-200512060-00008 16330791
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2015 Číslo 12- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- Risks and Benefits of Nalmefene in the Treatment of Adult Alcohol Dependence: A Systematic Literature Review and Meta-Analysis of Published and Unpublished Double-Blind Randomized Controlled Trials
- Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled
- Police Killings and Police Deaths Are Public Health Data and Can Be Counted
- A Successful Failure: Missing the MDG4 Target for Under-Five Mortality in South Africa
- The Ebola Vaccine, Iatrogenic Injuries, and Legal Liability
- Progress in Medicine: Experts Take Stock
- Use of Viremia to Evaluate the Baseline Case Fatality Ratio of Ebola Virus Disease and Inform Treatment Studies: A Retrospective Cohort Study
- Acute Cardiovascular Events after Herpes Zoster: A Self-Controlled Case Series Analysis in Vaccinated and Unvaccinated Older Residents of the United States
- Moving Beyond “Food Deserts”: Reorienting United States Policies to Reduce Disparities in Diet Quality
- Public Health and International Partnerships in the Democratic People’s Republic of Korea
- Self-Administered Outpatient Antimicrobial Infusion by Uninsured Patients Discharged from a Safety-Net Hospital: A Propensity-Score-Balanced Retrospective Cohort Study
- Association between Regimen Composition and Treatment Response in Patients with Multidrug-Resistant Tuberculosis: A Prospective Cohort Study
- 10-y Risks of Death and Emergency Re-admission in Adolescents Hospitalised with Violent, Drug- or Alcohol-Related, or Self-Inflicted Injury: A Population-Based Cohort Study
- World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010
- Bariatric Surgery in the United Kingdom: A Cohort Study of Weight Loss and Clinical Outcomes in Routine Clinical Care
- Traditional and Emerging Lifestyle Risk Behaviors and All-Cause Mortality in Middle-Aged and Older Adults: Evidence from a Large Population-Based Australian Cohort
- Inequalities in Alcohol-Related Mortality in 17 European Countries: A Retrospective Analysis of Mortality Registers
- World Health Organization Estimates of the Global and Regional Disease Burden of 11 Foodborne Parasitic Diseases, 2010: A Data Synthesis
- A Molecular Host Response Assay to Discriminate Between Sepsis and Infection-Negative Systemic Inflammation in Critically Ill Patients: Discovery and Validation in Independent Cohorts
- World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Self-Administered Outpatient Antimicrobial Infusion by Uninsured Patients Discharged from a Safety-Net Hospital: A Propensity-Score-Balanced Retrospective Cohort Study
- Risks and Benefits of Nalmefene in the Treatment of Adult Alcohol Dependence: A Systematic Literature Review and Meta-Analysis of Published and Unpublished Double-Blind Randomized Controlled Trials
- World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010
- Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



